Abstract
A study was conducted to investigate saprobic fungal niches of Stachybotryaceae (Hypocreales) associated with leaves of Musa (banana) in China and Thailand. Three hyphomycetous taxa were collected during the dry season of 2018 and 2019. After a careful phenotypic characterization (both macro- and microscopically) and a phylogenetic tree reconstruction using a concatenated sequence dataset of internal transcribed spacer (ITS), calmodulin (cmdA), RNA polymerase II second largest subunit (rpb2), β-tubulin (tub2), and the translation elongation factor 1-alpha (tef1) gene regions, we report three species of Stachybotryaceae. Stachybotrys musae is introduced as a novel taxon from Yunnan, China, while S. microsporus is reported from Chiang Rai Province in Thailand on Musa. In addition, Memnoniella levispora is also reported from China for the first time.
Keywords: new species, fungi on banana, Musaceae, saprobes, Sordariomycetes
1. Introduction
In the past three decades, there have been several studies on saprobic fungi associated with tropical monocotyledonous hosts [1,2,3,4,5,6,7,8,9,10]. In addition, detailed taxonomic studies have been conducted to describe and document the saprobic fungi on Musa across South and South East Asia [11,12,13,14,15,16].
Stachybotryaceae accommodates 39 genera (including Memnoniella and Stachybotrys) in Hypocreales [17,18]. The taxonomic histories of Memnoniella and Stachybotrys are detailed in Wang et al. [19] and Lombard et al. [20]. An updated phylogeny for Stachybotryaceae was provided by Lombard et al. [20] using partial 28S large sub unit (LSU), internal transcribed spacer (ITS), rpb2, cmdA, tef1, and tub2 sequence data. Previously, Smith [21] and Wang et al. [19] stated that Memnoniella and Stachybotrys are congeneric. However, Lombard et al. [20] resurrected Memnoniella as a distinct genus in Stachybotryaceae. Lin et al. [22], Doilom et al. [23], Hyde et al. [17], and Mapook et al. [24] further supported the observations of Lombard et al. [20] and treated Memnoniella and Stachybotrys as two distinct genera. Hyde et al. [17] documented nine species of Memnoniella with DNA sequence data. Index Fungorum [25] documented 21 names of Memnoniella, but ten were transferred to other genera i.e., Brevistachys and Stachybotrys, in Stachybotryaceae [17,24,26]. Hyde et al. [17] listed 88 species of Stachybotrys on the basis of Species Fungorum [27]. Currently, 30 taxa of Stachybotrys have DNA sequence data in GenBank.
The asexual morph of Stachybotrys has branched or unbranched, erect conidiophores bearing terminal, discrete, phialidic conidiogenous cells with unicellular conidia formed in chains or slimy masses [19,20,28,29]. Memnoniella shares a similar morphology with Stachybotrys [19,20,26,30] even though both genera are phylogenetically distinct. The conidia of Memnoniella occur on the surface as dry chains, while those in Stachybotrys occur as slimy masses [20]. However, much research has disregarded this dry or wet conidial disposition pattern while distinguishing Memnoniella and Stachybotrys [17,20,21,26].
Stachybotrys is common in soil, plant litter (hay, straw, cereal grains, and decaying plant debris), marine habitats, and air [19,20,23,24,26]. In addition, Stachybotrys has been detected on damp paper, cotton, linen, cellulose-based building materials (drywalls, wallpapers in indoor environments), water-damaged indoor buildings, and air ducts [5,17,19,28,31,32,33,34,35]. Most Stachybotrys species are cellulolytic saprobes [36], as well as plant pathogens [37,38] and endophytes [39,40,41,42,43]. Memnoniella species exhibit saprobic lifestyles and have been reported from dead plant materials and soil [20,39]. Some taxa of Memnoniella and Stachybotrys (M. echinata and S. chartarum) coexist in similar ecological habitats such as indoor environments [39]. Mainly, S. chartarum and a few other species of Stachybotrys (i.e., S. elegans and S. microsporus) have veterinary and medical importance as they produce several mycotoxins [44,45,46,47,48].
Many species of Memnoniella and Stachybotrys have been documented from China and Thailand. Lin et al. [23] provided a check list of Stachybotrys species recorded from different hosts and substrates in Thailand (S. albipes, S. bambusicola, S. chartarum, S. elegans, S. microsporus, S. nephrosporus, S. palmae, S. parvisporus, S. renisporus, S. ruwenzoriensis, S. sansevieriae, S. suthepensis, and S. theobromae). Stachybotrys aksuensis (Xinjiang), S. biformis (Shaanxi), S. littoralis (Guangdong), and S. yushuensis (Qinghai) were introduced from soil habitats in China [49]. In addition, S. nielamuensis [50] (Tibet), S. subcylindrosporus [33] (Hainan), S. variabilis [51] (Qinghai), S. yunnanensis [52] (Yunnan), and S. zhangmuensis [50] (Tibet) were described from China. Memnoniella chromolaenae, M. echinata and M. sinensis were reported from Yunnan Province, China and Thailand [24,39,53].
Photita et al. [11,12] and Farr and Rossman [54] documented Stachybotrys nephrosporus, S. ruwenzoriensis, and S. theobromae as saprobes on Musa from Thailand. Photita et al. [11] introduced S. suthepensis, which was saprobic on dead petioles of Musa acuminata from Chiang Mai, Thailand. In addition, S. chartarum [55] (Somalia) and S. globosus [56] (India) were found on Musa. Memnoniella dichroa (Thailand), M. echinata (Honduras, Japan), and M. subsimplex (Bermuda, Ghana, New Zealand, Sierra Leone) were also recorded on Musa [12,28,57,58].
Most Stachybotrys and a few Memnoniella species were introduced only on the basis of morphology [19]. The limitation of DNA sequence data in GenBank has restricted the delineation of species based on phylogeny. Wang et al. [19] and Lombard et al. [20] tried to address these research gaps and highlighted that many taxa of Stachybotryaceae are invalidly published. The toxicological health effects of S. chartarum are widely studied, but other taxa in the genus are not as well studied. Therefore, the need for a more comprehensive morpho-molecular taxonomic work on Stachybotrys and Memnoniella was recommended in recent studies [17,19,20].
We have been studying fungi associated with Musa [14,15,59]. The present study concentrates on saprobic Stachybotrys and Memnoniella niches on Musa from China and Thailand. We introduce Stachybotrys musae sp. nov. on Musa from China (Yunnan Province, Xishuangbanna), while Memnoniella levispora is reported from China (Yunnan) for the first time. Stachybotrys microspores is also reported from Chiang Rai Province, Thailand. Multi-locus phylogenetic analyses, morphological illustrations, and taxonomic discussions are provided for these taxa.
2. Materials and Methods
2.1. Sample Collection, Morphological Studies, and Isolation
Decaying leaves of an undetermined species of Musa with fungal structures were collected from Yunnan Province, China and Thailand during December and April of 2018 and 2019. Plant materials were transferred to the laboratory in small cardboard boxes and treated as outlined in Senanayake et al. [60].
Single-spore isolation was conducted following the methods outlined in Senanayake et al. [60]. Herbarium specimens were deposited in the Mae Fah Luang University Herbarium (Herb. MFLU), Chiang Rai, Thailand. Living cultures of each strain were deposited in the Culture Collection of Mae Fah Luang University (MFLUCC). Faces of Fungi [61] and MycoBank numbers (https://www.MycoBank.org (accessed on 18 January 2021)) were obtained for the novel taxon.
2.2. DNA Extraction, PCR Amplification, and Sequencing
DNA extraction, PCR amplification, and sequencing followed the methods outlined in Dissanayake et al. [62]. Five gene regions, including the internal transcribed spacer (ITS), partial calmodulin (cmdA), partial β-tubulin (tub2), translation elongation factor 1-alpha (tef1), and partial second largest subunit of the DNA-directed RNA polymerase II (rpb2), were amplified using primers ITS5/ITS4 [63], CAL-228F/CAL2Rd [64,65], Bt2a and Bt2b [66], EF1-728F/EF2 [65,67], and fRPB2-5f/fRPB2-7cR [68], respectively.
