Abstract
The axon initial segment (AIS) region is crucial for action potential initiation due to the presence of high-density AIS protein voltage-gated sodium channels (Nav). Nav channels comprise several serine residues responsible for the recruitment of Nav channels into the structure of AIS through interactions with ankyrin-G (AnkG). In this study, a series of computational experiments are performed to understand the role of AIS proteins casein kinase 2 and AnkG on Nav channel recruitment into the AIS. The computational simulation results using Virtual cell software indicate that Nav channels with all serine sites available for phosphorylation bind to AnkG with strong affinity. At the low initial concentration of AnkG and casein kinase 2, the concentration of Nav channels reduces significantly, suggesting the importance of casein kinase 2 and AnkG in the recruitment of Nav channels.
Keywords: Alzheimer's disease, ankyrin-G, axon initial segment, casein kinase-2, microtubules, voltage-gated potassium channel, voltage-gated sodium channel
Chinese Library Classification No. R448; R31; R741
Introduction
Communication between neurons through action potentials (AP) is necessary for the nervous system to perform its functions. An AP is characterized by the changes in membrane potential or membrane voltage due to the efflux and influx of cations. Several studies have confirmed that AP is initiated at the axon initial segment (AIS) (Lemaillet et al., 2003; Rasband, 2009; Kole and Stuart, 2012; Gulledge and Bravo, 2016), which is a non-myelinated region (Figure 1), with a length of 10 to 60 µm, at the beginning of an axon (Kole et al., 2008; Buffington and Rasband, 2011; Kole and Stuart, 2012; Leterrier et al., 2015; Jones and Svitkina, 2016; Nelson and Jenkins, 2017; Fan and Markram, 2019). The main proteins in the AIS are voltage-gated sodium channels (Nav), voltage-gated potassium channels (Kv), microtubules (MTs), casein kinase 2 (CK2), and ankyrin-G (AnkG). AnkG is an essential protein in the AIS because the recruitment of other proteins (Kv, Nav, neurofascin 186 [NF186], and neuronal cell adhesion molecule [NrCAM]) into the AIS depends on it (Hedstrom et al., 2008; Jones and Svitkina, 2016). To evaluate the importance of AIS in neurological processes and to show its relationships with various neurological diseases, it is necessary to understand the structural organization of the AIS region (Figure 1).
Figure 1.
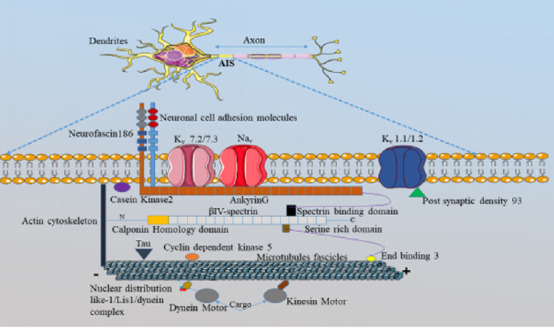
A schematic diagram of the AIS structure.
The AIS region of neurons consists of three membranes: the plasma membrane, the spectrin-actin membrane, and the microtubule related membrane. Each membrane carries out different functions that favor AP initiation at the AIS. AnkG acts as the master organizer and all the proteins in the AIS are directly or indirectly associated with it (Zhang and Bennet, 1998; Zhou et al., 1998; Hedstrom et al., 2008; Cunha and Mohler, 2009; Nelson and Jenkins, 2017). The initial part of AnkG is called the membrane-binding domain, to which the anchoring of Nav, and Kv channels (Kv 7.2/7.3) occurs (Nelson and Jenkins, 2017; Buffington and Rasband, 2011). At the AIS, the proteins binding with AnkG have specific amino acid sequence, known as the AIS motif, except for the Kv 1.1/1.2 channels (Schafer et al., 2009). Instead of AnkG, the recruitment of Kv 1.1/1.2 channels into the AIS depends on the post-synaptic density 93 (PSD 93) protein (Leterrier and Dargent, 2014; Yoshimura and Rasband, 2014). The membrane-binding domain is followed by a spectrin binding domain in the AnkG structure, and this is responsible for the binding between β-IV spectrin and AnkG (Jones and Svitkina, 2016). The microtubule fascicles in the AIS are also restricted by AnkG due to the interactions with an end binding 3 (EB3) protein (Nakata and Hirokawa, 2003; Jones and Svitkina, 2016). According to the experimental evidence, the high-density presence of Nav channels in the AIS is correlated to the AP initiation (Kole et al., 2012; Jones and Svitkina, 2016); and it depends not only on AnkG but also on casein kinase 2 (CK2)-facilitated phosphorylation (Brechet et al., 2008). The experimental investigations have also shown that CK2 plays a dynamic role in the AIS region, especially in the accumulation of Nav channels into the AIS (Brechet et al., 2008; Nishi et al., 2014; Xu and Cooper, 2015). AIS: Axon initial segment; AnkG: ankyrin-G; AP: action potential; Kv: voltage-gated potassium channel; MT: microtubule; Nav: voltage-gated sodium channel; NF186: neurofascin 186; NrCAM: neuronal cell adhesion molecule.
CK2, a serine/threonine-specific kinase, is acidophilic in nature and mediates phosphorylation of various proteins in the AIS (Meggio and Pinna, 2003). CK2 is constitutively active and does not require any second messenger or phosphorylation event to be activated (Meggio and Pinna, 2003; Bian et al., 2013). During the developmental stage, the early presence of CK2 in neurons has been observed, even before axon formation, and the experiments have shown the shortening of axons by 30% after CK2 inhibition (Ponce et al., 2011). CK2 plays a very critical role in AnkG-Nav binding to recruit Nav channels into the AIS facilitating the phosphorylation of Nav channels to increase their affinity towards AnkG (Rasband, 2008; Yamada and Kuba, 2016). Nav channels comprise a special type of amino acid sequence called the AIS motif, where the binding with AnkG takes place. The AIS motif contains four serine residues at different positions, S1112, S1123, S1124, and S1126 (Figure 2; Schafer et al., 2009). In addition to that, the AIS motif also contains the acidic residues: glutamate (E) and aspartate (D) residues are present at 1111 and 1113 positions respectively (Figure 2). All these residues have different functions in the AIS motif: the serine residues are responsible for the phosphorylation of Nav channels through CK2, and acidic residues (glutamate and aspartate) increase the affinity of phosphorylation process (Meggio and Pinna, 2003). All these serine sites are phosphorylated by CK2 and this increases the binding affinity of the Nav channels towards AnkG to restrict them at the AIS (Meggio and Pinna, 2003; Brechet et al., 2008; Bian et al., 2013).
Figure 2.
