Abstract
Simple Summary
Human papillomavirus (HPV) infection, smoking, and excessive alcohol consumption have been established as risk factors for the development of oropharyngeal squamous cell carcinoma (OPSCC). While the HPV epidemic has led to an increasing incidence of OPSCC, HPV-negative OPSCC cases associated with smoking and alcohol remain stable. As HPV-positive and -negative OPSCC present two distinct etiological, clinical, and prognostic entities, different treatment and follow-up strategies are being discussed. Still, specific surveillance strategies for HPV-positive OPSCC are lacking, as the risk of second primary tumors (SPT) in the era of HPV-associated OPSCC has not been comprehensively assessed. Our study investigated the frequency and localization of SPT of HPV-positive OPSCC, as well as their prognostic impact. We find that the SPT of HPV-positive OPSCC are less frequent than those of HPV-negative OPSCC, and they are also associated with higher survival rates. The localization of SPT of HPV-positive OPSCC did not differ from the localization of SPT of HPV-negative OPSCC.
Abstract
Purpose: To investigate the frequency, localization, and survival of second primary tumors (SPT) of oropharyngeal squamous cell carcinoma (OPSCC) depending on human papillomavirus (HPV) status. Methods: We performed a retrospective chart analysis of 107 OPSCC patients treated at the Zurich University Hospital from 2001 to 2010. Rate and localization of SPT after an index OPSCC were stratified according to smoking and HPV infection status. Results: In total, 57/91 (63%) included patients showed an HPV-associated OPSCC. Of these, 37/57 (64.9%) patients with an HPV-positive and 32/34 (94.1%) patients with an HPV-negative OPSCC were smokers. The median age at diagnosis of the SPT was 59.54 years (interquartile range 52.7–65.6). In addition, 8/57 (14%) HPV-positive and 13/34 (38.2%) HPV-negative patients developed SPT. The rate of SPT in patients with HPV-positive index tumors was significantly lower than in patients with HPV-negative OPSCC (p-value 0.01). Smokers showed significantly more SPT in the head and neck area than outside. The development of an SPT led to a significantly lower survival time in HPV-negative patients, while it did not significantly affect the survival time of HPV-positive patients. Conclusions: Patients with HPV-positive index tumors had a significantly lower risk of developing SPT than patients with HPV-negative tumors. If SPT developed, survival was significantly shorter in patients with HPV-negative tumors than with HPV-positive tumors.
Keywords: human papillomavirus, second primary, oropharyngeal squamous cell
1. Introduction
Oropharyngeal squamous cell carcinomas (OPSCC) are rare. Major risk factors for OPSCC include excessive tobacco and alcohol consumption [1]. In 1946, Slaughter [2] presented the model of field carcinogenesis, according to which the mucosa of the upper aerodigestive tract has an increased risk of oncogenic degeneration due to continuous exposure to noxious agents. Based on this observation, panendoscopy (laryngopharyngoscopy, bronchoscopy, and esophagoscopy) has been established for the workup of patients with newly diagnosed HNSCC to detect synchronous second primary tumors (SPT) [3]. SPT are observed in approximately 10–20% of patients with exposure to smoking or excessive alcohol consumption [4,5].
High-risk human papillomavirus (HR-HPV) infections have been known to cause genital tumors for about 3 decades [6]. About 2 decades ago, HPV infections were also recognized as a risk factor for OPSCC [1]. In developed countries, the incidence of all HNSCC has decreased—possibly due to declining tobacco exposure [7]. Meanwhile, the incidence of OPSCC has increased over the past three to four decades [8]. This specific incidence increase can be attributed to the increasing prevalence of oncogenic HPV infections in the oropharynx [9,10]. Thus, HPV has been identified as a new risk factor specific for OPSCC that shows little impact on other HNSCC [11]. HPV-associated OPSCC increasingly occur in individuals without the usual exposure to alcohol and tobacco [12].
HPV-induced OPSCC present an etiologically, molecularly, clinically, and prognostically distinct entity from tobacco- and alcohol-associated OPSCC [13,14,15,16]. SPT seem to occur less frequently in patients with HPV-positive OPSCC than in patients with HPV-negative OPSCC [17]. Therefore, a modulation of the follow-up strategy for HPV-positive OPSCC has to be considered.
The aim of this study was to analyze the frequency and localization as well as the prognostic significance of SPT after index OPSCC in relation to the risk factors HPV infection and smoking. Our research questions were the following: (i) How common are SPT in a consecutive cohort of patients with OPSCC? (ii) Are SPT more common in smokers than in nonsmokers? (iii) Does this frequency depend on HPV status? (iv) Does the location of SPT differ between smokers and nonsmokers or HPV-positive and HPV-negative patients? (v) What is the impact of the development of SPT on survival?
2. Materials and Methods
A retrospective analysis was performed using medical records of patients who were treated in a curative intent for newly diagnosed oropharyngeal carcinoma (OPSCC) at the Department of Otorhinolaryngology—Head and Neck Surgery at the University Hospital Zurich (USZ), Zurich, Switzerland between 2001 and 2010.
Inclusion criteria were an index tumor in the oropharynx. Exclusion criteria on the other hand were a non-evaluated HPV status. The starting point of observation was the diagnosis of the index tumor. Endpoint of observation was either last follow-up examination until January 2019 or death.
2.1. Analysis of Index Tumors
HPV positivity was determined according to an accepted algorithm [18], which includes p16 immunohistochemistry and PCR detection of HPV DNA (high-risk types). P16-positive and HPV DNA-negative tumors were considered HPV-negative.
Furthermore, age at diagnosis of the index tumor, gender, location of the index tumor, tobacco and alcohol consumption, occurrence of SPT, and disease progression (recurrence, death of patients) were obtained from medical records. Death was distinguished as due to index tumor, due to SPT, and independent of cancer. Survival rates were plotted using Kaplan–Meier survival curves, with the start of observation being the time of diagnosis of the index tumor.
Smokers were defined as ≥10 pack years, regardless of the time of tobacco use.
Alcohol abuse was defined as a history of >2 U/day for women and >3 U/day for men, respectively, with a standard unit (U) corresponding to 10 g of pure alcohol.
An SPT was defined as a second carcinoma in the upper aerodigestive tract, lung, or bladder, differing in site from the index tumor, which was unlikely to be a metastasis of the index carcinoma [19].
HPV types 16, 18, 33, 35, 56, and 59 were considered high-risk HPV types (HR-HPV) [20].
Age, sex, history of alcohol abuse, recurrence of index tumor, death from OPSCC-associated cancer, and development of second carcinomas were compared among the following three groups of patients: (1) HPV-positive nonsmokers, (2) HPV-positive smokers, and (3) HPV-negative patients.
2.2. Analysis of Second Tumors
Time to SPT occurrence was defined as time between diagnosis of index OPSCC and initial diagnosis of SPT.