The total volume of the PCR reaction was 25 μL and consisted of 12.5 μL of 2× Power Taq PCR Master Mix (a premix and ready to use solution, including 0.1 units/μL Taq DNA Polymerase, 500 μM dNTP Mixture each (dATP, dCTP, dGTP, dTTP), 20 mM Tris-HCL pH 8.3, 100 mM KCl, 3 mM MgCl2, stabilizer, and enhancer), 1 μL of each primer (10pM), 2 μL of genomic DNA template, and 8.5 μL of sterilized double-distilled water (ddH2O). The reaction was conducted by running for 40 cycles. The annealing temperatures followed Lombard et al. [20] and Samarakoon et al. [14,59]. The amplified PCR fragments were sent to a commercial sequencing provider (TsingKe Biological Technology Co., Beijing, China). Nucleotide sequence data obtained were deposited in GenBank.
2.3. Sequence Alignment
Obtained sequence data were primarily checked with the Basic Local Alignment Search Tool (BLAST) in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 June 2020)). BLAST results and initial morphological studies revealed that our isolates belong to Stachybotryaceae. Other sequences used in the analyses were obtained from GenBank according to recently published papers [19,20,23] (Table 1) and BLAST search results. The single-gene alignments were made using MAFFT v. 7.036 [69] (http://mafft.cbrc.jp/alignment/server/large.html (accessed on 22 June 2020)) using the default settings and later refined where necessary using BioEdit v. 7.0.5.2 [70].
Table 1.
Selected taxa with their corresponding GenBank accession numbers of Stachybotryaceae used in the phylogenetic analyses. Type strains are superscripted with T and newly generated sequence data are indicated in black bold.
| Taxa | Culture Collection | cmdA | ITS | rpb2 | TUB2 | tef1 |
|---|---|---|---|---|---|---|
| Achroiostachys aurantispora | DAOMC 225565 T | KU845784 | KU845804 | KU845840 | NA | KU845859 |
| Ac. betulicola | CBS 136397 T | KU845772 | KU845792 | KU845831 | KU845753 | KU845848 |
| Ac. humicola | CBS 868.73 T | KU845779 | KU845799 | KU845837 | KU845760 | KU845854 |
| Ac. levigata | CBS 185.79 T | KU845785 | KU845805 | KU845841 | KU845765 | KU845860 |
| Alfaria caricicola | CBS 113567 T | KU845976 | KU845983 | KU846001 | KU846014 | KU846008 |
| Al. ossiformis | CBS 324.54 T | KU845977 | KU845984 | KU846002 | KU846015 | KU846009 |
| Al. terrestris | CBS 477.91 T | KU845979 | KU845988 | KU846006 | KU846019 | KU846011 |
| Al. thymi | CBS 447.83 T | KU845981 | KU845990 | NA | KU846021 | KU846013 |
| Brevistachys globosa | CBS 141056 T | KU846024 | KU846038 | NA | KU846101 | KU846085 |
| Br. lateralis | CBS 141058 T | KU846027 | KU846043 | KU846074 | KU846106 | KU846090 |
| Br. ossiformis | CBS 696.73T | NA | KU846044 | NA | KU846107 | NA |
| Br. subsimplex | ATCC 32888 T | NA | AF205439 | NA | NA | NA |
| Br. variabilis | CBS 141057 | KU846030 | KU846047 | KU846076 | KU846110 | KU846093 |
| Cymostachys coffeicola | CPC 25009 | NA | KU846053 | NA | NA | NA |
| Cy. coffeicola | CBS 252.76 T | KU846035 | KU846052 | KU846081 | KU846113 | KU846097 |
| Cy. fabispora | CBS 136180 T | KU846036 | KU846054 | KU846082 | KU846114 | KU846098 |
| Globobotrys sansevieriicola | CBS 138872 T | NA | KR476717 | NA | KR476794 | KR476793 |
| Grandibotrys pseudotheobromae | CBS 136391 | NA | KU846136 | KU846189 | KU846242 | KU846215 |
| Gr. pseudotheobromae | CBS 136170 T | NA | KU846135 | KU846188 | KU846241 | KU846216 |
| Gr. xylophilus | CBS 136179 T | KU846115 | KU846137 | KU846190 | NA | KU846217 |
| Melanopsamma pomiformis | CBS 101322 T | KU846032 | KU846049 | KU846078 | NA | NA |
| Me. xylophila | CBS 100343 T | KU846034 | KU846051 | KU846080 | NA | KU846096 |
| Memnoniella brunneoconidiophora | CBS 109477 | NA | KU846138 | KU846192 | KU846243 | KU846218 |
| M. brunneoconidiophora | CBS 136191 T | KU846116 | KU846139 | KU846193 | KU846244 | KU846219 |
| M. dichroa | CBS 526.50 | KU846117 | KU846140 | KU846194 | NA | KU846220 |
| M. dichroa | ATCC 18913 T | NA | AF081472 | NA | NA | NA |
| M. echinata | CBS 304.54 | KU846120 | KU846143 | KU846197 | NA | NA |
| M. echinata | CBS 343.50 | KU846121 | KU846144 | KU846198 | KU846246 | NA |
| M. echinata | CBS 216.32 T | KU846119 | KU846142 | KU846196 | KU846245 | KU846222 |
| M. ellipsoidea | CBS 136199 | KU846127 | KU846150 | KU846204 | KU846252 | KU846230 |
| M. ellipsoidea | CBS 136200 | KU846128 | KU846151 | KU846205 | KU846253 | KU846231 |
| M. ellipsoidea | CBS 136201 T | KU846129 | KU846152 | KU846206 | KU846254 | KU846232 |
| M. humicola | CBS 463.74 T | KU846130 | KU846154 | KU846208 | NA | KU846234 |
| M. levispora | Menlev3308 | NA | KF626495 | NA | NA | NA |
| M. levispora | Memno0407 | NA | KF626494 | NA | NA | NA |
| M. levispora | MFLUCC 20-0189 | NA | MW477993 | NA | MW480236 | NA |
| M. longistipitata | ATCC 22699 T | NA | AF081471 | NA | NA | NA |
| M. oenanthes | CBS 388.73 | NA | KU846156 | KU846210 | NA | NA |
| M. oenanthes | ATCC 22844 T | NA | AF081473 | NA | NA | KU846236 |
| M. pseudonilagirica | CBS 136405 T | KU846132 | KU846157 | KU846211 | KU846257 | NA |
| M. putrefolia | CBS 136171 | KU846133 | KU846159 | KU846213 | KU846259 | KU846238 |
| M. putrefolia | CBS 101177 T | NA | KU846158 | KU846212 | KU846258 | KU846239 |
