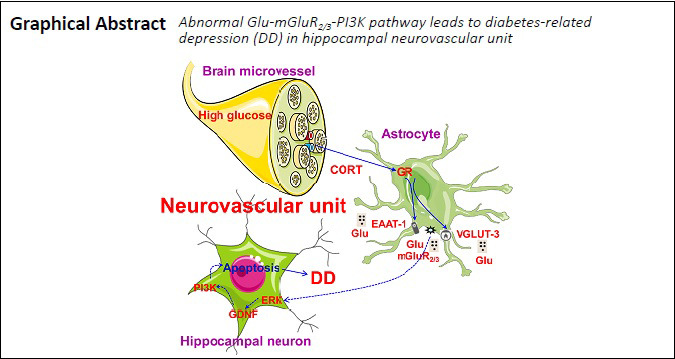
Keywords: diabetes-related depression, factor, hippocampus, in vitro, neurovascular unit, pathways, protein
Abstract
Our previous studies have shown that glutamate and hippocampal neuron apoptosis are key signals and direct factors associated with diabetes-related depression, and structural and functional damage to the hippocampal neurovascular unit has been associated with diabetes-related depression. However, the underlying mechanism remains unclear. We hypothesized that diabetes-related depression might be associated with the glutamate (Glu)/metabotropic glutamate receptor2/3 (mGluR2/3)/phosphoinositide 3-kinase (PI3K) pathway, activated by glucocorticoid receptors in the hippocampal neurovascular unit. To test this hypothesis, rat hippocampal neurovascular unit models, containing hippocampal neurons, astrocytes, and brain microvascular endothelial cells, were treated with 150 mM glucose and 200 µM corticosterone, to induce diabetes-related depression. Our results showed that under conditions of diabetes complicated by depression, hippocampal neurovascular units were damaged, leading to decreased barrier function; elevated Glu levels; upregulated glucocorticoid receptor, vesicular glutamate transporter 3 (VGLUT-3), and metabotropic glutamate receptor 2/3 (mGluR2/3) expression; downregulated excitatory amino acid transporter 1 (EAAT-1) expression; and alteration of the balance of key proteins associated with the extracellular signal-regulated kinase (ERK)/glial cell-derived neurotrophic factor (GDNF)/PI3K signaling pathway. Moreover, the viability of neurons was dramatically reduced in the model of diabetes-related depression, and neuronal apoptosis, and caspase-3 and caspase-9 expression levels, were increased. Our results suggest that the Glu/mGluR2/3/PI3K pathway, induced by glucocorticoid receptor activation in the hippocampal neurovascular unit, may be associated with diabetes-related depression. This study was approved by the Laboratory Animal Ethics Committee of The First Hospital of Hunan University of Chinese Medicine, China (approval No. HN-ZYFY-2019-11-12) on November 12, 2019.
Chinese Library Classification No. R446; R749.4; R587.1
Introduction
According to the World Health Organization, in 2017, approximately 425 million people, worldwide, suffer from diabetes mellitus. Diabetes has been associated with many complications, such as eye disease, kidney disease, and depression (Ziegler and Neu, 2018). Individuals who suffer from diabetes mellitus are at higher risk of developing depressive symptoms, including low mood, anhedonia, sleep disorders, and suicidal tendencies than individuals without diabetes mellitus (Islam et al., 2015). Moreover, epidemiological studies have shown that almost one in four adults with type 2 diabetes mellitus experience depression, which represents a higher incidence than that observed for non-diabetic patients (Khaledi et al., 2019). Moreover, diabetes-related depression (DD) causes great economic burden and a waste of medical resources. Thus, revealing the pathogenesis of DD is considered an urgent need.
The pathogenesis of DD is complicated, and the exact mechanism underlying DD has yet to be identified. The hippocampus is recognized as a vital tissue that is affected by diabetes mellitus and is also associated with depression (Ho et al., 2013). In our previous study, we demonstrated that the apoptotic process was controlled by the balance among apoptotic proteins (Bax, caspase-8, and Bcl-2) within the hippocampal neurons of DD model rats (Wang et al., 2015b). These findings indicated that the apoptosis of hippocampal neurons may be involved in triggering DD. Moreover, our preliminary studies showed that the abnormal upregulation of the glutamate (Glu) receptor metabotropic Glu receptor 2/3 (mGluR2/3) was closely related to the activation of the glucocorticoid receptor (GR) and an imbalance in Glu transporter expression (Liu et al., 2018). Furthermore, we also found that the activation of mGluR2/3, caused by high Glu levels, resulted in hippocampal neuron apoptosis (Liu et al., 2019a).
The association between cortisol and Glu and their destructive impacts on the hippocampus has drawn public attention. Although our previous studies have confirmed that Glu-mGluR2/3 signaling due to elevated Glu contents was involved in the regulation of apoptosis among hippocampal neurons in DD model rats (Liu et al., 2019a); however, the origins of excess Glu and the roles played by cortisol and Glu remain to be clarified. Evidence has shown that interactions between cortisol and Glu are of great importance for cognitive dysfunction syndromes, such as depression and electroconvulsive therapy (Reisner, 2003; Andrews and Reisner, 2017). Mimicking DD conditions (DD model, 150 mM glucose and 200 µM corticosterone) results in neuronal apoptosis. Cortisol and Glu are involved in the process of neuronal apoptosis, and studying the mechanism underlying apoptosis may reveal the pathogenesis of DD. In addition, our previous studies have shown that the expression levels of caspase-3 and mGluR2/3 were significantly higher in the hippocampus, and Glu levels were elevated in DD model rats, with increased numbers of terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling-positive hippocampal neurons (Liu et al., 2019a). However, the mechanisms underlying these changes remain unclear. Clarifying the mechanism through which the apoptosis of hippocampal neurons is regulated by Glu-mGluR2/3 in the hippocampal neurovascular unit (NVU) can improve our understanding of DD pathogenesis, which may explain hippocampal damage and dysfunction.
