Abstract
Mesothelin (MSLN) represents an attractive molecule for targeted cancer therapies. To identify tumors that might benefit from such therapies, tissue microarrays including 15,050 tumors from 122 different tumor types and 76 healthy organs were analyzed for MSLN expression by immunohistochemistry. Sixty-six (54%) tumor types showed at least occasional weak staining, including 50 (41%) tumor types with at least one strongly positive sample. Highest prevalence of MSLN positivity had ovarian carcinomas (serous 97%, clear cell 83%, endometrioid 77%, mucinous 71%, carcinosarcoma 65%), pancreatic adenocarcinoma (ductal 75%, ampullary 81%), endometrial carcinomas (clear cell 71%, serous 57%, carcinosarcoma 50%, endometrioid 45%), malignant mesothelioma (69%), and adenocarcinoma of the lung (55%). MSLN was rare in cancers of the breast (7% of 1138), kidney (7% of 807), thyroid gland (1% of 638), soft tissues (0.3% of 931), and prostate (0 of 481). High expression was linked to advanced pathological tumor (pT) stage (p < 0.0001) and metastasis (p < 0.0001) in 1619 colorectal adenocarcinomas, but unrelated to parameters of malignancy in 1072 breast-, 386 ovarian-, 174 lung-, 757 kidney-, 171 endometrial-, 373 gastric-, and 925 bladder carcinomas. In summary, numerous important cancer types with high-level MSLN expression might benefit from future anti-MSLN therapies, but MSLN’s prognostic relevance appears to be limited.
Keywords: mesothelin, multi-tumor tissue micro array, immunohistochemistry
1. Introduction
The mesothelin (MSLN) gene, located at chromosome 16p13.3, encodes for a membranous precursor glycoprotein that is subsequently cleaved into the soluble 31kD protein megakaryocyte potentiating factor (MPF) and the 40kD membrane-bound protein MSLN [1,2,3]. MSLN was first described as a membrane protein expressed in normal and neoplastic mesothelial cells, but subsequent studies demonstrated a broader expression pattern [1,4,5,6,7,8,9]. The function of MSLN is not fully understood. In normal cells, MSLN does not seem to be essential as a homozygous MSLN mutant mouse lacking MSLN protein developed and reproduced normally [10]. However, MSLN has been identified as a specific binding protein of cancer antigen (CA125) that mediates cell adhesion [11,12]. This interaction was suggested to play a role in the development of peritoneal metastasis [11,13]. In cell line and animal experiments, MSLN overexpression was shown to activate the PI3K/AkT, NFκB, and MAPK/ERK pathways, to hinder apoptosis and to promote cell proliferation, migration, and metastasis [14,15,16,17,18,19,20,21].
MSLN is expressed in only few normal tissues but has been found to be overexpressed in various tumor types at a relevant frequency [4,5,6,7,8,9,22]. Therefore, and due to its membranous location, MSLN represents an attractive molecule for target-specific cancer therapies. Targeted therapies use drugs to inhibit specific genes and proteins that are involved in tumor cell growth. Several therapeutic approaches, including adaptive immunotherapy (CAR-T cells, TC-210 T cells), monoclonal antibodies (Amatuximab/MORAb-009), recombinant immunotoxins (SS1P, LMB-100/RG7787), antibody-drug conjugates (Anetumab Ravtansine/BAY94-9343, DMOT4039A, BAY2287411, BMS-986148, h7D9.v3), listeria monocytogene induced anti-tumor immune response (CRS-207, JNJ-64041757), and immunocytokines (IL12-SS1) have provided encouraging data in animal models and/or clinical phase I and II trials [23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Which tumor entities might benefit most from anti-MSLN therapies is difficult to predict since the literature on MSLN expression is controversial for many tumor entities. For example, MSLN positivity has been described in 38% to 69% of lung adenocarcinomas [37,38,39], 17% to 100% of pancreatic adenocarcinomas [40,41], 2% to 68% of colorectal carcinomas [5,9,42], 55% to 100% of serous carcinomas of the ovary [43,44], 21% to 78% of gastric adenocarcinomas [5,9,45], and 3% to 36% of breast carcinomas [9,46,47]. These conflicting data are likely to be caused by the use of different antibodies, immunostaining protocols, and criteria to categorize MSLN immunostaining in these studies.
To better understand the prevalence and significance of MSLN expression in cancer, a comprehensive study analyzing a large number of neoplastic and non-neoplastic tissues under highly standardized conditions is needed. Therefore, MSLN expression was analyzed in more than 15,000 tumor tissue samples from 122 different tumor types and subtypes, as well as 76 non-neoplastic tissue categories, by immunohistochemistry (IHC) in a tissue microarray (TMA) format in this study.
2. Material and Methods
2.1. Tissue Microarrays (TMAs)
The normal tissue TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on one slide). The cancer TMAs contained a total of 15,050 primary tumors from 122 tumor types and subtypes. Detailed histopathological data on grade, pathological tumor stage (pT), or pathological lymph node status (pN) were available from 7625 tumors (cancers of the colon, bladder, ovarian, endometrium, lung, stomach, breast, and kidney tumors). Clinical follow-up data were available from 1178 breast cancer, 865 kidney cancer, and 254 bladder cancer patients with a median follow-up time of 49/39/14 months (range 1–88/1–250/1–77). No data on previous therapies were available. The composition of both normal and cancer TMAs is described in detail in the results section. All samples were from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described earlier in detail [48,49]. In brief, one tissue spot (diameter: 0.6 mm) was transmitted from a cancer containing donor block (≥70% cancer cells) in an empty recipient paraffin block. The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes, as well as patient data analysis, has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09, 25 January 2010). All work has been carried out in compliance with the Helsinki Declaration.
2.2. Immunohistochemistry (IHC)
Freshly prepared TMA sections were immunostained on one day in one experiment. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series, and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in pH 9 DakoTarget Retrieval SolutionTM (Agilent, Santa Clara, CA, USA; #S2367). Endogenous peroxidase activity was blocked with Dako Peroxidase Blocking SolutionTM (Agilent, CA, USA; #52023) for 10 min. Primary antibody specific against MSLN protein (mouse monoclonal, MSVA-235, MS Validated Antibodies, Hamburg, Germany) was applied at 37 °C for 60 min at a dilution of 1:150. Bound antibody was then visualized using the EnVision KitTM (Agilent, CA, USA; #K5007) according to the manufacturer’s directions. The sections were counterstained with hemalaun. A trained pathologist scored all tissue spots and marked tissue spots with questionable findings for revision by a second pathologist. For normal tissues, the staining intensity of positive cells was semi-quantitively recorded (+, ++, +++). For tumor tissues, the percentage of MSLN positive tumor cells was estimated and the staining intensity was semi-quantitatively recorded (0, 1+, 2+, 3+). For statistical analyses, the staining results were categorized into four groups as follows: Negative: no staining at all, weak staining: staining intensity of 1+ in ≤70% or staining intensity of 2+ in ≤30% of tumor cells, moderate staining: staining intensity of 1+ in >70%, staining intensity of 2+ in > 30% but in ≤70% or staining intensity of 3+ in ≤30% of tumor cells, strong staining: staining intensity of 2+ in >70% or staining intensity of 3+ in >30% of tumor cells.
2.3. Statistics
Statistical calculations were performed with JMP 14 software (SAS Institute Inc., Cary, NC, USA). Contingency tables and the chi²-test were performed to search for associations between MSLN and tumor phenotype. Survival curves were calculated according to Kaplan-Meier. The Log-Rank test was applied to detect significant differences between groups. A p-value of ≤0.05 was defined as significant.
3. Results
3.1. Technical Issues
A total of 12,679 (84.2%) of 15,050 tumor samples were interpretable in the TMA analysis. The remaining 2371 (15.8%) samples were not analyzable due to the lack of unequivocal tumor cells or loss of the tissue spot during the technical procedures. On the normal tissue TMA, a sufficient number of samples was always interpretable per tissue to determine MSLN expression.
3.2. MSLN Immunostaining in Normal Tissues
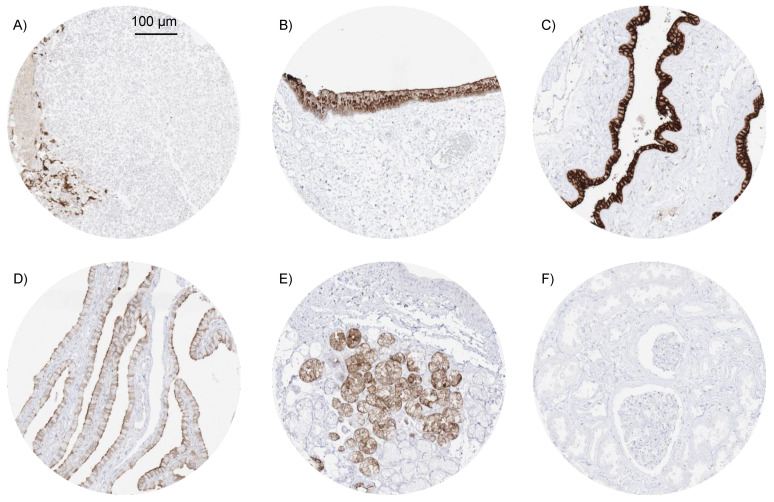
In normal tissues, the strongest MSLN expression was observed in the squamous epithelium of tonsil crypts (Figure 1A), where a fraction of cells (intermediate to superficial cell layers) showed strong (+++) MSLN staining. Strong MSLN immunostaining was also seen in some cells and cell groups of the rectal mucosa (+++), the anal transitional epithelium (+++) (Figure 1B) where staining was often particularly prominent in superficial mucinous cells, amnion cells (+++) (Figure 1C) and some chorion cells (++) of the mature placenta, and some elements of corpuscles of Hassall’s of the thymus (++). A somewhat weaker MSLN staining was seen in scattered cells and groups of cells of endocervical mucosa (++) and endometrium (++), epithelial cells of fallopian tube (apical cell border and cilia; ++) (Figure 1D), some intermediate (neck) cells of the stomach antrum (+), some scattered glands in sublingual (Figure 1E) and Brunner glands, few cells of respiratory epithelium (goblet cells;++), some cells or groups of cells in bronchial glands (++), seminal vesicle (+; not in all samples), and in the cytoplasm of few cells of the adenohypophysis (+). MSLN immunostaining was absent in endothelium and media of the aorta, heart muscle, striated muscle, tongue muscle, myometrium of the uterus, muscular wall of the appendix, esophagus, ileum, kidney pelvis, and urinary bladder, corpus spongiosum of the penis, ovarian stroma, fat, skin, hair follicles and sebaceous glands of the skin, non-keratinizing squamous epithelium from the lip, oral cavity, ectocervix, and the esophagus, urothelium of the kidney pelvis and urinary bladder, spleen, antrum and corpus of the stomach, gallbladder epithelium, liver, kidney (Figure 1F), epididymis, testis, lung, decidua cells, cerebellum, cerebrum, salivary glands, prostate, breast, adrenal gland, and lymphatic tissue.