Frequency and location of SPT after an index OPSCC were determined in relation to smoking and HPV status. Among SPT, synchronous tumors (detected simultaneously or within 6 months of the index tumor diagnosis) were distinguished from metachronous tumors (detected >6 months after the index tumor) [21]. In addition, the development of SPT of HPV-positive and HPV-negative patients was plotted over time.
2.3. Statistics
Statistical analyses of the data were performed using Stata 13. Patient characteristics were evaluated using T-test and Fisher’s exact test of group means controlled for unequal variances of individual characteristics. For evaluation of statistical significance of continuous variables such as age, the T-test was applied, while for discrete variables, Fisher’s exact test was applied. Statistical significance was defined at the 5% level of significance, i.e., p < 0.05.
To evaluate the effect of HPV status, UICC stage (Union internationale contre le cancer), and therapy mode, age, sex, tobacco, and alcohol abuse on the development of SPT, HPV status, and death from cancer, multivariate Cox regression analyses were performed.
For survival analysis, we used Kaplan–Meier survival curves and controlled for significant differences between groups using log-rank tests. Additionally, a multivariate cox regression analysis was performed to evaluate the influence of age, gender, tobacco and alcohol abuse, HPV status, index-UICC stage, therapy mode of the index tumor, and development of an SPT on survival.
2.4. Ethics
Ethical approval by the Zurich Ethics Committee for retrospective analysis of patient data is available (KEK-ZH-No. 2013-0019).
3. Results
3.1. Patient Characteristics
After exclusion of 16 patients due to undefined HPV status, the analyzed cohort consisted of 91 patients. Table 1 summarizes the patient characteristics. Follow-up of the initial carcinoma occurred for a mean of 57.17 months (4.76 years, interquartile range 31.08–65.25 years) from diagnosis.
Table 1.
Patient characteristics. The p-values related to age are calculated by T-test (columns 5 and 6, marked in italics). The other group comparisons of columns 5 and 6 were performed with two-sided Fisher’s exact test. Values statistically significant at the 5% significance level are marked with *. Abbreviations: − = negative, + = positive, chemo = chemotherapy, IQR = interquartile range, RT = radiotherapy.
| Patient Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Total | HPV+ Nonsmokers | HPV+ Smokers | HPV− Patients | p-Values HPV+ vs. HPV− (n = 57 vs. n = 34) | p-Values Smokers vs. Nonsmokers (n = 69 vs. n = 22) | ||
| n = 91 | n = 20 | n = 37 | n = 34 | ||||
| Age at diagnosis of index tumor | Median (y) | 59.54 | 58.2 | 58.7 | 57.6 | 0.483 | 0.646 |
| IQR | 52.7–65.6 | 52.8–66.7 | 51.0–67.3 | 53.8–65.1 | |||
| Sex | |||||||
| male | n (%) | 68 (74.7%) | 14 (70%) | 28 (75.7%) | 26 (76.5%) | 0.809 | 0.413 |
| female | n (%) | 23 (25.3%) | 6 (30%) | 9 (24.3%) | 8 (23.4%) | ||
| History of alcohol abuse | n (%) | 28 (30.8%) | 0 | 9 (24.3%) | 19 (55.9%) | 0.000 * | 0.000 * |
| Index-OPSCC localization |
|||||||
| Tonsils | n (%) | 79 (86.8%) | 19 (95%) | 34 (91.9%) | 26 (76.6%) | 0.050 * | 0.281 |
| Base of tongue | n (%) | 9 (9.9%) | 1 (5%) | 3 (8.1%) | 5 (14.7%) | 0.286 | 0.446 |
| Soft palate | n (%) | 1 (1.1%) | - | - | 1 (2.9%) | - | - |
| Pharyngeal wall | n (%) | 2 (2.2%) | - | - | 2 (5.9%) | - | - |
| Death due to OPSCC or associated cancer | n (%) | 17 (18.7%) | 2 (10%) | 5 (13.4%) | 10 (29.4%) | 0.054 | 0.226 |
| Index-UICC stadium | |||||||
| I | n (%) | 15 (16.5%) | 3 | 5 | 7 | 0.56 | 1 |
| II | n (%) | 17 (18.7%) | 5 | 6 | 6 | 1 | 0.345 |
| III | n (%) | 11 (12.1%) | 0 | 4 | 7 | 0.093 | 0.06 |
| IVa | n (%) | 47 (51.6%) | 12 | 22 | 13 | 0.055 | 0.47 |
| IVb | n (%) | 1 (1.1%) | 0 | 0 | 1 | - | - |
| Treatment | |||||||
| Surgery | n (%) | 91 (100%) | 20 (100%) | 37 (100%) | 34 (100%) | 1 | 1 |
| RT ± chemo | n (%) | 60 (65.9%) | 13 (65%) | 25 (67.6%) | 21 (61.8%) | ||
In total, 57/91 OPSCC (62.6%) showed p16-overexpression and positivity for HR-HPV DNA, as detected by PCR. Therefore, these tumors were considered as HPV-positive tumors. With 89.5% (51/57 HPV-positive tumors), HPV16 was the most frequently identified HPV type. Other HPV types detected were HPV18, HPV 33, HPV 35, HPV 56, and HPV 59.
More than 90% of the index tumors of HPV-positive patients were located in the tonsils, as shown in Table 1. The index tumors of HPV-negative patients were located in the tonsil (76.5%), base of tongue (14.7%), pharyngeal wall (5.9%), and soft palate (2.9%).
The mean age of all patients with OPSCC was 59.54 years and comparable among the three groups (HPV-positive smokers, HPV-positive nonsmokers, and HPV-negative smokers).
Independent of HPV status, OPSCC patients were more likely to be male (74.7%) and smokers (72.9%). HPV-positive patients smoked significantly less than HPV-negative patients (p-value 0.002). Only 2/34 patients with HPV-negative tumors were nonsmokers and therefore, they were not separately analyzed.
There were no patients with history of alcohol abuse among nonsmokers, whereas 9/37 (24.3%) of the HPV-positive smokers and 19/32 (59.3%) of the HPV-negative smokers had a history of alcohol abuse. This difference in alcohol consumption between smokers and nonsmokers was statistically highly significant (p-value 0.0001).
3.2. Frequency of Second Primary Tumors
In the overall cohort, 21/91 (23.1%) OPSCC patients developed an SPT during the observation period (Table 2): 4 (20%) HPV-positive nonsmokers, 4 (10.8%) HPV-positive smokers, and 13 (38.2%) HPV-negative patients. HPV-negative OPSCC patients developed significantly more SPT than HPV-positive patients (p-value 0.011, Fisher’s exact test), whereas there was no difference between HPV-positive nonsmokers and HPV-positive smokers.
Table 2.
