Abstract
The reaction of 5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 3 with formaldehyde solution and primary aromatic amines or 1-substituted piperazines, in ethanol at room temperature yielded the corresponding N-Mannich bases 3-arylaminomethyl-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thiones 4a–l or 3-[(4-substituted piperazin-1-yl)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thiones 5a–d, respectively. The in vitro inhibitory activity of compounds 4a–l and 5a–d was assessed against pathogenic Gram-positive, Gram-negative bacteria, and the yeast-like pathogenic fungus Candida albicans. The piperazinomethyl derivatives 5c and 5d displayed broad-spectrum antibacterial activities the minimal inhibitory concentration (MIC) 0.5–8 μg/mL) and compounds 4j, 4l, 5a, and 5b showed potent activity against the tested Gram-positive bacteria. In addition, the anti-proliferative activity of the compounds was evaluated against prostate cancer (PC3), human colorectal cancer (HCT-116), human hepatocellular carcinoma (HePG-2), human epithelioid carcinoma (HeLa), and human breast cancer (MCF7) cell lines. The optimum anti-proliferative activity was attained by compounds 4l, 5a, 5c, and 5d.
Keywords: 1,3,4-oxadiazoles; N-Mannich bases; antimicrobial activity; anti-proliferative activity
1. Introduction
The 1,3,4-Oxadiazoles are an important class of heterocyclic compounds with diverse pharmacological properties [1,2,3]. The 1,3,4-Oxadiazole nucleus represents an essential building unit in several drugs including the broad spectrum antibacterial drug furamizole [4], the antiretroviral drug raltegravir [5], the anticancer agent zibotentan [6], the anti-obesity/antidiabetic agent AZD 3988 [7], and the antihypertensive drugs tiodazosin [8], and nesadipil [9].
In addition, there is a growing interest in the chemotherapeutic activities of 2,5-disubstituted-1,3,4-oxadiazoles as antibacterial [10,11,12], antifungal [13,14,15], antitubercular [16,17], and antiviral [18,19,20,21] agents. Moreover, 1,3,4-Oxadiazole-2(3H)-thiones, their thioether derivatives and 3-aminomethyl analogues (N-Mannich bases) are the most interesting for their anticancer activities [21,22]. The 1,3,4-Oxadiazole derivatives exert their anticancer activities via different mechanisms, such as targeting epidermal growth factor receptors (EGFR) [23], vascular endothelial growth factor receptors (VEGF) [24], focal-adhesion kinase (FAK) [25,26], histone deacetylases (HDAC) [27,28], methionine aminopeptidase (MetAP) [29], NF-κB (nuclear factor κB) [30], poly(ADP-ribose) polymerase (PARP-1) [31], thymidine phosphorylase (TP) [32], telomerase [33], thymidylate synthase (TS) [34], zinc-finger protein 143 (ZNF143) [35], and tubulin polymerase [36].
Furthermore, 1,3,4-oxadiazoles were proved to exhibit potent anti-inflammatory [37,38,39], antioxidant [40], antidiabetic [41,42], and monoamine oxidase (MAO) inhibitory activities [43,44]. Besides, 1,3,4-oxadiazole derivatives are highly attractive compounds in the development of organic light-emitting diodes (OLEDs) [45,46].
Furthermore, 3,4-dimethoxyphenyl moiety represents an essential motif in various chemotherapeutic agents with anticancer [47,48,49,50,51,52], antibacterial [53,54], and antiviral [55] activities.
Motivated by the above-mentioned findings and following an ongoing interest in the pharmacological [56] and structural properties [57,58,59] of 1,3,4-oxadiazole-2(3H)-thione N-Mannich bases, we report herein the synthesis, characterization, antibacterial, antifungal, and anti-proliferative activities of related series of 5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione N-Mannich bases.
2. Results and Discussion
2.1. Chemical Synthesis
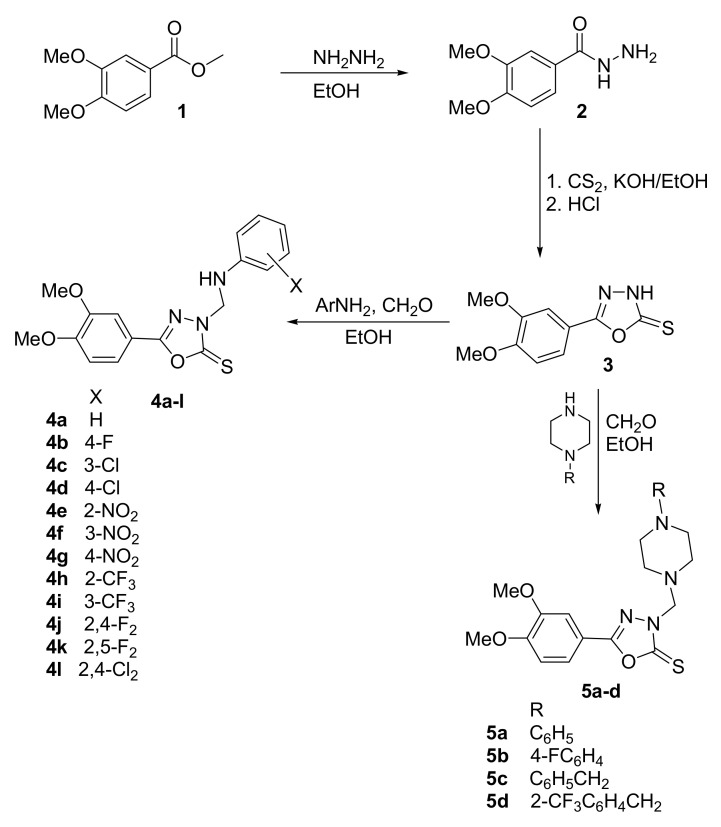
The 3,4-Dimethoxybenzohydrazide 2 was prepared from the commercially-available methyl 3,4-dimethoxybenzoate 1 via treatment with hydrazine in ethanol [60]. 1,3,4-Oxadiazole-2(3H)-thione 3 was obtained via reaction of the carbohydrazide 2 with carbon disulfide in ethanolic potassium hydroxide as previously described [61]. Furthermore, 1,3,4-Oxadiazole-2(3H)-thiones were reported to undergo aminomethylation through reaction with primary aromatic amines and formaldehyde to yield the corresponding N-Mannich bases [62]. Consequently, treatment of 3 with formaldehyde solution and various primary aromatic amines or 1-substituted piperazines, in ethanol at room temperature yielded their corresponding 3-arylaminomethyl 4a–l or 3-piperazinomethyl 5a–d N-Mannich bases, respectively, in good yields (Scheme 1, Table 1).
Scheme 1.
Synthesis of compounds 4a–l and 5a–d.
Table 1.
Crystallization solvents, melting points (M.P.), yield percentages, molecular (Mol.) formulae, and molecular weights (Wt.) of compounds 4a–l and 5a–d.
| Compound No. | X/R | Crystallization Solvents | M.P. (°C) | Yield (%) | Mol. Formula (Mol. Wt.) |
|---|---|---|---|---|---|
| 4a | H | EtOH/H2O | 147–149 | 82 | C17H17N3O3S (343.40) |
| 4b | 4-F | EtOH/H2O | 135–137 | 84 | C17H16FN3O3S (361.39) |
| 4c | 3-Cl | EtOH | 143–145 | 77 | C17H16ClN3O3S (377.85) |
| 4d | 4-Cl | EtOH | 166–168 | 79 | C17H16ClN3O3S (377.85) |
| 4e | 2-NO2 | EtOH/CHCl3 | 211–213 | 88 | C17H16N4O5S (388.40) |
| 4f | 3-NO2 | EtOH/CHCl3 | 181–183 | 85 | C17H16N4O5S (388.40) |
| 4g | 4-NO2 | EtOH/CHCl3 | 222–224 | 92 | C17H16N4O5S (388.40) |
| 4h | 2-CF3 | EtOH/H2O | 220–222 | 90 | C18H16F3N3O3S (411.40) |
| 4i | 3-CF3 | EtOH/H2O | 206–208 | 86 | C18H16F3N3O3S (411.40) |
| 4j | 2,4-F2 | EtOH | 169–171 | 92 | C17H15F2N3O3S (379.38) |
| 4k | 2,5-F2 | EtOH | 212–214 | 90 | C17H15F2N3O3S (379.38) |
| 4l | 2,4-Cl2 | EtOH | 227–229 | 94 | C17H15Cl2N3O3S (412.29) |
| 5a | C6H5 | EtOH | 151–153 | 85 | C21H24N4O3S (412.51) |
| 5b | 4-FC6H4 | EtOH | 118–120 | 78 | C21H23FN4O3S (430.50) |
| 5c | C6H5CH2 | EtOH/H2O | 121–123 | 75 | C22H26N4O3S (426.53) |
| 5d | 2-CF3C6H4CH2 | EtOH | 141–143 | 89 | C23H25F3N4O3S (494.53) |
The structures of compounds 4a–l and 5a–d were confirmed by elemental analyses, 1H NMR and 13C NMR spectral data.