An NVU consists primarily of microvessels, astrocytes, neurons, and the extracellular matrix and is defined as a completely functional and structural unit of the brain (Iadecola, 2004; Li et al., 2019). Current studies examining the pathological basis of diabetes mellitus and depression have focused on the NVU (Najjar et al., 2013; Bogush et al., 2017). Our previous study also indicated that the pathogenesis of DD was strongly associated with the abnormal increase in Glu levels and hippocampal neuronal apoptosis in the NVU (Liu et al., 2019a); however, the definite mechanism remains to be elucidated. Therefore, in the current study, we hypothesized that the pathogenesis of DD might involve the Glu/mGluR2/3/phosphoinositide 3-kinase (PI3K) signaling pathway, which is activated by GR in the hippocampal NVU. We used an in vitro model of DD and agonists and antagonists for key signaling pathway proteins, to test this hypothesis.
Materials and Methods
Animals
Specific-pathogen-free Sprague-Dawley rats [E17 embryos from pregnant females (n = 4; for hippocampal neuronal cultures), 3-day-old rats (n = 6; for hippocampal astrocytic cultures), and 9-day-old rats (n = 5; for brain microvascular endothelial cell cultures)] were purchased in Hunan Slac Jingda Laboratory Animal Co., Ltd., Changsha, China (license No. SCXK (Xiang) 2019-0004). All rats were allowed free access to food and water. All experiments were approved by the Laboratory Animal Ethics Committee of The First Hospital of Hunan University of Chinese Medicine, China (approval No. HN-ZYFY-2019-11-12) on November 12, 2019.
Establishment of the hippocampal NVU, in vitro, and cell identification
Hippocampal neurons, astrocytes, and brain microvascular endothelial cells from rats were isolated, purified, and cultured, as described in our previous study (Liu et al., 2019a). The hippocampal NVU model was established as described in our previous study (Liu et al., 2019a). Briefly, as shown in Figure 1, after neurons were seeded into a 24-well culture plate, for 3–5 days, purified astrocytes were seeded on the outer sides of the well inserts, and the well inserts were placed into matching 24-well plates. After 2 days of co-culture, purified brain microvascular endothelial cells were seeded on the inner side of the well inserts. The NVU, consisting of neurons, astrocytes, and brain microvascular endothelial cells, was successfully established (Xue et al., 2013). The identification of neurons, astrocytes, and brain microvascular endothelial cells was performed using specific markers, including neuron-specific enolase, glial fibrillary acidic protein, and platelet endothelial cell adhesion molecule-1/CD31, respectively, via immunocytochemical staining (Liu et al., 2019a). In brief, cells were fixed with 4% paraformaldehyde, for 30 minutes. Afterward, they were incubated in 0.25% Triton X-100, for 15 minutes, and blocked in 5% bovine serum albumin for 30 minutes, before incubation with different primary antibodies (neuron-specific enolase: 1:100, Cat# BA0535, Boster, Wuhan, China; glial fibrillary acidic protein: 1:200, Cat# BA0056, Boster; platelet endothelial cell adhesion molecule-1/CD31: 1:100, Cat# BA2966, Boster). After incubation at 4°C overnight, cells were incubated with anti-rabbit IgG conjugated to fluorescein isothiocyanate (1:400; Cat# SA00003-2; Proteintech, Chicago, IL, USA) at 37°C for 30 minutes. Then, the cell nucleus was stained with 4′,6-diamidino-2-phenylindole, for 20 minutes. Images were captured using a high-content analysis system (PerkinElmer, Boston, MA, USA) and an inverted fluorescence microscope (Olympus, Tokyo, Japan).
Figure 1.
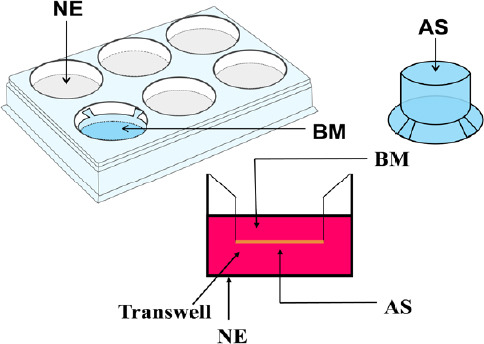
Diagram of the in vitro hippocampal NVU system.
Astrocytes were seeded in the outer side of the matching Transwell inserts, after neurons were seeded into a 24-well culture plate and cultured for 3–5 days. After 2 days of co-culture, brain endothelial cells were seeded on the inner side of the matching well inserts. The hippocampal NVU system could be successfully established after these three cell types were co-cultured for 3 days. AS: Astrocyte; BM: brain microvascular endothelial cell; NE: neuron; NVU: neurovascular unit.
Modeling of the hippocampal NVU under DD conditions and pharmacological interventions
DD conditions were established, as described in our previous study (Liu et al., 2019a). In brief, 150 mM glucose (Solarbio, Beijing, China) and 200 µM corticosterone (Sigma, St. Louis, MO, USA) were added to each well, for 18 hours, to establishing an in vitro DD model. After DD model was successfully established, the GR blocker RU-486 (1 µM; Cat# M8046; Sigma), the GR agonist dexamethasone (5 µM; Cat# D8040; Solarbio), the mGluR2/3 receptor blocker LY341495 (0.1 µM; Cat# A3577; Apexbio, Houston, TX, USA), and the mGluR2/3 receptor agonist LY379268 (1 µM; Cat# ab120196; Abcam, Cambridge, UK), were added to the transwell, individually, for 18 hours, and the effects of each intervention on protein expression, apoptosis, and cell survival in the in vitro hippocampal NVU model were assessed. A hippocampal NVU not subjected to DD condition was used as a control group.
Determination of hippocampal NVU barrier function
The barrier function was detected, as described in our previous study (Liu et al., 2019a). Briefly, a Millipore resistor (Millipore, Boston, MA, USA) was used to measure transendothelial electrical resistance. The permeability within 4 hours, between the inner and outer levels, was measured using a vernier caliper (Chiib, Shanghai, China).
