Abstract
Vitamins D have various biological activities, as well as intestinal calcium absorption. There has been recent concern about insufficient vitamin D intake. In addition to vitamins D2 and D3, there are lesser-known vitamins D4–D7. We synthesized vitamins D5–D7, which are not commercially available, and then evaluated and compared the mixed micelles-solubilized vitamins D uptake by Caco-2 cells. Except for vitamin D5, the uptake amounts of vitamins D4–D7 by differentiated Caco-2 cells were similar to those of vitamins D2 and D3. The facilitative diffusion rate in the ezetimibe inhibited pathway was approximately 20% for each vitamin D type, suggesting that they would pass through the pathway at a similar rate. Lysophosphatidylcholine enhanced each vitamin D uptake by approximately 2.5-fold. Lysophosphatidylcholine showed an enhancing effect on vitamin D uptake by reducing the intercellular barrier formation of Caco-2 cells by reducing cellular cholesterol, suggesting that increasing the uptakes of vitamins D and/or co-ingesting them with lysophosphatidylcholine, would improve vitamin D insufficiency. The various biological activities in the activated form of vitamins D4–D7 were estimated by Prediction of Activity Spectra for Substances (PASS) online simulation. These may have some biological activities, supporting the potential as nutritional components.
Keywords: Caco-2 cells, intercellular barrier formation, intestinal uptake, lysophosphatidylcholine, PASS, mixed micelles, vitamin D
1. Introduction
Vitamin D is required for intestinal absorption of calcium and phosphorus and has an important role in maintaining homeostasis of bone. Vitamin D deficiency causes rickets for children and osteomalacia for adults. In the elderly, vitamin D is an important nutritional component for prevention of osteoporosis, frailty, and locomotive syndrome.
Geographical and seasonal factors are well known to limit the biosynthesis of vitamin D3 by sun exposure. Especially in high latitudes > 35, the irradiation intensity of sunlight UV is weak, which is thought to reduce biosynthesis of vitamin D3 to insufficient quantities [1]. In addition, sunscreen with a high sun protection factor significantly inhibits vitamin D3 biosynthesis regardless of latitude, even in summer [2]. Aging also causes a decrease in the ability to produce 7-dehydrocholesterol, a vitamin D3 precursor [3]. Conversely, sunlight also has risks to the appearance and health of humans. UV reportedly can induce skin cancer [4], wrinkle formation [5,6,7], macular degeneration of the retina, and cataracts [8], suggesting that a vitamin D supply that is too dependent on sunlight may cause vitamin D deficiency due to habits, lifestyle, geography, and aging.
On the other hand, vitamin D is found in some foods. The anti-rickets component in cod liver oil was revealed to be vitamin D3 in 1936 [9]. However, fish oil has a flavor and odor problem, so direct intake has not generally been preferred. Other than cod liver oil, rats studies in the 1920s showed that coconut oil [10], alfalfa, and clover [11] contained an anti-rickets component, but the identity of the component was not unknown. UV irradiation has an anti-rickets effect not only in humans and animals, but also in foods. Although wheat, lettuce, cottonseed oil, linseed oil [12], yeast [13], cow and goat milk [14], lard, olive oil [15], and malt [16] did not exhibit anti-rickets activity by themselves, exposure of these foods to UV irradiation produced anti-rickets activity, but vitamin D had not yet been discovered at the time of these studies. These foods were thought to contain some precursors that were converted to vitamin D by UV. In 1926, ergosterol contained in mushrooms or fungi was found to be a precursor of vitamin D2 [17].
Even before the isolation of vitamin D2, fortified foods containing anti-rickets factor (vitamin D2) produced by UV irradiation to foods were developed and helped prevent onset of the disease [18]. However, foods that naturally contain vitamin D are very limited among readily available foods and at present include fish, shellfish, mushrooms, and eggs. Since vitamin D is usually not found in fruits and vegetables, for vegans and vegetarians and even people who eat meat and seafood, their eating habits may not ingest enough vitamin D from their diets. In fact, a large-scale cohort study showed that 81.3% of Japanese were vitamin D deficient [19]. In mothers, it has been found to affect subsequent fetal development [20,21]. Therefore, it is important to keep vitamin D levels inside the body by somehow continuously ingesting vitamin D from foods. We believe that this can be achieved by two methods: (i) increasing the intake of diverse foods containing vitamin D and (ii) enhancing the intestinal absorption of vitamin D (in other words, improved intestinal absorption even with few intake opportunities).
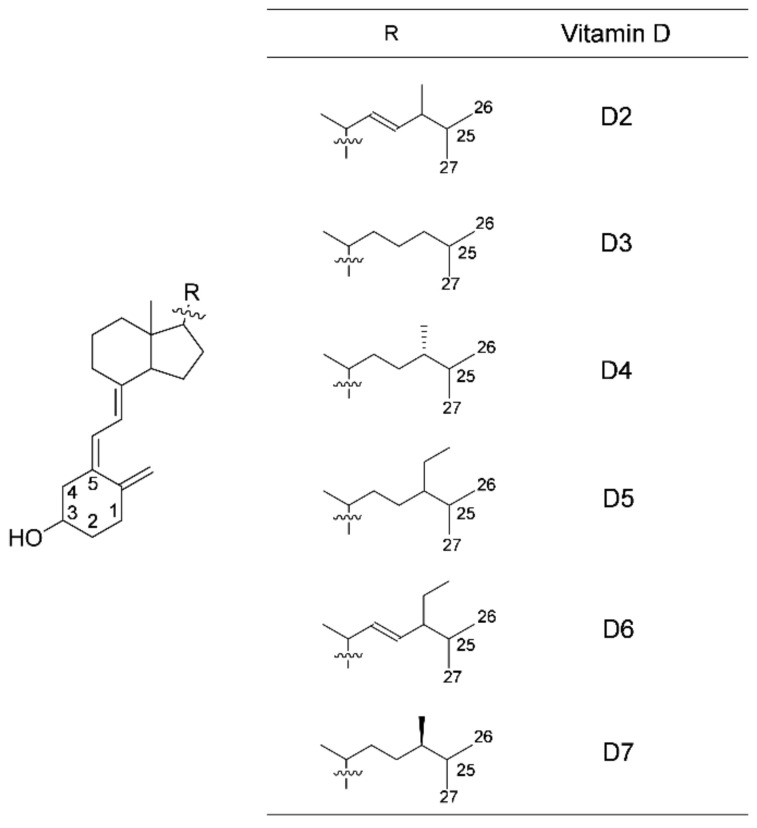
(i): In addition to vitamins D2 and D3, there are vitamins D4–D7, although they are not well known (Figure 1). There are many unclear points about what kinds of foods contain these forms and in what amounts. Ingestion of various types of vitamin D from diverse foods may lead to increased opportunities for intake of vitamin D. Vitamin D has poor bioavailability as do other fat-soluble components, such as carotenoids. After ingesting foods with certain compounds, such as carotenoids, vitamin D is also considered to be released from the matrix of food in the digestive tract and incorporated into an emulsion dispersed with bile and fats derived from the food. By pancreatic juice action, vitamin D is solubilized in mixed bile salt micelles that are absorbed in the intestinal tract. We have previously studied the intestinal absorption of carotenoids [22,23,24,25,26] and, here, have tried to apply a similar method to the study of intestinal absorption of vitamin D. Unfortunately, vitamins D5–D7 are not commercially available. Therefore, we have recently obtained these vitamins D by organic synthesis from commercial plant sterols as starting materials via a 7-dehydro form [27]. In the present study, to investigate the intestinal absorption of six types of vitamin D, which were commercially available vitamins D2–D4 and organically synthesized vitamins D5–D7, we evaluated and compared the uptake of vitamin D solubilized in mixed micelles composed of taurocholate, monoacylglycerol, fatty acid, and phospholipid by intestinal model Caco-2 cells.
Figure 1.
Chemical structures of vitamins D. The numbers in the structural formula indicate the locations of the carbons.
(ii): Enhancing the intestinal absorption of vitamin D that is, adopting an efficient absorption method, even if the intake opportunity is small, would help prevent vitamin D deficiency. Our group has previously evaluated 28 kinds of polar lipids, such as glycerophospholipids and glyceroglycolipids, involved in the uptake and/or secretion of carotenoids [22,23,24,25,28]. Among the 28 kinds, 1-16:0-2-OH-sn-glycerol-3-phosphocholine (lysophosphatidylcholine) showed the strongest enhancing effect. We have also revealed that cell-cell adhesion/cell-matrix adhesion was involved in the mechanism of the enhancing effect [24,25]. In other words, uptake and secretion were enhanced by the influence of lysophosphatidylcholine on cells other than mixed micelles. This finding suggests that the enhancing effect of lysophosphatidylcholine also can be applied to fat-soluble components other than carotenoids. In the present study, we also determined if lysophosphatidylcholine raises the uptake of vitamin D by Caco-2 cells. Regarding the enhancing effect mechanism, we previously predicted that lysoglycerolipids decreased cellular cholesterol and regulated cell-cell adhesion [24,25]. In the present study, we measured changes in cellular cholesterol concentrations by lysophosphatidylcholine in mixed micelles.
In addition to the above (i) and (ii), the following was studied.
In addition to the anti-rickets activity, in recent years, various biological functions of vitamin D have been attracting attention. A low vitamin D blood level or deficiency reportedly is linked with a heightened risk of various diseases/illnesses, including pregnancy diseases [29,30,31,32], virus diseases [33,34,35,36,37,38], cancers [39,40,41,42,43,44,45,46,47,48], vascular diseases [49,50,51,52], pulmonary diseases [53,54,55,56,57,58], metabolic disorders [59,60,61,62,63], cognition disorders [64,65,66,67,68,69], psychiatric disorders [70,71,72,73], reduction of exercise capacity [74,75], and reproductive function [76,77]. A relationship between maternal plasma levels of vitamin D during pregnancy and development of attention-deficit hyperactivity disorder-like symptoms in offspring has also been suggested [78,79]. It is also believed that the immunomodulatory effects of vitamin D may improve the clinical signs of coronavirus disease 2019 (COVID-19) patients at this very moment [80]. However, vitamin D itself has no biological activity but can exert its function after being metabolically converted; that is, it is activated by introducing an OH group at the C25-location in the liver and at the C1-location in the kidney. The amounts obtained in organic synthesis of vitamins D5–D7 used in the present study were very small. To subject these vitamins D to any functional test, the vitamin D needs to be further converted into an activated form, but it was difficult for us to secure adequate amounts. Therefore, we estimated the biological activities of vitamin D by using Prediction of Activity Spectra for Substances (PASS) online software, as we have used in the past [81]. However, there is no intention here to regard activated vitamins D as drugs, but only to discuss the biological activities after ingestion as food components.
2. Materials and Methods
2.1. Materials
Vitamin D2 was purchased from Nacalai Tesque (Kyoto, Japan). Vitamin D3 was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Vitamins D5–D7 were organically synthesized as described in our previous report [27]. Vitamin D4, β-sitosterol, vitamin K1, Trolox, fetal bovine serum (FBS), and 1-16:0-2-OH-sn-glycerol-3-phosphocholine (lysophosphatidylcholine) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The FBS was heat-inactivated and then used. Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Nissui Pharmaceutical (Tokyo, Japan). The other chemicals and solvents were of reagent grade.
2.2. Cell Culture and Differentiation
Caco-2 cells were purchased from the American Type Culture Collection (Rockville, MD, USA). DMEM containing 10% FBS, 4 mM L-glutamine, penicillin and streptomycin (40 units/mL and 40 μg/mL, respectively), and nonessential amino acids (0.1 mM) were used as the culture medium. The cells were grown in the culture medium at 37 °C in a humidified atmosphere of 5% CO2 in air and then were cultured by passaging two times per week. We used differentiated Caco-2 cells, which have been widely used as a human intestinal model, for vitamin D uptake experiments. To induce differentiation of Caco-2 cells, the cells were seeded in culture plate of 24-well at a density of 5 × 104 cells per well containing 0.5 mL of the culture medium. Then, the medium was changed with fresh medium two times per week for 20–22 d. We have previously reported that the cell monolayers obtained by this method had sufficient cell-cell adhesion [24].