2.2. In Vitro Antibacterial and Antifungal Activities
The in vitro growth inhibitory activity of compounds 4a–l and 5a–d was evaluated towards the standard Gram-positive bacterial strains Staphylococcus aureus American type culture collection (ATCC) 6571, Bacillus subtilis ATCC 5256 and Micrococcus luteus ATCC 27141, Gram-negative bacterial strains Escherichia coli ATCC 8726, and Pseudomonas aeruginosa ATCC 27853, and the yeast-like pathogenic fungus Candida albicans MTCC 227. The initial screening was performed by the semi-quantitative agar-disc diffusion method using Müller-Hinton agar medium [63]. The results of the preliminary screening of compounds 4a–l and 5a–d (200 μg/disc); the antibacterial antibiotics Gentamicin sulfate, Ampicillin trihydrate, and the antifungal drug Clotrimazole (100 μg/disc); and the calculated log P values (Clog P) are depicted in Table 2.
Table 2.
In vitro activity of compounds 4a–l and 5a–d (200 μg/8 mm disc); the broad-spectrum antibacterial drugs Gentamicin sulfate, Ampicillin trihydrate, and the antifungal drug Clotrimazole (100 μg/8 mm disc) against Staphylococcus aureus American type culture collection (ATCC) 6571 (SA), Bacillus subtilis ATCC 5256 (BS), Micrococcus luteus ATCC 27,141 (ML), Escherichia coli ATCC 8726 (EC), Pseudomonas aeruginosa ATCC 27,853 (PA), and the yeast-like pathogenic fungus Candida albicans MTCC 227 (CA).
| Comp. No. |
C log Pc | Diameter of Growth Inhibition Zone (mm) a | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | ||
| 4a | 3.701 | 14 | 13 | 15 | - | - | 10 |
| 4b | 4.146 | 15 | 16 | 17 | - | - | 12 |
| 4c | 4.716 | 14 | 17 | 13 | - | - | - |
| 4d | 4.716 | 15 | 13 | 14 | - | - | - |
| 4e | 4.242 | 12 | 11 | 10 | - | - | 13 |
| 4f | 4.092 | 11 | 12 | - | - | - | 12 |
| 4g | 4.092 | 13 | 12 | 11 | - | - | 16 |
| 4h | 5.113 | 15 | 12 | - | - | - | - |
| 4i | 5.113 | 16 | 12 | - | - | - | - |
| 4j | 4.395 | 19 (4) b | 12 | 16 | 12 | 13 | 11 |
| 4k | 4.395 | 17 | 14 | 15 | 14 | - | 12 |
| 4l | 5.535 | 19 (4) b | 21 (2) b | 18 (16) b | 15 | 12 | 11 |
| 5a | 3.789 | 23 (1) b | 26 (1) b | 21 (1) b | 14 | 13 | - |
| 5b | 4.103 | 20 (8) b | 23 (1) b | 20 (1) b | 16 | 17 | - |
| 5c | 4.712 | 26 (1) b | 30 (0.5) b | 22 (1) b | 19 (4) b | 18 (4) b | - |
| 5d | 5.595 | 28 (1) b | 29 (1) b | 26 (1) b | 22 (1) b | 20 (2) b | - |
| Gentamicin sulfate | 27 (1) b | 26 (2) b | 20 (2) b | 22 (0.5) b | 21 (0.5) b | NT | |
| Ampicillin trihydrate | 22 (2) b | 23 (1) b | 20 (2) b | 16 (8) b | 16 (8) b | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 (4) b | |
a (-): inactive (inhibition zone < 10 mm), b Figures shown in parentheses represent the minimal inhibitory concentration (MIC) values (μg/mL), c Calculated using the CS ChemOffice Ultra version 8.0, CambridgeSoft, Cambridge, MA, USA), NT: not tested. SA, Staphylococcus aureus; BS, Bacillus subtilis; ML, Micrococcus luteus; EC, Escherichia coli; PA, Pseudomonas aeruginosa; CA, Candida albicans. High activity (>18 mm) values have been bolded for emphasis.
The results revealed that potent antibacterial activity was displayed by the compounds 4j, 4l, 5a, 5b, 5c, and 5d, which displayed growth inhibition zones ≥ 18 mm against one or more of the tested microorganisms. In addition, compounds 4a, 4b, 4c, 4d, 4h, 4i, and 4k showed medium activity (growth inhibition zones 14–17 mm) and compounds 4e, 4f, and 4g showed poor antibacterial activity (growth inhibition zones 10–13 mm) against the tested microorganisms. In general, the activity against the tested Gram-positive bacteria is higher than the activity against the tested Gram-negative bacteria. The optimal antibacterial activity was attained by compounds 5c and 5d, which showed potent and broad-spectrum antibacterial activity against all the tested bacterial strains. The inhibitory activity of the compounds against Candida albicans was generally lower than their antibacterial activity, compounds 4g displayed medium activity, and all other compounds were either poorly active or inactive compared with Clotrimazole.
The minimal inhibitory concentrations (MICs) of the most active compounds 4j, 4l, 5a, 5b, 5c, and 5d, and the antibacterial antibiotics Gentamicin sulfate, Ampicillin trihydrate, and the antifungal drug Clotrimazole were determined by the microdilution susceptibility method in Müller–Hinton broth and Sabouraud liquid medium [64]. The MIC values were highly consistent with their growth inhibition zones.
Based on the results of the antibacterial activity, it could be concluded that the activity of the piperazinomethyl N-Mannich bases 5a–d is superior to their arylaminomethyl analogues 4a–l. Considering the anilinomethyl analogue 4a as the basic structure of the arylaminomethyl analogues 4a–l, the antibacterial activity of the monohalo derivatives 4b–d was slightly improved against the tested Gram-positive bacteria. Meanwhile, the antibacterial activity of the nitro derivatives 4e–g was greatly declined with general improvement of the antifungal activity. Despite the high lipophilicity of the trifluoromethyl derivatives 4h and 4i, the compounds only retained moderate activity against Staphylococcus aureus and lacked activity against the tested Gram-positive bacteria and Candida albicans. Introduction a difluoro- or dichlorophenyl moieties (compounds 4j–l) greatly enhanced the Gram-positive antibacterial activity and these derivatives endowed moderate or marginal activity against the tested Gram-negative bacteria and Candida albicans.
The replacement of the arylaminomethyl moiety with a piperazinomethyl moiety greatly enhanced the antibacterial activity and the piperazinomethyl derivatives 5a–d exhibited higher potency and broader antibacterial spectrum compared to their arylaminomethyl analogues 4a–l, with no antifungal activity. In addition, the antibacterial activity of the piperazinomethyl derivatives seems correlated to their lipophilicity as the optimum antibacterial activity against the tested Gram-negative bacteria was displayed by compounds 5c and 5d.
2.3. In Vitro Anti-proliferative Activity
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay [65,66] was adopted to evaluate the in vitro anti-proliferative activity of compounds 4a–l and 5a–d against five human cancer cell lines namely; prostate cancer (PC3), human colorectal cancer (HCT-116), human hepatocellular carcinoma (HePG-2), human epithelioid carcinoma (HeLa) and human breast cancer (MCF7) cell lines. The results of the anti-proliferative activity of compounds 4a–l, 5a–d, and the anticancer drug Doxorubicin [67] are displayed in Table 3.