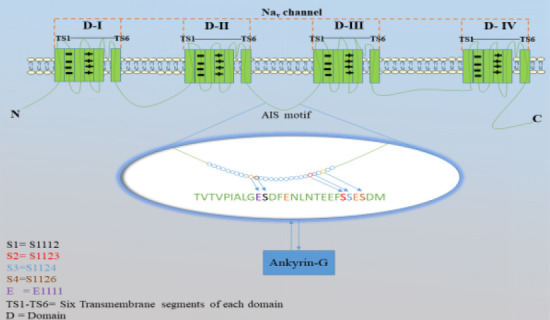
A schematic representation of the AIS motif within Nav channels.
The Nav channels comprise of four domains (D-I–IV), and each domain contains six transmembrane segments (TS1–TS6). The AIS motif (enlarged area) is located between the II and III domains of the Nav channels, and is made up of different 27 amino acids, with three main ones: serine (S) at the positions 1112, 1123, 1124, and 1126; glutamate (E) at the positions 1111 and 1115; and aspartate (D) at the position, 1113. Serine is important because the phosphorylation of Nav channels is accomplished by CK2, and CK2 is specific for serine or threonine. The presence of negative residues, such as glutamate and aspartate is also significant because they provide the support for the CK2-mediated phosphorylation of serine. Moreover, glutamate and aspartate assist CK2 in the identification of serine as a target for phosphorylation. This figure was produced using Servier Medical Art (https://smart.servier.com). AIS: Axon initial segment; AnkG: ankyrin-G; Nav: voltage-gated sodium channel.
Any alteration in neuronal transmission can cause various neurological conditions, such as Alzheimer’s disease (AD), epilepsy, schizophrenia and bipolar disorder (Buffington and Rasband, 2011; Kaphzan et al., 2011; Bi et al., 2012; Harty et al., 2013; Rueckert et al., 2013; Peltola et al., 2016). AD is the most common neurodegenerative disease in humans; thus, it is the most common cause of dementia and, as yet, there is no known cure for AD (Hardy and Higgins, 1992; Maccioni et al., 2010; Craig et al., 2011; Karran et al., 2011; Checler and Turner, 2012; Alzheimer’s Association, 2013; Kametani and Hasegawa, 2018). Several studies suggest the correlation of AD with AIS proteins and have shown the loss of AnkG and Nav channels in AD brain samples (Kim et al., 2007; Sun et al., 2014). Cleavage of Nav channel is associated with increase in Amyloid β peptides (Aβ) production, a protein responsible for the amyloid plaques in AD patients (Kim et al., 2007; Kovacs et al., 2010). Moreover, loss of AnkG from AIS structure could play a critical role in AD pathogenesis by disturbing the protein trafficking within the neuron by altering the function of molecular motors (Kinesin and Dynein) (Kim et al., 2007; Sun et al., 2014).
Biological systems are complex as their features can be explained using different methods, and this makes them very difficult to study and to predict their behaviors (Bailey et al., 2002). However, computational models can be used to understand the plausible molecular mechanisms in an organism at the molecular level if we carefully develop the questions to be investigated. As we mentioned before, AP initiation is associated with the high number of Nav channels in the AIS, and these channels are phosphorylated by CK2 at their serine sites before their accumulation in the AIS. Further, AnkG recruits the phosphorylated Nav channels in this region. The dynamics of the phosphorylated channels, CK2, and AnkG would have crucial effects on AP initiation, and any impairment of these interactions would perhaps explain the experimentally observed link of AnkG to AD pathogenesis. Hence, we develop a mathematical model to investigate the role of serine-specific phosphorylation of Nav channels by CK2 before the accumulation of Nav channels into the AIS. Also, we test the effect of changes in the initial concentration of CK2 and AnkG on the accumulation of Nav channels at the AIS. Here, we suggest the AIS association with Alzheimer’s disease (AD) pathogenesis by reporting CK2 mediated serine specific phosphorylation is necessary for the recruitment of Nav channels into the AIS through AnkG. CK2 mediated phosphorylation increases the binding affinity with AnkG. Moreover, initial concentration of both AnkG and CK2 is also important and can disturb the final Nav channel population in AIS. According to the literature, alteration in Nav channels is associated with amyloid β peptides main component of amyloid plaques in AD patients (Kim et al., 2007; Kovacs et al., 2010).
Materials and Methods
Development of AIS models
It is necessary to make simplifying assumptions in the development of the model so that we can understand the interactions between the proteins within AIS. By using Virtual cell (version 7.2.0, Uconn Health, USA) software with currently available experimental data and insights (see References), the following assumptions are made:
1) The AIS region is considered as a cylinder with a length of 40 µm and a diameter of 1.5 µm (Gulledge and Bravo, 2016). A total of 12 species are present in our model which includes four different Nav channels (NaA, NaB, NaC, and N1111), four AnkG species (G1, G2, G3, and G4) and four CK2 species (C1, C2, C3, and C4).
2) In this model, four types of Nav channels are considered based on different phosphorylation events within the channels. We assume five phosphorylation conditions: no site phosphorylation, single-site phosphorylation, double-site phosphorylation, triple-site phosphorylation and four-site phosphorylation (Figure 3). The no site phosphorylation means that all serine sites are in the non-phosphorylated state. We do not consider any Nav species in this model as not being phosphorylated because without any serine phosphorylation, Nav-AnkG interactions are impossible (Brechet et al., 2008). Similarly, none of the Nav species are assumed to be in the single-site phosphorylation condition as well, because AnkG does not bind to the Nav channels with single-site phosphorylation (Brachet et al., 2008). Four Nav species in the model are assumed based on double-site phosphorylation, triple-site phosphorylation, and four-site phosphorylation. Due to the lack of literature on the concentration of Nav channels before its restriction into the AIS, we estimate that the concentration of each of NaA, NaB, NaC, and N1111 as 3.32 × 10–12µM.
Figure 3.
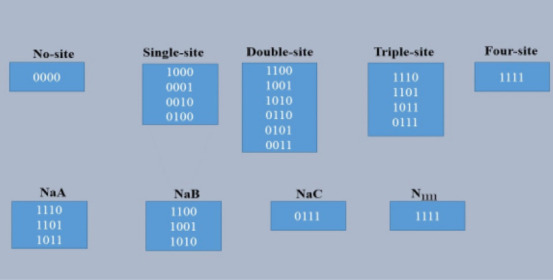
Classification of the Nav species in the AIS model.
None of the Nav species in the model is in the no phosphorylation or single-site phosphorylation conditions. In the double-site phosphorylation, two serine sites are phosphorylated, and there is a total of six combinations. Out of the six, only the first three combinations are considered for Nav-AnkG binding. These three combinations are considered as one and called NaB in the model. Combinations in the double phosphorylation condition, other than first three (NaB), are ignored based on the absence of an S1 site because the affinity of Nav channel binding with AnkG is high in the presence of the S1 site (Fache et al., 2004; Brechet et al., 2008). In the triple-site phosphorylation conditions, all combinations favor the interaction of Nav-AnkG. We, therefore, consider these first three combinations because of the presence of S1 and assume them as another Nav species (NaA) in the model; the fourth combination of the triple phosphorylation condition is taken as the third Nav species (NaC) even in the absence of an S1 site because of the phosphorylation of the remaining three serine sites (S2, S3, and S4) can favor Nav-AnkG interactions but with a low binding affinity (Brechet et al., 2008). The fourth Nav species (N1111) in the model is based on the presence of all serine sites being in the phosphorylated state.