Overview of the number and locations of SPT of HPV-positive nonsmokers, HPV-positive smokers, and HPV-negative patients. The table compares the number of SPT, as well as the number of SPT at specific locations, between HPV-positive and HPV-negative patients, smokers and nonsmokers, and between HPV-positive nonsmokers and HPV-positive smokers using a two-sided Fisher’s exact test. Results statistically significant at the 5% significance level are marked with *. Abbreviations: HPV = human papillomavirus, NS = nonsmokers, S = smokers, + = positive, − = negative, SPT = second primary tumors.
| Number and Localization of SPT | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Total Cohort n = 91 |
HPV+ NS n = 20 |
HPV+ S n = 37 |
HPV− n = 34 |
p-Values HPV+ vs HPV− (n = 57 vs. n = 34) |
p-Values S vs. NS (n = 69 vs. n = 22) |
p-Values HPV+ NS vs. HPV+ S (n = 20 vs. n = 37) |
|
| SPT | |||||||
| Yes | 21 (23.1%) | 4 (20%) | 4 (10.8%) | 13 (38.2%) | 0.011 * | 0.772 | 0.432 |
| SPT localization | |||||||
| Oral cavity | 4 (4.4%) | 1 (5%) | 0 | 3 (8.8%) | - | - | - |
| Oropharynx | 5 (5.5%) | 0 | 2 (5.4%) | 3 (8.8%) | 0.965 | 0.020 * | 0.429 |
| Larynx | 3 (3.3%) | 0 | 1 (2.7%) | 2 (5.9%) | - | - | - |
| Hypopharynx | 2 (2.2%) | 0 | 0 | 2 (5.9%) | - | - | - |
| Esophagus | 1 (1.1%) | 0 | 0 | 1 (2.9%) | - | - | - |
| Lung | 6 (6.6%) | 3 (15%) | 1 (2.7%) | 2 (5.9%) | 0.146 | 0.053 | 0.486 |
Using a multivariate Cox regression analysis, we examined the influence of HPV-positivity, smoking, alcohol, age, gender, UICC stage, and the therapy mode for the index tumor, on the development of SPT. The results of the multivariate Cox regression are shown in Table 3. Factors such as smoking status, alcohol abuse, age, and gender of the patients showed no significant influence on the development of SPT in this analysis. HPV positivity, on the other hand, was with an HR of 0.344 (95% CI 0.149–0.791, p = 0.012), which was statistically significantly inversely correlated with the development of SPT.
Table 3.
Multivariate Cox regression for SPT development. Table 3 shows the results of the multivariate Cox regression of SPT (0 = no SPT, 1 = SPT) on the independent variables of tumor HPV positivity (negative = 0, positive = 1), smoking status (0 = <10 py, 1 = ≥10 py), history of alcohol abuse (0 = no alcohol abuse, 1 = alcohol abuse), age (in years), sex (0 = male, 1 = female), UICC stage (1 = stage I, 2 = stage II, 3 = stage III, 4 = stage IVa, 5 = stage IVb), and therapy mode (0 = surgery only, 1 = RT ± chemo). * indicates statistical significance at the 5% significance level. Since alcohol use is unknown in three patients, 88 observations are included instead of 91. Abbreviations: Chemo = chemotherapy, CI = confidence interval, HR = hazard ratio, py = pack years, RT =radiotherapy, UICC = Union internationale contre le cancer.
| Multivariate Cox Regression for SPT Development | SPT Development | |
|---|---|---|
| Parameter | HR (95% CI) |
p-Value |
| Tumor HPV-positivity | 0.362 (0.155–0.847) |
0.019 * |
| Smoking (>10 py) | 0.723 (0.239–2.190) |
0.566 |
| Alcohol abuse | 0.995 (0.456–2.170) |
0.989 |
| Age | 0.981 (0.940–1.023) |
0.365 |
| Gender | 0.370 (0.092–1.488) |
0.161 |
| UICC stage | 0.908 (0.659–1.251) |
0.556 |
| Therapy mode | 0.911 (0.403–2.058) |
0.823 |
| Number of observations | 88 | |
3.3. Distribution of Second Primary Tumors over Time
Six of 21 (28.6%) SPT appeared synchronously with the index tumor, while 15/21 (71.4%) appeared metachronously. The synchronous SPT were located in the oropharynx (2 SPT), hypopharynx (2 SPT), larynx (1 SPT), and lung (1 SPT).
Metachronous SPT appeared after an average of 68.9 months (range: 11–190 months, IQR 20–101 months). The majority (60%) of the metachronous SPT was located inside the head and neck area (Table 4): oral cavity (4 SPT), oropharynx (3 SPT), and larynx (2 SPT).
Table 4.
Number of SPT in and outside the head and neck area in HPV-positive and HPV-negative patients. The p-value compares HPV-positive and HPV-negative patients regarding SPT localization in the head and neck area. The p-value was calculated using Fisher’s exact test.
| SPT Localizations | Total n = 21 (%) |
HPV-Positive (n = 8) |
HPV-Negative (n = 13) |
p-Value |
|---|---|---|---|---|
| Head and neck | 14 (66.7%) | 4 (50%) | 10 (76.9%) | 0.346 |
| outside head and neck | 7 (33.3%) | 4 (50%) | 3 (23.1%) |
One case showed a metachronous SPT in the esophagus, and 5 were located in the lung.
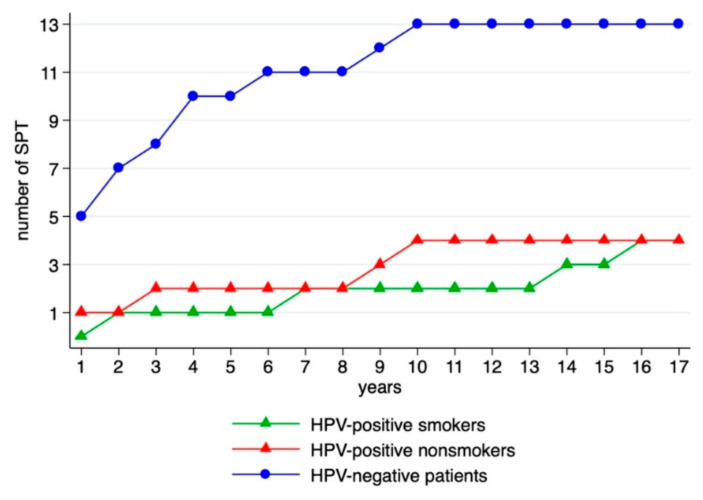
The number of SPT and timely distribution of SPT occurrence in relation to the HPV status are depicted in Figure 1.
Figure 1.
Distribution of SPT over time.
Metachronous SPT of HPV-positive patients occurred after an average of 91.6 months, while the SPT of HPV-negative patients were diagnosed after 49 months on average. Although this difference is striking, it did not reach statistical significance (p-value of 0.161, Table 5).
Table 5.
Time of occurrence of metachronous SPT.
| Time of Occurrence of Metachronous SPT (Months) | HPV-Positive (n = 7 SPT) |
HPV-Negative (n = 8 SPT) |
p-Value |
|---|---|---|---|
| Average | 91.6 | 49 | |
| Median | 90 | 39.5 | 0.161 |
| IQR | 22–154 | 17–77 |
Notably, two of the HPV-positive SPT were diagnosed after more than 12 and 15 years, respectively (larynx and oropharynx).
