Abstract
Data on Epstein–Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH) in adults in the United States remain very limited. A cluster of four cases of EBV-HLH was observed in a 4-month period at a tertiary center in Los Angeles County (LA County) and the clinical and molecular characteristics identified in these cases are being described. EBV typing, immunophenotypic and molecular genetic studies were performed. Diagnostic criteria that may be used to identify EBV as a cause of HLH in adults are also being suggested. Finally, the crude incidence rate for HLH in LA County was determined and was compared to the worldwide crude incidence rate for HLH. The cases each occurred in young male adult residents of California and were associated with evidence of EBV reactivation and ferritin levels of >20,000 μg/L. A higher rate of cases of EBV-HLH in 2010 was found at UCLA Medical Center than for 2007–2009 (4.9/10,000 hospital discharges vs. 0.14/10,000 hospital discharges, respectively; P = 0.0017). The cases were associated with EBV type 1, and the insertion of the codon CTC (leucine) was found in numerous of the EBNA-2 gene sequences. The annual incidence of secondary, non-familial HLH was estimated to be 0.9 cases per million persons >15 years of age in LA County. Although EBV-HLH is a rare disease, the incidence in adults in Western countries may be underestimated.
Keywords: Epstein–Barr virus, hemophagocytic lymphohistiocytosis, California
INTRODUCTION
Hemophagocytic lymphohistiocytosis (HLH) is a rare disease characterized by clinical signs and symptoms of extreme inflammation and limited population-based incidence and prevalence data are available [Imashuku, 2011]. Epstein–Barr virus (EBV) is the viral infection associated most commonly with HLH [Imashuku, 2011]. However, the majority of data regarding the epidemiology of EBV-related HLH (EBV-HLH) is from Asia, and there is limited published data on the incidence of HLH in the United States. To our knowledge, only 13 well-characterized cases of EBV-associated HLH have been described in the United States. However, none of these cases described the molecular characteristics of the virus and host associated with the EBV-HLH phenotype [Quintanilla-Martinez et al., 2000; Halasa et al., 2003; Lindemann and Greene, 2005; Mischler et al., 2007; Rouphael et al., 2007; Brodkin et al., 2008; Kaza et al., 2008; Belyea et al., 2010]. Although EBV-HLH is a rare disease, the incidence in adults in the United States may be underestimated.
An unusual cluster of four cases of EBV-HLH was recently observed at UCLA Medical Center. All cases were in young male residents of California who had evidence of prior EBV infection. The clinical data from these four cases are described and the incidence of HLH in Los Angeles County (LA County) was estimated. Both serology and quantitative polymerase chain reaction (qPCR) were used to identify cases of HLH potentially associated with EBV infection. Additionally, the molecular characteristics of the EBV viruses associated with this unusual cluster of EBV-HLH cases are described. Diagnostic criteria that may be used to identify EBV as a cause of HLH in adults are proposed. Delays in diagnosis of EBV-associated HLH can lead to worse prognosis of patients with this syndrome [Fisman, 2000; Filipovich, 2009]. Thus, given the severity of illness, clinicians should be aware about EBV-HLH in adults and this study contributes to further understanding of this clinical entity.
MATERIALS AND METHODS
Definitions
For the purposes of this investigation, an EBV-associated HLH case was defined as a patient diagnosed with HLH by the Histiocyte Society 2004 case definition [Henter et al., 2007] with concurrent active or prior EBV infection based on serology or qPCR test results. In this investigation, active EBV infection was defined as positive EBV IgM serology or an EBV DNA viral load >10 copies/ml whole blood, as determined by qPCR in patients who were not receiving immunosuppressive agents at the time of the PCR. Persons with <10 EBV copies/ml whole blood by qPCR, and positive IgG but negative IgM serologies for EBV, were classified as having prior (latent) EBV infection [Gulley and Tang, 2008]. Patients with a history of active EBV infection (documented by serology or PCR) within the 3 months prior to admission were also included. Cases were classified as “definite” (6 points), probable (3–5 points), or possible (1–2 points) EBV-associated HLH using a point system derived from a review of the literature and EBV studies, with points assigned for EBV viral load, the duration of viremia, and presence of EBV in tissue. Points were subtracted for possible alternate etiologies for HLH and/or history of exposure to other viruses during the period of onset of HLH (Table I). In view of the very limited data from research studies regarding EBV-HLH, mostly based on case reports, the 5,000 copies/ml and 1 month of documented EBV viremia (>10 copies/ml) were used as thresholds to define high (assigned 2 points) versus low (assigned 1 point) levels of EBV viremia and prolonged (assigned 2 points) versus short (assigned 1 point) duration of documented EBV viremia, respectively. Finally 2 points were assigned if there was presence of EBV in tissue (e.g., bone marrow). On the other hand, points were subtracted if there was evidence (history, serologies, PCR) of exposure to other virus associated with HLH [Rouphael et al., 2007] or alternative etiologies for HLH (Table I).
TABLE I.
Classification Criteria for Determination of EBV Infection as the Primary Process Causing HLH
| Parameter | Points |
|---|---|
| EBV load (PCR) <5,000 copies/ml | 1 |
| EBV load (PCR) >5,000 copies/ml | 2 |
| Duration of EBV viremia <1 month | 1 |
| Duration of EBV viremia >1 month | 2 |
| Presence of EBV in tissues | 2 |
| Evidence (history, serologies, PCR) of exposure to other virus associated with HLH | −1 |
| Alternative etiologies for HLH | −1 |
EBV, Epstein–Barr virus; HLH, hemophagocytic lymphohistiocytosis; PCR, polymerase chain reaction.
