Abstract
Simple Summary
With the increasing pressure to address the problems of bacterial resistance and drug residues, medicinal herbs are gradually taking a more important role in animal production. Scutellaria baicalensis is a common and widely used Chinese medicinal herb. The main bioactive compounds in the plant are baicalein and baicalin. These compounds have many biological functions including anti-oxidation, antipyretic, analgesic, anti-inflammatory, antiallergic, antimicrobial, immunomodulatory, and antitumor effects. S. baicalensis and its extracts can effectively promote animal growth, improve the production performance of dairy cows, reduce the stress and inflammatory response, and have effective therapeutic effects on diseases caused by bacteria, viruses, and other pathogenic microorganisms. This paper summarizes the biological function of S. baicalensis and its application in sustainable animal production to provide a reference for future application of S. baicalensis and other medicinal herbs in animal production and disease treatment.
Abstract
Drugs have been widely adopted in animal production. However, drug residues and bacterial resistance are a worldwide issue, and thus the most important organizations (FAO, USDA, EU, and EFSA) have limited or banned the use of some drugs and the use of antibiotics as growth promoters. Natural products such as medicinal herbs are unlikely to cause bacterial resistance and have no chemical residues. With these advantages, medicinal herbs have long been used to treat animal diseases and improve animal performance. In recent years, there has been an increasing interest in the study of medicinal herbs. S. baicalensis is a herb with a high medicinal value. The main active compounds are baicalin and baicalein. They may act as antipyretic, analgesic, anti-inflammatory, antiallergenic, antimicrobial, and antitumor agents. They also possess characteristics of being safe, purely natural, and not prone to drug resistance. S. baicalensis and its extracts can effectively promote the production performance of livestock and treat many animal diseases, such as mastitis. In this review, we summarize the active compounds, biological functions, and applications of S. baicalensis in the production of livestock and provide a guideline for the application of natural medicines in the production and treatment of diseases.
Keywords: Scutellaria baicalensis, extract, sustainable animal production, medicinal herb, feed
1. Introduction
Food from animal sources provides the human body with protein, fat, minerals, vitamin A, B vitamins, and other nutrients, thus giving animal products an important role in the food supply worldwide. Improving the health and production of food animals would also benefit human health. Manufactured chemical and antibiotic feed additives are widely used in animal husbandry, resulting in growing problems with antibiotic resistance and chemical residues, which are becoming limiting factors in animal husbandry [1,2,3,4]. Concerns over these issues have prompted various organizations and countries in the world to introduce policies for banning or restricting the use of chemicals and antibiotics as feed additives [5]. The “one health” approach is used worldwide [6,7]. Future actions and the World Health Organization action plan against antimicrobial resistance are based on best practices in implementing and monitoring the “one health” plans, supporting novel solutions to prevent and treat infections, thereby increasing the efforts in terms of combating antimicrobial resistance and related risks worldwide [8]. Medicinal herbs, with their unique advantages as natural products, might be one of the solutions to these issues in animal production.
Medicinal herbs and their extracts are natural, efficient with few side effects, and have minimal risk of inducing bacterial resistance [9]. At present, the application of medicinal herbs and their extracts in animal production is still at the emerging stage. It has been proven that medicinal herbs can effectively improve the utilization of feed protein, and the performance of growth and reproduction in animals [10,11], modulate rumen fermentation towards high efficiency [12,13,14], increase feed intake and digestibility by affecting feed intake behaviors and the secretion of digestive enzymes [14], alleviate the effects of stress by enhancing antioxidant ability [15,16], and consequently improve the health status of animals. Furthermore, the antimicrobial effects of medicinal herbs can also be used for the prevention and treatment of diseases. Thus, medicinal herbs as alternative natural feed additives to replace chemicals and antibiotics have attracted much attention [17].
Scutellaria baicalensis is an herb that has roots of high medicinal value. It has the functions of clearing heat and detoxification, purging fire and drying dampness, improving fertility, and hemostasis. It is also widely used in clinical practice. This paper reviews the research progress on the use of S. baicalensis and its extracts in the sustainable production and health of animals. The biological functions of S. baicalensis and its extracts and their applications in animal production and disease prevention are briefly described to form a reference for the future research and application of herbal preparations in animal production.
2. Scutellaria baicalensis and Its Active Components
Scutellaria baicalensis, also known as Chinese skullcap or Baikal skullcap, is a perennial herb of the family Lamiaceae. It mainly grows at an altitude of 60–2000 m in sandy soil on sunny slope land. The plant is natively distributed in East Asia and widely cultivated in European and American countries. China is the main producer of S. baicalensis for medicinal use [18]. The dry roots of the plant are often used as medicine. The chemical components of S. baicalensis roots are mainly flavonoids, anthraquinones, lignin, organic acids, volatile oils, and other compounds. Hereafter, S. baicalensis roots are sometimes referred to as S. baicalensis. The characteristic components of S. baicalensis are baicalin, baicalein, scutellarin, and scutellarin. Baicalin is a monomer active component extracted from the roots of S. baicalensis. Baicalein is the aglycon form of baicalin, which is a typical component of S. baicalensis [19].
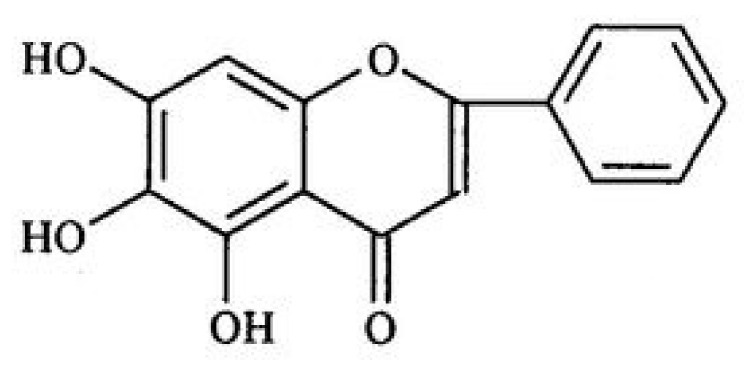
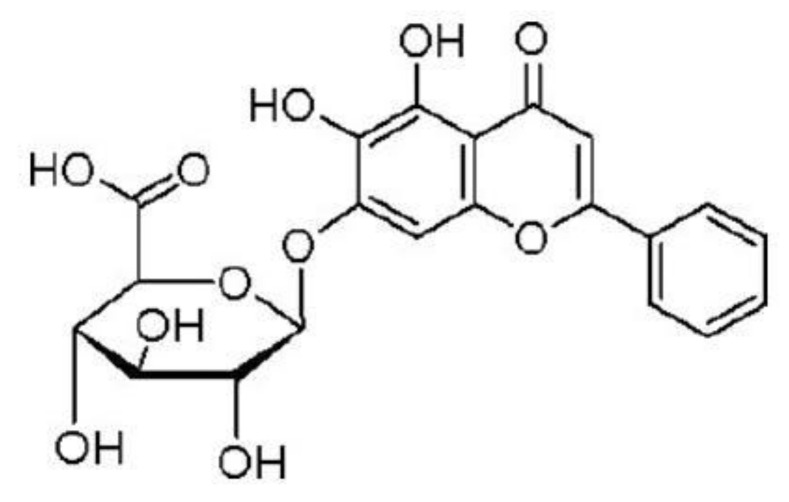
Baicalein and baicalin are the main active compounds of S. baicalensis, which are flavonoids. Baicalin is the glucuronide of baicalein [19]. The molecular formula of baicalein is C15H10O5 with a relative molecular mass of 270.24, and baicalein can be crystallized as yellow needles and is easily soluble in alcohol, acetone, and slightly soluble in chloroform. The chemical structure is shown in Figure 1. The molecular formula of baicalin is C21H18O11, with a relative molecular weight of 446.36. Pure baicalin exists as light yellow crystal needles and is soluble in hot acetic acid, insoluble in acetone, methanol, and ethanol, and almost insoluble in water. The molecular structure is shown in Figure 2.
Figure 1.
Molecular structure of baicalein. The IUPAC name of baicalein is 5,6,7-Trihydroxy-2-phenylchromen-4-one, or also known as 5,6,7-trihydroxyflavone.
Figure 2.
Molecular structure of baicalin. The IUPAC name of baicalin is (2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenylchromen-7-yl)oxy-3,4,5-trihydroxy-tetrahydropyran-2-carboxylic acid, or also known as baicalein 7-O-glucuronide.
3. Biological Functions of S. baicalensis
S. baicalensis has a long history as a medicinal herb due to its extensive biological and pharmacological activities. Its use in treating lung and liver diseases was first recorded in Sheng Nong’s herbal classic during the Han Dynasty in China. S. baicalensis has a cooling effect and is bitter in taste. It has antimicrobial, anti-inflammatory, antipyretic, and analgesic effects and improves animal growth performance (Table 1). It plays an important role in livestock and poultry production [20]. Baicalin, as the most abundant flavonoid in the roots of S. baicalensis, is stable in acidic environments and organic solvents but unstable in alkaline environments and in the plant. The absorption rate of baicalin is low via oral administration. However, baicalein formed from enzymatic hydrolysis of baicalin in the intestinal tract is easily absorbed into the blood and then is chemically converted into baicalin and other metabolites in the liver showing biological activities [21,22].
Table 1.
