Abstract
Simple Summary
The camel milk market was limited for a long time by its almost exclusive self-consumption use in nomadic camps. Significant development has been observed for the past two or three decades, including internationally, boosted by its reputation regarding its health effects for regular consumers. Such emergence has led the stakeholders in the sector to offer diversified products corresponding to the tastes of increasingly urbanized consumers, more sensitive to “modern” products. Thus, traditionally drunk in raw or naturally fermented form, camel milk has undergone unprecedented transformations such as pasteurization, directed fermentation, cheese or yoghurt processing, and manufacture of milk powder for the export market. However, the specific characteristics of this milk (composition, physical properties) mean that the technologies applied (copied from technologies used for cow milk) must be adapted. In this review, some technological innovations are presented, enabling stakeholders of the camel milk sector to satisfy the demand of manufacturers and consumers.
Abstract
Camel milk is a newcomer to domestic markets and especially to the international milk market. This recent emergence has been accompanied by a diversification of processed products, based on the technologies developed for milk from other dairy species. However, technical innovations had to be adapted to a product with specific behavior and composition. The transformation of camel milk into pasteurized milk, fermented milk, cheese, powder, or other products was supported, under the pressure of commercial development, by technological innovations made possible by a basic and applied research set. Some of these innovations regarding one of the less studied milk sources are presented here, as well as their limitations. Technical investigations for an optimal pasteurization, development of controlled fermentation at industrial scale, control of cheese technology suitable for standardized production, and improvements in processes for the supply of a high-quality milk powder are among the challenges of research regarding camel milk.
Keywords: camel, milk technology, pasteurization, cheesemaking, powder milk, fermentation
1. Introduction
For a long time, only fresh camel milk was consumed by pastoralists and was regarded as a gift for the hosts coming under the tent of the nomads. Consequently, it was not considered a commodity and its sale was often taboo. Moreover, it did not undergo any transformation, except for fermentation [1] to prolong its shelf life in desert conditions where the cold chain could not be respected. The introduction of camel milk to the regular market at a national or even international level is a recent feature [2]. Such development of the camel milk market was concomitant with a deepening of the knowledge of its fine composition [3] and of its transformation processes, allowing the marketing of a more diversified dairy product [4]. Recent findings are effectively available, allowing this important product renowned for its true or supposed “medicinal” virtues [5] to remove camel milk from marginality. Indeed, the production of camel milk at a world level is experiencing a considerable annual growth, exceeding 8% in the period 2009–2019 [6], testifying to the growing interest for this product.
Moreover, despite its proximity with cow milk in term of gross composition (fat, protein, lactose, and mineral proportions), camel milk shows many specificities [3] such as functional proteins, predominant medium-chain fatty acids in fat matter, low lactose intolerance, and richness in vitamin C or iron, among others. These proper characteristics have an obvious impact on the “behavior” of camel milk during processing.
Thus, the present paper proposes the state of the art regarding knowledge on camel milk processing by focusing on four main dairy products having different success on the market, i.e., pasteurized milk, fermented milk, camel cheese, and camel milk powder. Other products will rapidly emerge. In addition to the review regarding the current status of knowledge on camel milk processing, the more recent studies (2019–2020) focusing on this topic are presented in Table 1.
Table 1.
The main recent references (2019–2021) regarding camel milk processing. KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates; USA, United States of America.
| Processing | Reference | Country | Topic |
|---|---|---|---|
| Pasteurization | Zhang et al., 2020 [7] | China | Fouling characterization |
| Lajnaf et al., 2020 [8] | Tunisia | Foaming properties | |
| Li et al., 2020 [9] | China | Protein profile | |
| Yehia et al., 2020 [10] | KSA | Heat-resistant Staphylococcus aureus | |
| Bragason et al., 2020 [11] | Ethiopia | Antibacterial properties | |
| Fermentation | Gammoh et al., 2020 [12] | Jordan | Modification of functional properties |
| Sobti et al., 2021 [13] | UAE | Effect of pectin and alginate | |
| Mortazavi et al., 2021 [14] | Iran | Enrichment of fermented milk with pomegranate peel | |
| Zhadyra et al., 2021 [15] | China | Microflora diversity | |
| Sharma et al., 2021 [16] | KSA | Probiotic properties | |
| Ayyash et al., 2021 [17] | UAE | Exopolysaccharide impact | |
| Soleymanzadeh, 2019 [18] | Iran | Novel β-casein in fermented milk | |
| Edalati et al., 2019 [19] | Iran | Antagonistic effects of probiotic bacteria | |
| Cheese | Mbye et al., 2020 [20] | UAE | Physicochemical properties, sensory quality, and coagulation behavior |
| Belkheir et al., 2020 [21] | Algeria | Effect of starter on volatile and sensory profiles | |
| El-Hatmi et al., 2020 [22] | Tunisia | Cheese fortification by Allium roseum | |
| Spray-dried and freeze-dried | Perusko et al., 2021 [23] | Serbia | Maillard reaction products |
| Ho et al., 2019 [24] | Australia | Physicochemical properties with accelerated storage | |
| Deshval et al., 2020 [25] | India | Functional and morphological properties | |
| Zouari et al., 2020 [26] | Tunisia | Physical and biochemical properties | |
| Zouari et al., 2020 [27] | Tunisia | Microstructure and chemical composition | |
| Yoghurt | Kamal-Eldin et al., 2020 [28] | UAE | Properties of mixed camel/cow yoghurt |
| Buchilina and Aryana, 2021 [29] | USA | Yoghurt with monk fruit | |
| Atwaa et al., 2020 [30] | Egypt | Production of stirred camel yogurt | |
| Chen et al., 2019 [31] | China | Trisodium citrate and transglutaminase treatment of acid gel | |
| Cream | Kashaninejad and Razavi, 2020 [32] | Iran | Properties and color |
2. Pasteurized Milk
2.1. Current Global Conditions for Pasteurization of Camel Milk
Pasteurization of camel milk is a commonly used technique in camel countries. Nevertheless, the conditions of pasteurization implemented by each holder were often decided without considering the specificity of camel milk, with the rules being mainly based on the standard issued for pasteurization of cow milk. Some data regarding the conditions of camel milk pasteurization in scientific literature are quite variable: 60 °C for 30 min [33]; 75 °C for 15 s [34]; 63 °C for 30 min [35,36]. At the same time, many private companies in the United Arab Emirates (UAE), Saudi Arabia, Mauritania, Kazakhstan, Algeria, Tunisia, Morocco, and Niger are producing and selling pasteurized camel milk on the local market. All of these companies apply different conditions for pasteurization. It is important to mention that national/regional/international standards are not yet elaborated for camel milk or they are simply copied from cow milk. In some camel countries, no standard is proposed by the government or, at least, it is suggested to apply the same conditions as for bovine milk.
