Abstract
Aim
Coronavirus-19 disease (COVID-19) is a widespread condition in nursing home (NH). It is not known whether COVID-19 is associated with a higher risk of death than residents without COVID-19. Therefore, the aim of this study was to assess whether COVID-19 is associated with a higher mortality rate in NH residents, considering frailty status assessed with the Multidimensional Prognostic Index (MPI).
Methods
In this retrospective study, made in 31 NHs in Venice, Italy, the presence of COVID-19 was ascertained with a nasopharyngeal swab. Frailty was evaluated using the MPI, modified according to the tools commonly used in our NHs. A Cox’s regression analysis was used reporting the results as hazard ratios (HRs) with 95% confidence intervals (CIs), using COVID-19 as exposure and mortality as outcome and stratified by MPI tertiles. Similar analyses were run using MPI tertiles as exposure.
Results
Overall, 3946 NH residents (median age = 87 years, females: 73.9%) were eligible, with 1136 COVID-19 + . During a median follow-up of 275 days, higher values of MPI, indicating frailer people, were associated with an increased risk of mortality. The incidence of mortality in COVID-19 + was more than doubled than COVID-19- either in MPI-1, MPI-2 and MPI-3 groups. The presence of COVID-19 increased the risk of death (HR = 1.85; 95% CI 1.59–2.15), also in the propensity score model using MPI as confounder (HR = 2.48; 95% CI 2.10–2.93).
Conclusion
In this retrospective study of NH residents, COVID-19 was associated with a higher risk of all-cause mortality than those not affected by COVID-19 also considering the different grades of frailty.
Keywords: COVID-19, Nursing home, Prognosis, Multidimensional prognostic index, Frailty comprehensive geriatric assessment
Introduction
The spread of coronavirus-19 disease (COVID-19) is becoming unstoppable meeting the necessary epidemiological criteria to be declared a pandemic [1]. We know that COVID-19 is caused by the SARS-CoV 2, a type of coronavirus, of which the first human infection was identified in Wuhan province, China [2]. At the end of March 2021, more than 124 million people were officially affected by COVID-19, with more than 2.7 milion deaths in the world [3]. The epidemiological data so far indicated that COVID-19 could be considered as a condition typical of older people [4]. In fact, mortality rates are extremely high in older persons and the prevalence of COVID-19 is higher in older persons compared to the younger ones [5].
A particular interest was given to the COVID-19 outbreak in nursing homes (NHs) for several reasons [6–8]. First, NHs commonly include people that can be considered frail (e.g. for the presence of severe dementia) or disabled [9]. Moreover, these structures have a high proportion of people with relevant social problems, e.g., they are without relatives or they cannot live alone at their homes since they are disabled. All these conditions are, per se, considered to increase mortality risk. Finally, even if less than 10% of all COVID-19 cases are observed in NH in the world, NH and assisted living facilities residents and staff accounted for more than one third of the all deaths recorded [10, 11].
Given this background, it is important to precisely estimate the weight and the importance of COVID-19 in increasing mortality not only in community-dwelling older people, but also in NH setting. For example, in young persons, the COVID-19 pandemic was associated with a significant increase in mortality, being similar to the unintentional opioid overdoses occurring during 2018 in the United States [12]. Similar figures are present for older people [13]. Unfortunately, these epidemiological information are not available for NH people, a setting in which COVID-19 is a widespread condition, as mentioned before [14]. Moreover, the data available so far have shown the importance of frailty for prognosis in older people having COVID-19 [15–17], but these data are mainly based on community-dwelling and hospitalized older people and not in NHs [18–20].
The aim of this study was to assess whether COVID-19 is associated with a higher mortality rate in older persons living in NH, also taking in consideration frailty status assessed with the Multidimensional Prognostic Index (MPI), a common tool for stratifying prognosis and for clinical decision-making in geriatric medicine [21].
Methods
Participants
For the aims of this work, we considered all NH residents in the area of Venice, Italy. This entity is in an area of 1406 square Km in Veneto Region, North-East Italy, with about 650,000 inhabitants. This area includes 31 NHs hosting approximately 3850 beds.
At the end of March in response to the increased local awareness of COVID-19 in NH setting, the Veneto Region proposed periodical screening assessments with portable nasopharyngeal swabs for both residents and NH personnel [22]. The period of which study referred was from 01st March to 31st December 2020.
