Abstract
Objectives:
In older patients hospitalized with acute decompensated heart failure (ADHF), to assess the prevalence of frailty, its association with physical function, quality-of-life (QOL), cognition, and depression, and investigate more efficient detection methods.
Background:
In contrast to the outpatient population with chronic HF, much less is known regarding frailty in older, hospitalized patients with ADHF.
Methods:
Older hospitalized patients (n=202) with ADHF underwent assessment of frailty (by Fried criteria), short physical performance battery (SPPB), six-minute walk distance (6-WMD), QOL (Kansas City Cardiomyopathy Questionnaire), cognition (Montreal Cognition Assessment), and depression (Geriatric Depression Screen, GDS). The associations of frailty with these patient-centered outcomes were assessed using adjusted linear regression models. Novel strategies to identify frailty were examined.
Results:
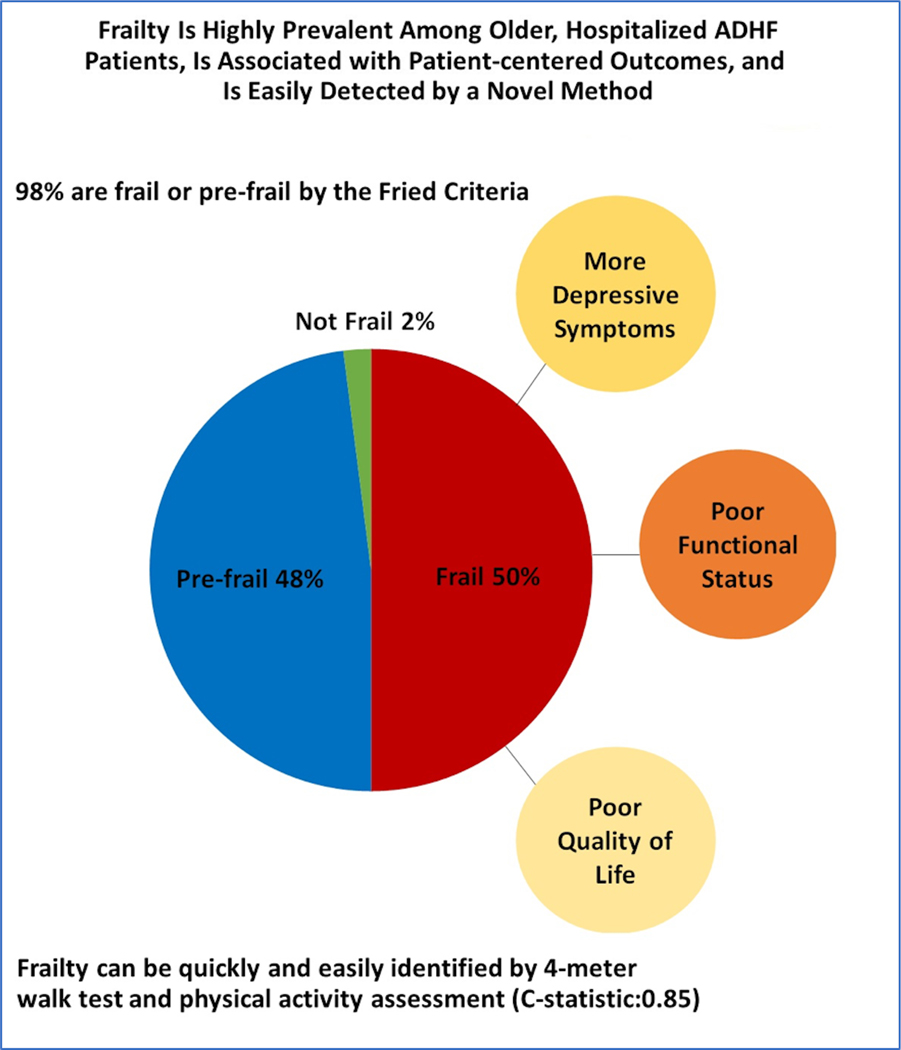
Fifty percent of older, hospitalized ADHF patients were frail; 48% were pre-frail; and 2% were non-frail. Female sex, co-morbidity burden, and prior HF hospitalization were significantly associated with higher likelihood of frailty. Frailty was associated with significantly worse SPPB score (5±2.2 versus 7±2.4), 6-MWD (143±79 versus 241±99 meters), QOL (35±19 versus 46±21) and more depression (GDS score: 5.5±3.5 versus 4.2±3.3) but similar cognition. These associations were unchanged after adjustment for age, sex, race, body mass index. Slow gait speed plus low physical activity discriminated frailty status well (C-statistic=0.85).
Conclusions:
Ninety-eight percent of older, hospitalized ADHF patients are frail or pre-frail. Frailty (vs. pre-frail status) is associated with worse physical function, QOL, comorbidity and depression. The simple 4-meter walk test combined with self-reported physical activity may quickly and efficiently identify frailty in older ADHF patients.