Table 3.
In vitro anti-proliferative activity of the tested compounds 4a–l, 5a–d, and Doxorubicin expressed as IC50 values against prostate cancer (PC3), human colorectal cancer (HCT-116), human hepatocellular carcinoma (HePG-2), human epithelioid carcinoma (HeLa), and MCF7 human cancer cell line.
| Comp. No. |
IC50 (µM) a | ||||
|---|---|---|---|---|---|
| PC3 | HCT-116 | HePG-2 | HeLa | MCF7 | |
| 4a | 63.94 ± 3.8 | 53.17 ± 3.3 | 47.23 ± 3.1 | 28.31 ± 2.0 | 55.34 ± 2.8 |
| 4b | 71.80 ± 4.0 | 77.52 ± 4.1 | 56.34 ± 3.3 | 49.47 ± 3.0 | 68.26 ± 3.4 |
| 4c | 84.52 ± 4.8 | 39.44 ± 2.6 | 78.86 ± 4.1 | 44.69 ± 2.8 | 33.86 ± 2.3 |
| 4d | 78.35 ± 4.5 | 48.30 ± 3.0 | 73.80 ± 3.9 | 54.02 ± 3.2 | 40.52 ± 2.5 |
| 4e | >100 | 92.11 ± 4.9 | >100 | 75.61 ± 3.9 | 88.33 ± 3.9 |
| 4f | 74.67 ± 4.4 | 69.38 ± 3.8 | 58.41 ± 3.5 | 57.26 ± 2.5 | 65.35 ± 3.2 |
| 4g | >100 | 92.11 ± 4.9 | >100 | 75.61 ± 3.9 | 88.33 ± 3.9 |
| 4h | 59.48 ± 3.5 | 35.01 ± 2.7 | 42.74 ± 2.9 | 31.72 ± 2.2 | 29.10 ± 2.1 |
| 4i | 59.48 ± 3.5 | 35.01 ± 2.7 | 42.74 ± 2.9 | 31.72 ± 2.2 | 29.10 ± 2.1 |
| 4j | 95.61 ± 5.1 | 64.07 ± 3.5 | 86.45 ± 4.5 | 61.98 ± 3.5 | 59.87 ± 2.7 |
| 4k | 75.22 ± 4.1 | 61.07 ± 3.4 | 77.40 ± 3.6 | 49.55 ± 3.2 | 29.56 ± 3.6 |
| 4l | 34.60 ± 2.3 | 19.95 ± 1.8 | 17.42 ± 1.4 | 10.96 ± 1.1 | 12.97 ± 1.0 |
| 5a | 52.53 ± 3.3 | 27.49 ± 2.3 | 36.08 ± 2.5 | 24.09 ± 1.8 | 17.80 ± 1.3 |
| 5b | >100 | 89.26 ± 4.6 | 91.78 ± 4.9 | 67.53 ± 3.7 | 79.16 ± 3.6 |
| 5c | 23.92 ± 1.9 | 14.69 ± 1.2 | 11.93 ± 1.0 | 9.50 ± 0.8 | 6.49 ± 0.4 |
| 5d | 38.02 ± 2.5 | 32.81 ± 2.6 | 22.91 ± 1.6 | 18.37 ± 1.4 | 24.33 ± 1.9 |
| Doxorubicin | 8.87 ± 0.6 | 5.23 ± 0.3 | 4.50 ± 0.2 | 5.57 ± 0.4 | 4.17 ± 0.2 |
a IC50 values presented as the mean ± SD of three separate determinations. Significant values (<25 µm) have been bolded for emphasis.
According to the anti-proliferative activity results, the tested compounds exhibited variable degrees of activity against the tested cancer cell lines. In general, the activity against MCF-7 and HeLa seems higher than PC-3, HCT-116, and HePG-2. In addition, the activity of the piperazinomethyl analogues is higher than their arylaminomethyl analogues. The optimal activity was attained by compounds 4l, 5a, 5c, and 5d with IC50 < 25 μM against the tested cell lines.
Within the arylaminomethyl analogues 4a–l, the compounds were inactive against PC3 cell lines (IC50 > 100 μM) or weakly active (IC50 = 51–100 μM) except compounds 4l, which retained moderate activity (IC50 34.60 μM). The anti-proliferative activity of the arylaminomethyl analogues 4a–l seems dependent on the aryl substituents, the 2,4-dichlorophenyl is the optimal substituents while the nitrophenyl substitution at 2- or 4- position (compounds 4e,g) greatly deteriorated the anti-proliferative activity. The anilinomethyl analogue 4a, monohalophenylaminomethyl analogues 4b–d, the trifluoromethylphenyl aminomethyl analogues 4h,i and the difluorophenylaminomethyl analogues 4j,k analogues only retained moderate anti-proliferative activity (IC50 = 26–50 μM) or poor activity (IC50 = 51–100 μM).
Concerning the anti-proliferative activity of the piperazinomethyl derivatives 5a–d, the 4-phenylpiperazinomethyl derivative 5a exhibited potent activity against HeLa and MFC7 cell lines, moderate activity against HCT-116 cell lines and weak activity against PC3 cell lines. Substitution of the phenyl group of compound 5a with 4-fluorophenyl group (compound 5b) dramatically deteriorated the anti-proliferative activity. The optimum antiproliferative activity was attained by the 4-benzylpiperazinomethyl derivative 5c, which showed potent activity against all the tested cell lines. Replacement of the benzyl group of compound 5c with 2-trifluoromethylbenzyl group (compound 5d) reduced the anti-proliferative activity against the tested cancer cell lines.
3. Materials and Methods
3.1. General Information
Melting points (°C, uncorrected) were determined in open glass capillaries using a Barnstead 9100 electro-thermal melting point apparatus. Nuclear magnetic resonance (NMR) spectra were determined in DMSO-d6 on a JEOL ECA 500 III at 500.16 MHz for 1H and 125.77 MHz for 13C. Elemental analyses (C, H, N, and S) were in agreement with the proposed structures within ± 0.4% of the theoretical values (Table S1). Monitoring of the reactions and checking of the purity of the final products were carried out by thin layer chromatography (TLC) using silica gel pre-coated aluminum sheets (60 F254; Merck) and visualization with ultraviolet light (UV) at 365 and 254 nm and/or stained with an anisaldehyde solution and a phosphomolybdic acid solution. All chemicals and solvents were purchased from Alfa Aesar (Germany), and used without additional purification. The reference drugs Gentamicin sulfate (CAS 1405-41-0), Ampicillin trihydrate (CAS 7177-48-2), Clotrimazole (CAS 23593-75-1) and Doxorubicin (CAS 23214-92-8) were purchased from Sigma-Aldrich Chemie GmbH (Germany). Compound 3 was prepared according to the previously reported procedure [61].
3.2. Synthesis of 3-(Arylaminomethyl)-5-(3,4-Dimethoxyphenyl)-1,3,4-Oxadiazole-2(3H)-Thiones 4a–l and 3-[(4-Substituted Piperazin-1-yl)Methyl]-5-(3,4-Dimethoxyphenyl}-1,3,4-Oxadiazole- 2(3H)-Thiones 5a–d
The appropriate primary aromatic amine or 1-substituted piperazine (0.01 mole) and 37% formaldehyde solution (1.0 mL) were added to a hot solution of 5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 3 (1.19 g, 5.0 mmole), in ethanol (10 mL), and the mixture was stirred at room temperature for 5 h and allowed to stand overnight. Water (5 mL) was then added drop-wisely to the reaction mixture with continuous stirring for one hour. The separated precipitate was filtered, washed with water, dried, and crystallized.
3-Anilinomethyl-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4a. Fine colorless needle crystals. 1H NMR: δ 3.84–3.90 (m, 7H, OCH3 & NH), 6.01–6.09 (m, 2H, CH2), 6.69 (t, 1H, Ar-H, J = 7.0 Hz), 6.91 (d, 2H, Ar-H, J = 8.5 Hz), 7.08–7.33 (m, 5H, Ar-H). 13C NMR: δ 55.6, 55.8 (OCH3), 57.9 (CH2), 108.4, 112.1, 112.9, 114.0, 117.9, 120.0, 129.1, 145.3, 149.1, 152.4 (Ar-C), 159.0 (Oxadiazole C5), 175.3 (C=S).
