Small Aδ and C nerve fibers of the sensory and autonomic nervous systems constitute 70–90% of peripheral nerve fibers including corneal nerves (Muller et al., 2003). Corneal nerves originate from the ophthalmic branch of the trigeminal nerve and enter the cornea at the limbus radially from all directions toward the central cornea at the level of anterior and middle stroma. The subepithelial nerve plexus lies at the interface between the Bowman layer and anterior stroma. They then divide into smaller branches and turn 90° toward Bowman’s layer (Muller et al., 2003), travelling between Bowman’s layer and the basal epithelial layer and forming the sub-basal nerve plexus (Muller et al., 2003). Innervation of the cornea is comprised almost entirely of unmyelinated type C nerve fibers, with the fiber width ranging between 0.2–2.0 μm. Corneal nerves not only provide important sensory function but also maintain the functional integrity of the ocular surface by releasing trophic substances that promote corneal epithelial homeostasis and by activating brainstem circuits that stimulate reflex tear production and blinking (Marfurt et al., 2010). Disruption of corneal nerves result in reduced or absent corneal sensations as well as negative impacts on the ocular surface integrity and tear film dynamics, leading to dry eye symptoms, corneal epithelial breakdown, and neurotrophic keratopathy (Al-Aqaba et al., 2019).
Corneal nerve morphology and distribution can be visualized with different types of staining techniques (Al-Aqaba et al., 2019). Conventional staining techniques, such as gold chloride, silver staining and methylene blue, are now less commonly used due to the high level of technical skill required and low sensitivity to show nerve patterns (Al-Aqaba et al., 2019). Acetylcholinesterase (AChE) staining and immunohistochemical staining with anti-class β III tubulin marker are two most common techniques used (Figure 1A and B). Corneal nerve axons contain cholinesterase enzymes; they play a role in maintaining the ionic gradient during propagation of the nerve impulse along the axons (Al-Aqaba et al., 2019). In a cornea with neovascularization, AChE staining may be confusing as AChE activity is also present in blood vessels (Al-Aqaba et al., 2019). β III tubulin is a microtubule element that is found almost exclusively in neurons (Al-Aqaba et al., 2019). It is used extensively to study the corneal nerves distribution or patterns. Corneal nerve features demonstrated by both these techniques show good correlation, although AChE staining is able to show more details such as nerve perforation sites in sub-Bowman’s layer and limbal nerve corpuscles (Al-Aqaba et al., 2019). These staining techniques, however, can only be applied for in vitro or ex vivo corneas.
Figure 1.
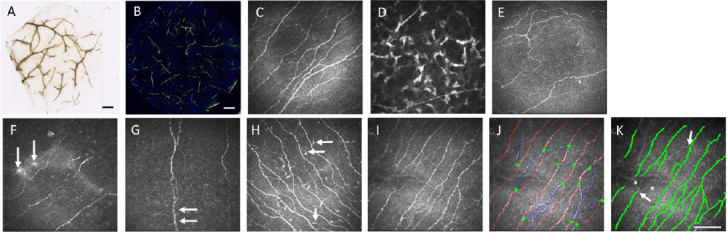
Corneal nerve plexus demonstrated by staining techniques and IVCM.
Corneal nerve bundles stained with acetylcholinesterase (A) and anti-class β III tubulin marker (B) on an intrastromal lenticule extracted from small incision lenticule extraction (SMILE) procedure showing the neurite distribution inside a lenticule (A: rabbit lenticule, Courtesy: Dua HS; B: human lenticule). Scale bars: 500 μm. In vivo confocal microscopy (IVCM) images of corneal sub-basal nerve plexus (C), subepithelial nerves (D), nerve plexus with increased tortuosity (E), micro-neuromas (F; arrows), nerve beading (G, arrows), and dendritic cells (H, arrows). Representative images of raw IVCM image (I), and images with sub-basal nerves marked manually with CCMetrics software (J) and automatically with ACCMetrics software (K). In manual analysis, red lines indicate nerve fibers, blue lines indicate nerve branches, and green dots indicate branching points. There is underestimation in the automated analysis in which low-contrast nerves (asterisks) and corresponding branching points (arrows) are not marked. Scale bar: 100 μm. Figure 1 is sourced from the authors’ unpublished data.
In vivo confocal microscopy (IVCM) has been used extensively to image corneal nerves as well as live cellular architecture in vivo. The use of tandem-scanning confocal microscope has been discontinued due to the scattering of light source, and slit-scanning confocal microscope does not provide sufficient details in nerve plexus (Jalbert et al., 2003). Hence laser scanning confocal microscope has become the dominant system in clinical use. laser scanning confocal microscope provides good image contrast, with image lateral resolution of 1–2 μm, axial resolution of 5–10 μm, and magnification of 600–800 times (Jalbert et al., 2003). These favourable characteristics have widened the applications of IVCM and tremendously enhanced the knowledge of corneal nerves in health and diseases.