| M. sinensis | YMF 1.05582 T | MK772065 | MK773576 | MK773575 | MK773574 | NA |
| Peethambara sundara | CBS 521.96 | NA | KU846470 | KU846508 | KU846550 | KU846530 |
| Pe. sundara | CBS 646.77 T | NA | KU846471 | KU846509 | KU846551 | KU846531 |
| Sirastachys castanedae | CBS 136403T | KU846555 | KU846660 | KU846887 | KU847096 | KU846992 |
| Si. phaeospora | CBS 100155 T | KU846560 | KU846666 | KU846891 | KU847102 | KU846995 |
| Si. phyllophila | CBS 136169 T | KU846566 | KU846672 | KU846897 | KU847108 | KU846999 |
| Stachybotrys aloicolus | CBS 137941 | KU846571 | KJ817889 | KU846902 | KJ817887 | NA |
| S. aloicolus | CBS 137940 T | KU846570 | KJ817888 | KU846901 | KJ817886 | NA |
| S. chartarum | CBS 129.13 | NA | KM231858 | KM232434 | KM232127 | KM231994 |
| S. chartarum | CBS 215.92 | NA | KU846680 | KU846905 | KU847116 | KU847003 |
| S. chartarum | CBS 363.49 | NA | KU846681 | KU846906 | KU847117 | KU847004 |
| S. chartarum | CBS 182.80 T | NA | KU846679 | KU846904 | KU847115 | KU847005 |
| S. chlorohalonatus | CBS 328.37 | KU846619 | KU846725 | KU846950 | KU847160 | KU847048 |
| S. chlorohalonatus | CBS 109283 | KU846622 | KU846728 | KU846953 | KU847163 | KU847049 |
| S. chlorohalonatus | CBS 251.89 | KU846618 | KU846724 | KU846949 | KU847159 | KU847052 |
| S. chlorohalonatus | CBS 109285 T | KU846623 | KU846729 | KU846954 | KU847164 | KU847053 |
| S. dolichophialis | DAOMC 227011 | KU846628 | KU846734 | KU846958 | KU847169 | NA |
| S. limonisporus | CBS 136165 | KU846630 | KU846736 | KU846960 | KU847171 | KU847058 |
| S. limonisporus | CBS 128809 T | KU846629 | KU846735 | KU846959 | KU847170 | KU847059 |
| S. microsporus | CBS 186.79 | KU846631 | KU846737 | DQ676580 | KU847172 | NA |
| S. microsporus | ATCC 18852 T | NA | AF081475 | NA | NA | NA |
| S. microsporus | MFLUCC 20-0190 | NA | MW477992 | NA | MW480235 | MW480237 |
| S. musae | MFLUCC 20-0152 | MW480231 | MW477991 | MW480229 | MW480233 | NA |
| S. musae | MFLUCC 20-0188T | MW480232 | MW477990 | MW480230 | MW480234 | NA |
| S. phaeophialis | KAS 525 T | KU846632 | KU846738 | KU846962 | KU847173 | NA |
| S. reniformis | ATCC 18839 | NA | AF081476 | NA | NA | NA |
| S. reniformis | CBS 136198 | NA | KU846740 | NA | NA | KU847063 |
| S. reniformis | CBS 976.95 | KU846633 | KU846739 | KU846963 | KU847174 | KU847064 |
| S. subsylvaticus | CBS 126205T | KU846634 | KU846741 | KU846964 | KU847175 | KU847076 |
Abbreviations of culture collections—ATCC: American Type Culture Collection, United States of America (USA); CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Working collection of Pedro Crous housed at CBS; DAOMC: Agriculture and Agri-Food Canada, Canadian Collection of Fungal Cultures, Canada; KAS: Collection of K.A. Seifert; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; NA: sequence data are not available in GenBank.
2.4. Phylogenetic Analyses
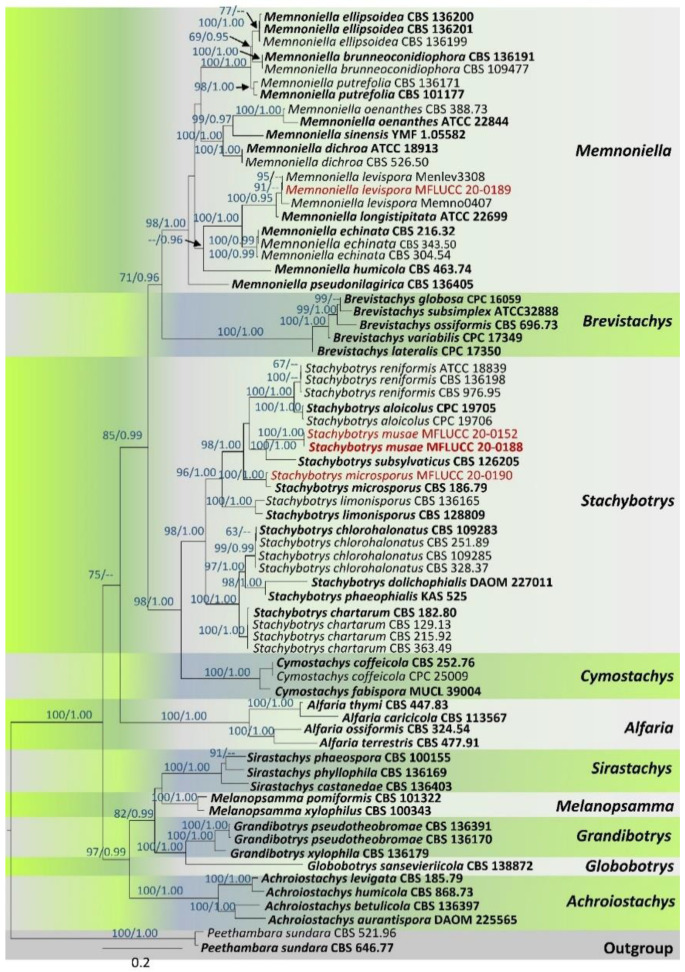
Maximum likelihood (ML) trees were generated using the RAxML-HPC2 on XSEDE (8.2.8) [71,72] in the CIPRES Science Gateway platform [73] using the GTR + I + G model of evolution. The latter model was selected independently for each locus of the dataset using MrModeltest v. 3.7 under the Akaike information criterion (AIC) [62]. Bootstrap supports were obtained by running 1000 pseudo-replicates. Maximum-likelihood bootstrap values equal to or greater than 60% are given above each node of the phylogenetic tree (Figure 1).
Figure 1.
Maximum likelihood tree revealed by RAxML analyses of internal transcribed spacer (ITS), cmdA, rpb2, tub2, and tef1 sequence dataset of selected genera in Stachybotryaceae showing the phylogenetic position of Stachybotrys musae (MFLUCC 20-0152, MFLUCC 20-0188), S. microsporus (MFLUCC 20-0190), and Memnoniella levispora (MFLUCC 20-0189). Maximum likelihood bootstrap supports (≥60%) and Bayesian posterior probabilities (≥0.95 BYPP) are given above the branches, respectively. The tree is rooted with Peethambara sundara (CBS 646.77 and CBS 521.96) (Stachybotry-aceae). Strains generated in this study are indicated in red. Ex-type strains are indicated in black bold. The scale bar represents the expected number of nucleotide substitutions per site.
A Bayesian analysis was conducted with MrBayes v. 3.1.2 [74] to evaluate posterior probabilities (PPs) [75,76] by Markov chain Monte Carlo sampling (MCMC). Two parallel runs were conducted using the default settings but with the following adjustments: four simultaneous Markov chains were run for 2,000,000 generations, trees were sampled every 100th generation, and 20,001 trees were obtained in total. The first 4000 trees, representing the burn-in phase of the analyses, were discarded to enter the high probability region, where the states of the Markov chain are more representative of the sampling distribution. The remaining 16,001 trees were used for calculating PPs in the majority rule consensus tree. Branches with Bayesian posterior probabilities (BYPPs) equal to or greater than 0.95 are indicated above each node of the phylogenetic tree (Figure 1). The tree was visualized with the FigTree v1.4.0 program [77] and reorganized in Microsoft PowerPoint (2013).