3-[(4-Fluorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4b. Fine colorless needle crystals. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.49 (d, 2H, CH2, J = 7.0 Hz), 6.89–6.94 (m, 2H, Ar-H), 6.99–7.06 (m, 2H, Ar-H), 7.21–7.25 (m, 1H, Ar-H), 7.32 (d, 1H, Ar-H, J = 1.5 Hz), 7.51 (d, 1H, Ar-H, J = 1.5 Hz). 13C NMR: δ 55.7, 55.8 (OCH3), 58.3 (CH2), 108.4, 112.1, 114.0 (d, JC-F = 5.0 Hz), 115.6 (d, JC-F = 23.0 Hz), 119.1 (d, JC-F = 7.0 Hz), 120.1, 141.9, 149.1, 152.4, 155.4 (d, JC-F = 232.5 Hz) (Ar-C), 159.0 (Oxadiazole C5), 175.4 (C=S).
3-[(3-Chlorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4c. Fine colorless needle crystals. 1H NMR: δ 3.87–3.88 (m, 7H, OCH3 & NH), 5.50 (d, 2H, CH2, J = 7.0 Hz), 6.73 (d, 1H, Ar-H, J = 1.5 Hz), 6.87 (d, 1H, Ar-H, J = 1.5 Hz), 7.02–7.05 (m, 1H, Ar-H), 7.17–7.19 (m, 1H, Ar-H), 7.33 (d, 1H, Ar-H, J = 1.5 Hz), 7.51 (d, 1H, Ar-H, J = 1.5 Hz), 7.55 (t, 1 H, Ar-H, J = 7.0 Hz). 13C NMR: δ 55.6, 55.8 (OCH3), 57.5 (CH2), 108.4, 111.8, 112.1, 112.5, 113.9, 117.5, 120.1, 130.7, 133.8, 147.1, 149.1, 152.4 (Ar-C), 159.1 (Oxadiazole C5), 175.4 (C=S).
3-[(4-Chlorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4d. Fine colorless needle crystals. 1H NMR: δ 3.87–3.88 (s, 7H, OCH3 & NH), 5.49 (d, 2H, CH2, J = 7.0 Hz), 6.93 (d, 2H, Ar-H, J = 9.0 Hz), 7.20–7.22 (m, 2H, Ar-H), 7.32 (d, 1 H, Ar-H, J = 1.5 Hz), 7.42 (d, 1 H, Ar-H, J = 8.0 Hz), 7.50 (d, 1 H, Ar-H, J = 1.5, 8.0 Hz). 13C NMR: δ 55.7, 55.8 (OCH3), 57.8 (CH2), 108.4, 112.1, 113.9, 114.5, 120.1, 121.5, 128.8, 144.4, 149.1, 152.4, 159.1 (Oxadiazole C5), 175.4 (C=S).
3-[(2-Nitrophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4e. Pale yellow block crystals. 1H NMR: δ 3.85–3.89 (m, 7H, OCH3 & NH), 5.77 (d, 2H, CH2, J = 7.0 Hz), 6.89–6.95 (m, 1H, Ar-H), 7.16–7.21 (m, 1H, Ar-H), 7.44–7.48 (m, 1H, Ar-H), 7.49–7.54 (m, 1H, Ar-H), 7.65–7.70 (m, 1H, Ar-H), 8.16 (d, 1H, Ar-H, J = 1.5 Hz), 8.85–8.91 (m, 1H, Ar-H). 13C NMR: δ 55.7, 55.8 (OCH3), 56.6 (CH2), 108.5, 112.1, 115.4, 117.9, 119.8, 120.0, 125.4, 126.5, 136.6, 142.2, 149.2, 152.5 (Ar-C), 159.3 (Oxadiazole C5), 175.5 (C=S).
3-[(3-Nitrophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4f. Pale yellow block crystals. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.59 (d, 2H, CH2, J = 7.0 Hz), 7.19 (d, 1H, Ar-H, J = 9.0 Hz), 7.32–7.36 (m, 1H, Ar-H), 7.45 (t, 1 H, Ar-H, J = 8.0 Hz), 7.51 (d, 1H, Ar-H, J = 1.5 Hz), 7.55 (d, 1H, Ar-H, J = 1.5 Hz), 7.87–7.94 (m, 2H, Ar-H). 13C NMR: δ 55.6, 55.8 (OCH3), 57.4 (CH2), 106.9, 108.4, 112.0, 112.4, 113.9, 119.6, 120.1, 130.2, 146.9, 148.8, 149.1, 152.4 (Ar-C), 159.0 (Oxadiazole C5), 175.5 (C=S).
3-[(4-Nitrophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4g. Pale yellow block crystals. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.61 (d, 2H, CH2, J = 7.0 Hz), 7.07 (d, 2H, Ar-H, J = 9.0 Hz), 7.19 (d, 1H, Ar-H, J = 8.5 Hz), 7.33 (d, 1H, Ar-H, J = 2.0 Hz), 7.52 (d, 1H, Ar-H, J = 2.0 Hz), 8.12 (d, 2H, Ar-H, J = 9.0 Hz). 13C NMR: δ 55.7, 55.8 (OCH3), 56.7 (CH2), 108.5, 112.1, 112.5, 113.9, 120.2, 126.0, 138.1, 149.1, 152.1, 152.5 (Ar-C), 159.2 (Oxadiazole C5), 175.5 (C=S).
3-[(2-Trifluoromethylphenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4h. Colorless prism crystals. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.61 (d, 2H, CH2, J = 6.5 Hz), 6.89 (t, 2H, Ar-H, J = 8.0 Hz), 7.24 (d, 2H, Ar-H, J = 9.0 Hz), 7.31–7.37 (m, 2H, Ar-H), 7.50–7.52 (m, 2H, Ar-H). 13C NMR: δ 55.7, 55.8 (OCH3), 57.3 (CH2), 108.5, 112.0, 114.6, 115.0, 116.7, 117.8, 119.8, 120.1, 126.6, 133.0, 133.7, 149.1, 152.1 (Ar-C & CF3), 160.6 (Oxadiazole C5), 177.2 (C=S).
3-[(3-Trifluoromethylphenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4i. White amorphous powder. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.56 (d, 2H, CH2, J = 7.5 Hz), 7.02 (d, 1H, Ar-H, J = 7.5 Hz), 7.17–7.21 (m, 1H, Ar-H), 7.31–7.34 (m, 2H, Ar-H), 7.37–7.42 (m, 1H, Ar-H), 7.51 (d, 1H, Ar-H, J = 1.5 Hz), 7.71 (t, 1 H, Ar-H, J = 7.5 Hz). 13C NMR: δ 55.6, 55.8 (OCH3), 57.4 (CH2), 108.3, 109.0, 112.0, 113.9, 115.4, 117.0, 120.1, 123.3, 125.5, 130.1, 146.2, 149.1, 152.4 (Ar-C & CF3), 159.0 (Oxadiazole C5), 175.5 (C=S).
3-[(2,4-Difluorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4j. Colorless needle crystals. 1H NMR: δ 3.86-3.90 (m, 7H, OCH3 & NH), 5.52 (d, 2H, CH2, J = 7.0 Hz), 6.95–7.04 (m, 2H, Ar-H), 7.17–7.21 (m, 2H, Ar-H), 7.32 (d, 1H, Ar-H, J = 1.5 Hz), 7.49–7.52 (m, 1H, Ar-H). 13C NMR: δ 55.6, 55.8 (OCH3), 57.9 (CH2), 104.0 (d, JC-F = 23.05 Hz), 108.4, 111.0 (d, JC-F = 19.0 Hz), 112.0, 113.8 (d, JC-F = 8.5 Hz), 113.9, 120.1, 130.2 (d, JC-F = 13.0 Hz), 149.1, 152.4, 154.3 (d, JC-F = 225.5 Hz), 159.7 d, JC-F = 237.5 Hz) (Ar-C), 159.2 (Oxadiazole C5), 175.4 (C=S).
