The current pandemic of COVID-19 caused by the SARS-CoV-2 virus that started in the city of Wuhan in China in December 2019 has infected over 23.9 million people globally and caused over 819,000 deaths (as on August 26, 2020). The elderly and those with comorbid conditions have the greatest risk of developing the severe form of the disease that manifests with pneumonia and severe acute respiratory syndrome. There is no definite treatment for COVID-19 as of now. Prevention of viral transmission by rapid isolation and disease containment measures such as social distancing, hand hygiene, and wearing of masks have limitations. Over 100 different vaccines are in the development phase in different parts of the world, but it is difficult to predict how many of them will succeed, and even when they do, it will be a long time until they will be made available for everyone. There is therefore an urgent need to find new drugs that are effective against COVID-19. Developing new drugs from scratch is not a practical solution because it not only costs millions of dollars, but also takes a long time (usually a decade).
Repositioning (re-purposing) of already approved drugs for other medical conditions is one way of overcoming this urgent need. At the moment, there are at least 50 such drugs that are being investigated for COVID-19 through more than 300 ongoing clinical trials in different parts of the world. Some of these drugs include antivirals that had been developed for other viruses (remdesivir, ribavirin, favipiravir, amantadine, etc.,), anti-inflammatory that have been used for other chronic inflammatory disorders (hydroxychloroquine, chloroquine, etc.,) and biologicals developed for other inflammatory states (tocilizumab, etc). While some of these drugs have shown modest benefit in the preliminary results, for example, remdesivir, the final results of many such clinical trials are still awaited with much expectation.
FIVE CASE REPORTS THAT SHOWED UNUSUAL BENEFICIAL EFFECTS OF INHALED CICLESONIDE IN COVID-19
On March 9, 2020, Iwabuchi et al. from Kanagawa Prefectural Ashigarakami Hospital in Japan submitted a case-series of three elderly male and female patients admitted to their hospital with COVID-19 pneumonia and respiratory failure, who were successfully treated with inhaled ciclesonide (800–1200 mcg/day).[1] All the three elderly patients had travelled in the Diamond cruise ship and presented with fever and high-resolution computed tomography scan showing features of typical COVID pneumonia (peripheral ground glass opacities) [Figure 1]. Their symptoms were not getting relieved despite giving them all available drugs, including lopinavir/ritonavir. The hospital did not have ventilators, neither could they transfer these patients to other hospitals because of lack of availability of beds. In this situation of crisis, the physicians discussed at their “Emergency Expansion Meeting” about the possibility of starting inhaled ciclesonide as a therapeutic trial. They had heard about the potential role of ciclesonide as an antiviral against SARS-CoV-2 along with its known anti-inflammatory effects, and therefore started these three patients on ciclesonide. All the patients recovered rapidly without requiring a ventilator.
Figure 1.
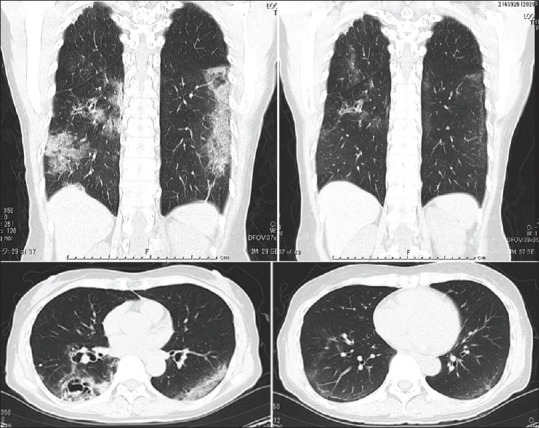
Sagittal and coronal high-resolution computed tomography images of a COVI-19 patient showing pneumonia that resolved after starting inhaled ciclesonide (obtained from reference no.1)
On the March 27, 2020, Nakajima et al.[2] from the Yokohama City University Hospital from the same city of Kanagawa in Japan submitted a case report of a 64-year-old, SARS-CoV-2-positive Japanese taxi driver who was hospitalized for fever, cough, and breathing difficulty, who was again successfully treated with ciclesonide. His computed tomography (CT) scan showed the classical bilateral peripheral ground glass opacities with infiltrating shadows consistent with COVID-19 pneumonia. The combination of lopinavir and ritonavir did not improve his condition and he had to be put on a ventilator. Having heard the success story of the three patients above, they started the patient empirically on inhaled ciclesonide. To their surprise, his oxygenation improved the next day and he was gradually weaned off the ventilator, extubated, and discharged home.
