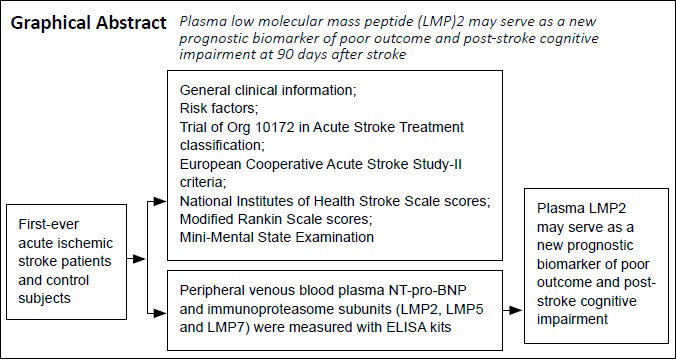
Keywords: behavior, biomarker, brain, clinical trial, cognitive impairment, immune function, neurological function, protein, stroke
Abstract
Many blood biomarkers are reportedly helpful for predicting post-stroke cognitive impairment (PSCI), but no biomarkers are widely used in clinical practice. The purpose of this study was to investigate the association between the plasma immunoproteasome and patients’ 90-day prognosis after first-ever acute ischemic stroke. In our prospective, single-center study, 259 patients with first-ever acute ischemic stroke were enrolled from the Department of Neurology, Fujian Provincial Hospital, China, from March to September 2014. Of these, 27 patients (10.4%) had unfavorable outcomes as assessed by the Modified Rankin Scale (scores of 3–6). The National Institutes of Health Stroke Scale score on admission, plasma N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels, and immunopro-teasome subunit (low molecular mass peptide [LMP]2, LMP5, and LMP7) levels were significantly higher in the unfavorable outcome group than in the favorable outcome group. To predict unfavorable outcomes, the optimal cutoff points were National Institutes of Health Stroke Scale score > 12, NT-pro-BNP level > 1883.5 pg/mL, and LMP2 level > 841.4 pg/mL. Of the 193 patients that were able to complete the Mini-Mental State Examination at 90 days post-stroke, 66 patients (34.2%) had PSCI. Plasma levels of NT-pro-BNP and LMP2 were higher in patients with PSCI than in those without PSCI. To predict PSCI, the optimal cutoff values were age > 70.5 years and LMP2 level > 630.5 pg/mL. These findings indicate that plasma LMP2 may serve as a new prognostic biomarker of poor outcome and PSCI at 90 days after stroke. This study was approved by the Ethics Committee of Fujian Provincial Hospital, Provincial Clinical Medical College of Fujian Medical University (approval No. K2014-01-003) on January 15, 2014.
Chinese Library Classification No. R446.1; R741; R392.3
Introduction
Acute ischemic stroke (AIS) is one of the leading causes of morbidity and mortality worldwide. Growing evidence indicates that many factors are closely related to an increased risk of poor outcome (Zhu et al., 2018a; Zhou et al., 2019; Zhang et al., 2020). However, although many biomarkers have been previously reported to have high statistical power for predicting the outcomes of stroke patients (Tóth et al., 2017; Zhu et al., 2018b), almost none of them have yet been accepted for use in clinical practice. Ideally, biomarkers should be closely related to the pathophysiological mechanisms of stroke, and provide a reliable predictive value of outcome.
Immunity and inflammation participate in the pathogenesis of ischemic stroke (Lee et al., 2018; Mo et al., 2020). A number of inflammatory mediators have been linked to stroke outcome and may be used as biomarkers in the future (Gori et al., 2017; Xu et al., 2018). The immunoproteasome is a subtype of proteasome, and contains three major catalytic subunits: β1i (also known as low molecular mass peptide 2 [LMP2]), β2i (also known as LMP5), and β5i (also known as LMP7). Recent studies have revealed a novel interaction between the immunoproteasome and glia-mediated inflammatory responses, which results in a pro-inflammatory environment (Basler et al., 2014; Chen et al., 2015). Our previous research has confirmed that the immunoproteasome participates in the inflammatory pathophysiological mechanisms of ischemic stroke, and that short hairpin RNA-mediated inhibition of immunoproteasome LMP2 significantly reduces cerebral infarction volume, attenuates inflammatory reactions, and promotes angiogenesis (Chen et al., 2015, 2018). Clinically, high plasma levels of immunoproteasomes are helpful for the early prediction of hemorrhagic transformation (HT) (Chen et al., 2017). However, the specific details of the association between the immunoproteasome and stroke out-come remain unclear.
Post-stroke cognitive impairment (PSCI) is common among stroke patients and sometimes occurs immediately after stroke. However, there is usually a delay of several days to months between stroke onset and the appearance of cognitive impairment (Mijajlović et al., 2017; Guo et al., 2020). It is therefore important to find a reliable biomarker that predicts PSCI. Recently, many blood biomarkers have been reported as helpful for predicting PSCI (Wu et al., 2020; Zhang and Bi, 2020), but the studies in this field have not yet been able to achieve a consensus. Interestingly, recent research suggests that the immunoproteasome is implicated in the regulation of anxiety, memory, and cognitive impairment (Orre et al., 2013; Gorny et al., 2019). Furthermore, both the expression and proteasome activity of the immunoproteasome are significantly upregulated in reactive glia surrounding β-amyloid plaques (Orre et al., 2013). However, less is known about the association between plasma immunoproteasomes and PSCI. Therefore, in this prospective single-center study, we aimed to explore the association between plasma immunoproteasomes and 90-day prognosis in first-ever AIS patients. We hope that these results will help to provide prognostic information about outcomes for AIS patients.
Participants and Methods
Participants
Between March and September 2014, 316 consecutive patients were prospectively admitted to the Department of Neurology, Fujian Provincial Hospital, within 72 hours of ischemic stroke symptom onset.