Figure 1.
Mesothelin (MSLN) immunostaining in normal tissues. Among normal tissues, MSLN immunostaining is particularly strong in a fraction of squamous epithelial cells in tonsil crypts (A), the anal transitional epithelium (B) and amnion cells (C). A somewhat weaker MSLN staining is seen at the apical cell border and in cilia of fallopian tube epithelium (D) and in a fraction mucinous cells (often grouped together) in sublingual glands (E). MSLN immunostaining was consistently lacking in the kidney (F).
3.3. MSLN Immunostaining in Neoplastic Tissues
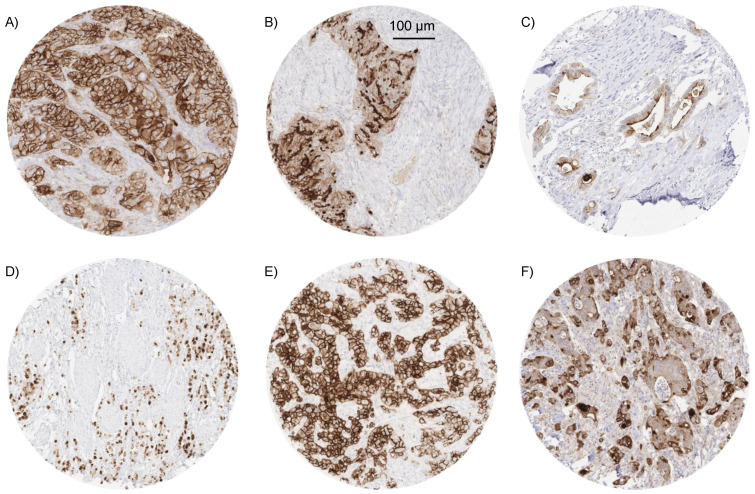
A significant MSLN immunostaining was observed in 2413 (19.0%) of 12,679 analyzable tumors, including 8.0% with weak, 3.4% with moderate, and 7.6% with strong staining intensity. The staining pattern was variable. Most positive tumors showed a predominantly apical membranous MSLN staining, often accompanied by a less intense cytoplasmic coloration. Other tumors showed a pure membranous staining or a predominantly cytoplasmic positivity. Representative images are shown in Figure 2. At least an occasional weak MSLN positivity was detected in 66 of 122 (54.1%) different tumor types and tumor subtypes and 50 (41.0%) tumor types and subtypes had at least one tumor sample exhibiting strong positivity. The highest frequencies of MSLN positivity were seen in different subtypes of ovarian (65% to 97%) and endometrium (45% to 71%) carcinomas, pancreatic adenocarcinoma (75% and 81%), malignant mesothelioma (69%), and adenocarcinoma of the lung (55%). Rare or absent MSLN positivity was observed in different subtypes of breast tumors (0% in 50 phyllodes, 0.9% in 294 lobular, 5.3% in 27 tubular, 6.6% in 1391 invasive no special type, and 10.9% in 58 mucinous subtypes), renal cell (0% in 177 oncocytomas, 1.6% in 131 chromophobe, 6.6% in 858 clear cell, and 8.7% in 255 papillary subtypes), and thyroid carcinomas (0% to 1.4%), as well as soft tissue tumors (0.3%), and adenocarcinomas of the prostate (0%). A detailed description of the immunostaining results is given in Table 1 and Figure 3.
Figure 2.
Pattern of MSLN immunostaining in cancer. MSLN immunostaining was found to be strong and predominantly membranous (A) or predominantly situated at the apical/luminal cell border (B) in two serous high-grade ovarian cancers, variable but predominantly apical in a cholangiocellular carcinoma (C), strong and predominantly cytoplasmic in a diffuse type adenocarcinoma of the stomach (D), strong and predominantly membranous in a malignant (epitheloid) mesothelioma (E), and weak to moderate, cytoplasmic and membranous in a colorectal adenocarcinoma (F).
Table 1.
MSLN immunostaining in human tumors.
| MSLN Immunostaining | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tumor Entity | on TMA (n) | Analyzable (n) | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | Positive (%) | |
| Tumors of the skin | Pilomatrixoma | 35 | 31 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Basal cell carcinoma | 88 | 83 | 98.8 | 1.2 | 0.0 | 0.0 | 1.2 | |
| Benign nevus | 29 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the skin | 90 | 84 | 95.2 | 4.8 | 0.0 | 0.0 | 4.8 | |
| Malignant melanoma | 48 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Merkel cell carcinoma | 46 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 110 | 105 | 80.0 | 11.4 | 3.8 | 4.8 | 20.0 |
| Squamous cell carcinoma of the pharynx | 60 | 57 | 80.7 | 5.3 | 8.8 | 5.3 | 19.3 | |
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 121 | 83.5 | 9.1 | 5.0 | 2.5 | 16.5 | |
| Pleomorphic adenoma of the parotid gland | 50 | 35 | 57.1 | 20.0 | 5.7 | 17.1 | 42.9 | |
| Warthin tumor of the parotid gland | 49 | 45 | 46.7 | 31.1 | 11.1 | 11.1 | 53.3 | |
| Basal cell adenoma of the salivary gland | 15 | 15 | 66.7 | 33.3 | 0.0 | 0.0 | 33.3 | |
| Tumors of the lung, pleura, and thymus | Squamous cell carcinoma of the lung | 127 | 71 | 74.6 | 18.3 | 5.6 | 1.4 | 25.4 |
| Adenocarcinoma of the lung | 250 | 174 | 44.8 | 19.0 | 13.8 | 22.4 | 55.2 | |
| Small cell carcinoma of the lung | 20 | 16 | 93.8 | 0.0 | 6.3 | 0.0 | 6.3 | |
| Mesothelioma, epitheloid | 39 | 25 | 36.0 | 12.0 | 4.0 | 48.0 | 64.0 | |
| Mesothelioma, other types | 76 | 65 | 29.2 | 10.8 | 4.6 | 55.4 | 70.8 | |
| Thymoma | 29 | 28 | 96.4 | 0.0 | 0.0 | 3.6 | 3.6 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 78 | 75 | 88.0 | 4.0 | 2.7 | 5.3 | 12.0 |
| Squamous cell carcinoma of the vulva | 130 | 123 | 89.4 | 7.3 | 0.8 | 2.4 | 10.6 | |
| Squamous cell carcinoma of the cervix | 130 | 125 | 57.6 | 21.6 | 8.0 | 12.8 | 42.4 | |
| Endometrioid endometrial carcinoma | 236 | 220 | 54.5 | 22.3 | 11.8 | 11.4 | 45.5 | |
| Endometrial serous carcinoma | 82 | 69 | 43.5 | 17.4 | 10.1 | 29.0 | 56.5 | |
| Carcinosarcoma of the uterus | 48 | 46 | 50.0 | 21.7 | 10.9 | 17.4 | 50.0 | |
| Endometrial carcinoma, high grade, G3 | 13 | 13 | 61.5 | 23.1 | 0.0 | 15.4 | 38.5 | |
| Endometrial clear cell carcinoma | 8 | 7 | 28.6 | 28.6 | 14.3 | 28.6 | 71.4 | |
| Endometrioid carcinoma of the ovary | 115 | 100 | 23.0 | 28.0 | 19.0 | 30.0 | 77.0 | |
| Serous carcinoma of the ovary | 567 | 511 | 2.7 | 5.1 | 8.4 | 83.8 | 97.3 | |
| Mucinous carcinoma of the ovary | 97 | 80 | 28.8 | 37.5 | 18.8 | 15.0 | 71.3 | |
| Clear cell carcinoma of the ovary | 54 | 42 | 16.7 | 33.3 | 19.0 | 31.0 | 83.3 | |
| Carcinosarcoma of the ovary | 47 | 43 | 34.9 | 16.3 | 11.6 | 37.2 | 65.1 | |
| Brenner tumor | 9 | 9 | 55.6 | 11.1 | 0.0 | 33.3 | 44.4 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 1391 | 1138 | 93.4 | 2.8 | 0.8 | 3.0 | 6.6 |
| Lobular carcinoma of the breast | 294 | 215 | 99.1 | 0.0 | 0.9 | 0.0 | 0.9 | |
| Medullary carcinoma of the breast | 26 | 26 | 76.9 | 11.5 | 7.7 | 3.8 | 23.1 | |
| Tubular carcinoma of the breast | 27 | 19 | 94.7 | 0.0 | 0.0 | 5.3 | 5.3 | |
| Mucinous carcinoma of the breast | 58 | 46 | 89.1 | 4.3 | 0.0 | 6.5 | 10.9 | |
| Phyllodes tumor of the breast | 50 | 36 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 48 | 72.9 | 16.7 | 8.3 | 2.1 | 27.1 |
| Adenomatous polyp, high-grade dysplasia | 50 | 46 | 56.5 | 30.4 | 8.7 | 4.3 | 43.5 | |
| Adenocarcinoma of the colon | 1882 | 1186 | 58.6 | 29.0 | 6.9 | 5.5 | 41.4 | |
| Adenocarcinoma of the small intestine | 10 | 6 | 0.0 | 50.0 | 16.7 | 33.3 | 100.0 | |
| Gastric adenocarcinoma, diffuse type | 176 | 164 | 51.2 | 14.6 | 11.6 | 22.6 | 48.8 | |
| Gastric adenocarcinoma, intestinal type | 174 | 170 | 60.6 | 18.8 | 11.2 | 9.4 | 39.4 | |
| Gastric adenocarcinoma, mixed type | 62 | 59 | 50.8 | 25.4 | 10.2 | 13.6 | 49.2 | |
| Adenocarcinoma of the esophagus | 133 | 75 | 58.7 | 14.7 | 18.7 | 8.0 | 41.3 | |
| Squamous cell carcinoma of the esophagus | 124 | 71 | 78.9 | 14.1 | 4.2 | 2.8 | 21.1 | |
| Squamous cell carcinoma of the anal canal | 91 | 86 | 75.6 | 14.0 | 2.3 | 8.1 | 24.4 | |
| Cholangiocarcinoma | 114 | 105 | 78.1 | 4.8 | 4.8 | 12.4 | 21.9 | |
| Hepatocellular carcinoma | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Ductal adenocarcinoma of the pancreas | 130 | 64 | 25.0 | 12.5 | 21.9 | 40.6 | 75.0 | |
| Pancreatic/Ampullary adenocarcinoma | 58 | 26 | 19.2 | 19.2 | 23.1 | 38.5 | 80.8 | |