The biological functions and application of Scutellaria baicalensis and its active compounds.
| Type of Product | Active Components | Biological Functions | Application | Target | References |
|---|---|---|---|---|---|
| Active compound | Baicalin | Antioxidation | Thymus protection | Chicken | [23,24,25] |
| Antipyretic effect | Reducing mastitis incidence | Dairy cow, rat | [26,27,28,29] | ||
| Analgesic effect | - | Rat | [30,31,32] | ||
| Anti-inflammation | Pyemiapyaemia Cardiovascular disease 1 |
Human, mouse | [33] [34] |
||
| Antibacterial effect | Escherichia coli | Dairy cow | [35,36,37,38] | ||
| Antitumor activity | Pancreatic cancer 1 Liver cancer 1 Colon cancer 1 Leukemia 1 |
Human | [39] [40] [41] [42] |
||
| Baicalein | Antioxidation | Protection against H2O2-induced oxidative injury of neuronal cells | Human | [19] [23,43,44,45] |
|
| Heart protection | Chicken | ||||
| Anti-inflammation | Treatment of skin diseases | Mouse | [46] | ||
| Antimicrobial effect | Staphylococcus aureus and E.coli | Animals | [47,48] | ||
| Candida | [49,50] | ||||
| Antitumor | Induction of tumor cell death | Human | [51] | ||
| Wogonin | Cartilage protection | Treatment of arthritis 1 | Mouse | [52] | |
| Anti-inflammation | Treatment of skin diseases | Animals | [53] | ||
| Antivirus | Treatment of avian influenza | Chicken | [54] | ||
| Extract | S. baicalensis extract | Antibacterial effect | Klebsiella pneumoniae and Pseudomonas aeruginosa | - | [55,56] |
| Antivirus | Avian infectious laryngotracheitis, tick borne encephalitis 1 | Animals | [57,58] | ||
| Root | S. baicalensis | Antiallergic effect | Treatment of pruritus | Animals | [59,60,61,62,63,64,65] |
| Inhibition of angiogenesis | Treatment of cancer 1 | Human | [66] |
1 Indicates potential in practical application.
3.1. Antioxidation
Flavonoids have significant antioxidant activity. There are two antioxidant mechanisms of flavonoids. One is a direct hydrogen pumping reaction mechanism. The antioxidant molecule loses the phenolic hydroxyl hydrogen atom to generate phenolic oxygen radicals, and its antioxidant activity depends on the ease of phenolic hydroxyl O-H bond breaking. The other one is a single electron transfer mechanism. The antioxidant transfers one electron to the reactive oxygen radicals to generate the flavonoid radical cation [67]. The A-ring of baicalin contains an o-diphenol structure, whereas the molecular structure of baicalein contains three hydroxyl groups [68]. The key to baicalin’s antioxidant mechanism is the regulation of nuclear factor-erythroid 2-p45 derived factor 2 (Nrf2). Nrf2 is the core transcription factor that regulates the cellular oxidative response. It can induce the activity of the antioxidative reactive protein (Ares) and thus inhibit the formation of oxidative free radicals (ROS), consequently reducing oxidative stress to keep the stability of the intracellular environment [25]. Baicalein can scavenge free radicals by decarboxylation in the body to regulate oxidative stress. Baicalein has a strong scavenging effect on free radicals such as alkane peroxide and superoxide anion [45].
Gao et al. [23] detected free radicals using the electron spin resonance (ESR) technology and found that baicalin and baicalein have a strong scavenging effect on hydroxyl radicals, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, and alkyl peroxide radicals. In addition, baicalein can inhibit xanthine oxidase activity and thus inhibit the production of oxygen free radicals during its metabolism [19]. Peroxides (such as hydrogen peroxide) can induce neuronal cell injury. Baicalin and baicalein have significant protective effects on the oxidative injury of human neuronal cells induced by H2O2, and baicalein has stronger antioxidant activity than baicalin [43]. The free radical scavenging and antioxidant effects of baicalin and baicalein can be used to effectively treat free radical and oxidative stress-related diseases.
To protect the heart, baicalein can concentration-dependently reduce hypoxia reoxygenation induced myocardial death and apoptosis. Further studies revealed that the cardioprotective effects of baicalein were mediated through μ- and δ-, but not κ-opioid receptors and their associated signal transduction pathways, such as protein kinase C and ATP-sensitive potassium (KATP) channels [44]. Baicalin can effectively attenuate oxidative stress and apoptosis by activating the Nrf2 signaling pathway, thereby protecting the thymus from structural and functional damage mediated by mycoplasma infection [24].
3.2. Antipyretic and Analgesic Effects
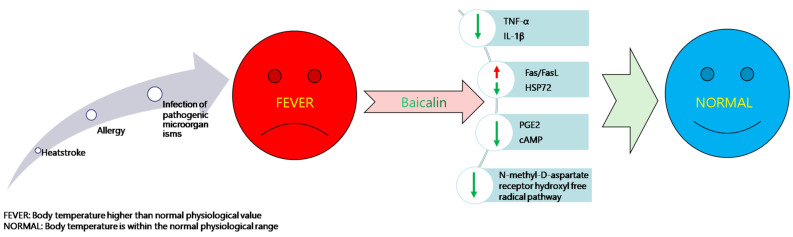
Baicalin has a significant antipyretic effect (Figure 3), and the mechanism underlying the effect may be related to the reduction of the contents of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in a significant dose-dependent manner [27]. The antipyretic effect of baicalin is via inhibiting the hydroxyl radical pathway of the N-methyl-D-aspartate receptor in the hypothalamus and accumulating TNF-α during fever. Studies have shown that baicalin can play an antipyretic role by reducing the concentration of prostaglandin E2 (PGE2) and cyclic adenosine monophosphate (cAMP) in the hypothalamus [28]. Other studies have found that baicalin reduces heat-stress-induced apoptosis by regulating the Fas/FasL pathway and upregulating heat shock protein 72 (HSP72) expression in bovine testicular Sertoli cells [29]. In addition to its antipyretic effect, baicalin also has a substantial analgesic effect [30]. It is found that baicalin has anti-inflammatory and analgesic effects in the studies on the evaluation of its effects on inflammation, pain, and edema [31]. Pain-related animal models were used to evaluate the analgesic activity of UP446 (a standard bioflavonoid component of baicalin and catechol). It was found that the pain sensitivity of hyperalgesic animals induced with carrageenan pretreatment reduced by 39.5% after an oral administration at 150 mg UP446/kg body weight. In the writhing test and formalin test, a single dose of UP446 orally administered at 100 mg/kg body weight showed 58% and 72% inhibition of pain sensitivity, respectively [32].
Figure 3.
Schematic diagram of the antipyretic mechanism of baicalin. FEVER: Body temperature is higher than the normal physiological value. NORMAL: Body temperature is within the normal physiological range.
3.3. Anti-Inflammatory and Antiallergic Effects
S. baicalensis and its extracts have a strong anti-inflammatory function, participate in the regulation of a variety of inflammatory factors, and also have cartilage protection effects [52]. Baicalin and baicalein treat skin diseases by regulating enzymes and pro-inflammatory factors such as interleukin-6, oxygenase-1, TNF-α, and cell adhesion factors [33,46,53]. In vitro experiments showed that baicalin has a protective effect against lipopolysaccharide (LPS)-induced inflammatory injury in mammary epithelial cells and can play an anti-inflammatory role by inhibiting nuclear factor kappa-B (NF-κB) activation and mitogen-activated protein kinase (p38) phosphorylation, and upregulating HSP72 to alleviate LPS-induced inflammation and cell apoptosis in mammary epithelial cells [69,70]. A study on rats found that baicalein can inhibit the release of interleukin-8 and the synthesis of cyclooxygenase-2 and increase the formation of heat shock protein 70 to improve the anti-inflammatory ability of the body, consequently blocking the inflammatory injury caused by inflammatory factors [71]. Baicalin can inhibit the production of inflammatory factors TNF-α, IL-1β, Interleukin-6 (IL-6), Interleukin-17 (IL-17), matrix metalloprotein-9 (MMP-9), and regulate NF-κB signaling pathway to have anti-inflammatory effects [72].
Baicalein and baicalin can also inhibit vascular permeability, the adsorption and migration ability of leukocytes, and inhibit the production and release of inflammatory mediators, providing a reference and theoretical basis for the development of drugs to treat cardiovascular-related diseases [73]. In addition, baicalin can reduce inflammation and edema as a dual inhibitor of cyclooxygenase and 5-lipoxygenase [34]. S. baicalensis exerts antiallergic effects mainly by inhibiting the mast cell degranulation process and inhibiting the release of the histamine slow-reacting substance of anaphylaxis (SRS-A). This may alleviate the itching, gastrointestinal contraction, and other symptoms caused by type I, II, and IV allergic reactions in animals, without apparent side-effects [59,60,61,62,63,64,65].
3.4. Antimicrobial Effect
The plant extract of S. baicalensis has a broad-spectrum inhibitory effect on the growth of bacteria, including mycoplasma and spirochetes type bacteria, fungi, and viruses. Baicalin and baicalein are the active compounds that inhibit the growth of bacteria by destroying the nucleic acid formation of bacteria and altering the energy metabolism of bacteria, as well as inhibiting the formation of biofilms of bacteria such as Klebsiella pneumoniae and Pseudomonas aeruginosa [74]. In experiments evaluating the inhibitory effect of baicalin on milk-derived Escherichia coli, it was found that baicalin had an inhibitory effect on E. coli in vitro [35]. After the use of baicalin, the sensitivity of most strains to other antimicrobial agents was enhanced [36].
In addition, S. baicalensis and its extract have a bactericidal effect on Helicobacter pylori, Staphylococcus aureus, and other pathogenic bacteria [55,56]. Baicalin in conjunction with penicillin and ciprofloxacin has a synergistic effect on the treatment of penicillin-resistant S. aureus and methicillin-resistant S. aureus (MRSA) [37,38]. Baicalein can prevent the formation of bacterial biofilms and disrupt the biofilms and consequently reduce the production of staphylococcal enterotoxin A and α-hemolysin, thereby inhibiting the growth of S. aureus [47]. In addition, baicalein can reduce the pathogenic ability of bacteria such as S. aureus and E. coli by disrupting their cell wall integrity, reducing bacterial enzymatic activities, and inhibiting bacterial energy production and nucleotide synthesis [48]. It was found that the extract of S. baicalensis has significant antifungal effects, such as Candida albicans, Aspergillus fumigatus, Hydramycetes, etc. Baicalein and wogonin have strong antifungal activities, which may induce programmed apoptosis of the fungal cells via excessive production of reactive oxygen species [49]. In addition, the combination of baicalein with fluconazole and other antifungal drugs can more effectively treat fungal infections such as Candida [50]. The extract of S. baicalensis has an in vitro inhibitory effect on tick-borne encephalitis virus via direct inhibition of the adsorption and intracellular replication of tick-borne encephalitis virus [57]. S. baicalensis also has marked therapeutic effects on viral diseases such as influenza, infectious bronchitis, and viral diarrhea [54,58]. Relevant studies have shown that baicalin has a polyphenolic hydroxyl structure and prevents glyoxal-induced cystatin aggregation by affecting its aggregation process, which opens a new way for the treatment of protein misfolding diseases [75]. In addition, baicalin extract has potential applications in the fields of anti-fibrosis, anti-cancer, anti-aging, anti-depression, and immune regulation [76,77,78,79,80].
