Sox9 – gliogenesis and beyond: Neurons and glial cells are the major neuroectodermal cell types of the vertebrate central nervous system (CNS). Their generation from common progenitor cells takes place mostly during embryonic and early postnatal development. After closure of the neural tube, neural epithelial progenitor cells (NEPs) establish the ventricular zone (VZ). By asymmetrical cell division, NEPs first give rise to neuronal precursor cells (NPs) that then differentiate into various types of neurons. Later, NEPs predominantly produce glial precursor cells that become either astroglia or oligodendroglia.
The initiation of gliogenesis requires the activity of several transcription factors. One of them is the high-mobility-group-domain protein Sox9. Following its Notch-dependent induction, Sox9 induces further transcriptional regulators and cooperation partners for gliogenesis in NEPs such as Nfia (Deneen et al., 2006; Kang et al., 2012). When Sox9 is deleted in NEPs of the spinal cord of the mouse embryo, neurons continue to be produced even beyond their regular birth date at the expense of glial cells, whose generation is strongly decreased (Stolt et al., 2003). This has led to the assumption that Sox9 functions as a neuron-glia switch. Additionally, Sox9 has been postulated to be already crucial for the induction of glial competence in NEPs, possibly by prebinding glial regulatory regions and thereby allowing their future activation (Klum et al., 2018). By doing so, Sox9 is required for transforming NEPs into multipotent neural stem cells (Scott et al., 2010). In the mouse cerebellum, Sox9 appeared particularly important for suppression of neurogenesis (Vong et al., 2015).
Sox9, NEPs and neuronal cells: To understand how Sox9 functions relate to each other, we generated a mouse model that allowed temporally controlled, Cre-induced and tetracycline-sensitive CNS expression of a Sox9 transgene, and analyzed spinal cord development (Vogel et al., 2020). The chosen Brn4::Cre led to precocious Sox9 expression in NEPs of the VZ at embryonic day (E) 9.0, approximately one day before the start of endogenous Sox9 expression (Figure 1). As a consequence of the first premature and then elevated Sox9 expression, several striking alterations were observed in the developing spinal cord, including a strong reduction in overall size and total cell numbers coupled to a dramatic increase in apoptosis. From early stages onwards, the VZ lost its epithelial organization. NEP survival was not impaired.
Figure 1.
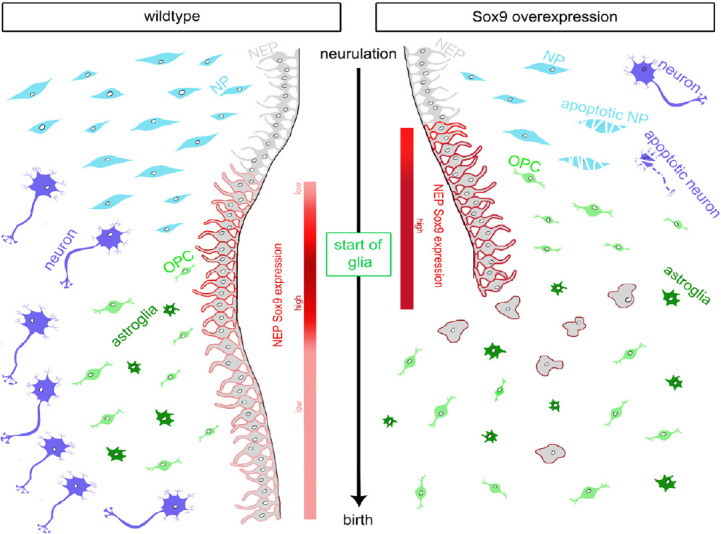
Sox9 in the developing wildtype spinal cord (left) and functional consequences of premature and increased Sox9 expression (right) during the period from neurulation (top) to birth (bottom).
Normal onset of gliogenesis and Sox9 expression levels in NEPs during embryogenesis are indicated in the middle. Time of appearance, relative number and increased susceptibility to apoptosis are shown for each cell type. NEPs: Neural epithelial progenitor cells.
At E9.0, high Sox9 levels are usually observed in the most dorsal part of the neural tube where premigratory neural crest cells reside. In these cells, Sox9 is responsible among others for initiating an epithelial-to-mesenchymal transition as a precondition for emigration (Cheung et al., 2005). It is thus conceivable, that precocious and elevated Sox9 levels in NEPs might have similar effects and cause NEPs to undergo an epithelial-to-mesenchymal transition. NEPs may even be transformed into cells with neural crest like properties. However, the absence of surplus cells with typical neural crest markers argues against such a cell conversion upon Sox9 overexpression in the transgenic spinal cord.