2.3. Preparation of Mixed Micelles Containing Vitamin D
The mixed micelles were prepared as described in our previous reports [22,23,24,25]. All of the components, i.e., taurocholate, monoolein, oleic acid, lysophosphatidylcholine, and vitamin D, were dissolved in an appropriate solvent. Portions of these solvent solutions were moved to glass tubes. The solvent was dried up with argon gas and further centrifugal evaporator. The dry matter was dispersed into FBS-free DMEM (same as the culture medium described above but without FBS). To remove any vitamin D not solubilized in the micelles, the resulting solution was passed through a filter (the pore size was 0.2-μm). The materials of mixed micelle and their concentrations were taurocholate (2 mM), monoolein (100 μM), oleic acid (33 μM), lysophosphatidylcholine (0 or 50 μM), and vitamin D (1 μM), respectively, unless otherwise stated. The concentrations were same as those used in our previous study [22,23,24]. Before adding the mixed micelles to the Caco-2cells, the initial concentration of vitamin D was checked as follows. One part of the mixed micelles was diluted with four parts of methylene chloride/methyl alcohol (1:4, v/v), and then a portion (30 μL) was analyzed by high-pressure liquid chromatography (HPLC) as explained in the “2.8. HPLC Analysis” subsection below. The initial concentration throughout all experiments was 1.04 ± 0.06 μM.
2.4. Evaluation of Vitamin D Uptake by Differentiated Caco-2 Cells from Mixed Micelles
Differentiated Caco-2 cell monolayers were washed two times with FBS-free DMEM (0.5 mL) and then treated with medium consisting of mixed micellar vitamin D (0.5 mL) at 37 °C for 2 h in a CO2 incubator. After treatment, the following operations were continued to measure i) the amount of vitamin D uptake by the cells and ii) the residual amounts of mixed micellar vitamin D in the medium.
(i): The cells were washed with 0.5 mL of 10 mM taurocholate in phosphate-buffered saline (PBS) and then washed two times with PBS. They were homogenized in 1 mL PBS containing 0.01 mL of 0.2 mM Trolox/methyl alcohol, which is an antioxidant, by using a probe-type sonicator. An aliquot (0.8 mL) of the cell homogenate was mixed with 0.2 mL of 1 μM vitamin K1/ethyl alcohol, which was used as an internal standard. Subsequently, ethyl alcohol (0.6 mL), ethyl acetate (0.8 mL), and n-hexane (0.8 mL) were added, and the mixture was shaken with a Vortex mixer after each addition. The upper layer was collected, and ethyl acetate and n-hexane were added to the lower layer and shaken in the same manner. This upper layer was combined with the first upper layer. The combined upper layers were dried in a centrifugal evaporator. The extract was dissolved in 200 μL of methylene chloride/methyl alcohol/H2O (9:81:10, v/v/v), and an aliquot (40 μL) was then subjected to HPLC as described in the “2.8. HPLC Analysis” subsection below. The recovery of the vitamin D spiked into a suspension of differentiated Caco-2 cells was 100.3% ± 1.2% (n = 4). To determine the cellular protein content, an aliquot (50 μL) of the cell homogenate was subjected to the Lowry method [82].
(ii): The micellar vitamin D decreased by agglomeration might have influenced the uptake of vitamin D from mixed micelles to cells because only micellar vitamin D could be taken up by Caco-2 cells, as shown by the agglomeration of carotenoids described in our previous report [22]. Parts of the medium collected at the end of the incubation were passed through a filter, as described above. One part filtrate was diluted with four parts methylene chloride/methyl alcohol (1:4, v/v), and then 40-μL was injected for HPLC analysis as described in the “2.8. HPLC Analysis” subsection below.
2.5. Evaluation of Facilitated Diffusion on Vitamin D Uptake by Differentiated Caco-2 Cells from Mixed Micelles
Although involvement of facilitated diffusion in intestinal absorption of vitamin D3 has been previously reported [83], other vitamins D have not been tested. In the present study, we evaluated the involvement of facilitated diffusion in the cellular uptake of each mixed micellar vitamin D2–D7 by using ezetimibe, an inhibitor of the protein Niemann-Pick C1-like 1 (NPC1L1) [84]. Ezetimibe was dissolved in dimethyl sulfoxide (DMSO), and then was added at a final concentration of 150 μM to the culture medium. At this time, the final concentration of DMSO was 1.5% (v/v). The differentiated Caco-2 cells were preincubated at 37 °C for 2 h with ezetimibe-containing culture medium (0.5 mL). The cells were washed two times with FBS-free DMEM (0.5 mL) and then treated with the medium containing mixed micellar vitamin D (0.5 mL) for 2 h, as described above. After treatment, cellular vitamin D, residual mixed micellar vitamin D, and the cellular protein content were evaluated as described in the previous subsection.
2.6. Impact of Cell-Cell Adhesion/Cell-Matrix Adhesion in Caco-2 Cells on Vitamin D Uptake
Since 2001, we have reported that lysophospholipids enhance the uptake of micellar carotenoids in Caco-2 cells [22,23,24,85]. In 2015, we demonstrated that cell-cell adhesion and cell-matrix adhesion were involved in the mechanism of its enhancing effect [24]. In the present study, using the same method, we examined whether the mechanism for the enhancing effect of lysophosphatidylcholine could be adapted to vitamin D, as well. To evaluate the impact of the degree of cell-cell adhesion/cell-matrix adhesion on vitamin D uptake by cells, we prepared in two different ways: i) dispersed Caco-2 cells without cell-cell adhesion/cell-matrix adhesion and ii) Caco-2 cells with insufficient cell-cell adhesion.
(i): The dispersed cells were prepared by trypsin treatment. After collecting the cells in the culture medium, the number of cells was counted and diluted with the medium. The cells were washed two times with FBS-free DMEM and then seeded in culture plate of 24-well for suspension cultures (SC plate; Greiner Bio-One, Frickenhausen, Germany) at a density of 15 × 104 cells per well containing the FBS-free DMEM (0.5 mL).
(ii): The cells that had insufficient cell-cell adhesion were prepared by reducing the culture period from 20–22 d for full differentiation to <7 d after the seeding described above.
Subsequent operations are common to the two models of cells. The medium consisting of mixed micellar vitamin D (0.5 mL) was added to adhered cells or dispersed cells in individual wells containing FBS-free DMEM (0.5 mL), resulting in a total medium volume of 1.0 mL/well, and the final start concentrations of mixed micellar components were one-half the initial concentration. The cells were incubated in the same conditions as described above, i.e., in a CO2 incubator at 37 °C for 2 h, and then the cells were washed with 2 mM taurocholate/PBS, followed by two times with PBS. The vitamin D uptake and residual mixed micellar vitamin D were evaluated as the previous subsection, but the filtrate of residual mixed micellar vitamin D was diluted with a 2x-volume of H2O. The cellular protein content was also determined as above.
2.7. Evaluation of Cellular Cholesterol in the Differentiated Caco-2 Cells Treated with the Mixed Micelles Containing Lysophosphatidylcholine
Mixed micelles were prepared and treated with the cells in the same manner as in the experiment using differentiated Caco-2 cells, but the concentration of lysophosphatidylcholine was varied from 0 to 250 μM, and the micelles did not include vitamin D. After treatment, the cells were washed and homogenized. The saponification reaction was performed as follows. A portion (0.8 mL) of the cell homogenate was mixed with 200 μL of 40 μM β-sitosterol/ethyl alcohol as an internal standard, 85 μL of 10 M KOH, and 0.6 mL ethyl alcohol and then incubated at 60 °C for 2 h under argon gas. After cooling the reaction solution, cholesterol was extracted with ethyl acetate and n-hexane in the same manner as the extraction of vitamin D described above. The extract was additionally washed with 0.8 mL H2O before the solvent completely dried and then dissolved in 200 μL of methylene chloride/methyl alcohol/H2O (9:81:10, v/v/v). An aliquot (40 μL) was then subjected to HPLC as described in the “2.8. HPLC Analysis” subsection below. The cellular protein content was also determined as above.
2.8. HPLC Analysis
Vitamin D and cholesterol were analyzed by using the HPLC system and columns which were the same as in our previous report [24]. Mobile phase X was ethanenitrile/methyl alcohol/H2O (75:15:10, v/v/v), mobile phase Y was methyl alcohol/ethyl acetate (70:30, v/v), and mobile phase Z was methyl alcohol/isopropyl alcohol (70:30, v/v). These mobile phases contained 0.1% ammonium acetate. An isocratic analysis was performed at a flow rate of 0.2 mL/min with mobile phase X/Y (30:70, v/v) for vitamin D or mobile phase X/Z (30:70, v/v) for cholesterol. Vitamin D and cholesterol were quantified from the peak area at 265 nm and 210 nm, respectively, by using the respective calibration curve of the authentic standard.
To minimize degradation of vitamin D by light irradiation, all experiments were carried out under dim yellow light.
2.9. Statistical Methods
The data were analyzed by one-way analysis of variance, followed by the t-test, Dunnett test, or Tukey–Kramer test. p-values < 0.05 were considered significant.
2.10. Estimation of Biological Activity of Vitamin D by Online Software Simulation
According to the homepage (http://www.pharmaexpert.ru/passonline/, accessed on 26 March 2021), the biological activity of organic compounds can be predicted by PASS online software. Vitamin D is metabolized into 1,25-di(OH)-vitamin D of an activated form through a two-step metabolism. Thus, in this study, the biological activity of activated form was predicted by PASS online software, as we have previously examined with another component [81]. The result was output in both Pa values (probability to be active) and Pi values (probability to be inactive). The closer the Pa value is to 1, the higher the activity. The larger the Pi value, the more inactive it is. The biological activities shown by the Pa and Pi values among vitamins D2–D7 were compared.
3. Results
Mixed micelles without and with lysophosphatidylcholine were designated NoPC mixed micelles and lysoPC mixed micelles, respectively. In our previous studies on the carotenoid absorption by Caco-2 cells, the concentration of residual micellar carotenoids after incubation was lower than the theoretical value, especially in NoPC mixed micelles [22,23,24]. One concern was that the reduction of micellar carotenoids might reduce the carotenoid transfer from micelles into cells; in other words, this decrease might be a factor affecting cellular uptake. However, we previously investigated this issue and dispelled the concern [24,25]. In the present study, as well, the decrease in micellar vitamin D during incubation did not seem to affect the cellular uptake, but, as a precaution, the residual micellar vitamin D concentration was monitored.
3.1. Vitamin D Uptake by Differentiated Caco-2 Cells and Effect of Lysophosphatidylcholine on Uptake
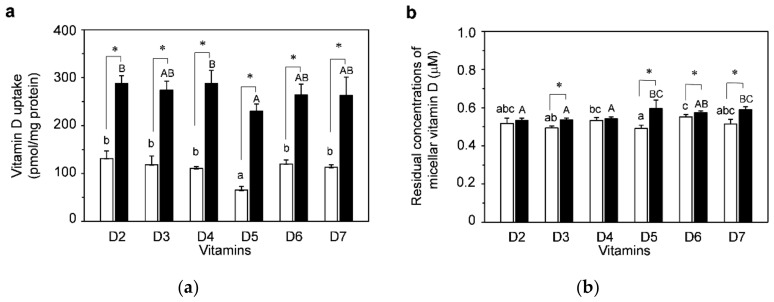
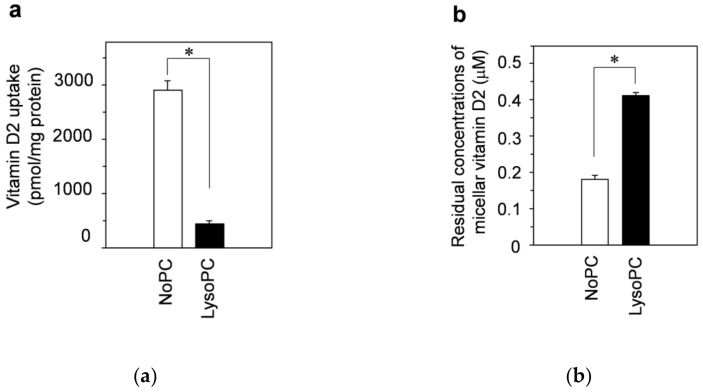
Figure 2a shows a comparison of uptakes of vitamins D2–D7 solubilized in NoPC mixed micelles (open bars) and lysoPC mixed micelles (filled bars) by differentiated Caco-2 cells. The uptake amount was lower by vitamin D5 than by other vitamins D in the NoPC mixed micelles. Compared with NoPC, lysophosphatidylcholine markedly enhanced all of the vitamin D uptakes (2.5 times on average for six vitamin D species). The vitamin D uptakes in lysoPC mixed micelles showed a trend similar to that in NoPC mixed micelles; that is, vitamin-D5 uptake tended to be lower than that of other vitamins D, but not statistically significantly different from that of vitamins D3, D6, and D7.