The inclusion criteria were as follows: first-ever ischemic stroke patients without previous cognitive impairment. Neurologists diagnosed AIS based on each patient’s clinical history as well as brain computed tomography (CT) and magnetic resonance imaging (MRI) scans (Hasan et al., 2018). The exclu-sion criteria were as follows: patients who had previously received oral anticoagulants or heparin, or who received thrombolytic therapy after ischemic stroke onset; patients with previous stroke; patients with a transient ischemic attack, or intracerebral or subarachnoid hemorrhage; patients with hypogly-cemia, electrolyte disturbance, severe cardiac abnormality (e.g., chronic cardiac failure, cardiomyopathy, or myocardial infarction), inflammatory or infectious diseases, epilepsies, hematological diseases, cancer, severe liver or renal failure; and patients who were previously dependent on care and/or had dementia. Thirty healthy subjects (19 men and 11 women; aged 68.1 ± 9.6 years) were also recruited as normal controls.
The study was approved by the Ethics Committee of Fujian Provincial Hospital, Shengli Clinical Medical College of Fujian Medical University (approval No. K2014-01-003) on January 15, 2014 (Additional file 1 (366.1KB, pdf) ). All participants or their legal guardians provided written informed consent.
Assessment of neurological function
Neurological symptoms were examined on admission and every day during each patient’s hospital stay. These symptoms were measured using the National Institutes of Health Stroke Scale (NIHSS) (Hasan et al., 2018). The short-term neurological functional outcome was determined at 90 days post-stroke using the modified Rankin Scale (MRS) (Broderick et al., 2017). The possible MRS scores range from 0–6; for this study, we classified patients into favorable outcome (MRS 0–2) or unfavorable outcome (MRS 3–6) subgroups. PSCI was assessed using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). PSCI was defined as MMSE < 27 (Mijajlović et al., 2017). MMSE was performed during the hospital stay (as the baseline MMSE) and at 90 days after stroke onset. Patients with severe dysphasia/aphasia, poor vision, or hearing impairment were not able to complete the cognitive testing; therefore, only 193 patients successfully completed the MMSE at 90 days post-stroke.
Clinical data collection
All subjects underwent comprehensive clinical evaluation, including blood routine, fibrinogen, and biochemical analyses, as well as electrocardiographs (Chen et al., 2017). Stroke etiology was classified according to the Trial of Org 10172 in Acute Stroke Treatment criteria (Adams et al., 1993), as follows: large artery atherosclerosis, cardioembolic, small vessel disease, and other or uncertain causes. Furthermore, the subjects were divided into groups according to the size of the infarction observed on CT or MRI (based on the methods of Pullicino et al. (1996); Pullicino formula: infarct volume = length × width × number of layers × thickness of layer/2): the large infarction group (> 10 cm3), medium infarction group (4.1–10 cm3), and small infarction group (≤ 4 cm3) (Chen et al., 2012). Lesion locations were divided into supratentorial or subtentorial infarctions, as identified by CT/MRI. HT was identified with repeated CT or MRI examinations performed 5 ± 2 days after stroke onset, or immediately in the case of clinical neurological deterioration (Chen et al., 2017). The type of HT was classified according to the European Cooperative Acute Stroke Study-II criteria (Hacke et al., 1998). Symptomatic HT or clinical deterioration caused by HT was defined as an increase of ≥ 4 points in the NIHSS (Yaghi et al., 2017) in combination with a visible HT on brain CT or MRI. All image analysis was conducted by a neuroradiologist who was blinded to this study, and the participants in the normal control group were also analyzed.
Blood sampling and laboratory measurements
Peripheral venous blood samples from the cubital veins were collected on admission, within 3 days of AIS onset (Chen et al., 2017). Ethylenediaminetetraacetic acid tubes were used to collect the blood samples. The plasma was immediately separated by centrifuging at 3000 r/min for 15 minutes at 4°C and stored at –80°C until analysis. Plasma LMP2, LMP5, and LMP7 levels were measured using quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kits (human LMP2, LMP5, and LMP7 ELISA kits) from Shanghai Meilian Biological Technology Co., Ltd. (Shanghai, China) (Chen et al., 2017). Plasma N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels were tested using human NT-pro-BNP ELISA kits (Westang, Shanghai, China). The kits were used as per the manufac-turers’ instructions. Detection was performed by researchers blinded to this study. All blood samples were tested in duplicate wells, and average values were analyzed.
Statistical analysis
Discrete variables are expressed as frequencies (percentages), continuous variables are expressed as the mean ± standard deviation (SD), and non-normally distributed variables are reported as medians with interquartile ranges (IQRs). Differences between categorical variables were assessed using the Fisher’s exact test or chi-squared test. Statistical intergroup differences were analyzed using the two independent samples t-test, one-way analysis of variance, and least significant difference test, as well as the Mann-Whitney U test or Kruskal-Wallis H test. A multivariate logistic regression analysis was performed to identify any independent factors of stroke outcome. Receiver operating characteristic (ROC) curve analysis was used to determine the sensitivity, specificity, and optimal diagnostic values of NIHSS scores, LMP2 levels, NT-pro-BNP levels, and age for stroke outcome and PSCI. The correla-tions between age and NT-pro-BNP and immunoproteasomes were investigated using Spearman’s correlation analysis. All statistical analyses were performed using SPSS (version 19; IBM, Armonk, NY, USA). P < 0.05 was considered to be significant.
Results
Clinical characteristics of first-ever ischemic stroke patients
In this study, 57 patients were lost at the 90-day follow-up; thus, 259 ischemic stroke patients were finally enrolled. The study flow chart is shown in Figure 1. Table 1 indicates the demographic and clinical characteristics of patients with both favorable and unfavorable outcomes. Briefly, 27 patients (10%) had poor outcomes, and the medians (IQRs) of MRS scores were 0 (0–1) for the favorable outcome group and 3 (3–4) for the unfavorable outcome group (P < 0.001). The mean age was higher in the unfavorable outcome group than the favorable outcome group (P = 0.033). In addition, there were significant differences between the favorable and unfavorable outcome groups in NIHSS scores on admission, percentage of atrial fibrillation, symptomatic HT, stroke etiology, and infarct volume (P < 0.05). In contrast, sex, peripheral blood plasma fibrinogen, creatinine, blood uric acid, glycosylated hemoglobin, and a history of hypertension, diabetes mellitus, hyperlipidemia, and drug treatment were not significantly different between the favorable and unfavorable outcome groups (P > 0.05; Table 1).