2.2. Indicators of Camel Milk Pasteurization
The pasteurization procedure for camel milk should have its own conditions and indicators. Indeed, a preliminary study showed that alkaline phosphatase (ALP), traditionally used for cow milk [37], was not a convenient indicator of successful pasteurization of camel milk, because camel ALP is heat-resistant, still showing activity at 90 °C [38]. Loiseau et al. [39] suggested using glutamyltranspeptidase (75 °C for 30 s) or leucine arylamidase (75 °C for 28 s or 80 °C for 7 s) as an indicator of pasteurization for camel milk. If camel milk must be pasteurized at 72 °C for 20 min, the most appropriate indicator could be gamma-glutamyl transferase (GGT) according to Wernery et al. [40]. However, later in 2011, Lorenzen et al. [41] concluded that GGT was still present in pasteurized camel milk, whereas lactoperoxidase (LPO) could be a more appropriate indicator of pasteurization. Tayefi-Nasrabadi et al. [42] confirmed that camel LPO was less heat-resistant than bovine LPO. Until now, no sufficiently in-depth studies have been done in this field, although pasteurized camel milk was introduced to the international market. Such doubts on a convenient indicator of pasteurization for camel milk are a constraint for the establishment of an international standard. Thus, the pasteurization of camel milk at an industrial scale is possibly achieved in the wrong way and its heat treatment may be incorrect.
2.3. Impact of Pasteurization on Physical Properties of Camel Milk
The characteristics of camel milk flow in pipes during the pasteurization process, the cleaning procedures, and the conditions of transfer and pumping also need to be studied. The behavior of fat globules and casein micelles in camel milk is different from that in cow milk, presenting an absolute viscosity of 1.72 mPa·s at 20 °C vs. 2.04 mPa·s at the same temperature in bovine milk. Thus, in dairy plants, the camel milk should not necessarily be transferred at 20 °C. According to Kherouatou et al. [43], camel milk in fresh conditions (without acidification) can resist some mechanical strains without changing its microstructure. The apparent viscosity was quite stable (between 1.6 and 2.0 mPa·s) at pH values between 5.2 and 6.7. Therefore, camel milk can be manipulated in dairy plants, e.g., pumping, agitation, skimming, homogenization, and bottling, for producing a pasteurized product.
Few studies have focused on the sensory characteristics of heat-treated camel milk. Comparing three treatment conditions (63 °C for 30 min, 72 °C for 15 s, and 100.5 °C for 10 min), Lund et al. [44] observed lower taste score, texture, and overall acceptability in treated samples of camel milk compared to control (nontreated); however, there was surprisingly no significant difference between pasteurization protocols, although the milk at 63 °C/30 min had the highest mean sensory score. However, these authors did not check the hygienic level of their samples. Indeed, to compare the different protocols, it is important to cross the data regarding pasteurization conditions and sensory characteristics with the hygienic level of the raw milk.
Some studies regarding behavior during heat treatment observed that camel milk could give an important amount of dry deposit on a stainless-steel plate during the pasteurization process from 60 to 90 °C for 1 h or 2 h [45]. This study showed that such a deposit is probably not of protein origin, because free thiol groups are in lower quantity than for cow milk treatment. A similar design of experiment with camel whey protein showed that, after 63 °C, whey proteins started to be denatured; this was especially evident at 98 °C [46]. It is possible that camel milk is producing a higher quantity of “milk stones”, i.e., the deposit of milk residues accumulated in an insufficiently cleansed dairy equipment where bacteria can be multiplied, contributing to bad flavors in milk. To the best of our knowledge, there are no available data in the literature regarding this aspect.
2.4. Camel Milk Protein Behavior Following Heat Treatment
In-depth studies on camel milk started relatively recently, especially regarding its technological properties. Even if some data on camel milk composition date from 1905 [47], research regarding the heat treatment impact on camel milk components started being implemented at the end of the 1980s. Thus, in a first trial, camel milk was heated at 63, 80, or 90 °C for 30 min. The rate of heat denaturation of whey proteins was twofold lower than for cow whey proteins [48]. After this study, another trial focused on heat coagulation at 100–130 °C in a pH range of 6.3–7.1. Only the 100 °C variant showed relative stability at pH 7, comparable to cow milk [49].
Because milk stability during heat treatment is the most important point and because the size of casein micelles could impact milk preservation in a homogeneous solution, the micelle size of camel milk was measured before and after pasteurization. They are broader than those of cow and human milk. However, an important point must be kept in mind, i.e., the samples were analyzed 36 h after sampling [50]. Regarding the mineralization and citrate quantity in camel casein micelles, the proportions were significantly different than for cow casein micelles after pasteurization. It was observed that, at pH 5, significant changes occurred in the dromedary casein micelle structure from milk to coagulum [51].
Some studies [35,52] stated that the pasteurization of camel milk could change its chemical composition. However, in these experiments, the microbial quality of raw camel milk was not assessed, no standardized starter culture was used, and the microbial contamination risk was not taken in account. Yet, in trials where microbiological status was tested before processing, pasteurization at 63 °C for 30 min improved the bacteriological quality of camel milk without changes in the composition compared to raw camel milk [36,53].
The observation of camel whey protein using SDS-PAGE showed that some proteins sensitively decrease after heat treatment, albeit to a lesser extent than for cow proteins. The pattern of camel whey proteins and the global composition of proteins are not the same for camel and cow milk. Accordingly, the major proteins of whey in bovine are serum albumin (SA), α-lactalbumin (α-La), and β-lactoglobulin (β-Lg), whereas those for camel are SA, α-La, and three other fractions not reported in cow milk [48,54,55,56,57].
In a recent study [58], the effect of heat treatment according to different protocols (65 °C for 30 min, 72 °C for 30 s, 75 °C for 5 min, 85 °C for 5 min, or 90 °C for 5 min) on camel whey proteins was assessed comparatively to cow milk. A lower denaturation of α-lactalbumin was observed in camel milk; after treatment at 90 °C for 5 min, 67% α-lactalbumin remained intact vs. only 5% in cow milk. However, camel serum albumin (CSA) was not totally detected after treatment at 85 °C for 5 min, although this was a less rapid process than with cow serum albumin, which disappeared after 75 °C for 5 min [58]. Similar figures were observed in a previous paper [45].
Some authors tried quantifying the changes in the concentration of camel whey proteins during the pasteurization process. However, debatable results occurred especially because different methods were used, needing clarification. Indeed, the quantification of whey proteins in camel milk can be done using electrophoresis [59], radial immunodiffusion [56,60,61,62], high-performance liquid chromatography [56], fast protein liquid chromatography [60], and the more recent techniques of liquid chromatography/tandem mass spectrometry and liquid chromatography/electrospray ionization mass spectroscopy [57].
The heat resistance of camel milk can also be revealed by the heat coagulation time [59]. Compositional difference plays an important role in heat resistance, particularly the absence of β-lg, different ratios in the casein complex (higher quantity of αs1-, αs2-, and β-caseins and lower quantity of κ-casein compared to cow milk [63]), and the presence of a higher quantity of other whey proteins. Whey proteins include three protein fractions described as common fractions of immunoglobulins (IgG1, IgG2, and IgG3), α-lactalbumin, lactophorin (which is closely related to the bovine proteose peptone component 3 (PP3)), the innate immunity peptido-glycan recognition protein (PGRP), and the whey acidic protein (WAP) [64].