Figure 2.
Uptake of vitamin D solubilized in mixed micelles by differentiated Caco-2 cells and the effects of lysophosphatidylcholine. Differentiated Caco-2 cells were incubated for 2 h with vitamin D in mixed micelles without lysophosphatidylcholine and mixed micelles with lysophosphatidylcholine, called NoPC mixed micelles (open bars) and lysoPC mixed micelles (filled bars), respectively. (a) Vitamin D uptake by differentiated Caco-2 cells. (b) Residual concentrations of vitamin D in the mixed micelles (micellar vitamin D) after 2-h incubation with the cells. Data are presented as the means ± standard deviation of four wells in a single experiment. Replicate experiments showed a similar trend. Statistical analyses were performed between NoPC mixed micelle and lysoPC mixed micelle in same vitamin D, among 6 kinds of vitamins D in NoPC mixed micelles, and among 6 kinds of vitamins D in lysoPC mixed micelles in each graph. The asterisk indicates a value with significant difference as determined by unpaired t-test (p < 0.05). Values not sharing a common letter were significantly different as determined by Tukey–Kramer test (p < 0.05).
Figure 2b shows the residual concentrations of micellar vitamin D in the medium after treatment of the Caco-2 cells with vitamin D. More than half of their initial concentrations remained in all vitamins D micelles. In vitamins D3, D5, and D7 micelles, the amount was statistically higher for lysoPC than for NoPC.
3.2. Possible Involvement of Facilitated Diffusion in Vitamin D Uptake
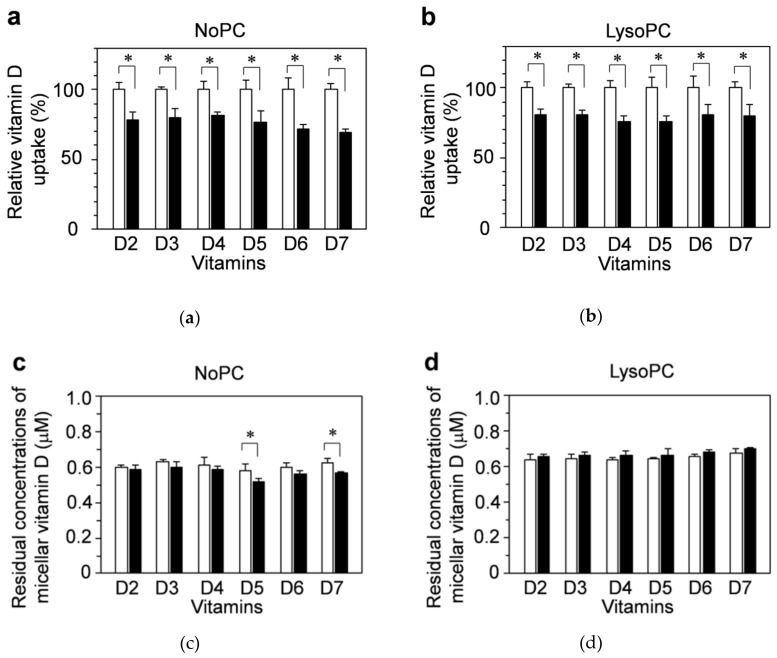
We evaluated the involvement of facilitated diffusion in the cellular uptake of vitamins D2–D7 by using an inhibitor (ezetimibe) that blocked part of the diffusion. Differentiated Caco-2 cells were pretreated with culture medium containing vehicle (1.5% DMSO) alone as a control (open bar) or 150 μM ezetimibe (filled bar) for 2 h, and then NoPC mixed micelles (a,c) or lysoPC mixed micelles (b,d) containing vitamin D were added to the cells. The vitamin D uptake amount was calculated relative to the control. Ezetimibe inhibited the uptakes of vitamins D2–D7 by approximately 20%. Similar trends were shown for the NoPC mixed micelles (Figure 3a) and lysoPC mixed micelles (Figure 3b).
Figure 3.
Inhibitory effects of ezetimibe on the uptake of vitamin D solubilized in mixed micelles by differentiated Caco-2 cells. Differentiated Caco-2 cells were pretreated for 2 h with the vehicle (1.5% dimethyl sulfoxide (DMSO), open bars) alone or with 150 μM ezetimibe (filled bars), which is an inhibitor of the protein Niemann-Pick C1-like 1 (NPC1L1), and then incubated for 2 h with vitamin D in NoPC mixed micelles (left side) and lysoPC mixed micelles (right side). (a,b): Vitamin D uptake by differentiated Caco-2 cells. (c,d): Residual concentrations of micellar vitamin D after 2-h incubation with the cells. Data are presented as the means ± standard deviation of four wells in a single experiment. Replicate experiments showed a similar trend. Statistical analysis was performed to compare each vitamin D with and without ezetimibe. The asterisk indicates a value with significant difference as determined by unpaired t-test (p < 0.05).
There were no differences in the concentrations of residual micellar vitamin D except for vitamins D5 and D7 in the NoPC mixed micelles (Figure 3c,d).
3.3. Impact of Cell-Cell Adhesion/Cell-Matrix Adhesion on Vitamin D Uptake by Caco-2 Cells
We previously reported on the mechanism by lysoglycerolipids, enhanced the cellular uptake of carotenoids [24]. Briefly, the mechanism underlying the enhancing effect involved cell-cell adhesion/cell-matrix adhesion rather than cell membrane permeability. In the present study, we investigated the impact of cell-cell adhesion/cell-matrix adhesion on vitamin D uptake by using a similar approach.
As shown in Figure 2a, differences were observed in the uptake amounts among the six types of vitamin D in both NoPC mixed micelles and lysoPC mixed micelles. The effect of cell-cell adhesion/cell-matrix adhesion that contributed to the difference in uptake among these vitamins D was evaluated in dispersed Caco-2 cells, which were prepared by trypsin treatment. Because the dispersed cells had no cell-cell adhesion/cell-matrix adhesion, we could assess its effect by comparison with the results obtained with differentiated Caco-2 cells.
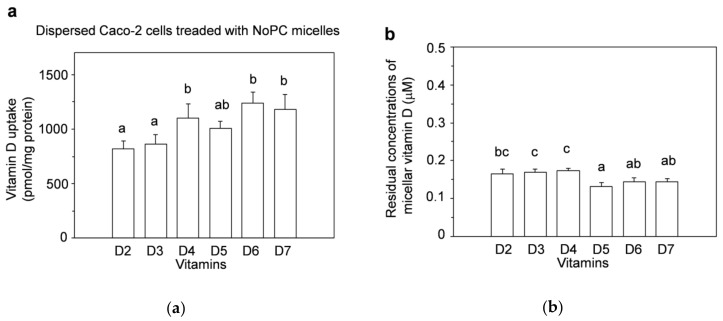
The uptake by dispersed Caco-2 cells (Figure 4a) was different from that of differentiated Caco-2 cells that were cultured for 20–22 d (Figure 2a), there was no tendency toward decreased vitamin-D5 uptake, and the uptake amounts of vitamins D4, D6, and D7 were higher than those of vitamins D2 and D3 (Figure 4a).
Figure 4.
Uptake of vitamin D solubilized in mixed micelles by dispersed Caco-2 cells. Dispersed Caco-2 cells without cell-cell adhesion/cell-matrix adhesion were incubated for 2 h with vitamin D in NoPC mixed micelles. The cells were prepared by dispersing the cells with trypsin. (a) Vitamin D uptake by dispersed Caco-2 cells. (b) Residual concentrations of the micellar vitamin D after 2-h incubation with the cells. Data are presented as the means ± standard deviation of four wells in a single experiment. Replicate experiments showed a similar trend. Statistical analysis was performed among all six samples in each graph. Values not sharing a common letter were significantly different as determined by Tukey–Kramer test (p < 0.05).
As described in the Materials and Methods section, the initial concentration of vitamin D in the mixed micelles was now half that of those in the differentiated Caco-2 cells because of the 2-fold dilution. However, the absolute amounts contained in the medium were the same in both experiments. The residual concentrations of micellar vitamin D in the medium tended to be lower in vitamins D5–D7, but approximately ≥0.3 μM remained in all types of mixed micelles (Figure 4b).
3.4. Cell-Cell Adhesion/Cell-Matrix Adhesion for Enhancing Effects of Micellar Lysophosphatidylcholine on Vitamin D Uptake: Examination in Dispersed Cells
Then we investigated the participation of cell-cell adhesion/cell-matrix adhesion in the enhancing effects of lysophosphatidylcholine on vitamin D uptake by using the dispersing Caco-2 cells. In the following experiments, vitamin D2 was used as a representative.
The open bars and filled bars in Figure 5a show the vitamin-D2 uptake amounts from the NoPC mixed micelles and lysoPC mixed micelles, respectively. The effect of lysophosphatidylcholine on the vitamin-D2 uptake by dispersed Caco-2 cells was much different from that of the differentiated Caco-2 cells. No enhancing effect of lysophosphatidylcholine on vitamin-D2 uptake by dispersed cells was observed. The vitamin-D2 uptake from lysoPC mixed micelles was approximately one-sixth that from NoPC mixed micelles.
Figure 5.
Effects of lysophosphatidylcholine on the uptake of vitamin D2 solubilized in mixed micelles by dispersed Caco-2 cells. Dispersed Caco-2 cells without cell-cell adhesion/cell-matrix adhesion were incubated for 2 h with vitamin D2 in NoPC mixed micelles and lysoPC mixed micelles. (a) Vitamin-D2 uptake by dispersed Caco-2 cells. (b) Residual concentrations of micellar vitamin D2 after 2-h incubation with the cells. Data are presented as the means ± standard deviation of four wells in a single experiment. Replicate experiments showed a similar trend. Statistical analysis was performed among all six samples in each graph. The asterisks indicate significant differences as determined by unpaired t-test (p < 0.05).
The residual concentrations of micellar vitamin D2 in the medium were lower in NoPC mixed micelles than in lysoPC mixed micelles (Figure 5b).