Figure 1.
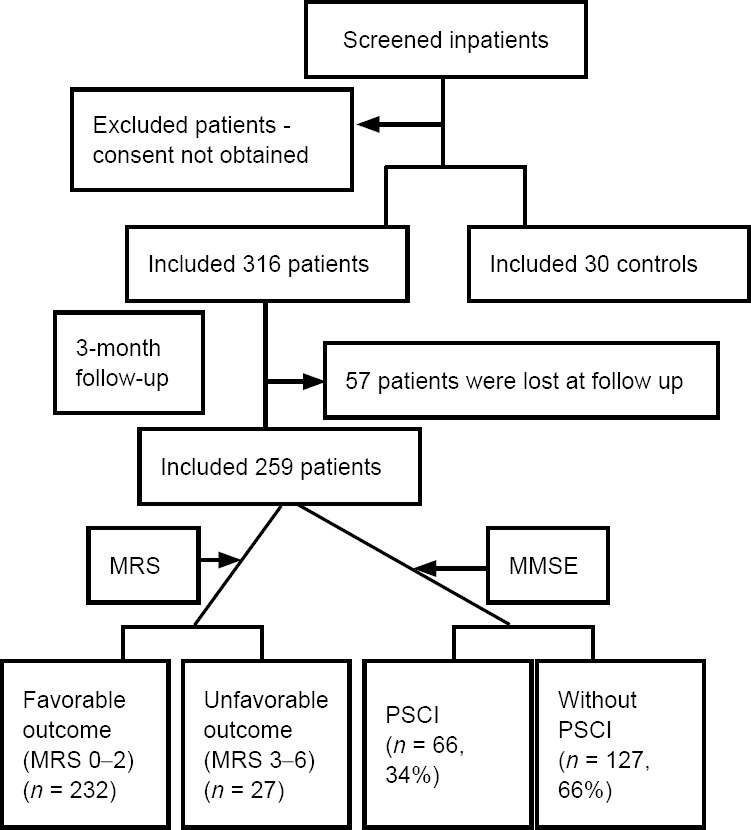
Study flow chart
MMSE: Mini-Mental State Examination; MRS: Modified Rankin Scale; PSCI: post-stroke cognitive impairment.
Table 1.
Demographic and clinical characteristics of patients with favorable and unfavorable outcomes
| Total (n = 259) | Favorable outcome (MRS 0–2) (n = 232) | Unfavorable outcome (MRS 3–6) (n = 27) | P-value | |
|---|---|---|---|---|
| MRS | 0(0–1) | 3(3–4) | < 0.001 | |
| Age (yr) | 65.3±12.5 | 64.7±12.7 | 70.1±9.0 | 0.033 |
| NIHSS on admission | 6(3–9) | 5(3–8) | 14(12–16) | < 0.001 |
| Male | 167(64.5) | 151(65.1) | 16(59.3) | 0.533 |
| Hypertension | 200(77.2) | 180(77.6) | 20(74.1) | 0.635 |
| Diabetes mellitus | 67(25.9) | 58(25) | 9(33.3) | 0.358 |
| Hyperlipidemia | 91(35.1) | 84(36.2) | 7(25.9) | 0.395 |
| Atrial fibrillation | 47(18.1) | 33(14.2) | 14(51.9) | < 0.001 |
| Hemorrhagic transformation | 24(9.3) | 12(5.2) | 12(44.4) | 0.009 |
| Symptom hemorrhagic transformation | 1(0.4) | 8(3.0) | ||
| Non -symptom hemorrhagic transformation | 11(4.4) | 4(1.5) | ||
| Stroke etiology (TOAST classification) | < 0.001 | |||
| Large artery atherosclerosis | 102(39.5) | 91(39.2) | 11(40.7) | |
| Cardioembolism | 58(22.4) | 34(14.7) | 14(51.9) | |
| Small vessel disease | 92(35.5) | 91(39.2) | 1(3.7) | |
| Other/unknown | 17(6.6) | 16(6.9) | 1(3.7) | |
| Infarct volume | < 0.001 | |||
| Large infarction | 28(10.8) | 10(4.4) | 18(66.7) | |
| Middle infarction | 119(45.9) | 111(47.8) | 8(29.6) | |
| Small infarction | 112(43.2) | 111(47.8) | 1(3.7) | |
| Plasma fibrinogen (g/L) | 3.9±1.3 | 3.8±1.4 | 4.3±1.3 | 0.156 |
| Creatinine (μM) | 72.7±30.5 | 76.7±27.3 | 72.3±31.0 | 0.476 |
| Blood uric acid (μM) | 294.1±107.9 | 296.4±106.9 | 274.4±109.3 | 0.314 |
| Glycosylated hemoglobin (%) | 6.7±1.6 | 6.7±1.9 | 6.7±1.8 | 0.65 |
| Drug treatment | ||||
| Antihypertensive agents | 194(74.9) | 176(75.8) | 18(66.7) | 0.112 |
| Antidiabetes drugs | 51(19.7) | 54(23.3) | 7(25.9) | 0.181 |
| Hypolipidemic Agents | 81(31.2) | 76(32.8) | 5(18.5) | 0.45 |
| Antithrombotic drugs | 252(97.3) | 227(97.8) | 25(92.6) | 0.158 |
Discrete variables are expressed as frequencies (percentages) and analyzed using the Fisher's exact test or chi-squared test. Continuous variables are expressed as the mean ± SD and analyzed using the two independent samples t-test. Non-normally distributed variables are reported as medians with interquartile ranges and analyzed using the Mann-Whitney U test. MRS: Modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; TOAST: trial of ORG 10172 in acute stroke treatment.