3.5. Antitumor Activity
Baicalin is able to induce the apoptosis of tumor cells. Baicalin could promote apoptosis in pancreatic cancer cells (SW1990 cell line) via dose-dependent upregulation of the expression of mitochondrial Bax (Bcl2-associated Xprotein) and cleavage-type enzymes caspase-3, and p53, consequently significantly decreasing B-cell lymphoma/lymphoma 2 (Bcl-2) protein levels, possibly activating c-Jun N-terminal kinase/forkhead box protein O1/Bcl-2 interacting mediator of cell death (JNK/Foxo1/BIM) pathway [39]. Baicalin can induce apoptosis in hepatoma cell lines HepG2 and SMMC-7721 cells [40]. It can also induce colon cancer cell apoptosis through microRNA-217/dickkopf (miR-217/DKK1)-mediated inhibition of the wingless-related integration (Wnt) signaling pathway [41]. Moreover, the combination of baicalin with drugs such as hexamethylene bis-acetamide (HMBA) showed promising efficacy against leukemia [42].
Baicalin can inhibit tumor invasion and metastasis. Baicalin effectively inhibits the invasion, migration, and adhesion abilities of multiple tumors by inhibiting the expression levels and activities of mitochondrial membrane potential 2 (MMP2) and mitochondrial membrane potential 9 (MMP9) [81]. Phosphorylation of AMP-activated protein kinase (AMPK) leads to mitochondrial membrane potential (MMP) expression and promotes tumor invasion and metastasis [82].
Baicalin inhibits non-small cell lung cancer migration and invasion by down-regulating MMP expression through activation of the mammalian target of rapamycin (mTOR) and silencing information regulator1/AMP-activated protein kinase (SIRT1/AMPK) signaling pathways [83]. In addition, baicalin also has the ability to induce cancer cell cycle arrest [84,85], overcome the drug resistance of tumor cells [86], and modulate tumor-associated inflammatory microenvironment [70,87].
Baicalein inhibits tumor cell development by modulating different metabolic signaling pathways, as well as decreasing tumor growth and metastasis rates, significantly decreasing CD31 (an endothelial cell marker) and α-smooth muscle actin (α-SMA, a parietal cell marker) expression and inducing cell death in tumor tissues [51].
Angiogenesis is the key process to promote cancer, and S. baicalensis has anti-angiogenesis activity in vitro. Liu et al. [66] evaluated the potential of baicalin and baicalein as antiangiogenic agents through the analysis of chicken chorioallantoic membrane (CAM) and the culture of human umbilical vein endothelial cells (HUVEC) in vitro. The study showed that baicalin and baicalein had the potential of antiangiogenesis and inhibited the migration of endothelial cells and the differentiation of endothelial cells into tubular branch networks in a dose-dependent manner [66]. Wang et al. [88] also determined the activity of angiogenesis through the proliferation of blood vessels on the chicken CAM model and cultured bovine aortic endothelial cells (BAEC). The study indicated that S. baicalensis could significantly inhibit the activity of angiogenesis.
Wogonoside is an active component of S. baicalensis. It can inhibit the migration and angiogenesis of HUVEC stimulated by LPS, as well as the microvascular sprouting of rat aortic rings in vitro. It may also have potential therapeutic value for diseases related to inflammation and angiogenesis [89]. Oroxylin A (one of the active components of S. baicalensis and one of the metabolites of baicalin in vivo) can also inhibit LPS-induced angiogenesis and may affect the lipopolysaccharide/Toll-like receptor 4 (LPS/TLR4) signaling pathway [90]. Wogonin also inhibits H2O2-induced angiogenesis by inhibiting the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and NF-κB signaling pathway [91].
4. Application of S. baicalensis in Sustainable Animal Production for Better Performance
Medicinal herb extracts have been used as feed additives, veterinary medicines, and environment-friendly disinfectants [92] with support from the public due to their natural, safe, and effective characteristics. As an important medicinal herb, S. baicalensis can enhance the immunity of the body, reduce allergic reactions, protect the liver, and treat the body’s peroxidative reactions [19]. S. baicalensis could play an important role in the improvement of animal growth and production performance. Table 2 summarizes the main effects of S. baicalensis in animal production.
Table 2.
The main effects of Scutellaria baicalensis on animals.
| Type of Animal | Age of Animals | Source | Dose or Concentration | Main Effects | References |
|---|---|---|---|---|---|
| Broilers | 7–42 d | Baicalein | 100–200 mg/kg diet | Improve growth performance, immunity, and antioxidant activity | [93] |
| Broilers | 1–42 d | S. baicalensis roots | 5, 10, 15 g/kg diet | Increase broiler body weight and feed conversion efficiency | [94] |
| Turkey hens | 42–63 d | S. baicalensis extract | 0.009, 0.018, 0.036 mL/kg BW 1 | Alter the contents of sodium, potassium, calcium, magnesium, copper, zinc, and iron in plasma and affect meat quality | [95] |
| Laying hens | - | S. baicalensis extract | 5 g/kg diet | Increase egg weight, decrease microbial content in the cecum, reduce the amount of propylene glycol in eggs and delay lipid oxidation in eggs | [96] |
| Chicken | 9–24 d | Curcuma longa and S. baicalensis extracts | 2 g/kg diet | Reduce intestinal inflammation and improve production performance | [97] |
| Broilers | 1–42 d | S. baicalensis and Lonicera japonica extracts | 500 mg/kg diet | Improve growth performance, promote the development of immune organs, and improve the antioxidant function | [98] |
| Broilers | 1–49 d | Baicalin | 20 mg/kg diet | Improve growth performance, blood parameters, nutrient digestibility, and meat quality | [99] |
| Weaned piglets | 28–56 d | Fermented Scutellaria | 1.5 mg/kg diet | Enhance appetite, increase average daily intake, reduce feed-to-weight ratio and diarrhea rate, and improve the feed reward | [100] |
| Weaned piglets | - | S. baicalensis extract | - | Improve growth performance and manipulate intestinal microflora | [101,102,103,104] |
| Weaned piglets | 28–35 d | Baicalin | 10 mg/kg BW, 1 time/d, 5 d; intramuscular injection | Prevent swine edema disease | [105] |
| Piglets | 5–25 d | Baicalin | 212.5 mg/time, 2 times/d, 5 d; oral administration | Prevent piglet diarrhea | [106] |
| Pregnant and lactating sows | - | Mixed herbs containing S. baicalensis | - | Decrease weight loss and improve litter performance | [107] |
| Dairy cows | - | S. baicalensis extract | 100 g/kg diet | Reduce incidence of mastitis and improve milk yield | [26] |
1 BW = body weight.
4.1. Poultry
Zhou et al. [93] evaluated the effects of baicalein on growth performance, immunity, and antioxidant activity of broilers at doses of 100 and 200 mg/kg diet. Compared with the basal diet, the baicalein-supplemented diet had no significant effect on the average daily feed intake but significantly increased body weight, average daily gain, and feed conversion efficiency of broilers at the age of 21–42 days and 7–42 days. The best growth performance was observed at the dose of 200 mg/kg diet. Compared with the control group, baicalein significantly increased CD3+/CD4+ and CD3+/CD8+ ratio, interferon γ (IFN-γ) concentration, anti-IB antibody titer, and spleen index. Total cholesterol, triglyceride, and low-density lipoprotein cholesterol were significantly decreased after baicalein intake compared with basal diet, while superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) activities in serum were increased with baicalein supplementation, and total antioxidant capacity (T-AOC) activity, total superoxide dismutase (T-SOD), and GSH-Px levels in liver tissues were significantly increased, while the intake of baicalein was significantly decreased. Malondialdehyde levels in serum and meat tissues were reduced. Baicalein can be used as an effective natural feed additive in broiler diets, and a 100–200 mg/kg diet is evaluated as the optimal dose. Króliczewska et al. [94] evaluated the effect of the roots of S. baicalensis on the production performance of broilers and found that the addition of S. baicalensis roots to the diet (5–15 mg/kg diet) was able to increase broiler body weight and feed conversion efficiency but had no effects on the quality and chemical composition of broiler leg muscles.
The addition of S. baicalensis extracts to the drinking water of turkeys (0.009, 0.018, and 0.036 mL/kg bodyweight) was able to change the concentrations of sodium, potassium, calcium, magnesium, copper, zinc, and iron in plasma, showing an upward trend in the concentrations of calcium and magnesium and a downward trend in the concentration of sodium, potassium, copper, zinc, and iron [95]. In addition, the dietary supplementation of S. baicalensis extracts to laying hens at a dose of 5 g/kg diet effectively increased the weight of the eggs, decreased the microbial content in the cecum, reduced the amount of propylene glycol, and delayed lipid oxidation in the eggs [96]. Fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, and S. baicalensis) at doses of 0.5–2 g/kg diet can be used as an alternative to reduce the use of antimicrobial agents in broilers for improved production performance [108].
Medicinal herbs are often applied in the form of a composite preparation after the assessment of compatibility. Varmuzova et al. [97] found that Curcuma (Curcuma longa) extract alone was not enough to reduce intestinal inflammation caused by heat stress. However, the mixture of C. longa and S. baicalensis plant extracts as feed additives reduced intestinal inflammation caused by a high air temperature or by Salmonella enteritidis, reduced the counts of Salmonella in cecum-midgut, and had no negative effects on body weight or humoral immune response. Using the 16S rRNA sequencing technique, it was found that the dietary supplementation of the two plant extracts had no effects on microbial diversity. However, if the plant extract supplements are provided to chickens infected with S. enteritidis, Enterococcus faecalis, and Lactobacillus spp., the bacterial genera with known positive effects on intestinal health are actively selected. Therefore, the supplementation of chicken feed with Curcuma and Scutellaria plant extracts can be used in poultry production to effectively reduce intestinal inflammation and improve chicken production performance [97]. Liu and Kim [109] found that the addition of S. baicalensis and Lonicera japonica extracts to the feed at a dose of 0.25–0.5 g/kg diet alleviated the detrimental effects of seasonal heat stress on the production performance of laying hens. Lv et al. [98] found that the dietary supplementation of S. baicalensis and L. japonica extracts could improve the growth performance of broilers, promote the development of immune organs, and improve the antioxidant function. Their results showed that the addition of 500 mg plant extracts/kg of diet could increase the average daily weight gain at the age of 21–42 days and 1–42 days, increase feed conversion efficiency, increase the thymus and bursa index of 42-day-old broilers, increase the activity of serum catalase, and decrease the level of malondialdehyde in 21- and 42-day-old broilers.