The study was approved by our local Ethical Committee.
COVID-19 diagnosis
A nasopharingeal swab test with an RT-PCR assays (Copan UTM System, Copan, Italy) for the identification of SARS-CoV-2 was administered to all NH residents.
Defining frailty using the MPI
A version of the MPI, slightly modified from the original version [23], using tests commonly used in our NHs for clinical follow-up of the residents was used. [24, 25]
Briefly, 9 domains, including 55 different questions, were considered: (1) age, (2) sex, (3) main diagnosis, (4) nursing care needs (VIP), (5) cognitive status (VCOG), evaluated by the Short Portable Mental Status Questionnaire (SPMSQ) [26], (6) pressure sores risk (VPIA), evaluated by the Exton-Smith Scale [27], (7) activities of daily living (VADL) and (8) mobility (VMOB) evaluated by the Barthel Index [28], and (9) social support (VSOC) [29]. To calculate this MPI, we used a weighted sum of each individual domain, taking as outcome mortality after 1 year [29].
We finally divided the participants according to two MPI cut-offs in tertiles, i.e. 0.31 and 0.40 considering in MPI-1 (robust) the lowest tertile (MPI 0–0.31), MPI-2 (pre-frail) the middle tertile (MPI 0.31–0.40) and MPI-3 (frail) the highest tertile (MPI > 0.40).
Outcomes
The primary outcome of our research was mortality. For NH residents positive to COVID-19, the follow-up period was calculated from the positivity to the nasopharingeal swab test until the date of death or the last observation made on 31st December 2020. For NH residents, the date of the beginning was posed on 01st March. The data regarding mortality are collected routinely as administrative data. The follow-up period was in median = 275 days, ranging from 0 to 295 days.
Statistical analysis
Continuous variables were evaluated in term of means and standard deviation (SD), after checking their normality. For categorical variables, relative frequencies (%) were reported. Parametric univariate tests (p values were referred to Fisher exact for frequencies and t Test for means) were used for evaluating possible association according to positivity or not to COVID-19.
The association between COVID-19 and mortality was made using different approaches. First, we reported the incidence of the outcome of interest, per 1000 persons-days overall and in subjects with different grade of frailty, as assessed by MPI. Moreover, we assessed the effect of COVID-19 with mortality using a Cox’s regression analysis, unadjusted and using a propensity score model with age, sex, nursing care needs, cognitive status, pressure sores risk, activities of daily living, mobility, social support, the needing of care assistants, the main medical diagnosis, using a 1:1 nearest-neighbor propensity score matching. The covariate balance for the treated and matched control groups was tested by Student’s t tests and Chi-squared tests for continuous and categorical variables, respectively. Multivariable Cox regression analysis, adjusting for propensity score quintiles, was conducted to assess the association between COVID-19 and mortality. The results were consequently reported as hazard ratios (HRs) with their 95% confidence intervals (95%CI). Similar analyses were run, by MPI tertiles used as stratifying factor and as exposure.
All analyses were performed using the SPSS 21.0 for Windows (SPSS Inc., Chicago, Illinois). All statistical tests were two-tailed and statistical significance was assumed for a p value < 0.05.
Results
Sample selection
Among 4316 NH residents, 51 aged less than 60 years, 318 did not have sufficient data for MPI calculation, and 1 participant did not report data regarding mortality. Finally, 3946 NH residents (median age = 87 years, range: 60–105; females: 73.9%) were eligible.
Baseline characteristics
Among 3946 participants initially enclosed, 1136 reported a diagnosis of COVID-19 (overall prevalence rate = 28.8%).
Table 1 shows the baseline characteristics according to the presence or not of COVID-19. NH residents with COVID-19 did not differ in terms of mean age (p = 0.85) or female sex (p = 0.08) compared to people never affected by COVID-19 (n = 2810). Moreover, the prevalence of dementia, immobilization syndrome and cardiovascular disease was similar between COVID-19 positive and negative. NH residents with COVID-19 did not differ compared to their counterparts in any of the MPI domain (activities of daily living, nursing care needs, mobility, pressure sore risk, social support, cognitive status) leading to a similar MPI score (0.36 ± 0.13 in COVID-19 + vs. 0.35 ± 0.13 in COVID-19−, p = 0.46) (Table 1).