Determination of EBV infection as the primary process causing HLH: definite: 6 points; probable: 3–5 points; possible: 1–2 points.
Low levels of EBV viremia were defined as EBV titers <5,000 copies/ml based on the reviewed literature of previous EBV-HLH cases.
High levels of EBV viremia were defined as EBV titers >5,000 copies/ml based on the reviewed literature of previous EBV-HLH cases.
Short duration of documented EBV viremia was defined as <1 month based on the reviewed literature of previous EBV-HLH cases.
Prolonged duration of documented EBV viremia was defined as >1 month based on the reviewed literature of previous EBV-HLH cases.
Alternative etiologies for HLH may include presence of other factors well known to be associated with HLH, such as other viral, fungal infection, immunodeficiency.
Patients
Patients diagnosed and treated for HLH were prospectively identified between December 2009 and March 2010 at UCLA Medical Center, Los Angeles, CA. Patients with possible cases of HLH also were identified by searching for discharge ICD-9 code 288.4, the diagnosis code for “Hemophagocytic syndromes,” “Familial HLH,” “infection-associated Histiocytic syndromes,” and “Macrophage activation syndrome.” Data were collected by review of the electronic medical records. This study was approved by the UCLA Institutional Review Board.
Review of the Literature
To identify reports of EBV-associated HLH, PubMed was searched through March 2011 using the search terms “lymphohistiocytosis,” “hemophagocytosis,” “HLH,” “Epstein–Barr virus,” alone and in all combinations. In addition, the references cited in these articles were examined to identify additional reports.
Determination of Crude Incidence Rate for HLH in Los Angeles County
In collaboration with LA County Department of Public Health a baseline crude incidence rate for HLH in LA County was established by examination of all ICD-9 coded discharge data for LA County acute care facilities in 2007 and 2008. All patients with any discharge diagnosis of 288.4 were included and the search was limited to 2007 and 2008 because ICD-9 code 288.4 was first used on October 1, 2006 and data were not available for 2009 at the time of this study. The cases were stratified by age <15 and ≥15 years and presence or absence of family history of HLH in an attempt to distinguish familial HLH (age <15 years and/or family history of HLH) from secondary HLH (age ≥15 years and negative family history of HLH). To estimate the baseline rate of ICD-9 coded acquired HLH in LA County, all hospital discharges during 2007–2008 with code 288.4 were examined and incidence rates per 1,000,000 population for the two age strata were calculated.
Determination of Crude Incidence Rate for HLH Referred to UCLA Medical Center
To identify number of HLH patients seen at UCLA Medical Center and investigate whether referral bias could explain the reported cluster of EBV-HLH cases, all hospital discharges from UCLA Medical Center were searched for ICD-9 code 288.4 usage between January 2007 and March 2010. Rates of EBV-HLH per 10,000 hospital discharges were compared using chi-squared test.
EBV Detection
Peripheral blood and bone marrow samples were obtained at the time of first diagnosis of HLH from four patients prospectively identified in 2010. DNA was extracted from patient whole blood using the MagnaPure (Roche Molecular Systems; Pleasanton, CA) and EBV DNA was detected and quantitated using the Roche LightCycler® EBV Quantitative IVD, which detects the lmp-2 gene.
EBV Typing: EBNA-2 and LMP-1 Sequence Determination
EBV typing was performed by sequence analysis of the EBV nuclear antigen 2 (EBNA-2) gene [Aitken et al., 1994]. Briefly, EBNA-2 was amplified from remnant EBV-positive DNA extracts from HLH patients, by typical PCR methods, using the primers E2p1 (5′-AGGGATCCCTGGACACAAGA-3′) and E2p2 (5′-TGGTGCTGCTGGTGGTGGCAAT-3′), as previously described [Mendes et al., 2008]. DNA extracts obtained from 10 EBV positive, HLH-negative patients seen at UCLA Medical Center in the same months and of similar ethnic groups as the four HLH case patients were also included in the analysis, as controls. Sequence determination was performed using an ABI 3130xl (Applied Biosystems, Foster City, CA) and the sequence data were analyzed by assembly of the forward and reverse sequences into a consensus EBNA-2 sequence for each patient, and compared to that of B95–8 (type 1 EBV sequence) and Ag876 (type 2 EBV sequence) by the Basic Local Alignment Search Tool (BLAST, NCBI). Latent membrane protein-1 (lmp-1) gene sequences were determined using a similar approach, but using the primers, lmp1-a (5′-CCT TTG GCT CCT CCT GTT TC-3′) and lmp1-b (5′-TAA TAC GAC TCA CTA TAG GG-3′), described by Tabata et al. [2000]. Lmp-1 sequences were compared to that of B95–8 EBV by BLAST.
In Situ Hybridization for EBV and Immunophenotypic Studies
In situ hybridization (ISH) for EBV early RNA-1 (EBER1) was performed on fixed paraffin-embedded sections as previously described [Quintanilla-Martinez et al., 2000]. ISH was performed first, followed by immunohistochemistry as previously described [Quintanilla-Martinez et al., 2000].
Molecular Genetic Studies
Genomic DNA from the bone marrow samples was analyzed for clonal rearrangements of the immunoglobulin heavy chain (IgH) genes and the T-cell receptor (TCR)γ genes by PCR and Southern blot [Quintanilla-Martinez et al., 2000].