3. Results
3.1. Phylogenetic Analyses
The combined ITS, cmdA, rpb2, tub2, and tef1 matrix comprised 70 sequences that represent selected genera in Stachybotryaceae. The best scoring RAxML tree is presented (Figure 1) with a final ML optimization likelihood value of −38,213.091. The matrix had 1833 distinct alignment patterns with 35.79% undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.229585, C = 0.291579, G = 0.254548, T = 0.224288; substitution rates were as follows: AC = 1.228527, AG = 3.573013, AT = 1.331197, CG = 0.93385, CT = 5.411134, GT = 1.0; the proportion of invariable sites was I = 0.400993; the gamma distribution shape parameter was α = 1.130129. All trees (ML and BYPP) obtained from the combined ITS, cmdA, rpb2, tub2, and tef1 dataset were equal in topology and did not show any notable deviation from Lin et al. [23] and Lombard et al. [20]. Isolates of the new species, Stachybotrys musae (MFLUCC 20-0152 and MFLUCC 20-0188), clustered sister to S. subsylvaticus (CBS 12620) as a monophyletic lineage with a strong statistical support (ML = 100%, BYPP = 1.00). The new strain MFLUCC 20-0190 constituted a strongly supported monophyletic clade with S. microsporus (CBS 186.79) (ML = 100%, BYPP = 1.00). In addition, the new strain MFLUCC 20-0189 grouped with Memnoniella levispora (Menlev3308 and Memno0407) (ML = 91%, BYPP = 0.94) with moderate statistical support.
3.2. Taxonomy
3.2.1. Stachybotrys musae Samarakoon & Chomnunti, sp. nov.
MycoBank No.—MB 838529; FoF Number—FoF 09574.
Etymology—Name reflects the host genus Musa, from which the novel taxon was originally isolated.
Holotype—MFLU 20-0626.
Saprobic on dead leaves of Musa sp. Sexual morph: undetermined. Asexual morph: colonies on the substrate surface: effuse, usually black or blackish green. Mycelium: superficial, with light brown, septate, 5.6–7.4 μm (= 6.5 μm, n = 30) wide hyphae, sometimes forming ropes. Stroma: none. Setae and hyphopodia: absent. Conidiophores: 45–94 × 2.6–3.9 μm (= 71.4 × 3.2 μm, n = 30) macronematous, mononematous, usually unbranched, and rarely branched, often with a distinct sub-hyaline shoe-shaped base 7–9 × 3–5.7 μm (= 8.4 × 5.2 μm, n = 20). Conidiophores: usually straight or flexuous, often curved near the base, straight toward the tip, multi-septate, often with 1–7 septa, sometimes more than seven septa, hyaline or sub-hyaline at the base, pale olivaceous brown toward apex, smooth or slightly verrucose at maturity, sometimes sub-hyaline, granulate on the surface, terminating with a crown of phialides at the apex. Conidiogenous cells: monophialidic, 10–13 × 3–5 μm (= 11.8 × 4.4 μm, n = 20), discrete, in groups of 4–6 at the apex of each conidiophore, broadly fusiform, with a minute collarette at the tip. Conidia: simple, unicellular, smooth, aggregated in large, slimy, often black and glistening heads. Immature conidia: hyaline, acute at one end, rounded at the other end, spherical. Mature conidia: 5–7.5 × 4–7 μm (= 7.1 × 5.6 μm, n = 40), ellipsoidal, acute or rounded at both ends, dark brown, blackish brown or black, smooth or verrucose, sometimes covered with dark granules.
Culture characteristics—Conidia germinated on potato dextrose agar (PDA) after 48 h; germ tubes produced from germ pores. Colonies grew on PDA reaching 2 cm diameter after 3 weeks in light conditions at 25 °C, mostly immersed mycelium, slimy and minutely dense, middle of the colony orange and pinkish orange at the periphery. Radially or unevenly striated; colonies have a wrinkled appearance from the top. Conidial formation was observed only in mature cultures rarely and minutely.
Material examined—China, Yunnan Province, Xishuangbanna, on a dead leaf of Musa sp., 19 December 2018, D.N. Wanasinghe, BNSWN8 (MFLU 20-0626, holotype), living cultures MFLUCC 20-0188 (ex-type strain) and MFLUCC 20-0152.
Notes—Based on BLASTn searches of ITS, cmdA, rpb2, and tub2 sequence data, Stachybotrys musae (Figure 2) showed a high similarity (cmdA = 84.34%, ITS = 94.29%, tub2 = 89.13%, and rpb2 = 90.07%) to S. subsylvaticus (CBS 126205). In the multigene phylogeny, S. musae clustered sister to S. subsylvaticus with ML = 100%, BYPP = 1.00 statistical support (Figure 1). Moreover, ITS sequence comparison revealed 4.94% base pair differences (without gaps) between S. musae and S. subsylvaticus. Stachybotrys musae (Figure 2) differs from S. subsylvaticus in having notably curved hyaline to olivaceous brown conidiophores, while those of S. subsylvaticus are straight to slightly flexuous and mostly hyaline to sub-hyaline [20]. The conidiophores of S. subsylvaticus are usually 1–4-septate, whereas S. musae has 1–7-septate or even more than 7-septate conidiophores. In addition, S. musae has distinct sub-hyaline shoe-shaped conidiophore bases that are absent in S. subsylvaticus. The apex of the phialidic conidiogenous cells of S. subsylvaticus is sub-hyaline to pale olivaceous brown, while S. musae has completely hyaline phialides. When considering the culture characteristics, the colonies on PDA of S. subsylvaticus are buff to pale luteous, whereas S. musae produces characteristic pinkish orange colonies on PDA. In our multigene analysis, S. musae has a close phylogenetic affinity to S. aloicolus and S. reniformis. However, S. aloicolus has allantoid to fusiform conidia containing 1–2 oil droplets [78]. Stachybotrys reniformis bears tuberculate and often globose conidia [19]. These specific features are absent in S. musae. Based on distinct morphological characteristics and significant statistical support from our molecular phylogenetic studies, S. musae is introduced herein as a new species on Musa from Xishuangbanna, Yunnan Province, China.
Figure 2.
Stachybotrys musae (MFLU 20–0626, holotype). (a) Conidiophores on the substrate surface; (b–d,g,h,j,m) conidiophores with attached conidia; (e,f) conidiophores; (i) conidiophore with monophialidic conidiogenous cells; (k) mycelium; (l) mass of conidia and conidiophores; (n) conidia; (o) colonies on PDA after 8 weeks. Scale bars: (a) = 500 μm; (j) = 200 μm; (c–i,l,m) = 50 μm; (b,f,g) = 25 μm; (n,k) = 5 μm.
3.2.2. Stachybotrys microsporus (B.L. Mathur & Sankhla) S.C. Jong & E.E. Davis
Saprobic on dead leaf petiole of Musa sp. Sexual morph: undetermined. Asexual morph: hyphomycetous. Colonies on the substrate surface are black and hairy. Conidiophores: macronematous, mononematous, often simple, erect, straight or mostly flexuous, irregularly or sympodially branched, 20–50 × 1.3–3.1 μm (= 32.4 × 2 μm, n = 20) at the base, tapering to 0.6–1.4 μm wide (= 0.94 μm, n = 20) near the apex, smooth, thick-walled, septate, hyaline at base, olivaceous brown at apex, bearing a crown of phialides at the tip. Conidiogenous cells: 3.7–7.1 × 2.5–3.1 μm (= 5.3 × 2.8 μm, n = 20), monophialidic, discrete, determinate, terminal, obovoid, with peripheral ones somewhat curved, smooth, sub-hyaline. Conidia: 7.7–14.2 × 5.1–9.8 μm (= 9.3 × 7.5 μm, n = 40) unicellular, simple, often aggregated as large glistening heads in black, when young elliptical, rounded at both ends, becoming globose, and often having pointed ends at maturity, roughened at surface, dark brown to black.
Culture characteristics—Conidia germinated on PDA after 36 to 48 h. Colonies grew on PDA reaching 2–2.5 cm diameter after 3 weeks in light conditions at 25 °C; slow-growing, flat, sparse, mycelium is completely immersed, pink, radially striated or wrinkled. Sporulation was not observed in cultures.
Material examined—Thailand, Chiang Rai Province, Mae Sai District, on dead leaf petiole of Musa sp., 20 April 2019, B. C. Samarakoon, BNS 30 (MFLU 20-0628), living culture MFLUCC 20-0190.
Substrates and known distribution—Soil (China and India), on Arachis hypogaea (Nigeria), decaying wood and sub shrubs (karst areas in Thailand), Solanum lycopersicum (Canada) [19,20,23,79].