2.5. Sterilized Milk
Sterilization of camel milk using an ultra-high-temperature (UHT) treatment is yet to be achieved, despite private companies trying to establish an appropriate method. Some studies on the heat resistance of whey and casein proteins, fat globules, vitamins, or other compounds of camel milk are expected to contribute to a technical solution [42,48,49,52,55,65,66,67].
After UHT treatment, camel milk presents a separation into two phases. During heat treatment at 65°/30 min, 72°/30 s, 75°/5 min, 85°/5 min, 90 °C/5 min [48] or at 63, 80 and 90 °C for 30 min and 72 °C for 15 s [52], it was observed that the whey proteins were overly sensitive and started denaturing. To stabilize camel milk proteins after UHT treatment, different protocols including the addition of chemicals (sodium hydroxide, calcium chloride, ĸ-casein from cow, sodium dihydrogen phosphate anhydrous, disodium hydrogen orthophosphate, or ethylenediaminetetraacetic salt) were tested but with disappointing results [68]. Further in-depth studies need to be implemented before being able to produce UHT camel milk.
However, it is possible to obtain sterilized milk after reconstitution of liquid milk using camel milk powder. This camel dairy product is available on the market in the Middle East. Moreover, ultrafiltration to yield concentrated skim camel milk was tested experimentally [69]. By using an α-alumina ultrafiltration membrane (pore size 1.4 µm) at an operating temperature of 50 °C, it was possible to obtain a product with an extended shelf-life up to 60 days and to decrease the germ count by 99%. The low filtration temperature did not damage the heat-sensitive proteins [70].
2.6. Antimicrobial Activity and Pasteurization of Camel Milk
Many people believe that camel milk has sufficient natural antimicrobial activity to preserve it from natural adulteration at ambient temperature for a long time. As such, they often consider that camel milk collection does not require the same requirements in terms of hygiene practices. Therefore, in many cases, potential microbial contamination is not checked before implementing the trials. Nevertheless, it is important to recall the results obtained by Sela et al. [71], whereby the thermal death time of Escherichia coli in camel milk was the same as in cow milk. The presence of E. coli in camel milk is common [72] and flouting hygienic rules will provoke digestive disorders in consumers. At the same time, raw and pasteurized camel milk has the capacity to inhibit Cronobacter sakazakii. As this bacterium can grow in powder milk, some authors think that it could be possible to use it in the production of infant formula [73], but such a possibility requires further investigation.
Obviously, pasteurization prolongs the shelf-life of milk and several data were obtained using different protocols. In the study of Lund et al. [44], quality was maintained after storage at ambient temperature for 24 h for raw milk vs. 76 h for milk treated at 100 °C/10 min, 64 h at 63 °C/30 min, and at 72 °C/15 s. The bacterial level appeared higher (2.77 log colony-forming units (CFU)/mL) with a protocol of 72 °C/15 s than 75 °C/10 min (2.65 log CFU/mL) and 65 °C/30 min (2.57 log CFU/mL), with the lowest content (2.45 log CFU/mL) being observed with a protocol of 80 °C/5 min [74].
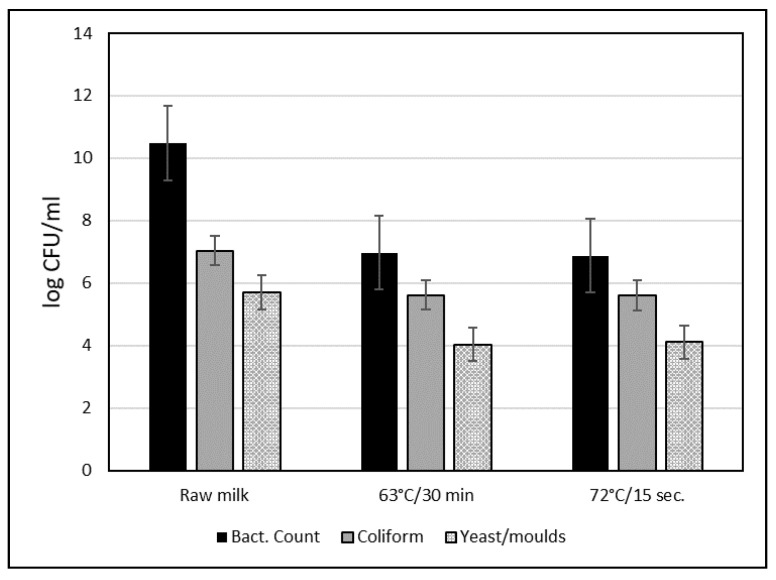
In their study assessing the effect of two protocols (63 °C/30 min and 72 °C/15 s), on total bacterium, coliform, and mold counts, Mohamed and El-Zubeir [75] found that the first protocol was non-significantly more efficient in terms of total bacterial and coliform count, but less in terms of yeast and mold count (Figure 1). However, the protocols were applied on highly contaminated milk. Accordingly, it is worth reiterating that pasteurization does not guarantee sterilization and requires respecting the hygienic rules at the milking, storage, and transport stages of the raw material to correct process the milk.
Figure 1.
Changes in total bacterium, coliform, and yeast/mold count in camel milk before (raw) and after heat treatment (from [75]).
Camel milk pasteurization is achieved at an industrial scale, and pasteurized camel milk can be directly provided to consumers with a shelf-life of around a week. Some dairy plants have extensive experience to producing pasteurized camel milk, for example, Tiviski (Mauritania), Camelicious (UAE), Al-Watania (KSA), and Tedjane (Algeria). However, further research is necessary, especially to study the rheological properties of raw and pasteurized camel milk, which are poorly documented.
3. Fermented Milk
The fermentation process is commonly used for the preservation of food. The process of milk fermentation, including camel milk, is a traditional ancestral method all over the world. It consists of the transformation of lactose into lactic acid by the natural microflora in milk dominated by lactic bacteria and, in some cases, by yeasts. To understand this process, numerous investigations on the microflora of raw camel milk were performed. As many fermented products from camel milk have been produced by spontaneous fermentation for centuries, we start our analysis of the literature by considering the global microflora (pathogenic or otherwise) of raw camel milk.
3.1. Microflora of Raw Camel Milk
3.1.1. Nonpathogenic Microflora in Raw Milk
The normal microflora in camel milk has been widely investigated, and the results testify to high diversity across countries. In Tunisia, [76] found in raw camel milk 2.7 × 102 CFU/mL of total mesophilic aerobic bacteria, 1.7 CFU/mL × 102 of yeasts/molds, and 1.3 × 103 CFU/mL of lactic acid bacteria (LAB). In total, 60 LAB strains were isolated. In camel milk samples from Morocco [77,78], 120 bacterial strains were isolated and identified as LAB by morphological and biochemical characterizations, as well as 16S ribosomal RNA (rRNA) gene amplicon sequencing. Among the described strains, some having probiotic properties were detected. Some of those strains were isolated in raw camel milk [79]. They belong to the genus Lactobacillus (L. acidophilus, L. rhamnosus, L. gasseri, and L. delbrueckii) [80]. Other strains belonging to the same genus (L. plantarum, L. pentosus, and Lactococcus lactis) were reported by Yateem et al. [81]. The diversity of microflora in camel milk also includes yeast as observed in Algeria where 12 species, dominated by Trichosporon asahii, Pichia fermentans, and Rhodotorula mucilaginosa, were identified [82].
