Abstract
Several mechanisms are involved in the biological control of plant pathogens by the soil-borne Trichoderma spp. fungi. The aim of this study was to characterize a new strain of Trichoderma as a potential biological control agent to control the postharvest anthracnose of chili pepper caused by Colletotrichum gloeosporioides. A total of nine strains of Trichoderma spp. were screened for their antifungal activity using a dual culture assay against C. gloeosporioides. Trichoderma koningiopsis PSU3-2 was shown to be the most effective strain, with a percentage inhibition of 79.57%, which was significantly higher than that of other strains (p < 0.05). In the sealed plate method, T. koningiopsis PSU3-2 suppressed the growth of C. gloeosporioides by 38.33%. Solid-phase microextraction (SPME) was applied to trap volatiles emitted by T. koningiopsis PSU3-2, and the GC/MS profiling revealed the presence of antifungal compounds including azetidine, 2-phenylethanol, and ethyl hexadecanoate. The production of cell-wall-degrading enzymes (CWDEs) was assayed through cell-free culture filtrate (CF) of PSU3-2, and the enzyme activity of chitinase and β-1,3-glucanase was 0.06 and 0.23 U/mL, respectively, significantly higher than that in the control (p < 0.05). Scanning electron microscopy of the mycelium incubated in cell-free CF of T. koningiopsis PSU3-2 showed the abnormal shape of C. gloeosporioides hyphae. Application of T. koningiopsis PSU3-2 by the dipping method significantly reduced the lesion size (p < 0.05) after inoculation with C. gloeosporioides compared to the control, and there was no disease symptom development in T. koningiopsis PSU3-2-treated chili pepper. This study demonstrates that T. koningiopsis PSU3-2 is an effective antagonistic microorganism and a promising biocontrol agent against postharvest anthracnose of chili pepper, acting with multiple mechanisms.
Keywords: in vitro tests; β-1,3-glucanase; chitinase; electron microscopy; GC/MS profiling
1. Introduction
Rhizosphere soil has long been considered as the main source of isolation of useful beneficial microorganisms [1,2]. At present, numerous soil fungi isolated from soil are employed as biological control agents, especially fungi in the genus Trichoderma. Trichoderma species are widely used to control numerous plant pathogens and reduce disease severity [3,4], due to their capacity for nutrient and space competition [5,6], parasitism [7], secretion of antimicrobial metabolites [7,8,9,10], activation of defense responses [11,12], and promotion of plant growth [8,9,13]. Moreover, metabolites, such as volatile organic compounds (VOCs), secreted from the Trichoderma species have been applied to promote plant growth [8,9,14]. Application of the Trichoderma species has been used to reduce the disease severity of leaf spots on lettuce [12] and sugar beet [15], as well as brown spots on rice [16]. Biological control presents low human health risks, as well as an environmentally friendly method without the excessive use of chemical fungicides in various crops.
Anthracnose is a common plant disease characterized by dark, sunken lesions on fruits, leaves, and stems containing conidia [17]. The causal agents of this disease, identified as Colletotrichum spp., reduce both the quality and the quantity of a harvest yield. Disease severity increases during the rainy season, as conidia of Colletotrichum are splashed and dispersed onto fresh fruit, resulting in secondary infection [18]. Anthracnose disease caused by Colletotrichum spp. has been reported to negatively impact the cultivation and production of mangoes [19,20], bananas [21], tomatoes [22], and chili peppers [23].
Chili anthracnose is a major constraint in chili production leading to huge losses, especially postharvest anthracnose, which causes the decay of chili pepper in tropical and subtropical regions [24,25]. Developing biological management strategies to control chili anthracnose may benefit disease management in chili peppers. This study, therefore, aimed to explore the potential of Trichoderma spp. isolated from soil as a biocontrol agent through dipping application. Multiple mechanisms of Trichoderma strains were tested for antifungal activity against Colletotrichum gloeosporioides.
2. Materials and Methods
2.1. Source of Trichoderma Species and Colletotrichum gloeosporioides
A total of nine Trichoderma strains, namely, Trichoderma asperelloides PSU-P1 [9], TSU1 [26], Trichoderma asperellum T76-14 [10], T. koningiopsis PSU3-2 (GenBank accession no. LC600711 and LC600712), and Trichoderma sp. PSU1-1, Tri1-1, Tri1-2, Tri2-1, and Tri2-2, were obtained from the Culture Collection of Pest Management (CCPM), Faculty of Natural Resources, Prince of Songkla University, whereas Colletotrichum gloeosporioides causing postharvest anthracnose of chili pepper was obtained from the Department of Agriculture, Ministry of Agriculture and Cooperatives, Bangkok, Thailand. Trichoderma and C. gloeosporioides were cultured on potato dextrose agar (PDA) (Himedia, Mumbai, India) at 28 ± 2 °C for 3 days before bioassays.