Keywords: Acute heart failure, Frailty, Functional status, Quality of life, Aging
Introduction
Frailty is a geriatric syndrome characterized by decreased functional reserve and heightened vulnerability to pathophysiological stressors due to multisystem impairment that involves upregulation of inflammatory pathways, endocrine dysregulation, and sarcopenia.(1) There is growing recognition of the importance of frailty in heart failure (HF), particularly those who are older (>60 years). Outpatients with chronic HF have a relatively high prevalence of frailty and in that population, frailty is associated with higher risk of adverse outcomes, including mortality and rehospitalization.(2,3) However, much less is known regarding frailty among hospitalized patients with acute decompensated HF (ADHF), particularly older ADHF patients who are the most vulnerable due to a plethora of underlying age-related changes that reduce reserve capacity and due to much higher comorbidity burden, and who have the highest rates of clinical events. In the Frail-HF study, Vidan et al.(2) demonstrated the independent association between frailty and worse 1-year clinical outcomes among older hospitalized HF patients. However, few data exist regarding the association of frailty with patient-centered outcomes among older patients hospitalized with ADHF. This is particularly relevant considering the growing importance of patient-centered outcomes such as functional capacity and quality-of-life for clinical care as endpoint in clinical trials.(4)
A major factor underlying our limited understanding of frailty in older patients with ADHF is that it is rarely assessed in routine clinical care. Although several frailty assessments methods have been described,(1) many are cumbersome, time intensive, and have not been fully validated in older patients with ADHF. Thus, there is a need for a simple, effective, and efficient screening tool to identify frailty in older patients with ADHF. A better understanding of the relationships of frailty with patient-centered outcomes, and availability of a simpler means to identify frailty in hospitalized ADHF patients would facilitate future efforts to incorporate frailty assessment into care pathways and to develop and test novel interventions to mitigate frailty and its contributions to adverse outcomes in this vulnerable older ADHF population, who continue to suffer from high rates of clinical events and poor quality of life following hospitalization.(5–7)
We therefore sought to address these important knowledge gaps by examining baseline data from the first 202 consecutively enrolled patients in the multi-center Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial (NCT02196038). We assessed clinical and historical variables associated with frailty based on the Fried criteria. We also examined associations between frailty and multiple measures of physical function, quality-of-life, depression, and cognitive function. Finally, we investigated simplified methods for efficiently identifying frailty specifically among older patients hospitalized with ADHF.
Methods
Study Population
The present analysis included the first 202 consecutively enrolled participants in the REHAB-HF trial between 9/2014 and 02/2017.(6,8) REHAB-HF is an ongoing multicenter, randomized, attention-controlled trial evaluating a novel physical rehabilitation intervention among older patients hospitalized with ADHF. The study protocol, recruitment strategy, and inclusion and exclusion criteria for REHAB-HF have been published previously.(6) The study participants were older (≥60 years) patients hospitalized with ADHF who could perform basic activities of daily living independently and ambulate ≥4m, and were planned for discharge to home. Both HF with preserved ejection fraction(EF) (EF ≥45%) and HF with reduced EF (EF <45%) were included. Patients with end-stage HF, severe valvular disease, advanced dementia, end-stage renal disease, or terminal illness were excluded. The objective criteria for ADHF included at least 2 signs of decompensated HF (pulmonary congestion by x-ray or clinical exam, elevated jugular venous pressure, elevated natriuretic peptide levels, lower extremity edema), at least 1 symptom of decompensated HF (exertional dyspnea, fatigue, paroxysmal nocturnal dyspnea, or orthopnea), and use of ADHF therapies such as diuretics, vasodilators, and inotropes. ADHF diagnosis is confirmed by a REHAB-HF investigator, board-certified cardiologist with expertise in HF. Informed consent was obtained from all patients and the study was approved by the Institution Review Boards of all participating centers.
Frailty Definition
Frailty status (frail, pre-frail, or non-frail) of the study participants was determined based on the widely-accepted and validated Fried criteria as described previously(9–11) using the following five criteria: 1) unintentional weight loss in the last year; 2) self-reported exhaustion; 3) weakness assessed by the grip strength using a hand dynamometer; 4) slowness assessed by gait speed during a 4-meter walk test; and 5) low physical activity assessed by the Short Form-12 Physical Composite Score (SF-12 PCS). The cutoffs for meeting each of these criteria are shown in supplemental Table-1. The participants were identified frail, pre-frail, and non-frail if ≥3, 1–2, and none of the criteria for frailty were met at baseline assessment, respectively. Gait speed assessed using a simple 4-meter walk test and grip strength were also investigated as alternative single-item frailty measures,(1) as discussed further below.
Assessment of physical function, QOL, and cognitive function
All measurements were conducted by trained personnel using standardized protocols after initial treatment and stabilization of ADHF symptoms. Physical function was assessed using the short-physical performance battery (SPPB) score, 6-minute walk distance (6-MWD), and handgrip strength in accordance with standardized protocols. The SPPB score is a well-established and reproducible measure of physical function in older adults and the primary outcome for the REHAB-HF trial.(6,12) It consists of 3 components – standing balance, gait speed, and timed repeated chair rises. Each component is scored on a scale of 0 to 4 and combined for a total score of upto 12 with a lower score indicating greater functional impairment. 6-MWD was measured with participants walking in an unobstructed hallway. Handgrip strength was measured using a hand dynamometer.