Cheng et al. [110] studied the anti-inflammatory effect of baicalin on LPS-induced chicken liver inflammation and its molecular mechanism. Histopathological changes, serum biochemical analysis, nitric oxide (NO) level, and myeloperoxidase activity showed that baicalin pretreatment alleviated LPS-induced liver inflammation. ELISA and qPCR analysis showed that baicalin dose-dependently inhibited the formation of IL-1β, IL-6, and TNF-α. In addition, baicalin significantly decreased the mRNA expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Molecular studies showed that baicalin pretreatment inhibited TLR4 expression and the activation of the NF-kB signaling pathway. Baicalin pretreatment down-regulated TLR4 expression and inhibited NF-kB activation, thus had a protective effect on chicken liver from LPS-induced inflammation. Ishfaq et al. [111] also showed that baicalin treatment could effectively prevent Mycoplasma gallinarum-induced inflammation, apoptosis, and energy metabolism dysfunction, providing a basis for new therapeutic targets to control M. gallinarum infection. However, Króliczewska et al. [112] found that excessive supplementation of S. baicalensis roots (5–15 g/kg diet) may cause immunosuppression and may have a negative impact on the development of immune organs. S. baicalensis roots inhibited the formation of radially segmented nuclei, showing anti-metastatic properties and phagocytosis of chicken heterophils.
Dietary supplementation of S. baicalensis extracts improves growth performance, blood parameters, nutrient digestibility, and meat quality of broilers [113]. The combined use of S. baicalensis extract and Zn in the feed not only improved the quality of broilers but also showed antioxidant capacity [114]. Liang et al. [99] examined the effects of baicalin on the growth performance and intestinal bacterial community of broiler chickens at different doses supplemented to the basal diet and found that the average body weight of broilers in all treatment groups was increased compared with the control group. Among different treatments, the dose of 10 mg baicalein/kg diet had the highest body weight and the supplementation of 5 mg/kg baicalein to the diet increased the average daily feed intake. Compared with the control group, the number of E. coli and Salmonellas were decreased and the number of Lactobacillus and Bifidobacterium were increased in all experimental groups. The addition of an appropriate dose (10 mg/kg diet) of baicalein in the diet could promote the growth of broilers and modulate the intestinal microbial community. Wang et al. [115] studied the potential alleviative effect of combined plant extracts of L. japonica and S. baicalensis (active ingredients are chlorogenic acid and baicalin) on intestinal damage and bacterial dysbiosis caused by Salmonella pullorum. It was found that this preparation could effectively alleviate the intestinal damage and the loss of animal performance caused by S. pullorum, for which the regulation of intestinal microbial composition by the plant extracts is an important mechanism of action.
4.2. Swine
Li and Diao [100] found that the addition of fermented S. baicalensis to the diet could enhance the appetite of weaned piglets at the age of 28 days, increase the average daily intake, reduce the feed-to-weight ratio and diarrhea rate, and improve the feed conversion rate. Among the different doses, a dose of 1.5 g/kg diet had the best effect on daily intake. With this dosage, feed intake and daily weight gain were increased by 13% and 33%, and the feed-to-weight ratio and diarrhea rate decreased by 15% and 31% compared with the control group, respectively.
S. baicalensis and its extracts can effectively improve the growth performance and manipulate the intestinal microflora of pigs. Zhao et al. [101] evaluated the dietary supplementation of fermented medicinal plants (FMP) consisting of Gynura procumbens, R. glutinosa and S. baicalensis in weaned piglets. It was found that FMP additives significantly increased the average daily weight gain, average daily feed intake, the ratio of body weight to feed, and the apparent total tract digestibility of dry matter, nitrogen, and gross energy, and decreased the concentrations of ammonia, total thiol, and hydrogen sulfide. In addition, the diarrhea rate of piglets was reduced with the addition of FMP. Thus, FMP can be used to improve growth performance and nutrient digestibility, mitigate harmful gas emissions from feces, and reduce the early diarrhea rate of weaned piglets.
The addition of FMP containing S. baicalensis to the diet of fattening pigs was able to improve body weight gain, feed conversion efficiency, and digestibility and mitigate harmful gas emissions [116,117]. The combination of S. baicalensis, Gardenia jasminoides, and lactic acid bacteria can accelerate the clearance time of bacteria in feces, improve the immunity of infected pigs, and regulate the enzymatic activity of intestinal microorganisms in order to convert herbal compounds into active compounds [102]. Therefore, this mixture as a feed additive can be a potential preventive agent for Salmonella infection.
The addition of S. baicalensis extract to the diet at 1000 mg/kg diet can effectively alleviate diarrhea in weaned piglets and may reduce the expression of inflammatory cytokines by inhibiting the nuclear factor kappa-B/mitogen activated protein kinase (NF-κB/p38) signaling pathway, thereby improving intestinal health [103]. Baicalin in combination with aluminum can reduce piglet diarrhea. Genomic analysis indicated that the baicalin aluminum complex preparation could modulate the gut microbiota of diarrheal piglets and was associated with flagellar assembly, bacterial chemotaxis, lipopolysaccharide biosynthesis, ATP binding cassette transporters, biosynthesis of amino acids, and phosphotransferase systems [104]. The addition of mixed medicinal herbs containing S. baicalensis to the diet of pregnant and lactating sows can lead to decreased weight loss after delivery and can improve litter performance [107].
In addition, baicalin improves parthenogenetically activated and in vitro fertilized pig embryos by inhibiting the production and apoptosis of reactive oxygen species, regulating mitochondrial activity, and activating sonic hedgehog signaling [118].
4.3. Ruminants
S. baicalensis extracts can alter the microbial flora in the rumen of ruminants, thereby promoting the fermentation of forage in the rumen [119]. S. baicalensis has evident antipyretic effects and has been incorporated into many medicinal herb preparations [120], and thus has great potential for reducing the impact of heat stress in intensive and large-scale ruminants farming. Baicalin can be used as an anti-apoptotic agent to alleviate heat stress-induced apoptosis. Guo et al. [29] examined the effects of baicalin on heat stress-induced apoptosis of bovine Sertoli cells using flow cytometry and found that pretreatment with baicalin at 1, 10, and 20 μg/mL significantly reduced apoptosis induced by heat stress at 43 °C for 1 h. There was a dose-dependent relationship between baicalin concentration and the rate of cell survival.
Systemic inflammation is more common in early lactation dairy cows and can result in a decline in milk production. S. baicalensis contains flavonoids with anti-inflammatory and antioxidant effects. Olagaray et al. [26] incorporated S. baicalensis extracts into pelleted feed and provided them to lactating Holstein dairy cows at a dose of 10 g extracts/d (containing 3.3 g of flavonoid baicalin/d), which resulted in a reduced incidence of mastitis. A long-term supplementation (60 days) significantly improved milk yield in the whole lactation period, while short-term (5 days) administration did not change the milk production. The mechanisms underlying the improvement of lactation performance warrant further study.
5. Application of S. baicalensis in Disease Prevention and Treatment
Medicinal herbal preparations are mainly natural plant extracts, which can not only be used as feed ingredients but also have significant anti-parasitic, antibacterial, anti-viral, and other effects due to the effective bioactive compounds. Thus, they are of great value in the prevention and treatment of animal diseases.
Liao et al. [105] studied the therapeutic effect of baicalein injection on artificially infected swine edema disease in weaned piglets aged 28–35 days. Intraperitoneal injection of Vibrio cholerae O139 bacteria suspension was used to establish the swine edema disease model of artificially infected pigs, and different doses of S. baicalensis extract were injected for the treatment of the disease. It was found that the doses of 0.2 and 0.4 mL S. baicalensis extract/kg body weight significantly increased the weight gain rate of the experimental animals. The weight gain rate and total effective rate were 96% and 90%, respectively. This indicates that the injection of S. baicalensis extract could effectively treat the edema disease of artificially infected pigs.
Baicalin can effectively inhibit Mycoplasma gallisepticum induced immune deficiency and attenuate inflammatory response and apoptosis in the chicken bursa of Fabricius. Baicalin attenuates the levels of pro-inflammatory factors, and suppresses NF-κB expression at the protein and miRNA levels, alleviating reduction in M. gallisepticum induced CD8+ cells and bacterial burden in the bursa [121]. In addition, treatment with baicalin (450 mg/kg BW for 5 days) could effectively alleviate the extent of lesions in lung and tracheal tissues, alveolar space, and mucosal layer thickening were restored, cilia were gradually restored, and the IL-17 signaling pathway-related genes were significantly reduced. The activities of cytokines and chemokines (CXCL1, CXCL2, MMP1, GMCSF, and MUC5AC) were decreased significantly. Baicalin was able to effectively treat co-infection caused by M. gallinarum and E. coli [122]. Baicalin can resolve intestinal dysbacteriosis caused by H9N2 subtype avian influenza infection by modulating lactic acid bacteria, thus preventing the high mortality caused by the secondary infection of Escherichia. Baicalin had beneficial effects for clinical prevention and control of H9N2 Subtype Avian infection and secondary bacterial infections and inflammation by inhibiting the loss of intestinal structure and improving antioxidant capacity, affecting blood biochemical indexes, and inhibiting the production of inflammatory factors [123]. Baicalin effectively treats polyserositis caused by Glaesserella parasitis infection by alleviating the downregulation of mRNA for tight junctions, preventing the abnormalities, and maintaining the integrity of tight junctions, and is a potential natural medicine for the prevention and treatment of G. parasitis [124].