2.2. Dual Culture Assay
Nine strains of Trichoderma spp. were screened for antifungal activities on the mycelial growth of C. gloeosporioides through a dual culture assay on PDA plates [27]. An agar plug of a 5-day-old C. gloeosporioides colony was placed on the side of 9 cm Petri dishes, with an agar plug of each Trichoderma sp. placed on the opposite side 5 cm from the pathogen. PDA plates with pathogen alone served as the control. The experiment was designed according to a complete randomized block (CRD) with five replicates and repeated twice. The tested plates were incubated at ambient temperature (28 ± 2 °C) for 7 days. Colony radii of C. gloeosporioides were measured, and the percentage inhibition was calculated using the method of Rahman et al. [28], as given in Equation (1).
| (1) |
where R1 is the radial growth of C. gloeosporioides in control, and R2 is the radial growth of C. gloeosporioides with treatment.
2.3. Volatile Antifungal Bioassay and Solid-Phase Microextraction GC/MS Analysis
The effect of volatiles emitted by Trichoderma spp. was examined using the sealed plate method [10,29]. The most effective Trichoderma isolate was cultured in a 20 mL chromatography vial, 20 mm in diameter (PerkinElmer, Waltham, MA, USA), and incubated at 28 ± 2 °C for 10 days. Volatiles emitted by Trichoderma were trapped by solid-phase microextraction (SPME) fibers and inserted into the injection port of an SQ8 gas chromatograph (PerkinElmer, Waltham, MA, USA). GC/MS conditions adhered to the method previously described by Phoka et al. [9] and Intana et al. [10]. Total volatiles released from Trichoderma were tentatively identified by a computer search of the National Institute of Standards and Technology (NIST) Mass Spectral Library Search Chromatogram.
2.4. Liquid-Phase Cultivation and Enzyme Assay
The effective Trichoderma spp. were cultivated in potato dextrose broth (PDB) and incubated at 28 ± 2 °C for 5 days according to the method of Wonglom et al. [6]. The PDB-cultured Trichoderma spp. were filtrated with a 0.45 µm Minisart® Syringe Filter (Sigma-Aldrich, St. Louis, MO, USA) and used as cell-free culture filtrate (CF). An enzyme assay was conducted to confirm that the cell-free CF of Trichoderma spp. contained cell-wall-degrading enzymes (CWDEs) responsible for the fungal cell-wall degradation, while chitinase and β-1,3-glucanase activities were assayed with 3,5-dinitrosalicylic acid (DNS), as suggested by Miller [30]. Reaction mixtures containing colloidal chitin were used as the substrate in the chitinase assay, whereas mixtures containing laminarin (Sigma-Aldrich, St. Louis, MO, USA) were used as the substrate in the β-1,3-glucanase assay. An assay with PDB alone served as the control. Reducing sugar released in the test reaction mixtures was measured using an ultraviolet/visible light (UV/Vis) spectrophotometer UV5300 (METASH, Shanghai, China) at 550 and 575 nm for β-1,3-glucanase and chitinase, respectively. Enzymes were assayed in three replicates, and the experiments were repeated twice.
2.5. Scanning Electron Microscopy
To test the effect of cell-free CF on fungal mycelia morphology, a scanning electron microscope (SEM) was utilized according to the method of Baiyee et al. [12]. A mycelial plug (0.5 cm) of a 7-day-old colony of C. gloeosporioides was incubated in the cell-free CF of effective Trichoderma strains at 37 °C for 1 h, whereas the control was incubated with PDB only. The mycelial plugs were fixed in 3% glutaraldehyde at 4 °C for 24 h and then dehydrated in a 30%, 50%, 60%, 70%, 80%, 90%, and 100% alcohol series, three times each. The samples were coated with gold and observed using a JSM-580 LV SEM (JEOL, Peabody, MA, USA) at the Science Equipment Center, Prince of Songkla University, Songkhla, Thailand.
2.6. In Vivo Test
A spore suspension of effective Trichoderma was prepared, and the concentration was adjusted with sterile distilled water (DW) to 1 × 106 conidia/mL. A spore suspension of the Colletotrichum sp. was prepared in the same manner. Chili peppers were surface-disinfected with 70% ethanol, dipped in the spore suspension of Trichoderma spp., and air-dried in a laminar airflow cabinet. Chili peppers dipped in DW alone and the spore suspension of the Colletotrichum sp. served as the negative and positive controls, respectively. Then, 20 mL spore suspensions of C. gloeosporioides were sprayed onto the chili peppers after being dipped in the spore suspension of Trichoderma for 24 h and incubated in a moist box for 5 days, at which time the lesion development of all treated chili peppers was measured. Each treatment included five chili peppers (five replicates), and each experiment was repeated three times.
2.7. Statistical Analysis
The results regarding fungal inhibition, the enzyme assay, and lesion development were subjected to one-way analysis of variance (ANOVA). Statistically significant differences among treated samples were determined by Tukey’s test.