Detection of immunopositivity against GR, vesicular Glu transporter 3, excitatory amino-acid transporter 1 and mGluR2/3 in the astrocytes of the hippocampal NVU
After the establishment of the DD model, RU-486 and dexamethasone treatments were applied to the NVU, and astrocytes in the NVU were washed with phosphate-buffered saline, three times, followed by fixation with 4% paraformaldehyde, for 30 minutes. Astrocytes were then treated with 0.25% Triton X-100, for 15 minutes, and blocked in 5% bovine serum albumin, for 30 minutes, before incubation with primary antibodies, overnight, in the dark, in a humidified container, at 4°C. The following antibodies were used: rabbit anti-GR polyclonal antibody (1:100; Cat# 24050-1-AP; Proteintech), rabbit anti-vesicular Glu transporters 3 (VGLUT-3) polyclonal antibody (1:100; Cat# ab23977; Abcam), rabbit anti-excitatory amino-acid transporter 1 (EAAT-1) polyclonal antibody (1:100; Cat# ab416; Abcam), and rabbit anti-mGluR2/3 polyclonal antibody (1:200; Cat# ab254078; Abcam). The astrocytes were then incubated with anti-rabbit IgG, conjugated to fluorescein isothiocyanate (1:400; Cat# SA00003-2; Proteintech) at 37°C for 30 minutes, and stained with 4′,6-diamidino-2-phenylindole (1:800; Cat# ab228549; Abcam) at room temperature for 15 minutes. A high-content analysis system was used to analyze protein expression.
Detection of Glu content in the hippocampal NVU
The cell supernatant from the hippocampal NVU was collected after the establishment of the DD model. The level of Glu was measured using an enzyme-linked immunosorbent assay kit (R&D, Minneapolis, MN, USA).
Detection of immunopositivity against extracellular signal-regulated kinase, glial cell-derived neurotrophic factor, and PI3K in the neurons of the hippocampal NVU
After the establishment of the DD model, RU-486, dexamethasone, LY341495, and LY379268 treatment was applied to hippocampal neurons in the NVU, similar to the method used in astrocytes. Neurons were incubated with rabbit anti-extracellular signal-regulated kinase (ERK; 1:100; Cat# 16443-1-AP; Proteintech), rabbit anti-glial cell-derived neurotrophic factor (GDNF; 1:200; Cat# ab18956; Abcam) and rabbit anti-PI3K (1:100; Cat# 20584-1-AP; Proteintech) at 4°C overnight. Cells were then incubated with anti-rabbit IgG, conjugated to fluorescein isothiocyanate (1:400; Cat# SA00003-2; Proteintech) at 37°C for 30 minutes, and stained with 4′,6-diamidino-2-phenylindole (1:800; Cat# ab228549; Abcam), at room temperature for 15 minutes. A high-content analysis system was used to analyze protein expression.
Detection of cell survival among neurons in the hippocampal NVU
After DD model establishment and treatment with RU-486, dexamethasone, LY341495, or LY379268, neurons were incubated with 10 µL Cell Counting Kit-8 (Bioss, Beijing, China) for 4 hours at 37°C and 5% CO2. Afterward, cell survival was measured at 450 nm, using a microplate reader (Thermo, Waltham, MA, USA).
Detection of neuronal apoptosis in the hippocampal NVU
After DD model establishment and treatment with RU-486, dexamethasone, LY341495, or LY379268, neurons in the NVU were fixed with 4% paraformaldehyde, 0.2% Triton X-100, and 0.3% H2O2. Then, apoptosis among hippocampal neurons was observed by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling staining (Roche, Basel, Switzerland) and examined by microscopy (Olympus).
Western blot assay was used to detect the apoptosis-associated proteins caspase-3 and caspase-9. The total protein content was detected by bicinchoninic acid assay. Proteins were separated on polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Membranes were blocked in skimmed milk and probed with rabbit anti-caspase-3 (1:1000; Cat# 19677-1-AP; Proteintech), rabbit anti-caspase-9 (1:1000; Cat# 10380-1-AP; Proteintech), and rabbit anti-β-actin (1:3000; Cat# 20536-1-AP; Proteintech), at 4°C overnight. The blots were washed twice with Tris-buffered saline before incubation with anti-rabbit secondary antibody-horseradish peroxidase (1:400; Cat# BM5180; Boster), at room temperature for 1 hour. Finally, the expression levels of apoptosis-associated proteins were quantified, using AlphaEase FC (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation (SD), and calculated for each test group. All data were analyzed by SPSS 16.0 (SPSS, Chicago, IL, USA). A one-way analysis of variance, followed by Dunnett’s post hoc test, was performed for comparisons among groups. P < 0.05 was considered significant.
Results
Cell morphological characteristics and the identification of the hippocampal NVU
As shown in Figure 2A1–3, the neuronal somas were clearly visible, with crisscrossing dendrites, weaving a rich neural network. Astrocytes possessed intensive and slender dendrites. Microvascular endothelial cells presented with the typical pavement structural characteristics. Furthermore, the cytoplasmic compartments of neurons, astrocytes, and brain microvascular endothelial cells were demonstrated the expression of neuron-specific enolase, glial fibrillary acidic protein, and platelet endothelial cell adhesion molecule-1/CD31, respectively. Immunofluorescent staining results indicated that the cultured cells were the targeted cells, which could be ascribed to specific cell types (Figure 2B1–3).
Figure 2.
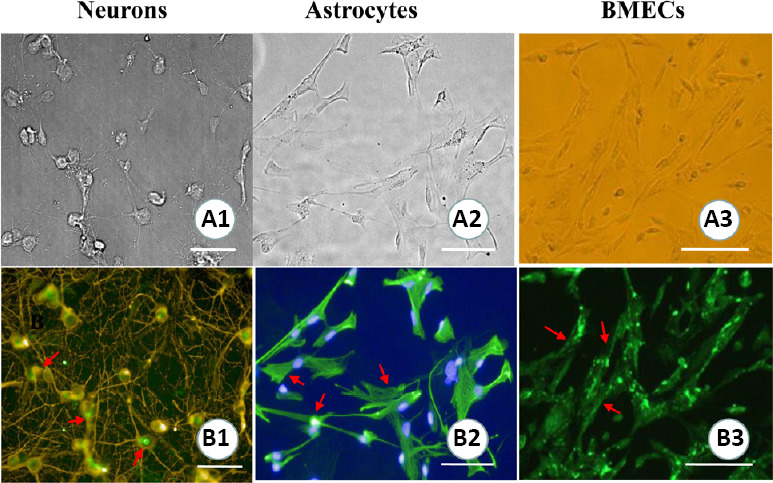
Cell morphological characteristics and the identification of a hippocampal neurovascular unit.