Baicalin showed significant antibacterial activity against E. coli in vitro. In a study by Zhao et al. [36], the minimum inhibitory concentration of baicalin against E. coli isolated from mastitis in dairy cattle was 4000 µg/mL, and antimicrobials such as streptomycin, ciprofloxacin, and ampicillin had synergistic effects in combination with baicalin. The combinations could significantly increase the susceptibility to E. coli. Therefore, baicalin may be used as an antimicrobial agent alone or in combination with antibiotics to treat E. coli caused mastitis in dairy cows. It was found that panton-valentine leucocidin (PVL)-killing interleukin produced by S. aureus was one of the causes of dairy cow mastitis. Apoptosis and necrosis of bovine mammary epithelial cells were associated with PVL, while baicalin could inhibit interleukin-producing S. aureus and consequently reduce apoptosis. Thus, baicalin has great potential for use in the treatment of mastitis caused by S. aureus [125]. High-yielding dairy cows are prone to oxidative reactions which are closely associated with inflammation. Baicalin has considerable anti-inflammatory and antioxidant effects on LPS-induced inflammatory damage of mammary epithelial cells in dairy cows. It can be used to prevent oxidative metabolic disorders in dairy cows and has been effectively applied in clinical practice [69,126].
Baicalin is widely used to treat viral diseases such as bovine viral diarrhea and enteritis [127]. Previous studies have shown that the cure rate of the decoction of S. baicalensis in conjunction with Fructus gardeniae on bovine diarrhea was 95%, and the cure rate in vivo was 75% [128]. In addition, the decoction of S. baicalensis in conjunction with F. gardeniae can directly inhibit the bovine viral diarrhea virus in vitro [129]. Lv et al. [106] showed that oral administration of baicalin at medium and low doses (850 and 425 mg/d for 5 days) could effectively treat piglet diarrhea and found that baicalin could inhibit inflammatory exudation and reduce the release of inflammatory cytokines, which might be one of the mechanisms of its therapeutic effect. Baicalin showed potent activity against Newcastle disease virus and direct killing effect against Newcastle disease, capable of inhibiting the infection of chicken embryo fibroblasts and blocking the intracellular Newcastle disease virus, and has the potential to be used as a pharmaceutical ingredient [130].
Baicalin had inhibitory effects on duck hepatitis virus (duck hepatitis A virus type 1, DHAV-1). An in vitro mechanistic study revealed that baicalin inhibited the propagation of DHAV-1 by interfering with the viral replication and release of the virus. An in vivo mechanistic study showed that the antioxidant and immunological enhancement functions of baicalin played a crucial role in its therapeutic effects against duck viral hepatitis. Baicalin may serve as a potential agent for the treatment of duck viral hepatitis [131].
6. Conclusions
S. baicalensis contains a variety of active compounds with multiple biological functions, such as antioxidation and bacteriostatic and anti-inflammatory effects. Thus, it has extensive potential in the production and health of food animals. At present, S. baicalensis is sometimes used in animal husbandry production in the form of composite preparations. The chemical composition of the composite preparations is complicated, which makes it challenging to elucidate mechanisms of actions and results in unstable effects. Therefore, it is particularly important to study monomer compounds present in medicinal herbs. Currently, most studies on the biological activity and pharmacological effects of baicalin, baicalein, and other extracts of S. baicalensis are conducted with laboratory animals and few studies have been performed in animal production and clinical application. There is an urgent need to strengthen the research and application of baicalin and other herbal active compounds in clinical practice. The substitution of antibiotics by natural herbs and their active compounds has become a trend in the future research and development of animal feed, with great potential in practice.
Author Contributions
Conceptualization, X.S. and B.Y.; writing—original draft preparation, W.L., B.Y., H.Q., J.Y., and X.S.; writing—review and editing, X.S.; supervision, B.Y. and X.S.; project administration, X.S.; funding acquisition, X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Jilin Agricultural Science and Technology University, grant number 2018:5001 and by the Development of Science and Technology of Jilin Province, grant number 20200602016ZP.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the collection, analyses, or interpretation of data collected from the literature; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wallmann J. Antimicrobial resistance: Challenges ahead. Vet. Rec. 2014;175:323–324. doi: 10.1136/vr.g5953. [DOI] [PubMed] [Google Scholar]
- 2.Phillips I., Casewell M., Cox T., De Groot B., Friis C., Jones R., Nightingale C., Preston R., Waddell J. Antibiotic use in animals. J. Antimicrob. Chemother. 2004;53:885. doi: 10.1093/jac/dkh149. [DOI] [PubMed] [Google Scholar]
- 3.Bren L. Battle of the bugs: Fighting antibiotic resistance. FDA Consum. 2002;36:28–34. [PubMed] [Google Scholar]
- 4.Walton J.R. Antibiotic residues in meat. Br. Vet. J. 1987;143:485–486. doi: 10.1016/0007-1935(87)90034-0. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulou C., Leach N., Eckford S. Waste milk feeding, animal by-products regulations and antibiotic resistance. Vet. Rec. 2013;172:166. doi: 10.1136/vr.f783. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. WHO; Geneva, Switzerland: 2017. [PubMed] [Google Scholar]
- 7.European Food Safety Authority (EFSA) ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals: Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA J. 2017;15:e04872. doi: 10.2903/j.efsa.2017.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprarulo V., Giromini C., Rossi L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal. 2021;15:100064. doi: 10.1016/j.animal.2020.100064. [DOI] [PubMed] [Google Scholar]
- 9.Liu S.H., Chuang W.C., Lam W., Jiang Z., Cheng Y.C. Safety surveillance of traditional Chinese medicine: Current and future. Drug Saf. 2015;38:117–128. doi: 10.1007/s40264-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storlien T.M., Prestløkken E., Beauchemin K.A., McAllister T.A., Iwaasa A., Harstad O.M. Supplementation with crushed rapeseed causes reduction of methane emissions from lactating dairy cows on pasture. Anim. Prod. Sci. 2017;57:81–89. doi: 10.1071/AN15287. [DOI] [Google Scholar]
- 11.Shan C.H., Guo J., Sun X., Li N., Yang X., Gao Y., Qiu D., Li X., Wang Y., Feng M., et al. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J. Anim. Sci. 2018;96:4444–4457. doi: 10.1093/jas/sky270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W.J., Wang S.P., Luo D.M., Zhao X.L., Yin M.J., Zhou C.F., Liu G.W. Effect of Chinese herbal medicines on rumen fermentation, methanogenesis and microbial flora in vitro. S. Afr. J. Anim. Sci. 2019;49:63–70. doi: 10.4314/sajas.v49i1.8. [DOI] [Google Scholar]
- 13.Zhu Z., Song Z.H., Cao L.T., Wang Y., Zhou W.Z., Zhou P., Zuo F.Y. Effects of traditional Chinese medicine formula on ruminal fermentation, enzyme activities and nutrient digestibility of beef cattle. Anim. Sci. J. 2018;89:661–671. doi: 10.1111/asj.12978. [DOI] [PubMed] [Google Scholar]
- 14.Patra A.K., Saxena J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie Van Leeuwenhoek. 2009;96:363–375. doi: 10.1007/s10482-009-9364-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Xie H., Liu F., Wang Y. Production performance, immunity, and heat stress resistance in Jersey cattle fed a concentrate fermented with probiotics in the presence of a Chinese herbal combination. Anim. Feed Sci. Technol. 2017;228:59–65. doi: 10.1016/j.anifeedsci.2017.03.015. [DOI] [Google Scholar]
- 16.Wang H.F., Yang W.R., Wang Y.X., Yang Z.B., Cui Y.H. The study on the effects of Chinese herbal mixtures on growth, activity of post-ruminal digestive enzymes and serum antioxidant status of beef cattle. Agric. Sci. China. 2011;10:448–455. doi: 10.1016/S1671-2927(11)60024-2. [DOI] [Google Scholar]
- 17.Abdallah A., Zhang P., Zhong Q., Sun Z. Application of traditional chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019;20:54–64. doi: 10.2174/1389200219666180523102920. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Cao B., Bai C., Li G., Mao M. Predicting suitable cultivation regions of medicinal plants with Maxent modeling and fuzzy logics: A case study of Scutellaria baicalensis in China. Environ. Earth Sci. 2016;75:1–12. doi: 10.1007/s12665-015-5133-9. [DOI] [Google Scholar]
- 19.Zhao T., Tang H., Xie L., Zheng Y., Ma Z., Sun Q., Li X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019;71:1353–1369. doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]
- 20.Xing J., Chen X., Sun Y., Luan Y., Zhong D. Interaction of baicalin and baicalein with antibiotics in the gastrointestinal tract. J. Pharm. Pharmacol. 2005;57:743–750. doi: 10.1211/0022357056244. [DOI] [PubMed] [Google Scholar]
- 21.Huang T., Liu Y., Zhang C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 2019;44:159–168. doi: 10.1007/s13318-018-0509-3. [DOI] [PubMed] [Google Scholar]
- 22.Xiao L., Wang F., Li H.D., Zhao X.Y. Pharmacokinetic study on baicalin of Qingkailing injection in rats. Zhongguo Zhong Yao Za Zhi. 2007;32:2534–2538. [PubMed] [Google Scholar]