3.5. Cell-Cell Adhesion/Cell-Matrix Adhesion for Enhancing Effects of Lysophosphatidylcholine on Vitamin D Uptake: Examination Using Adherent Caco-2 Cells with Insufficient Cell-Cell Adhesion
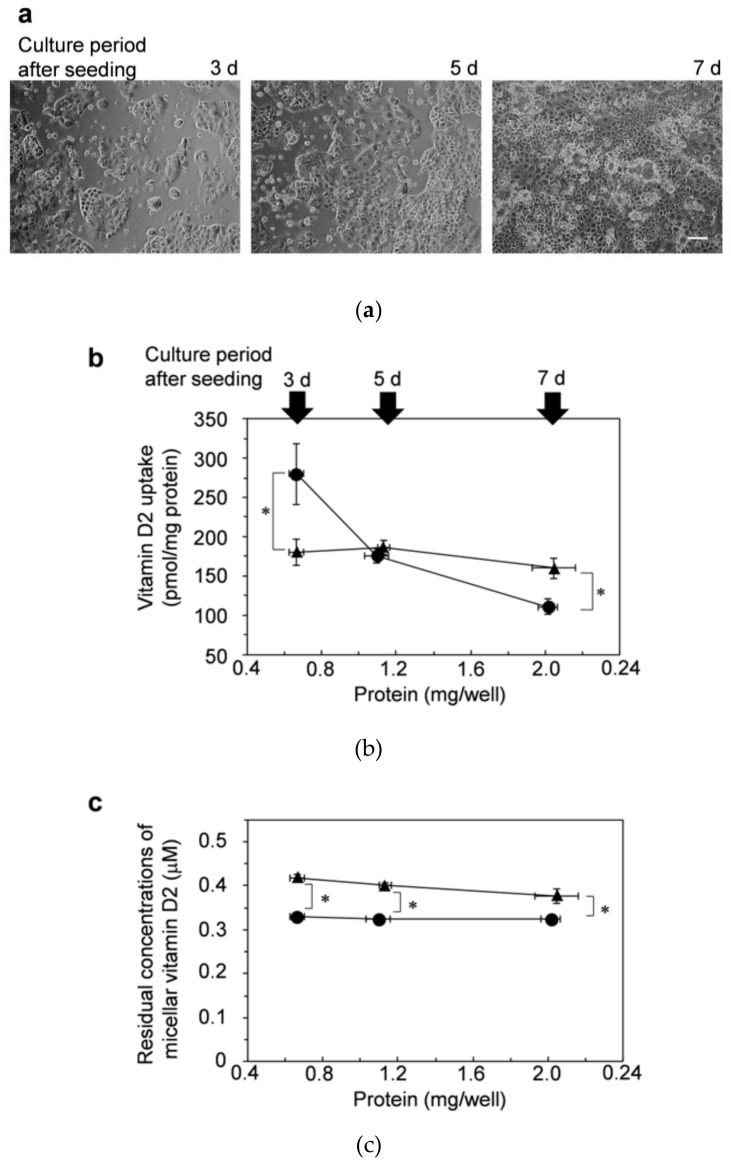
In addition to evaluations in dispersed cells, we evaluated the uptake of vitamin D2 in cells with insufficient cell-cell adhesion. The cells were cultured for 3, 5, and 7 d after seeding into 24-well plates and then incubated with vitamin D2 solubilized in mixed micelles.
As shown in Figure 6a, the cells adhered to the bottom of a plate, but there is a distance between the cell colonies cultured for 3 d. The distance was shortened after 5 d of culture. Finally, the cells reached confluence after cultivating for 7 d. Then, the cells cultured for 3 d and 5 d had insufficient cell-cell adhesion.
Figure 6.
Effects of lysophosphatidylcholine on the uptake of vitamin D2 by Caco-2 cells with insufficient cell-cell adhesion. Caco-2 cells with insufficient cell-cell adhesion/cell-matrix adhesion were prepared by cultivating for 3, 5, and 7 d after seeding in the culture plate. (a) These cells were observed by phase-contrast microscopy (100×). The white line in the photo of 7 d indicates the scale bar (100 μm). The adhered Caco-2 cells were then incubated for 2 h with vitamin D2 in NoPC mixed micelles (circles) and lysoPC mixed micelles (triangles). (b) Vitamin-D2 uptake by the adherent Caco-2 cells. (c) Residual concentrations of the micellar vitamin D2 after 2-h incubation with the cells. Data are presented as the means ± standard deviation of four wells in a single experiment. Replicate experiments showed a similar trend. Statistical analysis was performed between two mixed micelles at the same cultivation time (3, 5, and 7 d). The asterisk indicates a value with significant difference as determined by unpaired t-test (p < 0.05).
The results shown by using cells cultured for 3 d (Figure 6b) were also much different from those shown by using the differentiated cells, as shown in Figure 2a. The amount of vitamin D uptake was significantly higher by cells cultured for 3 d from NoPC mixed micelles (circle) than those from lysoPC mixed micelles (triangle). However, the uptake from NoPC mixed micelles decreased as the culture time increased to 5 d and 7 d. In contrast, the amounts of vitamin D2 uptake from lysoPC mixed micelles showed little change (Figure 6b). There was no difference in the uptake amounts between NoPC mixed micelles and lysoPC mixed micelles in cells cultured for 5 d. Finally, in the cells cultured for 7 d, the amounts of vitamin D2 uptake from lysoPC mixed micelles were significantly higher than those from NoPC mixed micelles, similar to the tendency of the results by the differentiated Caco-2, as shown in Figure 2a.
The residual concentrations of micellar vitamin D2 in the medium was lower in NoPC mixed micelles (circle) than in lysoPC mixed micelles (triangle) in cell-culture periods from 3–7 d (Figure 6c).
3.6. Effect of Lysophosphatidylcholine in Mixed Micelles on Cellular Cholesterol Amounts in Differentiated Caco-2 Cells
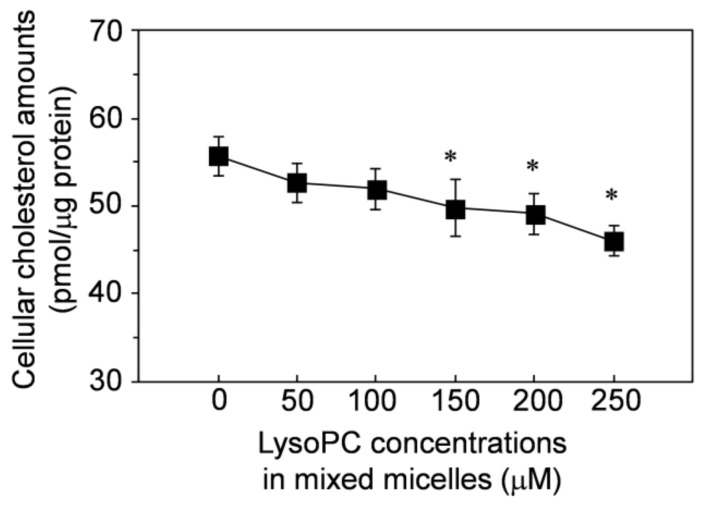
Lysophosphatidylcholine may regulate the amount of cholesterol originally contained in cells (cellular cholesterol), thereby affecting cell-cell adhesions and enhancing vitamin D uptake. We evaluated whether lysophosphatidylcholine in mixed micelles affected the amounts of cellular cholesterol. As shown in Figure 7, the amounts of cellular cholesterol decreased depending on the concentration of lysophosphatidylcholine in the mixed micelles. Lysophosphatidylcholine at >150 μM significantly decreased the cellular cholesterol and even concentrations <100 μM still tended to decrease the cellular cholesterol, but the decrease was not significant.
Figure 7.
Reducing effect of lysophosphatidylcholine in mixed micelles on cellular cholesterol amounts in the differentiated Caco-2 cells. The differentiated Caco-2 cells were incubated for 2 h with the mixed micelles containing lysophosphatidylcholine at 0–250 μM. The mixed micelles contained no vitamin D. Data are presented as the means ± standard deviation of four wells in a single experiment. Replicate experiments showed a similar trend. The asterisks indicate significant differences from the value of lysophosphatidylcholine at 0 μM as determined by Dunnett’s test (p < 0.05).
3.7. Estimation of Biological Activities of 1,25-Di(OH)-Vitamin D
Ingested vitamin D is absorbed from the intestinal tract and a portion is metabolized to 1,25-di(OH)-vitamin D by hydroxylation of the C25-location in the liver and further hydroxylation of the C1-location in the kidney. We analyzed the biological activities of the respective activated forms of vitamins D2–D7 by PASS online software simulation.
The PASS simulation showed the diverse biological activities that vitamins D possess, as described in the introduction section. However, only the biological activities associated with vitamin D as an anti-rickets factors are listed here in Table 1. Since PASS cannot distinguish between vitamins D4 and D7 (these chemical structures are shown in Figure 1), the same results were output. Among the five types of vitamin D (D2, D3, D4/D7, D5, and D6), the one with the highest Pa value was marked with “a,” and the runner-up was “b.” For each vitamin D, Pa was output at a higher value for bone-/calcium regulation-related activities (Table 1). In “vitamin” and “bone-formation stimulant”, the Pa value was higher for vitamin D5 than for vitamins D2 and D3. Vitamin D6 had the highest Pa value after vitamin D2 in “calcium regulator”, “vitamin D-like”, and “vitamin D receptor agonist”.
Table 1.
The predicted biological activities of 1, 25-di(OH)-vitamin D.
| Vitamin D2 | Vitamin D3 | Vitamin D4/D7 ∗ | Vitamin D5 | Vitamin D6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Activity | Pa 1 | Pi 2 | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi |
| Anti-osteoporotic | 0.982 a | 0.003 | 0.973 b | 0.003 | 0.969 | 0.003 | 0.965 | 0.003 | 0.970 | 0.003 |
| Bone diseases treatment | 0.981 a | 0.003 | 0.976 b | 0.003 | 0.968 | 0.003 | 0.966 | 0.003 | 0.966 | 0.003 |
| Vitamin | 0.977 b | 0.000 | 0.975 | 0.000 | 0.936 | 0.000 | 0.978 a | 0.000 | 0.976 | 0.000 |
| Hyperparathyroidism treatment | 0.946 a | 0.000 | 0.933 b | 0.000 | 0.883 | 0.000 | 0.882 | 0.000 | 0.901 | 0.000 |
| Calcium regulator | 0.902 a | 0.001 | 0.876 | 0.002 | 0.869 | 0.002 | 0.870 | 0.002 | 0.880 b | 0.001 |
| Vitamin D-like | 0.869 a | 0.000 | 0.757 | 0.000 | 0.578 | 0.000 | 0.791 | 0.000 | 0.844 b | 0.000 |
| Vitamin D receptor agonist | 0.816 a | 0.000 | 0.693 | 0.000 | 0.680 | 0.000 | 0.686 | 0.000 | 0.738 b | 0.000 |
| Bone formation stimulant | 0.578 | 0.004 | 0.598 | 0.004 | 0.628 b | 0.003 | 0.634 a | 0.003 | 0.627 | 0.003 |
1,2 Pa (probability to be active) and Pi (probability to be inactive) values were estimated by PASS (Prediction of Activity Spectra for Substances) online. The closer the Pa value is to 1, the higher the activity. The larger the Pi value, the more inactive it is. ∗ These isomers cannot be distinguished by PASS. a: Highest activity among vitamins D. b: Second highest activity among vitamins D. a,b: both judged by both Pa value and Pi value.
4. Discussion
To investigate the possibility of increasing the chance of vitamin D ingestion, we assumed the intestinal absorption of vitamins D4–D7 in addition to vitamins D2 and D3 and compared the uptake characteristics of these vitamins D solubilized in mixed micelles by using an intestinal cell model with differentiated Caco-2 cells. Except for vitamin D5, these vitamins D showed no significant differences in the amounts taken up by the cells (Figure 2). This result suggested that after vitamin D was solubilized in mixed bile salt micelles in the intestinal tract, vitamins D4, D6, and D7 were taken up by intestinal cells at levels similar to those for vitamins D2 and D3. In a previous study, we reported that the tendency of cellular uptake by Caco-2 for 11 kinds of carotenoids was close to their actual human bioavailability [85]. For example, α-carotene, β-carotene, lutein, β-cryptoxanthin, and zeaxanthin had a high uptake rate by Caco-2 cells, and in fact, these are typical carotenoids present in human blood [86]. On the other hand, neoxanthin and fucoxanthin had very low cellular uptake and are hardly absorbed by humans [86]. The results for vitamin D4–D7 uptake cannot be compared with their human bioavailability, since there are no human test results. This may be worth considering in future studies. In addition to the uptake, the study of vitamins D secretion using Transwell could also be an issue for future study.