3) The four phosphorylation sites in the AIS motifs (S1112, S1123, S1124 and S1126) are denoted by S1 = S1112, S2 = S1123, S3 = S1124, and S4 = S1126. All these sites within the Nav channels are responsible for equally strong interactions between the Nav channels and AnkG (Cantrell et al., 2018).
Here are the phosphorylation sequences for all four sites in the Nav species:
The phosphorylation of S1 is the first preference for CK2 because the regions near S1 (E, S1, D, F, and E) fulfill the criteria for the minimum consensus sequence (S/T, X, X, and D/E/pS/pT) for CK2 phosphorylation (Figure 4). The presence of acidic residues at the n + 1 and n + 3 positions makes S1 the most suitable site for phosphorylation by CK2 (Meggio and Pinna, 2003).
S3 also fulfills the minimum criterion for the consensus sequence (S/T, X, X, and D/E/pS/pT). S3 also has acidic residues at the n + 1 and n + 3 positions, similar to S1 (Figure 4). However, S3 is assumed to be the second preference after S1 because S1 is located near the N-terminal.
S4 is considered the third phosphorylation site because of the presence of an acidic residue (D) at the n + 1 position (Figure 4). The fourth, and final, phosphorylation site is S2 (S2, pS, E, pS, and D) because the region near S2 fulfills the minimum consensus sequence due to the presence of phosphorylated S or T at the n + 1 and n + 3 positions (Figure 4).
Figure 4.

Consensus sequence for CK2 mediated phosphorylation.
The AIS motif of Nav channels and the requirements for CK2-mediated phosphorylation. S represents serine residues; E refers to glutamate, and D refers to aspartate. D and E assist CK2 to find suitable serine for phosphorylation (Meggio and Pinna, 2003; Nishi et al., 2014). The positions of residues within the motif are represented by n – 1, n, n + 1, and so on. The potential targets of CK2 for phosphorylation are serine/threonine residues in a motif surrounded by any of the acidic residues, such as E and D. CK2-mediated phosphorylation of serine/threonine requires at least one acidic residue between the n – 4 to n + 7 positions (Meggio and Pinna, 2003; Nishi et al., 2014). The most favorable serine for CK2-mediated phosphorylation is when an acidic residue is present at the n + 3 position within a motif. In some instances, the phosphorylation by CK2 is enabled when an acidic residue is present at the n + 1 position and this is the second most preferred position after n + 3 (Meggio and Pinna, 2003; Nishi et al., 2014). The minimum consensus sequence for CK2 phosphorylation is S/T, X, X, D/E/pS/pT, where S/T is a serine or threonine in the nth position, followed by any type residue at the n + 1 and n + 2 positions. At the n + 3 position, D/E/pS/pT stands for aspartate (D) or glutamate (E) or phosphorylated serine/threonine (pS/ pT), respectively. Phosphorylated serine or threonine mimics the function of acidic residues and makes serine/threonine at the n position suitable for CK2 phosphorylation. The presence of basic residues (lysine, proline, and lysine) at the n + 1, n + 2, or n + 3 positions has a negative impact on CK2-mediated phosphorylation. In this figure, the presence of a negative residue (E) at n + 3 in the sequence makes S at the nth position suitable for phosphorylation by CK2 (Fache et al., 2004). S at the nth position is phosphorylated first because it is near the N-terminal when compared to the other serine residues. Similarly, other serine residues in the AIS motif can be identified as targets for CK2 by assuming them at n positions and by checking nearby for the presence of negative residues (D/E/pS/pT). AIS: Axon initial segment; CK2: casein kinase 2; Nav: voltage-gated sodium channel.
4) Phosphorylation of the Nav species (NaA, NaB, NaC, and N1111) by CK2 (C1, C2, C3, and C4) and its interaction with AnkG (G1, G2, G3, and G4) in this model are based on the mass action law.
5) In general, the phosphorylation is modeled using Michael-Menten kinetics (Wang and Wu, 2002) but due to rapid binding and unbinding of CK2 to the sites, we assume that the binding-unbinding can be modeled as elementary reactions and follows the mass action kinetics within the first few minutes; i.e., the serine related phosphorylation of Nav channels by CK2 is considered as a reversible elementary reaction. Because of the importance of the serine residues, the main aim is to understand the binding of CK2 with the Nav channels. Protein kinases have significant effects on the target protein they bind to. Protein kinases go on and off by auto-phosphorylation by binding with inhibitors or activator proteins or other small molecules. Their activity is highly regulated. However, this is not the case with CK2 due to its pleiotropic nature: CK2 does not require any mediators to activate because CK2 is always active. There is no evidence in the literature for any regulation of CK2 activity. CK2 not only phosphorylates the Nav and Kv channels but also AnkG. Another reason for considering the mass action law to model the phosphorylation of Nav species (NaA, NaB, NaC, and N1111) is the abundance of CK2 in the brain; therefore, we assume negligible competition of other species for CK2. In the absence of data, the phosphorylation of all four Nav species is assumed to occur at the same association (Kon) and dissociation rates (Koff).
6) In the brain, more than 300 substrates are available for CK2-mediated phosphorylation (Meggio and Pinna, 2003; Nishi et al., 2014). We have no data on the exact number of CK2 molecules required for phosphorylation of the Nav channels. As CK2 present in abundance, we assume that the total CK2 concentration is 5.28 × 10–8 (µM). Specifically, for this model, total CK2 concentration is divided into four equal concentrations designated as C1, C2, C3, and C4 to phosphorylate NaA, NaB, NaC, and N1111, respectively.
7) According to the literature, the total number of Nav channels present in the AIS after binding with AnkG is 100–300 molecules/µm (Kole and Stuart, 2012). On the other hand, according to Srinivasan et al. (1988), AnkG numbers are ten times higher than the Nav channels (Srinivasan et al., 1988). Based on this observation, in this model initial AnkG concentration is assumed as 1.32 × 10–13µM. Similar to CK2 species, for this model, we divide AnkG species into four species named as G1, G2, G3 and G4 with the equal concentration of 3.32 × 10–14µM; G1, G2, G3, and G4 bind with the phosphorylated form of NaA, NaB, NaC, and N1111, respectively.
Equations in the AIS model
The schematic of the model is shown in Figure 5. All parameters in the model, including their initial concentrations and rate constants, were estimated in accordance with the literature (Srinivasan et al., 1988; Meggio and Pinna, 2003; Ubersax and Ferrell, 2007; Brechet et al., 2008; Kole et al., 2008; Tables 1 and 2).
Figure 5.