3. Results
3.1. Antifungal Activity of Trichoderma spp.
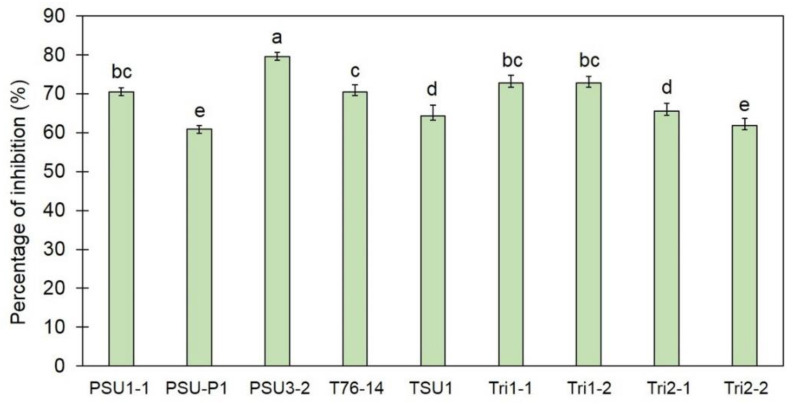
After incubation for 7 days, a smaller growth of C. gloeosporioides was observed in the dual culture plate than in the control plate. Nine strains of Trichoderma spp. inhibited the fungal growth of C. gloeosporioides in dual culture plates with inhibition percentages ranging from 60.84 to 79.57% (Figure 1). T. koningiopsis PSU3-2 was shown to be the most effective strain, with a percentage inhibition of 79.57%, statistically higher than that of other strains (p < 0.05) in this assay (Figure 1); therefore, the T. koningiopsis PSU3-2 strain was selected for further bioassays.
Figure 1.
Percentage inhibition of Trichoderma spp. against Colletotrichum gloeosporioides. Different letters indicate statistically significant differences among treatments (p < 0.05) using Tukey’s test.
3.2. Production of Volatile Antifungal Compounds
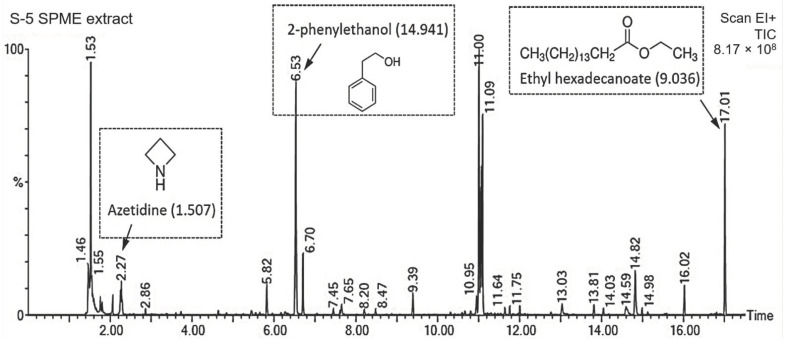
The sealed plate method showed that T. koningiopsis PSU3-2 inhibited the fungal growth of C. gloeosporioides, with a percentage inhibition of 38.33%. This result reveals that T. koningiopsis PSU3-2 produced volatile organic compounds which were responsible for suppressing the mycelial growth of C. gloeosporioides in vitro. A total of 16 volatile compounds were detected in T. koningiopsis PSU3-2 through GC/MS analysis. The volatile compounds contained carbon numbers ranging from C1 (fluoro(trinitro)methane) to C20 (ethyl (E)-octadec-9-enoate). The major compounds found in this study were 2-phenylethanol followed by fluoroethane and 1-oxacyclotetradeca-4,11-diyne, with percentage peak areas of 14.94, 12.85, and 11.588%, respectively (Table 1). According to previous literature reviews, only three compounds were reported as volatile antifungal compounds (VOCs), namely, azetidine (1.507% peak area), 2-phenylethanol (14.941%), and ethyl hexadecanoate (9.036%). Figure 2 shows the mass spectrum of volatile antifungal compounds and their structures. No major peaks were observed in PDA alone, which served as the control group.
Table 1.
International Union of Pure and Applied Chemistry (IUPAC) names of volatile compounds produced by T. koningiopsis PSU3-2 identified through solid-phase microextraction (SPME)/GC/MS analysis.
| Retention Time | IUPAC Name | Percentage Match | Percentage Area | Formula |
|---|---|---|---|---|
| 1.463 | fluoro(trinitro)methane | 95 | 4.2 | CFN3O6 |
| 1.528 | fluoroethane | 78.9 | 12.851 | C2H5F |
| 2.274 | azetidine | 89.9 | 1.507 | C3H7N |
| 5.824 | 3-isopropyl-5-methylhexan-2-one | 71.8 | 1.581 | C10H20O |
| 6.534 | 2-phenylethanol | 91.8 | 14.941 | C8H10O |
| 6.71 | (4-nitrophenyl) heptanoate | 79.2 | 3.181 | C13H17NO4 |
| 7.65 | 3-methylidene-1,2-dihydroindene | 88.2 | 0.541 | C10H10 |
| 9.389 | (E)-2,5,6-trimethylhept-4-en-3-one | 74.9 | 1.096 | C10H18O |
| 10.95 | 1-oxacyclotetradeca-4,11-diyne | 75.2 | 0.976 | C13H18O |
| 11 | 1-oxacyclotetradeca-4,11-diyne | 76.7 | 11.588 | C13H18O |
| 11.09 | 1-oxacyclotetradeca-4,11-diyne | 77.4 | 7.882 | C13H18O |
| 11.75 | 2,4-di-tert-butylphenol | 77.4 | 0.41 | C14H22O |
| 13.03 | cyclohex-2-en-1-ylmethylbenzene | 70.5 | 0.809 | C13H16 |
| 13.81 | 2,2-dimethyl-3-(3-methylpenta-2,4-dienyl)oxirane | 80 | 0.53 | C10H16O |
| 14.59 | (9E,12E)-octadeca-9,12-dienoic acid | 80.2 | 1.131 | C18H32O2 |
| 14.82 | ethyl (E)-octadec-9-enoate | 81.5 | 3.631 | C20H38O2 |
| 16.02 | ethyl pentadecanoate | 83.2 | 1.452 | C17H34O2 |
| 17.01 | ethyl hexadecanoate | 85.9 | 9.036 | C18H36O2 |
Figure 2.