(A1–3) Cell morphologies of hippocampal neurons, astrocytes, and brain microvascular endothelial cells (BMECs). (B1–3) The identification of neurons, astrocytes, and BMECs (immunofluorescence staining). Arrows show the positive expression of neuron-specific enolase (B1), glial fibrillary acidic protein (B2), and platelet endothelial cell adhesion molecule-1/CD31 (B3). Scale bars: 100 µm.
Barrier function in the NVU is impaired by DD conditions
The barrier function of the NVU was assessed by performing transendothelial electrical resistance and permeability assays. As shown in Figure 3A, transendothelial electrical resistance was reduced in the hippocampal NVU after DD establishment, compared with that in the control group (P < 0.01). Moreover, the permeability within 4 hours increased under simulated DD conditions compared with control conditions (P < 0.01; Figure 3B). These findings suggested that the barrier function of the NVU was impaired under DD conditions.
Figure 3.
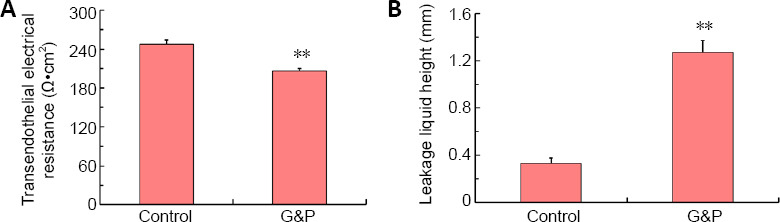
Transendothelial electrical resistance and leakage liquid height in the neurovascular unit are impaired by diabetes-related depression conditions.
(A) Transendothelial electrical resistance was detected by a resistor. (B) Leakage liquid height was detected by a vernier caliper. Data are shown as the mean ± SD (n = 3). **P < 0.01, vs. control group (one-way analysis of variance followed by Dunnett’s post hoc test). Experiments were performed in triplicate. G&P: Glucose and corticosterone, i.e., simulated diabetes-related depression conditions.
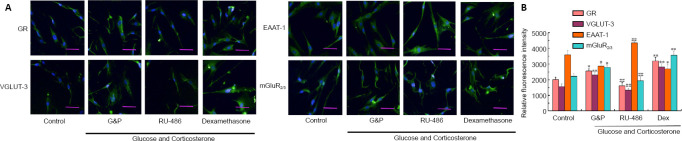
Aberrant immunopositivity against of GR, VGLUT-3, and mGluR2/3 in astrocytes of the hippocampal NVU after DD model establishment
Immunopositivity against GR, Glu transporters, and Glu receptors was detected by immunofluorescence staining. As shown in Figure 4, the immunopositivity against GR (P < 0.05), VGLUT-3 (P < 0.01), and mGluR2/3 (P < 0.05) increased, whereas immunopositivity against EAAT-1 (P < 0.05) significantly decreased after DD model establishment compared with control conditions. The GR agonist aggravated the upregulation of GR (P < 0.01), VGLUT-3 (P < 0.01), and mGluR2/3 (P < 0.01), as determined by increased immunopositivity, and downregulated EAAT-1 immunopositivity (P < 0.05). The upregulation of GR, VGLUT-3, and mGluR2/3, and the downregulation of EAAT-1 were reversed by RU-486 treatment (P < 0.01). These results revealed that DD conditions adversely affected the functions of Glu transporters and the expression levels of the Glu receptor mGluR2/3 in astrocytes of the hippocampal NVU, which may be associated with the GR activation.
Figure 4.
Aberrant immunopositivity against GR, glutamate transporters, and glutamate receptor in hippocampal neurovascular unit astrocytes after diabetes-related depression induction.
(A) Typical images showing GR, VGLUT-3, EAAT-1, and mGluR2/3 immunopositivity (immunofluorescence staining). Positive detection of GR, VGLUT-3, EAAT-1, and mGluR2/3 appear in green, stained by fluorescein isothiocyanate. Simulated DD conditions (G&P) resulted in the upregulation of GR, VGLUT-3, and mGluR2/3 and the downregulation of EAAT-1 compared with control conditions. The upregulation of GR, VGLUT-3, and mGluR2/3 and the downregulation of EAAT-1 could be reversed by RU-486 treatment relative to the G&P group. Dexamethasone aggravated the changes in immunopositive detection among these proteins. Scale bars: 100 µm. (B) Quantitative analysis of GR, VGLUT-3, EAAT-1, and mGluR2/3 immunopositivity. Data are expressed as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. control group; ##P < 0.01, vs. model group (G&P) (one-way analysis of variance followed by Dunnett’s post hoc test). Experiments were performed in triplicate. Dex: Dexamethasone, i.e., glucocorticoid receptor agonist; EAAT-1: excitatory amino-acid transporter 1; GR: glucocorticoid receptor; G&P: glucose and corticosterone, i.e., simulated diabetes-related depression conditions; mGluR2/3: metabotropic glutamate receptor 2/3; RU-486: mifepristone, i.e., glucocorticoid receptor blocker; VGLUT-3: vesicular glutamate transporter 3.
Levels of Glu in the hippocampal NVU increase after DD model establishment
Figure 5 shows the levels of Glu in the hippocampal NVU after DD model establishment. The levels of Glu under simulated DD conditions in the dexamethasone treatment group were significantly higher than those in the control group (P < 0.01). These increases were significantly inhibited by RU-486 treatment (P < 0.05). Therefore, we can conclude that the simulated DD conditions increased the levels of Glu in the hippocampal NVU, which may be associated with the activation of GR.
Figure 5.
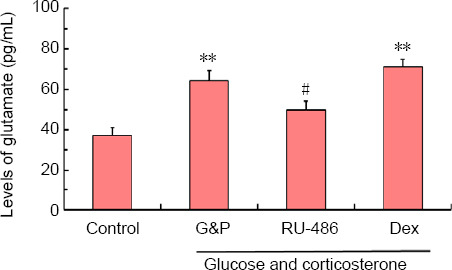
Levels of glutamate in the hippocampal NVU after the simulation of diabetes-related depression, as detected by enzyme-linked immunosorbent assay.
Data are shown as the mean ± SD (n = 4). **P < 0.01, vs. control group; #P < 0.05, vs. model group (G&P) (analysis of variance). Experiments were performed in triplicate. Dex: Dexamethasone, i.e., glucocorticoid receptor agonist; G&P: glucose and corticosterone, i.e., simulated diabetes-related depression conditions; RU-486: Mifepristone, i.e., glucocorticoid receptor blocker.