Notes—Stachybotrys microsporus (strain MFLUCC 20-0190) grouped with S. microsporus (strain CBS 186.79) with strong statistical support (Figure 1). All strains of S. microsporus described in Wang et al. [19] and Lin et al. [23] have a similar morphology (i.e., hyaline, sympodially or irregularly branched conidiophores with tapering apices) with our collection (MFLU 20-0628) (Figure 3). On the basis of DNA sequence data of a Brazil collection, Santos [80] reported that S. globosus is conspecific with S. microsporus. However, S. globosus was described from India, and neither an ex-type strain nor an epitype strain exists for this species. It is recommended to obtain DNA from the holotype or the ex-type of S. globosus to validate the conspecificity with S. microsporus. Previously, S. globosus was documented on Musa from India without molecular justifications [56]. Hence, in this study, we report S. microsporus on Musa from Thailand with morphological evidences and DNA sequence data.
Figure 3.
Stachybotrys microsporus (MFLU 20–0628). (a,b) Conidiophores on the substrate surface; (c,d,f) conidiophores; (e,g–i,k) conidiogenous cells with attached conidia; (j,l–o) conidia. Scale bars: (a,b) = 500 μm; (f) = 30 μm; (i) = 50 μm; (e,g,h) = 20 μm; (c,d,j–m) = 15 μm; (n,o) = 10 μm.
3.2.3. Memnoniella levispora Subram
Saprobic on dead leaf petiole of Musa sp. Sexual morph: undetermined. Asexual morph: colonies on the substrate surface, gregarious, scattered, superficial, black, powdery and bouquet-like. Conidiophores: 43.6–60 × 2.5–4.7 μm (= 48.7 × 3.7 μm, n = 20) at the base, 5–7 μm wide at swollen apex, straight or flexuous, macronematous, unbranched, bearing a crown of phialides at the apex, minutely verrucose at base, often covered in part with dark granules to black olivaceous at lower half, thick-walled, 1–3-septate. Conidiogenous cells: 4–6.9 × 2.3–3.1 μm ( = 5.8 × 2.6 μm, n = 20) phialidic, sub-hyaline, short and narrow at apex, clavate, ampulliform, cylindrical or broadly fusiform, without collarettes. Conidial heads: arising from conidiogenous cells, convex, round at apex and flat at base, black. Conidia: 2.5–4.3 × 1.5–3.6 μm ( = 3.5 × 2.2 μm, n= 20), in unbranched chains, simple, spherical to subspherical, often flattened in a plane or hemispherical, gray, dark brown to black and smooth.
Culture characteristics—Conidia germinated on PDA after 24 h. Germ tubes were produced from germ pores. Colonies grew on PDA reaching 16–21 mm diameter after 3 weeks in light conditions at 25 °C, slow-growing, crenated, flat or effuse, moderately fluffy, medium sparse, aerial, white from above, pale yellowish from below.
Material examined—China, Yunnan Province, Xishuangbanna, on dead leaf of Musa sp., 18 December 2018, D.N. Wanasinghe, BNSWN6 (MFLU 20-0627), living culture MFLUCC 20-0189.
Substrates and known distribution—on Morus (India), Oryza sativa (Cuba), Roystonea regia (Cuba), Sanchezia (India, Pakistan), Tectona grandis (Thailand) [19,22,28,81,82].
Notes— Our strain, MFLUCC 20-0189, grouped with strains identified as Memnoniella levispora (Menlev3308 and Memno0407) in GenBank with moderate statistical support (ML = 91%, BYPP = 0.94) (Figure 1). The morphological descriptions of M. levispora given in Wang et al. [19] and Doilom et al. [22] share similar features such as the bouquet-like fungal colonies and catenate, numerous conidia, with our strain (Figure 4). Memnoniella levispora was documented on Musa sp. from India by Munjal and Kapoor [83] using only morphological data. We report M. levispora as a saprobe on Musa sp. for the first time from Yunnan, China as a new geographical record based on morpho-molecular data. We observed that molecular data available in GenBank represent neither an ex-type strain nor an epitype strain of M. levispora. Hence, we highly recommend re-examining the Indian holotype to see the possibility of sequencing or epitypify the species with a new collection.
Figure 4.
Memnoniella levispora (MFLU 20–0627). (a) Conidiophores on the substrate surface; (b–g) conidiophores and conidia; (h,l) conidiogenous cells and conidia; (i–k,m,n) conidia. Scale bars: (a) = 500 μm; (b,c,e,f) = 50 μm; (d,g–l) = 20 μm; (i–k,m,n) = 10 μm.
4. Discussion
Taxonomic evidence for the new species is further strengthened by a comparison of Stachybotrys taxa previously described from Musa based only on morphology. Stachybotrys suthepensis was described from a dead petiole of Musa acuminata by Photita et al. [11]. However, S. suthepensis differs from S. musae in having significantly verruculose, ellipsoid to cylindrical conidia which are rounded at the ends. Conidia of our new collection are not verruculose and ellipsoidal in shape with acute ends. In addition, the conidiophores of S. musae are notably curved compared to those formed by S. suthepensis. Molecular data of S. suthepensis are not available in GenBank for a comparison with our strain.
Stachybotrys chartarum, S. kampalensis, S. nephrosporus, and S. theobromae are distinct from the new species according to morpho-molecular data. Stachybotrys ruwenzoriensis, for which no DNA sequence data are available in Genbank, differs in having obovoid phialides and notably verrucose, globose to subglobose conidia. Stachybotrys yunnanensis was recorded from the same geographical region (Yunnan, Yunnan Province, China) as S. musae but differs in both morphology and phylogeny.
Stachybotrys bambusicola differs from the new species in having pink conidia [84]. In S. longisporus [20], the distinct conidiophore base is globular shaped, whereas, in S. musae, it is shoe-shaped. The conidiogenous cells of S. longispora do not have collarettes compared with those of S. musae. The conidiophore base of S. nephrodes [85] is similar to S. musae, but the conidial shape is different from our new species in being reniform. Stachybotrys reniverrucosa [35] also has notably curved conidiophores like S. musae, but both species can be easily differentiated by the conidial shape.
Many Stachybotrys taxa lack ex-type strains, and holotypes are often difficult to locate. Sequence data for several species are lacking in GenBank. Some species were established, described, and identified solely using ITS sequence data. However, constructing phylogenies only based on ITS data will not result in good tree topologies in Stachybotrys. Multiple sequence alignments combined with protein-coding regions result in well-resolved phylogenies with well-separated clades for Memnoniella and Stachybotrys (Figure 1). We noted the lack of other protein-coding gene regions (i.e., cmdA, rpb2, tub2, and tef1) in GenBank for many extant species of Stachybotrys. Differentiating Memnoniella and Stachybotrys has been problematic for over 50 years, and it was finally resolved by Lombard et al. [20]. Several genera in Stachybotryaceae are similar in morphology but have different molecular data [20]. Therefore, further taxa of Stachybotryaceae should be collected and isolated, and new sequence data should be generated for a better taxonomic resolution.
Acknowledgments
Samantha C. Karunarathna thanks CAS President’s International Fellowship Initiative (PIFI) under grant 2020FYC0002 for funding his postdoctoral research and the National Science Foundation of China (NSFC, project code 31851110759) for partially funding this work. Rungtiwa Phookamsak thanks CAS President’s International Fellowship Initiative (PIFI) for young staff (grant No. Y9215811Q1), the National Science Foundation of China (NSFC) project code 31850410489 (grant No. Y81I982211), and Chiang Mai University for their partial support of this research work. Dhanushka N. Wanasinghe thanks CAS President’s International Fellowship Initiative (PIFI) for funding his postdoctoral research (number 2021FYB0005), the Postdoctoral Fund from Human Resources and Social Security Bureau of Yunnan Province, and the National Science Foundation of China (grant No. 41761144055) for financial support. The authors thank the Thailand research grants entitled “The future of specialist fungi in a changing climate: baseline data for generalist and specialist fungi associated with ants, Rhododendron species, and Dracaena species (grant No. DBG6080013) and “Impact of climate change on fungal diversity and biogeography in the Greater Mekong Sub region (grant No. RDG6130001). Binu C. Samarakoon offers her sincere gratitude to G.C. Ren, Janith Vishvakeerthi, Asanka Bandara, Sajini Chandrasiri, Digvijayini Bhundun, and Erandi Weeragalle for the valuable support they have given. Chiang Mai University is thanked for partially supporting this research work.