Comparisons of plasma biomarkers in first-ever ischemic stroke patients with favorable and unfavorable outcomes
The concentrations of plasma NT-pro-BNP, LMP2, LMP5, and LMP7 levels on admission were significantly higher in AIS patients compared with the normal control group (P < 0.001) (Table 2). Furthermore, the levels of plasma NT-pro-BNP, LMP2, LMP5, and LMP7 were higher in the unfavorable outcome group than in the favorable outcome group (P < 0.001; Table 2). In addition, there was a positive correlation between age and NT-pro-BNP levels (rs = 0.143, P = 0.021); however, there were no correlations between age and LMP2 (rs = 0.050, P = 0.422), LMP5 (rs = 0.026, P = 0.678), or LMP7 (rs = 0.034, P = 0.583) levels (Figure 2).
Table 2.
Comparisons of plasma biomarker levels (pg/mL) among normal controls and first-ever ischemic stroke patients with favorable and unfavorable outcomes
| Total (n = 259) | Favorable outcome (MRS 0–2) (n = 232) | Unfavorable outcome (MRS 3–6) (n = 27) | Normal group (n = 30) | P-value | |
|---|---|---|---|---|---|
| NT-pro-BNP | 397.0 (92.4–1027.0) | 306.5 (82.9–780.0) | 2359.5 (2022.4–3379.0) | 227.6 (125.7–440.1) | < 0.001 |
| LMP2 | 641.8 (368.4–860.3) | 614.0 (319.5–798.7) | 1131.9 (714.4–1473.0) | 221.5 (163.8–290.4) | < 0.001 |
| LMP5 | 410.1 (316.3–585.6) | 397.4 (306.8–553.9) | 684.9 (372.2–896.9) | 246.1 (182.5–283.6) | 0.002 |
| LMP7 | 293.0 (211.9–425.9) | 279.1 (203.6–383.0) | 652.8 (360.2–780.2) | 208.2 (155.1–256.9) | < 0.001 |
Data are expressed as medians with interquartile ranges and analyzed using the Kruskal–Wallis H test. LMP: Low molecular mass peptide; MRS: Modified Rankin Scale; NT-pro-BNP: N-terminal pro-B-type natriuretic peptide.
Figure 2.
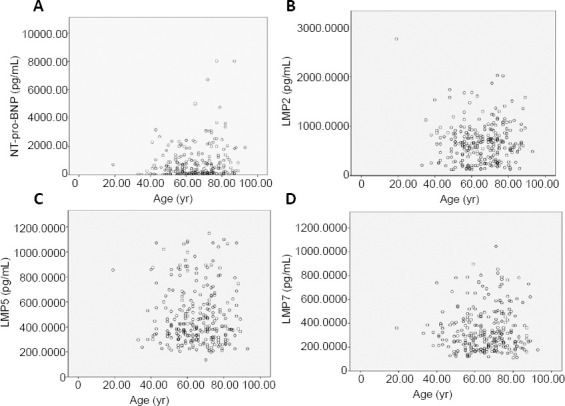
Correlation analysis between age and NT-pro-BNP, LMP2, LMP5, or LMP7 levels in first-ever ischemic stroke patients.
(A) There was a positive correlation between age and NT-pro-BNP levels (rs = 0.143, P = 0.021). (B) There was no correlation between age and LMP2 levels (rs = 0.050, P = 0.422). (C) There was no correlation between age and LMP5 levels (rs = 0.026, P = 0.678). (D) There was no correlation between age and LMP7 levels (rs = 0.034, P = 0.583). The correlations were analyzed using Spearman’s correlation analysis. LMP: Low molecular mass peptide; NT-pro-BNP: N-terminal pro-B-type natriuretic peptide.
Logistic regression analysis of stroke outcome biomarkers in first-ever ischemic stroke patients
Multivariate logistic regression analysis showed that NIHSS scores on admission (P = 0.041), NT-pro-BNP levels (P = 0.043), and LMP2 levels (P = 0.047) were independent predictors of stroke outcome, as evaluated by MRS scores (Table 3). ROC curve analysis was performed to estimate the sensitivity, specificity, and predictive values of NIHSS scores, LMP2 levels, and NT-pro-BNP levels for stroke outcome. The optimal cutoff points were as follows: NIHSS score > 12 (sensitivity 81.5%, specificity 94.4%, area under the curve [AUC] 0.953, 95% confidence interval [CI] 0.913–0.994), NT-pro-BNP level > 1883.5 pg/mL (sensitivity 88.9%, specificity 93.1%, AUC 0.960, 95% CI 0.937–0.982), and LMP2 level > 841.4 pg/mL (sensitivity 70.4%, specificity 78.9%, AUC 0.824, 95% CI 0.738–0.909) (P < 0.001).
Table 3.
Multivariate logistic regression analysis to identify independent factors associated with unfavorable outcomes in first-ever ischemic stroke patients
| Variable | Exp(B) | 95% confidence interval | P-value |
|---|---|---|---|
| Age | 0.906 | 0.714–1.151 | 0.418 |
| Atrial fibrillation | 0.002 | 0–2.375 | 0.085 |
| NIHSS on admission | 1.444 | 0.944–2.258 | 0.041 |
| Symptomatic hemorrhagic transformation | 0.043 | 0–72.988 | 0.384 |
| Infarct volume | 0.777 | 0.016–37.507 | 0.899 |
| NT-pro-BNP | 1.006 | 1.000–1.011 | 0.043 |
| LMP2 | 1.005 | 1.000–1.011 | 0.047 |
| LMP5 | 0.992 | 0.983–1.002 | 0.1 |
| LMP7 | 1.106 | 0.999–1.032 | 0.061 |
LMP: Low molecular mass peptide; NIHSS: National Institutes of Health Stroke Scale; NT-pro-BNP: N-terminal pro-B-type natriuretic peptide.
Prevalence and factors associated with PSCI at 90 days in first-ever ischemic stroke patients
The overall frequency of PSCI at 90 days was 34.2% (66 patients). The MMSE scores after 90 days were 20.05 ± 1.67 in patients with PSCI and 27.94 ± 1.39 in patients without PSCI (P < 0.001; (Table 4). There were significant differences in MRS score, age, NIHSS score on admission, percentage of atrial fibrillation, symptomatic HT, infarction location, and infarct volume between patients with PSCI and patients without PSCI (P < 0.05). The association of PSCI with infarction location was largely related to the presence of supratentorial lesions. No other differences were observed between patients with and without PSCI (P > 0.05; Table 4).