3.4. Influence of Second Primary Tumors on Survival
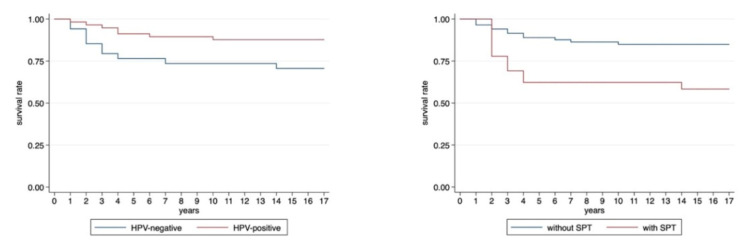
Overall, 17/91 (18.7%) OPSCC patients died: 2 HPV-positive nonsmokers, 5 HPV-positive smokers, and 10 HPV-negative patients. Patients with HPV-positive OPSCC showed significantly better disease-specific survival than patients with HPV-negative OPSCC (5 year survival 91.2% for HPV-positive vs. 76.5% for HPV-negative patients; p < 0.0001, Figure 2), as shown by Kaplan–Meier analysis.
Figure 2.
Kaplan–Meier survival plots of HPV-positive and HPV-negative patients. (Log-rank test: p-value = 0.037) and of patients with and without SPT (Log-rank test: p-value = 0.02).
Five out of 21 (23.8%) OPSCC patients with SPT and 12/70 (17.1%) patients without SPT died. OPSCC patients without SPT had significantly better disease-specific survival than OPSCC patients with SPT (5-year survival 88.5% without SPT vs. 69.2% with SPT; Fisher’s exact test: p-value < 0.0001, Figure 2).
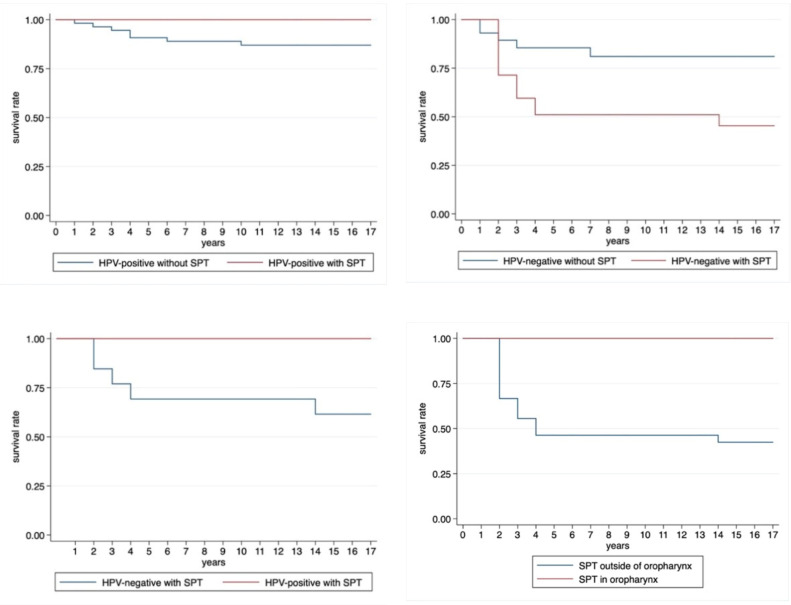
Out of 57 HPV-positive OPSCC patients, seven (12.3%) died from the index tumor and eight (14%) developed an SPT from which no one died within the observation period. There was no significant difference in survival in the HPV-positive patient group with SPT compared to HPV-positive patients without (Log-rank test: p-value = 0.514, Figure 3).
Figure 3.
Kaplan–Meier survival plots of HPV-positive patients with and without SPT. (Log-rank test: p-value = 0.514), Kaplan–Meier survival plot of HPV-negative patients with and without SPT (Log-rank test: p-value = 0.033), of HPV-negative and HPV-positive patients with SPT (Log-rank test: p-value = 0.054), and of patients with SPT in and outside the oropharynx (Log-rank test: p-value = 0.054).
Out of 34 HPV-negative patients, 5/21 (23.8%) died without SPT and 5/13 (38.5%) died with SPT. The disease-specific 5-year survival rate was statistically significantly higher in HPV-negative patients without SPT (83.3%) than in HPV-negative patients with SPT (51.4%, Fisher’s exact test: p-value < 0.0001).
Figure 3 also shows the Kaplan–Meier survival plot of the 21 patients with SPT, analyzed by the HPV positivity of the index tumor. There was a trend for a worse survival in HPV-negative patients developing SPT compared to HPV-positive patients with SPT (log-rank test: p-value = 0.054).
Interestingly, none of the patients with a SPT in the oropharynx died during the time of observation. This stands in contrast to 23.8% deaths among patients with an SPT outside the oropharynx. However, the difference was not statistically significant (Kaplan–Meier survival plot in Figure 3, log-rank test: p-value 0.105).
As shown in Table 6, neither therapy mode nor UICC stage of the index tumor showed significant influence on OPSCC-associated survival.
Table 6.
Multivariate Cox regression for death in association with OPSCC. Table 6 shows the influence of the following variables on death due to OPSCC and/or SPT after OPSCC: tumor HPV positivity (negative = 0, positive = 1), smoking status (0 = <10 py, 1 = ≥10 py), alcohol status (0 = no alcohol abuse, 1 = alcohol abuse), age (in years), SPT development (0= no SPT, 1 = SPT), therapy mode (1 = surgery, 2 = RT, 3 = surgery + RT, 4 = surgery + RT + chemotherapy, 5 = surgery + chemotherapy), and UICC stage of the index OPSCC (1 = stage I, 2 = stage II, 3 = stage III, 4 = stage IVa, 5 = stage IVb). Abbreviations: CI = confidence interval, HR = hazard ratio, py = pack years, RT = radiotherapy.
| Multivariate Cox Regression for Death Associated with OPSCC | Death Associated with OPSCC | |
|---|---|---|
| Parameter | HR (95% CI) |
p-Value |
| Tumor HPV-positivity | 0.519 0.733–1.459) |
0.195 |
| Smoking (>10 py) | 1.634 (0.372–7.182) |
0.515 |
| Alcohol abuse | 1.248 (0.462–3.373) |
0.662 |
| Age | 1.024 (0.981–1.069) |
0.276 |
| SPT development | 0.978 (0.404–2.364) |
0.960 |
| Treatment of index tumor | 1.034 (0.733–1.459) |
0.850 |
| Index UICC stage | 0.871 (0.580–1.308) |
0.505 |
| Number of observations | 88 | |
4. Discussion
SPT of HPV-associated OPSCC have been investigated in search of optimal diagnostic workup strategies [22,23]. As shown by Hsu et al. [24], SPT present a major obstacle improving the long-term survival of patients with OPSCC. Therefore, SPT are of imminent importance to follow-up of OPSCC patients. Still, to our knowledge, this is the first study investigating the long-term survival of HPV-positive OPSCC patients with and without SPT.