Author Contributions
Conceptualization, B.C.S., D.N.W., S.C.K. and J.B.; data curation, B.C.S., R.P. and S.C.K.; formal analysis, B.C.S. and D.N.W.; funding acquisition, P.C., S.C.K. and R.P.; investigation, B.C.S. and D.N.W.; methodology, B.C.S., D.N.W. and P.C.; project administration, S.C.K., P.C. and D.N.W.; supervision, P.C., S.C.K. and S.L.; writing—original draft, B.C.S., D.N.W., P.C., R.P. and S.C.K.; writing—review and editing, J.B. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Chiang Mai University, the National Science Foundation of China projects 31851110759, 31850410489, and 41761144055, “Impact of climate change on fungal diversity and biogeography in the Greater Mekong Sub-region RDG6130001”, and “The future of specialist fungi in a changing climate: baseline data for generalist and specialist fungi associated with ants, Rhododendron species, and Dracaena species, Grant No: DBG6080013.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences generated in this study are deposited in GenBank (Table 1). The finalized alignment and tree were submitted to TreeBASE (submission ID: 27607, http://www.treebase.org/ (accessed on 18 January 2021)). Morphological data are available at FigShare (https://doi.org/10.6084/m9.figshare.13602710, https://doi.org/10.6084/m9.figshare.13602719.v1, and https://doi.org/10.6084/m9.figshare.13602767 (accessed on 20 January 2021)). Specimens were deposited in the Mae Fah Luang University (MFLU) Herbarium, Chiang Rai, Thailand. Living cultures and DNA sequence data with chromatograms were deposited in the Culture Collection of Mae Fah Luang University (MFLUCC) Chiang Rai, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhilabutra W., McKenzie E.H.C., Hyde K.D., Lumyong S. Fungi on the grasses, Thysanolaena latifolia and Saccharum spontaneum, in Northern Thailand. Mycosphere. 2010;1:301–314. [Google Scholar]
- 2.Hyde K.D., Fröhlich J., Taylor J.E. Fungi from palms. XXXVI. Reflections on unitunicate ascomycetes with apiospores. Sydowia. 1998;50:21–80. [Google Scholar]
- 3.Konta S., Maharachchikumbura S.S.N., Senanayake I.C., McKenzie E.H.C., Stadler M., Boonmee S., Phookamsak R., Jayawardena R.S., Senwanna C., Hyde K.D., et al. A new genus Allodiatrype, five new species and a new host record of diatrypaceous fungi from palms (Arecaceae) Mycosphere. 2020;11:239–268. doi: 10.5943/mycosphere/11/1/4. [DOI] [Google Scholar]
- 4.Thambugala K.M., Wanasinghe D.N., Phillips A.J.L., Camporesi E., Bulgakov T.S., Phukhamsakda C., Ariyawansa H.A., Goonasekara I.D., Phookamsak R., Dissanayake A., et al. Mycosphere notes 1-50: Grass (Poaceae) inhabiting Dothideomycetes. Mycosphere. 2017;8:697–796. doi: 10.5943/mycosphere/8/4/13. [DOI] [Google Scholar]
- 5.Thongkantha S., Lumyong S., McKenzie E.H.C., Hyde K.D. Fungal saprobes and pathogens occurring on tissues of Dracaena lourieri and Pandanus spp. in Thailand. Fungal. Divers. 2008;30:149–169. [Google Scholar]
- 6.Tibpromma S., Hyde K.D., McKenzie E.H.C., Bhat D.J., Phillips A.J.L., Wanasinghe D.N., Samarakoon M.C., Jayawardena R.S., Dissanayake A.J., Tennakoon D.S., et al. Fungal diversity notes 840–928: Micro-fungi associated with Pandanaceae. Fungal. Divers. 2018;93:1–160. doi: 10.1007/s13225-018-0408-6. [DOI] [Google Scholar]
- 7.Wong M.K.M., Hyde K.D. Diversity of fungi on six species of Gramineae and one species of Cyperaceae in Hong Kong. Mycol. Res. 2001;105:1485–1491. doi: 10.1017/S0953756201004695. [DOI] [Google Scholar]
- 8.Phookamsak R., Norphanphoun C., Tanaka K., Dai D.Q., Luo Z.L., Liu J.K., Su H.Y., Bhat D.J., Bahkali A.H., Mortimer P.E., et al. Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov. and Astrosphaeriellopsis, gen. nov. Fungal. Divers. 2015;74:143–197. doi: 10.1007/s13225-015-0352-7. [DOI] [Google Scholar]
- 9.Liu J.K., Phookamsak R., Dai D.Q., Tanaka K., Jones E.B.G., Xu J.C., Chukeatirote E., Hyde K.D. Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa. 2014;181:1–33. doi: 10.11646/phytotaxa.181.1.1. [DOI] [Google Scholar]
- 10.Dai D.Q., Phookamsak R., Wijayawardene N.N., Li W.J., Bhat D.J., Xu J.C., Taylor J.E., Hyde K.D., Chukeatirote E. Bambusicolous fungi. Fungal. Divers. 2017;82:1–105. doi: 10.1007/s13225-016-0367-8. [DOI] [Google Scholar]
- 11.Photita W., Lumyong P., McKenzie E.H.C., Hyde K.D., Lumyong S. Memnoniella and Stachybotrys species from Musa acuminata. Cryptogam. Mycol. 2003;24:147–152. [Google Scholar]
- 12.Photita W., Lumyong P., McKenzie E.H.C., Hyde K.D., Lumyong S. Saprobic fungi on dead wild banana. Mycotaxon. 2003;85:345–356. [Google Scholar]
- 13.Photita W., Lumyong S., Lumyong P., Ho W.H., McKenzie E.H.C., Hyde K.D. Fungi on Musa acuminata in Hong Kong. Fungal. Divers. 2001;6:99–106. [Google Scholar]
- 14.Samarakoon B.C., Phookamsak R., Wanasinghe D.N., Chomnunti P., Hyde K.D., McKenzie E.H.C., Promputtha I., Xu J.C., Li Y.J. Taxonomy and phylogenetic appraisal of Spegazzinia musae sp. nov. and S. deightonii (Didymosphaeriaceae, Pleosporales) on Musaceae from Thailand. Mycokeys. 2020;70:19–37. doi: 10.3897/mycokeys.70.52043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samarakoon B.C., Wanasinghe D.N., Samarakoon M.C., Phookamsak R., McKenzie E.H.C., Chomnunti P., Hyde K.D., Lumyong S., Karunarathna S.C. Multi-gene phylogenetic evidence suggests Dictyoarthrinium belongs in Didymosphaeriaceae (Pleosporales, Dothideomycetes) and Dictyoarthrinium musae sp. nov. on Musa from Thailand. Mycokeys. 2020;71:101–118. doi: 10.3897/mycokeys.71.55493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somrithipol S. A synnematous species of Dictyoarthrinium from Thailand. Mycologia. 2007;99:792–796. doi: 10.1080/15572536.2007.11832542. [DOI] [PubMed] [Google Scholar]
- 17.Hyde K.D., Norphanphoun C., Maharachchikumbura S.S.N., Bhat D.J., Jones E.B.G., Bundhun D., Chen Y.J., Bao D.F., Boonmee S., Calabon M.S., et al. Refined families of Sordariomycetes. Mycosphere. 2020;11:305–1059. doi: 10.5943/mycosphere/11/1/7. [DOI] [Google Scholar]
- 18.Wijayawardene N.N., Hyde K.D., Al-Ani L.K.T., Tedersoo L., Haelewaters D., Rajeshkumar K.C., Zhao R.L., Aptroot A., Leontyev D.V., Saxena R.K., et al. Outline of fungi and fungus-like taxa. Mycosphere. 2020;11:1060–1456. doi: 10.5943/mycosphere/11/1/8. [DOI] [Google Scholar]
- 19.Wang Y., Hyde K.D., McKenzie E.H.C., Jiang Y.L., Li D.W., Zhao D.G. Overview of Stachybotrys (Memnoniella) and current species status. Fungal. Divers. 2015;71:17–83. doi: 10.1007/s13225-014-0319-0. [DOI] [Google Scholar]