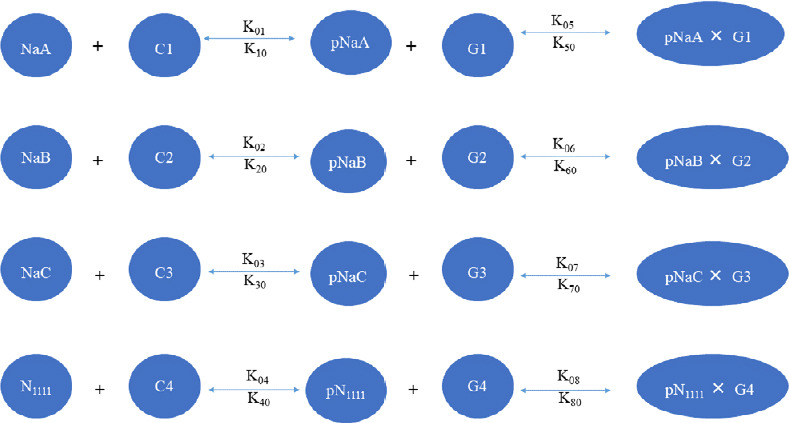
Model for the accumulation of Nav channels in the axon initial segment.
The model contains 12 different species: CK2 (C1, C2, C3, C4), Nav (NaA, NaB, NaC, N1111), and AnkG (G1, G2, G3, G4); and sixteen rate constants. A total of eight reactions are used to model the phosphorylation (p) of Nav species through CK2 and the binding of phosphorylated Nav species to AnkG. AnkG: ankyrin-G; CK2: casein kinase 2; Nav: voltage-gated sodium channel.
Table 1.
Initial concentration of all species considered in the axon initial segment model
| Protein | Initial concentration (µM) | Source |
|---|---|---|
| NaA | 3.32 × 10–12 | Kole et al. (2008) |
| NaB | 3.32 × 10–12 | Kole et al. (2008) |
| NaC | 3.32 × 10–12 | Kole et al. (2008) |
| N1111 | 3.32 × 10–12 | Kole et al. (2008) |
| C1 | 1.32 × 10–8 | Meggio and Pinna (2003) |
| C2 | 1.32 × 10–8 | Meggio and Pinna (2003) |
| C3 | 1.32 × 10–8 | Meggio and Pinna (2003) |
| C4 | 1.32 × 10–8 | Meggio and Pinna (2003) |
| G1 | 3.32 × 10–14 | Srinivasan et al. (1988) |
| G2 | 3.32 × 10–14 | Srinivasan et al. (1988) |
| G3 | 3.32 × 10–14 | Srinivasan et al. (1988) |
| G4 | 3.32 × 10–14 | Srinivasan et al. (1988) |
Table 2.
Rate constants used in the axon initial segment model with their biological meaning
| Rate constants | Biological meaning | Values | Source |
|---|---|---|---|
| K01 | Association rate for NaA and C1 | 1 × 1012 (µM/s) | Ubersax and Ferrell (2007) |
| K10 | The dissociation rate of pNaA into NaA and C12 | 1 × 106 (/s) | Ubersax and Ferrell (2007) |
| K02 | Association rate for NaB and C2 | 1 × 1012 (µM/s) | Ubersax and Ferrell (2007) |
| K20 | The dissociation rate of pNaB into NaB and C2 | 1 × 106 (/s) | Ubersax and Ferrell (2007) |
| K03 | Association rate for NaC and C3 | 1 × 1012 (µM/s) | Ubersax and Ferrell (2007) |
| K30 | The dissociation rate of pNaC into NaC and C3 | 1 × 106 (/s) | Ubersax and Ferrell (2007) |
| K04 | Association rate of N1111 and C4 | 1 × 1012 (µM/s) | Ubersax and Ferrell (2007) |
| K40 | The dissociation rate of pN1111 into N1111 and C4 | 1 × 106 (/s) | Ubersax and Ferrell (2007) |
| K05 | Association rate of pNaA and G1 | 1.71 × 109 (µM/s) | Brechet et al. (2008) |
| K50 | The dissociation rate of pNaA × G1 into pNaA and G1 | 3.3 × 10–3 (/s) | Brechet et al. (2008) |
| K06 | Association rate of pNaB and G2 | 8.2±2.6 × 108 (µM/s) | Brechet et al. (2008) |
| K60 | Dissociation rate of pNaB × G2 into pNaB and G2 | 3.4±0.2 × 10–3 (/s) | Brechet et al. (2008) |
| K07 | Association rate of pNaC and G3 | 8.9±0.2 × 108 (µM/s) | Brechet et al. (2008) |
| K70 | The dissociation rate of pNaC × G3 into pNaC and G3 | 3.3±0.4 × 10–3 (/s) | Brechet et al. (2008) |
| K08 | Association rate of pN1111 and G4 | 4.24 × 109 (µM/s) | Brechet et al. (2008) |
| K80 | The dissociation rate of pN1111 × G4 into pN1111 and G4 | 2.1±0.8 × 10–3 (/s) | Brechet et al. (2008) |
Phosphorylation of Nav species by CK2
Equations 1 to 20 models the dynamic changes in all Nav species (NaA, NaB, NaC and N1111) after phosphorylation mediated by CK2 (C1, C2, C3, and C4) based on ordinary differential equations. The phosphorylated forms of the Nav species are denoted as pNaA, pNaB, pNaC and pN1111. [NaA], [NaB], [NaC], [N1111], [pNaA], [pNaB], [pNaC], [pN1111], [C1], [C2], [C3] and [C4] represent the concentration of the species. The phosphorylation of all Nav species by CK2 species in Equation (1), (6), (11), and (16) are achieved at specific association rates (K01, K02, K03 and K04) and dissociation rates (K10, K20, K30 and K40).
NaA+C1 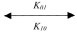 pNaA (1)
pNaA (1)
d[NaA]/dt = –K01[NaA][C1] + K10[pNaA] (2)
d[C1]/dt = –K01[NaA][C1] + K10[pNaA] (3)
d[pNaA]/dt = K01[NaA][C1] – K10[pNaA] (4)
d[NaA]/dt = d[C1]/dt = –{d[pNaA]/dt} (5)
NaB+C2 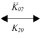 pNaB (6)
pNaB (6)
d[NaB]/dt = –K02[NaB][C2] + K20[pNaB] (7)
d[C2]/dt = –K02[NaB][C2] + K20[pNaB] (8)
d[pNaB]/dt = K02[NaB][C2] – K20[pNaB] (9)
d[NaB]/dt = d[C2]/dt= –{d[pNaB]/dt} (10)
NaC+C3 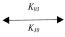 pNaC (11)
pNaC (11)
d[NaC]/dt = –K03[NaC][C3] + K30[pNaC] (12)
d[C3]/dt = –K03[NaC][C3] + K30[pNaC] (13)
d[pNaC]/dt = K03[NaC][C3] – K30[pNaC] (14)
d[NaC]/dt = d[C3]/dt =–{d[NaC]/dt} (15)
N1111+C4 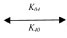 pN1111 (16)
pN1111 (16)
d[N1111]/dt = –K04[N1111] [C4] + K40[pN1111] (17)
d[C4]/dt = –K04[N1111] [C4] + K40[pN1111] (18)
d[pN1111]/dt = K04[N1111] [C4] – K40[pN1111] (19)
d[N1111]/dt = d[C4]/dt= –{d[pN1111]/dt} (20)
Binding of phosphorylated Nav species with AnkG
Equations 21 to 40 describe the accumulation of Nav species at the AIS after binding with AnkG. The ordinary differential equations for binding between the phosphorylated form of Nav species (pNaA, pNaB, pNaC and pN1111) and AnkG (G1, G2, G3, G4) are written using the mass action law. [pNaA], [pNaB], [pNaC], [pN1111], [pNaA × G1], [pNaB × G2], [pNaC × G3], [pN1111× G4], [G1], [G2], [G3] and [G4] represent the concentrations of the species. The binding of phosphorylated Nav species with AnkG species in equations 21, 26, 31 and 36 is accomplished through the association rates (K05, K06, K07 and K08) and the dissociation rates (K50, K60, K70 and K80).