Our study compares the frequency, localization, and timely distribution of SPT development in HPV-positive and HPV-negative OPSCC patients. Additionally, a comprehensive analysis of the influence of risk factors on SPT development and SPT localization is given. We report a significantly lower rate of SPT in HPV-positive OPSCC compared to HPV-negative OPSCC. Our survival analysis showed a significantly negative impact of SPT on survival in HPV-negative patients, while it did not significantly affect the survival time of HPV-positive patients. Due to the small number of SPT, especially in the cohort of HPV-positive patients, no firm conclusions on SPT localization can be made.
The focus of the latest research on HPV-associated OPSCC has been on possible therapy modifications [25]. With the presented results, we hope to extend the discussion to follow-up protocol for HPV-positive OPSCC patients.
4.1. Patient Characteristics
The three main risk factors for OPSCC are smoking, alcohol abuse, and HPV infection [26]. Indicating the impact of HPV infections, our cohort showed 63% HPV-associated OPSCC. Older literature had found HPV-associated OPSCC to be present in a group of patients who are younger than HPV-negative OPSCC patients and have less exposure to noxious agents. [12]. However, more recent results from the US show a “moderation of increasing incidence in younger individuals and a shift in the burden to older individuals” [27]. Consistent with the increasing number of HR-HPV infections in the oropharynx [8], HPV-positive nonsmokers (all without alcohol abuse) accounted for a remarkable 22% of our cohort. In addition, HPV-positive patients smoked significantly less than HPV-negative patients. The fraction of smokers among HPV-negative OPSCC patients was high at 94.1%. Excessive alcohol consumption was only found in smokers and was more prevalent in HPV-negative patients. The association of heavy tobacco and alcohol abuse is well known in the literature [28].
Contrary to other studies [13,29], in our study cohort, HPV-positive patients were not younger than HPV-negative patients.
4.2. Localization of Index Tumors
Consistent with literature [30,31], the vast majority of HPV-positive index OPSCC in our cohort was located in the tonsils (93%) and the base of the tongue (7%). This supports the suggestion of the predilection of HPV-associated tumors in the lymphoid tissue of Waldeyer’s ring [32,33,34].
In contrast, HPV-negative patients showed a broader distribution with index tumors in tonsil (76.6%), base of tongue (14.7%), pharyngeal wall (5.9%), and soft palate (2.9%).
4.3. Frequency and Localization of Second Primary Tumors
In this study, the definition of SPT was based on Warren and Gates [19], even though the latest and more exact definition of SPT goes back to Braakhuis et al. [35]. Due to the retrospective nature of this study, data on genetic patterns of the SPT were not available. Therefore, the genetic differentiation of SPT from recurrence and metastasis, as suggested by Braakhuis et al. [35], was not applicable. Table 7 provides an overview over the localization and histology of all SPT in our cohort.
Table 7.
Overview over localization and histological entity of the tumors of HPV-positive and HPV-negative patients who developed an SPT.
| HPV Positivity | Localization of Index Tumor | Localization SPT | Histology of SPT |
|---|---|---|---|
| Positive | tonsil | lung | Squamous cell carcinoma, with growth pattern distinct from index tumor |
| Tonsil | Lung | Squamous cell carcinoma | |
| Base of tongue (right) | Vallecula (left) | Squamous cell carcinoma | |
| Base of tongue | Lung | Small cell lung carcinoma | |
| Oropharynx | Lung | Squamous cell carcinoma | |
| Tonsil | Vocal cord | Squamous cell carcinoma | |
| Tonsil | Side of the tongue | Squamous cell carcinoma | |
| Tonsil (left) | Tonsil (right) | unknown | |
| Negative | Oropharynx | Thyroid gland | Papillary thyroid carcinoma |
| Oropharynx | Side of the tongue | Squamous cell carcinoma, p16-negative | |
| Oropharynx | Lung | adenocarcinoma | |
| Tonsil | Base of tongue | Squamous cell carcinoma, p16-negative | |
| Tonsil (right) | Vallecula (left) | Squamous cell carcinoma | |
| Oropharynx | Soft palate | High grade dysplasia | |
| Base of tongue (right) | Larynx (left) | Squamous cell carcinoma | |
| Tonsil (left) | Hypopharynx (right) | Squamous cell carcinoma | |
| Tonsil | Lung | Squamous cell carcinoma | |
| Tonsil | Hypopharynx | Squamous cell carcinoma | |
| Tonsil (right) | Tonsil (left) | Squamous cell carcinoma (p16-positive) | |
| Tonsil | Hypopharynx | Squamous cell carcinoma | |
| Tonsil | Esophagus | Squamous cell carcinomas/adeno |
As hypothesized, HPV-positive patients developed significantly fewer SPT (15.79%) than HPV-negative patients (38.24%) [17,21,36]. Similarly, Martel et al. [15] performed a study with 412 OPSCC patients, including T and N category as additional risk factors, and they found only HPV status showing a significant impact on the occurrence of SPT. The authors found a 4.5-fold increased risk of developing SPT in HPV-positive patients with heavy abuse of noxious agents or HPV-negative patients with moderate or no abuse of noxious agents, compared with HPV-positive patients without exposure to noxious agents. The risk of HPV-negative patients with severe noxious exposure was even increased by a factor of 13.2 compared with HPV-positive patients without exposure to extrinsic carcinogens. This illustrates the lower SPT risk of HPV-positive OPSCC, even with the influence of tobacco/alcohol abuse.
Contrary to expectations, we did not find a significantly increased incidence of SPT in smokers compared to nonsmokers, even with statistical control for age, sex, HPV status, and alcohol abuse (Table 3). Putting this result into perspective, Table 8 showed a significant influence of smoking on the occurrence of SPT in the head and neck area. We attributed the nonsignificant influence of smoking on SPT development in Table 3 to the small number of cases in our cohort. Milliet et al. [36] found alcohol and tobacco consumption, as well as a negative HPV status to be significant predictors for SPT development in OPSCC patients.
Table 8.