On IVCM evaluation, sub-basal nerves appear as well-defined, densely-anastomosed, thin and linear structures with homogenous reflectivity and Y-shaped or H-shaped branches (Figure 1C). The subepithelial nerve plexus is usually of low contrast with the appearance of some varicosities or beads that are sparsely distributed in the anterior stroma (Figure 1D). Stromal nerves are present in the anterior and mid stroma and are thick and bright structure with no internal structure details being visible on IVCM.
With growing interest in research on corneal nerves, development of reliable methods to quantify nerve parameters are imperative, to monitor the nerve plexus changes with time, to allow accurate evaluation on nerve damage and recovery following surgery or treatment, to stratify corneal neuropathy, and to ensure comparisons across research studies. The analytical software ranges from being fully manual, semi-automated, to being fully automated, all of which produce a multitude of metrics related to corneal nerve morphology.
CCMetrics (University of Manchester, UK) software utilizes an algorithm to derive nerve statistics, including corneal nerve fiber density (CNFD), corneal nerve fiber length (CNFL), corneal nerve branch density (CNBD) and nerve fiber tortuosity, after users manually trace the visible nerves using the in-built manual drawing module. It is therefore highly user-dependent and labor-intensive. Given its subjective element in the measurements, it requires the expert to discern the nerve fibers, particularly those nerves with poor contrast, and the analysis is prone to have intra-observer and inter-observer inconsistencies. The intra-/inter-observer intra-class correlation is approximately 0.51–0.81/0.39–0.81 for CNFD, 0.72–0.76/0.68–0.78 for CNFL, and 0.44–0.67/0.32–0.63 for CNBD, based on our experiences and literature (Ostrovski et al., 2015). Neuron J (National Institute of Health, USA) is a semi-automated nerve-tracing software. It provides automated tracing by identifying the beginning and the optimal path of the nerve, and then allows manual editing for those nerves incorrectly identified or missed out by the software.
Due to the time-consuming nature of manual and semi-automated software, there is a need to develop an automated tool to enhance the efficiency of nerve analysis and to eliminate the process for training personnel for analysis. The automated software has to consist of two processes: nerve fiber detection and nerve fiber quantification. ACCMetrics software (University of Manchester, UK) is the most commonly used tool, and it saves the image analysis time from 15–20 minutes per image for manual analysis, to 15 seconds for automated analysis. Hence it is particularly useful for large studies that contain a high volume of IVCM images. Corneal nerves are firstly detected via a dual model feature descriptor combined with a neural network classifier which distinguishes nerve fibers from background noise and underlying tissue. The software then quantifies the morphometric nerve parameters, including CNFD, CNFL, CNBD, nerve fiber total branch density, nerve fiber area, nerve fiber width, nerve fiber orientation histogram, nerve fiber width histogram, and nerve fibre fractal dimension (CFracDim). Patients with diabetic corneal neuropathy, dry eye diseases, corneal dystrophy, keratoconus, or those who have undergone refractive surgery, have been shown to have decreased CNFD, CNFL and CNBD (Agca et al., 2015; Ostrovski et al., 2015; Al-Aqaba et al., 2019; Giannaccare et al., 2019). CFracDim is a metric that indicates the spatial loss of nerves: a high CFracDim value corresponds to an evenly distributed complex nerve fiber structure that likely belongs to a healthy subject (Chen et al., 2018). A reduction in CFracDim has been reported in patients with diabetic corneal neuropathy (Chen et al., 2018).
Among the nerve metrics, CNFD and CNFL are the most validated parameters, and have been shown to have good reproducibility (Chin et al., 2020). CNFL is considered as the most consistent parameter to evaluate the status of sub-basal nerve plexus. Both CNFD and CNFL assessed by manual and automated tools are generally highly correlated, but an underestimation is noted when using the automated software (Chin et al., 2020). This may be due to the inability of automated software to define finer, out-of-focus, fainter and low-contrast nerve fibers, compared to the manual method (Figure 1). This also explains why CNBD measurement is susceptible to measurement errors as sometimes 2 crossing fibers against a noisy background can be interpreted as a single branching fiber in the automated software or vice versa (Chin et al., 2020). Due to the difference in the algorithm and inherent measurement bias in each analytic system, we feel that the values obtained with different software are therefore not interchangeable across different studies. The optimal analytical tool chosen depends on the study design and aims.
Increased nerve tortuosity is observed in a variety of corneal pathologies such as dry eye disease, corneal edema, diabetic corneal neuropathy, or herpetic corneal diseases (Al-Aqaba et al., 2019). Nerve tortuosity has been categorized and quantified with subjective and objective grading scales. The former subjectively grade the nerve tortuosity from grade 0 (almost straight) to grade 5 (very tortuous, showing abrupt and frequent changes in nerve fiber direction; Oliveira-Soto and Efron score). The latter is available in the CCMetrics software, and it mathematically computes the fiber tortuosity and derives a tortuosity coefficient (0–1). Research on the development of novel tortuosity estimation paradigm that has more discriminative tortuosity features is ongoing.