QOL assessment was performed using 3 complementary assessments: 1) the KCCQ (overall and physical limitation score); 2) the EuroQOL-5D-5L questionnaire (EQ-5D-5L); and 3) the SF-12 Mental Composite Score (SF-12 MCS). The SF-12 is a 12-item questionnaire which provides a Physical (SF-12 PCS) and Mental Composite Score (SF-12 MCS); the SF-12 PCS was used to define physical activity frailty criteria (and is therefore not used as a QOL measure in this analysis), while the SF-12 MCS was used as a measure of emotional QOL.(6) Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) test. Depressive symptoms among study participants were assessed using the Geriatric Depression Scale (GDS) 15-item survey with score of >5 indicating depression.(6)
Statistical Analysis
The proportion of hospitalized patients with ADHF that were frail, pre-frail, and non-frail was determined using the Fried frailty criteria. Owing to the small number of patients that were non-frail (N=4, 2%), the analysis focused on comparisons between the pre-frail vs. frail groups. The baseline characteristics of frail and pre-frail ADHF patients were reported as mean (±standard deviations) and frequency (%) for continuous and categorical variables respectively. Chi-square test and t-test were used for the between-group comparisons of the categorical and continuous variables respectively. Negative binomial regression was used for count data (e.g., hospitalizations, falls). Participant characteristics that differed between frail and pre-frail status (at the p<0.10 level) were entered into a logistic regression model with a backward selection method to identify clinical predictors of frail vs. pre-frail status. A significance level of 0.05 was used for effect variable removal during the selection process.
Adjusted linear regression models were also constructed to determine the association of frailty phenotype and its individual components with outcomes of 6-MWD, QOL measures, and cognitive function measures. Separate models were constructed for each outcome and exposure variables of interest and adjusted for age, sex, race, body mass index (BMI), and total comorbidities. The SPPB score and physical health components of the SF-12 were not included in this analysis due to overlap with the Fried criteria used in this study. The sample size of our study (202) provided 80% power to detect correlations as low as 0.20 between frailty status and other patient-centered outcomes.
Finally, receiver operating characteristic curves were created to assess how each frailty criteria identified frailty versus pre-frailty using area under the curve (C-statistic). The two criteria with highest discrimination index (C-statistic) were combined in a model to assess how this improved frailty identification. Additional receiver operating characteristic curves were constructed for gait speed using simplified cutoffs ranging from 0.4–1.0 m/s at intervals of 0.2 m/s based on the previously reported thresholds for meaningful associations with functional status.(13) All statistical analysis were performed using SAS version 7.1 (SAS Institute Inc., NC, USA).
Results
Frailty burden in ADHF patients
Based on the standard Fried criteria for frailty, 50%(N=101) of participants were classified as frail, 48% (N=97) as pre-frail, and only 2%(N=4) as non-frail (Central Illustration). The most common frailty criteria met among the frail and the pre-frail patients with ADHF was slowness (95% in frail and 49% in pre-frail) and exhaustion (92% in frail and 63% in pre-frail).
Central Illustration:
Synopsis of key findings from this study.
Clinical characteristics between frail vs. pre-frail patients with ADHF
Female sex, chronic kidney disease, higher co-morbidity burden, and HF hospitalization (but not all-cause hospitalizations) in the six months prior to enrollment were associated with being frail in the unadjusted analysis. There were no differences in age, EF, NYHA class, and use of HF therapies among pre-frail vs. frail participants (Table-1). In logistic regression, significant predictors of frailty were female sex (odds ratio [95% CI]: 2.69[1.47–4.93], p=0.001); prior HF hospitalization (2.19[1.09–4.39], p=0.027); and overall co-morbidity burden (1.21[1.04–1.41], p=0.012).
Table 1:
Baseline Characteristics, Comorbidities, and Medications by Frailty Status of Older Hospitalized Patients with Acute Decompensated Heart Failure
| Characteristics | Pre-Frail (n=97) | Frail (n=101) | p-value |
|---|---|---|---|
| Age, years | 71.1 ± 7.3 | 72.9 ± 7.7 | 0.091 |
| Women | 43 (44%) | 65 (64%) | 0.005 |
| Non-white | 54 (56%) | 48 (48%) | 0.25 |
| BMI, kg/m2 | 32.8 ± 7.7 | 33.9 ± 9.8 | 0.39 |
| Ejection Fraction <45% | 56 (58%) | 47 (47%) | 0.11 |
| New York Heart Association Class | |||
| II | 16 (16%) | 13 (13%) | 0.72 |
| III | 50 (52%) | 52 (51%) | |
| IV | 23 (24%) | 22 (22%) | |
| Patients with previous hospitalization within 6 months | 37 (38%) | 49 (48%) | 0.14 |
| Patients with previous HF hospitalizations within 6 months | 18 (19%) | 34 (34%) | 0.016 |
| Comorbidities | |||
| Hypertension | 89 (92%) | 94 (93%) | 0.73 |
| Hyperlipidemia | 69 (71%) | 69 (68%) | 0.67 |
| Diabetes mellitus | 54 (56%) | 54 (53%) | 0.76 |
| Atrial fibrillation | 39 (40%) | 52 (51%) | 0.11 |
| CAD (previous MI, PCI, or CABG) | 37 (38%) | 35 (35%) | 0.61 |
| Peripheral vascular disease | 13 (13%) | 13 (13%) | 0.91 |
| Chronic obstructive pulmonary disease | 25 (25%) | 37 (37%) | 0.10 |
| Obstructive sleep apnea | 29 (30%) | 31 (31%) | 0.90 |
| Chronic kidney disease | 25 (26%) | 41 (41%) | 0.027 |
| Stroke | 18 (19%) | 14 (14%) | 0.37 |
| Arthritis / Connective tissue disease | 40 (41%) | 53 (53%) | 0.11 |
| Cancer | 17 (18%) | 24 (24%) | 0.28 |
| Dementia/Cognitive impairment | 1 (1%) | 3 (3%) | 0.33 |
| Depression | 13 (13%) | 20 (20%) | 0.23 |
| Total Comorbidities | 5.8 ± 1.9 | 6.5 ± 2.2 | 0.013 |
| Current medications | |||
| Loop diuretic | 91 (95%) | 93 (92%) | 0.44 |
| Beta blocker | 80 (83%) | 85 (84%) | 0.88 |
| ACE or ARB | 61 (63%) | 64 (63%) | 0.82 |
| Aldosterone antagonist | 21 (22%) | 15 (15%) | 0.20 |
| Digoxin | 8 (8%) | 5 (5%) | 0.34 |
Values presented as frequency (%) or mean ± standard deviation, unless otherwise noted.
Abbreviations: ACE – angiotensin converting enzyme, ARB – angiotensin II receptor blocker, BMI – body mass index, CAD – coronary artery disease, CABG – coronary artery bypass graft; HF – heart failure, MI – myocardial infarction, PCI – percutaneous coronary intervention
N=4 (2%) of participants were classified as non-frail (met 0 criteria)
Physical function, Cognition, & QOL between frail vs. pre-frail patients with ADHF
Frail participants had significant impairment in physical function with a lower mean SPPB score across all domains and significantly decreased 6-MWD compared to pre-frail participants. 99% of frail and 85% of pre-frail participants had a SPPB score <10 (P-value<0.001), a cutoff associated with significantly higher risk of disability and mortality in older adults.(12) Gait speed was also significantly decreased in frail vs. pre-frail participants. In contrast, grip strength was significantly lower in frail (vs. pre-frail) women but comparable among men across the two groups. We observed a high burden of mild cognitive impairment (MoCA<26) in both frail and pre-frail study participants (82% vs. 73%; P-value: 0.23). There was also no significant association between frailty status and cognitive performance on the MoCA scale. (Table-2)
Table 2:
Physical Function, Quality of Life, and Cognition by Frailty Status of Hospitalized Patients with Acute Decompensated Heart Failure
| Physical Function Variable | Pre-Frail (n=97) | Frail (n=101) | p-value |
|---|---|---|---|
| SPPB total score | 7.0 ± 2.4 | 5.0 ± 2.2 | <0.001 |
| Balance score | 2.9 ± 1.2 | 2.4 ± 1.3 | 0.001 |
| 4-meter walk score | 2.7 ± 1.0 | 1.8 ± 0.8 | <0.001 |
| Chair stand score | 1.4 ± 1.2 | 0.8 ± 0.9 | <0.001 |
| SPPB < 10 | 82 (85%) | 100 (99%) | <0.001 |
| Grip Strength (kg) | |||
| Male (n=89) | 30.0 ± 7.9 | 27.8 ± 9.1 | 0.21 |
| Female (n=105) | 23.2 ± 7.4 | 18.1 ± 5.8 | <0.001 |
| Gait speed, meters/second | 0.70 ± 0.22 | 0.50 ± 0.16 | <0.001 |
| 6MWD, meters | 221 ± 99 | 143 ± 79 | <0.001 |
| Quality of Life | |||
| KCCQ Overall Summary Score | 46 ± 21 | 35 ± 19 | <0.001 |
| KCCQ Physical Limitation Score | 53 ± 23 | 42 ± 23 | <0.001 |
| Short Form-12 MCS | 44 ± 15 | 44 ± 14 | 0.78 |
| EQ-5D-5L Components | |||
| Mobility | 2.3 ± 1.0 | 2.7 ± 1.0 | 0.008 |
| Self-care | 1.6 ± 0.8 | 1.8 ± 0.9 | 0.14 |
| Usual Activities | 2.5 ± 1.2 | 2.9 ± 1.2 | 0.018 |
| Pain/Discomfort | 2.1 ± 1.0 | 2.7 ± 1.1 | 0.001 |
| Anxiety/Depression | 1.7 ± 0.9 | 1.9 ± 1.0 | 0.09 |
| Thermometer/VAS (0–100) | 62 ± 21 | 53 ± 24 | 0.008 |
| Geriatric Depression Scale Score | 4.2 ± 3.3 | 5.5 ± 3.5 | 0.009 |
| GDS > or equal to 5 | 39 (40%) | 54 (53%) | 0.06 |
| MoCA Total Score | 22.0 ± 4.5 | 21.4 ± 4.5 | 0.94 |
| MOCA < 26 | 73 (75%) | 83 (82%) | 0.23 |
Values presented as mean ± standard deviation or frequency (%) unless otherwise indicated.
Abbreviations: 6MWD – six minute walk distance, EQ-5D-5L – EuroQol, KCCQ – Kansas City Cardiomyopathy Questionnaire, MCS – mental composite score, PCS – physical composite score, SPPB – Short Physical Performance Battery, VAS – visual analog scale, MoCA- Montreal Cognitive Assessment
Frail (vs. pre-frail) participants had significantly lower HF-specific (KCCQ) and general QOL (EQ-5D-5L). General QOL was reduced in frail patients in multiple domains including mobility, usual activities, and higher pain/discomfort level (Table-2). Furthermore, there was a trend for higher depression prevalence among frail participants, with 53% frail and 40% pre-frail patients demonstrating a GDS score of 5 or higher suggestive of depression (P-value=0.06). The associations between frailty and impairment in physical function (6-MWD), lower QOL measures, and higher burden of depression were also consistent in adjusted analysis accounting for age, sex, race, and BMI.(Table-3) Among the individual components of the Fried phenotype, slowness, as determined by gait speed, was strongly associated with physical function measures, and exhaustion and physical activity were associated with QOL and depression burden (Supplemental Table-2).
Table 3:
Adjusted Association of Frailty Phenotype, Gait Speed, and Grip Strength with Measures of Physical Function, Quality-of-life, and Cognition
| Frail vs. Pre-frail | Gait Speed* | Grip Strength† | ||||
|---|---|---|---|---|---|---|
| Parameter estimate | p-value | Parameter estimate | p-value | Parameter estimate | p-value | |
| 6MWD, meters | −61 ± 12 | <0.001 | 28 ± 2 | <0.001 | 13 ± 5 | 0.015 |
| KCCQ Overall Score | −12 ± 3 | <0.001 | 1 ± 1 | 0.09 | 0 ± 1 | 0.71 |
| KCCQ Physical Limitation Score | −11 ± 3 | 0.002 | 3 ± 1 | 0.007 | 1 ± 1 | 0.67 |
| SF-12 MCS | −1 ± 2 | 0.98 | 0 ± 1 | 0.43 | 0 ± 1 | 0.57 |
| EQ-5D-5L Components | ||||||
| Mobility | 0.3 ± 0.1 | 0.051 | −0.1 ± 0.0 | <0.001 | −0.0 ± 0.1 | 0.92 |
| Self-care | 0.2 ± 0.1 | 0.24 | −0.1 ± 0.0 | 0.023 | −0.0 ± 0.1 | 0.52 |
| Usual Activities | 0.3 ± 0.2 | 0.08 | −0.1 ± 0.0 | 0.08 | −0.0 ± 0.1 | 0.95 |
| Pain/Discomfort | 0.5 ± 0.2 | 0.001 | −0.1 ± 0.0 | 0.025 | 0.0 ± 0.1 | 0.57 |
| Anxiety/Depression | 0.2 ± 0.1 | 0.13 | −0.1 ± 0.0 | 0.14 | −0.0 ± 0.1 | 0.54 |
| VAS (0–100) | −9 ± 3 | 0.008 | 0.6 ± 0.8 | 0.42 | 3 ± 1 | 0.05 |
| Geriatric Depression Scale Score | 1.3 ± 0.5 | 0.009 | −0.2 ± 0.1 | 0.044 | −0.4 ± 0.2 | 0.08 |
| MoCA Total Score | −0.4 ± 0.7 | 0.54 | 0.4 ± 0.2 | 0.012 | 0.5 ± 0.3 | 0.043 |
Adjusted for age, sex, BMI, race, and comorbidities
per 0.10 meter/second change in gait speed.
per 6 kg change in handgrip strength. Abbreviations: 6MWD – six-minute walk distance, EQ-5D-5L – EuroQol, KCCQ – Kansas City Cardiomyopathy Questionnaire, MoCA- Montreal Cognitive Assessment, MCS – mental composite score, VAS – visual analog scale.
Association of gait speed and handgrip strength with outcomes
Higher gait speed and handgrip strength were both significantly associated with higher 6MWD and better cognitive function in adjusted analyses. Higher gait speed, but not handgrip strength, was associated with higher HF-specific and overall QOL based on KCCQ physical limitation and EQ-5D-5L scores. (Table-3)
Simplified Frailty Assessment
Among the individual frailty criteria, slow gait speed (by 4-meter walk test) and low physical activity (by the SF-12 PCS) had the highest discrimination (C-statistic=0.73 for each) in distinguishing frail from pre-frail patients with ADHF based on the full, standard 5-item Fried criteria for frailty. (Table-4) When combined together, the C-statistic of slow gait speed and low physical activity was 0.85±0.02. The 4-meter walk test alone using a cut-point of 0.8 m/s was highly sensitive for identifying frailty (sensitivity=0.98, specificity=0.37; Table-4). When combined with low physical activity, a gait speed cutoff of 0.8m/s had a C-statistic of 0.83±0.03.
Table 4.
Discriminative Ability of Individual Components of the Frailty Criteria Based on the Fried Phenotype in Hospitalized Patients with Acute Decompensated Heart Failure
| C-Statistic (Area Under Curve) | Sensitivity | Specificity | |
|---|---|---|---|
| Fried Frailty Criteria* | |||
| Slowness (gait speed)† | 0.73 ± 0.03 | 95% | 51% |
| Weakness (handgrip strength) | 0.69 ± 0.03 | 53% | 85% |
| Weight Loss | 0.61 ± 0.03 | 40% | 81% |
| Exhaustion | 0.65 ± 0.03 | 92% | 37% |
| Low Physical Activity† | 0.73 ± 0.03 | 67% | 78% |
| Slowness + Low Physical Activity Combined† | 0.85 ± 0.02 | 62% | 91% |
| Additional Gait Speed Cutoffs | |||
| <1.0 m/s | 0.54 ± 0.01 | 100% | 7% |
| <0.8 m/s | 0.68 ± 0.03 | 98% | 37% |
| <0.7 m/s | 0.69 ± 0.03 | 88% | 50% |
| <0.6 m/s | 0.71 ± 0.03 | 73% | 69% |
| <0.4 m/s | 0.61 ± 0.28 | 31% | 91% |
| Gait speed <0.8 m/s + Low Physical Activity Combined | 0.83 ± 0.03 | 65% | 89% |
The cutoff for each component to meet the Fried frailty criteria are shown in Supplemental Table 1.
Slowness is defined using the 4-m walk test according to the Fried frailty criteria cutoff. Low physical activity is based on the Physical Composite Score of the SF-12 questionnaire (see supplemental Table 1).
The participants were identified as frail if they met 3 or more of the components of the Fried Frailty criteria listed above.
Discussion
We observed several important findings in our study. First, female sex, prior HF hospitalization, and total comorbidity burden were independently associated with frailty status, whereas age and individual comorbidities were not. Second, frailty, as defined by the well-accepted and validated Fried criteria, was independently associated with worse physical performance across all domains (balance, mobility, strength and endurance), lower HF-specific and general QOL, and higher burden of depressive symptoms, but not with cognitive impairment. Finally, we explored simplified, more efficient frailty assessment strategies for older patients hospitalized with ADHF. Gait speed, assessed by a simple 4-meter walk test, was a highly sensitive and meaningful single-item assessment, retaining most associations with physical performance, QOL and depression seen with the full Fried frailty criteria. When combined with self-reported physical activity easily and quickly obtained from the SF-12, this simple 2-item assessment provided good discrimination of frail vs pre-frail (C-Statistic:0.85).
Our study findings add significantly to prior knowledge regarding the importance of frailty in HF patients, and particularly the large, important population of older patients hospitalized for ADHF.(2) The patient-centered outcomes associated with frailty in the present analysis, such as impaired physical function, and QOL, have independent clinical importance in addition to also being strong, independent predictors of rehospitalization, loss of independence, and all-cause mortality.(2,14) Furthermore, among frail older ADHF patients, the impairments to physical function (6MWD<150 meters, SPPB score<6; collectively assessing endurance, balance, mobility and strength) and QOL(KCCQ<40; global visual analog scale<55) were strikingly broad and severe, especially when considering that patients requiring placement in a nursing or rehabilitation facility were excluded.(6) Among individual components of the Fried frailty phenotype, low gait speed on the 4-meter walk test appeared to primarily account for the association of frailty with 6-MWD, and the physical inactivity and exhaustion components of the Fried frailty phenotype appeared to primarily account for associations with QOL and higher depression burden. Taken together, our study findings suggest that distinct aspects of frailty in older patients with ADHF may identify impairments across specific domains including functional status, mood, and quality of life and inform caregivers and facilitate design of novel interventions to address these impairments.
Frailty prevalence in our cohort of older, hospitalized HF patients (50% frail; 98% meeting at least one frailty criterion) was atleast double that observed among older outpatients with HF (15–25%).(3) Consistent with our observations, Vidan et al.(2) also reported a high prevalence of frailty (~75%) in FRAIL-HF, which studied an older (>70 years; mean age: 80 years) cohort of 450 hospitalized patients with HF from Europe. The present study builds upon the Vidan study and further adds to our understanding of frailty in ADHF by evaluating the associations of frailty measures with other patient-centered outcomes in a cohort not quite as old (mean age: 72 years) ADHF patients from the United States with higher burden of co-morbidities and higher proportion of patients with NYHA Class III/IV symptoms. The present study also explored a potentially more efficient, simple means of identifying frailty in ADHF patients that might be more amenable to routine clinical practice.
Several factors likely account for the high prevalence of frailty in older ADHF patients, including aging-related changes, the systemic effects of ADHF mediated through activation of inflammatory and neurohumoral pathways and hospital-associated immobility, which contribute to the “post-hospitalization syndrome”.(15) Older frail patients with ADHF may be especially vulnerable to this phenomenon, associated with significant functional decline during hospitalization that persist following discharge when the risk of adverse clinical events is highest. Lingering adverse effects of prior HF hospitalization can persist for months following discharge.(16,17) and, may help account for the higher rates of frailty among those hospitalized for HF within the 6-months prior to study enrollment. Overall burden of comorbidities also contributed significantly and independently to frailty. This is consistent with frailty models that incorporate a cumulative tally of comorbid conditions in identifying frailty.(10,18)
Comorbid burden has been shown in other studies to contribute significantly to adverse clinical outcomes in HF patients.(19) At least 50%of re-hospitalizations and deaths experienced by this population are attributable to non-cardiac causes, but no single comorbid condition is dominant.(20,21) Importantly, comorbid conditions also interact with HF, and collectively increase the risk of HF-related events as well.(22)
Surprisingly, age was not independently associated with frailty in our cohort. This is despite a relatively low age cut-off to define older adults (≥60 years) and a wide range of ages represented (range:60–92; mean:72.4±7.8 years). A similar finding has been reported among frail patients with advanced HF considered for LVAD and heart transplant.(23,24) Such patients share multiple characteristics with the present cohort, including recurrent HF hospitalizations, severely reduced QOL, and severe functional impairment, all independent predictors of adverse clinical events as previously noted. These findings support the need to formally assess frailty in a broad age-range of ADHF patients, particularly among women who were at more than double the risk of being frail compared to men.
Cognitive impairment, which is also highly prevalent in our cohort, did not appear associated with physical frailty. This may be related to the fact that the Fried frailty phenotype focuses primarily on impairments of physical function.(25) Cognition impairments provide independent prognostic value even after accounting for physical frailty based on the Fried criteria,(23) supporting that HF care models should address both cognitive and physical function impairments.(7)
Despite the high prevalence and prognostic implications of frailty in this high-risk population, it is not routinely assessed or addressed in current care models or disease management pathways for ADHF. This has been largely attributed to the cumbersome and resource intensive nature of the most common frailty assessment methods. The current findings suggest simplified and efficient frailty assessment strategies that may be more easily integrated into routine clinical practice. Gait speed, as measured by the simple, quick 4-meter walk test, was a highly effective screening test with 98% sensitivity using a cut-off of 0.8 m/s. Gait speed is also clinically meaningful, retained associations with patient-centered outcomes, and has been strongly linked to adverse clinical events.(2) Furthermore, gait speed is time efficient, simple to measure, requires no special equipment, and thus can be obtained on most patients, including older, patients with ADHF, as shown in this study. Combining gait speed with self-reported physical activity added specificity such that this simple 2-item assessment demonstrated high discriminative (C-statistic: 0.85) value for frailty vs pre-frailty as determined by the standard Fried criteria. The credibility of our findings with regard to efficient strategies for identification of frailty in older ADHF patients is supported by reports from the chronic HF and general geriatric populations that gait speed is a powerful predictor of a wide range of outcomes, including mortality, hospitalization, and nursing home placement.(26)
Another potential barrier to the integration of frailty assessments into clinical practice is the limited evidence about the clinical benefit of targeting frailty. The present study findings have important clinical implications in this regard, suggesting that frailty assessment among hospitalized ADHF patients identifies those with significant functional impairment. Such impairments persist following discharge when the risk of adverse clinical events is highest. This phenomenon, referred to as the “post-hospitalization syndrome”, has been the focus of recent health policy initiatives to avoid re-hospitalization.(15) Identifying patients who may be at the highest risk of disability and adverse events post-discharge can facilitate future efforts to develop novel interventions to address the adverse impact of frailty on outcomes.(2,12)
This study has several strengths, including comprehensive assessment of frailty using the full Fried criteria to compare with, multiple, detailed measures of physical function, QOL, depression, and cognition within a relatively large, diverse population of older patients with ADHF from across multiple sites and with uniform training in the assessment of these outcomes. The study addresses two key knowledge gaps: how different frailty assessments or screening instruments perform among patients with ADHF; and explores single, quick, and efficient measures of frailty status for use in this vulnerable population. Thus, this work makes several steps forward that are essential for the ultimate goal of identifying frailty in clinical practice and developing and testing interventions to mitigate it.
This study also has some limitations. The study cohort was limited to participants who were eligible for enrollment in a clinical trial. However, the trial enrollment criteria were broadly inclusive of older, sick, patients with multiple comorbidities, wide range of functional performance, and a strong representation of HF with both preserved and reduced ejection fraction, women and minorities, all typical of the general older ADHF population. Second, due to exclusion of patients who were planned for transition to rehabilitation or skilled nursing facilities, we may have underestimated the magnitude of impairment in hospitalized older ADHF patients. However, the severity of the impairments in performance noted across multiple domains of physical function and QOL as well as cognitive performance assessments support the robustness and generalizability of these observations. Third, cross-sectional analyses don’t allow assessment of associations between frailty and clinical events. Fourth, our novel methods to detect frailty should be confirmed in a separate prospective sample of ADHF patients. Fifth, although the samples size provided 80% power to detect correlations as low as 0.20, the study may have missed weaker correlations although correlations </=0.19are usually regarded to be of marginal clinical relevance. Sixth, although some of the measures, such as for example the 6-MWD and 4-meter walk test which is a component of the Fried Frailty Phenotype, may seem inherently related, correlations are not predestined. For example, 6-MWD is primarily a measure of endurance and is determined by cardiovascular reserve and can be limited by cardiovascular symptoms. In contrast, the 4-meter walk test measures mobility and integrates balance, coordination, neurosensory feedback, and is rarely affected by endurance and cardiovascular symptoms. Finally, the MoCA test is a screening instrument for cognitive impairment and not a diagnostic tool. More comprehensive neuropsychological testing is required to identify specific cognitive deficits and the associated clinical implications. Similarly, prevalence of depression may be underestimated by the GDS, which minimizes somatic symptoms of depression that may be confounded between depression and medical conditions common in older ages.
In conclusion, 98% of older hospitalized patients with ADHF met criteria for frailty or were pre-frail. In this population, frailty is independently associated with worse physical performance across all domains, lower quality-of-life, and higher burden of depression. Importantly, frailty can be quickly and efficiently identified in older ADHF patients by a simplified screening tool that incorporates assessment of gait speed using a 4-meter walk test and self-reported physical activity. Identification of frailty status in older ADHF patients may help enhance care in this high-risk population.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Among hospitalized patients with acute decompensated heart failure (ADHF), 98% met at least 1 of the Fried criteria of frailty and 50% met full criteria for frailty.
These findings demonstrate the importance of frailty for functional and patient-reported outcomes, identify risk factors for frailty specific to older patients with ADHF, and suggest a potential method for simplified, practical assessment of frailty that can be easily incorporated in clinical management of this high-risk patient population.
TRANSLATIONAL OUTLOOK:
- Future studies are needed to:
- confirm the novel, efficient methods to identify frailty in this population
- prospectively evaluate how routine frailty assessment in patients with ADHF may help guide tailored approaches to its management and improve clinical outcomes.
Acknowledgments
Funding:
This study was supported in part by the following research grant awards from the National Institutes of Health: R01AG045551 and R01AG18915. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine (DWK); the Claude D. Pepper Older Americans Independence Center NIH Grants P30AG021332 (DWK) and P30AG028716 (AMP); the OAIC Pepper National Coordinating Center NIH Grant U24 AG05964; and the Wake Forest Clinical and Translational Science Award, NIH Grant UL1TR001420.
Disclosures:
Dr. Kitzman has been a consultant for Abbvie, AstraZeneca, Merck, Novartis, Corvia Medical, Bayer, CinRx, Boehringer-Ingleheim, and St. Luke’s Medical Center in Kansas City, Kansas; received grant support from Novartis, Bayer, AstraZeneca, and St. Luke’s Medical Center in Kansas City, Kansas; and owns stock in Gilead Sciences. Dr. Mentz receives research support from Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, Luitpold, Medtronic, Merck, Novartis, Otsuka, and ResMed; honoraria from Abbott, Bayer, Janssen, Luitpold Pharmaceuticals, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, Luitpold, Merck and Boehringer Ingelheim. Dr. Whellan has research support from Amgen, CVR Global, Merck, NIH, Novartis, ResMed; and has been a consultant for Akros Pharmaceuticals, BDC Advisors, Cytokinetics, and Fibrogen.
Abbreviations
- ADHF
acute decompensated heart failure
- QOL
quality of life
- HF
heart failure
- SPPB
short physical performance battery
- 6-MWD
six-minute walk distance
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- SF-12
Short Form 12 item survey
- SF-12 PCS
Short Form 12 item survey Physical Composite Score
- SF-12 MCS
Short Form 12 item survey Mental Composite Score
- EQ-5D-5L
EuroQol 5-dimension questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afilalo J, Alexander KP, Mack MJ et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 2016;18:869–75. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Lupon J, Vidan MT et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2018;7:e008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/treatment-heart-failure-endpoints-drug-development-guidance-industry. 2019.
- 5.Reeves GR, Whellan DJ, O’Connor CM et al. A Novel Rehabilitation Intervention for Older Patients With Acute Decompensated Heart Failure: The REHAB-HF Pilot Study. JACC Heart Fail 2017;5:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves GR, Whellan DJ, Duncan P et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorodeski EZ, Goyal P, Hummel SL et al. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol 2018;71:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warraich HJ, Kitzman DW, Whellan DJ et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults >/=60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ Heart Fail 2018;11:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 10.McNallan SM, Chamberlain AM, Gerber Y et al. Measuring frailty in heart failure: a community perspective. Am Heart J 2013;166:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNallan SM, Singh M, Chamberlain AM et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail 2013;1:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guralnik JM, Ferrucci L, Pieper CF et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31. [DOI] [PubMed] [Google Scholar]
- 13.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther 2009;32:46–9. [PubMed] [Google Scholar]
- 14.Hornsby WE, Sareini MA, Golbus JR et al. Lower Extremity Function Is Independently Associated With Hospitalization Burden in Heart Failure With Preserved Ejection Fraction. J Card Fail 2019;25:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med 2013;368:100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders NA, Supiano MA, Lewis EF et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018;20:1570–1577. [DOI] [PubMed] [Google Scholar]
- 17.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004;292:2115–24. [DOI] [PubMed] [Google Scholar]
- 18.Dunlay SM, Park SJ, Joyce LD et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant 2014;33:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ather S, Chan W, Bozkurt B et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunlay SM, Redfield MM, Weston SA et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009;54:1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah KS, Xu H, Matsouaka RA et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 22.Mentz RJ, Kelly JP, von Lueder TG et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014;64:2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jha SR, Hannu MK, Chang S et al. The Prevalence and Prognostic Significance of Frailty in Patients With Advanced Heart Failure Referred for Heart Transplantation. Transplantation 2016;100:429–36. [DOI] [PubMed] [Google Scholar]
- 24.Joseph SM, Manghelli JL, Vader JM et al. Prospective Assessment of Frailty Using the Fried Criteria in Patients Undergoing Left Ventricular Assist Device Therapy. Am J Cardiol 2017;120:1349–1354. [DOI] [PubMed] [Google Scholar]
- 25.Joyce E, Howell EH, Senapati A, Starling RC, Gorodeski EZ. Prospective assessment of combined handgrip strength and Mini-Cog identifies hospitalized heart failure patients at increased post-hospitalization risk. ESC Heart Fail 2018;5:948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studenski S, Perera S, Patel K et al. Gait speed and survival in older adults. JAMA 2011;305:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.