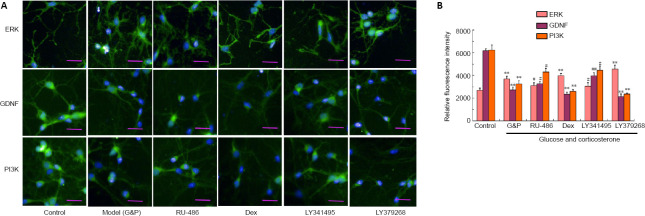
Aberrant immunopositivity against ERK, GDNF, and PI3K in the neurons of the hippocampal NVU after DD model establishment
Immunopositivity against ERK, GDNF, and PI3K were detected by immunofluorescence staining. As shown in Figure 6, simulated DD conditions resulted in the marked upregulation of ERK immunopositivity and the downregulation of GDNF and PI3K immunopositivity in hippocampal neurons of the NVU compared with the control conditions (P < 0.01 or P < 0.05). Immunopositivity against ERK, GDNF, and PI3K in the neurons of the hippocampal NVU, after DD model establishment, was significantly inhibited by both RU-486 and LY341495 treatments (P < 0.01 or P < 0.05). However, dexamethasone and LY379268 aggravated the disordered ERK, GDNF, and PI3K immunopositivity in the neurons of the hippocampal NVU after DD model establishment. These findings suggested that the simulated DD conditions adversely impacted imbalances in the ERK/GDNF/PI3K signaling pathway, such as the expression levels of the key proteins ERK, GDNF, and PI3K in the neurons of the hippocampal NVU, which may be associated with GR activation and the activation the Glu receptor mGluR2/3.
Figure 6.
Aberrant immunopositivity against ERK, BDNF, and PI3K in hippocampal neurovascular unit neurons after diabetes-related depression induction.
(A) Typical images showing ERK, BDNF, and PI3K immunopositivity (immunofluorescence staining). The detection of ERK, BDNF, and PI3K is shown in green, stained by fluorescein isothiocyanate. Simulated DD conditions (G&P) significantly upregulated ERK and down-regulated GDNF and PI3K when compared with the control conditions. The upregulation of ERK and the downregulation of GDNF and PI3K could be reversed by both RU-486 and LY341495 treatment when compared with the G&P group. However, dexamethasone and LY379268 aggravated the disordered expression patterns of these proteins. Scale bars: 100 µm. (B) Quantitative analysis of ERK, BDNF, and PI3K immunopositivity. Data are expressed as the mean ± SD (n = 3). **P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. model group (G&P) (one-way analysis of variance followed by Dunnett’s post hoc test). Experiments were performed in triplicate. BDNF: Brain-derived neurotrophic factor; Dex: dexamethasone, i.e., glucocorticoid receptor agonist; ERK: extracellular signal-regulated kinase; G&P: glucose and corticosterone, i.e., simulated diabetes-related depression conditions; LY341495: metabotropic glutamate receptor 2/3 receptor blocker; LY379268: metabotropic glutamate receptor 2/3 receptor agonist; PI3K: phosphoinositide 3-kinase; RU-486: Mifepristone, i.e., glucocorticoid receptor blocker.
Neuronal cell survival in the hippocampal NVU is reduced after DD model establishment
As shown in Figure 7, the cell viability of neurons under simulated DD conditions was markedly decreased compared with that in the corresponding control group (P < 0.01). This reduced cell viability was significantly inhibited by treatment with RU-486 and LY341495 (P < 0.05). In contrast, both dexamethasone and LY379268 aggravated declining cell survival after DD model establishment (P < 0.01). These results indicated that cell survival among the neurons in the hippocampal NVU after DD model establishment was associated with the activation of both GR and mGluR2/3.
Figure 7.
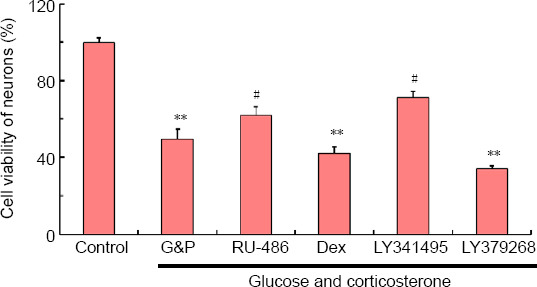
Cell survival among hippocampal neurovascular unit neurons after diabetes-related depression induction, as detected by the Cell Counting Kit-8 assay.
Data are expressed as the mean ± SD (n = 3). **P < 0.01, vs. control group; #P < 0.05, vs. model group (G&P) (one-way analysis of variance followed by Dunnett’s post hoc test). Experiments were performed in triplicate. Dex: Dexamethasone, i.e., glucocorticoid receptor agonist; G&P: glucose and corticosterone, i.e., simulated diabetes-related depression condition; LY341495: metabotropic glutamate receptor 2/3 receptor blocker; LY379268: metabotropic glutamate receptor 2/3 receptor agonist; RU-486: mifepristone, i.e., glucocorticoid receptor blocker.
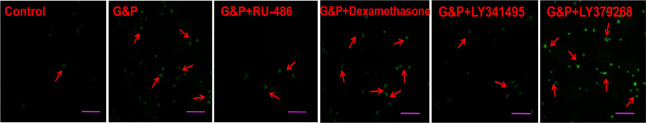
DD dramatically accelerates neuronal apoptosis in the hippocampal NVU
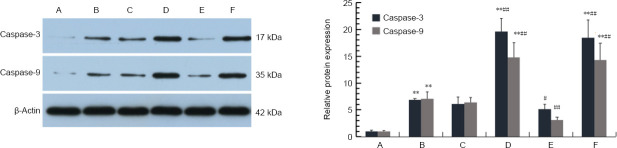
As shown in Figure 8, apoptosis among hippocampal neurons under simulated DD conditions was increased compared with control neurons. Furthermore, neuronal apoptosis was substantially reduced following pharmacological intervention with RU-486 and LY341495. However, both dexamethasone and LY379268 aggravated apoptosis among neurons in the hippocampal NVU. As shown in Figure 9, the results of the western blot analysis indicated that the expression levels of the apoptosis proteins caspase-3 and caspase-9 were markedly increased under simulated DD conditions compared with the corresponding control group (P < 0.01). Both dexamethasone and LY379268 increased the expression levels of caspase-3 and caspase-9 (P < 0.01). However, the upregulation of apoptotic proteins was significantly ameliorated after treatment with RU-486 and LY341495 (P < 0.05). These results suggested that apoptosis among hippocampal neurons was regulated by the Glu/mGluR2/3/PI3K pathway, induced by GR and mGluR2/3 activation in the hippocampal NVU.