As much as uptake, solubilization of vitamin D into mixed bile salt micelles is also an essential factor in its bioavailability. However, solubilization of vitamins D4–D7 in food is an issue for future research because the foods containing vitamins D4–D7 are not well known even now. Vitamin D2 is present in mushrooms. Vitamin D3 is mainly found in fish and shellfish; shiitake mushrooms [87]; and leaves of plants, such as tomatoes, eggplants, and zucchini [88,89]. Unfortunately, vitamin D3 was not found in these edible fruit parts. As mentioned above, in 1924, alfalfa was reported to contain an anti-rickets factor [11]. In 1984, the factors were identified as vitamins D2 and D3 [90]. However, plants cannot synthesize ergosterol. Vitamin D2 in plants has been identified as a contaminant derived from fungi (mold) [91]. Therefore, vitamin D3 is probably the main form in alfalfa. Although vitamin D4 was isolated in 1937 [92] at the same time as the isolation of vitamin D3 [93], it was not until 2012 that the foods containing it were revealed. Vitamin D4 is also contained in mushrooms [94]. Vitamin D5 and its precursor (7-dehydrositosterol) were reported to be present in Arabidopsis thaliana in 2018, and UV irradiation has been shown to increase its amount of vitamin D5 [95]. In addition, 7-dehydrositosterol in natural products is known to be present in Cyanidium caldarium (red algae) [96] and Rauwolfia serpentina [97]. These would have the potential to contain vitamin D5. There are few reports on what foods contain vitamins D6 and D7, but there are reports about the presence of their precursors in nature. Since 7-dehydrostigmasterol (vitamin D6 precursor) or 7-dehydrocampesterol (vitamin D7 precursor) is present in protists, such as trypanosomes [98] and amoeba [99] or in Critidia (in flagella) [98], vitamins D6 and D7 must be produced by UV irradiation in nature. Both stigmasterol and campesterol are typical plant sterols. In the future, their 7-dehydro forms, or vitamins D6 and D7, may be found in some foods, which would increase vitamin D intake opportunities.
Ergosterols and plant sterols are not absorbed in the intestine [100,101]. Like ergosterol, the 7-dehydro form of plant sterols will not be absorbed in the intestinal tract. If they are absorbed, they must be transported to the skin and exposed to UV irradiation to be converted to vitamin D forms. To the best of our knowledge, no such route has been reported. Therefore, we have to ingest the vitamin D form, not the precursor.
Intestinal absorption and accumulation of vitamin D seems to vary depending on the species. Both vitamins D2 and D3 are absorbed in humans, but the absorbability has not been determined because that of D2 equals that of D3 [87,102,103] or D3 > D2 [104,105] has been reported. Although fish ingest both vitamins D2 and D3 from plankton, most vitamin D3 is found in the body of the fish [106,107], and vitamin D2 is hard to accumulate. In chicks, vitamin D2 is less absorbed than vitamin D3 [108] or D4 [109]. In rats, vitamin D2 is slightly more easily absorbed than vitamin D3 [108], and vitamins D3 and D4 have similar levels of absorption [109]. Little information is available on the intestinal absorbability of vitamins D5–D7. The present study may be one of the hints of the little information.
The uptake of vitamin D5 tended to be smaller than that of other vitamins D. In addition to the uptake by differentiated Caco-2 cells, we evaluated the uptake by dispersed Caco-2 (Figure 4). The test with dispersed cells was originally intended to investigate the impact of cell-cell adhesions on cellular uptake, but simultaneously, we considered that this reflected the ease with which vitamin D could be transferred from mixed micelles to cell membrane lipids, as reported previously [24]. However, the uptake by dispersed cells did not match that by differentiated cells, and the dispersed cell experiment did not show that vitamin D5 was less likely than other vitamins D to be absorbed. The basic skeleton on the chemical structure of vitamin D is secosterol, and the types of vitamin D are determined by differences in their side chain structure (Figure 1). The side chains of vitamin D5 and β-sitosterol, a plant sterol, are the same. In the uptake test of plant sterols by Caco-2 cells, the order of increasing uptake was reported to be campesterol > β-sitosterol = brassicasterol ≥ stigmasterol [110]. The absorption characteristics may differ between vitamins D and plant sterols even with the same side chains. Thus, the trend of the vitamin D amounts taken up by differentiated cells may be caused by factors other than the transfer of vitamin D from mixed micelles to cell membrane lipids.
Niemann-Pick Cl-like 1 (NCP1L1) and scavenger receptor class B member 1 (SR-B1) are a transporter and a receptor, respectively, related to the intestinal absorption of cholesterol [111]. Reportedly, these cholesterol absorption-related factors are also involved in absorption of vitamin D3, which has the same side chain as cholesterol, in Caco-2 cells and mice [83]. Ezetimibe is widely used as an inhibitor of NCP1L1 [84]. We used ezetimibe to investigate the involvement of the facilitated diffusion pathway via NCP1L1 in vitamin D uptake. We found that the vitamin-D3 uptake was blocked by ezetimibe at an approximate rate of 20–30%, a finding similar to a previous one [83]. The uptakes of vitamins D2 and D4–D7 showed tendencies similar to that of vitamin D3 (Figure 3), suggesting that facilitated diffusion via the NCP1L1 pathway occurred in 20–30% of the uptakes of the vitamin D forms tested. Although the NCP1L1 pathway was not intervened in the low vitamin-D5 uptake by differentiated Caco-2 cells, the involvement of SR-B1 was not examined here but will be studied in the future.
Another possibility for low vitamin-D5 uptake by the differentiated Caco-2 cells is that an excretory transporter may be involved. Plant sterols are well known substrates for excretory transporters and ATP-binding cassette transporters G5/G8 (ABC G5/G8) [101]. Recently, vitamin-D3 absorption was reported to increase in mice deficient in ABC G5/G8 relative to that of the wild type [112], indicating that some vitamin D3 absorbed in the intestinal tract is excreted luminally by ABC G5/G8 in mice. Since the involvement of excretory transporters in other types of vitamin D is unknown, this point will also be studied in the future.
As mentioned above, foods containing vitamin D are limited. However, the deficiency of vitamin D intake due to low-intake opportunities might be recoverable by increasing the intestinal absorbability of vitamin D through the absorption-enhancing effect. We have shown in previous studies that lysoglycerolipids enhanced the intestinal absorption of carotenoids [22,23,24,25]. The applicability of the enhancing effect to vitamin D was examined in a present study. We demonstrated that lysophosphatidylcholine conspicuously enhanced the uptake of vitamin D by differentiated Caco-2 cells, suggesting that the enhancing effect is probably applicable to various fat-soluble components, including carotenoids and vitamins D. In a real diet, the use of phospholipids, such as phosphatidylcholine from egg yolk and soybeans, may increase the bioavailability of vitamin D because phospholipids derived from ingested food were hydrolyzed by pancreatic lipase to free fatty acids and lysophospholipids, such as lysophosphatidylcholine, in the human intestinal tract.
We think that lysophosphatidylcholine caused enhanced vitamin D uptake through the same mechanism underlying carotenoids uptake. We previously reported that the formation of cell-cell adhesion/cell-matrix adhesion is involved in the mechanism [24,25]. In the present study, we evaluated the enhancing effects of lysophosphatidylcholine on vitamin D uptake by cultivating two models of cells with adhesion conditions different from those of the differentiated cells: dispersed Caco-2 cells without cell-cell adhesion/cell-matrix adhesion, and adhered Caco-2 cells with insufficient cell-cell adhesion caused by low-density adherence to the culture plate.
In experiments with dispersed cells, the vitamin D uptake amount was markedly lower from mixed micelles with lysophosphatidylcholine than from those without lysophosphatidylcholine (Figure 5). This result showed two things: the transfer of vitamin D to cell membrane lipids was considerably more efficient from NoPC mixed micelles than from lysoPC mixed micelles, and cell-cell adhesion/cell-matrix adhesion was involved in the enhancing effect of lysophosphatidylcholine. Subsequently, we evaluated the vitamin D uptake by adhered cells with insufficient cell-cell adhesion. The uptake amounts from the NoPC mixed micelles were remarkably reduced depending on the growth of cell-cell adhesion, while those from the lysoPC mixed micelles were not changed. Consequently, when the cell-cell adhesion was fully developed, the trend of both uptake results was reversed, indicating that the growth of cell-cell adhesion reduced uptake. Together with our previous results [24], the enhancing effect of lysophosphatidylcholine would be applied not only to carotenoids but also to vitamins D or other fat-soluble components.
Lysophosphatidylcholine has been previously reported to reduce the transelectrical epithelial resistance (TEER) of Caco-2 cells; that is, to reduce the intercellular barrier formed by cell-cell adhesion [113,114,115,116]. As observed for lysophosphatidylcholine, methyl β-cyclodextrin [117,118,119], dodecylphosphocholine [120], and 2-alkoxy-3-alkylamidopropylphosphocholines [121] also reduced the TEER of Caco-2 cells, and they also enhanced the absorption of hydrophilic components, such as mannitol [121] and dextran [118,121]. At the same time, cellular cholesterol was removed from the cells [117,118,119], which would be involved in the enhancing effect on absorption. Removal of cellular cholesterol by lysophosphatidylcholine enhanced the expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and maintained cholesterol levels in the cells [122]. Cellular cholesterol contributed to the formation of the intercellular barrier formed by cell-cell adhesion [119]. The intestinal absorption of hydrophilic components was transported via paracellular diffusion [118,121], which is the intercellular barrier-to-basolateral side transport process.
On the other hand, intestinal absorption of hydrophobic compounds, such as carotenoids and vitamins D, has been shown to be transported via transcellular diffusion across the cell membrane [123]. In our previous study, the enhancing effect of lysophosphatidylcholine did not involve the TEER of Caco-2 cells on intestinal absorption of carotenoid [25]. These studies with transwells can examine secretion in addition to uptake, but conversely, they are not suitable for experiments with cells with immature cell-cell adhesion as we have shown here.
The present study also suggested that lysophosphatidylcholine enhanced the uptake of vitamins D by decreasing the intercellular barrier formation by removal of cholesterol from the cells in the transcellular pathway. We previously showed a figure of the candidate mechanism for the enhancing effect of lysophosphatidylcholine on uptake of carotenoids by Caco-2 cells [24], but we have not shown it again here. This mechanism can extend its application to other fat-soluble components, at least vitamins D, which would have been removable from mixed micelles across the region of the cytoplasmic membrane in dispersed Caco-2 cells. The components would be more easily transferred from NoPC mixed micelles than from lysoPC mixed micelles. In the case of adherent cells, cell-cell adhesion/cell-matrix adhesion would extremely decrease the region through which the components in NoPC mixed micelles could be moved to the cytoplasmic membrane. On the other hand, lysophosphatidylcholine would not decrease the moveable region of the components, which may have been caused by reduction of the formation of cell-cell adhesion/cell-matrix adhesion of Caco-2 cells due to loss of cellular cholesterol by lysophosphatidylcholine. Thus, the uptake level increased more by lysoPC mixed micelles than by NoPC mixed micelles in link with the adhesion growth.
In our previous study, to investigate the involvement of cell membrane permeability as a candidate mechanism for the enhancing effect of lysoglycerolipids, we examined the permeation-promoting effect of lysoglycerolipids by using 5(6)-carboxyfluorescein-encapsulated Phosphatidylcholine liposomes [24]. However, the results suggested that an alteration in the membrane permeability caused by lysoglycerolipids was not intervened in the enhancing effect of lysoglycerolipids. In addition, if the uptake amount was increased by increasing membrane permeability, it should also be increased in dispersed cells, which was not observed. Therefore, that candidate mechanism was dismissed.
Similar to our previous studies on carotenoid uptake [22,23,24,25], the present study also monitored the concentration of residual micellar vitamin D in the medium after incubation with Caco-2 cells because only micellar vitamin D can be taken up by cells. In the experiments with differentiated Caco-2 cells, the residual concentrations of micellar vitamin D or carotenoid tended to be higher in lysoPC mixed micelles than in NoPC mixed micelles. The enhancing effect of lysophosphatidylcholine may be due to the high concentration of residual micellar vitamin D. However, the trend of the results on the uptake of vitamin D by dispersed Caco-2 cells was reversed between NoPC mixed micelles and lysoPC mixed micelles (3 d vs. 7 d, respectively), and the residual concentrations of micellar vitamin D were almost constant (Figure 6c), indicating that the residual concentrations did not influence the enhancing effect of lysophosphatidylcholine under the present experimental conditions.