In our mouse model, the onset of Sox9 transgene expression could be postponed by tetracycline treatment until later stages of embryonic spinal cord development. When delayed until E10.5 or E12.5, the effect on NEPs was much milder and the integrity of the VZ was preserved. It is thus obvious that precocity rather than increase of Sox9 expression is the main trigger for the NEP alterations in our mouse model.
The observed decrease in total cell numbers after early stage overexpression of Sox9 was mostly attributable to a reduction in the number of neuronal precursors and neurons (Figure 1). Considering the fact, that apoptosis mostly occurred in neuronal cells it is reasonable to assume that continued Sox9 expression is incompatible with neurogenesis and causes these cells to die. In our mouse model, dorsal neurons suffered much more from Sox9 transgene expression than ventral neurons. Most likely, this is a consequence of the chosen Cre transgene as ventral neurons are already specified from NEPs before the onset of Brn4::Cre expression and did not express the Sox9 transgene or were protected from the detrimental effects of its expression, for instance due to changes in chromatin structure that limit Sox9 activity. This interpretation is supported by experiments where Sox9 transgene expression was suppressed by tetracycline treatment until E10.5 or E12.5. In these mice, many neuronal cells were able to undergo specification from NEPs before Sox9 expression. As a consequence, they survived and neuronal cell numbers were almost normal.
In conclusion, too early expression of Sox9 in NEPs disturbs their epithelial character. There also exists a sensitive period for neuronal populations, in which the presence of Sox9 interferes with survival. Decreased survival may be secondary to a failure to differentiate. However, our data do not allow a clear distinction between a primary effect on survival, differentiation or both.
Sox9 and glial cells: As expected from the drastic reduction of macroglial cells in previous loss-of-function studies (Stolt et al., 2003; Scott et al., 2010), Sox9 overexpression in our complementary gain-of-function approach led to strongly increased numbers of both oligodendroglial and astroglial cells in the embryonic spinal cord (Figure 1). This increase occurred even when Sox9 overexpression was postponed until E12.5. According to literature, Sox9 function in astroglial cells is intimately linked to interaction with Nfia, whereas its role in oligodendroglial cells depends on cooperation with the Olig2 transcription factor and crosstalk with its close relative Sox10 (Finzsch et al., 2008; Kang et al., 2012; Klum et al., 2018). In astroglial cells, Sox9 remains continuously present, whereas expression in oligodendroglial cells extinguishes upon differentiation (Stolt et al., 2003).
When starting at E9.0, oligodendroglial cells were not only present in increased numbers. They also appeared prematurely and were already spread widely throughout the spinal cord parenchyma when the first oligodendroglial precursor cells in controls were specified in the ventral VZ. Oligodendroglial cells are normally labelled by Olig2 and Sox10, two transcription factors that are constantly required during oligodendroglial development. Following Sox9 overexpression, the numbers of Sox10-expressing and Olig2-expressing cells were both dramatically increased. However, at early times there were substantially more Sox10- than Olig2-expressing cells. Differences had disappeared at later stages. During early neural crest development, Sox9 acts as a strong, direct inducer of Sox10 (Cheung et al., 2005). Therefore, it seems plausible to assume that high Sox9 levels also initiate Sox10 expression in early NEPs and that Sox10 in turn activates Olig2 expression. In support of such an assumption, Sox10-positive cells were found in closer vicinity to the VZ than Olig2-positive cells in those experiments where Sox9 overexpression was delayed and VZ integrity maintained. Induction of Sox10 prior to Olig2 is actually the reverse of what normally happens during oligodendroglial precursor cell specification. It may involve regulatory elements and circuits that are involved in maintaining Olig2 expression during later oligodendroglial development, including a Sox10-responsive enhancer approximately 33kb upstream the Olig2 gene (Weider et al., 2015).
During late stages of spinal cord development, numbers of differentiating oligodendrocytes were also elevated. However, the observed increase was surprisingly modest. Oligodendroglial cells normally downregulate their Sox9 expression prior to and as a prerequisite of differentiation (Stolt et al., 2003). It is thus conceivable that Sox9 overexpression by itself precludes efficient terminal differentiation. Alternatively, the embryonic spinal cord may simply not yet contain all permissive or provide the inductive signals required for oligodendrocyte differentiation.
We also observed an increased generation of astroglial cells. However, the increase in astroglial numbers was not as impressive as the rise in oligodendroglial cells independent of whether Sox9 expression started at E9.0 or E12.5. This was unexpected as Sox9 is normally an efficient inducer of astroglial fate and identity in VZ cells outside the oligodendroglia-generating region within the ventral VZ (Deneen et al., 2006). Considering that there are many more VZ cells outside than inside this region, we had assumed astrogliogenesis to prevail over oligodendrogliogenesis in our mouse model.