A fifth case study was reported by Ito et al.[3] of a 40-year-old, Sars-CoV-2-positive female patient from the Asahi Rosai Hospital in Aichi, Japan, with a COVID-19 pneumonia successfully treated with ciclesonide (1200 mcg/day) which was started on the 1st day along with Favipiravir.
Although the above five cases suggest an unusual beneficial role of inhaled ciclesonide in the management of COVID-19, it could be argued that this observation could have occurred by chance and that the patients were already receiving anti-viral drugs whose action may have kicked in later. Moreover, ciclesonide which is an inhaled corticosteroid may intuitively worsen the underlying viral infection, as steroids are known to do that. So, did ciclesonide really work against COVID-19, and if at all it did, what could have been the underlying molecular mechanism(s)?
Halpin et al.[4] reported an interesting observation that the prevalence of asthma among patients with SARS and COVID-19 seems surprisingly lower than among the general population, and he went on to suggest that either the lung disease, patients' behavior or, more likely, their treatments may have had some protective effects.
JAPANESE RESEARCHERS SHOW THAT CICLESONIDE INHIBITS SARS-COV-2 REPLICATION BY INTERACTING WITH THE VIRAL PROTEIN NONSTRUCTURAL PROTEIN 15
Matsuyama et al.[5] from the Department of Virology (I and III), National Institute of Infectious Diseases in Tokyo, Japan, were researching on the antiviral effects of different steroid molecules. In a report published on March 12, 2020, they showed that while cortisone, prednisone, dexamethasone, and fluticasone demonstrated no antiviral effects, mometasone and ciclesonide almost completely suppressed the growth of SARS-CoV-2 at low micromolar concentrations without affecting the host epithelial cell viability [Figure 2]. They also showed that ciclesonide and mometasone suppressed replication of other coronaviruses, HCoV-229E and SARS-CoV, but had no effect on rhino and influenza viruses. Ciclesonide also showed a slight but significant viral growth suppressing effect on the rubella virus. They subsequently went on to show that ciclesonide interacted with the nonstructural protein 15 (NSP15) of the SARS-CoV-2 virus, thereby inhibited its replication.
Figure 2.
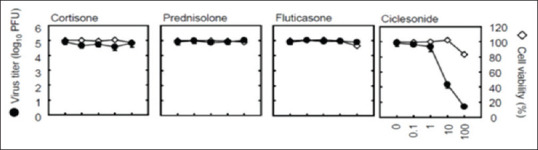
Ciclesonide showing anti-SARC-CoV-2 effects (open circles showing cell viability and closed circles showing SARS-CoV-2 viral titer (adapted from reference no. 5)
SOUTH KOREAN RESEARCHERS SHOW CICLESONIDE HAS ANTIVIRAL EFFECTS AGAINST MERS-COV AND SARC-COV-2
In 2003, the SARS-CoV, and in 2015, the MERS-CoV corona viruses caused significant morbidity and mortality in South Korea. A group of scientists working at the Respiratory Virus Laboratory, Institut Pasteur Korea, in Gyeonggi, South Korea, were researching on repurposing existing drugs for the treatment of MERS-CoV. Using bioinformatic tools, they screened 5,406 compounds containing the United States Food and Drug Administration (US FDA) approved drugs, bioactives, kinase inhibitors, and natural products to identify potential inhibitors of the MERS-CoV virus. Among these, 256 compounds demonstrated >70% MERS-CoV inhibition and >70% cell viability. These were further refined to 54 compounds that showed maximum therapeutic index. Among these, 12 were the US FDA approved drugs. Niclosamide (anti-helminthic) and ciclesonide (corticosteroid) showed the most promising anti-viral effects against MERS-CoV.[6]
More recently, the same group expanded their scope to cover the SARS-CoV-2 virus and in a subsequent publication[7] reported 24 drugs that had potential anti-viral activities. Among these, niclosamide and ciclesonide were again found to show very potent anti-viral activity against SARS-CoV-2 [Figure 3].
Figure 3.