Vitamin D exerts its function after being metabolically converted into an activated form. In this study, the biological activities of activated vitamin D were estimated by PASS online. Vitamin D originally had been identified as an anti-rickets activity factor. The activities of vitamins D2–D7 have long been investigated by performing a line test using rats. A comparison of vitamins D provided as a crystal showed that vitamins D2 and D3 had the highest activities and were similar [109], the activity of vitamin D4 ranged from 50–75% of that of vitamin D2 [92], and the activity of vitamin D5 was very low and 1/180th that of vitamin D3 [124]. Studies have also been conducted on UV-irradiated precursors, but not crystals. In this case, the activity may appear to be lower than when using crystals, probably because the conversion rate to the vitamin D form after UV irradiation would not be taken into consideration. The activity of vitamin D4 was 1/30th to 1/40th that of vitamin D2 [125], the activity of vitamin D5 was 1/70th that of vitamin D2 [126] or 1/40th that of vitamins D2 and D3 as UV irradiation precursors [127], and vitamin D6 showed little or no activity [126,127,128,129], and the activity of vitamin D7 was 1/10th that of vitamin D2 [130]. These reports collectively showed that vitamins D5–D7 had very low anti-rickets activities. However, it should be noted that these activities were limited to anti-rickets activity in rats.
The potencies of 1,25-di(OH)-vitamins D5 and D6 estimated by PASS online were not very low in terms of bone-related, calcium regulation-related, or vitamin D-like activity because they were significantly different from those assumed from previous reports of anti-rickets activity in rats, as described above. The activity of 1-OH-vitamin D5, but not of 1,25-di(OH)-vitamin D5, in actual rat studies has been reported [131]. 1-OH-Vitamin D3 and 1-OH-vitamin D2 were shown to be metabolized into 1,25-di(OH)-vitamin D as an activated form in human liver model cells [132] or in the human body [133]. Therefore, 1-OH-vitamin D5 would convert to the activated form in the liver. Although 1-OH-vitamin D5 was certainly less active than 1,25-di(OH)-vitamin D3 for increasing the blood concentration of calcium in rats, it still showed approximately one-sixth of the activity of 1,25-di(OH)-vitamin D3 [131]. The activity of 1-OH-vitamin D5 seems to be sufficiently high based on the report above stating that the anti-rickets activity of vitamin D5 was 1/70th–1/40th that of vitamins D3 or D2.
The biological activity of vitamin D may vary between species. In fact, in chicks, the anti-rickets activities were found to be D3 > D4 > D2, an order different from that in rats [109]. There are still many unclear points regarding the difference in anti-rickets activities among vitamins D in other animal species or humans. The strength of biological activities may not always match that of anti-rickets activity. Elucidation of these biological activities deserves future study.
5. Conclusions
We investigated the uptakes of six types of vitamins D2–D7 solubilized in mixed micelles by human intestinal model Caco-2 cells. Vitamins D2–D4 are commercially available. Vitamins D5–D7 were obtained by our organic synthesis. The vitamin D uptakes by differentiated Caco-2 cells did not differ markedly among the types of vitamin D except for vitamin D5. In other words, the uptake amounts of vitamins D4–D7 were similar to those of vitamins D2 and D3, suggesting that taking various types of vitamin D from a wide variety of foods eliminates vitamin D deficiency. Lysophosphatidylcholine enhanced vitamin D uptake, suggesting that the intestinal absorption of vitamin D is enhanced by using phospholipids for cooking or eaten together. Its mechanism involved cell-cell adhesion/cell-matrix adhesion. We also confirmed reduction of cellular cholesterol by micellar lysophosphatidylcholine; in other words, lysophosphatidylcholine showed an enhancing effect on vitamin D uptake by reducing the intercellular barrier formation in Caco-2 cells by reducing cellular cholesterol. In the future, further functional tests and in vivo intestinal absorption tests of vitamins D5–D7 would be useful. However, it is difficult to conduct such an experiment without synthesizing vitamins D5–D7 in large quantities. The anti-rickets activities of vitamins D5–D7 reportedly were lower than those of vitamins D2 and D3 in rats, but the PASS online estimation suggests that vitamins D other than vitamins D2 and D3 also have strong biological activities. Verification of the actual effects on the nutritional significance of vitamins D4–D7, including confirmation of metabolic conversion into activated forms, is a topic for future research.
Author Contributions
E.K.-N. designed the experiments, analyzed the data, and wrote the article. S.K. performed organic synthesis and instrumental analysis of vitamins D5–D7. E.K.-N. and S.K. performed PASS online simulation. E.K.-N. and M.H. performed experiments using culture cells. All authors discussed the results and helped critically revise the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Number 16K00896 and 19K05888.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miyauchi M., Hirai C., Nakajima H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J. Nutr. Sci. Vitaminol. 2013;59:257–263. doi: 10.3177/jnsv.59.257. [DOI] [PubMed] [Google Scholar]
- 2.Godar D.E., Pope S.J., Grant W.B., Holick M.F. Solar UV doses of young Americans and vitamin D3 production. Environ. Health Perspect. 2012;120:139–143. doi: 10.1289/ehp.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afzal S., Nordestgaard B.G., Bojesen S.E. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: A prospective cohort study. J. Investig. Dermatol. 2013;133:629–636. doi: 10.1038/jid.2012.395. [DOI] [PubMed] [Google Scholar]
- 5.Lee D.U., Weon K.Y., Nam D.Y., Nam J.H., Kim W.K. Skin protective effect of guava leaves against UV-induced melanogenesis via inhibition of ORAI1 channel and tyrosinase activity. Exp. Dermatol. 2016;25:977–982. doi: 10.1111/exd.13151. [DOI] [PubMed] [Google Scholar]
- 6.Rijken F., Bruijnzeel-Koomen C.A. Photoaged skin: The role of neutrophils, preventive measures, and potential pharmacological targets. Clin. Pharmacol. Ther. 2011;89:120–124. doi: 10.1038/clpt.2010.221. [DOI] [PubMed] [Google Scholar]
- 7.Rittié L., Fisher G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015;5:a015370. doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov I.V., Mappes T., Schaupp P., Lappe C., Wahl S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics. 2018;11:e201700377. doi: 10.1002/jbio.201700377. [DOI] [PubMed] [Google Scholar]
- 9.Brockmann H. Die Isolierung Des antirachitischen Vitamins aus Thunfischleberöl. Hoppe Seylers Z. Physiol. Chem. 1936;241:104–115. doi: 10.1515/bchm2.1936.241.1-3.104. [DOI] [Google Scholar]
- 10.McCollum E.V., Simmonds N., Becker J.E., Shipley P.G. Studies on experimental rickets. XXI. an experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922;53:293–312. doi: 10.1016/S0021-9258(18)85783-0. [DOI] [PubMed] [Google Scholar]
- 11.Shipley P.G., Kinney E.M., McCollum E.V. Studies on experimental rickets: XXIV. the effect of creation extracts of plant tissues of florid rickets. J. Biol. Chem. 1924;59:165–175. doi: 10.1016/S0021-9258(18)85309-1. [DOI] [Google Scholar]
- 12.Hess A.F., Weinstock M. Antirachitic properties imparted to inert fluids and to green vegetables by ultra-violet irradiation. J. Biol. Chem. 1924;62:301–313. doi: 10.1016/S0021-9258(18)85064-5. [DOI] [Google Scholar]
- 13.Hume E.M., Smith H.H., Smedley-Maclean I. The examination of yeast-fat for the presence of vitamins A and D before irradiation and of vitamin D after irradiation. Biochem. J. 1928;22:27–33. doi: 10.1042/bj0220027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenbock H., Hart E.B., Hoppert C.A., Black A. Fat-soluble vitamin: XXVI. The antirachitic property of milk and its increase by direct irradiation and by irradiation of the animal. J. Biol. Chem. 1925;66:441–449. doi: 10.1016/S0021-9258(18)84761-5. [DOI] [Google Scholar]
- 15.Steenbock H., Black A. fat-soluble vitamins: XXIII. The induction of growth-promoting and calcifying properties in fats and their unsaponifiable constituents by exposure to light. J. Biol. Chem. 1925;64:263–298. doi: 10.1016/S0021-9258(18)84925-0. [DOI] [Google Scholar]
- 16.Redman T. The hydrogen ion concentration and the calcium and phosphorus content of the faeces of rachitic children. Biochem. J. 1929;23:256–260. doi: 10.1042/bj0230256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windaus A., Hess A. Sterine und antirachitisches vitamin. Nachr. Ges. Wiss. Gött. 1926;1926:174–184. [Google Scholar]
- 18.Hess A.F. The rôle of activated milk in the anti-rickets campaign. Am. J. Public Health Nations Health. 1932;22:1215–1219. doi: 10.2105/AJPH.22.12.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimura N., Muraki S., Oka H., Morita M., Yamada H., Tanaka S., Kawaguchi H., Nakamura K., Akune T. Profiles of vitamin D insufficiency and deficiency in Japanese men and women: Association with biological, environmental, and nutritional factors and coexisting disorders: The ROAD study. Osteoporos. Int. 2013;24:2775–2787. doi: 10.1007/s00198-013-2372-z. [DOI] [PubMed] [Google Scholar]
- 20.Nakano S., Suzuki M., Minowa K., Hirai S., Takubo N., Sakamoto Y., Ishijima M., Hoshino E., Tokita A., Shimizu T. Current vitamin D status in healthy Japanese infants and young children. J. Nutr. Sci. Vitaminol. 2018;64:99–105. doi: 10.3177/jnsv.64.99. [DOI] [PubMed] [Google Scholar]
- 21.Yorifuji J., Yorifuji T., Tachibana K., Nagai S., Kawai M., Momoi T., Nagasaka H., Hatayama H., Nakahata T. Craniotabes in normal newborns: The earliest sign of subclinical vitamin D deficiency. J. Clin. Endocrinol. Metab. 2008;93:1784–1788. doi: 10.1210/jc.2007-2254. [DOI] [PubMed] [Google Scholar]
- 22.Kotake-Nara E., Yonekura L., Nagao A. Effect of glycerophospholipid class on the beta-carotene uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2010;74:209–211. doi: 10.1271/bbb.90665. [DOI] [PubMed] [Google Scholar]
- 23.Kotake-Nara E., Nagao A. Effects of mixed micellar lipids on carotenoid uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2012;76:875–882. doi: 10.1271/bbb.110777. [DOI] [PubMed] [Google Scholar]
- 24.Kotake-Nara E., Yonekura L., Nagao A. Glyceroglycolipids affect uptake of carotenoids solubilized in mixed micelles by human intestinal Caco-2 cells. Lipids. 2015;50:847–860. doi: 10.1007/s11745-015-4033-9. [DOI] [PubMed] [Google Scholar]
- 25.Kotake-Nara E., Yonekura L., Nagao A. Lysoglyceroglycolipids improve the intestinal absorption of micellar fucoxanthin by Caco-2 cells. J. Oleo Sci. 2015;64:1207–1211. doi: 10.5650/jos.ess15180. [DOI] [PubMed] [Google Scholar]
- 26.Kotake-Nara E., Hase M. Effect of dispersed form on the bioavailability of β-carotene from daily intake in humans. Biosci. Biotechnol. Biochem. 2020;84:2545–2557. doi: 10.1080/09168451.2020.1803728. [DOI] [PubMed] [Google Scholar]
- 27.Komba S., Kotake-Nara E., Tsuzuki W. Simultaneous synthesis of vitamins D2, D4, D5, D6, and D7 from commercially available phytosterol, β-sitosterol, and identification of each vitamin D by HSQC NMR. Metabolites. 2019;9:107. doi: 10.3390/metabo9060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonekura L., Tsuzuki W., Nagao A. Acyl moieties modulate the effects of phospholipids on beta-carotene uptake by Caco-2 cells. Lipids. 2006;41:629–636. doi: 10.1007/s11745-006-5013-x. [DOI] [PubMed] [Google Scholar]