Multivariate Cox regression for SPT development in the head and neck area. Table 8 describes the results of the multivariate Cox regression of all SPT with respect to localization in the head and neck area (0 = SPT outside head and neck, 1 = SPT in head and neck) regressed on the independent variables tumor HPV positivity (negative = 0, positive = 1), smoking status (0 = <10 py, 1 = ≥10 py), alcohol status (0 = no alcohol abuse, 1 = alcohol abuse), and age (in years). * indicates statistical significance at the 5% significance level. Abbreviations: CI = confidence interval, HR = hazard ratio, py = pack years.
| Multivariate Cox Regression for SPT Development in the Head and Neck | SPT in Head and Neck | |
|---|---|---|
| Parameter | HR (95% CI) |
p-Value |
| Tumor HPV-positivity | 1.102 (0.629–1.930) |
0.734 |
| Smoking (>10 py) | 3.513 (1.154–10.693) |
0.027 * |
| Alcohol abuse | 1.301 (0.730–2.319) |
0.372 |
| Age | 0.934 (0.886–0.985) |
0.012 * |
| Number of observations | 21 | |
In a literature review of the last 40 years (1979–2019), Coca-Pelaz et al. [5] found that SPT after HNSCC occurred most frequently in the head and neck region, followed by lung and esophagus. In our cohort, the head and neck area and the lung were also the most common location of SPT. The same distribution was shown for HPV-positive patients, independent of tobacco use as a risk factor. Exemplarily, an HPV-positive nonsmoker of our cohort developed a SPT in the oral cavity. This stands in contrast to the hypothesis of Rietbergen et al. [37] saying that SPT of HPV-positive OPSCC do not occur in the head and neck region due to absent field carcinogenesis. They examined the tumor-free resection margins of HPV-positive OPSCC and were unable to detect HPV16-E6 mRNA [37]. From this, they concluded that field carcinogenesis by HPV is unlikely. McGovern et al. [38] examined the mucosa between two adjacent HPV-positive OPSCC and were not able to detect HPV, either.
In our study, HPV-positive patients mostly showed metachronous SPT. This in agreement with the results of Xu et al. [23], who showed that the diagnostic workup of HPV-positive OPSCC detected less synchronous SPT than in HPV-negative OPSCC. Thus, field cancerization by HPV, as first described by Slaughter in 1946 [2], seems unlikely for HPV-associated tumors.
On the basis of the clonal relationship of first and second tumors [39], Rietbergen et al. [37] postulated that synchronous SPT of HPV-positive OPSCC result from the same infection as the index tumor. They also deduce that SPT arise from the migration of HPV-infected cells. A similar phenomenon has been described in women with cervical carcinoma and SPT in the lower genital tract (vulva and vagina) [40].
In a case report of a 46-year-old patient with three synchronous HPV-associated OPSCC and two cervical metastases, McGovern et al. [38] also commented on the phenomenon of multiple HPV-associated tumors. After excluding field carcinogenesis by HPV, McGovern et al. postulated two models for the development of second HPV-associated lesions: (1) multiple independent HPV infections leading to the development of multiple tumors, and (2) a primary HPV-associated tumor producing clonally related neoplasms that migrate to new localizations and appear as metachronous SPT.
The latter theory was supported by the findings of a case report of Joseph et al. [39], who showed four HPV-associated carcinomas of the tonsils with molecularly identical SPTs in the respective contralateral tonsil. In view of these data, the question arises as to the exact delineation between SPT and metastasis. A 100% genetic match would classify for a metastasis, even if it appeared with an exceptionally large temporal distance from the primary tumor.
Due to the small number of HPV-positive SPT in our cohort, we could not make robust statements on the frequency of HPV-associated SPT at individual sites. Still, the detected localizations, especially of HPV-positive SPT, are noteworthy.
In our cohort, looking specifically at SPT in the head and neck area, tumoral HPV positivity of the index tumor did not show any significant influence on the frequency of SPT, while smoking and age of the patient did. Thus, the risk of SPT in the head and neck area decreased with increasing age, while it increased in smokers.
This is consistent with the results of Peck et al. [22] who demonstrated that HPV-seropositive patients developed proportionally fewer SPT in traditionally tobacco-associated sites than HPV-seronegative patients. However, it is important to note that Peck et al. [22] determined HPV status using serum markers, which show exceptionally high specificity [41,42] but may precede tumor development by years [43].
4.4. Distribution of Second Primary Tumors over Time
Tumor follow-up was conducted over an average of 4.76 years after diagnosis, which is in line with other studies on the same topic [5]. Still, our cohort contained patients that were observed over 17 years.
As Kreimer et al. [43,44] found in serologic studies, more than 10 years can elapse between HPV infection and the appearance of oropharyngeal cancer.
From Figure 1, we find that the majority of SPT occurred metachronously; in particular, SPT of HPV-positive OPSCC. HPV-positive metachronous tumors were detected an average of 42.13 months later than HPV-negative ones, and two of the SPT did not appear until more than 13 years after diagnosis of the index tumor. Thus, it seems important to maintain follow-up over time, especially for HPV-positive OPSCC. To determine SPT risk at different points in time after diagnosis of the index OPSCC, studies with long-term follow-up of larger cohorts than ours are needed.
4.5. Prognostic Impact of SPT
The Kaplan–Meier plot in Figure 2 shows a significantly higher survival rate of HPV-positive patients compared to HPV-negative patients. This is consistent with the results of many studies and with the landmark paper by Ang et al. [45]. They showed a more than 50% lower mortality risk in HPV-positive versus HPV-negative OPSCC patients.
Looking at the impact of SPT on the prognosis of OPSCC patients, the consensus in the literature is the following: despite advances in therapy, the survival rate of OPSCC patients remains reduced, especially if SPT are developed [24,46]. Bhattacharyya and Nayak [47] found an up to 50% decreased survival rate in HNSCC patients with SPT compared to HNSCC patients without SPT. Milliet et al. [36], on the other hand, did not find a difference in overall survival between patients with and without SPT. In line with the results of Bhattacharyya and Nayak [47], our cohort also showed significantly worse survival of patients with SPT compared to patients without SPT. An analogous result was observed when looking at HPV-negative patients only, who showed significantly worse survival rates when SPT occurred (Figure 3). These findings were confirmed by the results of Martel et al. [15].
In contrast, in HPV-positive OPSCC patients, survival with SPT did not significantly differ from survival without SPT (Figure 3). The fact that survival in HPV-positive patients does not significantly worsen with the occurrence of SPT may be due to the better treatment response of HPV-positive tumors, as it has been shown in HPV-positive index tumors [48,49]. To our knowledge, no comparable study of survival with and without SPT has been published so far.
4.6. Strenghts and Limitations of the Study
This study is limited by the small number of cases for analysis of SPT. Since SPT are much less frequent in HPV-positive OPSCC than in HPV-negative OPSCC, we only recorded eight SPT of HPV-positive OPSCC. Thus, conclusions about the frequencies of specific SPT localizations are limited, but they provide a lead for larger prospective studies.
Retrospective analyses, as performed in this study, must be interpreted with caution due to the limited quality of retrospectively collected data, sample size limitations, and the potential for noncontrolled influencing factors.
Although this study focused on SPT of HPV-associated OPSCC, HPV status of the SPT was concluded solely based on the location and the HPV status of the index OPSCC. Therefore, uncertainty remains regarding the HPV status of SPT. The possibility remains, especially with tumors of the lung, that SPT are actually single metastases. Absolute certainty in this regard can only be obtained by genetic sequence analysis, which was not investigated in this study.