Some strains isolated from raw camel milk such as Leuconostoc mesenteroides have shown specific antimicrobial activity against Listeria [83]. A similar finding was observed with Enterococcus faecium [84]. Abo-Amer [85] described the ability of Lactobacillus acidophilus strains isolated from raw camel milk to produce substances with antimicrobial activities. In a study achieved in Iran, among 64 LAB strains, 11 (belonging to genus Enterococcus, Lactobacillus, or Pediococcus) presented significant antibacterial activity against Staphylococcus aureus subsp. aureus or Bacillus cereus [86]. In Kenya, the predominant strains belonged to species E. faecalis, S. agalactiae, Weisselia confuse, Rhodotorula mucilanginosa, Cryptococcus albidus, and Candida lusitaniae [87,88]. In Kazakhstan, natural microflora including lactic bacteria (E. durans, E. faecalis, E. faecium, Lb. casei, Lb. curvatus, Lb. kefiri, Lb. paracasei, Lb. sakei, Lc. lactis, and Lc. mesenteroides) and yeasts/molds (Kazakhstania unispora, Saccharomyces cerervisiae, and Kluyvermyces marxianus) were identified in dromedary and Bactrian milks [89,90]. In Bactrian milk collected in China [91], 72 strains of lactic bacteria were isolated including Lb. paracasei, E. italicus, E. durans, Lc. Lactis, W. confuse, and E. faecium.
Such diversity has high technological interest, with the microflora playing important role both in terms of antimicrobial activity as emphasized above and in terms of acidification, which is essential for fermented products and cheese processing. However, due to the antimicrobial properties of camel milk proteins being greater than those in cow milk [92] and, in some cases, due to the low hygienic status of camel milk samples, the acidification process appears to be slower than for cow milk [93]. The starters used in camel milk processing for fermentation or cheesemaking (mesophilic, thermophilic, or their mixture) led to an acidification rate at 37 °C between 33% and 79% lower than for cow milk [88].
3.1.2. Pathogenic Microflora of Raw Camel Milk
Substantial data have been dedicated to a preliminary bacteriological description of raw camel milk using different methods from all camel countries. Sela et al. [71] previously reported that camel milk could contain E. coli if elementary rules of hygiene were not applied. Camel milk could also contain other pathogenic strains such as Streptococcus or Staphylococcus species [94]. According to the latest study in Algeria, 58% of commercialized samples of raw camel milk were of satisfactory quality, 8.33% were acceptable, and 33.3% were unacceptable. However, in their samples, they did not find any Salmonella sp. and Shigella sp. Another study achieved in Sudan reported the presence of E. coli, Klebsiella spp., Pseudomonas spp., Proteus spp., Enterococcus spp., Micrococcus spp., Streptococcus spp., and Staphylococcus spp. [95]. The presence of coliforms can be observed despite the concurrent development of lactic acid bacteria. In Tunisia for example, a study on raw camel milk focused on the numeration of mesophilic count, total LAB, and coliforms, reporting values of 7 × 103, 1.37 × 102, and 1.8 × 101 CFU/mL, respectively [96]. Therefore, it is important to check all raw camel milk samples for microbiological quality before processing. Indeed, when significant changes in camel milk composition are observed during the process, these could be linked to the microbiological status of the raw samples. The lack of systematic checks of microbiological quality in initial raw milk could impact all processing steps.
3.2. Diversity of Fermented Camel Milk
The microflora biodiversity leads to a rich diversity of fermented beverages prepared from camel milk. Moreover, fermentation is one of the oldest methods of consuming camel milk. Camel milk producers living in different regions of the world have their own varieties of fermented products with specific taste, texture, and flavor. Each camel country has described their traditional fermented milk in terms of microbiological, physicochemical, and chemical properties, as well as volatile organic compound profiles in some cases. The most known fermented camel milk products described in the literature are shubat in Kazakhstan [97] and China [98], khoormog in Mongolia [99], garris in Sudan [100,101], suusac in Kenya [102], laben (lben) in Arabic countries [103], and ititu and dhanaan in Ethiopia [104,105] (Table 2). Other traditional fermented beverages based on a mixture of camel milk and water are available in Mauritania known as zrig [106], in Morocco known as Lfrik [107], and in Iran and Turkmenistan known as chal [108].
Table 2.
Some main characteristics of fermented camel milk from different countries.
| Product | References | TVM | LAB | YM | Coli. | pH |
|---|---|---|---|---|---|---|
| In log10 CFU/mL | ||||||
| Suusac | Lore et al. [109] | 9.03 | 6.77 | 2.05 | 1.00 | 6.0–4.25 |
| Jans et al. [110] | ND | 7.2–8.5 | ND | ND | 4.9 | |
| Garris | Hassan et al. [35] | ND | ND | ND | ND | 6.2–3.8 |
| Adelgadir et al. [111] | 7.11–8.36 | 7.34–8.66 | 6.05–7.79 | ND | 3.79–4.32 | |
| Shubat | Rahman et al. [98] | ND | 6.8–7.6 | 4.3–4.7 | ND | ND |
TVM: total viable microorganisms; LAB: lactic bacteria; YM: yeasts and molds; Coli: coliforms; CFU: colony-forming units; ND: not determined.
However, in most cases, the fermentation process occurs spontaneously upon using previously fermented milk to inoculate raw milk [112]. The microflora in the fermented product is consequently more diversified than that in the raw milk samples [113]. For example, traditional suusac (Kenyan fermented camel milk) contains 45 LAB and three yeasts as identified by API50CHL and API20AUX. LABs were mainly represented by Lactobacillus curvatus, Lb. plantarum, Lb. salivarius, Lactococcus raffinolactis, and Leuconostoc mesenteroides subsp. mesenteroides, and yeasts were mainly represented by Candida krusei, Geotrichum penicillatum, and Rhodotorula mucilaginosa [111]. Other strains such as E. faecalis, Lb. fermentum, Lc. lactis, Cryptococcus laurentii, Candida lusitaniae, Saccharomyces cerevisiae, Trichosporon mucoides, and T. cutaneum were also identified more recently [88]. Traditional garris from Sudan contains the following LAB based on the API39CHL identification system: Lactobacillus animalis, Lb. brevis, Lb. divergens, Lb. plantarum, Lb. rhamnosus, Lb. gasseri, Lb. paracasei, Lb. fermentum, Lactococcus raffinolactis, and Lc. alimentarium [114].