Table 4.
Characteristics and potential factors associated with cognitive status in first-ever ischemic stroke patients at 90 days after stroke
| Total (n = 193) | PSCI (n = 66, 34.2%) | Without PSCI (n = 127, 65.8% ) | P-value | |
|---|---|---|---|---|
| MMSE | ||||
| Baseline | 28.57±1.02 | 28.46±0.87 | 28.63±1.10 | 0.303 |
| After 90 d | 25.04±4.04 | 20.05±1.67 | 27.94±1.39 | < 0.001 |
| MRS | 0(0–1) | 1(0–2) | 0(0–1) | < 0.001 |
| Age (yr) | 67.7±10.9 | 75.9±8.3 | 63.5±9.7 | < 0.001 |
| Male | 123 (63.7) | 39 (59.1) | 84(66.1) | 0.348 |
| Hypertension | 148(76.7) | 48(72.7) | 100(78.7) | 0.373 |
| NIHSS on admission | 6(3–9) | 7(5–11) | 5(3–8) | 0.007 |
| Diabetes mellitus | 50(25.9) | 19(28.8) | 31(24.4) | 0.604 |
| Hyperlipidemia | 69(35.8) | 24(36.4) | 45(35.3) | 1 |
| Atrial fibrillation | 35(18.1) | 18(27.3) | 17(13.4) | 0.029 |
| Hemorrhagic transformation | 19(9.8) | 10(15.2) | 9(7.1) | 0.656 |
| Symptom hemorrhagic transformation | 4(0.4) | 5(3.0) | ||
| Non-symptom hemorrhagic transformation | 6(4.4) | 4(1.5) | ||
| Infarction location | 66(32.2) | 127(65.8) | < 0.001 | |
| Supratentorial | 64(97.0) | 98(77.2) | ||
| Subtentorial | 2(3.0) | 29(22.8) | ||
| Infarct volume | < 0.001 | |||
| Large infarction | 18(9.3) | 9(13.6) | 9(7.0) | |
| Middle infarction | 94(48.7) | 43(65.2) | 51(40.2) | |
| Small infarction | 81(42.0) | 14 (21.2) | 67(52.8) | |
| Plasma fibrinogen (g/L) | 3.9±2.7 | 4.4±4.2 | 3.7±1.1 | 0.156 |
| Hemoglobin (g/L) | 130.1±11.7 | 132.3±12.4 | 128.9±11.7 | 0.183 |
| Creatinine (μM) | 70.5±16.3 | 75.7±15.5 | 67.9±19.5 | 0.052 |
| Blood uric acid (μM) | 293.6±29.7 | 311.5±30.7 | 284.4±22.5 | 0.073 |
| Glycosylated hemoglobin (%) | 6.7±1.2 | 6.7±1.9 | 6.7±1.8 | 0.503 |
| Drug treatment | ||||
| Antihypertensive agents | 145(75.1) | 47(71.2) | 98(77.2) | 0.384 |
| Antidiabetes drugs | 44(22.8) | 16(24.2) | 28(22.1) | 0.722 |
| Hypolipidemic agents | 60(31.8) | 19(28.8) | 41(32.3) | 0.743 |
| Antithrombotic drugs | 187(96.9) | 63(95.5) | 124(97.6) | 0.413 |
Discrete variables are expressed as frequencies (percentages) and analyzed using the Fisher's exact test or chi-squared test. Continuous variables are expressed as the mean ± SD and analyzed using the two independent samples t-test. Non-normally distributed variables are reported as medians with interquartile ranges and analyzed using the Mann-Whitney U test. MMSE: Mini-Mental State Examination; MRS: Modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; PSCI: post-stroke cognitive impairment.
We next investigated the potential risk factors for PSCI. As shown in Table 5, the levels of NT-pro-BNP and LMP2 were higher in patients who developed PSCI than in those who did not develop PSCI (P < 0.05). Furthermore, a multivariable regression model revealed that age, infarction location, and LMP2 levels were independently associated with PSCI (Table 6). The optimal cutoff values for auxiliary diagnosis of PSCI were as follows: age > 70.5 years (sensitivity 81.8%, specificity 75.6%, AUC 0.844, 95% CI 0.784–0.904, P < 0.001) and LMP2 level > 630.5 pg/mL (sensitivity 69.1%, specificity 72.0%, AUC 0.829, 95% CI 0.747–0.911, P = 0.003).
Table 5.
Comparisons of plasma biomarker levels (pg/mL) between patients with and without PSCI
| Total (n = 193) | PSCI (n = 66) | Without PSCI (n = 127) | P-value | |
|---|---|---|---|---|
| NT-pro-BNP | 393.4 (103.4–1023.0) | 671.0 (132.9–1383.9) | 305.0 (84.0–876.0) | 0.027 |
| LMP2 | 645.8 (436.7–822.9) | 685.1 (536.8–1074.9) | 615.8 (322.9–764.7) | 0.003 |
| LMP5 | 408.6 (316.3–576.6) | 409.4 (315.3–675.0) | 403.8 (316.2–557.1) | 0.577 |
| LMP7 | 288.9 (212.4–435.7) | 304.1 (239.8–573.3) | 286.3 (197.6–393.9) | 0.052 |
Date are expressed as medians with interquartile ranges and analyzed using the Kruskal-Wallis H test. LMP: Low molecular mass peptide; NT-pro-BNP: N-terminal pro-B-type natriuretic peptide; PSCI: post-stroke cognitive impairment.
Table 6.
Multivariate logistic regression analysis to identify independent factors of PSCI
| Variable | Exp(B) | 95% confidence interval | P-value |
|---|---|---|---|
| MRS | 0.939 | 0.473–1.861 | 0.856 |
| Age | 1.163 | 1.108–1.222 | < 0.001 |
| Atrial fibrillation | 0.876 | 0.270–2.842 | 0.825 |
| NIHSS on admission | 0.948 | 0.781–1.152 | 0.593 |
| Infarction location | 7.493 | 1.285–43.708 | 0.025 |
| Infarct volume | 1.936 | 0.636–5.895 | 0.404 |
| NT-pro-BNP | 1.000 | 0.999–1.000 | 0.61 |
| LMP2 | 1.001 | 1.000–1.003 | 0.043 |
LMP2: Low molecular mass peptide 2; MRS: Modified Rankin Scale; NT-pro- BNP: N-terminal pro-B-type natriuretic peptide; PSCI: post-stroke cognitive impairment.