Figure 8.
Apoptosis of neurons in the hippocampal neurovascular unit, detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling.
Apoptosis of neurons in simulated DD conditions increased compared with control conditions. The number of apoptotic cells was markedly reduced by RU-486 and LY341495 treatment. However, both dexamethasone and LY379268 aggravated apoptosis among neurons. Arrows indicate apoptotic cells. Scale bars: 100 µm. Dex: Dexamethasone, i.e., glucocorticoid receptor agonist; G&P: glucose and corticosterone, i.e., simulated diabetes-related depression conditions; LY341495: metabotropic glutamate receptor 2/3 receptor blocker; LY379268: metabotropic glutamate receptor 2/3 receptor agonist; RU-486: mifepristone, i.e., glucocorticoid receptor blocker.
Figure 9.
Apoptotic protein expression in the hippocampal neurovascular unit, detected by western blot assay.
Simulated diabetes-related depression conditions resulted in the significant upregulation of caspase-3 and caspase-9 expression in the hippocampal NVU compared with control conditions. The upregulation of caspase-3 and caspase-9 was reversed by both RU-486 and LY341495 treatment compared with the untreated model. However, dexamethasone and LY379268 aggravated the upregulation of these two proteins. Data are expressed as the mean ± SD (n = 3). **P < 0.01, vs. control group. #P < 0.05, ##P < 0.01, vs. model group (G&P) (one-way analysis of variance followed by Dunnett’s post hoc test). A–F: Control, model (glucose and corticosterone, i.e., simulated diabetes-related depression conditions), RU-486 (glucocorticoid receptor blocker), dexamethasone, (glucocorticoid receptor agonist), LY341495 (mGluR2/3 receptor blocker), and LY379268 (mGluR2/3 receptor agonist). mGluR2/3: metabotropic glutamate receptor 2/3.
Discussion
The NVU, which primarily consists of microvessels, astrocytes, neurons, and extracellular matrix, was first introduced by Lo et al. (2003). Neurons, neurogliocytes, and brain microvascular endothelial cells in the NVU are interrelated and influence each other and maintain a dynamic balance between homeostasis and neurotransmitter release, which play essential roles in central nervous system diseases, such as DD (Han and Suk, 2005; Liu et al., 2011; Adriani et al., 2017). The current study examining DD primarily focused on the neuron, as a single target; however, few studies have examined neurons within the overall environment of the hippocampal NVU (Wang et al., 2015a; Liu et al., 2019a). Consequently, the concept of the NVU has not only revolutionized the understanding of central nervous system pathophysiology but has also provided a new perspective for studying the pathogenesis of DD.
DD occurs secondary to diabetes. The hippocampus is a key target of DD, which is caused by hippocampal damage (Ho et al., 2013; Lenart et al., 2019). Our previous studies elucidated that DD may be associated with structural and functional damage to the hippocampal NVU, after we determined that Glu concentrations and hippocampal neuron apoptosis were key signals and direct factors associated with DD development (Liu et al., 2019a). Furthermore, research has revealed that the permeability of the hippocampal blood-brain barrier was destroyed by high glucose concentrations and that high corticosterone concentrations in plasma can affect astrocytes through microvascular endothelial cells, affecting the structures and functions of astrocytes (Yang et al., 2015). The association between cortisol and Glu and their destructive impacts on the hippocampus have begun to receive attention (Reisner, 2003, 2013). Glu is an amino acid that acts as an excitatory neurotransmitter in the hippocampus. The amount of excitatory amino acid release may be proportional to memory and cognitive dysfunction. Cortisol is a type of glucocorticoid, and excessive cortisol is released in response to the dysregulation of the hypothalamic-pituitary-adrenal axis among patients with depression. The interaction between cortisol and Glu is of great importance for cognitive dysfunctional syndromes, such as depression, post-traumatic stress disorder, and memory deficits following electroconvulsive therapy (Andrews and Reisner, 2017). Evidence has shown that the stimulation of Glu and cortisol are not mutually exclusive (Chamberlin and Tsai, 1998; Reisner, 2003, 2013). On the one hand, the overstimulation of Glu receptors in the hippocampus can be exacerbated by high levels of glucocorticoids. Cortisol may maintain Glu receptors in an open position for longer periods of time and more frequently, which may augment glutamatergic hyperstimulation. On the other hand, glutamatergic stimulation can result in free radical formation, which can gradually disrupt cerebrovascular endothelial cells, accelerating the release of cortisol.
Elevated cortisol levels, in combination with increased glutamatergic stimulation, may have a particularly negative effect on memory, due to the excessive release of excitatory amino acids and the activation of their receptors. This effect not only supports the role of Glu and cortisol but also provides a useful model for DD condition-induced neuronal damage. Moreover, the expression levels of the Glu transporters EAAT-1 and VGLUT in astrocytes were affected by GR binding with corticosterone in astrocytes, resulting in the abnormal increase in intracellular glutamic acid levels (Xu et al., 2016). In this article, we found that simulated DD conditions adversely affected the barrier function of the NVU, including the dramatic reduction of transendothelial electrical resistance and increased permeability, within 4 hours. In addition, the expression levels of GR and VGLUT-3 were upregulated, whereas the level of EAAT-1 was downregulated. The abnormal expression patterns observed for GR, VGLUT-3, and EAAT-1 were reversed by the GR blocker (RU-486). Therefore, we speculated that the barrier function of cerebral microvascular endothelial cells was destroyed by high glucose concentrations in the hippocampal NVU. Subsequently, GR in astrocytes was activated by the massively elevated corticosterone contents, as a result of barrier dysfunction. The over-stimulation of GRs, which was activated by corticosterone, may result in disordered EAAT-1 and VGLUT in astrocytes, resulting in increased Glu concentrations. These results provided evidence regarding the origins of excess Glu and demonstrated the potential successive roles played by Glu and cortisol. Simultaneously, these findings indicated that simulated DD conditions adversely impact barrier function in the NVU and the expression of Glu transporters in NVU astrocytes, which may be closely associated with the activation of GR.