3-[(2,5-Difluorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4k. Colorless needle crystals. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.51 (d, 2H, CH2, J = 6.5 Hz), 7.02–7.07 (m, 1H, Ar-H), 7.12–7.16 (m, 1H, Ar-H), 7.18–7.21 (m, 1H, Ar-H), 7.33 (d, 1H, Ar-H, J = 1.5 Hz), 7.39–7.44 (m, 1H, Ar-H), 7.49–7.53 (m, 1H, Ar-H). 13C NMR: δ 55.7, 55.8 (OCH3), 57.1 (CH2), 100.5 (d, JC-F = 27.5 Hz), 103.2 (d, JC-F = 7.0 Hz), 108.4, 111.9, 112.0, 114.6, 115.6 (d, JC-F = 11.0 Hz), 119.8, 120.1, 149.1, 152.1, 159.0 (d, JC-F = 241.0 Hz) (Ar-C), 159.7 (Oxadiazole C5), 175.5 (C=S).
3-[(2,4-Dichlorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione4l. Fine colorless needle crystals. 1H NMR: δ 3.86–3.90 (m, 7H, OCH3 & NH), 5.58 (d, 2H, CH2, J = 7.0 Hz), 7.04–7.08 (m, 1H, Ar-H), 7.16–7.20 (m, 1H, Ar-H), 7.28–7.33 (m, 2H, Ar-H), 7.47–7.52 (m, 2H, Ar-H). 13C NMR: δ 55.7, 55.8 (OCH3), 57.4 (CH2), 108.5, 112.0, 113.8, 113.9, 119.1, 120.1, 121.7, 127.9, 128.8, 140.5, 149.1, 152.4 (Ar-C), 159.2 (Oxadiazole C5), 175.4 (C=S).
5-(3,4-Dimethoxyphenyl)-3-[(4-phenylpiperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione5a. Fine colorless needle crystals. 1H NMR: δ 2.89-2.99 (m, 4H, Piperazine-H), 3.13–3.23 (m, 4H, Piperazine-H), 3.89 (s, 6H, OCH3), 5.13 (s, 2H, CH2), 6.80 (t, 1H, Ar-H, J = 7.0 Hz), 6.96 (d, 2H, Ar-H, J = 8.0 Hz), 7.18–7.26 (m, 3H, Ar-H), 7.36–7.39 (m, 1H, Ar-H), 7.55 (d, 1H, Ar-H, J = 8.0 Hz). 13C NMR: δ 48.3, 49.6 (Piperazine-C), 55.7, 55.8 (OCH3), 69.7 (CH2), 108.5, 112.0, 114.1, 115.7, 119.0, 120.1, 128.9, 149.1, 151.0, 152.3 (Ar-C), 158.7 (Oxadiazole C5), 177.2 (C=S).
5-(3,4-Dimethoxyphenyl)-3-{4-[(4-fluorophenyl)piperazin-1-yl]methyl}-1,3,4-oxadiazole-2(3H)-thione5b. Colorless needle crystals. 1H NMR: δ 2.92–2.95 (m, 4H, Piperazine-H), 3.09–3.16 (m, 4H, Piperazine-H), 3.89 (s, 6H, OCH3), 5.12 (s, 2H, CH2), 6.96–6.98 (m, 2H, Ar-H), 7.05-7.07 (m, 1H, Ar-H), 7.08-7.19 (m, 1H, Ar-H), 7.21 (d, 1H, Ar-H, J = 1.5 Hz), 7.37 (d, 2H, Ar-H, J = 8.0 Hz). 13C NMR: δ 49.1, 49.6 (Piperazine-C), 55.7, 55.8 (OCH3), 69.7 (CH2), 108.5, 112.0, 114.2, 115.3 (d, JC-F = 21.5 Hz), 117.4 (d, JC-F = 7.0 Hz), 120.1, 147.0, 149.1, 152.3, 156.1 (d, JC-F = 236.5 Hz) (Ar-C),158.6 (Oxadiazole C5), 177.2 (C=S).
5-(3,4-Dimethoxyphenyl)-3-[(4-benzylpiperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione5c. White amorphous powder. 1H NMR: δ 2.30–2.50 (m, 4H, Piperazine-H), 2.74–2.88 (m, 4H, Piperazine-H), 3.50 (s, 2H, Benzylic CH2), 3.89 (s, 6H, OCH3), 5.05 (s, 2H, CH2), 7.19 (d, 1H, Ar-H, J = 9.0 Hz), 7.24–7.41 (m, 6H, Ar-H), 7.53 (d, 1H, Ar-H, J = 8.0 Hz). 13C NMR: δ 49.5, 52.3 (Piperazine-C), 55.7, 55.8 (OCH3), 61.9 (Benzylic CH2), 69.7 (CH2), 108.5, 112.0, 114.3, 120.0, 127.0, 128.1, 128.8, 137.8, 149.1, 152.2 (Ar-C), 158.6 (Oxadiazole C5), 177.2 (C=S).
5-(3,4-Dimethoxyphenyl)-3-{4-[(2-trifluorobenzyl)piperazin-1-yl]methyl}-1,3,4-oxadiazole-2(3H)-thione5d. Colorless needle crystals. 1H NMR: δ 2.38–2.52 (m, 4H, Piperazine-H), 2.78-2.90 (m, 4H, Piperazine-H), 3.64 (s, 2H, Benzylic CH2), 3.89 (s, 6H, OCH3), 5.06 (s, 2H, CH2), 7.20 (d, 1H, Ar-H, J = 8.0 Hz), 7.37 (d, 1H, Ar-H, J = 1.5 Hz), 7.47 (t, 1H, Ar-H, J = 7.0 Hz), 7.55 (d, 1H, Ar-H, J = 8.0 Hz), 7.64 (t, 1H, Ar-H, J = 8.0 Hz), 7.71 (d, 1H, Ar-H, J = 8.0 Hz), 7.76 (d, 1H, Ar-H, J = 8.0 Hz). 13C NMR: δ 49.7, 52.6 (Piperazine-C), 55.7, 55.8 (OCH3), 57.5 (Benzylic CH2), 69.8 (CH2), 108.5, 112.0, 114.2, 118.5, 120.1, 123.4, 125.7, 127.3, 130.3, 132.4, 137.2, 149.1, 152.3 (Ar-C & CF3), 158.6 (Oxadiazole C5), 177.2 (C=S).
4. Conclusions
Sixteen 1,3,4-oxadiazole-linked N-Mannich bases namely; 3-arylaminomethyl-5-(3,4- dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thiones 4a–l and 3-[(4-substituted piperazin-1- yl)-methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thiones 5a–d were synthesized and their structures were confirmed by 1H NMR, 13C NMR and elemental analysis. The in vitro inhibitory activity of compounds 4a–l and 5a–d was assessed against a panel of standard pathogenic Gram-positive bacteria, Gram-negative bacteria, and the yeast-like pathogenic fungus Candida albicans. Compounds 5c and 5d displayed potent broad spectrum antibacterial activities and compounds 4j, 4l, 5a, and 5b showed potent activity against the tested Gram-positive bacteria. The anti-proliferative activity of the compounds was evaluated against prostate cancer (PC3), human colorectal cancer (HCT-116), human hepatocellular carcinoma (HePG-2), human epithelioid carcinoma (HeLa), and human breast cancer (MCF7) cell lines. Compounds 4l, 5a, 5c, and 5d showed potent inhibition of cell proliferation in almost all the tested cancer cell lines. The prepared 1,3,4-oxadiazole-linked N-Mannich bases could be considered good antibacterial and anticancer drug candidates. The biological testing results are considered as preliminary and further investigations, including experimental and theoretical investigations for the exploration of their targets, are required for optimization of their chemotherapeutic activities.
Supplementary Materials
The micro-analytical data (C, H, N, and S), the experimental details of the determination of in vitro antimicrobial activity, in vitro anti-proliferative activity, and the NMR spectra can be found online.