RESULTS
Description of Cases
Of the four cases of HLH identified in 2010, one (patient 3) was classified as definite, one (patient 1) as probable, and two (patients 2 and 4) as possible EBV-associated HLH. The demographic, clinical, laboratory characteristics of the four cases are summarized in Figure 1, Tables II and III and in Supplemental Appendix (Supplemental Table I).
Fig. 1.
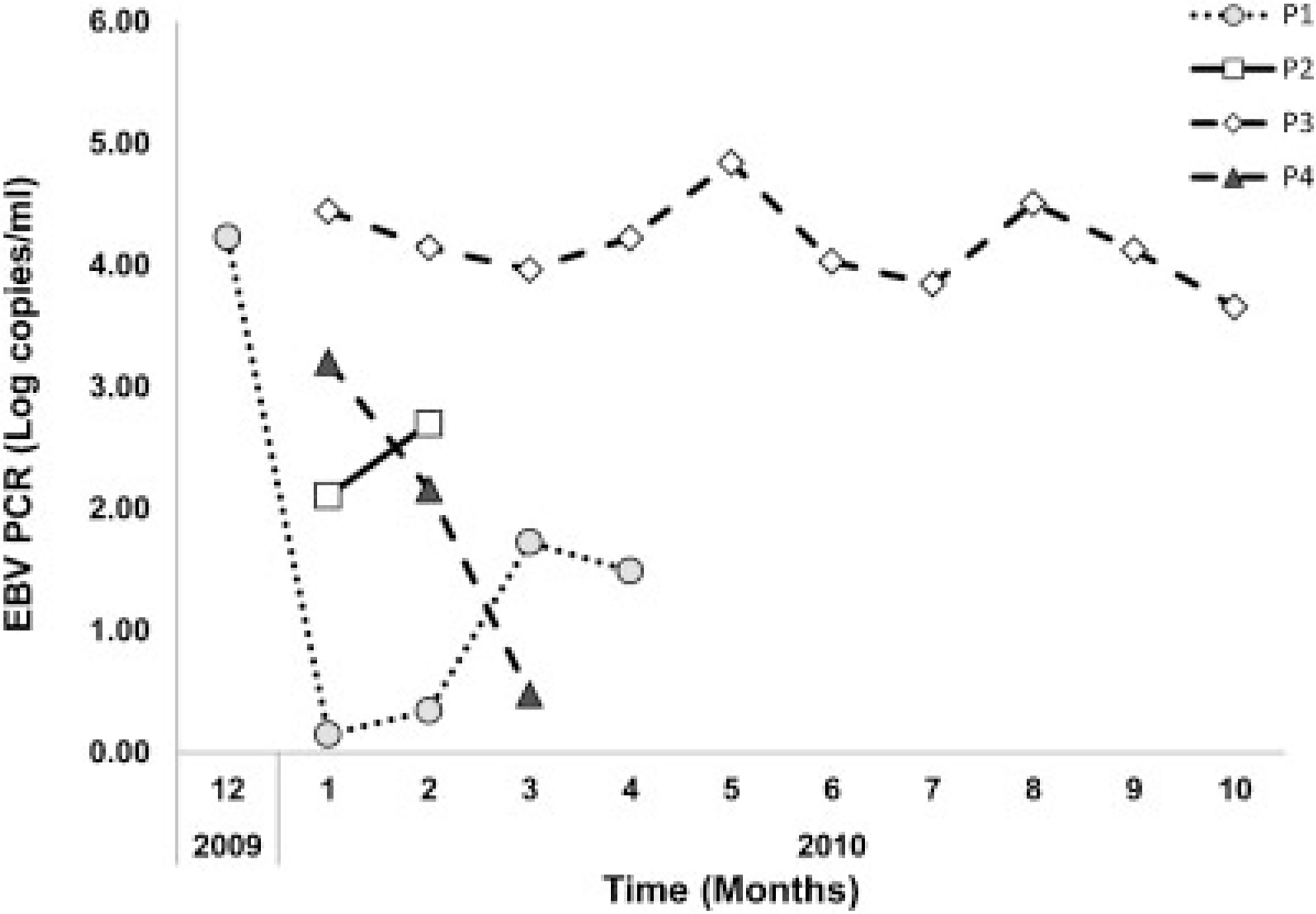
The detected EBV viral loads (log copies/ml) during the entire hospitalization is shown for all four cases.
TABLE II.
Cases of HLH at UCLA Medical Center Between December 2009-March 2010
| Case | Age | Sex | Race | Area of residence | Time of viral illness | Onset of systemic disease | Classification of EBV as cause of HLH (points) | Evidence of presence of other viruses | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | M | Asian | San Francisco | December 2009 | January 2010 | Probable (4) | HHV 6 IgG (+), HHV 6 PCR (-), CMV PCR (+) 3 months after hospitalization | Ganciclovir, IVIG, cyclosporine, rituximab, HLH 2004 protocol | Died |
| 2 | 33 | M | Caucasian | Los Angeles | October 2009 | January 2010 | Possible (2) | HHV 6 IgG (+), HHV 6 PCR (-), CMV PCR (+) in blood and bone marrow 2 months after hospitalization, H1N1 influenza on admission | None | Died |
| 3 | 23 | M | Hispanic | West Los Angeles | November-December 2009 | December 2009-January 2010 | Definite (6) | No | Ganciclovir, HLH 2004 protocol | Survived |
| 4 | 31 | M | Caucasian | West Los Angeles | November-December 2009 | December 2009-January 2010 | Possible (2) | HSV (+) PCR in blood and sputum 4 months after hospitalization, HHV 8 IgG (+) CMV IgG (+) | HLH 2004 protocol | Died |
CMV, cytomegalovirus; EBV, Epstein–Barr virus; HLH, hemophagocytic lymphohistiocytosis; HSV, herpes simplex virus; IVIG, intravenous immunoglobulin; NK, natural killer; PCR, polymerase chain reaction.