A strength of our study is the long observation period of up to 17 years.
5. Conclusions
HPV-positive OPSCC showed a lower risk of SPT than HPV-negative OPSCC. SPT of HPV-negative OPSCC occurred in the typical tobacco- and alcohol-associated sites. Significantly more HPV-negative patients died from index OPSCC or its SPT than HPV-positive patients. Thus, HPV positivity is a positive prognostic factor not only in the context of overcoming cancer but also in terms of long-term survival. Since SPT of HPV-positive OPSCC still occurred after more than 10 years, follow-up should not be reduced despite the lower SPT risk of HPV-positive OPSCC.
Author Contributions
Conceptualization, M.A.B.; methodology, M.A.B. and S.L.B.; software, S.L.B.; validation, M.A.B. and S.L.B.; formal analysis, S.L.B.; investigation, S.L.B.; resources, M.A.B. and S.L.B.; data curation, M.A.B., S.L.B. and G.B.M.; writing—original draft preparation, S.L.B.; writing—review and editing, S.L.B., M.A.B. and G.B.M.; visualization, S.L.B.; supervision, M.A.B.; project administration, M.A.B.; funding acquisition, M.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Kantonale Ethikkommission Zürich (protocol code 2013-0019, date of approval: 5 April 2013).
Informed Consent Statement
Due to the retrospective nature of the study with a significant number of patients that had already died, according to the judgement of the Ethics Committee, no written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gillison M.L., Koch W.M., Capone R.N., Spafford M., Westra W.H., Wu L., Zahurak M.L., Daniel R.W., Vigilone M., Symer D.E., et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter D.P. Multicentric origin of intra-oral carcinoma. Surgery. 1946;20:133–146. [PubMed] [Google Scholar]
- 3.Guardiola E., Pivot X., Dassonville O., Poissonnet G., Marcy P.-Y., Otto J., Poudenx M., Francois E., Bensadoun R.-J., Thyss A., et al. Is routine triple endoscopy for head and neck carcinoma patients necessary in light of a negative chest computed tomography scan? Cancer. 2004;101:2028–2033. doi: 10.1002/cncr.20623. [DOI] [PubMed] [Google Scholar]
- 4.Grégoire V., Lefebvre J.-L., Licitra L., Felip E. Squamous cell carcinoma of the head and neck:EHNS–ESMO–ESTRO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;21:184–186. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 5.Coca-Pelaz A., Rodrigo J.P., Suarez C., Nixon I.J., Mäkitie A., Sanabria A., Quer M., Strojan P., Bradford C.R., Kowalski L.P., et al. The risk of second primary tumors in head and neck cancer: A systematic review. Head Neck. 2020;42:456–466. doi: 10.1002/hed.26016. [DOI] [PubMed] [Google Scholar]
- 6.Schneider-Maunoury S., Croissant O., Orth G. Integration of human papillomavirus type 16 DNA sequences: A possible early event in the progression of genital tumors. J. Virol. 1987;61:3295–3298. doi: 10.1128/JVI.61.10.3295-3298.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King B., Dube S., Kaufmann R., Shaw L., Pechacek T. Vital signs: Current cigarette smoking among adults aged ≥18 years—United States, 2005–2010. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1207–1212. [PubMed] [Google Scholar]
- 8.Chaturvedi A.K., Engels E.A., Anderson W.F., Gillison M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi A.K., Anderson W.F., Lortet-Tieulent J., Curado M.P., Ferlay J., Franceschi S., Rosenberg P.S., Bray F., Gillison M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillison M.L., Alemany L., Snijders P.J., Chaturvedi A.K., Steinberg B.M., Schwartz S., Castellsagué X. Human papillomavirus and diseases of the upper airway: Head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30:34–54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 11.Castellsagué X., Alemany L., Quer M., Halec G., Quiros B., Tous S., Clavero O., Alos L., Biegner T., Szafarowski T., et al. HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst. 2016;108:403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 12.Ringström E., Peters E., Hasegawa M., Posner M., Liu M., Kelsey K.T. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–3192. [PubMed] [Google Scholar]
- 13.Gillison M.L., D’Souza G., Westra W.H., Sugar E., Xiao W., Begum S., Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 14.Rampias T., Sasaki C., Psyrri A. Molecular mechanisms of HPV induced carcinogenesis in head and neck. Oral Oncol. 2014;50:356–363. doi: 10.1016/j.oraloncology.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Martel M., Alemany L., Taberna M., Mena M., Tous S., Bagué S., Castellsagué X., Quer M., Leon X. The role of HPV on the risk of second primary neoplasia in patients with oropharyngeal carcinoma. Oral Oncol. 2017;64:37–43. doi: 10.1016/j.oraloncology.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Ragin C.C., Modugno F., Gollin S.M. The epidemiology and risk factors of head and neck cancer: A focus on human papillomavirus. J. Dent. Res. 2007;86:104–114. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 17.Boakye E.A., Buchanan P., Hinyard L., Stamatakis K., Osazuwa-Peters N., Simpson M.C., Schootman M., Piccirillo J.F. Risk and outcomes for second primary human papillomavirus–related and –unrelated head and neck malignancy. Laryngoscope. 2019;129:1828–1835. doi: 10.1002/lary.27634. [DOI] [PubMed] [Google Scholar]
- 18.Smeets S.J., Hesselink A.T., Speel E.M., Hasevoets A., Snijders P.J., Pawlita M., Meijer C.J.L.M., Braakhuis B.J.M., Leemans C.R., Brakenhoff R.H. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 19.Warren S., Gates O. Multiple primary tumors. Am. J. Cancer Res. 1932;16:1358–1414. [Google Scholar]
- 20.Munnoz N., Bosch F.X., De Sanjosé S., Herrero R., Castellsagué X., Shah K.V., Snijders P.J.F., Meijer C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 21.Morris L.G., Sikora A.G., Patel S.G., Hayes R.B., Ganly I. Second primary cancers after an index head and neck cancer: Subsite- specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J. Clin. Oncol. 2011;29:739–746. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peck B.W., Dahlstrom K.R., Gan S.J., Caywood W., Li G., Wei Q., Zafereo M.E., Sturgis E.M. Low risk of second primary malignancies among never smokers with human papillomavirus–associated index oropharyngeal cancers. Head Neck. 2013;35:794–799. doi: 10.1002/hed.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C.C., Biron V.L., Puttagunta L., Seikaly H. HPV Status and second primary tumours in Oropharyngeal Squamous Cell Carcinoma. Head Neck Surg. 2013;42:36. doi: 10.1186/1916-0216-42-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu S.-H., Wong Y.-K., Wang C.-P., Wang C.-C., Jiang R.-S., Chen F.-J., Liu S.-A. Survival analysis of patients with oral squamous cell carcinoma with simultaneous second primary tumors. Head Neck. 2013;35:1801–1807. doi: 10.1002/hed.23242. [DOI] [PubMed] [Google Scholar]