| Acinar cell carcinoma of the pancreas | 7 | 6 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Gastrointestinal stromal tumor (GIST) | 50 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the urinary system | Non-invasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 154 | 96.1 | 3.2 | 0.0 | 0.6 | 3.9 |
| Non-invasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 125 | 96.8 | 3.2 | 0.0 | 0.0 | 3.2 | |
| Non-invasive papillary urothelial carcinoma, pTa G3 | 187 | 130 | 96.9 | 2.3 | 0.0 | 0.8 | 3.1 | |
| Urothelial carcinoma, pT2-4 G3 | 940 | 838 | 87.5 | 8.0 | 2.5 | 2.0 | 12.5 | |
| Small cell neuroendocrine carcinoma of the bladder | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Sarcomatoid urothelial carcinoma | 25 | 22 | 90.9 | 9.1 | 0.0 | 0.0 | 9.1 | |
| Clear cell renal cell carcinoma | 858 | 807 | 93.4 | 3.7 | 1.6 | 1.2 | 6.6 | |
| Papillary renal cell carcinoma | 255 | 242 | 91.3 | 5.4 | 1.7 | 1.7 | 8.7 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 21 | 81.0 | 19.0 | 0.0 | 0.0 | 19.0 | |
| Chromophobe renal cell carcinoma | 131 | 124 | 98.4 | 0.8 | 0.8 | 0.0 | 1.6 | |
| Oncocytoma | 177 | 170 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 80 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 76 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 84 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adenocarcinoma of the prostate (recurrence) | 330 | 241 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Small cell neuroendocrine carcinoma of the prostate | 17 | 17 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Seminoma | 624 | 613 | 99.7 | 0.0 | 0.0 | 0.3 | 0.3 | |
| Embryonal carcinoma of the testis | 50 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Yolk sack tumor | 50 | 37 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Teratoma | 50 | 43 | 83.7 | 11.6 | 4.7 | 0.0 | 16.3 | |
| Squamous cell carcinoma of the penis | 80 | 76 | 93.4 | 5.3 | 0.0 | 1.3 | 6.6 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 114 | 104 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Papillary thyroid carcinoma | 392 | 358 | 99.2 | 0.8 | 0.0 | 0.0 | 0.8 | |
| Follicular thyroid carcinoma | 158 | 146 | 98.6 | 1.4 | 0.0 | 0.0 | 1.4 | |
| Medullary thyroid carcinoma | 107 | 91 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Anaplastic thyroid carcinoma | 45 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical adenoma | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical carcinoma | 26 | 25 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Phaeochromocytoma | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Appendix, neuroendocrine tumor (NET) | 22 | 14 | 92.9 | 0.0 | 7.1 | 0.0 | 7.1 | |
| Colorectal, neuroendocrine tumor (NET) | 10 | 10 | 70.0 | 30.0 | 0.0 | 0.0 | 30.0 | |
| Ileum, neuroendocrine tumor (NET) | 49 | 47 | 87.2 | 8.5 | 0.0 | 4.3 | 12.8 | |
| Lung, neuroendocrine tumor (NET) | 19 | 17 | 94.1 | 5.9 | 0.0 | 0.0 | 5.9 | |
| Pancreas, neuroendocrine tumor (NET) | 102 | 95 | 98.9 | 0.0 | 0.0 | 1.1 | 1.1 | |
| Colorectal, neuroendocrine carcinoma (NEC) | 11 | 9 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Gallbladder, neuroendocrine carcinoma (NEC) | 4 | 4 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pancreas, neuroendocrine carcinoma (NEC) | 13 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of hematopoietic and lymphoid tissues | Hodgkin Lymphoma | 103 | 101 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Non-Hodgkin Lymphoma | 62 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Small lymphocytic lymphoma, B-cell type (B-SLL/B-CLL) | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B cell lymphoma (DLBCL) | 114 | 114 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Follicular lymphoma | 88 | 87 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| T-cell Non Hodgkin lymphoma | 24 | 24 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mantle cell lymphoma | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Marginal zone lymphoma | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B-cell lymphoma (DLBCL) in the testis | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Burkitt lymphoma | 5 | 2 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of soft tissue and bone | Tenosynovial giant cell tumor | 45 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Granular cell tumor | 53 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyoma | 50 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Angiomyolipoma | 91 | 84 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Angiosarcoma | 73 | 66 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Dermatofibrosarcoma protuberans | 21 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 6 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyosarcoma | 87 | 84 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Liposarcoma | 132 | 116 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Malignant peripheral nerve sheath tumor (MPNST) | 13 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Myofibrosarcoma | 26 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Neurofibroma | 117 | 113 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Sarcoma, not otherwise specified (NOS) | 75 | 73 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Paraganglioma | 41 | 40 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Primitive neuroectodermal tumor (PNET) | 23 | 19 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 7 | 7 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Schwannoma | 121 | 111 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Synovial sarcoma | 12 | 11 | 72.7 | 27.3 | 0.0 | 0.0 | 27.3 | |
| Osteosarcoma | 43 | 36 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Chondrosarcoma | 39 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
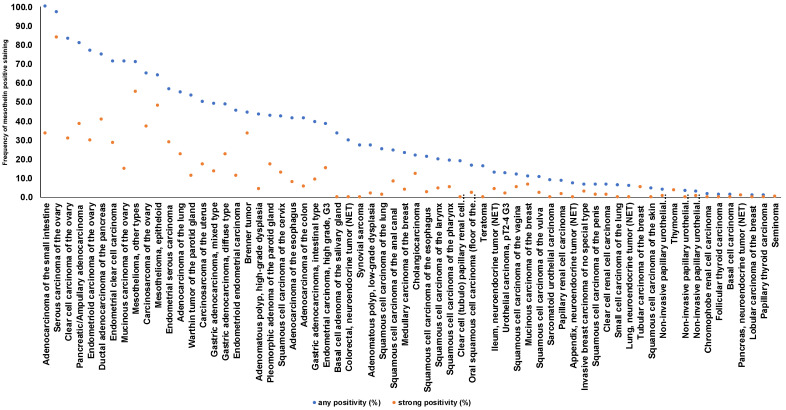
Figure 3.
Ranking order of MSLN immunostaining in cancers. Both the frequency of positive cases (blue dots) and the frequency of strongly positive cases (orange dots) are shown. Fifty-six additional tumor entities without any MSLN positive cases are not shown due to space restrictions.
3.4. MSLN Immunostaining, Tumor Phenotype, and Prognosis
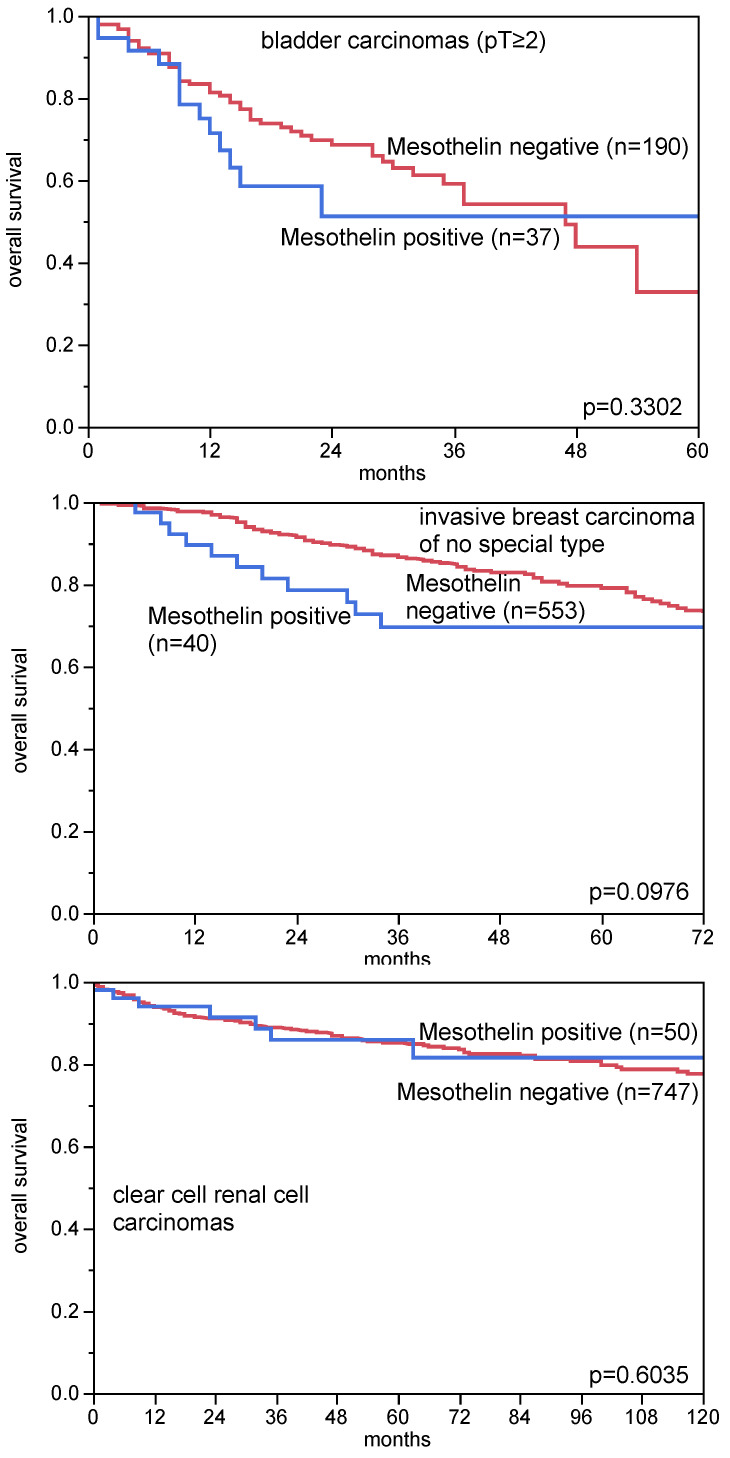
A comparison of MSLN immunostaining with pT, pN, and histological grade in 1619 colorectal adenocarcinomas, 1072 invasive breast carcinomas of no special type, 386 serous carcinomas of the ovary, 174 lung adenocarcinomas, 757 clear cell renal cell carcinomas, and 171 endometrioid endometrial, in 373 gastric, and in 925 bladder carcinomas revealed only a statistically significant association between MSLN immunostaining and pT stage, as well as pN status in colorectal cancer (p < 0.0001 each, Table 2). MSLN immunostaining was unrelated to overall survival in 227 bladder carcinomas (p = 0.3302; only pT ≥ 2), 593 invasive breast carcinomas of no special types (p = 0.0976), and 502 clear cell renal cell carcinomas (p = 0.3144, Figure 4). A significant association was found between positive MSLN immunostaining and RAS mutations in colorectal carcinomas (p = 0.0010), and triple negative invasive breast carcinomas of no special type (p < 0.0001, Table 2). There was also a strong tendency towards higher MSLN expression in HPV positive than in HPV negative squamous cell carcinomas (p = 0.0098, Table 3).