Our previous studies have found that simulated DD conditions adversely impact the barrier function of the NVU, resulting in abnormal expression patterns for GR and Glu transporters, following by the activation of the Glu receptor mGluR2/3 and elevated levels of Glu in astrocytes (Liu et al., 2018, 2019b). Furthermore, our findings showed that abnormal Glu-mGluR2/3 pathway activation can result in hippocampal neuronal apoptosis, which was found to be a direct factor associated with DD onset and may also be strongly associated with GR and mGluR2/3 activation (Liu et al., 2019b). Consequently, the present study implied that the underlying pathogenesis of DD is based on the activation of the Glu-mGluR2/3 pathway by GR and mGluR2/3 in the NVU.
Damage to hippocampal neurons can be caused by the excitatory toxicity associated with excessive Glu levels. The Glu contents dramatically increased after the abnormal expression of the Glu transporters VGLUT and EAAT-1 in astrocytes (Hubbard et al., 2016). Abnormally elevated Glu levels stimulated the over-expression of the Glu receptor mGluR2/3 (Losonczy et al., 2003). On the one hand, the synthesis and secretion of GDNF through the ERK signaling pathway was interrupted by the abnormal activation of the Glu receptor mGluR2/3 (Di Liberto et al., 2011). On the other hand, the expression levels of GDNF receptor growth differentiation factor-family receptor alpha-1 and -2 were downregulated, which also resulted from the activation of the Glu receptor mGluR2/3 (Chen et al., 2001). Moreover, GDNF, after binding growth differentiation factor-family receptor alpha-1, phosphorylated the proto-oncogene rearranged during transfection and regulated the PI3K/AKT signaling pathway (Gleason et al., 2013). In the present study, we observed increased levels of Glu in the hippocampal NVU after DD model establishment, and simulated DD conditions resulted in the upregulation of ERK and the downregulation of GDNF and PI3K in hippocampal neurons of the NVU. These effects were significantly inhibited by both the GR blocker RU-486 and the mGluR2/3 blocker LY341495 but were aggravated by the GR agonist dexamethasone and the mGluR2/3 receptor agonist LY379268. Therefore, these findings suggested that simulated DD conditions may adversely impact imbalances in the ERK/GDNF/PI3K signaling pathway, among key proteins expressed in the neurons of the hippocampal NVU, which may be associated with the activation of GR and mGluR2/3.
In our previous study, we demonstrated that hippocampal neuronal apoptosis was involved in triggering DD onset (Liu et al., 2019a). The PI3K/AKT signaling pathway, which is downstream of the mGluR2/3-GDNF pathway, affected the survival and apoptosis of hippocampal neurons by regulating cyclic adenosine monophosphate response element-binding protein, nuclear factor kappa B, glycogen synthase kinase, caspases, and their phosphorylated proteins (Tsai et al., 2014). Interestingly, in this study, we demonstrated that the cell viability of neurons was markedly decreased, whereas the apoptosis of neurons increased in the hippocampal NVU after DD model establishment. Furthermore, cell viability and the apoptosis of neurons were closely associated with GR and mGluR2/3 activation, which was consistent with our previous results in an animal DD model (Liu et al., 2019b). Thus, this study provides evidence that the Glu/mGluR2/3/PI3K pathway may be involved in the regulation of cell viability and neuronal apoptosis in the hippocampal NVU.
Although the in vitro study described in the present paper revealed that the pathogenesis of DD was associated with the Glu/mGluR2/3/PI3K signaling pathway, additional studies using the DD model rat should be repeated, and in vivo pharmacological approaches should be explored, to support these conclusions. Additionally, the exact mechanism and targets associated with DD that are regulated by the Glu/mGluR2/3/PI3K pathway remain to be uncovered, in subsequent experiments.
In conclusion, the findings of the present study implied that hippocampal neuron apoptosis was regulated by the Glu/mGluR2/3/PI3K signaling pathway, activated by GR in the hippocampal NVU and may be involved in the pathogenesis of DD. However, further studies elucidating the explicit mechanisms associated with this pathway remain necessary.
Additional file: Open peer review report 1 (80.8KB, pdf) .
Footnotes
P-Reviewer: Andrews CJ; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Giles L, Yu J, Song CP; T-Editor: Jia Y
Conflicts of interest: The authors declare that they have no conflict of interests.
Financial support: This study was supported by the National Natural Science Foundation of China, Nos. 81573965 (to YHW), 81874464 (to YHW); the Natural Science Foundation of Hunan Province of China, No. 2017JJ3241 (to JL); and the Education Department Scientific Research Foundation of Hunan Province of China, No. 17C1229 (to JL). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Laboratory Animal Ethics Committee of The First Hospital of Hunan University of Chinese Medicine, China (approved No. HN-ZYFY-2019-11-12) on November 12, 2019.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81573965 (to YHW), 81874464 (to YHW); the Natural Science Foundation of Hunan Province of China, No. 2017JJ3241 (to JL); and the Education Department Scientific Research Foundation of Hunan Province of China, No. 17C1229 (to JL).
Open peer reviewer: Christopher J Andrews, University of Queensland, Australia.