Table 1.
Descriptive analysis of nursing home residents, by presence of COVID-19
| Domain | COVID-19 − (n = 2810) | COVID-19 + (n = 1136) | P value |
|---|---|---|---|
| Age | 86.1 (7.9) | 86.1 (7.8) | 0.85 |
| Female sex (%) | 73.1 | 75.9 | 0.08 |
| Dementia (%) | 35.2 | 38.4 | 0.07 |
| Immobilization syndrome (%) | 18.4 | 20.4 | 0.10 |
| Cardiovascular disease (%) | 11.5 | 10.1 | 0.18 |
| VIP | 3.24 (5.78) | 2.95 (5.15) | 0.18 |
| VPIA | 4.15 (5.33) | 4.42 (5.64) | 0.15 |
| VCOG | 7.00 (2.86) | 7.01 (2.80) | 0.87 |
| VADL | 49.9 (13.5) | 49.8 (13.62) | 0.82 |
| VMOB | 33.1 (10.1) | 33.2 (10.1) | 0.97 |
| VSOC | 238 (11) | 239 (9) | 0.08 |
| MPI | 0.36 (0.13) | 0.35 (0.13) | 0.46 |
MPI Multidimensional Prognostic Index, VADL activities of daily living, VCOG cognitive functions, VIP nursing care needs, VMOB mobility, VPIA pressure sores risk, VSOC social support network
Mortality data
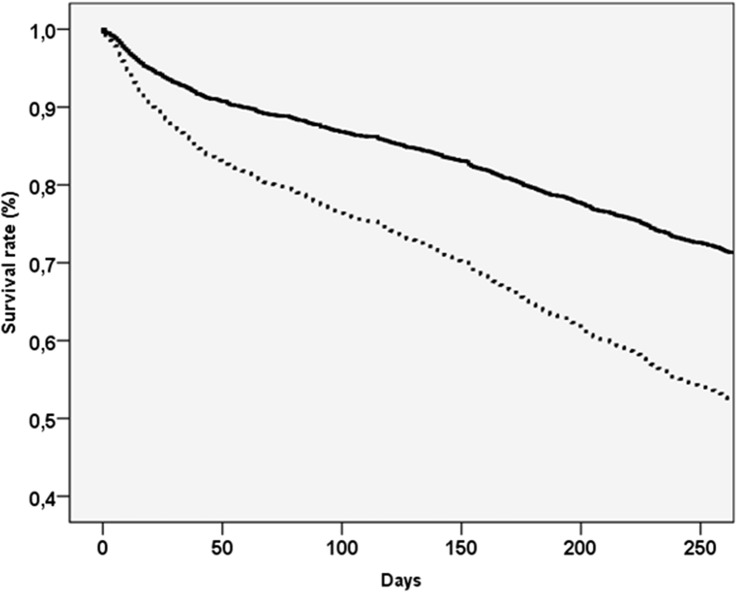
During the follow-up period that was in median 275 days, we recorded 1187 deaths. Table 2 shows the mortality data according to COVID-19 status. The incidence rate of mortality in people affected by COVID-19 + was more than doubled than in those with COVID-19− (3.00 vs. 1.27 per 1000 persons-days, p < 0.0001). In the sample as whole, the presence of COVID-19 increased the risk of death of 85% in the unadjusted model (HR = 1.85; 95%CI 1.59–2.15) and of 148% in the propensity score model (HR = 2.48; 95% CI 2.10–2.93). These findings are graphically reported in Fig. 1.
Table 2.