Such a complex microflora ecosystem in fermented milk could lead to very variable final products, hardly compatible with obtaining a product with standard organoleptic quality. For example, shubat, which is prepared mainly in Kazakhstan, Uzbekistan, Russia, the Xinjiang region of China, and the western part of Mongolia, is made by spontaneous fermentation, often leading to the production of gas and foam and sometimes resulting in a particularly acidic product, provoking some reluctance in urban consumers [115]. Moreover, spontaneous fermentation can be affected by the presence of pathogenic strains of E. coli because the initial pH is not sufficient to suppress their growth [116]. To solve these problems, it is convenient to use starter cultures, i.e., a preparation containing a limited number of identified live microbial strains (single or mixed) inoculated in raw milk. Such management of controlled fermentation can lead to the expected sensorial properties of the final product by contributing to flavor and texture adapted to the urban consumers’ taste. This also contributes to a standardized and safer product on the market. Unfortunately, despite the high biodiversity of camel milk microflora, starter cultures used in the camel industry are mainly taken from bovine milk. In Kazakhstan, among the 104 isolates from shubat samples, sampled in different regions of the country, 79 were maintained in a pure culture (71 bacteria and 8 yeasts), while three of the strains were selected because of their biochemical properties (Lactobacillus fermentum К5, Lactobacillus fermentum К6, and Lactobacillus plantarum К7), before being tested for their growth kinetics and characteristics to measure their produced biomass [117,118]. This was important for developing an industrial culture in fermenters. Then, each culture was tested for technological suitability, acidity, bacterial colonization, and organoleptic properties [118]. The transfer to an industrial level was achieved thanks to the acquisition of a high-capacity bioreactor, wherein the media used were selected based on optimal bacterial growth and a convenient ratio of cost/growth potential. The final product (packaged starters in powder after lyophilization) was commercialized in two volumes (5 and 10 g per pack). However, industrial transfer remains difficult and requires supplementary studies regarding the technological properties of the numerous strains available in natural fermented milk [119]. In addition to the commercial interest for the development of fermented milk with standardized organoleptic properties, there is the public health benefit of such products thanks to their potential probiotic effect. For example, camel milk inoculated with LAB strain Lactococcus lactis KX881782 isolated from raw camel milk showed higher α-glucosidase inhibition (antidiabetic effect), antioxidant activity, angiotensin-converting enzyme inhibition (antihypertensive effect), and antiproliferative activity (anticancer effect) than cow milk [120].
4. Camel Cheese
Technical innovations regarding fermented camel milk have been applied to beverages known from prehistoric times to extend their shelf-life [121]. Furthermore, the making of camel cheese itself was an innovation. Indeed, the difference in casein proportions (notably the lower content of κ-casein) between cow and camel milk should explain the clotting difficulties observed in this process: 3–4% κ-casein vs. 13–15% in cow milk [122]. Moreover, bovine chymosin used in the dairy industry does not allow the optimal clotting of casein micelles from camel milk, leading to a weak curd. Thus, obtaining a firm coagulum was the first challenge faced by camel scientists and dairy factories processing camel milk [123].
4.1. The Challenge of Coagulation
The first trials were achieved in the 1980s using bovine rennet enriched in chloride and calcium phosphate with Rhizomucor miehei as coagulant (commercialized under the name of Camifloc®); however, the coagulum remained fragile and brittle [124]. Different vegetal coagulants were also tested as extracts from Zingiber officinale [125] or from Moringa oleifera [126], as well as abomasum extracts from young or adult camel [127]. The solution to getting a firm curd followed the sequencing of camel chymosin achieved by Kappeler et al. [122], which allowed introducing the coding gene for camel chymosin into a mold (Aspergillus niger). Later, this recombinant enzyme was produced by Chr. Hansen© at an industrial scale and marketed from 2008 under the trade name Chymax-M1000®. However, despite a good curd being a necessary condition, it did not guarantee a “good” cheese adapted to the consumers’ preference in terms of taste, especially because camel cheese was often generated by scientists in a laboratory rather than cheese technicians in dairy plants, except in Mauritania [128].
With great variety being possible in cheese, many trials are necessary to propose a large panel of products to the consumers. Different cheeses based on the technology for making gruyere [129], mozzarella [130], or feta and halloumi [131] were tested, but the texture, taste, and flavor of the final product did not correspond to the bovine equivalent. Indeed, the behavior of the camel’s “proteinic–lipidic matrix” during cheese processing differs from cattle milk. Such discrepancies among milk from different dairy species require more fundamental investigations of rheological properties to understand the changes during the different steps of acidification, coagulation, draining, brining, and refining, as well as the effect of various starters and thermal treatments [123].
4.2. Rheological and Microstructural Studies
As camel cheesemaking is a recent achievement all over the world, basic studies on the rheology or microstructure of the final products remain scarce. Investigations on curd tension and syneresis were achieved on soft camel cheese [132]. Gel firmness and gelation properties were tested according to different levels of temperature and pH [133]. The parameters of cheese viscosity (storage and loss moduli, loss tangent) after camel milk coagulation by camel chymosin were also reported [134]. To the best of our knowledge, while several studies on texture measurements (hardness, adhesiveness) have been published (for example, [135]), the microstructure properties of camel cheese require more investigations. In Iran, the effects of different mixtures of coagulants (Rhizomucor miehei protease and camel chymosin) on the microstructure and rheological properties of white cheese were compared, reporting a more compact protein network and firmer structure in the cheeses made with camel chymosin, but they used cow milk as a model [136]. In other studies, a comparison of the viscoelastic structure and the global gelation properties at different temperatures between cow and camel milk showed the higher difficulties of getting cheese with pasteurized camel milk [137].
However, only studies achieved at a laboratory scale are available. True investigations at the industrial scale are lacking. Moreover, in the published literature regarding the physicochemical and rheological characteristics of camel cheese, the microflora (pathogenic or otherwise) was not systematically considered.
4.3. Comparative “Behavior” with Cow Cheese
A comparison of the changes observed in the “proteinic–lipidic matrix” of camel and cow milk during cheese processing is a common feature in scientific literature to understand the specificity of their respective “behavior”. However, this comparison is of low interest when the gross composition of each specific milk differs significantly. To avoid this, Konuspayeva et al. [131] adjusted cow milk to obtain the same fat and total protein concentrations. Although they got similar cheese raw yields (7.4 ± 0.15 vs. 7.3 ± 0.55 kg/100 kg for camel and cow milk, respectively) and calcium recovery, camel cheese presented a higher recovery in terms of total nitrogen, while cow cheese retained more fat. Significant differences were also observed in lactoserum composition: camel lactoserum contained more fat and total nitrogen (9.0 ± 1.73 g/kg and 9.21 ± 0.23 g/kg, respectively) compared to cow (7.7 ± 1.61 g/kg and 7.30 ± 0.02 g/kg, respectively) despite similar dry matter (68.9% ± 3.2% and 68.1% ± 1.15% in camel and cow whey, respectively).
Two main technological difficulties are faced in camel cheese processing: (i) the continuous removal of serum from curd, and (ii) the slow acidification of the curd. Indeed, contrary to cow cheese where ripening is started after curdling with the rapid appearance of crust formation, camel cheese is characterized by a weak crusting and a continuous loss of moisture, due to serum release, leading often to a very dry curd which hinders correct ripening. Such behavior can be favorable for some types of cheese (such as feta), but not for hard cheeses [123]. This slow acidification was first reported by Farah and Bachmann [137], who found that 10 h was necessary to decrease the pH from 6.6 to 5. Such a delay can be reduced at 36 °C compared to 20 °C [130]. Thermophilic starters such as Lactobacillus helveticus, L. lactis, or Streptococcus thermophilus, known for their high acidifying power, can also be used to improve the acidification process [131]. However, for the management of fermentation to get specific fermented products, camel cheese manufacturing does not use starters made with lactic bacteria isolated from camel milk. The challenge for scientists and cheesemakers could be the identification of LAB strains specific to camel milk, allowing the potential to provide the typical aroma of cheese and to enrich the variety of camel cheese proposed to consumers.
Investigations on the interactions between minerals and camel cheese processing are also lacking. It has been established that there was no effect of the addition of phosphate or calcium chloride on improving gelation by camel chymosin [130] contrary to cow milk [138]. There is also a lack of information regarding camel cheese in terms of the analysis of volatile organic compounds responsible for aroma which supporting the typicity of the cheeses. Currently, such an analytical approach has only been developed for cow cheese [139].
4.4. The Challenge of Industrial Development
Up to now, it seems that scientists and cheesemakers aim to make cheese by using the same methodology as for cow cheese. However, when feta- or mozzarella-type cheeses are prepared using camel milk, the results are often disappointing because the taste, texture, and consistency differ with respect to the same cheese made using cow milk. In the Middle East, where consumers prefer products with a neutral taste, it is difficult to expect the development of cheeses with a strong character as found in western Europe. To achieve products adapted to the local consumers, different trials were proposed, for example, cheese spread [140] or white soft cheese [141,142]. However, few cheeses have given rise to sensory analyses assessing the acceptability of the product by the local consumers [131]. Yet, such research would be useful for the dairy industry to develop convenient cheeses. To the best of our knowledge, few industrial initiatives have seen the light of day [128].
The industrial development of camel cheesemaking is limited not only by the technological difficulties, but also by the hygienic quality of raw milk as mentioned above, notably because it is difficult to coagulate camel milk after pasteurization, forcing manufacturers to work with raw milk. The use of halloumi-type cheese technology [131] is an interesting alternative because the coagulum is pasteurized in lactoserum for 10 min at 80 °C after pressing. A second difficulty is linked to the cost of camel cheese due to the high price of the primary matter [143]. An alternative could be the valorization of lactoserum, which represents 88% to 90% of the initial milk volume.
5. Camel Milk Powder
As outlined in Section 1, camel milk is a recent development on the international dairy market, made possible by the development of powder milk production, which is the best way to preserve this highly perishable product for later consumption. Moreover, camel milk is often produced in remote places far away from the consumption basin, whereby the only solution to transport a high quantity of milk is by removing the water it contains (88% to 90% of the weight). Another advantage of this process is the conservation of the nutritive value of liquid milk. To make camel milk powder, two main modern technologies are used: spray-drying (hot-drying) and lyophilization (freeze-drying).
5.1. The Drying Technologies
The first reported trial aimed at making camel milk powder was recent, where freeze-drying technology was implemented to study the thermal characteristics of camel milk and its main components [7]. However, these tests were carried out in a laboratory (not at an industrial scale) with a freeze-dryer, allowing drying from −40 to 20 °C with a vacuum of 100 Pa. The resulting powder was stabilized at 11.3% humidity. A second, more recent study [144] pursued similar objectives, namely, to assess the effect of freeze-drying on the nutritional properties of camel milk, i.e., how the procedure affects the fine composition of camel milk in comparison to fresh milk. Analyses indicated a relative stability of most components (including minerals and vitamins) and concluded that the nutritional properties of camel milk powder were maintained. However, this was also achieved using laboratory equipment with limited capabilities. Moreover, nothing was said about the solubility of the powder obtained.
The spray-drying process was also the subject of a few scientific publications. In a study comparing the physical properties of powdered camel and cow milk obtained by spraying, [145] used a two-step process; milk was first concentrated to 20–30% dry matter using a rotating evaporator at 80 °C and then passed through a sprayer. The equipment used (FT80/81 Tall Form Spray Dryer) allows treating small amounts with the same effects of an industrial sprayer. In their protocol, the drying conditions were as follows: air intake temperature of 200–220 °C, air outlet temperature between 98 and 105 °C, pump speed at 3–5 arbitrary units, and air outlet humidity between 1.2% and 5.8%. In their conclusion, the authors indicated that this process allows obtaining powder with less than 1.8% water, thus allowing a long shelf-life. The drying temperature should be well controlled, as too high temperature results in an increase in the insolubility index due to protein denaturation. Overall, the solubility of camel milk powder is lower than that of cow milk. Another criterion used by manufacturers to assess the quality of milk powder is fluidity. This is the ratio between the density of the untamped powder and its density when compacted. This fluidity appears to be lower for camel milk compared to cow milk but remains at a fairly good level [145]. In a recent study regarding the acid gelation of fresh and powdered camel milk acid [146], the dry-spraying process was achieved using a laboratory sprayer (Buchi B-290), allowing an air entry temperature of 190 °C and an output temperature of 90 °C with an input flow of 600 mL/h. In their publication, [147] also aimed to test the effect of spray-drying, i.e., how the temperature of the air intake (160, 140 and 120 °C), atomization pressure (800, 600, and 400 bars), and feeding flow (5.4 and 3 arbitrary units/s) affect the nutritional components of camel milk (vitamin C, fatty-acid profiles). The equipment used was the same mini-laboratory sprayer cited in previous publications. Powder yield increased with the highest air intake temperatures and the lowest feed flows. High temperatures and high spray pressures reduced vitamin C levels. Lastly, the atomization pressure increased the fatty-acid content.
5.2. Interests and Limitations of the Technologies Used for Spraying Camel Milk
The spray-drying method seems preferable to make camel milk powder for a better reconstitution of liquid milk, but the investment for the dairy industry is more important as it requires the procurement of a costly milk drying tower and sprayer. However, the powder obtained by freeze-drying (lyophilization) could be used by agro-food industries (pastry and chocolate factories).
Nevertheless, the main limit is that drying consumes a high level of energy. In the dairy industry, the spray-drying process has a higher energy demand per ton of end-product, despite the recent technical improvements and novel equipment decreasing the energy consumption per ton of finished product. Moreover, the high investment necessary for obtaining and using a drying tower to make milk powder requires a sufficient volume of raw matter, which is only possible in certain contexts such as large camel dairy farms or collecting centers with a large network of camel farms.
Lastly, it appears that trials on camel milk (in any case, those that are published) are limited in number and that industrial trials are poorly documented. It is also apparent that the spraying of camel milk requires an optimization of the input parameters (temperature, pressure, and flow) to maintain the nutritional properties of the product and the functional characteristics (solubility, hygroscopy, and fluidity) of the powder. As camel milk studies were all carried out using materials dealing with small quantities, the “translation” of these results to an industrial scale involving large volumes is not possible, although the practice of industrial hot spraying of camel milk is implemented in the United Arab Emirates, China, and Europe.
5.3. The Challenge for Camel Milk Powder Development
The main problem with camel milk during high-temperature heat treatment, as happens during spraying, is the denaturation of proteins (especially whey proteins), which explains the difficulty with obtaining UHT milk. To maintain powder milk in the best possible conditions, and to facilitate the solubility of the powder to replenish the liquid milk, the surface composition of the powder is essential [148]. This surface of the spray-dried emulsion is naturally composed of mostly fat (mostly triglycerides), in addition to some proteins. Thus, the denaturation of serum proteins at a high temperature increases the fatty surface content of the powder and makes it difficult to replenish liquid milk. For a better emulsion during this reconstruction, it is proposed to perform an “encapsulation” using sodium caseinates, thereby ensuring stability of the powder. Such encapsulation is improved by the presence of lactose. For example, surface fat decreases from 30% to less than 5% if lactose is present in a 1:1 ratio relative to sodium caseinate.
In Table 3, possible improvements are reported to obtain a powder of stable quality due to the specificities of camel milk.
Table 3.
Possible improvements to obtain camel milk powder with high quality.
| Process | Activity | Particularities | Comments |
|---|---|---|---|
| Raw camel milk (raw material) | Limit the number of suppliers or implement collecting centers with quality control | Low bacterial load (ideally <100 CFU/mL coliforms) Titratable acidity <16° Dornic | Without respect for hygiene, the milk may clot during powder processing and cause harmful fouling of the equipment |
| Concentration to remove some of the water (on average, there is 88% water in camel milk) | Remove at least 30% of the water using the principle of a “pressure cooker”, which is less expensive in terms of energy, saving atomization time and improving the quality of the final product | If hygienic standards are insufficient, risk of clotting | Clotted products are difficult to reuse, not only for the reconstruction of liquid milk, but also for the transformation into other products (cheese, yoghurt, fermented milk) |
| Homogenization to better emulsify | Make smaller fat and casein micelles to obtain a good-quality emulsion | Optional for better powder quality, a stabilizer can be added to the emulsion (caseinates), and lactose can possibly be removed to obtain an optimal 1:1 ratio of caseinates/lactose | This noncompulsory phase would result in a powder of better physical quality (solubility, fluidity, and low hygroscopic capacity) and stability over a longer period (preservation time) |
| Spray-drying | Optimize temperature (inlet/outlet), atomization pressure, and input flow to minimize energy costs and preserve the nutritional properties of camel milk | If hygienic standards are insufficient, the risk of appearance of poorly dried agglomerates will impact the commercial quality of the product | Data are available from suppliers |
| Packaging of camel milk powder | To preserve the quality of the finished product, it is advisable to use automated bagging | Working in a sterile atmosphere under strictly controlled humidity | This is an important step because any anomaly can involve all the previous steps (critical point in a Hazard Analysis Critical Control Point (HACCP) approach) Access to this part of the chain should be limited |
6. Other Products
6.1. Yogurt
Ample literature is available on the possibility of making yoghurt with camel milk [149,150]. Several strains of conventional lactic bacteria have been tested such as Lactobacillus bulgaricus or Streptococcus thermophilus [151], as well as L. acidophilus, L. casei, and Bifidobacteria [152].
However, the manufacture of camel milk yoghurt poses a texture problem, with the product appearing sticky and ultimately unpleasant to the palate [153]. Indeed, the viscosity of the product does not change during the gelling process compared to the milk of other dairy species. This constraint is related to protein composition [154] and to antibacterial factors naturally present in camel milk [155]. Another reason could be linked to the foaming properties of camel milk. The foam in this milk is stable, but it leads to a weak structure of the gel, which becomes unstable [156].
To obtain a better texture, trials with the addition of gelatin, alginate, or calcium were attempted [157], whereas ferments producing exo-polysaccharides were also used [158]. The application of a high-pressure treatment could have a positive effect on the texture, but no trials have been conducted to date with camel milk [159].
Other authors have attempted to improve the manufacture of camel milk yoghurt by mixing it with milk from other species [160] or by introducing 0.75% biosynthesized xanthan, albeit with moderate results in terms of organoleptic properties [161]. In any case, the final product corresponds at best to a “drinking yoghurt” without having the taste qualities, even when natural or synthetic aromas are added [162]. These difficulties explain why there is limited industrial production of camel milk yoghurt at present. Some researchers proposed frozen yogurt as a product that is between yogurt and ice cream [163]. The optimal composition from a texture point of view would be allowed with several ingredients such as fat (5%), sugar (13%), gelatin (0.5%), and 14% banana [164]; however, such a proposal has never gone beyond a laboratory scale.
6.2. Butter and Sweet
The fat in camel milk contains less than 0.5% butyric acid [165] compared to almost 5% in cow milk. In addition, fat cells are smaller than in cow milk [166]. As a result, butter yield is low [167] with disappointing organoleptic properties [167,168]. To obtain fat cells at the time of butter production, it is necessary to implement vigorous hot shaking (22–23 °C), which allows recovering about 80% of the fat [168]. Ghee (clarified butter), a popular product in India, has also been attempted using camel milk [169]; however, in addition to very low yield compared to buffalo or cow milk, the final product was found to be more susceptible to rancidity. Transformation into butter, therefore, does not seem fundamentally interesting in the context of an industrial valuation of camel milk. In fact, apart from trials in Ethiopia where butter consumption, including rancid butter for certain recipes, is popular, the production of camel butter has little future.
Making ice creams with different flavors is an easy technology. Ice cream made from camel milk is commercialized in the United Arab Emirates, Morocco, and Kazakhstan. The same technology is used as for other milks. Ice cream is highly popular among consumers and, above all, provokes less reluctance than other products. However, very few studies on the texture and sensory properties have been conducted [170].
There is no reference for processing camel milk into sweets. However, traditional products are available. For example, in Kazakhstan, a caramel called Balkailmak is obtained after a long thermal treatment of about 10 h at boiling temperature. The introduction of milk powder to chocolate as proposed in the Emirates can also be mentioned. A study on the acceptance of camel milk in a panel including 470 Emirati students showed a higher score with chocolate-flavored milk [171].
6.3. Non-Alimentary Processing of Camel-Milk
The manufacture of soaps and other cosmetic creams with camel milk is now common practice in many countries (Morocco, Mauritania, Saudi Arabia, India, Holland, China, Australia, etc.), whether on a semi-industrial scale or on a handicraft scale. China sells cabinets containing various cosmetic products from lipsticks to moisturizers, shampoos, and various lotions. The interest in the use of camel milk for the cosmetic industry benefits from the hypoallergenic properties of its proteins [172].
7. Conclusions
The “modernized” processing of camel milk is a recent feature compared to the milk from other dairy species. However, the technologies used to transform milk into pasteurized or fermented products, cheese or yoghurt, powder, or various sweets face two main challenges: (i) the systematic application of already proven technologies for cow milk is not necessarily suitable for camel milk and requires adaptations based on more fundamental research on the behavior of milk components during processing; (ii) the transfer of laboratory results already relatively numerous to an industrial scale remains insufficient, especially for products such as cheese or yoghurt, and it requires additional technical and economic analyses. The worldwide interest in camel milk, which is largely due to its expected health effect for consumers, is prompting basic research and development to continue investigations in order to translate technological innovations into products available on a large scale.