- 23.Gao Z., Huang K., Yang X., Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta. 1999;1472:643–650. doi: 10.1016/S0304-4165(99)00152-X. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Qiao Z., Hu W., Zhang W., Shah S.W.A., Ishfaq M. Baicalin mitigated Mycoplasma gallisepticum-induced structural damage and attenuated oxidative stress and apoptosis in chicken thymus through the Nrf2/HO-1 defence pathway. Vet. Res. 2019;50:83. doi: 10.1186/s13567-019-0703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Yang L. Molecular regulatory mechanism of Nrf2 antioxidant. Chin. J. Bioinform. 2018;16:1–6. doi: 10.3969/j.issn.1672-5565.201708001. [DOI] [Google Scholar]
- 26.Olagaray K.E., Brouk M.J., Mamedova L.K., Sivinski S.E., Liu H., Robert F., Dupuis E., Zachut M., Bradford B.J. Dietary supplementation of Scutellaria baicalensis extract during early lactation decreases milk somatic cells and increases whole lactation milk yield in dairy cattle. PLoS ONE. 2019;14:e0210744. doi: 10.1371/journal.pone.0210744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Ge X. Efffect of baicalin on antipyresis and influence on cytokine. Zhongguo Zhong Yao Za Zhi. 2010;35:1068–1072. doi: 10.4268/cjcmm20100829. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H.Y., Zhang F., Fan S.D. Effects of baicalin on contents of PGE2 and cAMP in hypothalamus of fever rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2002;18:139–141. [PubMed] [Google Scholar]
- 29.Guo X., Chi S., Cong X., Li H., Jiang Z., Cao R., Tian W. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod. Toxicol. 2015;57:196–203. doi: 10.1016/j.reprotox.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 30.Chen D.S., Cao J.G., Zhu B., Wang Z.L., Wang T.F., Tang J.J. Baicalin attenuates joint pain and muscle dysfunction by inhibiting muscular oxidative stress in an experimental osteoarthritis rat model. Arch. Immunol. Ther. Exp. Warsz. 2018;66:453–461. doi: 10.1007/s00005-018-0518-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.-K., Song Y.-K., Lim H.-H. Analgesic and anti-inflammatory effect of Scutellaria baicalensis. Korean J. Orient. Med. 2007;28:124–135. [Google Scholar]
- 32.Yimam M., Brownell L., Hodges M., Jia Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet Suppl. 2012;9:155–165. doi: 10.3109/19390211.2012.708713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J., Wang J., Sheng Y., Zou Y., Bo L., Wang F., Lou J., Fan X., Bao R., Wu Y., et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS ONE. 2012;7:e35523. doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnett B.P., Jia Q., Zhao Y., Levy R.M. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food. 2007;10:442–451. doi: 10.1089/jmf.2006.255. [DOI] [PubMed] [Google Scholar]
- 35.Simujide A.H., Aorigele C., Chun-Jie W., Manda B. Serotyping of Escherichia coli from healthy cattle and analyses of antimicrobial activities of Chinese herbal drugs on Escherichia coli strains with different serotypes in Hulunbeier, China. Minerva Biotecnol. 2011;23:65–70. [Google Scholar]
- 36.Zhao Q.Y., Yuan F.W., Liang T., Liang X.C., Luo Y.R., Jiang M., Qing S.Z., Zhang W.M. Baicalin inhibits Escherichia coli isolates in bovine mastitic milk and reduces antimicrobial resistance. J. Dairy Sci. 2018;101:2415–2422. doi: 10.3168/jds.2017-13349. [DOI] [PubMed] [Google Scholar]
- 37.Chan B.C., Ip M., Lau C.B., Lui S.L., Jolivalt C., Ganem-Elbaz C., Litaudon M., Reiner N.E., Gong H., See R.H., et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011;137:767–773. doi: 10.1016/j.jep.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 38.Qian M., Tang S., Wu C., Wang Y., He T., Chen T., Xiao X. Synergy between baicalein and penicillins against penicillinase-producing Staphylococcus aureus. Int. J. Med. Microbiol. 2015;305:501–504. doi: 10.1016/j.ijmm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Huang Q., Zhang J., Peng J., Zhang Y., Wang L., Wu J., Ye L., Fang C. Effect of baicalin on proliferation and apoptosis in pancreatic cancer cells. Am. J. Transl. Res. 2019;11:5645–5654. [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y., Pei M., Li L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int. J. Clin. Exp. Med. 2015;8:8958–8967. [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Y., Chen L., Guo S., Li Y. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol. Biol. Rep. 2019;46:1693–1700. doi: 10.1007/s11033-019-04618-9. [DOI] [PubMed] [Google Scholar]
- 42.Ren X., Zhang Y., Li C., Wang H., Jiang Z., Zhang Z., Guo Q., Song G., Bi K., Jiang G. Enhancement of baicalin by hexamethylene bisacetamide on the induction of apoptosis contributes to simultaneous activation of the intrinsic and extrinsic apoptotic pathways in human leukemia cells. Oncol. Rep. 2013;30:2071–2080. doi: 10.3892/or.2013.2684. [DOI] [PubMed] [Google Scholar]
- 43.Gao Z., Huang K., Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001;43:173–178. doi: 10.1006/phrs.2000.0761. [DOI] [PubMed] [Google Scholar]
- 44.Tu I.H., Yen H.T.D., Cheng H.W., Chiu J.H. Baicalein protects chicken embryonic cardiomyocyte against hypoxia-reoxygenation injury via μ- and δ- but not κ-opioid receptor signaling. Eur. J. Pharmacol. 2008;588:251–258. doi: 10.1016/j.ejphar.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Dai C.S., Tang S.S., Zhang Q.J., Li D.W., Xiao X.L. Biological functions of baicalin and baicalein and their application in animal production. China Feed. 2015;18:11–14. doi: 10.15906/j.cnki.cn11-2975/s.20150803. [DOI] [Google Scholar]
- 46.Yun M.Y., Yang J.H., Kim D.K., Cheong K.J., Song H.H., Kim D.H., Cheong K.J., Kim Y.I., Shin S.C. Therapeutic effects of baicalein on atopic dermatitis-like skin lesions of NC/Nga mice induced by dermatophagoides pteronyssinus. Int. Immunopharmacol. 2010;10:1142–1148. doi: 10.1016/j.intimp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y., Liu T., Wang K., Hou C., Cai S., Huang Y., Du Z., Huang H., Kong J., Chen Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the Quorum sensing system in vitro. PLoS ONE. 2016;11:e0153468. doi: 10.1371/journal.pone.0153468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farhadi F., Khameneh B., Iranshahi M., Iranshahy M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019;33:13–40. doi: 10.1002/ptr.6208. [DOI] [PubMed] [Google Scholar]
- 49.Da X., Nishiyama Y., Tie D., Hein K.Z., Yamamoto O., Morita E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019;9:1683. doi: 10.1038/s41598-019-38916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serpa R., França E.J.G., Furlaneto-Maia L., Andrade C., Diniz A., Furlaneto M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. J. Med. Microbiol. 2012;61:1704–1708. doi: 10.1099/jmm.0.047852-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H.C., Wang H., Shi K., Li J.M., Zong Y., Du R. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules. 2018;24:131. doi: 10.3390/molecules24010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith J.F., Starr E.G., Goodman M.A., Hanson R.B., Palmer T.A., Woolstenhulme J.B., Weyand J.A., Marchant A.D., Bueckers S.L., Nelson T.K., et al. Topical application of Wogonin provides a novel treatment of knee osteoarthritis. Front. Physiol. 2020;11:80. doi: 10.3389/fphys.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chi Y.S., Lim H., Park H., Kim H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003;66:1271–1278. doi: 10.1016/S0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 54.Seong R.K., Kim J.A., Shin O.S. Wogonin, a flavonoid isolated from Scutellaria baicalensis, has anti-viral activities against influenza infection via modulation of AMPK pathways. Acta Virol. 2018;62:78–85. doi: 10.4149/av_2018_109. [DOI] [PubMed] [Google Scholar]
- 55.Wu J., Hu D., Wang K.X. Study of Scutellaria baicalensis and baicalin against antimicrobial susceptibility of Helicobacter pylori strains in vitro. Zhong Yao Cai. 2008;31:707–710. [PubMed] [Google Scholar]
- 56.Qiu F., Meng L., Chen J., Jin H., Jiang L. In vitro activity of five flavones from Scutellaria baicalensisin combination with Cefazolin against methicillin resistant Staphylococcus aureus (MRSA) Med. Chem. Res. 2016;25:2214–2219. doi: 10.1007/s00044-016-1685-9. [DOI] [Google Scholar]
- 57.Leonova G.N., Shutikova A.L., Lubova V.A., Maistrovskaya O.S. Inhibitory activity of Scutellaria baicalensis flavonoids against tick-borne encephalitis virus. Bull. Exp. Biol. Med. 2020;168:665–668. doi: 10.1007/s10517-020-04776-y. [DOI] [PubMed] [Google Scholar]
- 58.Zhang T., Chen J., Wang C., Shi W., Li D. The therapeutic effect of Yinhuangerchen mixture on avian infectious laryngotracheitis. Poult. Sci. 2018;97:2690–2697. doi: 10.3382/ps/pey125. [DOI] [PubMed] [Google Scholar]
- 59.Shin H.S., Bae M.J., Choi D.W., Shon D.H. Skullcap (Scutellaria baicalensis) extract and its active compound, wogonin, inhibit ovalbumin-induced Th2-mediated response. Molecules. 2014;19:2536–2545. doi: 10.3390/molecules19022536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae M.J., Shin H.S., See H.J., Jung S.Y., Kwon D.A., Shon D.H. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci. Rep. 2016;6:32225. doi: 10.1038/srep32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim T.W., Song I.B., Lee H.K., Kim M.S., Ham S.H., Cho J.H., Lim J.H., Yun H.I. Assessment of dermal safety of Scutellaria baicalensis aqueous extract topical application on skin hypersensitivity. Planta Med. 2013;79:959–962. doi: 10.1055/s-0032-1328714. [DOI] [PubMed] [Google Scholar]