Total ion chromatogram of volatile compounds identified from T. koningiopsis PSU3-2 through GC/MS analysis. Peaks at 2.27, 6.53, and 17.01 min were tentatively identified as azetidine, 2-phenylethanol, and ethyl hexadecanoate, the structures of which are shown. Numbers in parentheses indicate the percentage of peak areas.
3.3. Cell-Wall-Degrading Enzyme Activities
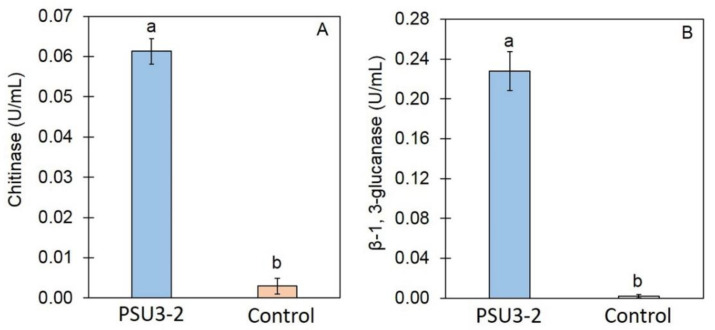
The activity of CWDEs, including chitinase and β-1,3-glucanase, was assayed through the cell-free CF of T. koningiopsis PSU3-2. The enzyme activity of chitinase and β-1,3-glucanase in the cell-free CF of T. koningiopsis PSU3-2 was 0.061 and 0.227 U/mL (Figure 3), respectively, significantly higher (p < 0.05) than that in the control (PDB alone).
Figure 3.
Cell-wall-degrading enzyme activities of cell-free culture filtrate (CF) of T. koningiopsis PSU3-2: (A) enzyme activity of β-1,3-glucanase; (B) enzyme activity of chitinase. Different letters indicate statistically significant differences among treatments (p < 0.05) using Tukey’s test.
3.4. Effect of Cell-Free CF on Fungal Mycelia
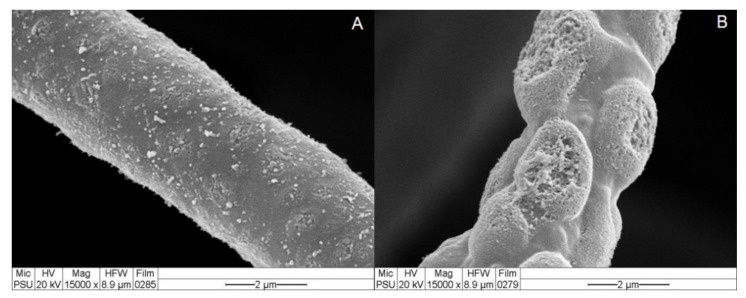
SEM analysis was conducted to confirm the nature of the cell-free CF of T. koningiopsis PSU3-2 containing CWDEs or antifungal compounds responsible for inhibiting the fungal growth of C. gloeosporioides. The SEM micrograph of the control (PDB alone) exhibited no morphological change in the fungal mycelia of the Colletotrichum sp. (Figure 4), whereas the fungal mycelia incubated in the cell-free CF of T. koningiopsis PSU3-2 displayed abnormal shapes and mycelial distortions (Figure 4).
Figure 4.
Effects of cell-wall-degrading enzymes on the fungal morphology of C. gloeosporioides (A) hypha of C. gloeosporioides incubated in potato dextrose broth alone; (B) hypha of C. gloeosporioides incubated in cell-free culture filtrate (CF) of T. koningiopsis PSU3-2.