- 20.Lombard L., Houbraken J., Decock C., Samson R.A., Meijer M., Reblova M., Groenewald J.Z., Crous P.W. Generic hyper-diversity in Stachybotriaceae. Persoonia. 2016;36:156–246. doi: 10.3767/003158516X691582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith G. Some new and interesting species of micro-fungi. Trans. Brit. Mycol. Soc. 1962;45:387–394. doi: 10.1016/S0007-1536(62)80077-1. [DOI] [Google Scholar]
- 22.Doilom M., Dissanayake A.J., Wanasinghe D.N., Boonmee S., Liu J.K., Bhat D.J., Taylor J.E., Bahkali A.H., McKenzie E.H.C., Hyde K.D. Microfungi on Tectona grandis (Teak) in Northern Thailand. Fungal. Divers. 2017;82:107–182. doi: 10.1007/s13225-016-0368-7. [DOI] [Google Scholar]
- 23.Lin C.G., McKenzie E.H.C., Bhat D.J., Ran S.F., Chen Y., Hyde K.D., Li D.W., Wang Y. Stachybotrys-like taxa from karst areas and a checklist of Stachybotrys-like species from Thailand. Mycosphere. 2016;7:1273–1291. doi: 10.5943/mycosphere/7/9/3. [DOI] [Google Scholar]
- 24.Mapook A., Hyde K.D., McKenzie E.H.C., Jones E.B.G., Bhat D.J., Jeewon R., Stadler M., Samarakoon M.C., Malaithong M., Tanunchai B., et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (siam weed) Fungal. Divers. 2020;101:1–175. doi: 10.1007/s13225-020-00444-8. [DOI] [Google Scholar]
- 25.Index Fungorum. [(accessed on 15 February 2021)]; Available online: http://www.indexfungorum.org.
- 26.Zheng H., Zhang Z., Liu D.Z., Yu Z.F. Memnoniella sinensis sp. nov., a new species from China and a key to species of the genus. Int. J. Syst. Evol. Microbiol. 2019;69:3155–3163. doi: 10.1099/ijsem.0.003605. [DOI] [PubMed] [Google Scholar]
- 27.Species Fungorum. [(accessed on 10 December 2020)]; Available online: http://www.speciesfungorum.org/Names/Names.asp.
- 28.Ellis M.B. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew; London, UK: 1971. [Google Scholar]
- 29.Seifert K.A., Morgan-Jones G., Gams W., Kendrick B. The Genera of Hyphomycetes. Volume 9. CBS Biodiversity Series; Utrecht, The Netherlands: 2011. p. 997. [Google Scholar]
- 30.Gond A.K., Ramanuj P., Sanjay P., Jamaluddin, Pandy A.K. New record of Memnoniella levispora subram on Ficus carica L. from India. J. New Biol. Rep. 2013;2:272–274. [Google Scholar]
- 31.Ellis M.B. More Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew; Surrey, UK: 1976. [Google Scholar]
- 32.Izabel T.D.S.S., Cruz A.C.R.D., Barbosa F.R., Ferreira S.M.L., Marques M.F.O., Gusmão L.F.P. The genus Stachybotrys (anamorphic fungi) in the semi-arid region of Brazil. Rev. Bras. Bot. 2010;33:479–487. doi: 10.1590/S0100-84042010000300010. [DOI] [Google Scholar]
- 33.Jie C.Y., Geng K., Jiang Y.L., Xu J.J., Hyde K.D., McKenzie E.H.C., Zhang T.Y., Bahkali A.H., Li D.W., Wang Y. Stachybotrys from soil in China, identified by morphology and molecular phylogeny. Mycol. Prog. 2013;12:693–698. doi: 10.1007/s11557-012-0878-y. [DOI] [Google Scholar]
- 34.Tang A.M.C., Hyde K.D., Corlett R.T. Diversity of fungi on wild fruits in Hong Kong. Fungal. Divers. 2003;14:165–185. [Google Scholar]
- 35.Whitton S.R., McKenzie E.H.C., Hyde K.D. Microfungi on the Pandanaceae: Stachybotrys, with three new species. N. Z. J. Bot. 2001;39:489–499. doi: 10.1080/0028825X.2001.9512752. [DOI] [Google Scholar]
- 36.Li Q.R., Jiang Y.L. Stachybotrys subreniformis, new from soil in China. Mycotaxon. 2011;115:171–173. doi: 10.5248/115.171. [DOI] [Google Scholar]
- 37.Pathak P.D., Chauhan R.K.S. Leaf spot disease of Crotolaria juncea L. caused by Stachybotrys atra Corda. Curr. Sci. 1976;45:567. [Google Scholar]
- 38.Zhao G.H., Li D.W., Jiang J.H., Peng J. First report of Stachybotrys chartarum causing leaf blight of Tillandsia tenuifolia in China. Plant Dis. 2010;94:1166. doi: 10.1094/PDIS-94-9-1166B. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H., Yang M.H., Zhuo F.F., Gao N., Cheng X.B., Wang X.B., Pei Y.H., Kong L.Y. Seven new cytotoxic phenylspirodrimane derivatives from the endophytic fungus Stachybotrys chartarum. RSCAdv. 2019;9:3520–3531. doi: 10.1039/C8RA10195G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busby P.E., Ridout M., Newcombe G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016;90:645–655. doi: 10.1007/s11103-015-0412-0. [DOI] [PubMed] [Google Scholar]
- 41.Cao R., Liu X., Gao K., Mendgen K., Kang Z., Gao J., Dai Y., Wang X. Mycoparasitism of endophytic fungi isolated from reed on soilborne phytopathogenic fungi and production of cell wall-degrading enzymes in vitro. Curr. Microbiol. 2009;59:584–592. doi: 10.1007/s00284-009-9477-9. [DOI] [PubMed] [Google Scholar]
- 42.Raghavendra A.K.H., Newcombe G. The contribution of foliar endophytes to quantitative resistance to Melampsora rust. New Phytol. 2013;197:909–918. doi: 10.1111/nph.12066. [DOI] [PubMed] [Google Scholar]
- 43.Yang X., Jin H., Xu L., Cui H., Xin A., Liu H., Qin B. Diversity and functions of endophytic fungi associated with roots and leaves of Stipa purpurea in an Alpine Steppe at Tibet plateau. J. Microbiol. Biotechnol. 2020;30:1027–1036. doi: 10.4014/jmb.2002.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason C.D., Rand T.G., Oulton M., MacDonald J.M., Scott J.E. Effects of Stachybotrys chartarum (atra) conidia and isolated toxin on lung surfactant production and homeostasis. Nat. Toxins. 1998;6:27–33. doi: 10.1002/(SICI)1522-7189(199802)6:1<27::AID-NT6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 45.Hossain M.A., Ahmed M.S., Ghannoum M.A. Attributes of Stachybotrys chartarum and its association with human disease. J. Allergy Clin. Immunol. 2004;113:200–208. doi: 10.1016/j.jaci.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Lichtenstein J.H.R., Molina R.M., Donaghey T.C., Amuzie C.J., Pestka J.J., Coull B.A., Brain J.D. Pulmonary responses to Stachybotrys chartarum and its toxins: Mouse strain affects clearance and macrophage cytotoxicity. Toxicol. Sci. 2010;116:113–121. doi: 10.1093/toxsci/kfq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyde K.D., Xu J.C., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S., et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal. Divers. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- 48.Jarvis B.B., Salemme J., Morals A. Stachybotrys toxins. 1. Nat. Toxins. 1995;3:10–16. doi: 10.1002/nt.2620030104. [DOI] [PubMed] [Google Scholar]