Diagnosis of HLH was made based on presence of at least 5 out of 8 established clinical criteria: 1—fever; 2—splenomegaly; 3—cytopenias (affecting >2 of 3 lineages in peripheral blood): hemoglobin <9 g/dl, platelets <100 × 109/L, neutrophils <1.0 × 109/L; 4—hypertriglyceridemia and/or hypofibrinogenemia: fasting triglycerides ≥265 mg/dl, fibrinogen ≤1.5 g/L; 5—hemo-phagocytosis in bone marrow or spleen or lymph nodes: no evidence of malignancy; 6—low or absent NK-cell activity (according to local lab reference); 7—ferritin ≥500 μg/L. Soluble IL-2 receptor >2,400 U/ml.
TABLE III.
Summary of EBV Study Results
| Case | VCA-IgM | VCA-IgG | EA-IgG | EBNA-IgG | Whole blood PCR (copies/ml) | EBV detected in bone marrow | EBV detected in other cells in bone marrow | EBER detection | LMP detection | EBV detection in other fluids |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (−) | (+) | ND | (+) | 1.4–17,000 | No | No | No | No | No |
| 2 | ND | ND | (−) | ND | 128–501 | Yes | T cells, histiocytes | Yes | No | No |
| 3 | (−) | (+) | (+) | (+) | 7,005–69,950 | Yes | T cells, NK cells histiocytes | Yes | No | CSF |
| 4 | (−) | (+) | (+) | (+) | 3–1,612 | No | No | No | No | No |
CSF, cerebrospinal fluid; EA, early antigen; EBER, EBV early RNA-1; EBV, Epstein–Barr virus; EBNA, Epstein–Barr virus nuclear antigen; LMP, latent membrane protein-1; ND, not done; VCA, viral capsid antigen.
All four cases occurred in adult males who were under 35 years of age and who were residing in California at the time of illness onset and following a viral prodrome with detectable EBV viremia (range maximal EBV whole blood copy number, 501–69,950/ml; Fig. 1; Appendix). Three of the four cases (Cases 1–3) met criteria for HLH and EBV infection. One case (Case 4) had HLH and a documented positive EBV PCR (whole blood EBV PCR of 1,612 copies/ml) approximately 1 month after the onset of his symptoms but his EBV PCR was <10 copies/ml at the time of diagnosis of HLH and thus the possible association of EBV with HLH remains unclear in this case. One case was a resident of San Francisco who was initially hospitalized in Las Vegas and three cases were residents of West Los Angeles and they all developed a viral illness between October and December of 2009. Of note, another non-EBV associated case of HLH was hospitalized at UCLA in January of 2010. EBV was considered to be involved in the development of HLH definitely (patient 3), probably (patient 1) and possibly (patients 2 and 4). Evidence of presence of other viruses during the prolonged hospitalization was documented for three cases (patients 1, 2, and 4). Serologic markers of prior EBV infection were detected in each of the three cases, when performed (positive EBV VCA IgG Ab and EBNA Ab). In the remaining case (patient 2), the only EBV serology performed was a negative EBV IgG early antigen. Due to the complexity of the cases presented herein, EBV was considered the definite cause of HLH in only one case (patient 3). This case was the only one in which there was persistent EBV viremia in high copy counts that is typical of EBV-HLH [Rouphael et al., 2007].
ICD-9 Coded Discharges for HLH at Acute Care HospitalsinLACountyandUCLAMedicalCenter
The estimated rate of secondary, non-familial HLH among persons aged ≥15 years in LA County was 0.4 cases/1,000,000 persons in 2007, 1.3 cases/1,000,000 persons in 2008, with a mean of 0.9 cases/1,000,000 persons during the period 2007–2008. The estimated rate of possibly familial HLH among persons aged ≤15 years in LA County was 2.8 cases/1,000,000 persons in 2007, 4.3 cases/1,000,000 persons in 2008, with a mean of 3.5 cases/1,000,000 persons during the period 2007–2008. The estimated rate of HLH in LA County was 3.2 cases/1,000,000 persons in 2007, 5.6 cases/1,000,000 persons in 2008, with a mean of 4.4 cases/1,000,000 persons during the period 2007–2008. In a study in Japan, the annual incidence of HLH was 1 in 800,000 persons per year or 1.25 cases/1,000,000 persons [Ishii et al., 2007]. Thus, the estimated HLH incidence rate in LA County was approximately 3.5-fold higher compared to a previously published HLH incidence rate [Ishii et al., 2007].
The rate of EBV-associated HLH at UCLA Medical Center was significantly higher in 2010 than 2007–2009 (4.9/10,000 hospital discharges vs. 0.14/10,000 hospital discharges, respectively; P = 0.0017). However these data should be interpreted with caution due to the small number of cases. In a study in Japan, the annual incidence of EBV-associated HLH was 0.4 cases/1,000,000 persons [Ishii et al., 2007]. Unfortunately no incidence rate of EBV-HLH could be calculated based on hospital discharge data.