3.5. Effect of Trichoderma on Lesion Development
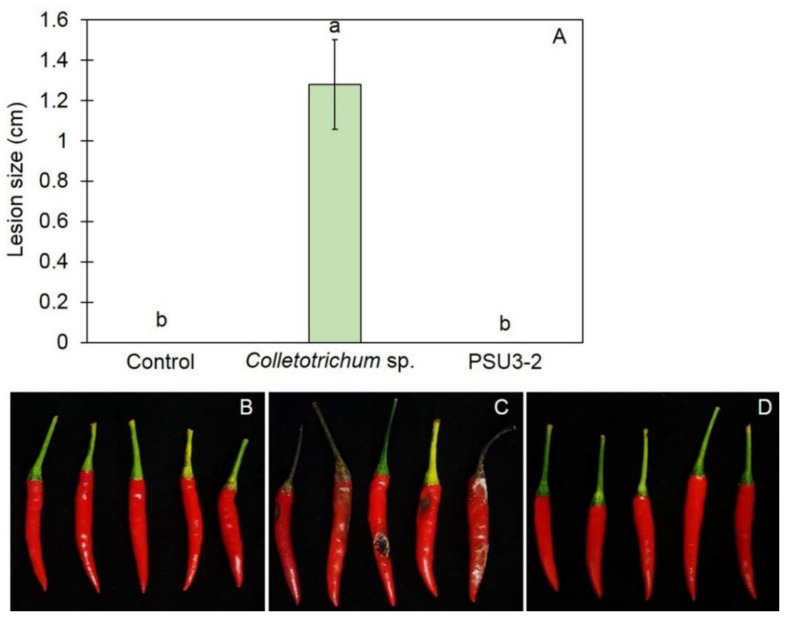
Treatment of T. koningiopsis PSU3-2 using the dipping method prior to inoculation with Colletotrichum sp. significantly reduced the size of anthracnose lesions (p < 0.05) analyzed for all chili peppers in all treatments. The lesion sizes developed on the chili pepper of the untreated control group, the Trichoderma PSU3-2-treated chili pepper, and C. gloeosporioides inoculation alone (control) were 0, 0, and 1.28 cm in diameter, respectively (Figure 5). There was no disease development in the T. koningiopsis PSU3-2-treated chili pepper fruit after incubation for 5 days.
Figure 5.
(A) Lesion sizes developed after inoculation with Colletorichum sp.; (B) chili pepper fruit inoculated with distilled water alone; (C) chili pepper fruit inoculated with Colletorichum sp. alone; (D) chili pepper fruit challenge inoculation with T. koningiopsis PSU3-2 and Colletorichum sp. Different letters indicate statistically significant differences among treatments (p < 0.05) using Tukey’s test.
4. Discussion
Postharvest anthracnose of chili pepper is reportedly caused by Colletotrichum spp., leading to a reduction in both the quality and the quantity of chili pepper production [24,25]. This study investigated the antifungal activity of Trichoderma spp. against postharvest anthracnose of chili pepper fruit. T. koningiopsis PSU3-2 effectively suppressed the fungal growth of the C. gloeosporioides, revealing a competition mechanism (Figure 1). This isolate was documented as being capable of emitting VOCs to restrict the mycelial growth of the C. gloeosporioides (Figure 3), along with overproduction of CWDEs leading to a morphological change in the C. gloeosporioides (Figure 4). Furthermore, treatment with T. koningiopsis PSU3-2 protected chili peppers from postharvest anthracnose decay (Figure 5).
The ability to compete for nutrients and space is commonly found in several Trichoderma spp. to overcome the growth of fungal pathogens through a dual culture assay [3,4,6,31]. In vitro studies revealed the competition mechanism of Trichoderma spp. against Sclerotium sclerotiorum [32], Rhizoctonia solani, Macrophomina phaseolina [33], and Curvularia oryzae [3]. Our findings in this study are in agreement with previous publications that found that T. koningiopsis PSU3-2 grew faster than the C. gloeosporioides, effectively inhibiting the growth of the C. gloeosporioides in PDA-assayed plates, thereby suggesting a competition mechanism involved in biocontrol activity (Figure 1).
VOCs have been reported as being produced and released by several Trichoderma species with a diversity of volatile compounds [31]. The VOCs emitted by Trichoderma species display multiple functions; they have antifungal properties, induce a defense response, and promote plant growth [8,9]. Among the 16 VOCs produced by T. koningiopsis PSU3-2, three compounds, namely, azetidine, 2-phenylethanol, and ethyl hexadecanoate, have been reported to have antimicrobial activity [34,35,36]. For instance, 2-phenylethanol emitted from T. asperellum T76-14 was reported to control the postharvest fruit rot of muskmelon [10]. Therefore, the VOCs of T. koningiopsis PSU3-2 containing azetidine, 2-phenylethanol, and ethyl hexadecanoate may be associated with the suppression of the mycelial growth of the C. gloeosporioides, suggesting the antibiosis mechanism of T. koningiopsis PSU3-2. Several Trichoderma species produce and secrete hydrolytic enzymes responsible for degrading the fungal cell wall. The main CWDEs produced by Trichoderma species are chitinase and β-1,3-glucanase [37]. Chitinase restricts fungal growth by degrading chitin, the major component within the fungal cell wall [38], whereas β-1,3-glucanase hydrolyzes β-glucan to oligosaccharide and glucose [39]. A combination of both enzyme activities strongly suppresses the growth of several plant fungal pathogens [4]. Our results demonstrate a high activity of CWDEs in the cell-free CF of T. koningiopsis PSU3-2 (Figure 3), possibly related to the inhibition of fungal growth. We confirmed through SEM analysis that the cell-free CF of T. koningiopsis PSU3 contained CWDEs, which caused lysis and distortion of the C. gloeosporioides hyphae (Figure 4). The ability to produce CWDEs capable of creating mycelial lysis (holes), further resulting in fungal penetration in the host fungi, suggests mycoparasitism [40]. Baiyee et al. [4] similarly observed high activities of chitinase and β-1,3-glucanase, which caused abnormal changes in the fungal mycelia. These findings may be the result of CWDEs or some type of antifungal compound released by T. koningiopsis PSU3-2. However, we only studied the effects of cell-free CF, and we did not observe other metabolites in this study.