- 29.Baird D.D., Hill M.C., Schectman J.M., Hollis B.W. Vitamin D and the risk of uterine fibroids. Epidemiology. 2013;24:447–453. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catherino W.H., Eltoukhi H.M., Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin. Reprod. Med. 2013;31:370–379. doi: 10.1055/s-0033-1348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodnar L.M., Simhan H.N., Catov J.M., Roberts J.M., Platt R.W., Diesel J.C., Klebanoff M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. 2014;25:207–214. doi: 10.1097/EDE.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burris H.H., Rifas-Shiman S.L., Kleinman K., Litonjua A.A., Huh S.Y., Rich-Edwards J.W., Camargo C.A., Jr., Gillman M.W. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2012;207:182.e1–182.e8. doi: 10.1016/j.ajog.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh E., Yin M.T. Continued interest and controversy: Vitamin D in HIV. Curr. HIV/AIDS Rep. 2018;15:199–211. doi: 10.1007/s11904-018-0401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 35.Urashima M., Mezawa H., Noya M., Camargo C.A., Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014;5:2365–2370. doi: 10.1039/C4FO00371C. [DOI] [PubMed] [Google Scholar]
- 36.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speeckaert M.M., Delanghe J.R. Association between low vitamin D and COVID-19: Don’t forget the vitamin D binding protein. Aging Clin. Exp. Res. 2020;32:1207–1208. doi: 10.1007/s40520-020-01607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornton K.A., Marín C., Mora-Plazas M., Villamor E. Vitamin D deficiency associated with increased incidence of gastrointestinal and ear infections in school-age children. Pediatr. Infect. Dis. J. 2013;32:585–593. doi: 10.1097/INF.0b013e3182868989. [DOI] [PubMed] [Google Scholar]
- 39.Garland C.F., Garland F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 40.Garland C.F., Garland F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 2006;35:217–220. doi: 10.1093/ije/dyi229. [DOI] [PubMed] [Google Scholar]
- 41.Egan K.M., Sosman J.A., Blot W.J. Sunlight and reduced risk of cancer: Is the real story vitamin D? J. Natl. Cancer Inst. 2005;97:161–163. doi: 10.1093/jnci/dji047. [DOI] [PubMed] [Google Scholar]
- 42.Giovannucci E., Liu Y., Rimm E.B., Hollis B.W., Fuchs C.S., Stampfer M.J., Willett W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 43.Grant W.B. Vitamin D status: Ready for guiding prostate cancer diagnosis and treatment? Clin. Cancer Res. 2014;20:2241–2243. doi: 10.1158/1078-0432.CCR-14-0369. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz G.G., Hulka B.S. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–1311. [PubMed] [Google Scholar]
- 45.Schwartz G.G. Vitamin D and intervention trials in prostate cancer: From theory to therapy. Ann. Epidemiol. 2009;19:96–102. doi: 10.1016/j.annepidem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Garland C.F., Garland F.C., Gorham E.D., Lipkin M., Newmark H., Mohr S.B., Holick M.F. The role of vitamin D in cancer prevention. Am. J. Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smedby K.E., Hjalgrim H., Melbye M., Torrång A., Rostgaard K., Munksgaard L., Adami J., Hansen M., Porwit-MacDonald A., Jensen B.A., et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J. Natl. Cancer Inst. 2005;97:199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 48.Weihkopf T., Becker N., Nieters A., Mester B., Deeg E., Elsner G., Blettner M., Seidler A. Sun exposure and malignant lymphoma: A population-based case-control study in Germany. Int. J. Cancer. 2007;120:2445–2451. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 49.Sluyter J.D., Camargo C.A., Jr., Stewart A.W., Waayer D., Lawes C.M.M., Toop L., Khaw K.T., Thom S.A.M., Hametner B., Wassertheurer S., et al. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: A randomized controlled trial substudy. J. Am. Heart Assoc. 2017;6:e006802. doi: 10.1161/JAHA.117.006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brøndum-Jacobsen P., Benn M., Jensen G.B., Nordestgaard B.G. 25-Hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012;32:2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- 51.Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K., Benjamin E.J., D’Agostino R.B., Wolf M., Vasan R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brøndum-Jacobsen P., Nordestgaard B.G., Schnohr P., Benn M. 25-Hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann. Neurol. 2013;73:38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 53.Afzal S., Lange P., Bojesen S.E., Freiberg J.J., Nordestgaard B.G. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax. 2014;69:24–31. doi: 10.1136/thoraxjnl-2013-203682. [DOI] [PubMed] [Google Scholar]
- 54.Færk G., Çolak Y., Afzal S., Nordestgaard B.G. Low concentrations of 25-hydroxyvitamin D and long-term prognosis of COPD: A prospective cohort study. Eur. J. Epidemiol. 2018;33:567–577. doi: 10.1007/s10654-018-0393-9. [DOI] [PubMed] [Google Scholar]
- 55.Azam F., Shaheen A., Arshad R. Frequency of hypovitaminosis D and its associated risk factors in newly diagnosed pulmonary tuberculosis patients. Pak. J. Med. Sci. 2016;32:480–484. doi: 10.12669/pjms.322.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pal M., Datta S., Mitra R. Tuberculosis is associated with low levels of vitamin D. World J. Pharm. Pharm. Sci. 2014;3:1449–1463. [Google Scholar]
- 57.Salahuddin N., Ali F., Hasan Z., Rao N., Aqeel M., Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selvaraj P., Harishankar M., Afsal K. Vitamin D: Immuno-modulation and tuberculosis treatment. Can. J. Physiol. Pharmacol. 2015;93:377–384. doi: 10.1139/cjpp-2014-0386. [DOI] [PubMed] [Google Scholar]
- 59.Munger K.L., Levin L.I., Massa J., Horst R., Orban T., Ascherio A. Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am. J. Epidemiol. 2013;177:411–419. doi: 10.1093/aje/kws243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soltesz G., Patterson C.C., Dahlquist G. Worldwide childhood type 1 diabetes incidence—What can we learn from epidemiology? Pediatr. Diabetes. 2007;8:6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 61.Lu L., Bennett D.A., Millwood I.Y., Parish S., McCarthy M.I., Mahajan A., Lin X., Bragg F., Guo Y., Holmes M.V., et al. Association of vitamin D with risk of type 2 diabetes: A Mendelian randomisation study in European and Chinese adults. PLoS Med. 2018;15:e1002566. doi: 10.1371/journal.pmed.1002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitri J., Dawson-Hughes B., Hu F.B., Pittas A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011;94:486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Von Hurst P.R., Stonehouse W., Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 64.Biström M., Alonso-Magdalena L., Andersen O., Jons D., Gunnarsson M., Vrethem M., Hultdin J., Sundström P. High serum concentration of vitamin D may protect against multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019;5:2055217319892291. doi: 10.1177/2055217319892291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldberg P. Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence. (A viewpoint) part 1: Sunlight, dietary factors and epidemiology. Int. J. Environ. Stud. 1974;6:19–27. doi: 10.1080/00207237408709630. [DOI] [Google Scholar]
- 66.Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 67.Afzal S., Bojesen S.E., Nordestgaard B.G. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimer’s Dement. 2014;10:296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 68.Mizwicki M.T., Menegaz D., Zhang J., Barrientos-Durán A., Tse S., Cashman J.R., Griffin P.R., Fiala M. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J. Alzheimer’s Dis. 2012;29:51–62. doi: 10.3233/JAD-2012-110560. [DOI] [PubMed] [Google Scholar]
- 69.Llewellyn D.J., Lang I.A., Langa K.M., Muniz-Terrera G., Phillips C.L., Cherubini A., Ferrucci L., Melzer D. Vitamin D and risk of cognitive decline in elderly persons. Arch. Intern. Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertone-Johnson E.R., Powers S.I., Spangler L., Brunner R.L., Michael Y.L., Larson J.C., Millen A.E., Bueche M.N., Salmoirago-Blotcher E., Liu S., et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am. J. Clin. Nutr. 2011;94:1104–1112. doi: 10.3945/ajcn.111.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grudet C., Malm J., Westrin A., Brundin L. Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology. 2014;50:210–219. doi: 10.1016/j.psyneuen.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 72.Milaneschi Y., Shardell M., Corsi A.M., Vazzana R., Bandinelli S., Guralnik J.M., Ferrucci L. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J. Clin. Endocrinol. Metab. 2010;95:3225–3233. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valipour G., Saneei P., Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: A systematic review and meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 2014;99:3863–3872. doi: 10.1210/jc.2014-1887. [DOI] [PubMed] [Google Scholar]
- 74.Sinha A., Hollingsworth K.G., Ball S., Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013;98:E509–E513. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- 75.Sohl E., van Schoor N.M., de Jongh R.T., Visser M., Deeg D.J., Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J. Clin. Endocrinol. Metab. 2013;98:E1483–E1490. doi: 10.1210/jc.2013-1698. [DOI] [PubMed] [Google Scholar]
- 76.Barassi A., Pezzilli R., Colpi G.M., Corsi Romanelli M.M., Melzi d’Eril G.V. Vitamin D and erectile dysfunction. J. Sex. Med. 2014;11:2792–2800. doi: 10.1111/jsm.12661. [DOI] [PubMed] [Google Scholar]
- 77.Bellastella G., Maiorino M.I., Olita L., Capuano A., Rafaniello C., Giugliano D., Esposito K. Vitamin D deficiency in type 2 diabetic patients with hypogonadism. J. Sex. Med. 2014;11:536–542. doi: 10.1111/jsm.12384. [DOI] [PubMed] [Google Scholar]
- 78.Daraki V., Roumeliotaki T., Koutra K., Chalkiadaki G., Katrinaki M., Kyriklaki A., Kampouri M., Margetaki K., Vafeiadi M., Papavasiliou S., et al. High maternal vitamin D levels in early pregnancy may protect against behavioral difficulties at preschool age: The Rhea mother-child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry. 2018;27:79–88. doi: 10.1007/s00787-017-1023-x. [DOI] [PubMed] [Google Scholar]
- 79.Morales E., Julvez J., Torrent M., Ballester F., Rodríguez-Bernal C.L., Andiarena A., Vegas O., Castilla A.M., Rodriguez-Dehli C., Tardón A., et al. Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology. 2015;26:458–465. doi: 10.1097/EDE.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 80.Sengupta T., Majumder R., Majumder S. Role of vitamin D in treating COVID-19-associated coagulopathy: Problems and perspectives. Mol. Cell Biochem. 2021;18:1–7. doi: 10.1007/s11010-021-04093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komba S., Kotake-Nara E., Machida S. Fucoxanthin derivatives: Synthesis and their chemical properties. J. Oleo Sci. 2015;64:1009–1018. doi: 10.5650/jos.ess15039. [DOI] [PubMed] [Google Scholar]