- 62.Yoon S.B., Lee Y.J., Park S.K., Kim H.C., Bae H., Kim H.M., Ko S.G., Choi H.Y., Oh M.S., Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009;125:286–290. doi: 10.1016/j.jep.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 63.Kim D.S., Son E.J., Kim M., Heo Y.M., Nam J.B., Ro J.Y., Woo S.S. Antiallergic herbal composition from Scutellaria baicalensis and Phyllostachys edulis. Planta Med. 2010;76:678–682. doi: 10.1055/s-0029-1240649. [DOI] [PubMed] [Google Scholar]
- 64.Cha J.-H., Kim H.-W., Kim S.G., Jung S.-H., Whang W.-K. Antioxidant and antiallergic activity of compounds from the aerial parts of Scutellaria baicalensis Georgi. Yakhak Hoeji. 2006;50:136–143. [Google Scholar]
- 65.Zhou Y.J., Wang H., Sui H.H., Li L., Zhou C.L., Huang J.J. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic Rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm. Res. 2016;65:603–612. doi: 10.1007/s00011-016-0943-0. [DOI] [PubMed] [Google Scholar]
- 66.Liu J.J., Huang T.S., Cheng W.F., Lu F.J. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int. J. Cancer. 2003;106:559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- 67.Xue Y., Zheng Y., An L., Dou Y., Liu Y. Density functional theory study of the structure-antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014;151:198–206. doi: 10.1016/j.foodchem.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 68.Tong R., Mehendale S.R., Wang C.Z., Shao Z., Yuan C.S. Comparison of antioxidant effects of various Scutellaria baicalensis fractions and the potential role of catalase upregulation. Am. J. Chin. Med. 2009;37:621–623. doi: 10.1142/S0192415X09007107. [DOI] [PubMed] [Google Scholar]
- 69.Yang W., Li H., Cong X., Wang X., Jiang Z., Zhang Q., Qi X., Gao S., Cao R., Tian W. Baicalin attenuates lipopolysaccharide induced inflammation and apoptosis of cow mammary epithelial cells by regulating NF-κB and HSP72. Int. Immunopharmacol. 2016;40:139–145. doi: 10.1016/j.intimp.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 70.Guo M., Zhang N., Li D., Liang D., Liu Z., Li F., Fu Y., Cao Y., Deng X., Yang Z. Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int. Immunopharmacol. 2013;16:125–130. doi: 10.1016/j.intimp.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Tian H., Wang X.P., Zhang F.L. Protective effects of baicalein on the gastric mucosa of rats with chronic atrophic gastritis. Tradit. Chin. Med. J. 2015;14:62–64. doi: 10.14046/j.cnki.zyytb2002.2015.05.025. [DOI] [Google Scholar]
- 72.Dinda B., Dinda S., DasSharma S., Banik R., Chakraborty A., Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Lee W., Ku S.K., Bae J.S. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation. 2015;38:110–125. doi: 10.1007/s10753-014-0013-0. [DOI] [PubMed] [Google Scholar]
- 74.Chen W., Li B., Li S., Ou Y.W., Ou Q. Effects of Scutellaria baicalensis on activity and biofilm formation of Klebsiella pneumoniae. Chin. Med. Sci. J. 2016;31:180–184. doi: 10.1016/S1001-9294(16)30048-7. [DOI] [PubMed] [Google Scholar]
- 75.Sohail A., Bhat W.F., Bhat S.A., Furkan M., Shah A., Bano B. Investigating the preventive effects of baicalin and gallocatechin against glyoxal-induced cystatin aggregation. J. Biomol. Struct. Dyn. 2018;36:3791–3802. doi: 10.1080/07391102.2017.1400470. [DOI] [PubMed] [Google Scholar]
- 76.Zeng Q., Zhang Y., Zhang W., Guo Q. Baicalein suppresses the proliferation and invasiveness of colorectal cancer cells by inhibiting Snail-induced epithelial-mesenchymal transition. Mol. Med. Rep. 2020;21:2544–2552. doi: 10.3892/mmr.2020.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song J., Zhou Y.Z., Pang Y.Y., Gao L., Du G.H., Qin X.M. The anti-aging effect of Scutellaria baicalensis Georgi flowers extract by regulating the glutamine-glutamate metabolic pathway in D-galactose induced aging rats. Exp. Gerontol. 2020;134:110843. doi: 10.1016/j.exger.2020.110843. [DOI] [PubMed] [Google Scholar]
- 78.Ren L., Liu W., Wang C., Yang Y., Huang X., Wang C., Li Y. The ancient Chinese formula Longdan Xiegan Tang improves antipsychotic-induced hyperprolactinemia by repairing the hypothalamic and pituitary TGF-β1 signaling in rats. J. Ethnopharmacol. 2020;254:112572. doi: 10.1016/j.jep.2020.112572. [DOI] [PubMed] [Google Scholar]
- 79.Nam J.E., Jo S.Y., Ahn C.W., Kim Y.S. Baicalin attenuates fibrogenic process in human renal proximal tubular cells (HK-2) exposed to diabetic milieu. Life Sci. 2020;254:117742. doi: 10.1016/j.lfs.2020.117742. [DOI] [PubMed] [Google Scholar]
- 80.Limanaqi F., Biagioni F., Busceti C.L., Polzella M., Fabrizi C., Fornai F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants. 2020;9:234. doi: 10.3390/antiox9030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Q., Xu H., Zhao X. Baicalin inhibits human cervical cancer cells by suppressing protein kinase C/signal transducer and activator of transcription (PKC/STAT3) signaling pathway. Med. Sci. Monit. 2018;24:1955–1961. doi: 10.12659/MSM.909640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han Y.H., Kee J.Y., Kim D.S., Mun J.G., Park S.H., Kim Y.J., Um J.Y., Hong S.H. Arctii Fructus inhibits colorectal cancer cell proliferation and MMPs mediated invasion via AMPK. Am. J. Chin. Med. 2017;45:1309–1325. doi: 10.1142/S0192415X17500720. [DOI] [PubMed] [Google Scholar]
- 83.You J., Cheng J., Yu B., Duan C., Peng J. Baicalin, a Chinese herbal medicine, inhibits the proliferation and migration of human non-small cell lung carcinoma (NSCLC) cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Med. Sci. Monit. 2018;24:2126–2133. doi: 10.12659/MSM.909627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diao X., Yang D., Chen Y., Liu W. Baicalin suppresses lung cancer growth by targeting PDZ-binding kinase/T-LAK cell-originated protein kinase. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y., Hong Z., Chen P., Wang J., Zhou Y., Huang J. Baicalin inhibits growth and induces apoptosis of human osteosarcoma cells by suppressing the AKT pathway. Oncol. Lett. 2019;18:3188–3194. doi: 10.3892/ol.2019.10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng A., Liang X., Zhu S., Liu C., Luo X., Zhang Q., Song L. Baicalin, a potent inhibitor of NF-κB signaling pathway, enhances chemosensitivity of breast cancer cells to docetaxel and inhibits tumor growth and metastasis both in vitro and in vivo. Front. Pharmacol. 2020;11:879. doi: 10.3389/fphar.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng L., Dong J., Yu W., Huang J., Liu B., Feng X. Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm. Pharmacol. Ther. 2010;23:411–419. doi: 10.1016/j.pupt.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Wang S., Zheng Z., Weng Y., Yu Y., Zhang D., Fan W., Dai R., Hu Z. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 2004;74:2467–2478. doi: 10.1016/j.lfs.2003.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y., Lu N., Ling Y., Gao Y., Wang L., Sun Y., Qi Q., Feng F., Liu W., Liu W., et al. Wogonoside inhibits lipopolysaccharide-induced angiogenesis in vitro and in vivo via toll-like receptor 4 signal transduction. Toxicology. 2009;259:10–17. doi: 10.1016/j.tox.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Song X., Chen Y., Sun Y., Lin B., Qin Y., Hui H., Li Z., You Q., Lu N., Guo Q. Oroxylin A, a classical natural product, shows a novel inhibitory effect on angiogenesis induced by lipopolysaccharide. Pharmacol. Rep. 2012;64:1189–1199. doi: 10.1016/S1734-1140(12)70915-5. [DOI] [PubMed] [Google Scholar]
- 91.Zhou M., Song X., Huang Y., Wei L., Li Z., You Q., Guo Q., Lu N. Wogonin inhibits H2O2-induced angiogenesis via suppressing PI3K/Akt/NF-κB signaling pathway. Vasc. Pharmacol. 2014;60:110–119. doi: 10.1016/j.vph.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Fan J.H., Yang Y.L., Gu H.B., Jiang Y.X., Li S.M., Dou X.H. Study on the germicidal efficacy and safety evaluation of a Chinese medicine semen disinfectant. Chin. J. Vet. Drug. 2020;54:41–48. [Google Scholar]
- 93.Zhou Y., Mao S., Zhou M. Effect of the flavonoid baicalein as a feed additive on the growth performance, immunity, and antioxidant capacity of broiler chickens. Poult. Sci. 2019;98:2790–2799. doi: 10.3382/ps/pez071. [DOI] [PubMed] [Google Scholar]
- 94.Króliczewska B., Zawadzki W., Skiba T., Kopec W., Kroliczewski J. The influence of baical skullcap root (Scutellaria baicalensis radix) in the diet of broiler chickens on the chemical composition of the muscles, selected performance traits of the animals and the sensory characteristics of the meat. Vet. Med. 2008;53:373–380. doi: 10.17221/1994-VETMED. [DOI] [Google Scholar]
- 95.Rusinek-Prystupa E., Lechowski J., Żukiewicz-Sobczak W., Sobczak P., Zawiślak K. Influence of citrosept addition to drinking water and Scutellaria baicalensis root extract on the content of selected mineral elements in blood plasma of Turkey hens. Ann. Agric. Environ. Med. 2014;21:595–600. doi: 10.5604/12321966.1120609. [DOI] [PubMed] [Google Scholar]
- 96.An B.K., Kwon H.S., Lee B.K., Kim J.Y., You S.J., Kim J.M., Kang C.W. Effects of dietary skullcap (Scutellaria baicalensis) extract on laying performance and lipid oxidation of chicken eggs. Asian-Australas. J. Anim. Sci. 2010;23:772–776. doi: 10.5713/ajas.2010.90517. [DOI] [Google Scholar]
- 97.Varmuzova K., Matulova M.E., Gerzova L., Cejkova D., Gardan-Salmon D., Panhéleux M., Robert F., Sisak F., Havlickova H., Rychlik I. Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella enteritidis infection. Poult. Sci. 2015;94:2049–2058. doi: 10.3382/ps/pev190. [DOI] [PubMed] [Google Scholar]