pNaA + G1 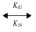 pNaA × G1 (21)
pNaA × G1 (21)
d[pNaA]/dt = –K05[pNaA][G1] + K50[pNaA × G1] (22)
d[G1]/dt = –K05[pNaA][G1]+K50[pNaA × G1] (23)
d[pNaA × G1]/dt = K05[pNaA][G1] – K50[pNaA × G1] (24)
d[pNaA]/dt = d[G1]/dt = –{d[pNaA × G1]/dt} (25)
pNaB + G2 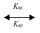 pNaB × G2 (26)
pNaB × G2 (26)
d[pNaB]/dt = –K06[pNaB][G2] + K60[pNaB × G2] (27)
d[G2]/dt = –K06[pNaB][G2] + K60[pNaB × G2] (28)
d[pNaB × G2]/dt = K06[pNaB][G2] – K60[pNaB × G2] (29)
d[pNaB]/dt = d[G2]/dt= –{d[pNaB × G2]/dt} (30)
pNaC + G3 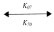 pNaC × G3 (31)
pNaC × G3 (31)
d[pNaC]/dt = –K07[pNaC][G3] + K70[pNaC × G3] (32)
d[G3]/dt = –K07[pNaC][G3] + K70[pNaC × G3] (33)
d[pNaC × G3]/dt = K07[pNaC][G3] – K70[pNaC × G3] (34)
d[pNaC]/dt = d[G3]/dt = –{d[pNaC × G3]/dt} (35)
pN1111 + G4  pN1111 × G4 (36)
pN1111 × G4 (36)
d[pN1111]/dt = –K08[pN1111] [G4] + K80[pN1111 × G4] (37)
d[G4]/dt = –K08[pN1111] [G4] + K80[pN1111 × G4] (38)
d[pN1111 × G4]/dt = K08[pN1111][ G4] – K80[pN1111 × G4] (39)
d[pN1111]/dt = d[G4]/dt = –{d[pN1111 × G4]/dt} (40)
Results
Using the computational model, we tested the role of serine site in the recruitment of Nav channels into the AIS to understand the nature of binding of all phosphorylated Nav species (NaA, NaB, NaC, and N1111) with AnkG species (G1, G2, G3, and G4) (Figure 6). The binding of pN1111 with G4 and achieves the highest concentration of the pN1111 recruited by G4 (2.64 × 10–15µM or equivalent to 1565 molecules within the AIS volume) among all Nav species. These results are supported by the study in which 100–300 Nav molecules/µm of the AIS are documented (Kole et al., 2008). The higher concentration of pN1111 after binding to G4 indicates that in the presence of all serine sites, the binding with AnkG takes place with a strong binding affinity, which corroborated with the experimental evidence (Fache et al., 2004; Brechet et al., 2008). This strong binding affinity is responsible for the high density of Nav channels at the AIS. Nav species other than pN1111 shows a dramatic decrease in their protein concentrations after binding with AnkG. After binding with G1, pNaA species has the second-highest concentration (7.2 × 10–16µM or equivalent to 433 molecules within the AIS volume). As mentioned in the assumptions, the fact that pNaA consists of three serine sites including S1 could be the reason for this significant reduction of pNaA as compared to pN1111. Remaining Nav species (pNaB and pNaC) after their respective binding with G2 and G3 show almost similar concentration but lesser than pN1111 and pNaA (3.8 × 10–16µM or equivalent to 205 molecules in the AIS volume, and 3.8 × 10–16µM or equivalent to 228 molecules in the AIS volume) respectively. Similar concentrations of pNaB and pNaC after binding with AnkG species demonstrate the importance of the S1 site in the AIS motif. Similar results are given by Brechet et al. (2008), where the concentration of the Nav channels after S1 mutation is the same as the Nav channels with a double site mutation in the presence of S1 (Brechet et al., 2008). The S1 site within the AIS motif of a Nav channel is surrounded by acidic residues, such as glutamate and aspartate. The presence of these acidic residues increases the importance of the S1 site by increasing its probability of being phosphorylated by CK2 as the first preference. This could be the reason for pNaB to show a similar behavior compared to the pNaC species (S1 absence).
Figure 6.
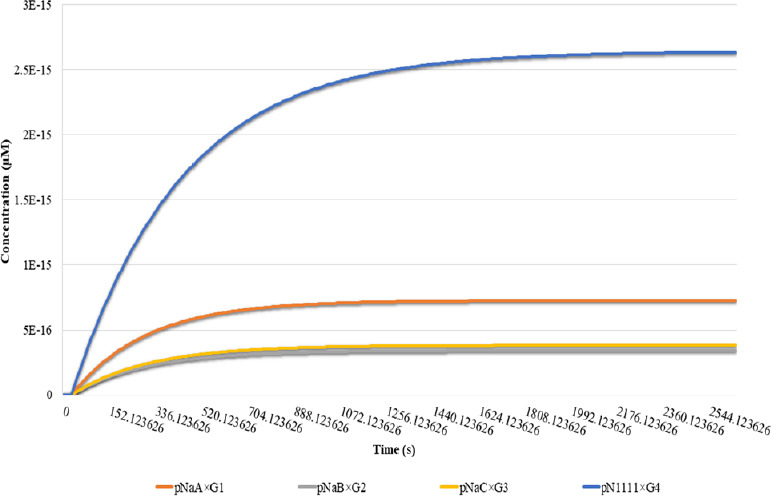
Binding of Nav species with AnkG species (G1, G2, G3, and G4) under different phosphorylation conditions.