The application of a Trichoderma spore suspension has been shown to successfully control several plant diseases [3,16,41]. Treatment with a spore suspension of Trichoderma spirale T76-1 reduced the disease severity of lettuce leaf spots caused by Corynespora cassiicola and Curvularia aeria [4]. Root dipping with a T. asperellum T1 spore suspension was reported to activate defense responses in lettuce against leaf spot disease [12]. Treatment with Trichoderma protected tomato plants from infection by Phytophthora nicotianae [42]. Jogaiah et al. [43] demonstrated that the application of a Trichoderma virens spore suspension mediated resistance in tomatoes against Fusarium wilt by activating the jasmonic and salicylic pathways. Our study showed that chili peppers dipped in a spore suspension of T. koningiopsis PSU3-2 displayed no anthracnose lesions (Figure 5). Therefore, the biological activity of T. koningiopsis PSU3-2 is able to limit fungal infections, thereby controlling postharvest anthracnose of chili pepper fruit.
5. Conclusions
This study revealed the potential of a new strain of T. koningiopsis PSU3-2 isolated from soil as a biocontrol agent against anthracnose of chili pepper fruit caused by a C. gloeosporioides. The ability to compete for nutrients and space (competition), the production of VOCs (antibiosis), and the production of CWDEs (mycoparasitism) were the main factors contributing to its success in controlling the postharvest anthracnose of chili pepper fruit. The potential to develop a biopesticide to control chili anthracnose using T. koningiopsis PSU3-2 needs to be verified in the near future.
Acknowledgments
The authors would like to specially thank the Prince of Songkla University and the Center of Excellence in Agricultural and Natural Resources Biotechnology (CoE-ANRB) phase 3 for the facilities, PerkinElmer Co. Ltd., Bangkok, Thailand for the GC/MS analysis, the Innovative Agriculture Research Center, Faculty of Agriculture, Chiang Mai University for partial support, and MDPI’s English editing service for English editing.
Author Contributions
Conceptualization, O.-U.R. and A.S.; methodology, O.-U.R., C.P., and K.P.; software, K.P.; validation, O.-U.R. and A.S.; formal analysis, K.P.; investigation, O.-U.R., C.P., and A.S.; resources, O.-U.R., and A.S.; data curation, K.P.; writing—original draft preparation, O.-U.R., C.P., and K.P.; writing—review and editing, A.S.; supervision and project administration, A.S.; funding acquisition, O.-U.R. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Prince of Songkla University annual government statement of expenditure under the Plant Genetic Conservation Project under the Royal initiative of Her Royal Highness Princess Maha Chakri Sirindhorn, Year 2019, grant number NAT620297S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mendes R., Garbeva P., Raaijmakers J.M. The rhizosphere microbiome: Significance of plant beneficial pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- 2.Abdelmoteleb A., González-Mendoza D. A novel Streptomyces rhizobacteria from desert soil with diverse anti-fungal properties. Rhizosphere. 2020;16:100243. doi: 10.1016/j.rhisph.2020.100243. [DOI] [Google Scholar]
- 3.Sunpapao A., Chairin T., Ito S. The biocontrol by Streptomyces and Trichoderma of leaf spot disease caused by Curvularia oryzae in oil palm seedlings. Biol. Control. 2018;123:36–42. doi: 10.1016/j.biocontrol.2018.04.017. [DOI] [Google Scholar]
- 4.Baiyee B., Pornsuriya C., Ito S., Sunapapo A. Trichoderma spirale T76-1 displays biocontrol activity on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol. Control. 2019;129:195–200. doi: 10.1016/j.biocontrol.2018.10.018. [DOI] [Google Scholar]
- 5.Sharma V., Salwan R., Sharma P.N. The comparative mechanistic aspects of Trichoderma and probiotic: Scope for future research. Physiol. Molec. Plant Pathol. 2017;100:84–96. doi: 10.1016/j.pmpp.2017.07.005. [DOI] [Google Scholar]
- 6.Wonglom P., Daengsuwan W., Ito S., Sunpapao A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanism involved. Physiol. Mol. Plant Pathol. 2019;107:1–7. doi: 10.1016/j.pmpp.2019.04.007. [DOI] [Google Scholar]
- 7.Bailey B.A., Bae H., Strem M.D., Crozier J., Thomas S.E., Samuels G.J., Vinyard B.T., Holmes K.A. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol. Control. 2008;46:24–35. doi: 10.1016/j.biocontrol.2008.01.003. [DOI] [Google Scholar]