EBV DNA Sequence Analysis
Single nucleotide polymorphisms (SNPs) in the EBNA-2 gene were used to strain type EBV, as previously described [Aitken et al., 1994]. EBV DNA isolated from the blood of the four HLH patients, and also from 10 patients with other non-hematological diagnoses, demonstrated 96% homology to the B95–8 EBNA-2 gene, the prototypical virus sequence for type 1 EBV. The SNPs identified in the EBNA-2 genes of the HLH patients were 99% identical to those found in the control patients, suggesting that no single sub-strain of EBV was associated with the HLH cases [Aitken et al., 1994; Schuster et al., 1996] (Supplemental Table II).
Tabata et al. [2000] previously related polymorphisms in the 3′ end of the lmp-1 gene with HLH patients in Japan. In particular, Tabata et al. noted a 30 nucleotide deletion at position 168285–168256. Sequence analysis of the lmp-1 gene from EBV DNA isolated from HLH and non-HLH patients did not reveal the 30-bp deletion in any patient (Fig. 2). However, various SNPs were identified in the lmp-1 genes, as compared to the B95–8 prototypical EBV lmp-1 sequence. These SNPs were predicted to encode 10 amino acid mutations (Fig. 2). Seven of the mutations were found in all four EBV-HLH patients, as well as in the lmp-1 gene sequences from EBV isolated from five non-HLH control patients. Interestingly, three of these mutations lie within the 10 amino acids found deleted in the Japanese HLH patients’ EBV DNA (Fig. 2). The remaining three mutations were found in the lmp-1 sequences from patients 1, 2, and 4 and one to three control patients (Fig. 2).
Fig. 2.

Lmp-1 amino acid sequence polymorphisms, predicted from lmp-1 gene sequences of HLH (case) and non-HLH (ctrl) patient EBV DNA. Amino acids 243–386 of B95.8 Lmp-1 sequence are shown; highlighted amino acids in B95.8 sequence represent the 10 amino acid deletion found by Tabata et al.
ISH for EBV
In situ hybridization for EBV using the EBER1 probe [Quintanilla-Martinez et al., 2000] showed striking positivity in the majority of the lymphoid cells in two cases (patients 2 and 3; Table III).
Immunophenotypic Findings
Immunophenotyping was performed on the bone marrow in two cases. The infiltrate in both cases was composed predominantly of hemophagocytic histiocytes and T cells (Table III).
Molecular Genetic Studies
Analysis by PCR of paraffin-embedded tissue from the bone marrow did not identify any clonal rearrangements of the TCR-γ genes in two cases (patients 1 and 3). PCR for IgH gene rearrangements showed a polyclonal pattern in these cases (Supplemental Table I).
DISCUSSION
HLH is a rare disorder and limited population-based data are available. Virus-associated hemophagocytic syndrome has been well described [Rouphael et al., 2007]. During a 4-month period, four cases of HLH related to EBV-reactivation in young adults were identified at UCLA Medical Center, a rate 35 times higher than for the prior 3-year period. No change in referral patterns over the prior 3-year period to explain this apparent cluster of cases of HLH was found. Evidence supporting a potential role of EBV reactivation in pathogenesis of HLH was present in all four cases, but no mutations in the EBV genomes that have been described with EBV-associated HLH were identified.
EBV is one of the most frequent pathogens found in infection-associated HLH [Rouphael et al., 2007]. EBV-associated HLH has been described most commonly in Asian populations [Imashuku, 2002]. In a study in Japan, the annual incidence of HLH was 1 in 800,000 persons per year and 90% of the HLH cases were secondary, giving an estimated annual incidence of 1.1 cases of secondary HLH per million persons per year [Ishii et al., 2007]. Of these secondary cases, approximately one-third were EBV-associated [Ishii et al., 2007], for an estimated annual incidence of 0.4 cases of EBV-associated HLH per million persons. Using this proportion, a similar annual incidence of EBV-associated HLH of 0.4 per 1 million population in LA County during 2007–2008 was estimated.
EBV-HLH cases can occur in non-Asians and in younger age groups [Sonke et al., 2008; Imashuku, 2009]. In a recent retrospective study of 70 HLH cases, the prevalence of HLH was estimated as 1.07 cases per 100,000 persons less than 18-year old but no information about potential EBV infection was provided in this study [Niece et al., 2010].
To our knowledge only 13 well-characterized cases of EBV-associated HLH have been described in the United States (Supplemental Table III) [Quintanilla-Martinez et al., 2000; Halasa et al., 2003; Lindemann and Greene, 2005; Mischler et al., 2007; Rouphael et al., 2007; Brodkin et al., 2008; Kaza et al., 2008; Belyea et al., 2010], and of these, four (30.8%) were Latino [Quintanilla-Martinez et al., 2000; Brodkin et al., 2008], four (30.8%) white [Quintanilla-Martinez et al., 2000; Halasa et al., 2003; Mischler et al., 2007; Rouphael et al., 2007], one (7.7%) Asian [Quintanilla-Martinez et al., 2000], and one (7.7%) African-American [Kaza et al., 2008]. Race/ethnicity was not reported in three (23.1%) of the cases [Lindemann and Greene, 2005; Belyea et al., 2010]. In four (30.8%) of these cases another cause, such as immunodeficiency [Halasa et al., 2003; Mischler et al., 2007; Kaza et al., 2008] or malignancy [Brodkin et al., 2008] was attributed as the primary cause of HLH. A seasonal pattern has been suggested for EBV-HLH, with cases more often occurring in the summer [Chen et al., 1991]. However, all cases in this study presented during winter.