Table 2.
MSLN immunostaining and tumor phenotype.
| MSLN Immunostaining | p Value | ||||||
|---|---|---|---|---|---|---|---|
| Analyzable (n) | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | |||
| endometrioid endometrial carcinoma | all cancers | 171 | 55.0 | 20.5 | 12.9 | 11.7 | |
| pT1 | 108 | 55.6 | 17.6 | 14.8 | 12.0 | 0.5077 | |
| pT2 | 24 | 58.3 | 25.0 | 4.2 | 12.5 | ||
| pT3-4 | 35 | 51.4 | 28.6 | 14.3 | 5.7 | ||
| pN0 | 50 | 42.0 | 24.0 | 22.0 | 12.0 | 0.6098 | |
| pN+ | 30 | 56.7 | 20.0 | 13.3 | 10.0 | ||
| serous high grade ovarian carcinoma | all cancers | 386 | 3.1 | 5.2 | 8.8 | 82.9 | |
| pT1 | 32 | 0.0 | 12.5 | 9.4 | 78.1 | 0.3821 | |
| pT2 | 42 | 2.4 | 7.1 | 4.8 | 85.7 | ||
| pT3 | 259 | 2.7 | 3.9 | 9.3 | 84.2 | ||
| pN0 | 81 | 1.2 | 3.7 | 9.9 | 85.2 | 0.0621 | |
| pN1 | 166 | 1.8 | 6.6 | 10.8 | 80.7 | ||
| Invasive breast carcinoma of no special type | all cancers | 1072 | 93.7 | 2.7 | 0.8 | 2.8 | |
| pT1 | 545 | 95.0 | 1.8 | 1.1 | 2.0 | 0.0262 | |
| pT2 | 406 | 93.8 | 2.5 | 0.5 | 3.2 | ||
| pT3-4 | 79 | 86.1 | 8.9 | 0.0 | 5.1 | ||
| G1 | 150 | 98.0 | 1.3 | 0.0 | 0.7 | <0.0001 | |
| G2 | 540 | 97.6 | 0.7 | 0.4 | 1.3 | ||
| G3 | 381 | 86.4 | 6.0 | 1.8 | 5.8 | ||
| pN0 | 544 | 92.5 | 2.5 | 1.8 | 3.3 | 0.0390 | |
| pN+ | 398 | 95.4 | 1.7 | 0.2 | 2.8 | ||
| non triple negative | 737 | 98.2 | 0.9 | 0.1 | 0.7 | <0.0001 | |
| Triple negative | 133 | 67.7 | 14.3 | 6.0 | 12.0 | ||
| Urinary bladder carcinoma | all cancers | 925 | 90.7 | 5.8 | 1.8 | 1.6 | |
| pTa G2 low | 154 | 96.1 | 3.2 | 0.0 | 0.6 | <0.0001 | |
| pTa G2 high | 125 | 96.8 | 3.2 | 0.0 | 0.0 | ||
| pTaG3 | 130 | 96.9 | 2.3 | 0.0 | 0.8 | ||
| pT ≥2 G3 | 792 | 87.8 | 8.1 | 2.3 | 1.9 | ||
| pN0 | 312 | 87.2 | 7.4 | 2.9 | 2.6 | 0.7125 | |
| pN+ | 170 | 83.7 | 9.0 | 4.5 | 2.8 | ||
| Clear cell renal cell carcinoma | all cancers | 757 | 93.4 | 4.0 | 1.6 | 1.1 | |
| pT1 | 450 | 93.3 | 4.0 | 1.3 | 1.3 | 0.8197 | |
| pT2 | 82 | 95.1 | 2.4 | 1.2 | 1.2 | ||
| pT3-4 | 219 | 92.7 | 4.6 | 2.3 | 0.5 | ||
| ISUP 1 | 241 | 92.9 | 3.7 | 2.5 | 0.8 | 0.6913 | |
| ISUP 2 | 250 | 92.0 | 4.8 | 1.2 | 2.0 | ||
| ISUP 3 | 211 | 95.3 | 3.3 | 0.9 | 0.5 | ||
| ISUP 4 | 45 | 93.3 | 4.4 | 2.2 | 0.0 | ||
| pN0 | 127 | 93.7 | 3.1 | 2.4 | 0.8 | 0.5127 | |
| pN+ | 19 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Gastric carcinoma | all cancers | 373 | 55.0 | 19.0 | 10.2 | 15.8 | |
| pT1-2 | 63 | 57.1 | 17.5 | 12.7 | 12.7 | 0.2490 | |
| pT3 | 122 | 60.7 | 18.9 | 9.0 | 11.5 | ||
| pT4 | 122 | 45.9 | 22.1 | 10.7 | 21.3 | ||
| pN0 | 83 | 65.1 | 13.3 | 8.4 | 13.3 | 0.0697 | |
| pN+ | 222 | 48.7 | 23.0 | 11.3 | 17.1 | ||
| Colorectal adenocarcinoma | all cancers | 1619 | 58.8 | 28.9 | 6.5 | 5.8 | |
| pT1 | 68 | 66.2 | 30.9 | 1.5 | 1.5 | <0.0001 | |
| pT2 | 323 | 68.4 | 22.9 | 5.9 | 2.8 | ||
| pT3 | 894 | 55.8 | 31.4 | 7.4 | 5.4 | ||
| pT4 | 322 | 56.5 | 27.3 | 5.3 | 10.9 | ||
| pN0 | 839 | 64.2 | 25.4 | 5.7 | 4.6 | <0.0001 | |
| pN+ | 752 | 52.5 | 33.0 | 7.3 | 7.2 | ||
| MMR proficient | 1114 | 58.6 | 29.0 | 6.9 | 5.5 | 0.7017 | |
| MMR deficient | 82 | 62.2 | 25.6 | 4.9 | 7.3 | ||
| RAS wildtype | 441 | 62.4 | 28.1 | 5.4 | 4.1 | 0.0010 | |
| RAS mutation | 345 | 49.0 | 34.5 | 9.0 | 7.5 | ||
| BRAF wildtype | 122 | 60.7 | 27.9 | 8.2 | 3.3 | 0.1063 | |
| BRAF V600E mutation | 21 | 47.6 | 19.0 | 19.0 | 14.3 | ||
| adenocarcinoma of the lung | all cancers | 174 | 44.8 | 19.0 | 13.8 | 22.4 | |
| pT1 | 83 | 45.8 | 19.3 | 14.5 | 20.5 | 0.8172 | |
| pT2 | 52 | 46.2 | 21.2 | 7.7 | 25.0 | ||
| pT3 | 28 | 42.9 | 17.9 | 17.9 | 21.4 | ||
| pT4 | 9 | 44.4 | 11.1 | 33.3 | 11.1 | ||
| pN0 | 95 | 51.6 | 14.7 | 14.7 | 18.9 | 0.1194 | |
| pN1 | 57 | 36.8 | 28.1 | 10.5 | 24.6 | ||
Abbreviation: pT: pathological tumor stage, pN: pathological lymph node status, G: grade, ISUP: International Society of Urological Pathology, MMR: mismatch repair.
Figure 4.
MSLN immunostaining and patient prognosis. All bladder cancer patients had at least pT2 cancers and were treated by cystectomy.
Table 3.
MSLN immunostaining and HPV status.
| MSLN Immunostaining | n | HPV Status | |||
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | ||||
| All cancers | negative | 427 | 56.7 | 43.3 | 0.0098 |
| positive | 93 | 41.9 | 58.1 | ||
| Oral squamous cell carcinoma | negative | 60 | 85.0 | 15.0 | 0.3061 |
| positive | 15 | 73.3 | 26.7 | ||
| Squamous cell carcinoma of the pharynx | negative | 43 | 44.2 | 55.8 | 0.0996 |
| positive | 3 | 18.2 | 81.8 | ||
| Squamous cell carcinoma of the larynx | negative | 47 | 83.0 | 17.0 | 0.4281 |
| positive | 12 | 91.7 | 8.3 | ||
| Squamous cell carcinoma of the cervix | negative | 48 | 10.4 | 89.6 | 0.6182 |
| positive | 28 | 14.3 | 85.7 | ||
| Squamous cell carcinoma of the vagina | negative | 26 | 50.0 | 50.0 | 1.0000 |
| positive | 4 | 50.0 | 50.0 | ||
| Squamous cell carcinoma of the vulva | negative | 71 | 70.4 | 29.6 | 0.0698 |
| positive | 8 | 20.0 | 80.0 | ||
| Squamous cell carcinoma of the penis | negative | 68 | 38.2 | 61.8 | 0.3435 |
| positive | 5 | 60.0 | 40.0 | ||
| Squamous cell carcinoma of the skin | negative | 37 | 97.3 | 2.7 | 0.8161 |
| positive | 1 | 100.0 | 0.0 | ||
| Squamous cell carcinoma of the anal canal | negative | 27 | 11.1 | 88.9 | 0.4237 |
| positive | 9 | 22.2 | 77.8 | ||
4. Discussion
The result of our normal tissue analysis for MSLN expression is consistent with a high potential of this protein as a therapeutic target. It is obvious that the risk for potential side effects of targeted therapies is connected to the target protein’s site and level of expression in normal tissues. MSLN expression was mostly seen in organs that are not vital for adult or old-aged people, such as tonsil, thymus, gallbladder, seminal vesicle, fallopian tube, uterus, and placenta. In the stomach, duodenum, rectum, and the anal canal, the fraction of MSLN expressing cells was so low that critical side effects might not occur, even if these rare cells were disturbed by drug effects. Our normal tissue results are largely consistent with RNA expression data derived from the FANTOM5 project [50,51] and the Genotype-Tissue Expression (GTEx) project [52] which are all summarized in the protein atlas (https://www.proteinatlas.org/ENSG00000102854-MSLN/tissue, accessed on 6 January 2021). These RNA data would suggest the lung as the organ, which might be most endangered by side effects of anti-MSLN therapies. A high MSLN RNA expression in the lung could be explained by strong MSLN immunostaining in goblet cells of respiratory epithelium and occasional positivity of bronchial glands. As the alveolar system did not show any MSLN expression, we would expect that possible lung side effects derived from therapeutic anti-MSLN antibodies would rather affect the bronchial system than the alveolar space.