That this is not the case may again be due to the robust Sox9-dependent Sox10 induction. Sox10 in turn not only promotes the oligodendroglial identity but also inhibits astroglial fate and properties in developing spinal cord glia by antagonizing Nfia function (Glasgow et al., 2014). Under experimental conditions, Sox10 can even convert astroglia into oligodendroglia (Mokhtarzadeh Khanghahi et al., 2018). An efficient generation of astroglial cells by Sox9 therefore requires conditions where Sox9 no longer induces strong Sox10 expression. It could for instance be envisaged that at some point during spinal cord development, the Sox10 locus is epigenetically silenced by chromatin modifications in NEPs. As a consequence, NEPs would lose their competence to become oligodendroglial cells and would instead acquire an astroglial fate in response to Sox9. Such a mechanism may also explain why astrogliogenesis from NEPs usually occurs after NEP-dependent oligodendrogliogenesis.
Summary and perspectives: Sox9 overexpression in the developing spinal cord has shown that its function is strongly influenced by timing, the exact expression levels and the cellular context. Depending on these parameters, Sox9 expression has different consequences. This accounts for the various functions previously ascribed to Sox9 during CNS development (Stolt et al., 2003; Scott et al., 2010; Vong et al., 2015). Premature expression in NEPs is not compatible with maintenance of their epithelial character. High amounts of Sox9 expression furthermore prevent proper neuronal development and instead promote both oligodendroglial and astroglial development by counteracting shut-off and promoting expression of glial genes. Again, timing and cellular context appear important as conditions that lead to Sox10 induction favour oligodendrogenesis, while efficient astrogliogenesis can only occur in the absence of Sox10.
While our analysis has yielded substantial insights into early developmental functions of Sox9 including its impact on NEP and glial fate specifications, there is little information yet on its role in already specified astroglial or oligodendroglial cells. Therefore it will be interesting in future experiments to study macroglial development in mice in which Sox9 is only transiently expressed during early times and then turned off again, or in mice in which Sox9 expression is confined selectively to later stages of astroglial or oligodendroglial development. All this can be done in our mouse model by making use of different Cre transgenes and exploiting the possibility of modulating Sox9 expression by tetracycline treatment.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, No. We1326/14 and We1326/15 to MW.
Footnotes
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Finzsch M, Stolt CC, Lommes P, Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor {alpha} expression. Development. 2008;135:637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- 4.Glasgow SM, Zhu W, Stolt CC, Huang TW, Chen F, LoTurco JJ, Neul JL, Wegner M, Mohila C, Deneen B. Mutual antagonism between Sox10 and NFIA regulates diversification of glial lineages and glioma subtypes. Nat Neurosci. 2014;17:1322–1329. doi: 10.1038/nn.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB, Graham BH, Foster AE, Novitch BG, Gronostajski RM, Deneen B. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 2012;74:79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klum S, Zaouter C, Alekseenko Z, Bjorklund AK, Hagey DW, Ericson J, Muhr J, Bergsland M. Sequentially acting SOX proteins orchestrate astrocyte- and oligodendrocyte-specific gene expression. EMBO Rep. 2018;19:e46635. doi: 10.15252/embr.201846635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokhtarzadeh Khanghahi A, Satarian L, Deng W, Baharvand H, Javan M. In vivo conversion of astrocytes into oligodendrocyte lineage cells with transcription factor Sox10; Promise for myelin repair in multiple sclerosis. PLoS One. 2018;13:e0203785. doi: 10.1371/journal.pone.0203785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gomez Gaviro MV, Booth S, Gao B, Cheah KS, Lovell-Badge R, Briscoe J. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13:1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 9.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel JK, Weider M, Engler LA, Hillgartner S, Schmitt C, Hermans-Borgmeyer I, Wegner M. Sox9 overexpression exerts multiple stage-dependent effects on mouse spinal cord development. Glia. 2020;68:932–946. doi: 10.1002/glia.23752. [DOI] [PubMed] [Google Scholar]
- 11.Vong KI, Leung CK, Behringer RR, Kwan KM. Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum. Mol Brain. 2015;8:25. doi: 10.1186/s13041-015-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weider M, Wegener A, Schmitt C, Küspert M, Hillgartner S, Bösl MR, Hermans-Borgmeyer I, Nait-Oumesmar B, Wegner M. Elevated in vivo levels of a single transcription factor directly convert satellite glia into oligodendrocyte-like cells. PLoS Genet. 2015;11:e1005008. doi: 10.1371/journal.pgen.1005008. [DOI] [PMC free article] [PubMed] [Google Scholar]