Author Contributions
Conceptualization, A.A.E.-E. and A.A.B.M.; methodology, L.H.A.-W., S.S.T., and H.M.H.; validation, H.M.H..; formal analysis, L.H.A.-W., A.A.B.M., and S.S.T.; investigation, L.H.A.-W. and H.M.H.; data curation, H.M.H. and S.S.T.; writing—original draft preparation, A.A.E.-E.; writing—review and editing, A.A.E.-E. and A.A.B.M.; supervision, L.H.A.-W., A.A.E.-E.; project administration, A.A.E.-E.; funding acquisition, L.H.A.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Research Groups Program (Grant No. RGP-1442-0010-4).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 4a–l and 5a–d are available from the corresponding author.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verma G., Khan M.F., Akhtar W., Alam M.M., Akhter M., Shaquiquzzaman M. A Review Exploring Therapeutic Worth of 1,3,4-Oxadiazole Tailored Compounds. Mini-Reviews Med. Chem. 2019;19:477–509. doi: 10.2174/1389557518666181015152433. [DOI] [PubMed] [Google Scholar]
- 2.De Oliveira C.S., Lira B.F., Barbosa-Filho J.M., Lorenzo J.G.F., De Athayde-Filho P.F. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules. 2012;17:10192–10231. doi: 10.3390/molecules170910192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumdar P., Pati A., Patra M., Behera R.K., Behera A.K. Acid Hydrazides, Potent Reagents for Synthesis of Oxygen-, Nitrogen-, and/or Sulfur-Containing Heterocyclic Rings. Chem. Rev. 2014;114:2942–2977. doi: 10.1021/cr300122t. [DOI] [PubMed] [Google Scholar]
- 4.Ogata M., Atobe H., Kushida H., Yamamoto K. In Vitro Sensitivity of Mycoplasmas Isolated from Various Animals and Sewage to Antibiotics and Nitrofurans. J. Antibiot. 1971;24:443–451. doi: 10.7164/antibiotics.24.443. [DOI] [PubMed] [Google Scholar]
- 5.Summa V., Petrocchi A., Bonelli F., Crescenzi B., Donghi M., Ferrara M., Fiore F., Gardelli C., Paz O.G., Hazuda D.J., et al. Discovery of Raltegravir, a Potent, Selective Orally Bioavailable HIV-Integrase Inhibitor for the Treatment of HIV-AIDS Infection. J. Med. Chem. 2008;51:5843–5855. doi: 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- 6.Shepard D.R., Dreicer R. Zibotentan for the treatment of castrate-resistant prostate cancer. Expert Opin. Investig. Drugs. 2010;19:899–908. doi: 10.1517/13543784.2010.491822. [DOI] [PubMed] [Google Scholar]
- 7.McCoull W., Addie M.S., Birch A.M., Birtles S., Buckett L.K., Butlin R.J., Bowker S.S., Boyd S., Chapman S., Davies R.D., et al. Identification, optimisation and in vivo evaluation of oxadiazole DGAT-1 inhibitors for the treatment of obesity and diabetes. Bioorganic Med. Chem. Lett. 2012;22:3873–3878. doi: 10.1016/j.bmcl.2012.04.117. [DOI] [PubMed] [Google Scholar]
- 8.Vardan S., Smulyan H., Mookherjee S., Eich R. Effects of tiodazosin, a new antihypertensive, hemodynamics and clinical variables. Clin. Pharmacol. Ther. 1983;34:290–296. doi: 10.1038/clpt.1983.170. [DOI] [PubMed] [Google Scholar]
- 9.Schlecker R., Thieme P.C. The synthesis of antihypertensive 3-(1,3,4-oxadiazol-2-yl)phenoxypropanolahines. Tetrahedron. 1988;44:3289–3294. doi: 10.1016/S0040-4020(01)85962-7. [DOI] [Google Scholar]
- 10.Zheng Z., Liu Q., Kim W., Tharmalingam N., Fuchs B.B., Mylonakis E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Futur. Med. Chem. 2018;10:283–296. doi: 10.4155/fmc-2017-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai N., Bhatt N., Somani H., Trivedi A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1,3,4-oxadiazoles. Eur. J. Med. Chem. 2013;67:54–59. doi: 10.1016/j.ejmech.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Luo Y., Wei C., Wu S., Wu R., Wang S., Hu D., Song B. Novel sulfone derivatives containing a 1,3,4-oxadiazole moiety: Design and synthesis based on the 3D-QSAR model as potential antibacterial agent. Pest Manag. Sci. 2020;76:3188–3198. doi: 10.1002/ps.5873. [DOI] [PubMed] [Google Scholar]
- 13.Xu H., Jia A., Hou E., Liu Z., Yang R., Yang R., Guo Y. Natural Product-Based Fungicides Discovery: Design, Synthesis and Antifungal Activities of Some Sarisan Analogs Containing 1,3,4-Oxadiazole Moieties. Chem. Biodivers. 2019;17:e1900570. doi: 10.1002/cbdv.201900570. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y.-Y., Shao W.-B., Zhu J.-J., Long Z.-Q., Liu L.-W., Wang P.-Y., Li Z., Yang S. Novel 1,3,4-Oxadiazole-2-carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. J. Agric. Food Chem. 2019;67:13892–13903. doi: 10.1021/acs.jafc.9b05942. [DOI] [PubMed] [Google Scholar]
- 15.Wani M.Y., Ahmad A., Shiekh R.A., Al-Ghamdi K.J., Sobral A.J. Imidazole clubbed 1,3,4-oxadiazole derivatives as potential antifungal agents. Bioorganic Med. Chem. 2015;23:4172–4180. doi: 10.1016/j.bmc.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 16.Sonawane A.D., Rode N.D., Nawale L., Joshi R.R., Joshi R.A., Likhite A.P., Sarkar D. Synthesis and biological evaluation of 1,2,4-triazole-3-thione and 1,3,4-oxadiazole-2-thione as antimycobacterial agents. Chem. Biol. Drug Des. 2017;90:200–209. doi: 10.1111/cbdd.12939. [DOI] [PubMed] [Google Scholar]
- 17.Desai N., Somani H., Trivedi A., Bhatt K., Nawale L., Khedkar V.M., Jha P.C., Sarkar D. Synthesis, biological evaluation and molecular docking study of some novel indole and pyridine based 1,3,4-oxadiazole derivatives as potential antitubercular agents. Bioorganic Med. Chem. Lett. 2016;26:1776–1783. doi: 10.1016/j.bmcl.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 18.Benmansour F., Eydoux C., Querat G., de Lamballerie X., Canard B., Alvarez K., Guillemot J.-C., Barral K. Novel 2-phenyl-5-[(E)-2-(thiophen-2-yl)ethenyl]-1,3,4-oxadiazole and 3-phenyl-5-[(E)-2-(thiophen-2-yl)ethenyl]-1,2,4-oxadiazole derivatives as dengue virus inhibitors targeting NS5 polymerase. Eur. J. Med. Chem. 2016;109:146–156. doi: 10.1016/j.ejmech.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 19.Wu W., Chen Q., Tai A., Jiang G., Ouyang G. Synthesis and antiviral activity of 2-substituted methylthio-5-(4-amino-2-methylpyrimidin-5-yl)-1,3,4-oxadiazole derivatives. Bioorganic Med. Chem. Lett. 2015;25:2243–2246. doi: 10.1016/j.bmcl.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 20.Lai H., Dou D., Aravapalli S., Teramoto T., Lushington G.H., Mwania T.M., Alliston K.R., Eichhorn D.M., Padmanabhan R., Groutas W.C. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: Potent inhibitors of Dengue and West Nile virus NS2B/NS3 proteases. Bioorganic Med. Chem. 2013;21:102–113. doi: 10.1016/j.bmc.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benassi A., Doria F., Pirota V. Groundbreaking Anticancer Activity of Highly Diversified Oxadiazole Scaffolds. Int. J. Mol. Sci. 2020;21:8692. doi: 10.3390/ijms21228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glomb T., Szymankiewicz K., Świątek P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules. 2018;23:3361. doi: 10.3390/molecules23123361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akhtar J., Siddiqui A.A., Khan A.A., Ali Z., Dewangan R.P., Pasha S., Yar M.S. Design, synthesis, docking and QSAR study of substituted benzimidazole linked oxadiazole as cytotoxic agents, EGFR and erbB2 receptor inhibitors. Eur. J. Med. Chem. 2017;126:853–869. doi: 10.1016/j.ejmech.