Diagnostic guidelines for HLH including clinical, laboratory, and histopathologic findings have been published by the Histiocyte Society in 2004 [Gupta et al., 2008]. In addition to fulfilling these diagnostic criteria the patient must also have at least one positive EBV test, including PCR and serology. Both serology and PCR were used to identify cases of HLH potentially associated with EBV infection.
However, EBV-specific serological assays are often uninformative in the diagnosis of EBV-related diseases [Rouphael et al., 2007]. Serologic testing for EBV suggested prior primary EBV infection and immunity in each of the four cases but could not be used to differentiate latent from reactivation infection [Rouphael et al., 2007].
The role of reactivation of latent EBV infection was supported by the finding of elevated genome copy numbers of EBV in whole blood by PCR in each of these four cases of HLH [Rouphael et al., 2007; Sonke et al., 2008]. Thus, quantitative determination of EBV genome copy numbers in peripheral blood is essential helping to determine not only the diagnosis, but also prognosis and efficacy of therapy [Rouphael et al., 2007; Sonke et al., 2008]. Higher viral loads typically are seen in EBV-associated HLH compared to infectious mononucleosis, and two of the cases had peak EBV viral loads in excess of 15,000 copies/ml [Rouphael et al., 2007]. However, it remains unclear what is the significance of the lower EBV viral loads for two patients, particularly in the later course of the disease, and whether EBV viral load in later stages may influence the prognosis of EBV-HLH.
Although B lymphocytes are the main target for EBV infection in infectious mononucleosis [Kasahara and Yachie, 2002], EBV genome also has been detected in other cells, including natural killer cells, in adult patients with EBV-HLH [Fox et al., 2010]. Consistent with other studies, histiocytes and T cells were found to account for the majority of EBER-1+cells in two EBV-HLH cases (patients 2 and 3), and NK cells were positive for EBV in one case (patient 3), consistent with a prior study of EBV-HLH cases in non-Asian patients [Fox et al., 2010]. In all four patients polyclonal population of lymphocytes in the bone marrow was found, similar to a recent study of 30 EBV-HLH cases in adults in which TCR-γ and IgH rearrangement were present in less than one-third of cases and did not have any clinical significance [Ahn et al., 2010].
EBV-infected T-cell populations showed a marked activation status with expression of HLA-DR and CD45RO [Kasahara and Yachie, 2002]. This phenotype may aid in differentiating EBV-HLH from infectious mononucleosis [Kasahara and Yachie, 2002]. Flow cytometry has been suggested as a means to monitor the clinical course of EBV-HLH and the dynamics of EBV-infected T cells [Kasahara and Yachie, 2002]. HLH also is associated with marked elevation in ferritin levels, but the impact of EBV infection and viral load on serum ferritin concentration was not examined.
The pathophysiology of acquired HLH remains incompletely understood [Rouphael et al., 2007], including whether a genetic predisposition and/or EBV-associated immune dysregulation underlies EBV-associated HLH. Host- and EBV-specific factors associated with EBV-HLH, including those that were observed in this study, are summarized in Supplemental Table IV. More studies are needed to determine the host and viral factors and that are associated with EBV-HLH.
Recent studies have focused on viral characteristics that may induce the phenotype of EBV-HLH. The higher incidence rates of EBV-HLH in Asian countries are theorized to be due to a more pathogenic strain of EBV [Tabata et al., 2000]. EBV typing by the EBNA-2 gene demonstrated all viruses in this study were EBV type 1, the predominant type found in EBV-infected immunocompetent individuals [Aitken et al., 1994; Schuster et al., 1996]. EBV type 1 can be further divided into sub-strains based on SNPs in the EBNA-2 gene [Aitken et al., 1994; Schuster et al., 1996], which may indicate the prevalent circulating EBV strain to which a particular patient was exposed (18). All 14 EBV, from 10 HLH-negative and 4 HLH-positive patients, showed 99% homology to each other suggestive that all isolates, including those from HLH and non-HLH patients, were of the same EBV sub-strain, which likely represents the sub-strain circulating in the Los Angeles area.
LMP-1 on the surface of EBV has been shown to lead to increased virulence of EBV and viral secretion from infected T lymphocytes [Kimura, 2006; Rouphael et al., 2007]. Tabata et al. [2000] previously described a 30-bp deletion in the 3′-end of the lmp-1 gene in EBV strains isolated from patients with HLH patients in Japan. No evidence of this deletion in sequence analysis of the lmp-1 gene in case or control specimens was found. While these findings may relate to an increased frequency of this deletion in EBV strains from Asian and European countries compared to North America, more recent reports describe lmp-1 gene variations in specimens from patients suffering a wide variety of EBV manifestations, including infectious mononucleosis [Tabata et al., 2000], questioning the significance of this deletion in the pathogenesis of EBV-HLH. Thus, this study is among the first studies, to our knowledge, that attempts to describe SNPs that are present in EBV-HLH cases in adults but the significance of these SNPs remains to be determined.