The successful analysis of more than 12,000 tumors identified various cancer types that might be particularly well suited for anti-MSLN drugs. Although the usage of tissue microarrays has disadvantages connected to the small size of the analyzed tissue spots (0.6 mm in diameter), including the risk of missing “relevant” tumor components in heterogenous tumors, or the impossibility to capture different tumor compartments (such as invasion front and tumor center) within one tissue spot, it allows for an unprecedented degree of experimental standardization across the analyzed tumors. Although different staining conditions may alter the absolute numbers of positive cancers, the relative ranking order of positive tumor types will remain unchanged. The cancer entities with highest prevalence and also highest levels of MSLN expression included all types of ovarian and endometrium carcinomas, pancreatic adenocarcinoma, malignant mesothelioma, and adenocarcinomas of the lung, stomach, esophagus, and the colorectum. High rates of MSLN expression have already been described for these tumor entities, although the results varied between studies. Previously described high MSLN positivity rates range from 55% to 100% in ovarian cancer [35,43,44,53], 59% to 76% in endometrium cancer [5,8], 57% to 100% in pancreatic adenocarcinoma [43,47,54], 45% to 100% in malignant mesothelioma [33,43,55,56], 38% to 69% of lung adenocarcinomas [37,38,39], 45% to 78% in stomach cancer [5,45,57,58], 29% to 46% in esophageal adenocarcinoma [6,59], and 30% to 68% of colorectal adenocarcinoma [5,8]. These cancers with a positivity rate of 40% or higher in our study appear to be the best candidates for targeted anti-MSLN therapy. Squamous cell carcinomas of various different sites of origin represent the next group of cancers that show MSLN expression at a relatively high frequency (10–40%). Clinical studies using adaptive immunotherapy (CAR-T cells), monoclonal antibodies (Amatuximab/MORAb-009), recombinant immunotoxins (SS1P, LMB-100/RG7787), antibody-drug conjugates (Anetumab, DMOT4039A), or listeria monocytogene induced anti-tumor immune response (CRS-207) therapies have so far focused on malignant mesothelioma, pancreatic adenocarcinoma, and carcinomas of the ovarian, lung, and breast [24,25,26,27,28,35,60,61,62,63].
It is of note that MSLN was initially suggested to represent a diagnostic marker for identification of tumors derived from the mesothelium [1]. Subsequent studies have however identified numerous other tumor entities with MSLN expression [5,6,7,8,9,22,33,35,36,37,38,39,40,41,42,43,44,45,46,47,53,54,55,56,57,58,59,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. That 66 of our 122 analyzed tumor entities contained MSLN positive cases demonstrates that a positive MSLN expression cannot be viewed as an argument for a specific tumor entity. However, a positive MSLN immunostaining might be considered an argument against a tumor origin from organs that never or very rarely gave rise to MSLN positive cancer cells, such as the prostate, thyroid, kidney, germ cell tumors, adrenal tumors, melanoma, many soft tissue tumor types, and hematologic neoplasms.
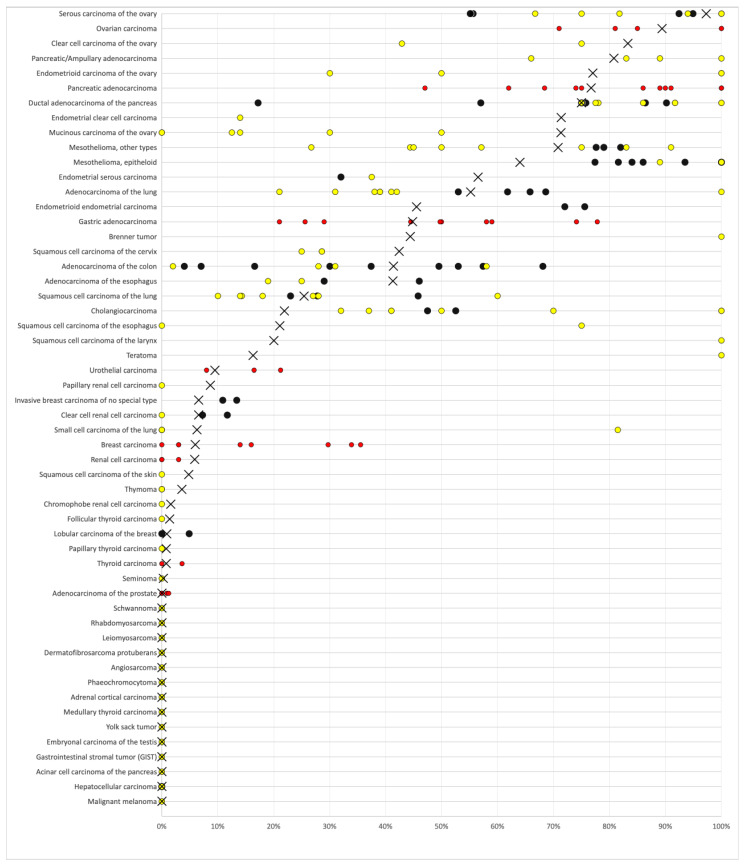
Our analysis of 1619 colorectal carcinomas identified significant associations with advanced pT stage and lymph node metastases. This is in line with data from 4 earlier studies all describing associations between high MSLN expression and parameters for cancer aggressiveness or poor patient prognosis [42,99,100,104]. However, the absence of statistical associations between MSLN expression and tumor phenotype and/or prognosis in carcinomas of the bladder, breast, ovary, endometrium, kidney, lung, and stomach argues against a major prognostic role of MSLN expression levels. In principle, this notion is consistent with the existing literature. Only 9 previous studies have earlier described associations between high MSLN expression and poor prognosis and/or unfavorable tumor phenotype in these tumor types [43,45,46,57,74,96,105,106,107], while there were 7 other studies which could not find associations with clinico-pathological parameters [5,44,47,58,64,65,92]. The concept of MSLN expression not representing a universal parameter of malignancy is also supported by the frequent MSLN expression in various benign tumors, including Brenner tumors of the ovary, as well as Warthin tumors, pleomorphic adenomas, and basal cell adenomas of the salivary glands. It is of note that high MSLN expression was linked to several key molecular features in the cancer types analyzed, such as triple negative breast cancer and RAS (KRAS or NRAS) mutations. This observation fits with known interactions of MSLN with relevant molecular pathways interacting with these molecular features, such as the MAPK/ERK pathway (interacting with MMP-7, MUC16, and ERK) [15,16,108], deregulation of HER2 expression [109], and the PI3K/AkT pathway (interacting with PI3K and MUC6) [16,108,110]. Importantly, all prevalences described in this study are specific to the reagents and the protocol used in our laboratory. It is almost certain that the use of different antibodies, protocols, and interpretation criteria have jointly caused highly diverse literature data on MSLN expression in cancer (summarized in Figure 5). It is well known that different antibodies designed for the same target protein can vary to a large extent in their binding properties and that protocol modifications greatly impact the rate of immunostained cases [111].
Figure 5.
Graphical comparison of MSLN data from this study (×) in comparison with the previous literature. Yellow dots are used for studies involving 1–50 cases, and black dots are used for studies involving more than 50 cases. Red dots are used for studies without subtype analyses. All studies are quoted in the list of references.
5. Conclusions
Our analysis of 12,679 cancers generated a ranking order of cancers according to their frequency of MSLN expression. Top ranked tumor entities, such as ovarian carcinomas, endometrium carcinomas, pancreatic adenocarcinomas, and malignant mesothelioma, thus, may be the best candidates for therapy with drugs targeting MSLN. Despite a link between MSLN positivity and aggressive colon cancer phenotype, the prognostic impact of MSLN expression appears to be low in many other tumor types. This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Acknowledgments
We are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
Author Contributions
S.W., T.K., A.H.M., R.S., and G.S. designed the study. P.G., N.G., M.L., A.M., A.M.L., C.F., K.M., C.B., P.L., T.S.C., F.J., J.R.I., K.J., R.U., W.W., S.S., S.M., E.B., R.H.K., and D.D. performed the immunohistochemical analyses and/or contributed to the pathological validation of the tumors, the tissue microarray construction, and data collection. M.K., N.C.B., C.H.-M., and R.S. carried out the data analyses. G.S., R.S., S.W., T.K., A.H.M., and M.K. wrote the first draft of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to the published, and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes, as well as patient data analysis, has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study (HmbKHG, §12).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The mesothelin (MSLN) antibody clone MSVA-235 was provided from MS Validated Antibodies GmbH (owned by a family member of GS).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang K., Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaguchi N., Hattori K., Oh-Eda M., Kojima T., Imai N., Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y. J. Biol. Chem. 1994;269:805–808. doi: 10.1016/S0021-9258(17)42180-6. [DOI] [PubMed] [Google Scholar]
- 3.Urwin D., Lake R.A. Structure of the Mesothelin/MPF Gene and Characterization of Its Promoter. Mol. Cell Biol. Res. Commun. 2000;3:26–32. doi: 10.1006/mcbr.2000.0181. [DOI] [PubMed] [Google Scholar]
- 4.Chang K., Pastan I., Willingham M.C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 5.Inaguma S., Wang Z., Lasota J., Onda M., Czapiewski P., Langfort R., Rys J., Szpor J., Waloszczyk P., Okoń K., et al. Comprehensive immunohistochemical study of mesothelin (MSLN) using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors with evaluation of its prognostic value in malignant pleural mesothelioma. Oncotarget. 2017;8:26744–26754. doi: 10.18632/oncotarget.15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez H., Rojas P.L., Yong K.-T., Ding H., Xu G., Prasad P.N., Wang J., Canto M., Eshleman J.R., Montgomery E.A., et al. Mesothelin is a specific biomarker of invasive cancer in the Barrett-associated adenocarcinoma progression model: Translational implications for diagnosis and therapy. Nanomed. Nanotechnol. Biol. Med. 2008;4:295–301. doi: 10.1016/j.nano.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ordóñez N.G. Value of Mesothelin Immunostaining in the Diagnosis of Mesothelioma. Mod. Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 8.Frierson H.F., Moskaluk C.A., Powell S.M., Zhang H., Cerilli L.A., Stoler M.H., Cathro H., Hampton G.M. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum. Pathol. 2003;34:605–609. doi: 10.1016/S0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 9.Dennis J.L., Hvidsten T.R., Wit E.C., Komorowski J., Bell A.K., Downie I., Mooney J., Verbeke C.S., Bellamy C., Keith W.N., et al. Markers of Adenocarcinoma Characteristic of the Site of Origin: Development of a Diagnostic Algorithm. Clin. Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 10.Bera T.K., Pastan I. Mesothelin Is Not Required for Normal Mouse Development or Reproduction. Mol. Cell. Biol. 2000;20:2902–2906. doi: 10.1128/MCB.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubbels J.A., Belisle J., Onda M., Rancourt C., Migneault M., Ho M., Bera T.K., Connor J., Sathyanarayana B.K., Lee B., et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko O., Gong L., Zhang J., Hansen J.K., Hassan R., Lee B., Ho M. A Binding Domain on Mesothelin for CA125/MUC. J. Biol. Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avula L.R., Rudloff M., El-Behaedi S., Arons D., Albalawy R., Chen X., Zhang X., Alewine C. Mesothelin Enhances Tumor Vascularity in Newly Forming Pancreatic Peritoneal Metastases. Mol. Cancer Res. 2019;18:229–239. doi: 10.1158/1541-7786.MCR-19-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharadwaj U., Marin-Muller C., Li M., Chen C., Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-alpha-induced apoptosis through Akt/PI3K/NF-kappaB activation and IL-6/Mcl-1 overexpression. Mol. Cancer. 2011;10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang M.C., Chen C.-A., Chen P.-J., Chiang Y.-C., Chen Y.-L., Mao T.-L., Lin H.-W., Chiang W.-H.L., Cheng W.-F. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem. J. 2012;442:293–302. doi: 10.1042/BJ20110282. [DOI] [PubMed] [Google Scholar]