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Ruel R., Thibeault C., L’Heureux A., Martel A., Cai Z.-W., Wei D., Qian L., Barrish J.C., Mathur A., D’Arienzo C., et al. Discovery and preclinical studies of 5-isopropyl-6-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(2-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrrolo[2,1-f][1,2,4]triazin-4-amine (BMS-645737), an in vivo active potent VEGFR-2 inhibitor. Bioorganic Med. Chem. Lett. 2008;18:2985–2989. doi: 10.1016/j.bmcl.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 25.Altıntop M.D., Sever B., Çiftçi G.A., Turan-Zitouni G., Kaplancıklı Z.A., Özdemir A. Design, synthesis, in vitro and in silico evaluation of a new series of oxadiazole-based anticancer agents as potential Akt and FAK inhibitors. Eur. J. Med. Chem. 2018;155:905–924. doi: 10.1016/j.ejmech.2018.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Sun J., Ren S.-Z., Lu X.-Y., Li J.-J., Shen F.-Q., Xu C., Zhu H.-L. Discovery of a series of 1,3,4-oxadiazole-2(3 H )-thione derivatives containing piperazine skeleton as potential FAK inhibitors. Bioorganic Med. Chem. 2017;25:2593–2600. doi: 10.1016/j.bmc.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Valente S., Trisciuoglio D., De Luca T., Nebbioso A., Labella D., Lenoci A., Bigogno C., Dondio G., Miceli M., Brosch G., et al. 1,3,4-Oxadiazole-Containing Histone Deacetylase Inhibitors: Anticancer Activities in Cancer Cells. J. Med. Chem. 2014;57:6259–6265. doi: 10.1021/jm500303u. [DOI] [PubMed] [Google Scholar]
- 28.Pidugu V.R., Yarla N.S., Bishayee A., Kalle A.M., Satya A.K. Novel histone deacetylase 8-selective inhibitor 1,3,4-oxadiazole-alanine hybrid induces apoptosis in breast cancer cells. Apoptosis. 2017;22:1394–1403. doi: 10.1007/s10495-017-1410-2. [DOI] [PubMed] [Google Scholar]
- 29.Sun J., Li M.-H., Qian S.-S., Guo F.-J., Dang X.-F., Wang X.-M., Xue Y.-R., Zhu H.-L. Synthesis and antitumor activity of 1,3,4-oxadiazole possessing 1,4-benzodioxan moiety as a novel class of potent methionine aminopeptidase type II inhibitors. Bioorganic Med. Chem. Lett. 2013;23:2876–2879. doi: 10.1016/j.bmcl.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 30.Mohan C.D., Anilkumar N.C., Rangappa S., Shanmugam M.K., Mishra S., Chinnathambi A., Alharbi S.A., Bhattacharjee A., Sethi G., Kumar A.P., et al. Novel 1,3,4-Oxadiazole Induces Anticancer Activity by Targeting NF-κB in Hepatocellular Carcinoma Cells. Front. Oncol. 2018;8:42. doi: 10.3389/fonc.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav N., Kumar P., Chhikara A., Chopra M. Development of 1,3,4-oxadiazole thione based novel anticancer agents: Design, synthesis and in-vitro studies. Biomed. Pharmacother. 2017;95:721–730. doi: 10.1016/j.biopha.2017.08.110. [DOI] [PubMed] [Google Scholar]
- 32.Ullah H., Rahim F., Taha M., Uddin I., Wadood A., Shah S.A.A., Farooq R.K., Nawaz M., Wahab Z., Khan K.M. Synthesis, molecular docking study and in vitro thymidine phosphorylase inhibitory potential of oxadiazole derivatives. Bioorganic Chem. 2018;78:58–67. doi: 10.1016/j.bioorg.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Zhu H., Yang Z.-M., Zhu H.-L. Synthesis, molecular modeling and biological evaluation of 2-aminomethyl-5-(quinolin-2-yl)-1,3,4-oxadiazole-2(3H)-thione quinolone derivatives as novel anticancer agent. Eur. J. Med. Chem. 2013;60:23–28. doi: 10.1016/j.ejmech.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Du Q.-R., Li D.-D., Pi Y.-Z., Li J.-R., Sun J., Fang F., Zhong W.-Q., Gong H.-B., Zhu H.-L. Novel 1,3,4-oxadiazole thioether derivatives targeting thymidylate synthase as dual anticancer/antimicrobial agents. Bioorganic Med. Chem. 2013;21:2286–2297. doi: 10.1016/j.bmc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Haibara H., Yamazaki R., Nishiyama Y., Ono M., Kobayashi T., Hokkyo-Itagaki A., Nishisaka F., Nishiyama H., Kurita A., Matsuzaki T., et al. YPC-21661 and YPC-22026, novel small molecules, inhibit ZNF143 activityin vitroandin vivo. Cancer Sci. 2017;108:1042–1048. doi: 10.1111/cas.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Aziz M., Metwally K.A., Gamal-Eldeen A.M., Aly O.M. 1,3,4-oxadiazole-2-thione Derivatives; Novel Approach for Anticancer and Tubulin Polymerization Inhibitory Activities. Anti-Cancer Agents Med. Chem. 2015;16:269–277. doi: 10.2174/1871520615666150907093855. [DOI] [PubMed] [Google Scholar]
- 37.Ozyazici T., Gurdal E.E., Orak D., Sipahi H., Ercetin T., Gulcan H.O., Koksal M. Synthesis, anti-inflammatory activity, and molecular docking studies of some novel Mannich bases of the 1,3,4-oxadiazole-2(3 H )-thione scaffold. Arch. der Pharm. 2020;353:e2000061. doi: 10.1002/ardp.202000061. [DOI] [PubMed] [Google Scholar]
- 38.Koksal M., Ozkan-Dagliyan I., Ozyazici T., Kadioglu B., Sipahi H., Bozkurt A., Bilge S.S. Some Novel Mannich Bases of 5-(3,4-Dichlorophenyl)-1,3,4-oxadiazole-2(3H )-one and Their Anti-Inflammatory Activity. Arch. der Pharm. 2017;350:1700153. doi: 10.1002/ardp.201700153. [DOI] [PubMed] [Google Scholar]
- 39.Dewangan D., Nakhate K., Tripathi D., Kashyap P., Dhongde H. Synthesis, Characterization and Screening for Analgesic and Anti-inflammatory activities of 2, 5-disubstituted 1, 3, 4-oxadiazole derivatives. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2015;14:138–145. doi: 10.2174/1871523014666150820100212. [DOI] [PubMed] [Google Scholar]
- 40.Ma L., Xiao Y., Li C., Xie Z.-L., Li D.-D., Wang Y.-T., Ma H.-T., Zhu H.-L., Wang M.-H., Ye Y.-H. Synthesis and antioxidant activity of novel Mannich base of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan. Bioorganic Med. Chem. 2013;21:6763–6770. doi: 10.1016/j.bmc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Taha M., Ismail N.H., Imran S., Wadood A., Rahim F., Saad S.M., Khan K.M., Nasir A. Synthesis, molecular docking and α-glucosidase inhibition of 5-aryl-2-(6′-nitrobenzofuran-2′-yl)-1,3,4-oxadiazoles. Bioorganic Chem. 2016;66:117–123. doi: 10.1016/j.bioorg.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Liao B.-R., He H.-B., Yang L.-L., Gao L.-X., Chang L., Tang J., Li J.-Y., Li J., Yang F. Synthesis and structure-activity relationship of non-phosphorus-based fructose-1,6-bisphosphatase inhibitors: 2,5-Diphenyl-1,3,4-oxadiazoles. Eur. J. Med. Chem. 2014;83:15–25. doi: 10.1016/j.ejmech.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Maccioni E., Alcaro S., Cirilli R., Vigo S., Cardia M.C., Sanna M.L., Meleddu R., Yanez M., Costa G., Casu L., et al. 3-Acetyl-2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoles: A New Scaffold for the Selective Inhibition of Monoamine Oxidase B. J. Med. Chem. 2011;54:6394–6398. doi: 10.1021/jm2002876. [DOI] [PubMed] [Google Scholar]