The effect of the presence and absence of serine sites within Nav channels is shown. Time in seconds on x-axis explains the simulation time and y-axis describe the concentration Nav channels (pNaA, pNaB, pNaC, and pN1111) after binding with AnkG (G1, G2, G3, and G4). The blue line represents the highest concentration of pN1111 due to the presence of all four serine sites for casein kinase 2-mediated phosphorylation. The orange line denotes the effect of the absence of two serine residues on the pNaA concentration. The concentration of the remaining two phosphorylated Nav species (pNaC and pNaB) are found to be at a similar level. However, their concentrations are lower than the concentrations of pN1111 and pNaA. AIS: Axon initial segment; AnkG: ankyrin-G; Nav: voltage-gated sodium channel.
Therefore, the availability of all serine sites for CK2-mediated phosphorylation is necessary for the regulation of the Nav channel population at the AIS to maintain the conditions suitable for AP initiation. Besides, any alteration in the phosphorylation conditions can cause a disturbance in the recruitment of Nav into the AIS by preventing Nav-AnkG binding. According to the literature, any alteration in the Nav channel densities at the AIS can increase the threshold voltage required for AP initiation. This disturbance in the AIS system can shift the AP initiation zone from AIS to a region such as the nodes of Ranvier with a high number of Nav channels after AIS (Lemaillet et al., 2003; Kole et al., 2008; Rasband, 2009; Gulledge and Bravo, 2016; Satake et al., 2017). However, NOR, as an AP initiator zone, cannot hold the voltage stress during AP due to its position away from the soma (Kole et al., 2008, 2012).
Effect of change in the initial concentrations of CK2 on Nav channel recruitment at AIS
To test the significance of the initial concentration of CK2 on the Nav channel recruitment at AIS, we phosphorylated N1111 species at ten different CK2 (C2) initial concentrations and observed its impact on the binding of pN1111 with AnkG (G4) (Figure 7). The initial concentration of C2 was taken within a range of 6.6 × 10–9µM to 1.98 × 10–8µM. According to the results, at low initial C2 concentration (6.6 × 10–9µM), lesser pN1111 molecules bound to G4 (1.38 × 10–15µM or equivalent to 833 molecules in the AIS volume). As we increased the initial C2 concentration, pN1111 molecules were also increased after binding with G4. These results indicate the dependency of binding affinity between Nav channels and AnkG on the CK2 concentration. Similar results were reported by various studies that supported the importance of CK2 (Brechet et al., 2008; Hsu et al., 2017). Hsu et al. (2017) showed that the inhibition of CK2 using 4, 5, 6, 7-tetrabromobenzotriazole significantly altered the binding of Nav channels with AnkG by eliminating their CK2-mediated phosphorylation. In addition to that, CK2 inhibition shortened the axons by 30% (Ponce et al., 2011). Our model and the experimental studies so far show that CK2 plays a critical role in the AIS by enhancing the binding affinity between Nav channels and AnkG (Brechet et al., 2008; Ponce et al., 2011; Hsu et al., 2017).
Figure 7.
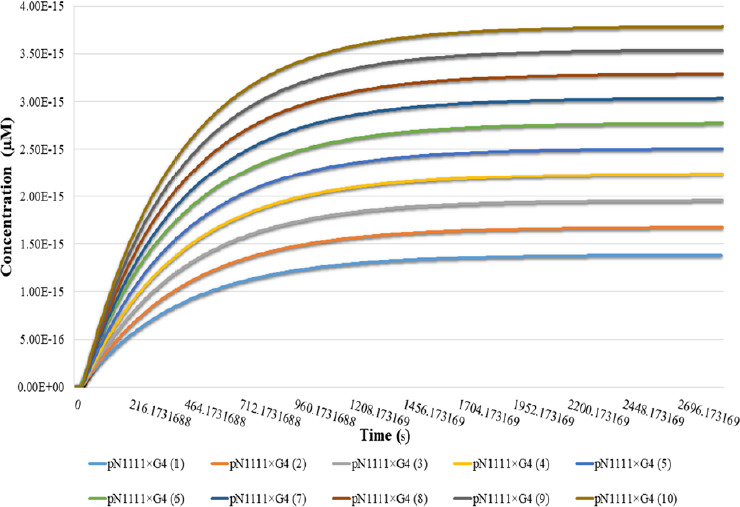
Effect of initial concentrations of CK2 on the Nav channels recruitment at AIS.
The effect of variation in initial CK2 concentration on the Nav-AnkG binding is shown. Time in seconds on x-axis explains the simulation time in seconds and y-axis describe the concentration of pN1111 after binding with AnkG (G4). Binding between pN1111 and G4 is tested at ten different initial CK2 concentration. In figure pN1111 × G4(1) represents the binding of pN1111 with G4 at a low initial CK2 concentration (1) and pN1111×G4 achieves the lowest concentration. However, as we increase the initial CK2 concentration, levels of pN1111- G4 binding product also increase. AIS: Axon initial segment; AnkG: ankyrin-G; Nav: voltage-gated sodium channel.
Effects of the initial concentrations of AnkG on Nav channel recruitment at AIS
To test the role of AnkG, we introduced ten new initial concentrations of G4 in the present model. The concentration range of G4 was set to from 1.66 × 10–14 to 4.98 × 10–14µM. Binding of G4 with pN1111 was taken into account to test the impact of AnkG on the binding of Nav channels into the AIS. According to the results (Figure 8), at the low initial concentration of G4, pN1111 levels were lower after binding with G4 (1.36 × 10–15µM ~806 molecules of pN1111 in the AIS). However, as we increased the G4 initial concentration, the concentration of pN1111 after binding with G4 increased. At the highest initial concentration of G4, pN1111 achieved the highest value after binding with G4 (3.96 × 1014µM /2394 molecules).
Figure 8.
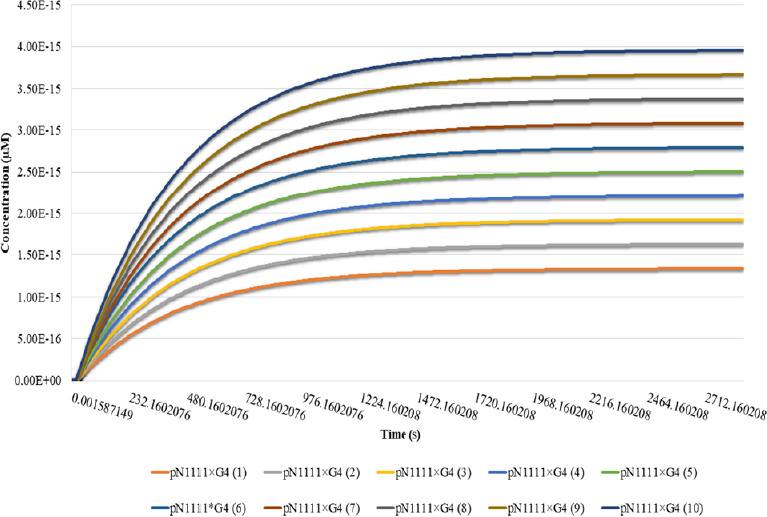
Effect of different concentrations of AnkG on the Nav channel recruitment at AIS.