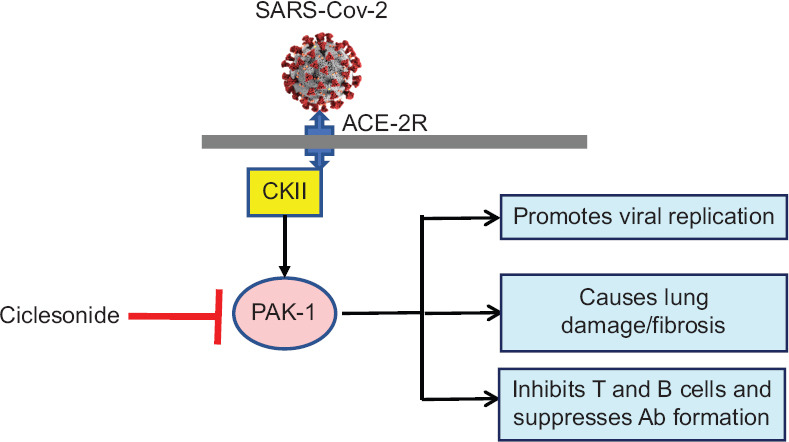
Possible mechanisms of action of ciclesonide in COVID-19 through inhibiting PAK-1. PAK-1 = Serine-threonine kinase, CKII = Kinase, ACE-2R = Angiotensin converting enzyme receptor-2
OTHER BIOINFORMATIC STUDIES IDENTIFY CICLESONIDE TO POSSESS ANTI-SARC-COV-2 EFFECTS
Pathak et al.[8] from the Gautam Buddha University, Noida, India, used a computer aided drug designing approach to identify novel candidates for repurposing for COVID-19 based on docking studies and selected drugs that were ranked for potential efficacy against COVID-19 based on their binding energy. Based on this in silico analysis, they identified rifampicin followed by ciclesonide as the most promising drugs for COVID-19, both of which showed better anti-viral effects than lopinavir and ritonavir.
Researchers from the University of Belgrade and Texas University used a novel in silico method to screen potential drugs for COVID-19. Among the 57 drugs that they identified, they reported that one of the best ranked allosteric inhibitors from their computational study was ciclesonide.[9]
Using computational studies, Sumalopao et al. from the Philippines also identified anti-viral properties of ciclesonide for SARS-CoV-2. At an IC50of 4.33 μM, ciclesonide inhibited SARC-CoV-2 and at an EC90of 6.3 μM it completely inhibited SARS-CoV-2 replication.[10] These concentrations are easily achieved by the drug in the lung following inhalation.
CICLESONIDE BLOCKS PAK-1 THE MAJOR PATHOGENIC KINASE ASSOCIATED WITH SARS-COV-2
PAK-1 is a major pathogenic kinase that is widely associated with a variety of disease states such as cancers, inflammation, viral infection, immunosuppression, and aging.[11] PAK-1 present in human cells gets activated by different viruses, including SARS-CoV-2, that not only leads to enhancing viral replication, but also releases key inflammatory mediators that cause lung damage and switches off the cell-mediated immunity so that antiviral antibody production is inhibited. PAK-1 inhibitors have therefore been suggested to have a potential role in the management of COVID-19. Some of the well-known PAK-1 inhibitors include propolis, melatonin, hydroxychloroquine, ivermectin, and ketorolac. Ciclesonide has been recently shown to possess PAK-1 inhibiting properties.[11] Ciclesonide has been recently shown to have anti-lung cancer properties, an effect that has been shown to be mediated through inhibiting PAK-1.[12]
POSSIBLE MECHANISMS OF ACTION OF CICLESONIDE FOR COVID-19
Pooling all the above observations, it is tempting to speculate that ciclesonide produces its anti-SARS-CoV-2 effects by the following molecular mechanisms (a) inhibition of NSP15, the structural protein present in SARS-CoV-2 that is associated with viral replication, survival and inhibition of synthesis of anti-viral interferons,[13](b) blocking of PAK-1, which inhibits SARS-CoV-2 viral replication, inhibits the pro-inflammatory pathways associated with lung damage and enhances the production of virus specific antibodies and (c) ciclesonide as a known corticosteroid has anti-inflammatory effects that suppresses the inflammatory mediators, such as interleukin 6 released from lung epithelial cells, thereby reducing cytokine storm, an immune inflammatory over-reaction.
WHAT IS CICLESONIDE?