- 16.Chang M., Chen C., Hsieh C., Lee C., Su Y., Hu Y., Cheng W. Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem. J. 2009;424:449–458. doi: 10.1042/BJ20082196. [DOI] [PubMed] [Google Scholar]
- 17.He X., Wang L., Riedel H., Wang K., Yang Y., Dinu C.Z., Rojanasakul Y. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol. Cancer. 2017;16:1–13. doi: 10.1186/s12943-017-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Servais E.L., Colovos C., Rodriguez L., Bograd A.J., Nitadori J.-I., Sima C., Rusch V.W., Sadelain M., Adusumilli P.S. Mesothelin Overexpression Promotes Mesothelioma Cell Invasion and MMP-9 Secretion in an Orthotopic Mouse Model and in Epithelioid Pleural Mesothelioma Patients. Clin. Cancer Res. 2012;18:2478–2489. doi: 10.1158/1078-0432.CCR-11-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uehara N., Matsuoka Y., Tsubura A. Mesothelin Promotes Anchorage-Independent Growth and Prevents Anoikis via Extracellular Signal-Regulated Kinase Signaling Pathway in Human Breast Cancer Cells. Mol. Cancer Res. 2008;6:186–193. doi: 10.1158/1541-7786.MCR-07-0254. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Wang L., Li D., Wang H.B., Chen Q.F. Mesothelin Promotes Invasion and Metastasis in Breast Cancer Cells. J. Int. Med Res. 2012;40:2109–2116. doi: 10.1177/030006051204000608. [DOI] [PubMed] [Google Scholar]
- 21.Zheng C., Jia W., Tang Y., Zhao H., Jiang Y., Sun S. Mesothelin regulates growth and apoptosis in pancreatic cancer cells through p53-dependent and -independent signal pathway. J. Exp. Clin. Cancer Res. 2012;31:84. doi: 10.1186/1756-9966-31-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ordóñez N.G. Application of Mesothelin Immunostaining in Tumor Diagnosis. Am. J. Surg. Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Bauss F., Lechmann M., Krippendorff B.-F., Staack R., Herting F., Festag M., Imhof-Jung S., Hesse F., Pompiati M., Kollmorgen G., et al. Characterization of a re-engineered, mesothelin-targeted Pseudomonas exotoxin fusion protein for lung cancer therapy. Mol. Oncol. 2016;10:1317–1329. doi: 10.1016/j.molonc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas A.R., Tanyi J.L., O’Hara M.H., Gladney W.L., Lacey S.F., Torigian D.A., Soulen M.C., Tian L., McGarvey M., Nelson A.M., et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019;27:1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan R., Alley E., Kindler H., Antonia S.J., Jahan T.M., Honarmand S., Nair N., Whiting C.C., Enstrom A., Lemmens E., et al. Clinical Response of Live-Attenuated, Listeria monocytogenes Expressing Mesothelin (CRS-207) with Chemotherapy in Patients with Malignant Pleural Mesothelioma. Clin. Cancer Res. 2019;25:5787–5798. doi: 10.1158/1078-0432.CCR-19-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan R., Blumenschein G.R., Jr., Moore K.N., Santin A.D., Kindler H.L., Nemunaitis J.J., Seward S.M., Thomas A., Kim S.K., Rajagopalan P. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 2020;38:1824–1835. doi: 10.1200/JCO.19.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan R., Kindler H.L., Jahan T., Bazhenova L., Reck M., Thomas A., Pastan I., Parno J., O’Shannessy D.J., Fatato P., et al. Phase II Clinical Trial of Amatuximab, a Chimeric Antimesothelin Antibody with Pemetrexed and Cisplatin in Advanced Unresectable Pleural Mesothelioma. Clin. Cancer Res. 2014;20:5927–5936. doi: 10.1158/1078-0432.CCR-14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q., Ghafoor A., Mian I., Rathkey D., Thomas A., Alewine C., Sengupta M., Ahlman M.A., Zhang J., Morrow B., et al. Enhanced efficacy of mesothelin-targeted immunotoxin LMB-100 and anti–PD-1 antibody in patients with mesothelioma and mouse tumor models. Sci. Transl. Med. 2020;12:eaaz7252. doi: 10.1126/scitranslmed.aaz7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H., Gao W., Ho M. Novel Immunocytokine IL12-SS1 (Fv) Inhibits Mesothelioma Tumor Growth in Nude Mice. PLoS ONE. 2013;8:e81919. doi: 10.1371/journal.pone.0081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanitis E., Poussin M., Hagemann I.S., Coukos G., Sandaltzopoulos R., Scholler N., Powell D.J. Redirected Antitumor Activity of Primary Human Lymphocytes Transduced With a Fully Human Anti-mesothelin Chimeric Receptor. Mol. Ther. 2012;20:633–643. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazzerini L., Jöhrens K., Sehouli J., Cichon G. Favorable therapeutic response after anti-Mesothelin antibody–drug conjugate treatment requires high expression of Mesothelin in tumor cells. Arch. Gynecol. Obstet. 2020;302:1255–1262. doi: 10.1007/s00404-020-05734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizukami T., Kamachi H., Fujii Y., Matsuzawa F., Einama T., Kawamata F., Kobayashi N., Hatanaka Y., Taketomi A. The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model. Oncotarget. 2018;9:33844–33852. doi: 10.18632/oncotarget.26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scales S.J., Gupta N., Pacheco G., Firestein R., French D.M., Koeppen H., Rangell L., Barry-Hamilton V., Luis E., Chuh J., et al. An Antimesothelin-Monomethyl Auristatin E Conjugate with Potent Antitumor Activity in Ovarian, Pancreatic, and Mesothelioma Models. Mol. Cancer Ther. 2014;13:2630–2640. doi: 10.1158/1535-7163.MCT-14-0487-T. [DOI] [PubMed] [Google Scholar]
- 34.Terwisscha van Scheltinga A.G., Ogasawara A., Pacheco G., Vanderbilt A.N., Tinianow J.N., Gupta N., Li D., Firestein R., Marik J., Scales S.J., et al. Preclinical Efficacy of an Antibody-Drug Conjugate Targeting Mesothelin Correlates with Quantitative 89Zr-ImmunoPET. Mol. Cancer Ther. 2017;16:134–142. doi: 10.1158/1535-7163.MCT-16-0449. [DOI] [PubMed] [Google Scholar]
- 35.Weekes C.D., Lamberts L.E., Borad M.J., Voortman J., McWilliams R.R., Diamond J.R., De Vries E.G.E., Verheul H.M., Lieu C.H., Kim G.P., et al. Phase I Study of DMOT4039A, an Antibody–Drug Conjugate Targeting Mesothelin, in Patients with Unresectable Pancreatic or Platinum-Resistant Ovarian Cancer. Mol. Cancer Ther. 2016;15:439–447. doi: 10.1158/1535-7163.MCT-15-0693. [DOI] [PubMed] [Google Scholar]
- 36.Yu L., Feng M., Kim H., Phung Y., Kleiner D.E., Gores G.J., Qian M., Wang X.W., Ho M. Mesothelin as a Potential Therapeutic Target in Human Cholangiocarcinoma. J. Cancer. 2010;1:141–149. doi: 10.7150/jca.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushitani K., Takeshima Y., Amatya V.J., Furonaka O., Sakatani A., Inai K. Immunohistochemical marker panels for distinguishing between epithelioid mesothelioma and lung adenocarcinoma. Pathol. Int. 2007;57:190–199. doi: 10.1111/j.1440-1827.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 38.Miettinen M., Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: An immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am. J. Surg. Pathol. 2003;27:150–158. doi: 10.1097/00000478-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Ordonez N.G. The immunohistochemical diagnosis of mesothelioma: A comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am. J. Surg. Pathol. 2003;27:1031–1051. doi: 10.1097/00000478-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Nahm C.B., Turchini J., Jamieson N., Moon E., Sioson L., Itchins M., Arena J., Colvin E., Howell V.M., Pavlakis N., et al. Biomarker panel predicts survival after resection in pancreatic ductal adenocarcinoma: A multi-institutional cohort study. Eur. J. Surg. Oncol. 2019;45:218–224. doi: 10.1016/j.ejso.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 41.Hassan R., Laszik Z.G., Lerner M., Raffeld M., Postier R., Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am. J. Clin. Pathol. 2005;124:838–845. doi: 10.1309/F1B64CL7H8VJKEAF. [DOI] [PubMed] [Google Scholar]
- 42.Shiraishi T., Shinto E., Mochizuki S., Tsuda H., Kajiwara Y., Okamoto K., Einama T., Hase K., Ueno H. Mesothelin expression has prognostic value in stage IotaIota/IotaIotaIota colorectal cancer. Virchows Arch. 2019;474:297–307. doi: 10.1007/s00428-018-02514-4. [DOI] [PubMed] [Google Scholar]