Discussion
The immunoproteasome has three specific catalytic subunits: LMP2, LMP5, and LMP7. We therefore do not fully understand the role of the immunoproteasome in ischemic stroke. It is generally believed that LMP2 is critical for proteasome activity, because LMP2 is essential for the proper assembly of the immunoproteasome (Groettrup et al., 1997). After cerebral ischemia, LMP2 and LMP7 are mainly present in astrocytes and microglia/macrophage cells, respectively (Chen et al., 2015). Given that astrocytes are the most abundant glial cells in the brain and are involved in the mechanisms of stroke pathophysiology, the present study focused on LMP2. The data from LMP5 and LMP7 showed no significance. We first demonstrated that higher plasma immunoproteasome LMP2 concentrations were significantly associated with worse functional outcomes at 90 days. Moreover, our data revealed that LMP2 is a novel high-risk factor for the development of PSCI, and may serve as an independent predictor of PSCI. Taken together, these results suggest that plasma LMP2 can act as a biomarker to predict PSCI at 90 days among first-ever AIS patients.
Several factors have been proposed to predict the prognosis of AIS. Many blood biomarkers, including NT-pro-BNP or BNP (Chen et al., 2012), microRNA (Mirzaei et al., 2018), and cytokines have been associated with poor outcome in ischemic stroke. Our previous study demonstrated that AIS patients with both elevated NT-pro-BNP levels (> 1583.50 pg/mL) and elevated NIHSS scores on admission (> 12.5) had a higher in-hospital mortality rate compared with other AIS patients (Chen et al., 2012). However, there was no consensus about blood biomarkers for predicting stroke outcome after patients were discharged from hospital. In the present study, we revealed that many factors, including age, NIHSS score on admission, percentage of atrial fibrillation, symptomatic HT, and stroke etiology, were higher in the unfavorable outcome group compared with the favorable outcome group in the acute phase. However, a multivariable regression model revealed that NIHSS scores on admission, NT-pro-BNP levels, and LMP2 levels were independent predictors of short-term outcome in stroke. In line with these findings, Sharma et al. (2006) reported that NT-pro-BNP levels also clearly predicted death at 120 days after acute stroke, and patients with poorer stroke outcomes displayed higher NT-pro-BNP concentrations in the acute phase. In addition, although the percentage of symptomatic HT was higher in the unfavorable outcome group in our study, it was not a prognostic indicator for stroke outcome. Together, our results demonstrate that NIHSS scores, NT-pro-BNP levels, and LMP2 levels are independent predictors of poor outcome at 90 days after stroke, as evaluated by the MRS. This finding might be useful for the future treatment and management of AIS.
Stroke survivors tend to develop cognitive impairment at least five to eight times more frequently than subjects who have not had a stroke (Srikanth et al., 2003). The acute tissue damage that can occur in stroke, especially in the left front temporal lobe or hippocampus, may affect cognition. It has been estimated that the overall frequency of PSCI and dementia at 3 months after stroke are 47.3% and 7.7%, respectively (Jacquin et al., 2014). In the current study, the percentage of PSCI at 90 days was 34.2%, although we did not divide PSCI into non-dementia PSCI and post-stroke vascular dementia subgroups. Risk factors such as age (over 65 years), education level (low), location of stroke (left hemisphere), and recurrent strokes have been strongly associated with PSCI (Mijajlović et al., 2017). Our multivariable analyses indicated that PSCI at 90 days was strongly associated with age, infarction location, and LMP2 levels. Indeed, subjects over 70 years old had a higher risk for stroke and PSCI, which was con-sistent with a previous report (Mijajlović et al., 2017). In addition, it has been reported that adults under the age of 50 years may have a better cognitive prognosis compared with older stroke patients (Brainin et al., 2015). The lesion location in stroke also has a strong correlation with PSCI. Data from both our and other studies suggest that patients with supratentorial ischemic strokes are more likely to develop PSCI, whereas patients with brainstem lesions are less likely to develop PSCI (Mijajlović et al., 2017). In addition, inflammatory markers and their relationship with post-stroke cognitive decline have recently gained attention (Mijajlović et al., 2017; Zhang and Bi, 2020). Our previous study showed that the immunoproteasome plays important roles in the inflammatory mechanisms of ischemic stroke (Chen et al., 2015), and we also revealed that high levels of plasma immunoproteasome subunits, such as LMP2, LMP5, and LMP7 proteins, are important predictors of early HT in AIS (Chen et al., 2017). Moreover, the present results suggest that elevated plasma LMP2 levels after stroke onset may be an independent risk factor of poor outcome, and might have prognostic value for PSCI among patients with ischemic stroke. The ROC analysis revealed that LMP2 > 841.4 pg/mL and LMP2 > 630.5 pg/mL potentially predict poor outcome and the risk of developing PSCI, respectively, in first-ever AIS pa-tients. Therefore, we propose that LMP2 may be used to predict functional outcomes in first-ever AIS patients, and to guide the clinical therapy of patients with stroke.
Limitations
Our study has certain limitations. First, the different periods from stroke onset to the collection of blood samples may have influenced the values of plasma protein concentrations. Although we have previously investigated the time course and cellular distribution of the immunoproteasome subunits LMP2 and LMP7 in the rat brain following cerebral ischemia-reperfusion (Chen et al., 2015), we do not know the precise temporal variations of the immunoproteasome in stroke patients. However, the brain’s inflammatory responses to ischemia involve time-dependent dynamic changes, and this should be taken into account when considering our results. Second, although the MMSE is the most commonly used cognitive screening test worldwide, it has several shortcomings for the detection of post-stroke vascular dementia (Delavaran et al., 2017). We used the MMSE as a relatively crude measure to assess cognitive impairment. However, our results would be more accurate if a more suitable method or tool existed for the assessment of cognitive impairment after stroke. Third, we did not perform any neuroimaging analyses correlating proteasome levels and stroke outcomes. Finally, this is a single-center study. The sample size was thus small, and the possibility of case selection bias cannot be ruled out. In a prospective study of 7158 subjects without previous memory disorders, it was reported that baseline logNT-pro-BNP levels were significantly associated with the risk of dementia in the entire study population (Tynkkynen et al., 2015). However, the present study did not reveal similar results; one possible explanation is that the number of patients with PSCI in our study was not large enough for accurate multivariable analyses. Thus, a larger multicenter study is required to further evaluate our findings.