References
- 1.Adriani G, Ma D, Pavesi A, Kamm RD, Goh EL. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip. 2017;17:448–459. doi: 10.1039/c6lc00638h. [DOI] [PubMed] [Google Scholar]
- 2.Andrews CJ, Reisner AD. Neurological and neuropsychological consequences of electrical and lightning shock: review and theories of causation. Neural Regen Res. 2017;12:677–686. doi: 10.4103/1673-5374.206636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol. 2017;12:593–601. doi: 10.1007/s11481-017-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamberlin E, Tsai GE. A glutamatergic model of ECT-induced memory dysfunction. Harv Rev Psychiatry. 1998;5:307–317. doi: 10.3109/10673229809003579. [DOI] [PubMed] [Google Scholar]
- 5.Chen AC, Eisch AJ, Sakai N, Takahashi M, Nestler EJ, Duman RS. Regulation of GFRalpha-1 and GFRalpha-2 mRNAs in rat brain by electroconvulsive seizure. Synapse. 2001;39:42–50. doi: 10.1002/1098-2396(20010101)39:1<42::AID-SYN6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Di Liberto V, Mudò G, Belluardo N. mGluR2/3 agonist LY379268, by enhancing the production of GDNF, induces a time-related phosphorylation of RET receptor and intracellular signaling Erk1/2 in mouse striatum. Neuropharmacology. 2011;61:638–645. doi: 10.1016/j.neuropharm.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Gleason SD, Li X, Smith IA, Ephlin JD, Wang XS, Heinz BA, Carter JH, Baez M, Yu J, Bender DM, Witkin JM. mGlu2/3 agonist-induced hyperthermia: an in vivo assay for detection of mGlu2/3 receptor antagonism and its relation to antidepressant-like efficacy in mice. CNS Neurol Disord Drug Targets. 2013;12:554–566. doi: 10.2174/18715273113129990079. [DOI] [PubMed] [Google Scholar]
- 8.Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2:409–423. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- 9.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346–1362. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubbard JA, Szu JI, Yonan JM, Binder DK. Regulation of astrocyte glutamate transporter-1 (GLT1) and aquaporin-4 (AQP4) expression in a model of epilepsy. Exp Neurol. 2016;283:85–96. doi: 10.1016/j.expneurol.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 12.Islam SM, Ferrari U, Seissler J, Niessen L, Lechner A. Association between depression and diabetes amongst adults in Bangladesh: a hospital based case-control study. J Glob Health. 2015;5:020406. doi: 10.7189/jogh.05.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaledi M, Haghighatdoost F, Feizi A, Aminorroaya A. The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 2019;56:631–650. doi: 10.1007/s00592-019-01295-9. [DOI] [PubMed] [Google Scholar]
- 14.Lenart L, Balogh DB, Lenart N, Barczi A, Hosszu A, Farkas T, Hodrea J, Szabo AJ, Szigeti K, Denes A, Fekete A. Novel therapeutic potential of angiotensin receptor 1 blockade in a rat model of diabetes-associated depression parallels altered BDNF signalling. Diabetologia. 2019;62:1501–1513. doi: 10.1007/s00125-019-4888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li CX, Wang XQ, Cheng FF, Yan X, Luo J, Wang QG. Hyodeoxycholic acid protects the neurovascular unit against oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Neural Regen Res. 2019;14:1941–1949. doi: 10.4103/1673-5374.259617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Wang YH, Li W, Liu L, Yang H, Meng P, Han YS. Structural and functional damage to the hippocampal neurovascular unit in diabetes-related depression. Neural Regen Res. 2019a;14:289–297. doi: 10.4103/1673-5374.244794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R, Zhang TT, Wu CX, Lan X, Du GH. Targeting the neurovascular unit: development of a new model and consideration for novel strategy for Alzheimer’s disease. Brain Res Bull. 2011;86:13–21. doi: 10.1016/j.brainresbull.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Zhao HQ, Meng P, Yang H, Xiang Y, Wang YH. Role of Glu-mGluR2/3-ERK signaling pathway in apoptosis of neurons in the prefrontal cortex of diabetic rats with depression. Zhongguo Yaolixue Tongbao. 2018;34:1662–1667. [Google Scholar]
- 19.Liu Z, Han Y, Zhao H, Luo W, Jia L, Wang Y. Glu-mGluR2/3-ERK signaling regulates apoptosis of hippocampal neurons in diabetic-depression model rats. Evid Based Complement Alternat Med. 2019b;2019:3710363. doi: 10.1155/2019/3710363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 21.Losonczy A, Somogyi P, Nusser Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J Neurophysiol. 2003;89:1910–1919. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- 22.Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisner AD. The electroconvulsive therapy controversy: evidence and ethics. Neuropsychol Rev. 2003;13:199–219. doi: 10.1023/b:nerv.0000009484.76564.58. [DOI] [PubMed] [Google Scholar]
- 24.Reisner AD. Possible mechanisms for delayed neurological damage in lightning and electrical injury. Brain Inj. 2013;27:565–569. doi: 10.3109/02699052.2013.766928. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CY, Chang AY, Chan JY, Chan SH. Activation of PI3K/Akt signaling in rostral ventrolateral medulla impairs brain stem cardiovascular regulation that underpins circulatory depression during mevinphos intoxication. Biochem Pharmacol. 2014;88:75–85. doi: 10.1016/j.bcp.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Yang H, Li W, Meng P, Han Y, Zhang X, Cao D, Tan Y. Zuogui Jiangtang Jieyu Formulation prevents hyperglycaemia and depressive-like behaviour in rats by reducing the glucocorticoid level in plasma and hippocampus. Evid Based Complement Alternat Med. 2015a;2015:158361. doi: 10.1155/2015/158361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YH, Chai S, Tan XW, Yang H, Meng P, Zhang XL. Effects of Zuogui Jiangtang Jieyu Prescription on expression of Bcl-2, Bax, Caspase 8 in hippocampus of diabetes mellitus rats with depression. Zhongguo Shiyan Fangji Xue Zazhi. 2015b;21:106–109. [Google Scholar]
- 28.Xu YL, Zhao HQ, Du Q, Yang H, Meng P, Wang YH. Effect of Zuogui Jiangtang Jieyu Formula on amino acid transporter expression in hippocampal astrocytes of diabetes mellitus rats with depression. Beijing Zhongyiyao Daxue Xuebao. 2016;39:470–475. [Google Scholar]
- 29.Xue Q, Liu Y, Qi H, Ma Q, Xu L, Chen W, Chen G, Xu X. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int J Biol Sci. 2013;9:174–189. doi: 10.7150/ijbs.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Du Q, Zhao HQ, Tan XW, Meng P, Wang YH. Effects of Zuogui Jiangtang Jieyu Formula on protein expression of CoIV, ZO-1, and α-SMA in hippocampal blood-brain barrier of diabetes mellitus rats with depression. Zhongcaoyao. 2015;46:3214–3218. [Google Scholar]
- 31.Ziegler R, Neu A. Diabetes in Childhood and Adolescence. Dtsch Arztebl Int. 2018;115:146–156. doi: 10.3238/arztebl.2018.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.