- 43.Illei P.B., Alewine C., Zahurak M., Cowan M.L., Montgomery E., Hassan R., Xiang L., Pastan I., Kelly R.J. Mesothelin Expression in Advanced Gastroesophageal Cancer Represents a Novel Target for Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2016;24:246–252. doi: 10.1097/PAI.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magalhaes I., Fernebro J., Own S.A., Glaessgen D., Corvigno S., Remberger M., Mattsson J., Dahlstrand H. Mesothelin Expression in Patients with High-Grade Serous Ovarian Cancer Does Not Predict Clinical Outcome But Correlates with CD11c+ Expression in Tumor. Adv. Ther. 2020;37:5023–5031. doi: 10.1007/s12325-020-01520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin S., Park S., Kim M.H., Nam C.M., Kim H., Choi Y.Y., Jung M.K., Choi H.J., Rha S.Y., Chung H.C. Mesothelin Expression Is a Predictive Factor for Peritoneal Recurrence in Curatively Resected Stage III Gastric Cancer. Oncologist. 2019;24:1108–1114. doi: 10.1634/theoncologist.2018-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y.R., Xian R.R., Ziober A., Conejo-Garcia J., Perales-Puchalt A., June C.H., Zhang P.J., Tchou J. Mesothelin expression is associated with poor outcomes in breast cancer. Breast Cancer Res. Treat. 2014;147:675–684. doi: 10.1007/s10549-014-3077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki T., Yamagishi Y., Einama T., Koiwai T., Yamasaki T., Fukumura‑Koga M., Ishibashi Y., Takihata Y., Shiraishi T., Miyata Y., et al. Membrane mesothelin expression positivity is associated with poor clinical outcome of luminal‑type breast cancer. Oncol. Lett. 2020;20:1. doi: 10.3892/ol.2020.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dancau A.-M., Simon R., Mirlacher M., Sauter G. Tissue Microarrays. Adv. Struct. Saf. Stud. 2016;1381:53–65. doi: 10.1007/978-1-4939-3204-7_3. [DOI] [PubMed] [Google Scholar]
- 49.Kononen J., Bubendorf L., Kallionimeni A., Bärlund M., Schraml P., Leighton S.B., Torhorst J., Mihatsch M.J., Sauter G., Kallionimeni O.-P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 50.Lizio M., The FANTOM Consortium. Harshbarger J., Shimoji H., Severin J., Kasukawa T., Sahin S., Abugessaisa I., Fukuda S., Hori F., et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:1–14. doi: 10.1186/s13059-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lizio M., Abugessaisa I., Noguchi S., Kondo A., Hasegawa A., Hon C.C., De Hoon M., Severin J., Oki S., Hayashizaki Y., et al. Update of the FANTOM web resource: Expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47:D752–D758. doi: 10.1093/nar/gky1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Consortium G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yen M.J., Hsu C.-Y., Mao T.-L., Wu T.-C., Roden R., Wang T.-L., Shih I.-M. Diffuse Mesothelin Expression Correlates with Prolonged Patient Survival in Ovarian Serous Carcinoma. Clin. Cancer Res. 2006;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 54.Liu H., Shi J., Anandan V., Wang H.L., Diehl D., Blansfield J., Gerhard G., Lin F. Reevaluation and Identification of the Best Immunohistochemical Panel (pVHL, Maspin, S100P, IMP-3) for Ductal Adenocarcinoma of the Pancreas. Arch. Pathol. Lab. Med. 2012;136:601–609. doi: 10.5858/arpa.2011-0326-OA. [DOI] [PubMed] [Google Scholar]
- 55.Ordóñez N.G., Sahin A.A. Diagnostic utility of immunohistochemistry in distinguishing between epithelioid pleural mesotheliomas and breast carcinomas: A comparative study. Hum. Pathol. 2014;45:1529–1540. doi: 10.1016/j.humpath.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Eguchi T., Kadota K., Mayor M., Zauderer M.G., Rimner A., Rusch V.W., Travis W.D., Sadelain M., Adusumilli P.S. Cancer antigen profiling for malignant pleural mesothelioma immunotherapy: Expression and coexpression of mesothelin, cancer antigen 125, and Wilms tumor 1. Oncotarget. 2017;8:77872–77882. doi: 10.18632/oncotarget.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Einama T., Homma S., Kamachi H., Kawamata F., Takahashi N., Taniguchi M., Kamiyama T., Furukawa H., Matsuno Y., Tanaka S., et al. Luminal membrane expression of mesothelin is a prominent poor prognostic factor for gastric cancer. Br. J. Cancer. 2012;107:137–142. doi: 10.1038/bjc.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baba K., Ishigami S., Arigami T., Uenosono Y., Okumura H., Matsumoto M., Kurahara H., Uchikado Y., Kita Y., Kijima Y., et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J. Surg. Oncol. 2011;105:195–199. doi: 10.1002/jso.22024. [DOI] [PubMed] [Google Scholar]
- 59.Rizk N.P., Servais E.L., Tang L.H., Sima C.S., Gerdes H., Fleisher M., Rusch V.W., Adusumilli P.S. Tissue and Serum Mesothelin Are Potential Markers of Neoplastic Progression in Barrett’s Associated Esophageal Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2012;21:482–486. doi: 10.1158/1055-9965.EPI-11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hassan R., Cohen S.J., Phillips M., Pastan I., Sharon E., Kelly R.J., Schweizer C., Weil S., Laheru D. Phase I Clinical Trial of the Chimeric Anti-Mesothelin Monoclonal Antibody MORAb-009 in Patients with Mesothelin-Expressing Cancers. Clin. Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kreitman R.J., Hassan R., Fitzgerald D.J., Pastan I. Phase I Trial of Continuous Infusion Anti-Mesothelin Recombinant Immunotoxin SS1P. Clin. Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsujikawa T., Crocenzi T., Durham J.N., Sugar E.A., Wu A.A., Onners B., Nauroth J.M., Anders R.A., Fertig E.J., Laheru D.A., et al. Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer. Clin. Cancer Res. 2020;26:3578–3588. doi: 10.1158/1078-0432.CCR-19-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassan R., Alewine C., Mian I., Spreafico A., Siu L.L., Gomez-Roca C., Delord J., Italiano A., Lassen U., Soria J., et al. Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer. 2020;126:4936–4947. doi: 10.1002/cncr.33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito T., Kajino K., Abe M., Sato K., Maekawa H., Sakurada M., Orita H., Wada R., Kajiyama Y., Hino O. ERC/mesothelin is expressed in human gastric cancer tissues and cell lines. Oncol. Rep. 2014;31:27–33. doi: 10.3892/or.2013.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parinyanitikul N., Blumenschein G.R., Wu Y., Lei X., Chavez-MacGregor M., Smart M., Gonzalez-Angulo A.M. Mesothelin Expression and Survival Outcomes in Triple Receptor Negative Breast Cancer. Clin. Breast Cancer. 2013;13:378–384. doi: 10.1016/j.clbc.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L., Niu Z., Zhang L., Liu X., Wang X., Li F., Wang Y. Clinicopathological Significance of Mesothelin Expression in Invasive Breast Cancer. J. Int. Med. Res. 2012;40:909–916. doi: 10.1177/147323001204000309. [DOI] [PubMed] [Google Scholar]
- 67.Swierczynski S.L., Maitra A., Abraham S.C., Iacobuzio-Donahue C.A., Ashfaq R., Cameron J.L., Schulick R.D., Yeo C.J., Rahman A., Hinkle D.A., et al. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum. Pathol. 2004;35:357–366. doi: 10.1016/j.humpath.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy D.M., Maitra A., Argani P., Rader A.E., Faigel D.O., Van Heek N.T., Hruban R.H., Wilentz R.E. Novel Markers of Pancreatic Adenocarcinoma in Fine-Needle Aspiration: Mesothelin and Prostate Stem Cell Antigen Labeling Increases Accuracy in Cytologically Borderline Cases. Appl. Immunohistochem. Mol. Morphol. 2003;11:238–243. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Argani P., Iacobuzio-Donahue C., Ryu B., Rosty C., Goggins M., Wilentz R.E., Murugesan S.R., Leach S.D., Jaffee E., Yeo C.J., et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin. Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 70.Frank R., Li S., Ahmad N.A., Sepulveda A.R., Jhala N.C. Mesothelin Expression in Pancreatic Mucinous Cysts. Am. J. Clin. Pathol. 2014;142:313–319. doi: 10.1309/AJCPDTTL2I5ECMFG. [DOI] [PubMed] [Google Scholar]
- 71.Jhala N., Jhala D., Vickers S.M., Eltoum I., Batra S.K., Manne U., Eloubeidi M., Jones J.J., Grizzle W.E. Biomarkers in Diagnosis of pancreatic carcinoma in fine-needle aspirates. Am. J. Clin. Pathol. 2006;126:572–579. doi: 10.1309/CEV30BE088CBDQD9. [DOI] [PubMed] [Google Scholar]
- 72.Dim D.C., Jiang F., Qiu Q., Li T., Darwin P., Rodgers W.H., Peng H.Q. The usefulness of S100P, mesothelin, fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fine-needle aspiration. Diagn. Cytopathol. 2011;42:193–199. doi: 10.1002/dc.21684. [DOI] [PubMed] [Google Scholar]
- 73.Hassan R., Kreitman R.J., Pastan I., Willingham M.C. Localization of Mesothelin in Epithelial Ovarian Cancer. Appl. Immunohistochem. Mol. Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 74.Han S.-H., Joo M., Kim H., Chang S. Mesothelin Expression in Gastric Adenocarcinoma and Its Relation to Clinical Outcomes. J. Pathol. Transl. Med. 2017;51:122–128. doi: 10.4132/jptm.2016.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le K., Wang J., Zhang T., Guo Y., Chang H., Wang S., Zhu B. Overexpression of Mesothelin in Pancreatic Ductal Adenocarcinoma (PDAC) Int. J. Med. Sci. 2020;17:422–427. doi: 10.7150/ijms.39012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibrahim D.A., Abouhashem N.S. Diagnostic value of IMP3 and mesothelin in differentiating pancreatic ductal adenocarcinoma from chronic pancreatitis. Pathol. Res. Pract. 2016;212:288–293. doi: 10.1016/j.prp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Gnemmi V., Leroy X., Triboulet J.-P., Pruvot F.-R., Villers A., Leteurtre E., Buob D. Pancreatic metastases of renal clear cell carcinoma: A clinicopathological study of 11 cases with special emphasis on the usefulness of PAX2 and mesothelin for the distinction from primary ductal adenocarcinoma of the pancreas. Anal. Quant. Cytopathol. Histopathol. 2013;35:157–162. [PubMed] [Google Scholar]
- 78.Glass J.P., Parasher G., Arias-Pulido H., Donohue R., Cerilli L.A., Prossnitz E.R. Mesothelin and GPR30 Staining Among a Spectrum of Pancreatic Epithelial Neoplasms. Int. J. Surg. Pathol. 2011;19:588–596. doi: 10.1177/1066896911409575. [DOI] [PubMed] [Google Scholar]