- 98.Lv H.Y., Li M., Wang Z.M., Liang W., Nie W., Guo Y.M. Effects of Lonicera japonica and Scutellaria baicalensis extracts on growth performance, immune organ development and antioxidant function of Broilers. Chin. J. Anim. Sci. 2020:1–9. doi: 10.19556/j.0258-7033.20200302-06. [DOI] [Google Scholar]
- 99.Liang Y., Ren C.C., Jing D., Teng Z.C., Bi H.M. Effects of flavonoids from Scutellaria baicalensis Georgi on growth performance and intestinal microflora of broilers. J. Tradit. Chin. Vet. Med. 2012;31:39–42. doi: 10.3969/j.issn.1000-6354.2012.04.013. [DOI] [Google Scholar]
- 100.Li G.Z., Diao X.P. Effect of adding fermented Scutellaria in feed on production performance of weaned piglets. China Feed. 2012;34:27–28. doi: 10.15906/j.cnki.cn11-2975/s.2012.12.007. [DOI] [Google Scholar]
- 101.Zhao P., Li H., Lei Y., Li T., Kim S., Kim I. Effect of fermented medicinal plants on growth performance, nutrient digestibility, fecal noxious gas emissions, and diarrhea score in weanling pigs. J. Sci. Food Agric. 2016;96:1269–1274. doi: 10.1002/jsfa.7217. [DOI] [PubMed] [Google Scholar]
- 102.Chang C.H., Chen Y.S., Chiou M.T., Su C.H., Chen D.S., Tsai C.E., Yu B., Hsu Y.M. Application of Scutellariae radix, Gardeniae fructus, and probiotics to prevent Salmonella enterica serovar choleraesuis infection in swine. Evid.-Based Complementary Altern. Med. 2013;2013 doi: 10.1155/2013/568528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang C., Wang Y., He X., Jiao N., Zhang X., Qiu K., Piao X., Yin J. The involvement of NF-κB/P38 pathways in Scutellaria baicalensis extracts attenuating of Escherichia coli K88-induced acute intestinal injury in weaned piglets. Br. J. Nutr. 2019;122:152–161. doi: 10.1017/S0007114519000928. [DOI] [PubMed] [Google Scholar]
- 104.Fu S., Zhuang F., Guo L., Qiu Y., Xiong J., Ye C., Liu Y., Wu Z., Hou Y., Hu C.A.A. Effect of baicalin-aluminum complexes on fecal microbiome in piglets. Int. J. Mol. Sci. 2019;20:2390. doi: 10.3390/ijms20102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao Y.J., Cui Y.M., Hu X.R., Zheng Y.W., Lu H.M., Huang D., Deng Y.H., Deng X.M. Treatment test of skullcap injection on the artificial infection of swine edema disease. Chin. J. Vet. Sci. 2016;36 doi: 10.16303/j.cnki.1005-4545.2016.05.24. [DOI] [Google Scholar]
- 106.Lv J.F., Wen A.Y., Ning K.J., Li L., Jiang J.P., Lu Z.X., Liu C., Ying R.H., Feng B.M. Effects and mechanism of baicalin for treating diarrheic piglets. Chin. J. Vet. Sci. 2016;36:1401–1405. doi: 10.16303/j.cnki.1005-4545.2016.08.26. [DOI] [Google Scholar]
- 107.Gheisar M.M., Cheong J.Y., Zhao P., Kim I.H. Evaluating the influence of dietary phytogenic blends on gestating and lactating sows and suckling piglets. Anim. Prod. Sci. 2018;50:2071–2075. doi: 10.1071/AN15447. [DOI] [Google Scholar]
- 108.Jeong J.S., Kim I.H. Effect of fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, scutellaria baicalensis) as alternative performance enhancers in broilers. J. Poult. Sci. 2015;52:119–126. doi: 10.2141/jpsa.0140061. [DOI] [PubMed] [Google Scholar]
- 109.Liu W.C., Kim I.H. Influence of extract mixture from Scutellaria baicalensis and Lonicera japonica on egg production, nutrient digestibility, blood profiles and egg quality in laying hens reared in hot humid season. Anim. Nutr. Feed Technol. 2017;17:137–146. doi: 10.5958/0974-181X.2017.00014.2. [DOI] [Google Scholar]
- 110.Cheng P., Wang T., Li W., Muhammad I., Wang H., Sun X., Yang Y., Li J., Xiao T., Zhang X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front. Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishfaq M., Zhang W., Hu W., Waqas Ali Shah S., Liu Y., Wang J., Wu Z., Ahmad I., Li J. Antagonistic effects of baicalin on Mycoplasma gallisepticum-induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infect. Drug Resist. 2019;12:3075–3089. doi: 10.2147/IDR.S223085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Króliczewska B., Graczyk S., Króliczewski J., Pliszczak-Król A., Miśta D., Zawadzki W. Investigation of the immune effects of Scutellaria baicalensis on blood leukocytes and selected organs of the chicken’s lymphatic system. J. Anim. Sci. Biotechnol. 2017;8:22. doi: 10.1186/s40104-017-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park J.H., Pi S.H., Kim I.H. Growth performance, blood profile, nutrient digestibility and meat quality of broilers fed on diets supplemented with Scutellaria baicalensis extract. Eur. Poult. Sci. 2016;80:1–10. doi: 10.1399/eps.2016.155. [DOI] [Google Scholar]
- 114.Liao X.D., Wen Q., Zhang L.Y., Lu L., Zhang L.Y., Luo X.G. Effect of dietary supplementation with flavonoid from Scutellaria baicalensis Georgi on growth performance, meat quality and antioxidative ability of broilers. J. Integr. Agric. 2018;17:1165–1170. doi: 10.1016/S2095-3119(17)61803-3. [DOI] [Google Scholar]
- 115.Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G., Qi G.H. Supplemental plant extracts from Flos lonicerae in combination with Baikal skullcap Attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeong J.S., Kim I.H. Effect of probiotic bacteria-fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, Scutellaria baicalensis) as performance enhancers in growing pigs. Anim. Sci. J. 2015;86:603–609. doi: 10.1111/asj.12331. [DOI] [PubMed] [Google Scholar]
- 117.Liu W.C., Pi S.H., Kim I.H. Effects of Scutellaria baicalensis and Lonicera japonica extract mixture supplementation on growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs. Ital. J. Anim. Sci. 2016;15:446–452. doi: 10.1080/1828051X.2016.1202736. [DOI] [Google Scholar]
- 118.Guo Q., Xuan M.F., Luo Z.B., Wang J.X., Han S.Z., Ri M.H., Choe Y.G., Hwang K.M., Yin X.J., Kang J.D. Baicalin improves the in vitro developmental capacity of pig embryos by inhibiting apoptosis, regulating mitochondrial activity and activating sonic hedgehog signaling. Mol. Hum. Reprod. 2019;25:538–549. doi: 10.1093/molehr/gaz036. [DOI] [PubMed] [Google Scholar]
- 119.Yausheva E.V., Duskaev G.K., Levakhin G.I., Nurzhanov B.S., Yuldashbaev Y.A., Rysaev A.F., Rakhmatullin S.G., Inchagova K.S. Evaluation of the effects of plant extracts on cattle rumen microbiome. IOP Conf. Ser. Earth Environ. Sci. 2019;341:012165. doi: 10.1088/1755-1315/341/1/012165. [DOI] [Google Scholar]
- 120.Gao X., Guo M., Li Q., Peng L., Liu H., Zhang L., Bai X., Wang Y., Li J., Cai C. Plasma metabolomic profiling to reveal antipyretic mechanism of Shuang-huang-lian injection on yeast-induced pyrexia rats. PLoS ONE. 2014;9:e100017. doi: 10.1371/journal.pone.0100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishfaq M., Zhang W., Liu Y., Wang J., Wu Z., Shah S.W., Li R., Miao Y., Chen C., Li J. Baicalin attenuated Mycoplasma gallisepticum-induced immune impairment in chicken bursa of Fabricius through modulation of autophagy and inhibited inflammation and apoptosis. J. Sci. Food Agric. 2021;101:880–890. doi: 10.1002/jsfa.10695. [DOI] [PubMed] [Google Scholar]
- 122.Wu Z., Fan Q., Miao Y., Tian E., Ishfaq M., Li J. Baicalin inhibits inflammation caused by coinfection of Mycoplasma gallisepticum and Escherichia coli involving IL-17 signaling pathway. Poult. Sci. 2020;99:5472–5480. doi: 10.1016/j.psj.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X., Zhao Q., Ci X., Chen S., Chen L., Lian J., Xie Z., Ye Y., Lv H., Li H., et al. Effect of baicalin on bacterial secondary infection and inflammation caused by H9N2 AIV infection in chickens. Biomed. Res. Int. 2020;2020:2524314. doi: 10.1155/2020/2524314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J., Zhang Z., Xu J., Ye C., Fu S., Hu C.A., Qiu Y., Liu Y. Protective effects of baicalin on peritoneal tight junctions in piglets challenged with Glaesserella parasuis. Molecules. 2021;26:1268. doi: 10.3390/molecules26051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jia F., Ma W., Zhang X., Wang D., Zhou X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 2020;103:2731–2742. doi: 10.3168/jds.2019-17619. [DOI] [PubMed] [Google Scholar]
- 126.Perruchot M.H., Gondret F., Robert F., Dupuis E., Quesnel H., Dessauge F. Effect of the flavonoid baicalin on the proliferative capacity of bovine mammary cells and their ability to regulate oxidative stress. PeerJ. 2019;7:e6565. doi: 10.7717/peerj.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng C., Pei T., Huang C., Chen X., Bai Y., Xue J., Wu Z., Mu J., Li Y., Wang Y. A novel systems pharmacology platform to dissect action mechanisms of traditional Chinese medicines for bovine viral diarrhea disease. Eur. J. Pharm. Sci. 2016;94:33–45. doi: 10.1016/j.ejps.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 128.Wang J.Y., Zhao Y.L., Qin J.H. Research on the efficacy of Chinese herbal compound for bovine viral diarrhea. Prog. Vet. Med. 2010;31:117–119. doi: 10.3969/j.issn.1007-5038.2010.11.029. [DOI] [Google Scholar]
- 129.Yang F., Yue H., Xu S.J., Luo T.T., Hou W. Estimation of the antivirus effects of Chinese herbal compound in vitro. J. Southwest Univ. Natl. Nat. Sci. Ed. 2009;35:529–531. doi: 10.3969/j.issn.1003-2843.2009.03.033. [DOI] [Google Scholar]
- 130.Jia Y., Xu R., Hu Y., Zhu T., Ma T., Wu H., Hu L. Anti-NDV activity of baicalin from a traditional Chinese medicine in vitro. J. Vet. Med Sci. 2016;78:819–824. doi: 10.1292/jvms.15-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Y., Yao F., Ming K., Shi J., Zeng L., Wang D., Wu Y., Hu Y., Liu J. Assessment of the effect of baicalin on duck virus hepatitis. Curr. Mol. Med. 2019;19:376–386. doi: 10.2174/1566524019666190405095301. [DOI] [PubMed] [Google Scholar]