- 49.Pan H., Kong J., Xu J., Zhang Y., Hang T. Four new species of Stachybotrys and a key to species of the genus known from soil in China. Mycosystema. 2014;33:785–792. [Google Scholar]
- 50.Wu Y.M., Zhang T.Y. Two new species of Stachybotrys from soil. Mycotaxon. 2009;109:461–464. doi: 10.5248/109.461. [DOI] [Google Scholar]
- 51.Wang H., Kong J., Wu Y., Zhang T. Notes on soil dematiaceous hyphomycetes from the Qaidam basin, Qinghai province, China I. Mycosystema. 2009;28:20–24. [Google Scholar]
- 52.Kong H.Z. Stachybotrys yunnanensis sp. nov. And Neosartorya delicata sp. nov. isolated from Yunnan, China. Mycotaxon. 1997;62:427–433. [Google Scholar]
- 53.Liu H., Zhang T. A preliminary report of soil dematiaceous hyphomycetes from the Yellow river delta I. Mycosystema. 2004;23:338–344. [Google Scholar]
- 54.Farr D.F., Rossman A.Y. Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. [(accessed on 15 December 2020)]; Available online: http://nt.ars-grin.gov/fungaldatabases/
- 55.Castellani E., Ciferri R. Prodromus Mycoflorae Africae Orientalis Italicae. Istituto Agricolo Coloniale Italiano; Florence, Italy: 1937. pp. 121–161. [Google Scholar]
- 56.Misra P.C., Srivastava S.K. Two undescribed Stachybotrys species from India. Trans. Brit. Mycol. Soc. 1982;78:556–559. doi: 10.1016/S0007-1536(82)80170-8. [DOI] [Google Scholar]
- 57.Matsushima T. Icones Microfungorum: A Matsushima Lectorum. Nippon printing Publishing Co.; Osaka, Japan: 1975. [Google Scholar]
- 58.McGuire J.U., Crandall B.S. Survey of Insect Pests and Plant Diseases of Selected Food Crops of Mexico, Central America and Panama. US Department of Agriculture; Washinghton, DC, USA: 1967. [Google Scholar]
- 59.Samarakoon S.M.B.C., Samarakoon M.C., Aluthmuhandiram J.V.S. The first report of Daldinia eschscholtzii as an endophyte from leaves of Musa sp.(musaceae) in Thailand. Asian J. Mycol. 2019;2:183–197. doi: 10.5943/ajom/2/1/9. [DOI] [Google Scholar]
- 60.Senanayake I.C., Rathnayaka A.R., Marasinghe D.S., Calabon M.S., Gentekaki E., Lee H.B., Hurdeal V.G., Pem D., Dissanayake L.S., Wijesinghe S.N., et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere. 2020;11:2678–2754. doi: 10.5943/mycosphere/11/1/20. [DOI] [Google Scholar]
- 61.Jayasiri S.C., Hyde K.D., Ariyawansa H.A., Bhat J., Buyck B., Cai L., Dai Y.C., Abd-Elsalam K.A., Ertz D., Hidayat I., et al. The faces of fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal. Divers. 2015;74:3–18. doi: 10.1007/s13225-015-0351-8. [DOI] [Google Scholar]
- 62.Dissanayake A.J., Bhunjun C.S., Maharachchikumbura S.S.M., Liu J.K. Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere. 2020;11:2652–2676. doi: 10.5943/mycosphere/11/1/18. [DOI] [Google Scholar]
- 63.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Volume 18. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 64.Groenewald J.Z., Nakashima C., Nishikawa J., Shin H.D., Park J.H., Jama A.N., Groenewald M., Braun U., Crous P.W. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 66.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/AEM.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Donnell K., Kistler H.C., Cigelnik E., Ploetz R.C. Multiple evolutionary origins of the fungus causing panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y.J.J., Whelen S., Benjamin D.H. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 69.Katoh K., Rozewicki J., Yamada K.D. Mafft online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 71.Stamatakis A. Raxml version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 73.Miller M.A., Pfeiffer W., Schwartz T. Creating the cipres science gateway for inference of large phylogenetic trees; Proceedings of the 2010 Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; New Orleans, LA, USA: IEEE; 2010. pp. 1–8. [Google Scholar]
- 74.Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 75.Rannala B., Yang Z.H. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996;43:304–311. doi: 10.1007/BF02338839. [DOI] [PubMed] [Google Scholar]
- 76.Zhaxybayeva O., Gogarten J.P. Bootstrap, bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genom. 2002;3:1–15. doi: 10.1186/1471-2164-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rambaut A. FigTree Tree Figure Drawing Tool Version 131, Institute of Evolutionary 623 Biology, University of Edinburgh. [(accessed on 24 May 2020)]; Available online: http://treebioedacuk/software/figtree/
- 78.Crous P.W., Shivas R.G., Quaedvlieg W., van der Bank M., Zhang Y., Summerell B.A., Guarro J., Wingfield M.J., Wood A.R., Alfenas A.C., et al. Fungal planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnston-Monje D., Loewen S., Lazarovits G. Mycobiomes of tomato plants with vine decline. Canad. J. Plant Pathol. 2017;39:184–200. doi: 10.1080/07060661.2017.1325938. [DOI] [Google Scholar]
- 80.Santos T.A.B. Relações Filogenéticas dos Complexos Stachybotrys-Memmoniella e Phalangispora-Speiropsis-Wiesneriomyces. Universidade Federal de Pernambuco; Pernambuco, Brazil: 2015. [Google Scholar]
- 81.Arnold G.R.W. Lista de Hongos Fitopatogenos de Cuba. Ministerio de Cultura, Editorial Cientifico-Tecnica Cuba; Havana, Cuba: 1986. [Google Scholar]
- 82.Barrios L.M., Pérez I.O. Nuevos registros de hongos en semillas de Oryza sativa en cuba. Manejo Integr. Plagas Agroecol. 2005;75:64–67. [Google Scholar]
- 83.Munjal R.L., Kapoor J.N. Some hyphomycetes from Himalayas. Mycopathol. Mycol. Appl. 1969;39:121–128. doi: 10.1007/BF02053485. [DOI] [Google Scholar]
- 84.Rifai M.A. Stachybotrys bambusicola sp. nov. Trans. Brit. Mycol. Soc. 1964;47:269–272. doi: 10.1016/S0007-1536(64)80062-0. [DOI] [Google Scholar]
- 85.McKenzie E.C. Dematiaceous hyphomycetes on Freycinetia (Pandanaceae). I., Stachybotrys. Mycotaxon. 1991;41:179–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences generated in this study are deposited in GenBank (Table 1). The finalized alignment and tree were submitted to TreeBASE (submission ID: 27607, http://www.treebase.org/ (accessed on 18 January 2021)). Morphological data are available at FigShare (https://doi.org/10.6084/m9.figshare.13602710, https://doi.org/10.6084/m9.figshare.13602719.v1, and https://doi.org/10.6084/m9.figshare.13602767 (accessed on 20 January 2021)). Specimens were deposited in the Mae Fah Luang University (MFLU) Herbarium, Chiang Rai, Thailand. Living cultures and DNA sequence data with chromatograms were deposited in the Culture Collection of Mae Fah Luang University (MFLUCC) Chiang Rai, Thailand.