The effect of variation in initial AnkG concentration on the Nav-AnkG binding is shown. Time in seconds on x-axis explains the simulation time in seconds and y-axis describe the concentration of pN1111 after binding with AnkG (G4). Binding between pN1111-G4 is tested at ten different AnkG initial concentrations. pN1111×G4(1) represents the binding of pN1111 with G4 at the lowest initial AnkG concentration (1) and pN1111×G4 binding achieved the lowest concentration. However, as we increase the initial AnkG concentration, levels of pN1111- G4 binding product also increases. AIS: Axon initial segment; AnkG: ankyrin-G; Nav: voltage-gated sodium channel.
The population of the Nav channels in the AIS region is higher than those in the soma region (Rasband, 2010). The high number of Nav channels in the AIS region is reported by Kole et al. (2008); their experimental results suggest that the density of Nav channels in this region is higher by 50-fold than that in the soma (Kole et al., 2008). The high number of Nav channels in the structure of the AIS supports the AIS skeleton in overcoming the load-induced during AP initiation. Moreover, due to their high number, Nav channels in the AIS region activate and deactivate much faster than the Nav channels present in the soma. Also, the voltage required to change the membrane potential in AIS is low compared with the soma (Ogawa and Rasband 2008; Yoshimura and Rasband, 2014). However, the functions of AnkG are not limited by Nav channel recruitment because, according to previous studies, AnkG is responsible for the recruitment of ion channels (Nav and Kv), molecular motors (kinesin and dynein) and CAMs (NF186 and NrCAM) into the AIS (Zhang and Bennet, 1998; Zhou et al., 1998; Hedstrom et al., 2008; Cunha and Mohler, 2009; Nelson and Jenkins, 2017). Moreover, the function of AnkG is also related to protein trafficking within the neurons because AnkG is connected with microtubules through the EB3 protein. While the kinesin and dynein motors travel through microtubules to facilitate the proper trafficking of the proteins, AnkG related mutations can cause disturbances in the transportation of the proteins within neurons (Sun et al., 2014). Moreover, dendritic proteins, such as integrin-β1 and microtubule associated protein-2, have been found in the axons after AnkG mutation indicating an alteration in protein trafficking (Hedstorm et al., 2008).
Possible role of AIS in AD pathogenesis
The AIS mutations are not only restricted to the reduced ability of a neuron to generate action potential but also are associated with the development of seizures and epilepsy (Hauser et al., 1986). The role of AIS in AD pathogenesis has been studied. Experimental evidence showed that patients with sporadic AD have the risk of developing seizures especially after the onset of dementia (Hauser et al., 1986). On the other hand, the studies using AD brain samples found the presence of disturbed Nav channels (Kim et al., 2007). Disturbed level of Nav channels has potentially been associated with elevated BACE-1 (β-site amyloid precursor protein (APP) cleavage enzyme-1). Increased BACE-1 possibly inhibits the trafficking of channels to the AIS by preventing the molecular motors to perform their functions (Kim et al., 2007). The increased level of BACE-1 precipitated in AD pathogenesis by increasing pathogenic Aβ production and is associated with the cleavage of Nav channel subunits (Kim et al., 2007; Kovacs et al., 2010). In mouse models of AD, the area around Aβ plaques showed synaptic losses, axonal swelling, and mutations in the neuronal network (Marin et al., 2016). Aβ plaques could cause damage near or around the region of AIS by targeting AnkG and βIV spectrin and decreasing the density and length of the AIS (Marin et al., 2016; Sohn et al., 2016). Disturbance in the AIS functions could be a consequence of the calpain-mediated proteolysis of AnkG and βIV spectrin due to the induction of Aβ (Buffington and Rasband, 2011; Marin et al., 2016). The low AnkG concentration can dismantle the structure of AIS by loosening the proteins anchored by AnkG. Dismantling the structure results in the disruption of neuronal polarity by abolishing the functions of the important proteins present in this region (Buffington and Rasband, 2011). The results are shown by Sun et al. (2014) support the relationship between AIS and AD by observing the low level of AnkG in AD transgenic mice. The lower level of AnkG could results in the alteration of Nav channel at the AIS.
Conclusion
The importance of the serine sites (S1, S2, S3, and S4) in the accumulation of Nav channels into the AIS to create suitable conditions for AP initiation has been shown through this modeling study which corroborates with the experimental findings. The results suggest that, in the presence of all the serine sites in Nav channels for CK2-mediated phosphorylation, the binding between the Nav channels and AnkG takes place with a strong binding affinity. We showed the significance of the initial concentrations of CK2 and AnkG on the recruitment of Nav channels. From the simulation results, we observed that the low initial levels of CK2 and AnkG reduced the population of the Nav channels at the AIS. At low levels of CK2, the Nav channels are not fully phosphorylated, which weakens the binding between the Nav channels and AnkG. Moreover, at low AnkG concentration, the AIS structural assembly dismantles which could create difficulties in the proper trafficking of proteins into the AIS such as Nav channels. Low levels of Nav channels increase the voltage required to initiate AP. Overall, these results indicate the potentially strong association of AIS proteins in AD pathogenesis. Future experimental investigation into the activity levels of AIS related CK2 in AD brains may shed light into the more confirmed role of CK2 in AD pathogenesis.
Footnotes
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: There are no competing interests.
Financial support: None.