The high mortality rate among patients with EBV-HLH is attributable, at least in part, to delays in diagnosis [Fisman, 2000; Filipovich, 2009]. Poor prognostic factors in EBV-HLH include older age, persistently elevated levels (>104 copies/ml) of cell-free EBV, chromosomal abnormalities, hereditary immunodeficiency, and reactivation rather than a primary EBV infection [Teramura et al., 2002; Ishii et al., 2007]. All patients in this study had evidence of reactivation of EBV infection, patient 2 had hereditary immunodeficiency (chronic granulomatous disease) and patient 3 had persistently elevated levels of cell-free EBV and chromosomal abnormalities. Despite these poor prognostic factors patient 3 was the only survivor.
EBV-specific therapy is ineffective in treating EBV-HLH, possibly because EBV responds poorly to antiviral agents. Antiviral therapy was used in two cases (patients 1 and 3) and led to significant reduction in EBV PCR titers in one case (patient 3). There is little data that antiviral therapy improves outcome in EBV-HLH [Ishii et al., 2007]. In mild cases, there may be a watch and wait “window” period, or the patient can be treated conservatively with corticosteroid and cyclosporine or intravenous immunoglobulins (IVIG) [Beutel et al., 2009]. This approach initially was utilized for patient 1 but did not improve his clinical picture. Rituximab may eradicate EBV from the circulation but not from the tissues and was used in patient 1, but had minimal effect on his clinical course [Kaza et al., 2008]. Three of the patients in this study received the HLH-2004 protocol [Imashuku et al., 2001]. One of the 3 patients initially responded to therapy but relapsed and then was lost to follow up. Bone marrow transplantation may be necessary for patients with refractory EBV-HLH [Imashuku et al., 2001] but the optimal timing of transplantation is often unclear, especially when a patient is responding clinically but has persistent high levels of EBV genome copies, as was seen with patient 3. Close follow-up is recommended in such patients [Beutel et al., 2009].
This study has a number of important limitations, including the retrospective, single-institution study design. The calculated incidence of HLH in LA County likely underestimates true incidence of the disease because of misclassification and misdiagnosis especially for cases with ICD-9 codes from other facilities. However, it remains unclear if the virologic characteristics of EBV-HLH described in young children, who usually present with primary EBV infection and thus have higher viral copy number, apply to the cases in this study that occurred in young adults with evidence of reactivation of EBV infection. Cases of HLH where the association with EBV infection was not as clear (e.g., patient 4) were also included, because of the rarity of the disorder and unusual temporal clustering of cases over a 4-month period.
Although the number of cases of EBV-associated HLH and secondary HLH that was detected during the study period did not exceed the threshold for public health concern, given the severity of illness, clinicians should be aware about this clinical entity. Although EBV-HLH is a rare disease, the incidence in adults in the United States may be underestimated, and, importantly, EBV-HLH can occur associated with EBV reactivation in EBV-immune individuals.
Supplementary Material
ACKNOWLEDGMENTS
We thank Caitlin Reed, MD, MPH (Epidemic Intelligence Service Officer, Centers for Disease Control and Prevention Los Angeles County Dept. of Public Health Acute Communicable Disease Control) for her role in the public health analysis.
Grant sponsor: Institutional Funds.
Footnotes
Disclaimers: none.
Additional supporting information may be found in the online version of this article.
REFERENCES
- Ahn JS, Rew SY, Shin MG, Kim Yang DH, Cho D, Kim SH, Bae SY, Lee SR, Kim YK, Kim HJ, Lee JJ. 2010. Clinical significance of clonality and Epstein–Barr virus infection in adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol 85: 719–722. [DOI] [PubMed] [Google Scholar]
- Aitken CS, Sengupta K, Aedes C, Moss DJ, Sculley TB. 1994. Heterogeneity within the Epstein–Barr virus nuclear antigen 2 gene in different strains of Epstein–Barr virus. J Gen Virol 75:95–100. [DOI] [PubMed] [Google Scholar]
- Belyea B, Hinson A, Moran C, Hwang E, Heath J, Barfield R. 2010. Spontaneous resolution of Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 55:754–756. [DOI] [PubMed] [Google Scholar]
- Beutel K, Gross-Wieltsch U, Wiesel T, Stadt UZ, Janka G, Wagner HJ. 2009. Infection of T lymphocytes in Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis in children of non-Asian origin. Pediatr Blood Cancer 53:184–190. [DOI] [PubMed] [Google Scholar]
- Brodkin DE, Hobohm DW, Nigam R. 2008. Nasal-type NK/T-cell lymphoma presenting as hemophagocytic syndrome in an 11-year-old Mexican boy. J Pediatr Hematol Oncol 30:938–940. [DOI] [PubMed] [Google Scholar]
- Chen RL, Su IJ, Lin KH, Lee SH, Lin DT, Chuu WM, Lin KS, Huang LM, Lee CY. 1991. Fulminant childhood hemophagocytic syndrome mimicking histiocytic medullary reticulosis. An atypical form of Epstein–Barr virus infection. Am J Clin Pathol 96:171–176. [DOI] [PubMed] [Google Scholar]
- Filipovich AH. 2009. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematol Am Soc Hematol Educ Program 127–131. [DOI] [PubMed] [Google Scholar]