Overall and subgroup analyses for nursing home residents by COVID-19 status, taking mortality as outcome: multivariate and propensity score quintiles adjusted models
| COVID-19 − | COVID-19 + | Estimatesb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frailty statusa | Number of deaths | Number of subjects | Incidence rate (per 1000) (95% CI) | Number of deaths | Number of subjects | Incidence rate (per 1000) (95% CI) | Unadjusted estimate (HR, 95%CI) | p value | Propensity Score modelc (HR, 95%CI) | p value |
| All sample | 901 | 2810 | 1.27 (1.19–1.36) | 286 | 1136 | 3.00 (2.66–3.37) | 1.85 (1.59–2.15) | < 0.0001 | 2.48 (2.10–2.93) | < 0.0001 |
| Robust (MPI lowest tertile) | 242 | 918 | 1.00 (0.89–1.13) | 83 | 398 | 2.57 (2.07–3.18) | 2.02 (1.50–2.72) | < 0.0001 | 1.91 (1.37–2.66) | < 0.0001 |
| Pre-frail (MPI middle tertile) | 289 | 944 | 1.21 (1.08–1.36) | 102 | 371 | 3.11 (2.55–3.80) | 2.07 (1.60–2.68) | < 0.0001 | 2.90 (1.73–4.86) | < 0.0001 |
| Frail (MPI highest tertile) | 370 | 948 | 1.60 (1.45–1.78) | 101 | 367 | 3.33 (2.74–4.07) | 1.60 (1.25–2.04) | < 0.0001 | 2.03 (1.38–2.99) | < 0.0001 |
aFrailty status was assessed using multidimensional prognostic index score tertiles values
bData are reported as hazard ratios (HRs) and their 95% confidence intervals (CIs)
cPropensity score was included in quintiles and based on multidimensional prognostic index (MPI) score that includes as domains age, sex, nursing care needs (VIP), cognitive status (VCOG), pressure sores risk (VPIA), activities of daily living (VADL), mobility (VMOB), social support (VSOC), the needing of care assistants (VIP), the main medical diagnosis
Fig. 1.
Survival curves, taking mortality as outcome, in the sample as whole for by presence (dashed line) or absence (continuous line) of COVID-19
Table 2 reports the data by COVID-19 status, according to frailty status. First, the incidence of mortality increased by frailty status, independently from the COVID-19 status (from 1.00 to 1.60 per 1000 persons-days in COVID-19− and from 2.57 to 3.33 per 1000 persons-days in COVID-19 +). Moreover, the presence of COVID-19 leads to an increased risk of death in all the MPI tertiles in both unadjusted (p for interaction across tertiles = 0.38) and propensity score models (p for interaction across tertiles = 0.26).
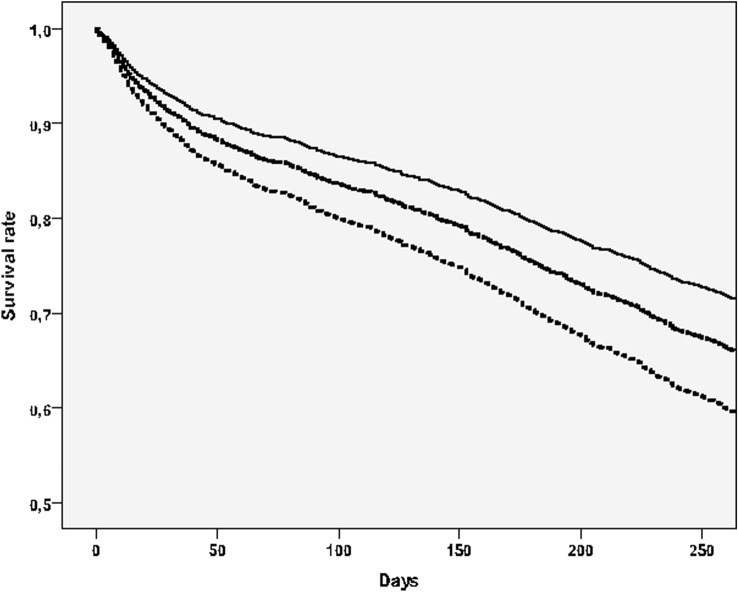
Finally, our study showed that frailty is associated with an increased risk of death in NH residents. As reported in Fig. 2, taking people in the lowest tertile of MPI as reference and after adjusting for the diagnosis of COVID-19, people in the middle (HR = 1.54; 95% CI 1.34–1.78; p < 0.0001) and in the highest (HR = 1.89; 95% CI 1.65–2.17; p < 0.0001) tertile carried a significant higher risk of mortality.
Fig. 2.