Quantification of other nerve features, like nerve dendritic cells, beading, sprouting or micro-neuromas (Figure 1E–H), still mainly relies on manual counting although there are some research-grade tools available for automatic counting. The long dendrites of dendritic cells sometimes mimic small nerve branches and lead to erroneous recognition. Beading of nerves are foci of hyper-reflective points, which are axon enlargements with a collection of mitochondria. They slightly protrude from the main truck and represents a natural response following nerve damage. Nerve regenerative activity following nerve denervation is manifested by sprouting from endbulbs and the formation of micro-neuromas, seen as abrupt swelling of injured nerve endings and neurite sprouting (Al-Aqaba et al., 2019).
The consequences of nerve denervation and regeneration may be reflected clinically on the ocular surface. Clinically, the corneal nerve parameters have been used as indicators for the evaluation of nerve status in the patients who have undergone refractive surgery (Patel abd McGhee, 2009; Agca et al., 2015), patients with neurotrophic keratopathy (Dua et al., 2018), dry eye (Patel and McGhee, 2009), or diabetic sensorimotor polyneuropathy (Pritchard et al., 2015). Moreover, the corneal nerve metrics provide predictive value in identifying high-risk patients, for example, patients at risk of developing severe neurotrophic keratopathy (Dua et al., 2018) or diabetic polyneuropathy (Pritchard et al., 2015). The changes of sub-basal nerve density have been reported to be correlated with changes in Schirmer’s test, corneal fluorescein staining, and corneal sensitivity (Benitez del Castillo et al., 2004). However, clinical function and nerve morphology may not correlate. Clinical symptoms can be present in the absence of visible nerve pathology and vice versa (Al-Aqaba et al., 2019). This comes from the fact that the current clinical evaluation on ocular surface integrity and sensitivity may be too coarse and not sensitive enough to reflect the changes in nerve status.
In summary, IVCM images can be used as a surrogate biomarker for the status of corneal nerves. The analysis of images provides information on the diagnosis of corneal neuropathy, disease severity and progression, and treatment responses. With the increasing popularity of research concerning corneal nerves and clinical applications of IVCM, there is a need to refine current image acquisition systems as well as analytic technology. The inherent limitation of current IVCM is it provides a small region of interest (< 500 μm2). Automated mapping or montaging techniques, as well as wide-field or large-area scanning of corneal sub-basal nerve plexus, have been proposed. However, at present these approaches are neither standardized nor commercially available. For analytic software, research work would need to focus in improving the accuracy and minimizing the discrepancies of automated measurements, by improving the algorithm and prespecified criteria for nerve detection, especially for those nerve fibers with low contrast on a relatively noisy background, or fine interconnecting branches with poor visibility. An artificial intelligence-based deep learning algorithm has attempted to segment the sub-basal nerve fibers to improve the quantitative capacity for nerve morphology. Post-scan imaging processing or different types of image filtering depending on the segment features to enhance local images may help. The analysis and acquisition time should be reasonably fast to make it suitable for clinical use.
Additional file: Open peer review report 1 (79.7KB, pdf) .
Footnotes
P-Reviewer: Lin Z; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Zhongjing Lin, Shanghai Jiao Tong University, China.
References
- 1.Agca A, Cankaya KI, Yilmaz I, Yildirim Y, Yasa D, Olcucu O, Demircan A, Demirok A, Yilmaz OF. Fellow eye comparison of nerve fiber regeneration after SMILE and femtosecond laser-assisted LASIK: a confocal microscopy study. J Refract Surg. 2015;31:594–598. doi: 10.3928/1081597X-20150820-04. [DOI] [PubMed] [Google Scholar]
- 2.Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762. doi: 10.1016/j.preteyeres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Graham J, Petropoulos IN, Ponirakis G, Asghar O, Alam U, Marshall A, Ferdousi M, Azmi S, Efron N, Malik RA. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Invest Ophthalmol Vis Sci. 2018;59:1113–1118. doi: 10.1167/iovs.17-23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin JY, Yang LWY, Ji AJS, Nubile M, Mastropasqua L, Allen JC, Mehta JS, Liu YC. Validation of the use of automated and manual quantitative analysis of corneal nerve plexus following refractive surgery. Diagnostics (Basel) 2020;10:493. doi: 10.3390/diagnostics10070493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, Shortt AJ, Geerling G, Nubile M, Figueiredo FC, Rauz S, Mastropasqua L, Rama P, Baudouin C. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–131. doi: 10.1016/j.preteyeres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Giannaccare G, Pellegrini M, Sebastiani S, Moscardelli F, Versura P, Campos EC. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefes Arch Clin Exp Ophthalmol. 2019;257:583–589. doi: 10.1007/s00417-018-04225-7. [DOI] [PubMed] [Google Scholar]
- 8.Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87:225–236. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 11.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in healthm, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 12.Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38:671–675. doi: 10.2337/dc14-2114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