Conclusions
Our findings suggest that elevated levels of plasma LMP2 during the acute phase of ischemic stroke are strongly associated with an increased risk of poor functional outcome and PSCI at 90 days post-stroke. Further research into this topic is warranted to reveal the underlying mechanisms and reduce the burden of unfavorable outcomes.
Additional file:
Additional file 1 (366.1KB, pdf) : Hospital Ethics Approval (Chinese).
Acknowledgments
The authors wish to thank all participants in the study.
Footnotes
C-Editor: Zhao M; S-Editor: Yu J, Li CH; L-Editor: Gardner B, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflict of interest.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81771250 (to XYC); the Natural Science Foundation of Fujian Province of China, Nos. 2013J01275 (to XYC), 2016J01432 (to XYC), 2018J01255 (to XZ); Young and Middle-aged Talents Training Project of Health and Family Planning Committee of Fujian Province, China, No. 2015-ZQN-JC-5 (to XYC), Joint Funds for the Innovation of Science and Technology of Fujian Province, China, No. 2017Y9065 (to XYC), and High-Level Hospital Foster Grants from Fujian Provincial Hospital, Fujian Province, China, No. 2020HSJJ07 (to XYC). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: This study was approved by the Ethics Committee of Fujian Provincial Hospital, Provincial Clinical Medical College of Fujian Medical University (approval No. K2014-01-003) on January 15, 2014.
Declaration of patient consent: The authors certify that they have obtained the consent forms from patients or their legal guardians. In the form, patients or their legal guardians have given their consent for the patients’ images and other clinical information to be reported in the journal. The patients or their legal guardians understand that patients’ names and initials will not be published.
Reporting statement: The writing and editing of the article were performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement.
Biostatistics statement: The statistical methods of this study were reviewed by the epidemiologist of Fujian Provincial Hospital, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices). Study protocol and informed consent form will be available immediately following publication, without end date. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81771250 (to XYC); the Natural Science Foundation of Fujian Province of China, Nos. 2013J01275 (to XYC), 2016J01432 (to XYC), 2018J01255 (to XZ); Young and Middle-aged Talents Training Project of Health and Family Planning Committee of Fujian Province, China, No. 2015-ZQN-JC-5 (to XYC), Joint Funds for the Innovation of Science and Technology of Fujian Province, China, No. 2017Y9065 (to XYC), and High-Level Hospital Foster Grants from Fujian Provincial Hospital, Fujian Province, China, No. 2020HSJJ07 (to XYC).
References
- 1.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Basler M, Mundt S, Muchamuel T, Moll C, Jiang J, Groettrup M, Kirk CJ. Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis. EMBO Mol Med. 2014;6:226–238. doi: 10.1002/emmm.201303543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brainin M, Tuomilehto J, Heiss WD, Bornstein NM, Bath PM, Teuschl Y, Richard E, Guekht A, Quinn T. Post-stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol. 2015;22:229–238. doi: 10.1111/ene.12626. e13-16. [DOI] [PubMed] [Google Scholar]
- 4.Broderick JP, Adeoye O, Elm J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke. 2017;48:2007–2012. doi: 10.1161/STROKEAHA.117.017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Wang Y, Fu M, Lei H, Cheng Q, Zhang X. Plasma immunoproteasome predicts early hemorrhagic transformation in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2017;26:49–56. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhan X, Chen M, Lei H, Wang Y, Wei D, Jiang X. The prognostic value of combined NT-pro-BNP levels and NIHSS scores in patients with acute ischemic stroke. Intern Med. 2012;51:2887–2892. doi: 10.2169/internalmedicine.51.8027. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Zhang X, Chen T, Jiang X, Wang X, Lei H, Wang Y. Inhibition of immunoproteasome promotes angiogenesis via enhancing hypoxia-inducible factor-1α abundance in rats following focal cerebral ischaemia. Brain Behav Immun. 2018;73:167–179. doi: 10.1016/j.bbi.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhang X, Wang Y, Lei H, Su H, Zeng J, Pei Z, Huang R. Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischemic stroke. Cell Death Dis. 2015;6:e1626. doi: 10.1038/cddis.2014.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delavaran H, Jönsson AC, Lövkvist H, Iwarsson S, Elmståhl S, Norrving B, Lindgren A. Cognitive function in stroke survivors: A 10-year follow-up study. Acta Neurol Scand. 2017;136:187–194. doi: 10.1111/ane.12709. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Gori AM, Giusti B, Piccardi B, Nencini P, Palumbo V, Nesi M, Nucera A, Pracucci G, Tonelli P, Innocenti E, Sereni A, Sticchi E, Toni D, Bovi P, Guidotti M, Tola MR, Consoli D, Micieli G, Tassi R, Orlandi G, et al. Inflammatory and metalloproteinases profiles predict three-month poor outcomes in ischemic stroke treated with thrombolysis. J Cereb Blood Flow Metab. 2017;37:3253–3261. doi: 10.1177/0271678X17695572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorny X, Säring P, Bergado Acosta JR, Kahl E, Kolodziejczyk MH, Cammann C, Wernecke KEA, Mayer D, Landgraf P, Seifert U, Dieterich DC, Fendt M. Deficiency of the immunoproteasome subunit β5i/LMP7 supports the anxiogenic effects of mild stress and facilitates cued fear memory in mice. Brain Behav Immun. 