Survival curves, taking mortality as outcome, by multidimensional prognostic index tertiles. Notes: the highest line indicated participants in the lowest MPI group (robust), the lowest those in the highest MPI group (frail)
Discussion
In this research, we found that in NH setting COVID-19 was associated with a significant higher risk of mortality, also when considering the presence of frailty, as estimated with the MPI score. Moreover, our paper further confirmed that frailty, as assessed by the MPI, increased the risk of mortality in a special context, such as NH.
Even if it is known that COVID-19 mortality linearly increases with age, limited evidence is available regarding the fact is COVID-19 is able or not to increase mortality in older persons resident in NH that can be considered frail per se. In this regard, few studies using resident-level data are available, often including data from a single facility or a small number of facilities, therefore, limiting the generalizability of these findings [14, 30–32]. Even if all these researches advanced our knowledge regarding mortality in NH during COVID-19 some limitations should be discussed. The most important is that these works did not have any control group; therefore, it is not possible to know if COVID-19 is able or not to increase mortality in this population characterized by a high mortality rate. Moreover, these papers did not consider the presence of a comprehensive geriatric assessment (CGA) tool, such as the MPI as we did. In our study, we tried to overcome these shortcomings including a control group never affected by COVID-19 during the follow-up period and comparable for the level of frailty. Our study, for the first time, showed that COVID-19 prevalence is not dependent on frailty and that COVID-19 leads to an increased risk of mortality not only in frailer subjects, but also in more robust ones. Our analyses, in fact, showed that people with and without COVID-19 are similar not only in terms of age, gender and comorbidities, but also relevant characteristics such as nursing care needs, cognitive status, pressure sores risk, activities of daily living, mobility, social support, and the needing of care assistance. Overall, our findings suggest that the presence of COVID-19 in NH practically doubled the risk of death in this setting, also when taking in account frailty, as evaluated by MPI.
To know that COVID-19 is able to significantly increase the risk of death in NH setting is, in our opinion, of clinical importance. Before our study, in fact, the sensation of several authors was that COVID-19 leaded to mortality only because frailer people were affected by this condition [30]. On the contrary, our study shows that COVID-19 is present in NH residents independently from the presence of frailty since the MPI score, at the baseline evaluation, was similar between COVID-19 + and COVID-19− residents. Moreover, the adjustment for a multidimensional score attenuated, but not nullified the association between COVID-19 and mortality in NH setting, indicating that COVID-19 is, unfortunately, an important cause of death in this population, independently from frailty.
From an epidemiological perspective, individuals resident in NH (such as in our case) are affected by advanced dementia (about one over three in our sample) and immobilization syndrome that often are present with dysphagia, associated with an increased risks of malnutrition, aspiration, bacterial pneumonia, and delirium, that further complicate the course of COVID-19 [14]. As widely known, these subjects generally require extensive assistance with the activities of daily living that put them close with many staff members who may be asymptomatically infected with viral strains from the community. [14, 33] However, again, our study suggests that the prevalence of COVID-19 is not dependent from the necessity of assistance in the NH (e.g., the Barthel Index score was similar between COVID-19 + and negative), suggesting that other research should be done regarding the risk factors for the difference in prevalence in NH.
Our findings should be considered in the light of certain limitations. First, the cause of death was not included in our analyses. Therefore, it is not possible to know the reasons why people affected by COVID-19 died more than their counterparts. Second, our study population resident in NH, who are traditionally frailer than people living in the community; therefore, our findings are not applicable to community-dwelling adults. Third, we did not have any information regarding the severity and the eventual therapy used in NHs, being administrative data. Fourth, even if we collected information until 31st December 2020, our NHs and Italy in general are assisting to a third wave of COVID-19 that can modify our findings. Fifth, our study is a retrospective research: therefore, a selection bias cannot be excluded.
In conclusion, in this retrospective study of Italian NH residents, COVID-19 was associated with a higher risk of all-cause mortality than those not affected by this condition, also in case of similar frailty scores. Our findings further suggest the importance to prevent the presence and the diffusion of COVID-19 in NHs that is associated per se with a high rate of mortality in this setting. Other prospective studies are needed to confirm our findings.
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement. This research is funded by an unrestricted grant of the SIGOT (Società Italiana Geriatria Ospedale e Territorio) that funded the data analysis and statistical elaborations.
Data availability
Available upon reasonable request to the corresponding author.
Code availability
Available upon reasonable request to the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This research was approved by the Ethical Committee of Venice.