Institutional review board statement: Ethical issues are not needed to be considered in undertaking this study.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Alzheimer’s Association (2013) 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Baley C, Lu H, Glinski G, Wheeler D, Hamilton P, Hendriksen M, Smith B. Using computer models to identify optimal conditions for flip-chip assembly and reliability. Circuit World. 2002;28:14–20. [Google Scholar]
- 3.Bi C, Wu J, Jiang T, Liu Q, Cai W, Yu P, Cai T, Zhao M, JiangY H, Sun ZS. Mutations of ANK3 identified by exome sequencing are associated with autism susceptibility. Hum Mutat. 2012;33:1635–1638. doi: 10.1002/humu.22174. [DOI] [PubMed] [Google Scholar]
- 4.Bian Y, Ye M, Wang C, Cheng K, Song C, Dong M, Pan Y, Qin H, Zou H. Global screening of CK2 kinase substrates by an integrated phosphoproteomics workflow. Sci Rep. 2013;3:1–7. doi: 10.1038/srep03460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bréchet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffington SA, Rasband MN. The axon initial segment in nervous system disease and injury. Eur J Neurosci. 2011;34:1609–1619. doi: 10.1111/j.1460-9568.2011.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantrell AR, Scheuer T, Catterall WA. Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons. J Neurosci. 2018;19:5301–5310. doi: 10.1523/JNEUROSCI.19-13-05301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Checler F, Turner AJ. Journal of Neurochemistry Special Issue on Alzheimer’s Disease: Amyloid cascade hypothesis - 20 years on’. J Neurochem. 2012;120(Suppl 1):iii–iv. doi: 10.1111/j.1471-4159.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- 9.Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci Biobehav Rev. 2011;35:1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Cunha SR, Mohler PJ. Ankyrin protein networks in membrane formation and stabilization. J Cell Mol Med. 2009;13(11-12):4364–4376. doi: 10.1111/j.1582-4934.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fache MP, Moussif A, Fernandes F, Giraud P, Garrido J, Dargent B. Endocytotic elimination and domain-selective tethering constitute a potential mechanism of protein segregation at the axonal initial segment. J Cell Biol. 2004;166:571–578. doi: 10.1083/jcb.200312155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan X, Markram H. A brief history of simulation neuroscience. Front Neuroinform. 2019;13:32. doi: 10.3389/fninf.2019.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulledge AT, Bravo JJ. Neuron morphology influences axon initial segment plasticity. eNeuro. 2016;3 doi: 10.1523/ENEURO.0085-15.2016. pii: ENEURO0085-152016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy J, Higgins G. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 15.Harty RC, Kim TH, Thomas EA, Cardamone L, Jones NC, Petrou S, Wimmer VC. Axon initial segment structural plasticity in animal models of genetic and acquired epilepsy. Epilepsy Res. 2013;105:272–279. doi: 10.1016/j.eplepsyres.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu WJ, Wildburger NC, Haidacher SJ, Nenov MN, Folorunso O, Singh AK, Chesson BC, Franklin WF, Cortez I, Sadygov RG, Dineley KT, Rudra JS, Taglialatela G, Lichti CF, Denner L, Laezza F. PPARgamma agonists rescue increased phosphorylation of FGF14 at S226 in the Tg2576 mouse model of Alzheimer’s disease. Exp Neurol. 2017;295:1–17. doi: 10.1016/j.expneurol.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SL, Svitkina TM. Axon initial segment cytoskeleton: architecture, development, and role in neuron polarity. Neural Plast. 2016;2016:6808293. doi: 10.1155/2016/6808293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci. 2018;12:25. doi: 10.3389/fnins.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of angelman syndrome. J Neurosci. 2011;31:17637–17648. doi: 10.1523/JNEUROSCI.4162-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 22.Katsuki T, Ailani D, Hiramoto M, Hiromi Y. Intra-axonal patterning: intrinsic compartmentalization of the axonal membrane in drosophila neurons. Neuron. 2009;64:188–199. doi: 10.1016/j.neuron.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ, Kovacs DM. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 25.Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs DM, Gersbacher MT, Kim D. Alzheimer’s secretases regulate voltage- gated sodium channels. Neurosci Lett. 2010;486:68–72. doi: 10.1016/j.neulet.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leterrier C, Dargent B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin Cell Dev Biol. 2014;27:44–51. doi: 10.1016/j.semcdb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Leterrier C, Potier J, Caillol G, Debarnot C, Rueda Boroni F, Dargent B. Nanoscale architecture of the axon initial segment reveals an organized and robust scaffold. Cell Rep. 2015;13:2781–2793. doi: 10.1016/j.celrep.2015.11.051. [DOI] [PubMed] [Google Scholar]
- 29.Maccioni RB, Farías G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch Med Res. 2010;41:226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Marin MA, Ziburkus J, Jankowsky J, Rasband MN. Amyloid-β plaques disrupt axon initial segments. Exp Neurol. 2016;281:93–98. doi: 10.1016/j.expneurol.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2. J FASEB. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 32.Moraru II, Schaff JC, Slepchenko BM, Blinov ML, Morgan F, Lakshminarayana A, Gao F, Li Y, Loew LM. Virtual Cell modelling and simulation software environment. IET Syst Biol. 2008;2:352–362. doi: 10.1049/iet-syb:20080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson AD, Jenkins PM. Axonal membranes and their domains: assembly and function of the axon initial segment and node of Ranvier. Front Cell Neurosci. 2017;11:1–17. doi: 10.3389/fncel.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishi H, Shaytan A, Panchenko AR. Physicochemical mechanisms of protein regulation by phosphorylation. Front Genet. 2014;5:1–10. doi: 10.3389/fgene.2014.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peltola MA, Kuja-Panula J, Liuhanen J, Võikar V, Piepponen P, Hiekkalinna T, Taira T, Lauri SE, Suvisaari J, Kulesskaya N, Paunio T, Rauvala H. AMIGO-Kv2. 1 potassium channel complex is associated with schizophrenia-related phenotypes. Bull. 2016;42:191–201. doi: 10.1093/schbul/sbv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platkiewicz J, Brette R. A threshold equation for action potential initiation. PLoS Comput Biol. 2010;6:e1000850. doi: 10.1371/journal.pcbi.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponce D, Muñoz A, Garrido JJ. Casein kinase 2 and microtubules control axon initial segment formation. Mol Cell Neurosci. 2011;46:222–234. doi: 10.1016/j.mcn.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- 40.Rasband MN. Na+ channels get anchored…with a little help. J Cell Biol. 2008;183:975–977. doi: 10.1083/jcb.200811086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasband MN. Converging on the origins of axonal ion channel clustering. PLoS Genet. 2009;5:e1000340. doi: 10.1371/journal.pgen.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rueckert EH, Barker D, Ruderfer D, Bergen SE, O’Dushlaine C, Luce CJ, Sheridan SD, Theriault KM, Chambert K, Moran J, Purcell SM, Madison JM, Haggarty SJ, Sklar P. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol Psychiatry. 2013;18:922–929. doi: 10.1038/mp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci. 2009;29:13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt-Hieber C, Jonas P, Bischofberger J. Action potential initiation and propagation in hippocampal mossy fibre axons. J Physiol. 2008;586:1849–1857. doi: 10.1113/jphysiol.2007.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohn PD, Tracy TE, Son HI, Zhou Y, Leite RE, Miller BL, Seeley WW, Grinberg LT, Gan L. Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Mol Neurodegener. 2016;11:47. doi: 10.1186/s13024-016-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988;333:177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- 47.Sun X, Wu Y, Gu M, Zhang Y. MiR-342-5p decreases ankyrin G levels in Alzheimer’s disease transgenic mouse models. Cell Rep. 2014;6:264–270. doi: 10.1016/j.celrep.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 48.Ubersax JA, Ferrell JE. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZX, Wu JW. Autophosphorylation of protein kinases. Biochem J. 2002;368:947–952. doi: 10.1042/BJ20020557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M, Cooper E C. An ankyrin-G N-terminal gate and protein kinase CK2 dually regulate binding of voltage-gated sodium and KCNQ2/3 potassium channels. J Biol Chem. 2015;290:16619–16632. doi: 10.1074/jbc.M115.638932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada R, Kuba H. Structural and functional plasticity at the axon initial segment. Front Cell Neurosci. 2016;10:250. doi: 10.3389/fncel.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimura T, Rasband MN. Axon initial segments: Diverse and dynamic neuronal compartments. Curr Opin Neurobiol. 2014;27:96–102. doi: 10.1016/j.conb.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Bennett V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J Cell Biol. 1998;142:1571–1581. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]