2019;80:35–43. doi: 10.1016/j.bbi.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci U S A. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo DX, Zhu ZB, Zhong CK, Bu XQ, Chen LH, Xu T, Guo LB, Zhang JT, Li D, Zhang JH, Ju Z, Chen CS, Chen J, Zhang YH, He J. Serum cystatin C levels are negatively correlated with post-stroke cognitive dysfunction. Neural Regen Res. 2020;15:922–928. doi: 10.4103/1673-5374.268928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 16.Hasan TF, Rabinstein AA, Middlebrooks EH, Haranhalli N, Silliman SL, Meschia JF, Tawk RG. Diagnosis and management of acute ischemic stroke. Mayo Clin Proc. 2018;93:523–538. doi: 10.1016/j.mayocp.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Jacquin A, Binquet C, Rouaud O, Graule-Petot A, Daubail B, Osseby GV, Bonithon-Kopp C, Giroud M, Béjot Y. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis. 2014;40:1029–1038. doi: 10.3233/JAD-131580. [DOI] [PubMed] [Google Scholar]
- 18.Lee GA, Lin TN, Chen CY, Mau SY, Huang WZ, Kao YC, Ma RY, Liao NS. Interleukin 15 blockade protects the brain from cerebral ischemia-reperfusion injury. Brain Behav Immun. 2018;73:562–570. doi: 10.1016/j.bbi.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, Kliper E, Shin YI, Kim YH, Choi S, Jung S, Lee YB, Sinanović O, Levine DA, Schlesinger I, Mead G, et al. Post-stroke dementia - a comprehensive review. BMC Med. 2017;15:11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei H, Momeni F, Saadatpour L, Sahebkar A, Goodarzi M, Masoudifar A, Kouhpayeh S, Salehi H, Mirzaei HR, Jaafari MR. MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. J Cell Physiol. 2018;233:856–865. doi: 10.1002/jcp.25787. [DOI] [PubMed] [Google Scholar]
- 21.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orre M, Kamphuis W, Dooves S, Kooijman L, Chan ET, Kirk CJ, Dimayuga Smith V, Koot S, Mamber C, Jansen AH, Ovaa H, Hol EM. Reactive glia show increased immunoproteasome activity in Alzheimer’s disease. Brain. 2013;136:1415–1431. doi: 10.1093/brain/awt083. [DOI] [PubMed] [Google Scholar]
- 23.Pullicino P, Snyder W, Munschauer F, Pordell R, Greiner F. Interrater agreement of computed tomography infarct measurement. J Neuroimaging. 1996;6:16–19. doi: 10.1111/jon19966116. [DOI] [PubMed] [Google Scholar]
- 24.Sharma JC, Ananda K, Ross I, Hill R, Vassallo M. N-terminal proBrain natriuretic peptide levels predict short-term poststroke survival. J Stroke Cerebrovasc Dis. 2006;15:121–127. doi: 10.1016/j.jstrokecerebrovasdis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Srikanth VK, Thrift AG, Saling MM, Anderson JF, Dewey HM, Macdonell RA, Donnan GA. Increased risk of cognitive impairment 3 months after mild to moderate first-ever stroke: a community-based prospective study of nonaphasic English-speaking survivors. Stroke. 2003;34:1136–1143. doi: 10.1161/01.STR.0000069161.35736.39. [DOI] [PubMed] [Google Scholar]
- 26.Tóth NK, Székely EG, Czuriga-Kovács KR, Sarkady F, Nagy O, Lánczi LI, Berényi E, Fekete K, Fekete I, Csiba L, Bagoly Z. Elevated factor VIII and von Willebrand factor levels predict unfavorable outcome in stroke patients treated with intravenous thrombolysis. Front Neurol. 2017;8:721. doi: 10.3389/fneur.2017.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tynkkynen J, Laatikainen T, Salomaa V, Havulinna AS, Blankenberg S, Zeller T, Hernesniemi JA. NT-proBNP and the risk of dementia: a prospective cohort study with 14 years of follow-up. J Alzheimers Dis. 2015;44:1007–1013. doi: 10.3233/JAD-141809. [DOI] [PubMed] [Google Scholar]
- 28.Wu JX, Xue J, Zhuang L, Liu CF. Plasma parameters and risk factors of patients with post-stroke cognitive impairment. Ann Palliat Med. 2020;9:45–52. doi: 10.21037/apm.2019.12.05. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Ye Z, Wang B, Huang Y, Zhou L, Liu C, Chen D, Sun M, Dai F, Guan S, Tang W. Tim-4 expression increases in ischemic stroke patients and is associated with poor outcome. J Neuroimmunol. 2018;316:1–6. doi: 10.1016/j.jneuroim.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, Mayer SA, Sheth KN, Schwamm LH. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e343–361. doi: 10.1161/STR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Saatman KE, Chen L. Therapeutic potential of natural compounds from Chinese medicine in acute and subacute phases of ischemic stroke. Neural Regen Res. 2020;15:416–424. doi: 10.4103/1673-5374.265545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Bi X. Post-stroke cognitive impairment: a review focusing on molecular biomarkers. J Mol Neurosci. 2020 doi: 10.1007/s12031-020-01533-8. doi:101007/s12031-020-01533-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, Ma MM, Fang JH, Zhao L, Zhou MK, Guo J, He L. Differences in brain-derived neurotrophic factor gene polymorphisms between acute ischemic stroke patients and healthy controls in the Han population of southwest China. Neural Regen Res. 2019;14:1404–1411. doi: 10.4103/1673-5374.253525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu M, Li N, Luo P, Jing W, Wen X, Liang C, Tu J. Peripheral blood leukocyte expression of lncRNA MIAT and its diagnostic and prognostic value in ischemic stroke. J Stroke Cerebrovasc Dis. 2018a;27:326–337. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Z, Xu T, Guo D, Huangfu X, Zhong C, Yang J, Wang A, Chen CS, Peng Y, Xu T, Wang J, Sun Y, Peng H, Li Q, Ju Z, Geng D, Chen J, Zhang Y, He J. Serum hepatocyte growth factor is probably associated with 3-month prognosis of acute ischemic stroke. Stroke. 2018b;49:377–383. doi: 10.1161/STROKEAHA.117.019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