- 79.Tabrizi A.D., Kalloger S.E., Köbel M., Cipollone J., Roskelley C.D., Mehl E., Gilks C.B. Primary Ovarian Mucinous Carcinoma of Intestinal Type: Significance of Pattern of Invasion and Immunohistochemical Expression Profile in a Series of 31 Cases. Int. J. Gynecol. Pathol. 2010;29:99–107. doi: 10.1097/PGP.0b013e3181bbbcc1. [DOI] [PubMed] [Google Scholar]
- 80.Kanner W.A., Galgano M.T., Stoler M.H., Mills S.E., Atkins K.A. Distinguishing Breast Carcinoma From Müllerian Serous Carcinoma With Mammaglobin and Mesothelin. Int. J. Gynecol. Pathol. 2008;27:491–495. doi: 10.1097/PGP.0b013e31817d5340. [DOI] [PubMed] [Google Scholar]
- 81.Pu R.T., Pang Y., Michael C.W. Utility of WT-1, p63, MOC31, mesothelin, and cytokeratin (K903 and CK5/6) immunostains in differentiating adenocarcinoma, squamous cell carcinoma, and malignant mesothelioma in effusions. Diagn. Cytopathol. 2008;36:20–25. doi: 10.1002/dc.20747. [DOI] [PubMed] [Google Scholar]
- 82.Leroy X., Farine M.-O., Buob D., Wacrenier A., Copin M.-C. Diagnostic value of cytokeratin 7, CD10 and mesothelin in distinguishing ovarian clear cell carcinoma from metastasis of renal clear cell carcinoma. Histopathology. 2007;51:874–876. doi: 10.1111/j.1365-2559.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 83.Galloway M.L., Murray D., Moffat D.F. The use of the monoclonal antibody mesothelin in the diagnosis of malignant mesothelioma in pleural biopsies. Histopathology. 2006;48:767–769. doi: 10.1111/j.1365-2559.2005.02279.x. [DOI] [PubMed] [Google Scholar]
- 84.Cao D., Ji H., Ronnett B.M. Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int. J. Gynecol. Pathol. 2005;24:67–72. [PubMed] [Google Scholar]
- 85.Ordóñez N.G. The diagnostic utility of immunohistochemistry in distinguishing between mesothelioma and renal cell carcinoma: A comparative study. Hum. Pathol. 2004;35:697–710. doi: 10.1016/j.humpath.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Pan C.-C., Chen P.C.-H., Chou T.-Y., Chiang H. Expression of calretinin and other mesothelioma-related markers in thymic carcinoma and thymoma. Hum. Pathol. 2003;34:1155–1162. doi: 10.1053/j.humpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Nomura R., Fujii H., Abe M., Sugo H., Ishizaki Y., Kawasaki S., Hino O. Mesothelin Expression Is a Prognostic Factor in Cholangiocellular Carcinoma. Int. Surg. 2013;98:164–169. doi: 10.9738/INTSURG-D-13-00001.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Showalter S.L., Huang Y.-H., Witkiewicz A., Costantino C.L., Yeo C.J., Green J.J., Langer R., Anderson D.G., Sawicki J.A., Brody J.R. Nanoparticulate delivery of diphtheria toxin DNA effectively kills Mesothelin expressing pancreatic cancer cells. Cancer Biol. Ther. 2008;7:1584–1590. doi: 10.4161/cbt.7.10.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Heek N.T., Maitra A., Koopmann J., Fedarko N., Jain A., Rahman A., Iacobuzio-Donahue C.A., Adsay V., Ashfaq R., Yeo C.J., et al. Gene expression profiling identifies markers of ampullary adenocarcinoma. Cancer Biol. Ther. 2004;3:651–656. doi: 10.4161/cbt.3.7.919. [DOI] [PubMed] [Google Scholar]
- 90.Liebig B., Brabletz T., Staege M.S., Wulfanger J., Riemann D., Burdach S., Ballhausen W.G. Forced expression of deltaN-TCF-1B in colon cancer derived cell lines is accompanied by the induction of CEACAM5/6 and mesothelin. Cancer Lett. 2005;223:159–167. doi: 10.1016/j.canlet.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Ordonez N.G., Ord N.G. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: A comparative study. Mod. Pathol. 2006;19:417–428. doi: 10.1038/modpathol.3800544. [DOI] [PubMed] [Google Scholar]
- 92.Regzedmaa O., Li Y., Li Y., Zhang H., Wang J., Gong H., Yuan Y., Li W., Liu H., Chen J. Prevalence of DLL3, CTLA-4 and MSTN Expression in Patients with Small Cell Lung Cancer. OncoTargets Ther. 2019;12:10043–10055. doi: 10.2147/OTT.S216362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Forest F., Patoir A., Col P.D., Sulaiman A., Camy F., Laville D., Bayle-Bleuez S., Fournel P., Habougit C. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: Prognostic implications. Pathology. 2018;50:635–641. doi: 10.1016/j.pathol.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Tan K., Kajino K., Momose S., Masaoka A., Sasahara K., Shiomi K., Izumi H., Abe M., Ohtsuji N., Wang T., et al. Mesothelin (MSLN) promoter is hypomethylated in malignant mesothelioma, but its expression is not associated with methylation status of the promoter. Hum. Pathol. 2010;41:1330–1338. doi: 10.1016/j.humpath.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Dainty L.A., Risinger J.I., Morrison C., Chandramouli G., Bidus M.A., Zahn C., Rose G.S., Fowler J., Berchuck A., Maxwell G.L. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol. Oncol. 2007;105:563–570. doi: 10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 96.Yildiz Y., Kabadayi G., Yigit S., Kucukzeybek Y., Alacacioglu A., Varol U., Taskaynatan H., Salman T., Oflazoglu U., Akyol M., et al. High expression of mesothelin in advanced serous ovarian cancer is associated with poor prognosis. J. BUON. 2019;24:1549–1554. [PubMed] [Google Scholar]
- 97.Drapkin R., Crum C.P., Hecht J.L. Expression of candidate tumor markers in ovarian carcinoma and benign ovary: Evidence for a link between epithelial phenotype and neoplasia. Hum. Pathol. 2004;35:1014–1021. doi: 10.1016/j.humpath.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 98.Zhao H., Davydova L., Mandich D., Cartun R.W., Ligato S. S100A4 protein and mesothelin expression in dysplasia and carcinoma of the extrahepatic bile duct. Am. J. Clin. Pathol. 2007;127:374–379. doi: 10.1309/37RTWYAEPABYY410. [DOI] [PubMed] [Google Scholar]
- 99.Inoue S., Tsunoda T., Riku M., Ito H., Inoko A., Murakami H., Ebi M., Ogasawara N., Pastan I., Kasugai K., et al. Diffuse mesothelin expression leads to worse prognosis through enhanced cellular proliferation in colorectal cancer. Oncol. Lett. 2020;19:1741–1750. doi: 10.3892/ol.2020.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiraishi T., Shinto E., Nearchou I.P., Tsuda H., Kajiwara Y., Einama T., Caie P.D., Kishi Y., Ueno H. Prognostic significance of mesothelin expression in colorectal cancer disclosed by area-specific four-point tissue microarrays. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 2020;477:409–420. doi: 10.1007/s00428-020-02775-y. [DOI] [PubMed] [Google Scholar]
- 101.Foda A.A.M., El-Hawary A.K., Hamed H. Aberrant Expression of Calretinin, D2–40 and Mesothelin in Mucinous and Non-Mucinous Colorectal Carcinomas and Relation to Clinicopathological Features and Prognosis. Pathol. Oncol. Res. 2016;22:725–732. doi: 10.1007/s12253-016-0060-y. [DOI] [PubMed] [Google Scholar]
- 102.Einama T., Kamachi H., Nishihara H., Homma S., Kanno H., Takahashi K., Sasaki A., Tahara M., Okada K., Muraoka S., et al. Co-Expression of Mesothelin and CA125 Correlates With Unfavorable Patient Outcome in Pancreatic Ductal Adenocarcinoma. Pancreas. 2011;40:1276–1282. doi: 10.1097/MPA.0b013e318221bed8. [DOI] [PubMed] [Google Scholar]
- 103.Kawamata F., Kamachi H., Einama T., Homma S., Tahara M., Miyazaki M., Tanaka S., Kamiyama T., Nishihara H., Taketomi A., et al. Intracellular localization of mesothelin predicts patient prognosis of extrahepatic bile duct cancer. Int. J. Oncol. 2012;41:2109–2118. doi: 10.3892/ijo.2012.1662. [DOI] [PubMed] [Google Scholar]
- 104.Kawamata F., Homma S., Kamachi H., Einama T., Kato Y., Tsuda M., Tanaka S., Maeda M., Kajino K., Hino O., et al. C-ERC/mesothelin provokes lymphatic invasion of colorectal adenocarcinoma. J. Gastroenterol. 2013;49:81–92. doi: 10.1007/s00535-013-0773-6. [DOI] [PubMed] [Google Scholar]
- 105.Hanaoka T., Hasegawa K., Kato T., Sato S., Kurosaki A., Miyara A., Nagao S., Seki H., Yasuda M., Fujiwara K. Correlation Between Tumor Mesothelin Expression and Serum Mesothelin in Patients with Epithelial Ovarian Carcinoma: A Potential Noninvasive Biomarker for Mesothelin-targeted Therapy. Mol. Diagn. Ther. 2017;21:187–198. doi: 10.1007/s40291-017-0255-2. [DOI] [PubMed] [Google Scholar]
- 106.Thomas A., Chen Y., Steinberg S.M., Luo J., Pack S., Raffeld M., Abdullaev Z., Alewine C., Rajan A., Giaccone G., et al. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget. 2015;6:11694–11703. doi: 10.18632/oncotarget.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bayoglu I.V., Kucukzeybek B.B., Kucukzeybek Y., Varol U., Yildiz I., Alacacioglu A., Akyol M., Demir L., Dirican A., Yildiz Y., et al. Prognostic value of mesothelin expression in patients with triple negative and HER2-positive breast cancers. Biomed. Pharmacother. 2015;70:190–195. doi: 10.1016/j.biopha.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 108.Muniyan S., Haridas D., Chugh S., Rachagani S., Lakshmanan I., Gupta S., Seshacharyulu P., Smith L.M., Ponnusamy M.P., Batra S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer. 2016;7:110–124. doi: 10.18632/genesandcancer.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Behaedi S., Landsman R., Rudloff M., Kolyvas E., Albalawy R., Zhang X., Bera T., Collins K., Kozlov S., Alewine C. Protein Synthesis Inhibition Activity of Mesothelin Targeting Immunotoxin LMB-100 Decreases Concentrations of Oncogenic Signaling Molecules and Secreted Growth Factors. Toxins. 2018;10:447. doi: 10.3390/toxins10110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen S.-H., Hung W.-C., Wang P., Paul C., Konstantopoulos K. Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation. Sci. Rep. 2013;3:srep01870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saper C.B. A Guide to the Perplexed on the Specificity of Antibodies. J. Histochem. Cytochem. 2008;57:1–5. doi: 10.1369/jhc.2008.952770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.