- 82.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 83.Reboul E., Goncalves A., Comera C., Bott R., Nowicki M., Landrier J.F., Jourdheuil-Rahmani D., Dufour C., Collet X., Borel P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011;55:691–702. doi: 10.1002/mnfr.201000553. [DOI] [PubMed] [Google Scholar]
- 84.Ono S., Matsuda J., Saito A., Yamamoto T., Fujimoto W., Shimizu H., Dateki S., Ouchi K. A case of sitosterolemia due to compound heterozygous mutations in ABCG5: Clinical features and treatment outcomes obtained with colestimide and ezetimibe. Clin. Pediatr. Endocrinol. 2017;26:17–23. doi: 10.1297/cpe.26.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugawara T., Kushiro M., Zhang H., Nara E., Ono H., Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001;131:2921–2927. doi: 10.1093/jn/131.11.2921. [DOI] [PubMed] [Google Scholar]
- 86.Kotake-Nara E., Nagao A. Absorption and metabolism of xanthophylls. Mar. Drugs. 2011;9:1024–1037. doi: 10.3390/md9061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keegan R.J., Lu Z., Bogusz J.M., Williams J.E., Holick M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Derm. Endocrinol. 2013;5:165–176. doi: 10.4161/derm.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prema T.P., Raghuramulu N. Vitamin D3 and its metabolites in the tomato plant. Phytochemistry. 1996;42:617–620. doi: 10.1016/0031-9422(95)00883-7. [DOI] [PubMed] [Google Scholar]
- 89.Aburjaia T., Al-Khalil S., Abuirjeie M. Vitamin D3 and its metabolites in tomato, potato, egg plant and zucchini leaves. Phytochemistry. 1998;49:2497–2499. doi: 10.1016/S0031-9422(98)00246-5. [DOI] [Google Scholar]
- 90.Horst R.L., Reinhardt T.A., Russell J.R., Napoli J.L. The isolation and identification of vitamin D2 and vitamin D3 from Medicago sativa (alfalfa plant) Arch. Biochem. Biophys. 1984;231:67–71. doi: 10.1016/0003-9861(84)90363-1. [DOI] [PubMed] [Google Scholar]
- 91.Jäpelt R.B., Jakobsen J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013;4:136. doi: 10.3389/fpls.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Windaus A., Trautmann G. Über das krystallisierte vitamin D4. Hoppe Seylers Z. Physiol. Chem. 1937;247:185–188. doi: 10.1515/bchm2.1937.247.4-5.185. [DOI] [Google Scholar]
- 93.Windaus A., Schenck F., Werder F.V. Über das antirachitisch wirksame Bestrahlungsprodukt aus 7-dehydro-cholesterin. Hoppe Seylers Z. Physiol. Chem. 1936;241:100–103. doi: 10.1515/bchm2.1936.241.1-3.100. [DOI] [Google Scholar]
- 94.Phillips K.M., Horst R.L., Koszewski N.J., Simon R.R. Vitamin D4 in mushrooms. PLoS ONE. 2012;7:e40702. doi: 10.1371/journal.pone.0040702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silvestro D., Villette C., Delecolle J., Olsen C.E., Motawia M.S., Geoffroy P., Miesch M., Jensen P.E., Heintz D., Schaller H. Vitamin D 5 in Arabidopsis thaliana. Sci. Rep. 2018;8:16348. doi: 10.1038/s41598-018-34775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seckbach J., Ikan R. Sterols and chloroplast structure of Cyanidium caldarium. Plant Physiol. 1972;49:457–459. doi: 10.1104/pp.49.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karmakar T., Chakraborty D.P. 7-Dehydrositosterol from Rauwolfia serpentina. Phytochemistry. 1983;22:608–609. doi: 10.1016/0031-9422(83)83065-9. [DOI] [Google Scholar]
- 98.Korn E.D., Von Brand T., Tobie E.J. The sterols of Trypanosoma cruzi and Crithidia fasciculata. Comp. Biochem. Physiol. 1969;30:601–610. doi: 10.1016/0010-406X(69)92137-9. [DOI] [PubMed] [Google Scholar]
- 99.Smith F.R., Korn E.D. 7-Dehydrostigmasterol and ergosterol: The major sterols of an amoeba. J. Lipid Res. 1968;9:405–408. doi: 10.1016/S0022-2275(20)42717-8. [DOI] [PubMed] [Google Scholar]
- 100.Schoenheimer R. New contributions in sterol metabolism. Science. 1931;74:579–584. doi: 10.1126/science.74.1928.579. [DOI] [PubMed] [Google Scholar]
- 101.Ajagbe B.O., Othman R.A., Myrie S.B. Plant sterols, stanols, and sitosterolemia. J. AOAC Int. 2015;98:716–723. doi: 10.5740/jaoacint.SGEAjagbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biancuzzo R.M., Young A., Bibuld D., Cai M.H., Winter M.R., Klein E.K., Ameri A., Reitz R., Salameh W., Chen T.C., et al. Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am. J. Clin. Nutr. 2010;91:1621–1626. doi: 10.3945/ajcn.2009.27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holick M.F., Biancuzzo R.M., Chen T.C., Klein E.K., Young A., Bibuld D., Reitz R., Salameh W., Ameri A., Tannenbaum A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trang H.M., Cole D.E., Rubin L.A., Pierratos A., Siu S., Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am. J. Clin. Nutr. 1998;68:854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 105.Heaney R.P., Recker R.R., Grote J., Horst R.L., Armas L.A. Vitamin D3 is more potent than vitamin D2 in humans. J. Clin. Endocrinol. Metab. 2011;96:E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 106.Takeuchi A., Okano T., Tanda M., Kobayashi T. Possible origin of extremely high contents of vitamin D3 in some kinds of fish liver. Comp. Biochem. Physiol. 1991;100:483–487. [Google Scholar]
- 107.Mattila P., Piironen V., Haapala R., Hirvi T., Uusi-Rauva E. Possible factors responsible for the high variation in the cholecalciferol contents of fish. J. Agric. Food Chem. 1997;45:3891–3896. doi: 10.1021/jf970243j. [DOI] [Google Scholar]
- 108.Imrie M.H., Neville P.F., Snellgrove A.W., DeLuca H.F. Metabolism of vitamin D2 and vitamin D3 in the rachitic chick. Arch. Biochem. Biophys. 1967;120:525–532. doi: 10.1016/0003-9861(67)90513-9. [DOI] [Google Scholar]
- 109.De Luca H.F., Weller M., Blunt J.W., Neville P.F. Synthesis, biological activity, and metabolism of 22,23-3H vitamin D4. Arch. Biochem. Biophys. 1968;124:122–128. doi: 10.1016/0003-9861(68)90310-X. [DOI] [PubMed] [Google Scholar]
- 110.Ito N., Ohtsubo T., Kusu F., Hakamata H. An ultra performance liquid chromatographic method for determining phytosterol uptake by Caco-2 cells. Anal. Biochem. 2012;421:86–91. doi: 10.1016/j.ab.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 111.Haikal Z., Play B., Landrier J.F., Giraud A., Ghiringhelli O., Lairon D., Jourdheuil-Rahmani D. NPC1L1 and SR-BI are involved in intestinal cholesterol absorption from small-size lipid donors. Lipids. 2008;43:401–408. doi: 10.1007/s11745-008-3172-7. [DOI] [PubMed] [Google Scholar]
- 112.Kiourtzidis M., Kühn J., Brandsch C., Stangl G.I. Vitamin D status of mice deficient in scavenger receptor class B type 1, cluster determinant 36 and ATP-binding cassette proteins G5/G8. Nutrients. 2020;12:2169. doi: 10.3390/nu12082169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakano T., Inoue I., Alpers D.H., Akiba Y., Katayama S., Shinozaki R., Kaunitz J.D., Ohshima S., Akita M., Takahashi S., et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G207–G214. doi: 10.1152/ajpgi.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sawai T., Usui N., Dwaihy J., Drongowski R.A., Abe A., Coran A.G., Harmon C.M. The effect of phospholipase A2 on bacterial translocation in a cell culture model. Pediatr. Surg. Int. 2000;16:262–266. doi: 10.1007/s003830050741. [DOI] [PubMed] [Google Scholar]
- 115.Sawai T., Drongowski R.A., Lampman R.W., Coran A.G., Harmon C.M. The effect of phospholipids and fatty acids on tight-junction permeability and bacterial translocation. Pediatr. Surg. Int. 2001;17:269–274. doi: 10.1007/s003830100592. [DOI] [PubMed] [Google Scholar]
- 116.Sawai T., Lampman R., Hua Y., Segura B., Drongowski R.A., Coran A.G., Harmon C.M. Lysophosphatidylcholine alters enterocyte monolayer permeability via a protein kinase C/Ca2+ mechanism. Pediatr. Surg. Int. 2002;18:591–594. doi: 10.1007/s00383-002-0860-x. [DOI] [PubMed] [Google Scholar]
- 117.Doi N., Tomita M., Hayashi M. Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab. Pharmacokinet. 2011;26:162–170. doi: 10.2133/dmpk.DMPK-10-RG-071. [DOI] [PubMed] [Google Scholar]
- 118.Lambert D., O’Neill C.A., Padfield P.J. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem. J. 2005;387:553–560. doi: 10.1042/BJ20041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lambert D., O’Neill C.A., Padfield P.J. Methyl-beta-cyclodextrin increases permeability of Caco-2 cell monolayers by displacing specific claudins from cholesterol rich domains associated with tight junctions. Cell. Physiol. Biochem. 2007;20:495–506. doi: 10.1159/000107533. [DOI] [PubMed] [Google Scholar]
- 120.Liu D.Z., LeCluyse E.L., Thakker D.R. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J. Pharm. Sci. 1999;88:1161–1168. doi: 10.1021/js990094e. [DOI] [PubMed] [Google Scholar]
- 121.Liu D.Z., Morris-Natschke S.L., Kucera L.S., Ishaq K.S., Thakker D.R. Structure-activity relationships for enhancement of paracellular permeability by 2-alkoxy-3-alkylamidopropylphosphocholines across Caco-2 cell monolayers. J. Pharm. Sci. 1999;88:1169–1174. doi: 10.1021/js9900957. [DOI] [PubMed] [Google Scholar]
- 122.Muir L.V., Born E., Mathur S.N., Field F.J. Lysophosphatidylcholine increases 3-Hydroxy-3-methylglutaryl-coenzyme A reductase gene expression in CaCo-2 cells. Gastroenterology. 1996;110:1068–1076. doi: 10.1053/gast.1996.v110.pm8612995. [DOI] [PubMed] [Google Scholar]
- 123.Yonekura L., Nagao A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007;51:107–115. doi: 10.1002/mnfr.200600145. [DOI] [PubMed] [Google Scholar]
- 124.Napoli J.L., Fivizzani M.A., Schnoes H.K., DeLuca H.F. Synthesis of vitamin D5: Its biological activity relative to vitamins D3 and D2. Arch. Biochem. Biophys. 1979;197:119–125. doi: 10.1016/0003-9861(79)90226-1. [DOI] [PubMed] [Google Scholar]
- 125.Windaus A., Langer R. Über das 22-dihydro-ergosterin. Eur. J. Org. Chem. 1933;508:105–114. doi: 10.1002/jlac.19345080111. [DOI] [Google Scholar]
- 126.Grab W. Die Auswertung der antirachitischen Wirksamkeit neuer Sterinderivate im Versuch an Ratten und Küken. Hoppe Seylers Z. Physiol. Chem. 1936;243:63–89. doi: 10.1515/bchm2.1936.243.1-3.63. [DOI] [Google Scholar]
- 127.Wunderlich W. Über das 7-Dehydro-sitosterin. Hoppe Seylers Z. Physiol. Chem. 1936;241:116–124. doi: 10.1515/bchm2.1936.241.1-3.116. [DOI] [Google Scholar]
- 128.Haslewood G.A. The action of light on substances related to ergosterol. Biochem. J. 1939;33:454–456. doi: 10.1042/bj0330454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Linsert O. Über das 7-Dehydro-stigmasterin. Hoppe Seylers Z. Physiol. Chem. 1936;241:125–128. doi: 10.1515/bchm2.1936.241.1-3.125. [DOI] [Google Scholar]
- 130.Ruigh W.L. 7-Dehydrocampesterol, a new provitamin D. J. Am. Chem. Soc. 1942;64:1900–1902. doi: 10.1021/ja01260a042. [DOI] [Google Scholar]
- 131.Mehta R.G., Moriarty R.M., Mehta R.R., Penmasta R., Lazzaro G., Constantinou A., Guo L. Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1alpha-hydroxyvitamin D5. J. Natl. Cancer Inst. 1997;89:212–218. doi: 10.1093/jnci/89.3.212. [DOI] [PubMed] [Google Scholar]
- 132.Strugnell S., Byford V., Makin H.L., Moriarty R.M., Gilardi R., LeVan L.W., Knutson J.C., Bishop C.W., Jones G. 1 alpha,24(S)-dihydroxyvitamin D2: A biologically active product of 1 alpha-hydroxyvitamin D2 made in the human hepatoma, Hep3B. Biochem. J. 1995;310:233–241. doi: 10.1042/bj3100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu G., Oettel K., Ripple G., Staab M.J., Horvath D., Alberti D., Arzoomanian R., Marnocha R., Bruskewitz R., Mazess R., et al. Phase I trial of 1alpha-hydroxyvitamin d(2) in patients with hormone refractory prostate cancer. Clin. Cancer Res. 2002;8:2820–2827. [PubMed] [Google Scholar]