Ciclesonide is an inhaled corticosteroid that was developed and patented by Altana, a German Pharma in 1990 and subsequently approved by the US FDA for use in patients with asthma in 2006. It was later bought by Astra Zeneca who sold it to Covis, a Swiss-based pharma company in 2018. It is a pro-drug that gets converted to its active metabolite, des-isobutyl ciclesonide in the lung by esterase enzymes present in the airway epithelial cells. It is a unique inhaled corticosteroid because it has a very small particle size (around 1 micron in mean aerodynamic diameter) and therefore has very high deposition (around 50%) in the distal portion of the lungs. The other traditional inhaled corticosteroids used in the treatment of asthma, such as budesonide and fluticasone have larger particle sizes and only a very small portion of these drugs get deposited in the distal portions of the lung. Due to high protein binding and rapid clearance, ciclesonide has one of the best safety profiles among inhaled corticosteroids. Despite being a very effective and safe inhaled corticosteroid for asthma, it got overshadowed by budesonide and fluticasone that were introduced earlier and had an accompanying long-acting beta-agonist that enhanced its efficacy. It is however, still widely used in the management of Asthma.
Having seen the successful case reports of ciclesonide in Japan and a strong underlying molecular mechanism to support its effects against COVID 19, many countries have initiated clinical trials for COVID-19 with inhaled ciclesonide, including the USA, UK, Sweden, South Korea, Japan, Australia, and India, the results of which are awaited eagerly. Whether ciclesonide will fulfill its expectation as a novel drug in the treatment of COVID-19, only time will tell.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: Report of three cases. J Infect Chemother. 2020;26:625–632. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajima K, Ogawa F, Sakai K, Uchiyama M, Oyama Y, Kato H, et al. A case of coronavirus disease 2019 treated with ciclesonide. Mayo Clin Proc. 2020;95:1296–7. doi: 10.1016/j.mayocp.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito K, Yokoyama T, Oguri A, Kato C, Horiuchi M, Kato M, et al. Case report: A case of COVID-19 pneumonia that did not worsen and was relieved by early administration of Favipiravir and Ciclesonide. 2020. [Last accessed on 2020 Aug 26]. Available from: http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200406.pdf .
- 4.Halpin DM, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–8. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawase M, Naro N, Shirato K, Ujike M, Kamitani W, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP-15. bioRxiv. 2020. [Last accessed on 2020 Aug 26]. Available from: https://www.biorxiv.org/content/10.1101/2020.03.11.987016v1.full.pdf .
- 6.Adzerikho RD, Aksentsev SL, Okun' IM, Konev SV. Letter: Change in trypsin sensitivity during structural rearrangements in biological membranes. Biofizika. 1975;20:942–4. [PubMed] [Google Scholar]
- 7.Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64:e00819–20. doi: 10.1128/AAC.00819-20. https://doi.org/10.1128/AAC.00819-20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathak M, Mishra A, Tripathi V. Rifampicin may be repurposed for COVID-19 treatment. Insights from an in-silico study, PREPRINT (Version 1) [Last accessed on 2020 Apr 14]. Available from: https://doi.org/10.21203/rs.3.rs-22546/v1 .
- 9.Sencanski M, Perovic V, Pajovic S, Adzic M, Paessler S, Glisic S. Drug repurposing for candidates SARS-CoV-2 main protease inhibitors by a novel in silico method. Chem Rxiv. 2020;25:3830. doi: 10.3390/molecules25173830. doi:10.3390/molecules25173830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumalapao DE. Elucidation of the physico-chemical properties of potential an clinically approved antiviral drugs. A search for effective therapies against SARS-CoV-2 infection. J Pure Appl Microbiol. 2020;14:1025–34. [Google Scholar]
- 11.Maruta H, He H. PAK-1 blockers; Potential therapeutics against COVID-19? Med Drug Discov. 2020 Jun;6:100039. doi: 10.1016/j.medidd.2020.100039. doi: 10.1016/j.medidd.2020. Epub 2020 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi HS, Kim SL, Kim JH, Lee DS. The FDA-approved anti-asthma medicine ciclesonide inhibits lung cancer stem cells through hedge-hog signalling-mediated SOX-2 regulation. Int J Mol Sci. 2020;21:1014. doi: 10.3390/ijms21031014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng X, Hackbart M, Mettekman RC, O'Brien A, Mielech AM, Yi G, et al. Coronavirus non-structural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. PNAS. 2017;114:E4251–60. doi: 10.1073/pnas.1618310114. [DOI] [PMC free article] [PubMed] [Google Scholar]