- 44.Distinto S., Meleddu R., Yanez M., Cirilli R., Bianco G., Sanna M.L., Arridu A., Cossu P., Cottiglia F., Faggi C., et al. Drug design, synthesis, in vitro and in silico evaluation of selective monoaminoxidase B inhibitors based on 3-acetyl-2-dichlorophenyl-5-aryl-2,3-dihydro-1,3,4-oxadiazole chemical scaffold. Eur. J. Med. Chem. 2016;108:542–552. doi: 10.1016/j.ejmech.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Wu X., Wang L., Hua Y., Wang C., Batsanov A.S., Bryce M.R. A carbazole-oxadiazole diad molecule for single-emitting-component white organic light-emitting devices (WOLEDs) Tetrahedron. 2014;70:2015–2019. doi: 10.1016/j.tet.2014.01.073. [DOI] [Google Scholar]
- 46.Kamtekar K.T., Monkman A.P., Bryce M.R. Recent Advances in White Organic Light-Emitting Materials and Devices (WOLEDs) Adv. Mater. 2010;22:572–582. doi: 10.1002/adma.200902148. [DOI] [PubMed] [Google Scholar]
- 47.Anticancer Research. Anticancer. Res. 2018;40:5035–5041. doi: 10.21873/anticanres. [DOI] [Google Scholar]
- 48.Karaaslan C., Duydu Y., Ustundag A., Yalcin C.O., Kaskatepe B., Goker H. Synthesis & Anticancer Evaluation of New Substituted 2-(3,4- Dimethoxyphenyl)benzazoles. Med. Chem. 2019;15:287–297. doi: 10.2174/1573406414666180711130012. [DOI] [PubMed] [Google Scholar]
- 49.Chitti S., Singireddi S., Reddy P.S.K., Trivedi P., Bobde Y., Kumar C., Rangan K., Ghosh B., Sekhar K.V.G.C. Design, synthesis and biological evaluation of 2-(3,4-dimethoxyphenyl)-6 (1,2,3,6-tetrahydropyridin-4-yl)imidazo[1,2-a]pyridine analogues as antiproliferative agents. Bioorganic Med. Chem. Lett. 2019;29:2551–2558. doi: 10.1016/j.bmcl.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Tan B.S., Tiong K.H., Muruhadas A., Randhawa N., Choo H.L., Bradshaw T.D., Stevens M.F., Leong C.-O. CYP2S1 and CYP2W1 Mediate 2-(3,4-Dimethoxyphenyl)-5-Fluorobenzothiazole (GW-610, NSC 721648) Sensitivity in Breast and Colorectal Cancer Cells. Mol. Cancer Ther. 2011;10:1982–1992. doi: 10.1158/1535-7163.MCT-11-0391. [DOI] [PubMed] [Google Scholar]
- 51.Aly O.M., Beshr E.A., Maklad R.M., Mustafa M., Gamal-Eldeen A. Synthesis, Cytotoxicity, Docking Study, and Tubulin Polymerization Inhibitory Activity of Novel 1-(3,4-Dimethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1 H -1,2,4-triazole-3-carboxanilides. Arch. der Pharm. 2014;347:658–667. doi: 10.1002/ardp.201400096. [DOI] [PubMed] [Google Scholar]
- 52.Ghorab M.M., Alsaid M.S., Nissan Y.M., Ashour A.E., Al-Mishari A.A., Kumar A., Ahmed S.F. Novel Sulfonamide Derivatives Carrying a Biologically Active 3,4-Dimethoxyphenyl Moiety as VEGFR-2 Inhibitors. Chem. Pharm. Bull. 2016;64:1747–1754. doi: 10.1248/cpb.c16-00614. [DOI] [PubMed] [Google Scholar]
- 53.Bansal S., Sinha D., Singh M., Cheng B., Tse-Dinh Y.-C., Tandon V. 3,4-Dimethoxyphenyl bis-benzimidazole, a novel DNA topoisomerase inhibitor that preferentially targets Escherichia coli topoisomerase I. J. Antimicrob. Chemother. 2012;67:2882–2891. doi: 10.1093/jac/dks322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felicetti T., Cannalire R., Burali M.S., Massari S., Manfroni G., Barreca M.L., Tabarrini O., Schindler B.D., Sabatini S., Kaatz G.W., et al. Searching for Novel Inhibitors of theS. aureusNorA Efflux Pump: Synthesis and Biological Evaluation of the 3-Phenyl-1,4-benzothiazine Analogues. ChemMedChem. 2017;12:1293–1302. doi: 10.1002/cmdc.201700286. [DOI] [PubMed] [Google Scholar]
- 55.Shcherbakov K.V., Artemyeva M.A., Burgart Y.V., Saloutin V.I., Volobueva A.S., Misiurina M.A., Esaulkova Y.L., Sinegubova E.O., Zarubaev V.V. 7-Imidazolyl-substituted 4′-methoxy and 3′,4′-dimethoxy-containing polyfluoroflavones as promising antiviral agents. J. Fluor. Chem. 2020;240:109657. doi: 10.1016/j.jfluchem.2020.109657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Emam A.A., Al-Deeb O.A., Al-Omar M., Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorganic Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 57.Al-Abdullah E.S., Sebastian S., Al-Wabli R.I., El-Emam A.A., Panicker C., Van Alsenoy C. Vibrational spectroscopic studies (FT-IR, FT-Raman) and quantum chemical calculations on 5-(Adamantan-1-yl)-3-[(4-fluoroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential chemotherapeutic agent. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014;133:605–618. doi: 10.1016/j.saa.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 58.Al-Omary F.A., Karakaya M., Sert Y., Haress N.G., El-Emam A.A., Çırak Ç. Structural and spectroscopic analysis of 3-[(4-phenylpiperazin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazole-2-thione with experimental (FT-IR, Laser-Raman) techniques and ab initio calculations. J. Mol. Struct. 2014;1076:664–672. doi: 10.1016/j.molstruc.2014.08.035. [DOI] [Google Scholar]
- 59.Al-Omary F.A., Mary Y.S., Panicker C.Y., El-Emam A.A., Al-Swaidan I.A., Al-Saadi A.A., Van Alsenoy C. Spectroscopic investigations, NBO, HOMO-LUMO, NLO analysis and molecular docking of 5-(adamantan-1-yl)-3-anilinomethyl-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential bioactive agent. J. Mol. Struct. 2015;1096:1–14. doi: 10.1016/j.molstruc.2015.03.049. [DOI] [Google Scholar]
- 60.Qadeer G., Rama N.H., Malik M.A., Raftery J. 3,4-Dimethoxybenzohydrazide. Acta Crystallogr. Sect. E Struct. Rep. Online. 2007;63:3026. doi: 10.1107/S1600536807025160. [DOI] [Google Scholar]
- 61.Abdullah M.A., Abuo-Rahma G.E.-D.A., Abdelhafez E.-S.M., Hassan H.A., El-Baky R.M.A. Design, synthesis, molecular docking, anti- Proteus mirabilis and urease inhibition of new fluoroquinolone carboxylic acid derivatives. Bioorganic Chem. 2017;70:1–11. doi: 10.1016/j.bioorg.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Goswami B.N., Kataky J.C.S., Baruah J.N., Nath S.C. Synthesis of 3,5-disubstituted 1,3,4-oxadiazole-2-thiones as potential fungicidal agents. J. Heterocycl. Chem. 1984;21:205–208. doi: 10.1002/jhet.5570210141. [DOI] [Google Scholar]
- 63.Woods G.L., Washington J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In: Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H., editors. Manual of Clinical Microbiology. American Society of Microbiology; Washington, DC, USA: 1995. [Google Scholar]
- 64.National Committee for Clinical Laboratory Standards . Approved standard document M-7A. NCCS; Villanova, PA, USA: 1985. [Google Scholar]
- 65.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 66.Berridge M., Tan A. Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 67.Tacar O., Sriamornsak P., Dass C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.