- 25.Nichols A.C., Lang P., Prisman E., Berthelet E., Tran E., Hamilton S., Wu J., Fung K., De Almeida J.R., Bayley A., et al. Treatment de-escalation for HPV-associated oropharyngeal squamous cell carcinoma with radiotherapy vs. trans-oral surgery (ORATOR2): Study protocol for a randomized phase II trial. BMC Cancer. 2020;20:125. doi: 10.1186/s12885-020-6607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillison M.L., Chaturvedi A.K., Anderson W.F., Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tota J.E., Best A.F., Zumsteg Z.S., Gillison M.L., Rosenberg P.S., Chaturvedi A.K. Evolution of the oropharynx cancer epidemic in the United States: Moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J. Clin. Oncol. 2019;37:1538–1546. doi: 10.1200/JCO.19.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batel P., Pessione F., Maitre C., Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1111/j.1360-0443.1995.tb03507.x. [DOI] [PubMed] [Google Scholar]
- 29.Stenmark M.H., Shumway D., Guo C., Vainshtein J., Mierzwa M., Jagsi R., Griggs J.J., Banerjee M. Influence of human papillomavirus on the clinical presentation of oropharyngeal carcinoma in the United States. Laryngoscope. 2018;127:2270–2278. doi: 10.1002/lary.26566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryerson A.B., Peters E.S., Coughlin S.S., Chen V.W., Gillison M.L., Reichman M.E., Wu X., Chaturvedi A.K., Kawaoka K. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113:2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 31.El-Mofty S.K., Lu D.W. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients a distinct clinicopathologic and molecular disease entity. Am. J. Surg. Pathol. 2003;27:1. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Klussmann J.P., Weissenborn S.J., Wieland U., Dries V., Kolligs J., Jungehuelsing M., Eckel H.E., Dienes H.P., Pfister H.J., Fuchs P.G. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::AID-CNCR10130>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Van Houten V.M., Snijders P.J., van den Brekel M.W., Kummer J.A., Meijer C.J., van Leeuwen B., Denkers F., Smeele L.E., Snow G.B., Brakenhoff R.H. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int. J. Cancer. 2001;93:232. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 34.Wilczynski S.P., Lin B.T., Xie Y., Paz I.B. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am. J. Pathol. 1998;152:145–156. [PMC free article] [PubMed] [Google Scholar]
- 35.Braakhuis B.J., Brakenhoff R.H., Leemans C.R. Second Field Tumors: A New Opportunity for Cancer Prevention? Oncologist. 2005;10:493–500. doi: 10.1634/theoncologist.10-7-493. [DOI] [PubMed] [Google Scholar]
- 36.Milliet F., Bozec A., Schiappa R., Viotti J., Modesto A., Dassonville O., Poissonnet G., Guelfucci B., Bizeau A., Vergez S., et al. Synchronous primary neoplasia in patients with oropharyngeal cancer: Impact of tumor HPV status. A GETTEC multicentric study. Oral Oncol. 2021;112:105041. doi: 10.1016/j.oraloncology.2020.105041. [DOI] [PubMed] [Google Scholar]
- 37.Rietbergen M.M., Braakhuis B.J., Moukhtari N., Bloemena E., Brink A., Sie D., Ylstra B., Baatenburg de Jong R.J., Snijders P.J.F., Brakenhoff R.H., et al. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumors. J. Oral Pathol. Med. 2014;43:137–142. doi: 10.1111/jop.12123. [DOI] [PubMed] [Google Scholar]
- 38.McGovern S.L., Williams M.D., Weber R.S., Sabichi A., Chambers M.S., Martin J.W., Chao C. Three synchronous HPV-associated squamous cell carcinomas of Waldeyer’s ring: Case report and comparison with Slaughter’s model of field cancerization. Head Neck. 2010;32:1118–1124. doi: 10.1002/hed.21171. [DOI] [PubMed] [Google Scholar]
- 39.Joseph A.W., Ogawa T., Bishop J.A., Lyford-Pike S., Chang X., Phelps T.H., Westra W.H., Pai S.I. Molecular etiology of second primary tumors in contralateral tonsils of human papillomavirus-associated index tonsillar carcinomas. Oral Oncol. 2013;49:244–248. doi: 10.1016/j.oraloncology.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinokurova S., Wentzensen N., Einenkel J., Klaes R., Ziegert C., Melsheimer P., Sartor H., Horn L.-C., Höckel M., von Knebel Doeberitz M. Clonal history of papillomavirus-induced dysplasia in the female lower genital tract. J. Natl. Cancer Inst. 2005;97:1816–1821. doi: 10.1093/jnci/dji428. [DOI] [PubMed] [Google Scholar]
- 41.Kuhs K.A., Anantharaman D., Waterboer T., Johansson M., Brennan P., Michel A., Willhauck-Fleckenstein M., Purdue M.P., Holcatova I., Ahrens W., et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: A pooled analysis. Cancer Epidemiol. Biomark. Prev. 2015;24:683–689. doi: 10.1158/1055-9965.EPI-14-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broglie M.A., Jochum W., Michel A., Waterboer T., Foerbs D., Schoenegg R., Stoeckli S.J., Pawlita M., Holzinger D. Evaluation of type-specific antibodies to high risk-human papillomavirus (HPV) proteins in patients with oropharyngeal cancer. Oral Oncol. 2017;70:43–50. doi: 10.1016/j.oraloncology.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Kreimer A.R., Ferreiro-Iglesias A., Nygard M., Bender N., Schroeder L., Hildesheim A., Robbins H.A., Pawlita M., Langseth H., Schlecht N.F., et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: Findings from the HPVC3 consortium. Ann. Oncol. 2019;30:1335–1343. doi: 10.1093/annonc/mdz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreimer A.R., Johansson M., Waterboer T., Kaaks R., Chang-Claude J., Drogen D., Overvad K., Quiros J.R., Gonzalez C.A., Sánchez M.J. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J. Clin. Oncol. 2013;31:2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tan P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz L.H., Ozsahin M., Zhang G.N., Touboul E., De Vataire F., Andolenko P., Lacau-Saint-Guily J., Laugier A., Schlienger M. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933–1938. doi: 10.1002/1097-0142(19941001)74:7<1933::AID-CNCR2820740718>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharyya N., Nayak V.K. Survival outcomes for second primary head and neck cancer: A matched analysis. Otolaryngol. Head Neck Surg. 2005;132:63–68. doi: 10.1016/j.otohns.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Fakhry C., Westra W.H., Li S., Cmelak A., Ridge J.A., Pinto H., Forastiere A., Gillison M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 49.Van Monsjou H.S., Balm A.J., van den Brekel M.M., Wreesmann V.B. Oropharyngeal squamous cell carcinoma: A unique disease on the rise? Oral Oncol. 2010;46:780–785. doi: 10.1016/j.oraloncology.2010.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons.