Abstract
Microbes operate their metabolic activities at a unicellular level. However, it has been revealed that a few metabolic activities only prove beneficial to microbes if operated at high cell densities. These cell density-dependent activities termed quorum sensing (QS) operate through specific chemical signals. In Gram-negative bacteria, the most widely reported QS signals are acylhomoserine lactones. In contrast, a novel QS-like system has been elucidated, regulating communication between microbes and plants through strigolactones. These systems regulate bioprocesses, which affect the health of plants, animals, and human beings. This mini-review presents recent developments in the QS and QS-like signal molecules in promoting plant health.
Keywords: acylhomoserine lactones, biocontrol agents, growth promoters, plant health, quorum sensing, strigolactones
1. Introduction
Plant–microbe interactions especially in the rhizoplane, rhizosphere, endosphere, and phyllosphere form discrete ecological units, the holobionts [1,2]. Here, the different organisms are largely free living, however, quite a few develop symbiotic relationships [3,4] or pathogenic relationships, by competing for the limited available resources [5]. Two well established groups of interactions are those involving plants and (i) bacteria, and (ii) fungi [6,7,8,9,10,11]. An interesting feature of these associations is the pivotal roles played by the microbes for the development of plants by providing nutrients, vitamins, energy minerals, and protecting them from pathogens [1,2,12,13,14,15]. A few well-studied plant holobionts have focused on agriculturally important species such as grains and legumes. Microbes including bacteria, viruses, archaea, fungi, and protists are members of these ecological units. Among these microbes the most beneficial for improving plant productivity have been recognized as (i) nitrogen-fixing bacteria belonging to genera such as Bacillus, Azotobacter, and Rhizobia, and (ii) fungi belonging to phyla Glomeromycota, Basidiomycota, and Ascomycota [16,17,18,19]. Thus, for maintaining soil fertility and enhancing crop yields, QS-mediated biological processes operating in nitrogen (N) fixing bacteria and phosphorus (P) up-taking AMFs can be exploited for substituting chemical fertilizers.
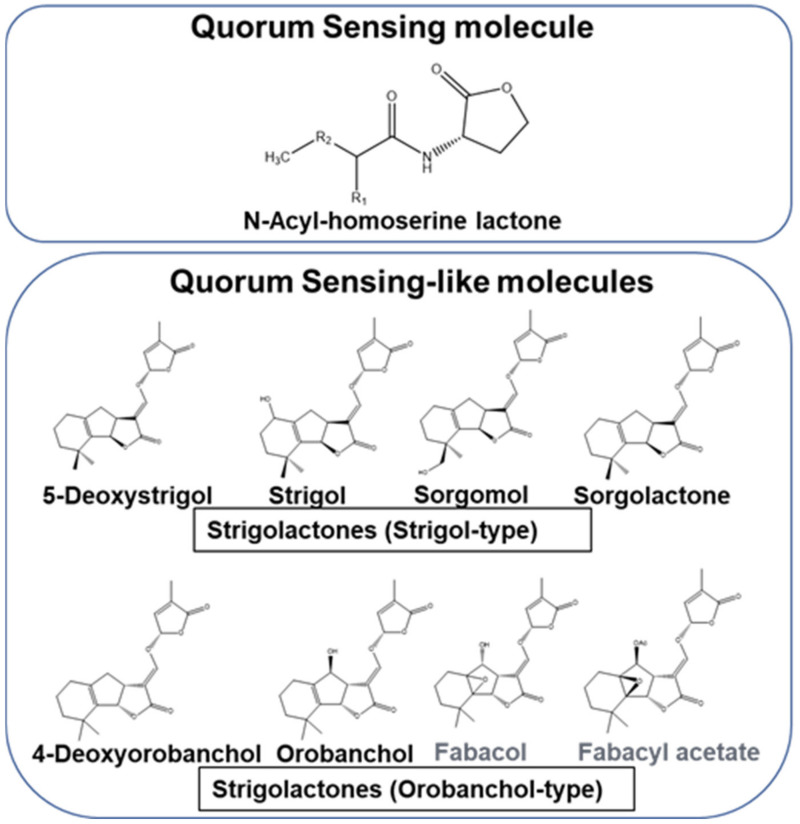
Microbial gene expression for producing biomolecules for metabolic activities and growth vary with the stage of their life cycle. It is necessary to synchronize the cell growth stage and transcription of genes for producing biomolecules in an economical and efficient manner. Certain microbes have the unique ability to express genes for specific processes only at high-cell density. This highly efficient system is termed as quorum sensing (QS) [20]. Microbes operate QS for (i) establishing a symbiotic relationship, (ii) evading competitors or stressful conditions by (a) undergoing sporulation, (b) forming biofilms, or (c) producing antimicrobial compounds [20,21]. The QS system (QSS) operation involves (a) synthesis of signal molecules, (b) their coupling to receptors, (c) binding of this complex to the promoter, and (d) gene transcription. QS signal molecules acylhomoserine lactones (AHLs) are found in Gram-negative bacteria (Figure 1), whereas in Gram-positive bacteria it is operated by autoinducing peptides, which act as autoinducers (AIs) [22,23,24]. AI-2 have been reported to act as a universal signal for QS-mediated gene expression [25]. Their primary roles are recorded in growth-promotion and protecting them from pathogens, bioremediation, and generating biofuels [26,27]. Thus, bacteria use AHLs to communicate among themselves. In contrast, Strigolactones (SLs) have been elucidated to operate as signal molecules for communication between plants and bacteria (Figure 1). It has been elucidated that SL mediated communication is a reminiscent of the bacterial QSS Their contributions in plant health and sustainability of economically important crops have been highlighted in this article.
Figure 1.
Chemical structure of quorum sensing and -like signal molecules: acylhomoserine and strigolactones. R1: H, OH or O. R2: (CH2)2–14. [22,23].
2. Plant Health Regulators
Plant growth depends on the presence of macro and minor nutrients. Limitation of any one of these elements in the rhizosphere, adversely affects plant growth and results in poor yields [28]. Among the primary macronutrients, nitrogen, phosphorus, and potassium are generally the most limiting since plants require these in large amounts to carry out major metabolic activities. Commercially available fertilizers are applied to circumvent these nutrients. However, biological processes are proving to be economical and ecofriendly. Bacteria possess AHL-mediated QSS, which regulates nitrogen fixation process. On the other hand, their symbiotic partners possess SL-mediated signalling system, which promote bacterial infection and nodulation process. Similarly, plant SL sensing system sends signals to fungi to establish symbiotic association for uptake of Pi. Together these systems can help in sustainable ecosystem and higher plant growth.
2.1. QS Signal Molecules-AHLs
Plant growth-promoting rhizobacteria (PGPR), help plants acquire nutrients, produce growth-promoters, and protect the host from pathogenic organisms [3,4,15]. QS-mediated nitrogen fixation in Rhizobium promotes leguminous plant growth. QS signal–N-(3-oxohexadecanoyl)-L-HSL of Sinorhizobium meliloti 1021 enhanced the production of auxins and flavonoids to encourage the development of Medicago truncatula seedlings [29]. The concentration and acyl chain length of AHLs has been found to induce adventitious roots and elongation in Arabidopsis and mung bean plants [30,31,32,33,34,35]. Burkholderia phytofirmans PsJN, another PGPR, colonizes Arabidopsis thaliana by promoting auxin signalling [36]. Engineering the QS-mediated synthesis of the phytohormone–indole acetic acid into a non-PGPR rhizobacterium Cupriavidus pinatubonensis JMP134, also improved root growth rate in A. thaliana. QS signal molecule, Pseudomonas quinolone signal, mediates the acquisition of ferric iron by producing siderophores. Iron deficiency created around the plant roots prevents competing pathogens’ growth [37,38,39]. The antifungal property of Burkholderia ambifaria against Rhizoctonia solani Pythium ultimum, and Candida albicans is due to its ability to produce aromatic sulfur compounds and ketones [40,41]. Enacyloxin IIa produced by B. ambifaria AMMDT, inhibit the growth of the pathogen Burkholderia multivorans [42]. In Pseudomonas chlororaphis PA23, QSS-PhzI/PhzR was responsible for the enhanced production of antibiotics pyrrolnitrin (PRN) and phenazine (PHZ) and degradative enzymes such as proteases [43]. It was inhibitory against Caenorhabditis elegans at 0.1 µg/mL and Sclerotinia sclerotiorum, causing lettuce drop disease [44,45]. P. chlororaphis O6 present in the rhizosphere inhibited the gall producing ability of root-knot nematodes (Meloidogyne spp.) [46]. These “green” nematicides acted as PGPR to promote plant health [46,47,48]. QS assists plant growth by producing biomolecules that enable them to acquire nutrition and tolerate stress, making them more competitive against pathogens [49].
The role of AHLs in inducing systemic resistance against plant pathogens has a significant impact on crop yield. Expression of chitinases and salicylic acid production in the leaves protects tomatoes pathogen Alternaria alternata [50]. QS-regulated biocontrol agents such as Serratia plymuthica protects: (i) Cucumis sativus L. against Botrytis cinereal, (ii) Phaseolus vulgaris L., and Lycopersicon esculentum L. against Pythium aphanidermatum [51]. Treating the roots of Hordeum vulgare L. and A. thaliana with N-(3-oxododecanoyl)-L-HSL enabled them to develop resistance against the phytopathogens (Blumeria graminis and Golovinomyces orontii) [51]. AHLs produced by S. meliloti in A. thaliana Col-0 plants’ rhizosphere restricted the leaf infection by Salmonella enterica [3]. AHLs were found to cause significant changes: 3.84-fold increase in dehydroascorbate reductase activity in barley shoots and a significant decrease (23%) in superoxide dismutase activity in barley roots [4]. Exposure of excised Arabidopsis roots to N-butanoyl-L-HSL leads to a twofold increase in the intracellular Ca2+; an intracellular messenger causing a significant increase in the transmembrane Ca2+ current [52]. The expression of AHL as biocontrol agents has been attributed to the accumulation of callose and phenols, deposition of lignin, enhanced oxylipins, and salicylic acid [53,54].
2.2. QS Like Signal Molecules—Strigolactones
Strigolatones (SLs) are hormones which are instrumental in regulating the growth and development of plants. The release of these carotenoid derivatives into the soil act as signal molecules to communicate their presence as a host for establishing symbiotic or parasitic relationships. Here, the most prominent responders are the arbuscular mycorrhizal (AM) fungi and the opportunistic root parasitic weeds. Plants produce hormones and use them as signal molecules to communicate with other organisms and to respond to environmental stimuli [6,55,56]. SLs can also trigger germination of seeds of root parasites, i.e., Alectra, Orobanche, Phelipanche, and Striga spp. [57,58]. SLs act as chemo-attractants allowing the weed to assimilate minerals [57,59,60]. This parasitism of crops by root parasitic plants cause dramatic yield losses in legumes, cereals, rapeseed, sunflower, and tomato [61]. Although SLs promote symbiosis of roots with bacteria and fungi, parasitic weeds exploit this opportunity for their survival [6]. Plants release SLs under P deficient conditions and induce hyphal branching in AM fungi, which supports survival of both [62,63,64,65]. SLs also act as shoot branching and high-tillering inhibitors [55,56]. In fact, it has been realized that SLs regulate different aspects of plant growth and development, including tolerance of biotic and abiotic stresses [22,66,67,68,69,70,71,72,73,74,75].
2.2.1. Biosynthesis and Structural Variation
Naturally occurring SLs have been extracted from various plant parts. Most of them are produced in the roots and axillary shoot buds [55,56,76]. Broadly these can be categorized into three groups. Canonical SLs have a tricycling lactone (ABC-ring) attached to a conserved lactone (butenolide, D-ring) [75]. Based on the stereochemistry of the B-/C-ring junction, these can be classified as strigol- and orobanchol- SLs. In the orobanchol-like SLs, the C-ring is in α orientation, whereas it is in β orientation in strigol-like SLs (Figure 1). In contrast, in the non-canonical SLs, the ABC-ring is replaced by different structural elements, such as epoxidation, hydroxylation, ketolation, and methylation [75,77]. A few representatives of the latter group are avenaol, heliolactone, methyl carlactonoate, and zealactone and zeapyranolactone [23]. Another group of signalling molecules related to SLs are the karrikins, which show similarity with respect to the conserved butanolide ring. These signaling molecules with a role in triggering seed germination in pioneer plants especially germinating after bush and forest fires have been perceived to participate in signal transduction pathways [78,79].
The synthesis of SLs in plants occurs through two routes: (i) mevalonate pathway, and (ii) the MEP (2-C-methyl-D-erythritol 4-phosphate) pathway. These were elucidated to take place in cytosol and plastids in maize seedlings using isoprenoid biosynthesis inhibitors: mevastatin and fosmidomycin [80]. The root exudate of maize seedlings treated with fluridone, a carotenoid biosynthesis inhibitor drastically reduced the Striga hermonthica seed germination [80]. Transcriptomic analysis has provided insights into the SL biosynthetic enzymes such as LATERAL BRANCHING OXIDOREDUCTASE (LBO), which in Arabidopsis is closely linked to MAX3 [81].
2.2.2. Mechanism of Action
The mechanism of action of SLs is quite similar to that of QS molecules. However, unlike QS signal molecules, SL-mediated communication is between different organisms. This density-dependent gene regulation reflects a reminiscent of the QS signal molecules, especially AHLs, found in a diverse range of bacteria [82]. Further, to categorize SLs as “truly” QS signal molecules, a few more criteria must be met: (i) must be actively exuded by host roots into the rhizosphere [83]; (ii) act extracellularly [77]; (iii) high specificity of response (observed in plants such as Arabidopsis, rice, and petunia but not fully elucidated in moss) [71,84,85]; (iii) response should be at physiological concentrations (recorded in moss) [86,87,88,89,90]; (iv) beneficial to the community; (v) a direct correlation between cell density and SLs concentration (not established as yet); and (vi) response must occur above a threshold concentration (demonstrated to some extent) [86].
2.2.3. Regulation of SL Production
Among the various factors responsible for plant growth and development, nutrient availability is the most critical. It has been realized that nitrogen (N) and Pi are among those nutrients, which are limiting in most soils. Plants have evolved to recruit symbiotic partners and modulate their root architecture to suit the relationship, such as nitrogen fixing bacterial nodules or AM fungi. These symbiotic partnerships help to increase N and Pi uptake [63]. In N and Pi limiting soils, biosynthesis of SLs was found to increase in red clover, rice, Arabidopsis, maize, and sorghum [9,91,92,93,94,95]. Effect of Pi concentration on production of heliolactone by sunflower roots, and SLs by Physcomitrella patens has been demonstrated using seed germination bioassays [73,96]. Beneficial effect of AM fungal (Rhizophagus irregularis) symbiotic relationships during nutrient deficiency conditions leading to enhanced production of SLs has been recorded in rice, M. truncatula, and tomato [97,98,99,100]. It may be remarked that biosynthesis of SLs, i.e., homeostasis is regulated by the presence of other plant hormones such as auxins, abscisic acid (ABA) and gibberellins (GAs) through a negative feedback mechanism [84,99,101,102,103,104,105,106].
2.3. Diversity of Roles of SLs
2.3.1. Outgrowth of Axillary Buds
Efforts to restrict unnecessary branching in economically important plants were made by inhibiting the outgrowth of axillary buds. The evidence of the production of such a plant growth regulator in the roots and its transport to shoots was initially obtained (i) using grafting studies, where a wild-type rootstock rescued the high-tillering, and (ii) by generating plant mutants with restricted branching phenotype in Arabidopsis, peas, rice, and petunia [107,108,109,110].
2.3.2. Regulating Branching
Non-vascular plants such as Marchantia (liverworts) and Physcomitrella (moss), exude SLs in the rhizosphere. It was observed that as a function of population density, SLs regulate colony extension and branching pattern of protonemal filaments. In fact, SLs are exuded under inorganic phosphate (Pi) limitation, leading to the optimization of uptake of Pi [9,111]. Within the plant, SLs inhibit side branching to decrease sink tissue. In addition, during the interaction with AM fungi, expansion in root surface enhances their capacity to mine Pi [86,112,113]. It helps the host to conserve energy by avoiding growth into regions depleted of Pi [86].
2.3.3. Symbiotic Relationships
The role of SLs in symbiotic association between beneficial rhizobia, AMFs and the plant roots seems to be instrumental in the growth and survival of around 80% of the land plants [62,64]. These symbiotic relationships between crop plants and microbes has been widely observed. The communication between the partners through SLs as signalling molecules, enable the host to carry out diverse endogenous and exogenous activities. These signal molecules regulate seed germination, and root development, especially its architecture, density, and hair length [66,114,115,116]. Elucidation of endogenous plant hormonal roles has been done by detecting hosts by parasitic plants and AMF [6,55,56].
AM Fungi
Plant–microbe association in the rhizosphere, especially for Pi uptake has been described by the symbiotic AMF [67,117]. The interaction is initiated by the exudation of SLs by the roots even at extremely low concentrations of 10−13 M [7]. They promote AM fungal growth leading to rhizobial symbiosis. Insights into the role of SLs in AM symbiosis has been elucidated using SL-deficient plant mutants [55,83,118,119,120]. It attracts and activates hyphal branching leading to enhanced metabolism (especially Pi) leading to generation of energy and plant growth [14,121,122]. SLs (5-deoxystrigol) as an active compound as exudates in the rhizosphere of Lotus japonicus L. were among the first indicators of this symbiotic signalling [6]. At biochemical level, this stimulation of spore germination, hyphal growth, and cell division is linked to higher respiratory activity and biogenesis of mitochondria [7,123,124,125]. It also promotes release of short chitin oligosaccharides and cytosolic calcium uptake by the hyphae [126,127]. An interesting observation was made in Lotus, where induction of CLAVATA3/ESR-related (CLE) peptide in the presence of high Pi levels downregulated SL biosynthesis. It thus negatively influenced the fungal colonization in AM [128]. Under Pi limiting conditions, exposure of wheat and tomato plants to a synthetic analogue of SLs (2’-epi-GR24), promoted the accumulation of SL [129]. It also influenced the root metabolic activities, which promoted the expression of metabolites associated with Pi limitation [130]. In addition, karrikin and carlactone-type SLs have been shown to induce hyphal branching in AFM [131,132]. AMF helps plants to overcome biotic and abiotic stresses by strengthening SL production. By protecting plants from pathogens and competing with other microbes for nutrient acquisition bacteria [133,134,135].
Nitrogen Fixation
Despite the presence of large quantities of molecular nitrogen (N2) in the air, plants cannot uptake it. However, many free-living and symbiotically associated microbes especially bacteria and algae can transform N2 to ammonia (NH3) and further into nitrites, nitrates, and organic acids [136]. The plants can easily take these up to meet their N needs. Free living nitrogen fixing bacteria present in the rhizosphere includes Azotobacter, Klebsiella, Clostridium, and Bacillus. On the other hand, Rhizobium spp. live in symbiotic relationships with plants for operating the N fixing activities with nitrogenase enzyme help [137]. Among the economically important crops, legumes roots establish a symbiotic relationship with Rhizobia, which induces special structures called nodules [8,10]. The process of symbiotic nitrogen fixation is initiated by the secretion of secondary metabolites, largely flavonoids, and isoflavonoids. These molecules act as signals for activating the nodulation (Nod) genes to produce Nod proteins and factors, such as lipochito-oligosaccharides. Nodule formation and its colonization are quite specific, e.g., S. meliloti–M. truncatula, Bradyrhizobium japonicum–Glycine max L. Merr. (soybean) [138,139,140,141]. Many plant hormones such as cytokinins participate in developing an efficiently N fixing nodule [142,143,144].
The role of SLs in nitrogen fixation was elucidated by applying chemically synthesized SL rac-GR24 to the leguminous plants’ roots. Strong evidence of the role of SLs in stimulating nodulation in Medicago sativa L., alfalfa was presented by observing an enhanced expression of nod genes of S. meliloti strain 1021, the symbiotic nitrogen-fixing partner. GR24 at concentrations ranging from 10−7, 10−5, and 10−3 M was influenced the expression of nodC gene as seen through β-galactosidase activity. An apparent enhancement in nodules per plant supported the role of SL [145]. Further evidence on SL’s role on nodulation was recorded in Pisum sativum L. (pea)-Rhizobium leguminosarum symbiosis. A SL-deficient rms1 mutant of pea treated with the synthetic analogue of GR24 elevated the number of nodules per g root dry weight, up from around 500 in the mutant to 900 in the treated plant [146,147]. Mutations induced in the genes responsible for SL biosynthesis in soybean and L. japonicus, led to drastic reduction in the number of nodules [148,149,150]. The role of SL biosynthesis and signalling was unequivocally shown in soybean (G. max). The expression of enzymes GmMAX1a and GmMAX4a responsible for SL biosynthesis was observed to be regulated by the infection by Rhizobia and their expression changed during nodule development. It also helped in increasing nodulation on soybean roots [151].
Although these studies strongly supported the role of SL in nodulation. However, there are a few exceptions, where the nodule number in the SL-insensitive ramosus4 (rms4) mutant were observed to increase [148]. Pretreatment of M. truncatula, with low doses of rac-GR24 (0.1 µM) stimulated nodulation. However, a negative impact was recorded at higher concentrations (2 and 5 µM). SL was influencing the early stages of nodule development and is thus critical for the symbiotic interaction [152]. The genetic makeup of the host also influences the nodulation response implying the specificity and sensitivity of SLs. Further MAX2/RMS4 along with certain other yet not identified ligands such as carotenoid-derived molecules and KARRIKIN-INSENSITIVE 2 (KAI2) are also perceived to be involved in this process [74]. In Arabidopsis, it has been observed that KAI2 recognizes the two stereoisomers of rac-GR24, one of them has a configuration similar to strigol, whereas the other is its enantiomer [153,154]. Correlation between SL biosynthesis and nodulation process could be elucidated through tissue-specific gene expression analysis [150,155,156,157]. rac-GR24 negatively affects the initiation of infection thread for rhizobial entry in M. truncatula. Pea plant carrying mutations in SL biosynthetic genes was observed to cause fewer infection threads compared to the than the parent strains [157]. This behaviour was not observed in certain mutants and in fact caused massive infection [11,152]. The Nod factor signaling pathway’s promoters were observed to be active in nodule formation and initiation of infection in M. truncatula nodules. Here, the role of cytokinin and auxin in increasing nodular mass has been speculated to be related to the process of autoregulation of nodulation (AON), which regulates nitrogen fixation [147,152].
2.3.4. Parasitic Relationships
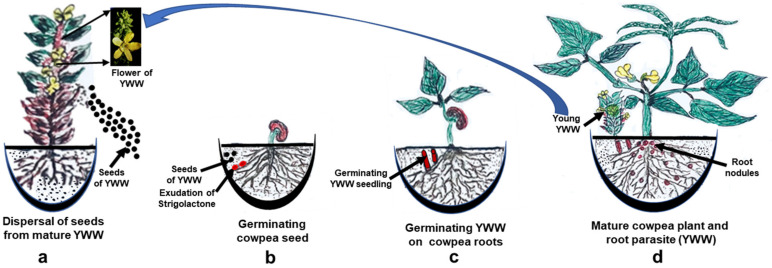
In contrast to developing symbiotic relationships, SLs also act as chemical signals, to establish a communication between weed and economically important crops (Figure 2). This relationship has a devasting effect on the crops’ productivity [158,159]. These signal molecules trigger seed germination in weeds such as Alectra, Orobanche, Phelipanche, and Striga spp. [57,58]. This synchronization between SLs and germination of seed of these parasitic plants ensure their long-term survival [160].
Figure 2.
Strigolactone (SL) signal dependent induction of seed germination of root parasite Alectra vogelii (Yellow witch weed, YWW) on Vigna unguiculata L. Walp. (cowpea). (a): Release of large number of small sized seeds from mature YWW plant; (b): Exudation of SLs by germinating cowpea roots attracting YWW seeds; (c): Germinating YW seed (underground portion lacking photosynthetic machinery) on cowpea roots; (d): YWW growing as parasite along with cowpea plant. YWW plant on maturity produce a large number of seeds which have very little reserve material and have very low survival rate.
2.3.5. Abiotic Stress
Abiotic stress conditions caused by alkalinity, salinity, drought, and temperature adversely affect yield. Chemical compounds (osmoprotectants and stimulants) such as betaine, glycine, and proline prove effective in overcoming stress and improve productivity [28]. However, rhizospheric microbes have been proving instrumental in fighting abiotic stress [161,162,163]. SLs have been proposed to stimulate resource allocation and as mediators for providing adaptive adjustments under abiotic stress conditions. Investigation of the relationship between L. japonicus and SLs under normal and stress conditions provided strong evidence between the two. Roots of SL-depleted plant, under P starvation and osmotic stress showed enhanced stomatal conductance. Their ability to resist drought caused by abscisic acid was also impaired due to slower stomatal closure. This proved that SLs play a significant role in drought resistance. The observation further supported that a rapid decline in SL concentration in the root exudates is recorded under osmotic stress. At the genetic level transcription of ABA biosynthetic gene LjNCED2 was downregulated on pre-treatment with exogenously supplied SLs [72]. Recent work on apple plant stressed due to excessive application of potash fertilizers has been a cause of concern [164,165]. Since, SLs have been known to enable plants to tolerate NaCl and drought stresses, attempts were made to exploit them for alleviating KCl stress [166]. Exogenous spray of SLs on Malus hupehensis Rehd. under KCl stress revealed enhancement in the activities of catalase and peroxidase enzymes, which eliminated reactive oxygen species production. It consequently promoted, expulsion of K+ and the accumulation of proline, which thus helped in maintaining osmotic balance i.e., ion homeostasis [166]. The relationship between SLs and drought in barley (H. vulgare) was established using a missense mutant of the gene responsible for the expression of SL-specific receptor HvD14. Its hyper-sensitivity to stress was evident in several factors including impaired photosynthesis, altered stomatal density, and disorganized chloroplast structure. In addition, it showed major changes in the expression of genes encoding for abscisic acid and SL signalling pathway [167]. Biosynthesis of SLs under Pi starvation was observed to confer moss, Physcomitrella patens with a resistance against phytopathogenic fungi [73]. The role of SL as a positive regulator of leaf senescence under Pi deficiency was observed by delaying leaf senescence in Arabidopsis oresara9 (ore9) mutant and Oryza sativa L. mutant DWARF3 (D3) [168,169]. It helps reallocation of nutrients from worn out tissues to younger developing tissues. SLs thus assist plants to survive under Pi deficiency.by re-programing its adaptation strategies [170]. The need is to seek microbes which can protect plants from stress, improve yield, and protecting them against pathogens [171,172].
2.4. Economic Significance
The significant risk is likely to affect crops such as oilseed rape and sunflower, potentially producing biofuels. Application of naturally occurring SLs (orobanchol and 5-deoxystrigol) and their synthetic analogues (GR7, a GR24 lacking the aromatic A-ring; Nijmegen-1) can induce suicidal seed germination in weeds [173,174,175,176,177]. Naturally occurring compounds, which can mimic SLs, include dehydrocostus lactone are produced by sunflower roots. It stimulates the germination Orobanche cumana, a root parasite affecting sunflower [178]. Thus, encouraging AM colonization and inducing suicidal germination in parasites provide an opportunity to curtail the crop productivity’s devasting effect [179]. Although SLs stimulate root germination even at extremely low concentrations of 10 pM [179,180,181]. However, their applications though promising, are limited by high production costs, low stability, and off-target effects [158,182,183].
3. Other Potential QS Signals
Burkholderia strains are known to regulate the synthesis of antifungal compounds through CepIR QS system. Burkholderia cenocepacia H111 produces an unusual bioactive compound fragin, a member of diazeniumdiolate, which inhibits microbial growth and even tumors. In silico analysis revealed that genes for biosynthesis of fragin, a metal chelator are regulated by valdiazen, a new class of cell signalling molecule. It autoregulates its own biosynthesis and many other genes in a cell density-dependent manner, a characteristic of QSS [184]. Based on the homology observed among ham operons, it was suggested that members of the genera Pseudomonas, Pandorea, and Burkholderia may use the diffusible signal molecule—valdiazen (valinol diazeniumdiolate), which autoregulates its own biosynthesis and also of fragin. Fragin is a metallophore and its metal chelating ability is responsible for its antifungal property [184,185,186].
4. Antibiotics as QS Signals
Microbes secrete a diverse range of small molecules in their natural environments. Microbes produce antibiotics as a weapon against their predators and to attack other microbes in the environment [187]. Most studies focus on using antibiotics at concentrations that are lethal for microbial growth [188]. However, it has been questioned if these molecules ever accumulate in the environment at concentrations that may prove inhibitory to microbial growth [189]. Hence, they may have additional roles, such as participating in bacterial communication [190,191]. These antibiotics at sub-inhibitory concentrations affect QS-mediated gene expression without altering bacterial growth [192]. A few studies have revealed that antibiotics can also serve as QS signal molecules below the minimal-inhibitory concentrations (MIC) [191,193]. Using antibiotics such as rifamycin and erythromycin at low concentrations, it was possible to measure gene transcription patterns in a few promoter-lux reporters that were engineered into Salmonella typhimurium strain ATCC 14028 library. Around 5% of the QS promoters were observed to affect the functions of many lux genes and others involved in DNA repair, virulence, and transport [194]. Aminoglycoside antibiotics were observed to enhance bacterial biofilm formation in Escherichia coli and Pseudomonas aeruginosa. The antibiotic tobramycin was the most effective in inducing biofilm formation, whereas others such as amikacin, streptomycin, and gentamycin could induce biofilm formation to a lesser extent, 75%, 66%, and 25%, respectively [187]. Effect of antibiotics at sub-inhibitory concentrations on the expression of thirteen QS-mediated virulence-related genes was reported in P. aeruginosa PA01. Vancomycin was observed to enhance the transcription of rhlAB and phzA2 by 10-fold. Consequently, a significant increase in the production of rhamnolipid and pyocyanin was recorded. Similar genotypic and phenotypic effects were also recorded using sub-inhibitory concentrations of ampicillin, azithromycin, and tetracycline [195]. Induction of different phenotypes was observed through the diffusion of different Rifampicin concentrations, which inhibits the transcription process, and Oligomycin A, with an ability to inhibit mitochondrial ATP synthases. The other phenotypic effects seen in Streptomyces coelicolor were (i) inhibition or acceleration of the development of aerial hyphae and (ii) undecylprodigiosin synthesis and inhibition of actinorhodin. These antibiotics affected QS-mediated biofilm formation at sub-inhibitory concentrations [193]. Similarly, biofilm formation in E. coli and P. aeruginosa was affected by sub-inhibitory concentrations of aminoglycoside antibiotics and β-lactam antibiotic, imipenem [187,196]. When used at concentrations like 1/4 to 1/8 of MIC (MIC of kanamycin, 8 µg/ mL), this antibiotic was observed to improve the QS-mediated violacein producing abilities of Chromobacterium violaceum. A few other antibiotics were also observed to show similar effects: tetracycline at 1/16 of MIC, erythromycin at 1/8 of MIC, amikacin at 1/4 of MIC and gentamycin at 1/2 of MIC [197]. Chitinase production, another QS-mediated activity, was reported to be significantly improved in the presence of antibiotics compared to control conditions. A negation of this enhanced activity in the presence of 20 µM of furanone compound 30 (C30), a known QS inhibitor, further established the role of antibiotics as QS signal [197,198]. Kanamycin at 1/6 MIC also showed a similar effect on biofilm formation [197]. Studies to elucidate the role of antibiotics at much lower concentrations using whole-transcriptome analysis of the marine bacterium Phaeobacter inhibens revealed an exciting role of broad-spectrum antibiotic tropodithietic acid (TDA). TDA was found to have the same regulatory functions as QS signal AHL. It was associated with LuxR-type transcriptional regulator for genes responsible for the expression of biofilm formation, motility, and antibiotic production [199]. Sub-inhibitory concentrations of natural and semisynthetic penicillins, such as oxacillin, ticarcillin, carbenicillin, and azlocillin, were shown to act as QS inducers for violacein production in C. violaceum strain NTCTC13274 (CV026), a mutant with an inability to synthesize AHLs [200].
5. Conclusions
Plant holobiont is a discrete ecological system where bacteria, fungi, and viruses interact to exchange nutrients, minerals, and produce bioactive molecules for mutual benefits. The most beneficial for improving the productivity of agricultural crops are the nitrogen-fixing bacteria and phosphate solubilizing mycorrhizal fungi. These biological processes can be most efficient if their expressions are well synchronized with the needs of the host and microbes. Here, the abilities of the plants and microbes to communicate with each other can be exploited for regulating specific gene expressions. Plants like pea, cow pea, and soybean exude signal molecules (SLs), which facilitate the entry of bacteria (S. meliloti), into the roots of M. truncatula leading to nodule formation. Within the nodule these bacteria express N fixing genes under the influence of QS signals (AHLs). Similarly, SLs attract fungi (R. irregularis) and promote their P solubilizing activity. Thus, using leguminous plants in combination with rhizobia and fungi, it is possible to enrich soil with N and P, which in turn will aid in improving the yield of other non-leguminous crop plants specially cereals. Thus, multiple organism approach may bring us closer to “nitrogen-fixing” cereals.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, V.C.K.; writing—review and editing, V.C.K., C.G., S.K.S.P., and J.-K.L.; funding acquisition, J.-K.L.; Project administration and Resources, J.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by Konkuk University in 2018. The sponsor(s) had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sánchez-Cañizares C., Jorrín B., Poole P.S., Tkacz A. Understanding the holobiont: The interdependence of plants and their microbiome. Curr. Opin. Microbiol. 2017;38:188–196. doi: 10.1016/j.mib.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Ugarelli K., Chakrabarti S., Laas P., Stingl U. The seagrass holobiont and its microbiome. Microorganisms. 2017;5:81. doi: 10.3390/microorganisms5040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández-Reyes C., Schenk S.T., Neumann C., Kogel K.H., Schikora A. N-acyl-homoserine lactones-producing bacteria protect plants against plant and human pathogens. Microb. Biotechnol. 2014;7:580–588. doi: 10.1111/1751-7915.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götz-Rösch C., Sieper C.T., Fekete A., Schmitt-Kopplin P., Hartmann A., Schröder P. Influence of bacterial N-acyl-homoserine lactones on growth parameters, pigments, antioxidative capacities and the xenobiotic phase II detoxification enzymes in barley and yam bean. Front. Plant Sci. 2015;6:205. doi: 10.3389/fpls.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickzad A., Déziel E. Adaptive significance of quorum sensing-dependent regulation of rhamnolipids by integration of growth rate in Burkholderia glumae: A trade-off between survival and efficiency. Front. Microbiol. 2016;7:1215. doi: 10.3389/fmicb.2016.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama K., Matsuzaki K., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;439:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 7.Besserer A., Puech-Pagés V., Kiefer P., Gomez-Roldan V., Jauneau A., Roy S., Portais J.C., Roux C., Bécard G., Séjalon-Delmas N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006;4:e226. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldroyd G.E.D., Downie J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 9.Kohlen W., Charnikhova T., Liu Q., Bours R., Domagalska M.A., Beguerie S., Verstappen F., Leyser O., Bouwmeester H., Ruyter-Spira C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldroyd G.E.D., Murray J.D., Poole P.S., Downie J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011;45:119–144. doi: 10.1146/annurev-genet-110410-132549. [DOI] [PubMed] [Google Scholar]
- 11.Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E.D., et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang Y.D., Liu X.G., Ma Y.X., Chernin L., Berg G., Gao K. Induction of systemic resistance, root colonisation and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur. J. Plant Pathol. 2009;124:261–268. doi: 10.1007/s10658-008-9411-1. [DOI] [Google Scholar]
- 13.Rosenberg E., Zilber-Rosenberg I. Microbes drive evolution of animals and plants: The hologenome concept. mBio. 2016;7:e01395. doi: 10.1128/mBio.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth R., Paszkowski U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 2017;39:50–56. doi: 10.1016/j.pbi.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez M., Torres M., Blanco L., Bejar V., Sampedro I., Llamas I. Plant growth-promoting activity and quorum quenching-mediated biocontrol of bacterial phytopathogens by Pseudomonas segetis strain P6. Sci. Rep. 2020;10:4121. doi: 10.1038/s41598-020-61084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Weger L.A., van der Vlugt C.I., Wijfjes A.H., Bakker P.A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J. Bacteriol. 1987;169:2769–2773. doi: 10.1128/JB.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerre P. Ergot alkaloids produced by endophytic fungi of the genus Epichloë. Toxins. 2015;7:773–790. doi: 10.3390/toxins7030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwelm A., Badstöber J., Bulman S., Desoignies N., Etemadi M., Falloon R.E., Gachon C.M.M., Legreve A., Lukes J., Merz U., et al. Not in your usual Top 10: Protists that infect plants and algae. Mol. Plant Pathol. 2018;19:1029–1044. doi: 10.1111/mpp.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begum N., Qin C., Ahanger M.A., Raza S., Khan M.I., Ashraf M., Ahmed N., Zhang L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019;10:1068. doi: 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 21.Saxena P., Joshi Y., Rawat K., Bisht R. Biofilms: Architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 2019;59:3–12. doi: 10.1007/s12088-018-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waters C.M., Bassler B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 23.Jia K.-P., Li C., Bouwmeester H.J., Al-babili S. Strigolactone biosynthesis and signal transduction. In: Koltai H., Prandi C., editors. Strigolactones–Biology and Applications. Springer International Publishing; Berlin/Heidelberg, Germany: 2019. p. 1. Chapter 1. [DOI] [Google Scholar]
- 24.Kalia V.C., Patel S.K.S., Kang Y.C., Lee J.-K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Whiteley M., Diggle S.P., Greenberg E.P. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551:313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalia V.C., Prakash J., Ray S., Koul S. Application of microbial quorum sensing systems for bioremediation of wastewaters. In: Kalia V.C., editor. Quorum Sensing and Its Biotechnological Applications. Springer Nature; Berlin/Heidelberg, Germany: 2018. pp. 87–97. Chapter 6. [DOI] [Google Scholar]
- 27.Prakash J., Kalia V.C. Application of quorum sensing systems in production of green fuels. In: Kalia V.C., editor. Quorum Sensing and Its Biotechnological Applications. Springer Nature; Berlin/Heidelberg, Germany: 2018. pp. 155–166. Chapter 10. [DOI] [Google Scholar]
- 28.Enebe M.C., Babalola O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018;102:7821–7835. doi: 10.1007/s00253-018-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathesius U., Mulders S., Gao M., Teplitski M., Caetano-Anolles G., Rolfe B.G., Bauer W.D. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA. 2003;100:1444–1449. doi: 10.1073/pnas.262672599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Rad U., Klein I., Dobrev P.I., Kottova J., Zazimalova E., Fekete A., Hartmann A., Schmitt-Kopplin P., Durner J. Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta. 2008;229:73–85. doi: 10.1007/s00425-008-0811-4. [DOI] [PubMed] [Google Scholar]
- 31.Bai X., Todd C.D., Desikan R., Yang Y., Hu X. N-3-oxodecanoyl-L-homoserine-lacto ne activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide dependent cyclic GMP signaling in mung bean. Plant Physiol. 2012;158:725–736. doi: 10.1104/pp.111.185769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin G., Liu F., Ma H., Hao S., Zhao Q., Bian Z., Jia Z., Song S. Two G-protein-coupled-receptor candidates, Cand2 and Cand7, in Arabidopsis root growth mediated by N-acyl-homoserine lactones, the bacterial quorum-sensing signals. Biochem. Biophys. Res. Commun. 2012;417:991–995. doi: 10.1016/j.bbrc.2011.12.066. [DOI] [PubMed] [Google Scholar]
- 33.Liu F., Bian Z., Jia Z., Zhao Q., Song S. The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-acyl-homoserine lactones, the bacterial quorum-sensing signals. Mol. Plant Microbe Interact. 2012;25:677–683. doi: 10.1094/MPMI-10-11-0274. [DOI] [PubMed] [Google Scholar]
- 34.Palmer A.G., Senechal A.C., Mukherjee A., Ane J.-M., Blackwell H.E. Plant responses to bacterial N-acyl-L-homoserine lactones are dependent on enzymatic degradation to L-homoserine. ACS Chem. Biol. 2014;9:1834–1845. doi: 10.1021/cb500191a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schikora A., Schenk S.T., Hartmann A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol. Biol. 2016;90:605–612. doi: 10.1007/s11103-016-0457-8. [DOI] [PubMed] [Google Scholar]
- 36.Zúñiga A., Poupin M.J., Donoso R., Ledger T., Guiliani N., Gutiérrez R.A., González B. Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol. Plant Microbe Interact. 2013;26:546–553. doi: 10.1094/MPMI-10-12-0241-R. [DOI] [PubMed] [Google Scholar]
- 37.Hazan R., He J., Xiao G., Dekimpe V., Apidianakis Y., Lesic B., Astrakas C., Déziel E., Lépine F., Rahme L.G. Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. PLoS Pathog. 2010;6:e1000810. doi: 10.1371/journal.ppat.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Tang H., Lin Z., Xu P. Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol. Adv. 2015;33:1484–1492. doi: 10.1016/j.biotechadv.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Rampioni G., Falcone M., Heeb S., Frangipani E., Fletcher M.P., Dubem J.-F., Visca P., Leoni L., Camara M., Williams P. Unravelling the genome-wide contributions of specific 2-alkyl-4-quinolones and PqsE to quorum sensing in Pseudomonas aeruginosa. PLoS Pathog. 2016;12:e1006029. doi: 10.1371/journal.ppat.1006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapalain A., Vial L., Laprade N., Dekimpe V., Perreault J., Déziel E. Identification of quorum sensing-controlled genes in Burkholderia ambifaria. MicrobiologyOpen. 2013;2:226–242. doi: 10.1002/mbo3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groenhagen U., Baumgartner R., Bailly A., Gardiner A., Eberl L., Schulz S., Weisskopf L. Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 2013;39:892–906. doi: 10.1007/s10886-013-0315-y. [DOI] [PubMed] [Google Scholar]
- 42.Mahenthiralingam E., Song L., Sass A., White J., Wilmot C., Marchbank A., Boaisha O., Paine J., Knight D., Challis G.L. Enacyloxins are products of an unusual hybrid modular polyketide synthase encoded by a cryptic Burkholderia ambifaria genomic island. Chem. Biol. 2011;18:665–677. doi: 10.1016/j.chembiol.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Morohoshi T., Yamaguchi T., Xie X., Wang W.-Z., Takeuchi K., Someya N. Complete genome sequence of Pseudomonas chlororaphis subsp. aurantiaca reveals a triplicate quorum-sensing mechanism for regulation of phenazine production. Microbes Environ. 2017;32:47–53. doi: 10.1264/jsme2.ME16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savchuk S.C., Fernando W.G.D. Effect of timing of application and population dynamics on the degree of biological control of Sclerotinia sclerotiorum by bacterial antagonists. FEMS Microbiol. Ecol. 2004;49:379–388. doi: 10.1016/j.femsec.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Nandi M., Selin C., Brassinga A.K.C., Belmonte M.F., Fernando W.G.D., Loewen P.C., de Kievit T.R. Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23 exhibits nematicidal and repellent activity against Caenorhabditis elegans. PLoS ONE. 2015;10:e0123184. doi: 10.1371/journal.pone.0123184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang B.R., Anderson A.J., Kim Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis O6 exhibits nematicidal activity against Meloidogyne hapla. Plant Pathol. J. 2018;34:35–43. doi: 10.5423/PPJ.OA.06.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang B.R., Anderson A.J., Kim Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis O6 is a key aphicidal metabolite. Can. J. Microbiol. 2019;65:185–190. doi: 10.1139/cjm-2018-0372. [DOI] [PubMed] [Google Scholar]
- 48.Nam H.S., Anderson A.J., Kim Y.C. Biocontrol efficacy of formulated Pseudomonas chlororaphis O6 against plant diseases and root-knot nematodes. Plant Pathol. J. 2018;34:241–249. doi: 10.5423/PPJ.NT.12.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zúñiga A., Fuente F., Federici F., Lionne C., Bônnet J., de Lorenzo V., González B. An engineered device for indoleacetic acid production under quorum sensing signals enables Cupriavidus pinatubonensis JMP134 to stimulate plant growth. ACS Synth. Biol. 2018;7:1519–1527. doi: 10.1021/acssynbio.8b00002. [DOI] [PubMed] [Google Scholar]
- 50.Schuhegger R., Ihring A., Gantner S., Bahnweg G., Knappe C., Vogg G., Hutzler P., Schmid M., Van Breusegem F., Eberl L., et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 51.Schikora A., Schenk S.T., Stein E., Molitor A., Zuccaro A., Kogel K.-H. N-acyl homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011;157:1407–1418. doi: 10.1104/pp.111.180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song S., Jia Z., Xu J., Zhang Z., Bian Z. N-butyryl-homoserine lactone, a bacterial quorum-sensing signaling molecule, induces intracellular calcium elevation in Arabidopsis root cells. Biochem. Biophys. Res. Commun. 2011;414:355–360. doi: 10.1016/j.bbrc.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 53.Schenk S.T., Stein E., Kogel K.H., Schikora A. Arabidopsis growth and defense are modulated by bacterial quorum sensing molecules. Plant Signal Behav. 2012;7:178–181. doi: 10.4161/psb.18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schenk S.T., Hernández-Reyes C., Samans B., Stein E., Neumann C., Schikora M., Reichelt M., Mithöfer A., Becker A., Kogel K.H., et al. N-acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell. 2014;26:2708–2723. doi: 10.1105/tpc.114.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.P., Letisse F., Matusova R., Danoun S., Portais J.C., et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 56.Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 57.Xie X., Yoneyama K., Yoneyama K. The strigolactone story. Annu. Rev. Phytopathol. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- 58.Yoneyama K., Kisugi T., Xie X., Yoneyama K. Molecular Microbial Ecology of the Rhizosphere. Volumes 1 and 2. Wiley/L; Hoboken, NJ, USA: 2013. Chemistry of strigolactones: Why and how do plants produce so many strigolactones? pp. 373–379. [DOI] [Google Scholar]
- 59.Delavault P., Simier P., Thoiron S., Véronési C., Fer A., Thalouarn P. Isolation of mannose 6-phosphate reductase cDNA, changes in enzyme activity and mannitol content in broomrape (Orobanche ramosa) parasitic on tomato roots. Physiol. Plant. 2002;115:48–55. doi: 10.1034/j.1399-3054.2002.1150105.x. [DOI] [PubMed] [Google Scholar]
- 60.Bouwmeester H.J., Matusova R., Zhongkui S., Beale M.H. Secondary metabolite signalling in host–parasitic plant interactions. Curr. Opin. Plant Biol. 2003;6:358–364. doi: 10.1016/S1369-5266(03)00065-7. [DOI] [PubMed] [Google Scholar]
- 61.Parker C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009;65:453–459. doi: 10.1002/ps.1713. [DOI] [PubMed] [Google Scholar]
- 62.Gutjahr C., Parniske M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu. Rev. Cell Dev. Biol. 2013;29:593–617. doi: 10.1146/annurev-cellbio-101512-122413. [DOI] [PubMed] [Google Scholar]
- 63.Gutjahr C. Phytohormone signaling in arbuscular mycorrhiza development. Curr. Opin. Plant Biol. 2014;20:26–34. doi: 10.1016/j.pbi.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Bonfante P., Genre A. Arbuscular mycorrhizal dialogues: Do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 2015;20:150–154. doi: 10.1016/j.tplants.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Khosla A., Nelson D.C. Strigolactones, super hormones in the fight against Striga. Curr. Opin. Plant Biol. 2016;33:57–63. doi: 10.1016/j.pbi.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Koltai H. Strigolactones are regulators of root development. New Phytol. 2011;190:545–549. doi: 10.1111/j.1469-8137.2011.03678.x. [DOI] [PubMed] [Google Scholar]
- 67.Brewer P.B., Koltai H., Beveridge C.A. Diverse roles of strigolactones in plant development. Mol. Plant. 2013;6:18–28. doi: 10.1093/mp/sss130. [DOI] [PubMed] [Google Scholar]
- 68.Ruyter-Spira C., Al-Babili S., Van Der Krol S., Bouwmeester H. The biology of strigolactones. Trends Plant Sci. 2013;18:72–83. doi: 10.1016/j.tplants.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Torres-Vera R., García J.M., Pozo M.J., López-Ráez J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014;15:211–216. doi: 10.1111/mpp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Ha C., Leyva-González M.A., Osakabe Y., Tran U.T., Nishiyama R., Watanabe Y., Tanaka M., Seki M., Yamaguchi S., Van Dong N. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA. 2014;111:851–856. doi: 10.1073/pnas.1322135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Babili S., Bouwmeester H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015;66:161–186. doi: 10.1146/annurev-arplant-043014-114759. [DOI] [PubMed] [Google Scholar]
- 72.Liu J., He H., Vitali M., Visentin I., Charnikhova T., Haider I., Schubert A., Ruyter-Spira C., Bouwmeester H.J., Lovisolo C. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta. 2015;241:1435–1451. doi: 10.1007/s00425-015-2266-8. [DOI] [PubMed] [Google Scholar]
- 73.Decker E.L., Alder A., Hunn S., Ferguson J., Lehtonen M.T., Scheler B., Kerres K.L., Wiedemann G., Safavi-Rizi V., Nordzieke S. Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol. 2017;216:455–468. doi: 10.1111/nph.14506. [DOI] [PubMed] [Google Scholar]
- 74.Waters M.T., Gutjahr C., Bennett T., Nelson D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017;68:291–322. doi: 10.1146/annurev-arplant-042916-040925. [DOI] [PubMed] [Google Scholar]
- 75.Jia K.P., Baz L., Al-Babili S. From carotenoids to strigolactones. J. Exp. Bot. 2018;69:2189–2204. doi: 10.1093/jxb/erx476. [DOI] [PubMed] [Google Scholar]
- 76.Koltai H., Dor E., Hershenhorn J., Joel D.M., Weininger S., Lekalla S., Shealtiel H., Bhattacharya C., Eliahu E., Resnick N., et al. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J. Plant Growth Regul. 2010;29:129–136. doi: 10.1007/s00344-009-9122-7. [DOI] [Google Scholar]
- 77.Wang Y., Bouwmeester H.J. Structural diversity in the strigolactones. J. Exp. Bot. 2018;69:2219–2230. doi: 10.1093/jxb/ery091. [DOI] [PubMed] [Google Scholar]
- 78.Nelson D.C., Riseborough J.A., Flematti G.R., Stevens J., Ghisalberti E.L., Dixon K.W., Smith S.M. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 2009;149:863–873. doi: 10.1104/pp.108.131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waters M.T., Scaffidi A., Flematti G.R., Smith S.M. The origins and mechanisms of karrikin signalling. Curr. Opin. Plant Biol. 2013;16:667–673. doi: 10.1016/j.pbi.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Matusova R., Rani K., Verstappen F.W., Franssen M.C., Beale M.H., Bouwmeester H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brewer P.B., Yoneyama K., Filardo F., Meyers E., Scaffidi A., Frickey T., Akiyama K., Seto Y., Dun E.A., Cremer J.E., et al. LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2016;113:6301–6306. doi: 10.1073/pnas.1601729113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ng W.L., Bassler B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kretzschmar T., Kohlen W., Sasse J., Borghi L., Schlegel M., Bachelier J.B., Reinhardt D., Bours R., Bouwmeester H.J., Martinoia E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–344. doi: 10.1038/nature10873. [DOI] [PubMed] [Google Scholar]
- 84.Yao R., Ming Z., Yan L., Li S., Wang F., Ma S., Yu C., Yang M., Chen L., Chen L., et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature. 2016;536:469–473. doi: 10.1038/nature19073. [DOI] [PubMed] [Google Scholar]
- 85.Bürger M., Mashiguchi K., Lee H.J., Nakano M., Takemoto K., Seto Y., Yamaguchi S., Chory J. Structural basis of karrikin and nonnatural strigolactone perception in Physcomitrella patens. Cell Rep. 2019;26:855–865.e5. doi: 10.1016/j.celrep.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Proust H., Hoffmann B., Xie X., Yoneyama K., Schaefer D.G., Yoneyama K., Nogue F., Rameau C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development. 2011;138:1531–1539. doi: 10.1242/dev.058495. [DOI] [PubMed] [Google Scholar]
- 87.Conn C.E., Bythell-Douglas R., Neumann D., Yoshida S., Whittington B., Westwood J.H., Shirasu K., Bond C.S., Dyer K.A., Nelson D.C. Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science. 2015;349:540–543. doi: 10.1126/science.aab1140. [DOI] [PubMed] [Google Scholar]
- 88.Toh S., Holbrook-Smith D., Stogios P.J., Onopriyenko O., Lumba S., Tsuchiya Y., Savchenko A., McCourt P. Structure-function analysis identifies highly sensitive strigolactone receptors in Striga. Science. 2015;350:203–207. doi: 10.1126/science.aac9476. [DOI] [PubMed] [Google Scholar]
- 89.Tsuchiya Y., Yoshimura M., Sato Y., Kuwata K., Toh S., Holbrook-Smith D., Zhang H., McCourt P., Itami K., Kinoshita T., et al. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science. 2015;349:864–868. doi: 10.1126/science.aab3831. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida S., Kim S., Wafula E.K., Tanskanen J., Kim Y.M., Honaas L., Yang Z., Spallek T., Conn C.E., Ichihashi Y., et al. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr. Biol. 2019;29:3041–3052.e4. doi: 10.1016/j.cub.2019.07.086. [DOI] [PubMed] [Google Scholar]
- 91.Yoneyama K., Xie X., Kusumoto D., Sekimoto H., Sugimoto Y., Takeuchi Y., Yoneyama K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- 92.Umehara M., Hanada A., Magome H., Takeda-Kamiya N., Yamaguchi S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010;51:1118–1126. doi: 10.1093/pcp/pcq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jamil M., Rodenburg J., Charnikhova T., Bouwmeester H.J. Pre-attachment Striga hermonthica resistance of New Rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytol. 2011;192:964–975. doi: 10.1111/j.1469-8137.2011.03850.x. [DOI] [PubMed] [Google Scholar]
- 94.Jamil M., Kanampiu F., Karaya H., Charnikhova T., Bouwmeester H. Striga hermonthica parasitism in maize in response to N and P fertilisers. Field Crop Res. 2012;134:1–10. doi: 10.1016/j.fcr.2012.03.015. [DOI] [Google Scholar]
- 95.Jamil M., Van Mourik T., Charnikhova T., Bouwmeester H. Effect of diammonium phosphate application on strigolactone production and Striga hermonthica infection in three sorghum cultivars. Weed Res. 2013;53:121–130. doi: 10.1111/wre.12003. [DOI] [Google Scholar]
- 96.Ueno K., Furumoto T., Umeda S., Mizutani M., Takikawa H., Batchvarova R., Sugimoto Y. Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower. Phytochemistry. 2014;108:122–128. doi: 10.1016/j.phytochem.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 97.Bonneau L., Huguet S., Wipf D., Pauly N., Truong H.N. Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favorable for arbuscular mycorrhizal symbiosis in Medicago truncatula. New Phytol. 2013;199:188–202. doi: 10.1111/nph.12234. [DOI] [PubMed] [Google Scholar]
- 98.Sun H., Tao J., Liu S., Huang S., Chen S., Xie X., Yoneyama K., Zhang Y., Xu G. Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J. Exp. Bot. 2014;65:6735–6746. doi: 10.1093/jxb/eru029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wen C., Zhao Q., Nie J., Liu G., Shen L., Cheng C., Xi L., Ma N., Zhao L. Physiological controls of chrysanthemum DgD27 gene expression in regulation of shoot branching. Plant Cell Rep. 2016;35:1053–1070. doi: 10.1007/s00299-016-1938-6. [DOI] [PubMed] [Google Scholar]
- 100.Stauder R., Welsch R., Camagna M., Kohlen W., Balcke G.U., Tissier A., Walter M.H. Strigolactone levels in dicot roots are determined by an ancestral symbiosis-regulated clade of the PHYTOENE SYNTHASE Gene Family. Front. Plant Sci. 2018;9:255. doi: 10.3389/fpls.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang L., Liu X., Xiong G., Liu H., Chen F., Wang L., Meng X., Liu G., Yu H., Yuan Y. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013;504:401. doi: 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sah S.K., Reddy K.R., Li J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016;4:571. doi: 10.3389/fpls.2016.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guillotin B., Etemadi M., Audran C., Bouzayen M., Bécard G., Combier J.P. Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. MicroTom) New Phytol. 2016;213:1124–1132. doi: 10.1111/nph.14246. [DOI] [PubMed] [Google Scholar]
- 104.Ito S., Yamagami D., Umehara M., Hanada A., Yoshida S., Sasaki Y., Yajima S., Kyozuka J., UeguchiTanaka M., Matsuoka M. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol. 2017;174:1250–1259. doi: 10.1104/pp.17.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vishwakarma K., Upadhyay N., Kumar N., Yadav G., Singh J., Mishra R.K., Kumar V., Verma R., Upadhyay R., Pandey M. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017;8:161. doi: 10.3389/fpls.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haider I., Andreo-Jimenez B., Bruno M., Bimbo A., Floková K., Abuauf H., Ntui V.O., Guo X., Charnikhova T., Al-Babili S. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018;69:2403–2414. doi: 10.1093/jxb/ery089. [DOI] [PubMed] [Google Scholar]
- 107.Foo E., Bullier E., Goussot M., Foucher F., Rameau C., Beveridge C.A. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell. 2005;17:464–474. doi: 10.1105/tpc.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stirnberg P., Furner I.J., Ottoline Leyser H. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007;50:80–94. doi: 10.1111/j.1365-313X.2007.03032.x. [DOI] [PubMed] [Google Scholar]
- 109.Hamiaux C., Drummond R.S., Janssen B.J., Ledger S.E., Cooney J.M., Newcomb R.D., Snowden K.C. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012;22:2032–2036. doi: 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 110.Zhou F., Lin Q., Zhu L., Ren Y., Zhou K., Shabek N., Wu F., Mao H., Dong W., Gan L., et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mayzlish-Gati E., De-Cuyper C., Goormachtig S., Beeckman T., Vuylsteke M., Brewer P.B., Beveridge C.A., Yermiyahu U., Kaplan Y., Enzer Y., et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 2012;160:1329–1341. doi: 10.1104/pp.112.202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kebrom T.H. Growing stem inhibits bud outgrowth–the overlooked theory of apical dominance. Front. Plant Sci. 2017;8:1874. doi: 10.3389/fpls.2017.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leyser O. Auxin signaling. Plant Physiol. 2018;176:465–479. doi: 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kapulnik Y., Delaux P.M., Resnick N., Mayzlish-Gati E., Wininger S., Bhattacharya C., Séjalon-Delmas N., Combier J.P., Bécard G., Belausov E., et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- 115.Kapulnik Y., Resnick N., Mayzlish-Gati E., Kaplan Y., Wininger S., Hershenhorn J., Koltai H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011;62:2915–2924. doi: 10.1093/jxb/erq464. [DOI] [PubMed] [Google Scholar]
- 116.Rochange S., Goormachtig S., Lopez-Raez J.A., Gutjahr C. The role of strigolactones in plant-microbe interactions. In: Koltai H., Prandi C., editors. Strigolactones–Biology and Applications. Springer International Publishing; Berlin/Heidelberg, Germany: 2019. p. 121. Chapter 4. [DOI] [Google Scholar]
- 117.Kapulnik Y., Koltai H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014;166:560–569. doi: 10.1104/pp.114.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kohlen W., Charnikhova T., Lammers M., Pollina T., Tóth P., Haider I., Pozo M.J., de Maagd R.A., Ruyter-Spira C., Bouwmeester H.J., et al. The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 2012;196:535–547. doi: 10.1111/j.1469-8137.2012.04265.x. [DOI] [PubMed] [Google Scholar]
- 119.Yoshida S., Kameoka H., Tempo M., Akiyama K., Umehara M., Yamaguchi S., Hayashi H., Kyozuka J., Shirasu K. The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol. 2012;196:1208–1216. doi: 10.1111/j.1469-8137.2012.04339.x. [DOI] [PubMed] [Google Scholar]
- 120.Kobae Y., Kameoka H., Sugimura Y., Saito K., Ohtomo R., Fujiwara T., Kyozuka J. Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant Cell Physiol. 2018;59:544–553. doi: 10.1093/pcp/pcy001. [DOI] [PubMed] [Google Scholar]
- 121.Smith S.E., Smith F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 122.Keymer A., Gutjahr C. Cross-kingdom lipid transfer in arbuscular mycorrhizal symbiosis and beyond. Curr. Opin. Plant Biol. 2018;44:137–144. doi: 10.1016/j.pbi.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 123.Salvioli A., Ghignone S., Novero M., Navazio L., Venice F., Bagnaresi P., Bonfante P. Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J. 2016;10:130–144. doi: 10.1038/ismej.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsuzuki S., Handa Y., Takeda N., Kawaguchi M. Strigolactone-induced putative secreted protein 1 is required for the establishment of symbiosis by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mol. Plant Microbe Interact. 2016;29:277–286. doi: 10.1094/MPMI-10-15-0234-R. [DOI] [PubMed] [Google Scholar]
- 125.Kamel L., Tang N., Malbreil M., San Clemente H., Le Marquer M., Roux C., Frei Dit Frey N. The comparison of expressed candidate secreted proteins from two arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Front. Plant Sci. 2017;8:124. doi: 10.3389/fpls.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Genre A., Chabaud M., Balzergue C., Puech-Pagès V., Novero M., Rey T., Fournier J., Rochange S., Bécard G., Bonfante P., et al. Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol. 2013;198:190–202. doi: 10.1111/nph.12146. [DOI] [PubMed] [Google Scholar]
- 127.Moscatiello R., Sello S., Novero M., Negro A., Bonfante P., Navazio L. The intracellular delivery of TAT-aequorin reveals calcium-mediated sensing of environmental and symbiotic signals by the arbuscular mycorrhizal fungus Gigaspora margarita. New Phytol. 2014;203:1012–1020. doi: 10.1111/nph.12849. [DOI] [PubMed] [Google Scholar]
- 128.Müller L.M., Flokova K., Schnabel E., Sun X., Fei Z., Frugoli J., Bouwmeester H.J., Harrison M.J. A CLE–SUNN module regulates strigolactone content and fungal colonization in arbuscular mycorrhiza. Nat. Plants. 2019;5:933–939. doi: 10.1038/s41477-019-0501-1. [DOI] [PubMed] [Google Scholar]
- 129.Besserer A., Becard G., Jauneau A., Roux C., Sejalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008;148:402–413. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gamir J., Torres-Vera R., Rial C., Berrio E., de Souza Campos P.M., Varela R.M., Macías F.A., Pozo M.J., Flors V., López-Ráez J.A. Exogenous strigolactones impact metabolic profiles and phosphate starvation signalling in roots. Plant Cell Environ. 2020;43:1655–1668. doi: 10.1111/pce.13760. [DOI] [PubMed] [Google Scholar]
- 131.Gutjahr C., Gobbato E., Choi J., Riemann M., Johnston M.G., Summers W., Carbonnel S., Mansfield C., Yang S.-Y., Nadal M., et al. Rice perception of symbiotic arbuscular mycorrhizal fungi requires the karrikin receptor complex. Science. 2015;350:1521–1524. doi: 10.1126/science.aac9715. [DOI] [PubMed] [Google Scholar]
- 132.Mori N., Nishiuma K., Sugiyama T., Hayashi H., Akiyama K. Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi. Phytochemistry. 2016;130:90–98. doi: 10.1016/j.phytochem.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 133.Pandey A., Sharma M., Pandey G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016;7:434. doi: 10.3389/fpls.2016.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mishra S., Upadhyay S., Shukla R.K. The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front. Physiol. 2017;7:691. doi: 10.3389/fphys.2016.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diagne N., Ngom M., Djighaly P.I., Fall D., Hocher V., Svistoonoff S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity. 2020;12:370. doi: 10.3390/d12100370. [DOI] [Google Scholar]
- 136.Seefeldt L.C., Hoffman B.M., Dean D.R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 2009;78:701–722. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dos Santos P.C., Fang Z., Mason S.W., Setubal J.C., Dixon R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genom. 2012;13:162. doi: 10.1186/1471-2164-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Radutoiu S., Madsen L., Madsen E., Jurkiewicz A., Fukai E., Quistgaard E., Albrektsen A., James E., Thirup S., Stougaard J. LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J. 2007;26:3923–3935. doi: 10.1038/sj.emboj.7601826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gough C., Cullimore J. Lipo-chitooligosaccharide signaling in endosymbiotic plant-microbe interactions. Mol. Plant Microbe. Interact. 2011;24:867–878. doi: 10.1094/MPMI-01-11-0019. [DOI] [PubMed] [Google Scholar]
- 140.Fliegmann J., Bono J.J. Lipo-chitooligosaccharidic nodulation factors and their perception by plant receptors. Glycoconj. J. 2015;32:455–464. doi: 10.1007/s10719-015-9609-3. [DOI] [PubMed] [Google Scholar]
- 141.Liu C., Murray J. The role of flavonoids in nodulation host-range specificity: An update. Plants. 2016;5:33. doi: 10.3390/plants5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murray J.D., Karas B.J., Sato S., Tabata S., Amyot L., Szczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–104. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- 143.Tirichine L., Sandal N., Madsen L.H., Radutoiu S., Albrektsen A.S., Sato S., Asamizu E., Tabata S., Stougaard J. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–107. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 144.Ferguson B.J., Mathesius U. Phytohormone regulation of legume-rhizobia interactions. J. Chem. Ecol. 2014;40:770–790. doi: 10.1007/s10886-014-0472-7. [DOI] [PubMed] [Google Scholar]
- 145.Soto M.J., Fernandez-Aparicio M.N., Castellanos-Morales V., García-Garrido J.M., Ocampo J.A., Delgado M.J., Vierheilig H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa) Soil Biol. Biochem. 2010;42:383–385. doi: 10.1016/j.soilbio.2009.11.007. [DOI] [Google Scholar]
- 146.Foo E., Davies N.W. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–1081. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- 147.Foo E., Ferguson B.J., Reid J.B. The potential roles of strigolactones and brassinosteroids in the autoregulation of nodulation pathway. Ann. Bot. 2014;113:1037–1045. doi: 10.1093/aob/mcu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Foo E., Yoneyama K., Hugill C.J., Quittenden L.J., Reid J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant. 2013;6:76–87. doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- 149.Liu J., Novero M., Charnikhova T., Ferrandino A., Schubert A., Ruyter-Spira C., Bonfante P., Lovisolo C., Bouwmeester H.J., Cardinale F. CAROTENOID CLEAVAGE DIOXYGENASE 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus Japonicus. J. Exp. Bot. 2013;64:1967–1981. doi: 10.1093/jxb/ert056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haq B.U., Ahmad M.Z., Ur Rehman N., Wang J., Li P., Li D., Zhao J. Functional characterization of soybean strigolactone biosynthesis and signaling genes in Arabidopsis max mutants and GmMAX3 in soybean nodulation. BMC Plant Biol. 2017;17:259. doi: 10.1186/s12870-017-1182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rehman N.U., Ali M., Ahmad M.Z., Liang G., Zhao J. Strigolactones promote rhizobia interaction and increase nodulation in soybean (Glycine max) Microb. Pathog. 2018;114:420–430. doi: 10.1016/j.micpath.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 152.De Cuyper C., Fromentin J., Yocgo R.E., De Keyser A., Guillotin B., Kunert K., Boyer F.D., Goormachtig S. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J. Exp. Bot. 2015;66:137–146. doi: 10.1093/jxb/eru404. [DOI] [PubMed] [Google Scholar]
- 153.Scaffidi A., Waters M.T., Sun Y.K., Skelton B.W., Dixon K.W., Ghisalberti E.L., Flematti G.R., Smith S.M. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 2014;165:1221–1232. doi: 10.1104/pp.114.240036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Flematti G.R., Scaffidi A., Waters M.T., Smith S.M. Stereospecificity in strigolactone biosynthesis and perception. Planta. 2016;243:1361–1373. doi: 10.1007/s00425-016-2523-5. [DOI] [PubMed] [Google Scholar]
- 155.Liu W., Kohlen W., Lillo A. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell. 2011;23:3853–3865. doi: 10.1105/tpc.111.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Zeijl A., Liu W., Xiao T.T., Kohlen W., Yang W.C., Bisseling T., Geurts R. The strigolactone biosynthesis gene DWARF27 is co-opted in Rhizobium symbiosis. BMC Plant Biol. 2015;15:260. doi: 10.1186/s12870-015-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McAdam E.L., Hugill C., Fort S., Samain E., Cottaz S., Davies N.W., Reid J.B., Foo E. Determining the site of action of strigolactones during nodulation. Plant Physiol. 2017;175:529–542. doi: 10.1104/pp.17.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vurro M., Boari A., Thiombiano B., Bouwmeester H. Strigolactones and parasitic plants. In: Koltai H., Prandi C., editors. Strigolactones–Biology and Applications. Springer International Publishing; Berlin/Heidelberg, Germany: 2019. p. 89. Chapter 3. [DOI] [Google Scholar]
- 159.Tippe D.E., Bastiaans L., van Ast A., Dieng I., Cissoko M., Kayeke J., Makokha D.W., Rodenburg J. Fertilisers differentially affect facultative and obligate parasitic weeds of rice and only occasionally improve yields in infested fields. Field Crop Res. 2020;254:107845. doi: 10.1016/j.fcr.2020.107845. [DOI] [Google Scholar]
- 160.Jia K.P., Kountche B.A., Jamil M., Guo X., Ntui V.O., Rüfenacht A., Rochange S., Al-Babili S. Nitro-phenlactone, a carlactone analog with pleiotropic strigolactone activities. Mol. Plant. 2016;9:1341–1344. doi: 10.1016/j.molp.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 161.Staudinger C., Mehmeti-Tershani V., Gil-Quintana E., Gonzalez E.M., Hofhans F., Bachmann G., Wienkoop S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteome. 2016;136:202–213. doi: 10.1016/j.jprot.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 162.Vurukonda S.S.K.P., Vardharajula S., Shrivastava M., Skz A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 163.Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hassini I., Martinezballesta M.D., Boughanmi N., Moreno D.A., Carvajal M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. 2017;226:141–151. doi: 10.1016/j.scienta.2017.08.030. [DOI] [Google Scholar]
- 165.Buvaneshwari S., Riotte J., Sekhar M., Sharma A.K., Helliwell R., Kumar M., Braun J.J., Ruiz L. Potash fertilizer promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci. Rep. 2020;10:3691. doi: 10.1038/s41598-020-60365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zheng X.D., Li Y.Q., Xi X.L., Ma C.Q., Sun Z.J., Yang X.Q., Li X.Y., Tian Y.K., Wang C.H. Exogenous strigolactones alleviate KCl stress by regulating photosynthesis, ROS migration and ion transport in Malus hupehensis Rehd. Plant Physiol. Biochem. 2021;159:113–122. doi: 10.1016/j.plaphy.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 167.Marzec M., Daszkowska-Golec A., Collin A., Melzer M., Eggert K., Szarejko I. Barley strigolactone signalling mutanthvd14.d reveals the role of strigolactones in abscisic acid-dependent response to drought. Plant Cell Environ. 2020;43:2239–2253. doi: 10.1111/pce.13815. [DOI] [PubMed] [Google Scholar]
- 168.Woo H.R., Chung K.M., Park J.H., Oh S.A., Ahn T., Hong S.H., Jang S.K., Nam H.G. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001;13:1779–1790. doi: 10.1105/TPC.010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yan H., Saika H., Maekawa M., Takamure I., Tsutsumi N., Kyozuka J., Nakazono M. Rice tillering dwarf mutant dwarf3 has increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death. Genes Genet. Syst. 2007;82:361–366. doi: 10.1266/ggs.82.361. [DOI] [PubMed] [Google Scholar]
- 170.Czarnecki O., Yang J., Weston D.J., Tuskan G.A., Chen J.-G. A dual role of strigolactones in phosphate acquisition and utilization in plants. Int. J. Mol. Sci. 2013;14:7681–7701. doi: 10.3390/ijms14047681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brun G. At the crossroads of strigolatcones and abscisic acid pathways: A role for miR156. Plant Cell Environ. 2020;43:1609–1612. doi: 10.1111/pce.13787. [DOI] [PubMed] [Google Scholar]
- 172.Visentin I., Pagliarani C., Deva E., Caracci A., Tureckova V., Novak O., Lovisolo C., Schubert A., Cardinale F. A novel strigolactone-miR156 module controls stomatal behaviour during drought recovery. Plant Cell Environ. 2020;43:1613–1624. doi: 10.1111/pce.13758. [DOI] [PubMed] [Google Scholar]
- 173.Zwanenburg B., Mwakaboko A.S., Reizelman A., Anilkumar G., Sethumadhavan D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009;65:478–491. doi: 10.1002/ps.1706. [DOI] [PubMed] [Google Scholar]
- 174.Dor E., Joel D.M., Kapulnik Y., Koltai H., Hershenhorn J. The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta. 2011;234:419–427. doi: 10.1007/s00425-011-1452-6. [DOI] [PubMed] [Google Scholar]
- 175.Kgosi R.L., Zwanenburg B., Mwakaboko A.S., Murdoch A.J. Strigolactone analogues induce suicidal seed germination of Striga spp. in soil. Weed Res. 2012;52:197–203. doi: 10.1111/j.1365-3180.2012.00912.x. [DOI] [Google Scholar]
- 176.Pandya-Kumar N., Shema R., Kumar M., Mayzlish-Gati E., Levy D., Zemach H., Belausov E., Wininger S., Abu-Abied M., Kapulnik Y., et al. Strigolactone analog GR24 triggers changes in PIN2 polarity, vesicle trafficking and actin filament architecture. New Phytol. 2014;202:1184–1196. doi: 10.1111/nph.12744. [DOI] [PubMed] [Google Scholar]
- 177.Lombardi C., Artuso E., Grandi E., Lolli M., Spirakys F., Priola E., Prandi C. Recent advances in the synthesis of analogues of phytohormones strigolactones with ring-closing metathesis as a key step. Org. Biomol. Chem. 2017;38:8218–8231. doi: 10.1039/C7OB01917C. [DOI] [PubMed] [Google Scholar]
- 178.Joel D.M., Chaudhuri S.K., Plakhine D., Ziadna H., Steffens J.C. Dehydrocostus lactone is exuded from sunflower roots and stimulates germination of the root parasite Orobanche cumana. Phytochemistry. 2011;72:624–634. doi: 10.1016/j.phytochem.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 179.Joel D.M., Bar H. The seed and the seedling. In: Joel D.M., Gressel J., Musselman L.J., editors. Parasitic Orobanchaceae. Springer; Berlin/Heidelberg, Germany: 2013. p. 147. Chapter 9. [DOI] [Google Scholar]
- 180.Kim H.I., Xie X., Kim H.S., Chun J.C., Yoneyama K., Nomura T., Takeuchi Y., Yoneyama K. Structure-activity relationship of naturally occurring strigolactones in Orobanche minor seed germination stimulation. J. Pestic. Sci. 2010;35:344–347. doi: 10.1584/jpestics.G10-17. [DOI] [Google Scholar]
- 181.Yoneyama K., Awad A.A., Xie X., Yoneyama K., Takeuchi Y. Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 2010;51:1095–1103. doi: 10.1093/pcp/pcq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zwanenburg B., Pospíšil T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant. 2013;6:38–62. doi: 10.1093/mp/sss141. [DOI] [PubMed] [Google Scholar]
- 183.Zwanenburg B., Ćavar Zeljković S., Pospíšil T. Synthesis of strigolactones, a strategic account. Pest Manag. Sci. 2016;72:15–29. doi: 10.1002/ps.4105. [DOI] [PubMed] [Google Scholar]
- 184.Jenul C., Sieber S., Daeppen C., Mathew A., Lardi M., Pessi G., Hoepfner D., Neuburger M., Linden A., Gademann K., et al. Biosynthesis of fragin is controlled by a novel quorum sensing signal. Nat. Commun. 2018;9:1297. doi: 10.1038/s41467-018-03690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morgan G.L., Li B. In vitro reconstitution reveals a Central role for the N-oxygenase PvfB in (Dihydro)pyrazine-N-oxide and valdiazen biosynthesis. Angew. Chem. Int. Ed. Engl. 2020;59:21387–21391. doi: 10.1002/anie.202005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sieber S., Mathew A., Jenul C., Kohler T., Bar M., Carrion V.J., Cazorla F.M., Stadler U., Hsieh Y.-C., Bigler L., et al. A volatile signal controls virulence in the plant pathogen Pseudomonas syringae pv. syringae and a strategy for infection control in organic farming. bioRxiv. 2020 doi: 10.1101/2020.09.18.279364. [DOI] [Google Scholar]
- 187.Hoffman L.R., D’Argenio D.A., MacCoss M.J., Zhang Z., Jones R.A., Miller S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 188.Berdy J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012;65:385–395. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- 189.Schertzer J.W., Boulette M.L., Whiteley M. More than signal: Non-signaling properties of quorum sensing. Trends Microbiol. 2009;17:189–195. doi: 10.1016/j.tim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 190.Davies J. Are antibiotics naturally antibiotics? J. Ind. Microbiol. Biotechnol. 2006;33:496–499. doi: 10.1007/s10295-006-0112-5. [DOI] [PubMed] [Google Scholar]
- 191.Linares J.F., Gustafsson I., Baquero F., Martinez J.L. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Davies J., Spoegelman G.B., Yim G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006;9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 193.Romero D., Traxler M.F., López D., Kolter R. Antibiotics as signal molecules. Chem. Rev. 2011;111:5492–5505. doi: 10.1021/cr2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goh E.B., Yim G., Tsui W., McClure J., Surette M.G., Davies J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. USA. 2002;99:17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shen L., Shi Y., Zhang D., Wei J., Surette M.G., Duan K. Modulation of secreted virulence factor genes by subinhibitory concentrations of antibiotics in Pseudomonas aeruginosa. J. Microbiol. 2008;46:441–447. doi: 10.1007/s12275-008-0054-x. [DOI] [PubMed] [Google Scholar]
- 196.Bagge N., Schuster M., Hentzer M., Ciofu O., Givskov M., Greenberg E.P., Høiby N. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and beta-lactamase and alginate production. Antimicrob. Agents Chemother. 2004;48:1175–1187. doi: 10.1128/AAC.48.4.1175-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu Z., Wang W., Zhu Y., Gong Q., Yu W., Lu X. Antibiotics at subinhibitory concentrations improve the quorum sensing behavior of Chromobacterium violaceum. FEMS Microbiol. Lett. 2013;341:37–44. doi: 10.1111/1574-6968.12086. [DOI] [PubMed] [Google Scholar]
- 198.Kalia V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 199.Beyersmann P.G., Tomasch J., Son K., Stocker R., Göker M., Wagner-Döbler I., Simon M., Brinkhoff T. Dual function of tropodithietic acid as antibiotic and signaling molecule in global gene regulation of the probiotic bacterium Phaeobacter inhibens. Sci. Rep. 2017;7:730. doi: 10.1038/s41598-017-00784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Deryabin D.G., Inchagova K.S. Subinhibitory concentrations of the penicillin antibiotics induce quorum-dependent violacein synthesis in Chromobacterium violaceum. Mikrobiologiya. 2017;86:448–454. doi: 10.1134/S0026261717040051. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.