Informed consent
All participants provided informed consent prior to their participation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2020) Coronavirus disease 19 (COVID-19): situation report, 72
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (2020) Coronavirus disease 2019 (COVID-19): situation report, 45
- 4.Lloyd-Sherlock PG, Kalache A, McKee M, et al. WHO must prioritise the needs of older people in its response to the covid-19 pandemic. BMJ. 2020;368:1. doi: 10.1136/bmj.m1164. [DOI] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 6.McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JK, Bayne G, Evans C, et al. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Healthy Longev. 2020;1:e21–e31. doi: 10.1016/S2666-7568(20)30012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trabucchi M, De Leo D. Nursing homes or besieged castles: COVID-19 in northern Italy. Lancet Psychiatry. 2020;7:387–388. doi: 10.1016/S2215-0366(20)30149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjia J, Rothman MR, Kiely DK, et al. Daily medication use in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58:880–888. doi: 10.1111/j.1532-5415.2010.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180:1336–1344. doi: 10.1001/jamainternmed.2020.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eurosurveillance Editorial Team Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Eurosurveillance. 2020;25:2003121. doi: 10.2807/1560-7917.ES.2020.25.10.2003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25–44 Years, March-July 2020. JAMA. 2020;325:785–787. doi: 10.1001/jama.2020.24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolf SH, Chapman DA, Lee JH. COVID-19 as the leading cause of death in the United States. JAMA. 2020;325:123–124. doi: 10.1001/jama.2020.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Int Med. 2021;1:1. doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellelli G, Rebora P, Valsecchi MG, et al. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020;1:1634–1636. doi: 10.1007/s00134-020-06087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Smet R, Mellaerts B, Vandewinckele H, et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:e200369–e200369. doi: 10.1001/jamahealthforum.2020.0369. [DOI] [PubMed] [Google Scholar]
- 19.Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2020;1:104324. doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X-M, Jiao J, Cao J. Frailty as a predictor of mortality among patients with COVID-19: a systematic review and meta-analysis. BMC Geriatr. 2021;21:1–11. doi: 10.1186/s12877-020-01943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilotto A, Custodero C, Maggi S, et al. A multidimensional approach to frailty in older people. Ageing Res Rev. 2020;60:101047. doi: 10.1016/j.arr.2020.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veronese N, Sbrogiò LG, Valle R, et al. Prognostic value of lung ultrasound in older nursing home residents affected by COVID-19. J Am Med Dir Assoc. 2020;21:1384–1386. doi: 10.1016/j.jamda.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11:151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veronese N, Stangherlin V, Mantoan P, et al. Frailty and risk of mortality and hospitalization in nursing home residents affected by COVID-19. Geriatr Care. 2021;7:9635. doi: 10.4081/gc.2021.9635. [DOI] [Google Scholar]
- 25.Pilotto A, Gallina P, Fontana A, et al. Development and validation of a multidimensional prognostic index for mortality based on a standardized multidimensional assessment schedule (MPI-SVaMA) in community-dwelling older subjects. J Am Med Dir Assoc. 2013;14:287–292. doi: 10.1016/j.jamda.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 27.Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon Bull Minist Health Public Health Lab Serv. 1966;25:238. [PubMed] [Google Scholar]
- 28.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 29.Gallina P, Saugo M, Antoniazzi M, et al. Validazione della scheda per la valutazione multidimensionale dell'anziano (SVAMA) Tendenze nuove. 2006;6:229–264. [Google Scholar]
- 30.Shi SM, Bakaev I, Chen H. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21:1378–1383.e1. doi: 10.1016/j.jamda.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham NS, Junghans C, Downes R, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81:411–419. doi: 10.1016/j.jinf.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bigelow BF, Tang O, Toci GR, et al. Transmission of SARS-CoV-2 involving residents receiving dialysis in a nursing home—Maryland, April 2020. Morb Mortal Wkly Rep. 2020;69:1089. doi: 10.15585/mmwr.mm6932e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbasi J. Abandoned nursing homes continue to face critical supply and staff shortages as COVID-19 toll has mounted. JAMA. 2020;324:123–125. doi: 10.1001/jama.2020.10419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon reasonable request to the corresponding author.
Available upon reasonable request to the corresponding author.