- 8.Wonglom P., Ito S., Sunpapao A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa) Fungal Ecol. 2020;43:100867. doi: 10.1016/j.funeco.2019.100867. [DOI] [Google Scholar]
- 9.Phoka N., Suwannarach N., Lumyong S., Ito S., Matsui K., Arikit S., Sunpapao A. Role of volatiles from the endophytic fungus Trichoderma asperelloides PSU-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi. 2020;6:341. doi: 10.3390/jof6040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intana W., Kheawleng S., Sunpapao A. Trichoderma asperellum T76-14 Released Volatile Organic Compounds against Postharvest Fruit Rot in Muskmelons (Cucumis melo) Caused by Fusarium incarnatum. J. Fungi. 2021;7:46. doi: 10.3390/jof7010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aamir M., Kashyap S.P., Zehra A., Dubey M.K., Singh V.K., Ansari W.A., Upadhyay R.S., Singh S. Trichoderma erinaceum bio-priming modulates the WRKYs defense programming in tomato against the Fusarium oxysporum f. sp. lycopersici (Fol) challenged condition. Front. Plant Sci. 2019;10:911. doi: 10.3389/fpls.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baiyee B., Ito S., Sunapapo A. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.) Physiol. Mol. Plant Pathol. 2019;106:96–101. doi: 10.1016/j.pmpp.2018.12.009. [DOI] [Google Scholar]
- 13.Zhang S., Gan Y., Xu B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016;7:1045. doi: 10.3389/fpls.2016.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinodkumar S., Indumathi T., Nakkeeran S. Trichoderma asperellum (NVTA2) as a potential antagonist for the management of stem rot in carnation under protected cultivation. Biol. Control. 2017;113:58–64. doi: 10.1016/j.biocontrol.2017.07.001. [DOI] [Google Scholar]
- 15.Galletti S., Burzi P.L., Cerato C., Marinello S., Sala E. Trichoderma as a potential biocontrol agent for Cercospora leaf spot of sugar beet. BioControl. 2008;53:917–930. doi: 10.1007/s10526-007-9113-1. [DOI] [Google Scholar]
- 16.Khalili E., Sadravi M., Naeimi S., Khosravi V. Biological control of rice brown spot with native isolates of three Trichoderma species. Braz. J. Microbiol. 2012;43:297–305. doi: 10.1590/S1517-83822012000100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaac S. Fungal Plant Interaction. Chapman and Hall Press; London, UK: 1992. p. 115. [Google Scholar]
- 18.Roberts P.D., Pernezny K.L., Kucharek T.A. Anthracnose Caused by Colletotrichum spp. on Pepper. [(accessed on 10 February 2021)];2001 Available online: http://edis.ifas.ufl.edu.
- 19.Liu L.P., Shu J., Zhang L., Hu R., Chen C.Q., Yang L.N., Lu B.H., Liu Y.N., Yu L., Wang X., et al. First report of postharvest anthracnose on mango (Mangifera indica) caused by Colletotrichum siamense in China. Plant Dis. 2017;101:833. doi: 10.1094/PDIS-08-16-1130-PDN. [DOI] [Google Scholar]
- 20.Li Q., Shu J., Tang L., Huang S., Guo T., Mo J., Ning P., Hsiang T. First report of mango leaf anthracnose caused by Colletotrichum asianum in Vietnam. Plant Dis. 2020;104:1558. doi: 10.1094/PDIS-09-19-1830-PDN. [DOI] [Google Scholar]
- 21.Zakaria L., Mahak S., Zakaria M., Salleh B. Characterisation of Colletotrichum species associated with anthracnose of banana. Trop. Life Sci. Res. 2009;20:119–125. [PMC free article] [PubMed] [Google Scholar]
- 22.Diao Y.Z., Zhang C., Lin D., Liu X.L. First report of Colletotrichum truncatum causing anthracnose of tomato in China. Plant Dis. 2014;98:678. doi: 10.1094/PDIS-05-13-0491-PDN. [DOI] [PubMed] [Google Scholar]
- 23.Oo M.M., Oh S.K. First report of anthracnose of chili pepper fruit caused by Colletotrichum truncatum in Korea. Plant Dis. 2019;104:564. doi: 10.1094/PDIS-09-19-1874-PDN. [DOI] [Google Scholar]
- 24.Ali A., Bordoh P.K., Singh A., Siddiqui Y., Droby S. Post-harvest development of anthracnose in pepper (Capsicum spp): Etiology and management strategies. Crop Protec. 2016;90:132–141. doi: 10.1016/j.cropro.2016.07.026. [DOI] [Google Scholar]