- Fisman DN. 2000. Hemophagocytic syndromes and infection. Emerg Infect Dis 6:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CP, Shannon-Lowe C, Gothard P, Kishore B, Neilson J, O’Connor N, Rowe M. 2010. Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis in adults characterized by high viral genome load within circulating natural killer cells. Clin Infect Dis 51:66–69. [DOI] [PubMed] [Google Scholar]
- Gulley ML, Tang W. 2008. Laboratory assays for Epstein–Barr virus-related disease. J Mol Diagn 10:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Weitzman S, Abdelhaleem M. 2008. The role of hemophagocytosis in bone marrow aspirates in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 50:192–194. [DOI] [PubMed] [Google Scholar]
- Halasa NB, Whitlock JA, McCurley TL, Smith JA, Zhu Q, Ochs H, Dermody TS, Crowe JE. 2003. Fatal hemophagocytic lymphohistiocytosis associated with Epstein–Barr virus infection in a patient with a novel mutation in the signaling lymphocytic activation molecule-associated protein. Clin Infect Dis 37:e136–e141. [DOI] [PubMed] [Google Scholar]
- Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. 2007. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48: 124–131. [DOI] [PubMed] [Google Scholar]
- Imashuku S 2002. Clinical features and treatment strategies of Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol 44:259–272. [DOI] [PubMed] [Google Scholar]
- Imashuku S 2009. Infection of T lymphocytes in non-Asian patients with Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 53:1359. [DOI] [PubMed] [Google Scholar]
- Imashuku S 2011. Treatment of Epstein–Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH); update 2010. J Pediatr Hematol Oncol 33:35–39. [DOI] [PubMed] [Google Scholar]
- Imashuku S, Kuriyama K, Teramura T, Ishii E, Kinugawa N, Kato M, Sako M, Hibi S. 2001. Requirement for etoposide in the treatment of Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol 19:2665–2673. [DOI] [PubMed] [Google Scholar]
- Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, Yamamoto K, Horiuchi H, Takada K, Ohshima K, Nakamura S, Kinukawa N, Oshimi K, Kawa K. 2007. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol 86:58–65. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Yachie A. 2002. Cell type specific infection of Epstein–Barr virus (EBV) in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Crit Rev Oncol Hematol 44:283–294. [DOI] [PubMed] [Google Scholar]
- Kaza U, Knight AK, Jeroudi M, Bocchini JA, Anga A, Bahna SL. 2008. A boy with fever, lymphadenopathy, hepatosplenomegaly, and lymphocytosis. Allergy Asthma Proc 29:216–220. [DOI] [PubMed] [Google Scholar]
- Kimura H 2006. Pathogenesis of chronic active Epstein–Barr virus infection: Is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol 16:251–261. [DOI] [PubMed] [Google Scholar]
- Lindemann TL, Greene JS. 2005. Persistent cervical lymphadenopathy in an adolescent with Epstein–Barr induced hemophagocytic syndrome: Manifestations of a rare but often fatal disease. Int J Pediatr Otorhinolaryngol 69:1011–1014. [DOI] [PubMed] [Google Scholar]
- Mendes TM, Oliveira LC, Yamamoto L, Del Negro GM, Okay TS. 2008. Epstein–Barr virus nuclear antigen-2 detection and typing in immunocompromised children correlated with lymphoproliferative disorder biopsy findings. Braz J Infect Dis 12:186–191. [DOI] [PubMed] [Google Scholar]
- Mischler M, Fleming GM, Shanley TP, Madden L, Levine J, Castle V, Filipovich AH, Cornell TT. 2007. Epstein–Barr virus-induced hemophagocytic lymphohistiocytosis and X-linked lymphoproliferative disease: A mimicker of sepsis in the pediatric intensive care unit. Pediatrics 119:e1212–e1218. [DOI] [PubMed] [Google Scholar]
- Niece JA, Rogers ZR, Ahmad N, Langevin AM, McClain KL. 2010. Hemophagocytic lymphohistiocytosis in Texas: Observations on ethnicity and race. Pediatr Blood Cancer 54:424–428. [DOI] [PubMed] [Google Scholar]
- Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M, Straus SE, Jaffe ES. 2000. Fulminant EBV(þ) T-cell lymphoproliferative disorder following acute/chronic EBV infection: A distinct clinicopathologic syndrome. Blood 96:443–451. [PubMed] [Google Scholar]
- Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. 2007. Infections associated with haemophagocytic syndrome. Lancet Infect Dis 7:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V, Ott G, Seidenspinner S, Kreth HW. 1996. Common Epstein–Barr virus (EBV) type-1 variant strains in both malignant and benign EBV-associated disorders. Blood 87:1579–1585. [PubMed] [Google Scholar]
- Sonke GS, Ludwig I, van Oosten H, Baars JW, Meijer E, Kater AP, de Jong D. 2008. Poor outcomes of chronic active Epstein–Barr virus infection and hemophagocytic lymphohistiocytosis in non-Japanese adult patients. Clin Infect Dis 47:105–108. [DOI] [PubMed] [Google Scholar]
- Tabata YS, Teramura T, Kuriyama K, Yagi T, Todo S, Sawada T, Imashuku S. 2000. Molecular analysis of latent membrane protein 1 in patients with Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis in Japan. Leuk Lymphoma 38:373–380. [DOI] [PubMed] [Google Scholar]
- Teramura T, Tabata Y, Yagi T, Morimoto A, Hibi S, Imashuku S. 2002. Quantitative analysis of cell-free Epstein–Barr virus genome copy number in patients with EBV-associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma 43:173–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