- 25.Diao Y.-Z., Zhang C., Liu F., Wang W.-Z., Liu L., Cai L., Liu X.-L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia. 2017;38:20–37. doi: 10.3767/003158517X692788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruangwong O.-U., Wonglom P., Suwannarach N., Kumla J., Thaochan N., Chomnunti P., Pitija K., Sunpapao A. Volatile Organic Compound from Trichoderma asperelloides TSU1: Impact on Plant Pathogenic Fungi. J. Fungi. 2021;7:187. doi: 10.3390/jof7030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo F.D.H., Padilla A.M.B., Morales G.G., Siller M.C., Herrera R.R., Gonzales C.A.N., Reyes F.C. In vitro antagonistic action of Trichoderma strains against Sclerotinia sclerotiorum and Sclerotium cepivorum. Am. J. Agric. Biol. Sci. 2011;6:410–417. doi: 10.3844/ajabssp.2011.410.417. [DOI] [Google Scholar]
- 28.Rahman M.A., Begum M.F., Alam M.F. Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Microbiology. 2009;37:277–285. doi: 10.4489/MYCO.2009.37.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennis C., Webster J. Antagonistic properties of species-groups of Trichoderma. Trans. Br. Mycol. Soc. 1971;57:41–48. doi: 10.1016/S0007-1536(71)80078-5. [DOI] [Google Scholar]
- 30.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 31.Stracquadanio C., Quiles J.M., Meca G., Cacciola S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma asperellum and Trichoderma atroviride in Liquid Medium. J. Fungi. 2020;6:263. doi: 10.3390/jof6040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matroudi S., Zamani M.R., Motallebi M. Antagonistic effects of three species of Trichoderma sp. on Sclerotium sclerotiorum, the causal agent of canola stem rot. Egypt. J. Biol. 2009;11:37–44. [Google Scholar]
- 33.Monteiro V., Silva R.N., Steindorff A., Costa F., Noronha E., Ricart C., de Sousa M., Vainstein M., Monteiro V., Ulhoa C. New insight in; Trichoderma harzianum antagonism of fungal plant pathogens by secreted protein analysis. Curr. Microbiol. 2010;61:298–305. doi: 10.1007/s00284-010-9611-8. [DOI] [PubMed] [Google Scholar]
- 34.Choi G.J., Jang K.S., Choi Y.H., Yu J.H., Kim J.-C. Antifungal activity of lower alkyl fatty acid esters against powdery mildews. Plant Pathol. J. 2010;26:360–366. doi: 10.5423/PPJ.2010.26.4.360. [DOI] [Google Scholar]
- 35.Angel L.P.L., Yusof M.T., Ismail I.S., Ping B.T.Y., Azni I.N.A.M., Kamarudin N.H., Sundram S. An in vitro study of antifungal activity of Trichoderma virens 7b and profile of its non-polar antifungal components released against Ganoderma boninense. J. Microbiol. 2016;54:732–744. doi: 10.1007/s12275-016-6304-4. [DOI] [PubMed] [Google Scholar]
- 36.Deep A., Kumar P., Narasimhan B., Lim S.M., Ramasamy K., Mishra R.K., Mani V. 2-Azetidine Derivatives: Synthesis, antimicrobial, anticancer, evaluation and qsar studies. Acta Pol. Pharm. 2016;73:65–78. [PubMed] [Google Scholar]
- 37.Asad S.A., Tabassum A., Hameed A., Hassan F., Afzal A., Khan S.A., Ahmed R., Shahzad M. Determination of lytic enzyme activities of indigenous Trichoderma isolates from Pakistan. Braz. J. Microbiol. 2015;46:1053–1064. doi: 10.1590/S1517-838246420140787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collinge D.B., Kragh K.M., Mikkelsen J.D., Nielsen K.K., Rasmussen U., Vad K. Plant chitinases. Plant J. 1993;3:31–40. doi: 10.1046/j.1365-313X.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- 39.Pitson S.M., Seviour R.J., McDougall B.M. Noncellulolytic fungal β-glucanase: Their physiological and regulation. Enzym. Microb. Technol. 1993;15:178–192. doi: 10.1016/0141-0229(93)90136-P. [DOI] [PubMed] [Google Scholar]
- 40.Sunpapao A. Antagonistic Microorganisms: Current Research and Innovations. Lambert Academic Publishing; Saarbrücken, Germany: 2020. p. 120. [Google Scholar]
- 41.Dawidziuk A., Popiel D., Kaczmarek J., Strakowska J., Jedryczka M. Optimal Trichoderma strains for control of stem canker of brassicas: Molecular basis of biocontrol properties and azole resistance. BioControl. 2016;61:755–768. doi: 10.1007/s10526-016-9743-2. [DOI] [Google Scholar]
- 42.La Spada F., Stracquadanio C., Riolo M., Pane A., Cacciola S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on tomato by modulating plant defense mechanisms and the expression of crinkler, necrosis-inducing Phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020;11:1–16. doi: 10.3389/fpls.2020.583539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jogaiah S., Abdelrahman M., Tran L.-S.P., Ito S. Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylicacid pathways. Mol. Plant Pathol. 2018;19:870–882. doi: 10.1111/mpp.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.