Despite these technological constraints, the global camel milk market is strongly changing. These changes are visible through two main structural innovations: (i) the emergence of intensive production systems under an entrepreneurial approach appearing more or less disconnected from pastoral dynamics; (ii) the development of periurban camel production systems, often initiated by pastoral breeders, contributing significantly to the urban supply in camel milk and keeping significant relationships with the pastoral economy. Such trends emphasize that the technical innovations in camel milk processing are coupled with socioeconomic dimensions. However, such changes raise the question of the sustainability of these new methods of producing milk and their environmental impact for the planet. Obviously, they contribute to a revaluation of the place of camel in national livestock economies, and it requires ensuring (i) the establishment of an international standard recognized by all the stakeholders, and (ii) the sustainability of “modernized” production and processing systems by avoiding the shortcomings noted in other modern livestock sectors.
Author Contributions
Bibliographical research and writing, G.K. and B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was achieved in the framework of MAK’IT call (Montpellier Advanced Knowledge Institute on Transitions) and was funded by the PRIMA program under grant agreement No1832, project “Boost the production, processing, and consumption of camel milk in the Mediterranean basin (CAMELMILK)”. The PRIMA program is supported by the European Union.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marsh A.J., Hill C., Paul Ross R., Cotter P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014:113–124. doi: 10.1016/j.tifs.2014.05.002. [DOI] [Google Scholar]
- 2.Faye B. The camel: New challenges for a sustainable development. Trop. Anim. Health Prod. 2016;48:689–692. doi: 10.1007/s11250-016-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-haj O.A., Kanhal H.A. Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J. 2010;20:811–821. doi: 10.1016/j.idairyj.2010.04.003. [DOI] [Google Scholar]
- 4.Konuspayeva G., Faye B. New Opportunities in the Camel Milk Valorization. In: Sghiri A., Kichou F., editors. Proceedings of the 5th Conference ISOCARD Recent Advances in Camelids Biology, Health and Production; Laâyoune, Morocco. 12–15 November 2018; Rabat, Morocco: Pub. IAV Hassan II; 2018. pp. 45–50. [Google Scholar]
- 5.Abdelgader A.G., Alhaider A.A. The unique medicinal properties of camel products: A review of the scientific evidence. J. Taibah Univ. Med Sci. 2016;11:98e103. doi: 10.1016/j.jtumed.2015.12.007. [DOI] [Google Scholar]
- 6.FAOstat. [(accessed on 23 March 2021)];2021 Available online: http://www.fao.org/faostat/en/#data/QL.
- 7.Zhang B.Y., Xu S., Villalobos-Santeli J.A., Huang J.Y. Fouling characterization of camel milk with comparison to bovine milk. J. Food Eng. 2020;285:11008. doi: 10.1016/j.jfoodeng.2020.110085. [DOI] [Google Scholar]
- 8.Lajnaf R., Zouari A., Trigui I., Attia H., Ayadi M.A. Effect of different heating temperatures on foaming properties of camel milk proteins: A comparison with bovine milk proteins, Int. Dairy J. 2020;104:104643. doi: 10.1016/j.idairyj.2020.104643. [DOI] [Google Scholar]
- 9.Li R.R., Yue H.T., Shi Z.Y., Shen T., Yao H.B., Zhang J.W., Gao Y., Yang J. Protein profile of whole camel milk resulting from commercial thermal treatment. LWT. 2020;134:110256. doi: 10.1016/j.lwt.2020.110256. [DOI] [Google Scholar]
- 10.Yehia H.M., Al-Masoud A.H., Alarjani K.M., Alamri M.S. Prevalence of methicillin-resistant (mecA gene) and heat-resistant Staphylococcus aureus strains in pasteurized camel milk, J. Dairy Sci. 2020;103:5947–5963. doi: 10.3168/jds.2019-17631. [DOI] [PubMed] [Google Scholar]
- 11.Bragason E., Berhe T., Dashe D., Sørensen K.I., Eshetu Guya M., Hansen E.B. Antimicrobial activity of novel Lactococcus lactis strains against Salmonella Typhimurium DT12, Escherichia coli O157:H7 VT− and Klebsiella pneumoniae in raw and pasteurised camel milk. Int. Dairy J. 2020;111:104832. doi: 10.1016/j.idairyj.2020.104832. [DOI] [Google Scholar]
- 12.Gammoh S., Alu’datt M.H., Tranchant C.C., Al-U’datt D.G., Alhamad M.N., Rababah T., Kubow S., Haddadin M.S.Y., Ammari Z., Maghaydah S., et al. Modification of the functional and bioactive properties of camel milk casein and whey proteins by ultrasonication and fermentation with Lactobacillus delbrueckii subsp. Lactis. LWT. 2020;129:109501. doi: 10.1016/j.lwt.2020.109501. [DOI] [Google Scholar]
- 13.Sobti B., Aljneibi A.H.A., Seraidy H.A.A., Alnaqbi A.A.H., Al Zain B., Ramachandran T., Hamed F., Kamal-Eldin A. Short communication: The effect of pectin and sodium alginate on labans made from camel milk and bovine milk. J. Dairy Sci. :2021. doi: 10.3168/jds.2020-19220. [DOI] [PubMed] [Google Scholar]
- 14.Mortazavi S.M., Jalali H., Hamidreza Ziaolhagh S. Production of a probiotic camel milk enriched with pomegranate peel powder. Iran. Food Sci. Technol. Res. J. 2021;16:123–132. [Google Scholar]
- 15.Zhadyra S., Han X., Anapiyayev B.B., Tao F., Xu P. Bacterial diversity analysis in Kazakh fermented milks Shubat and Ayran by combining culture-dependent and culture-independent methods. LWT. 2021;141:110877. doi: 10.1016/j.lwt.2021.110877. [DOI] [Google Scholar]
- 16.Sharma A., Lavania M., Singh R., Lal B. Identification and probiotic potential of lactic acid bacteria from camel milk. Saudi J. Biol. Sci. 2021;28:1622–1632. doi: 10.1016/j.sjbs.2020.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayyash M., Abu-Jdayil B., Itsaranuwat P., Almazrouei N., Galiwango E., Esposito G., Hunashal Y., Hamed F., Najjar Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020;333:127418. doi: 10.1016/j.foodchem.2020.127418. [DOI] [PubMed] [Google Scholar]
- 18.Soleymanzadeh N., Mirdamadi S., Mirzaei M., Kianirad M. Novel β-casein derived antioxidant and ACE-inhibitory active peptide from camel milk fermented by Leuconostoc lactis PTCC1899: Identification and molecular docking. Int. Dairy J. 2019;97:201–208. doi: 10.1016/j.idairyj.2019.05.012. [DOI] [Google Scholar]
- 19.Edalati E., Saneei B., Alizadeh M., Hosseini S.S., Zahedi Bialvaei A., Taheri K. Isolation of probiotic bacteria from raw camel’s milk and their antagonistic effects on two bacteria causing food poisoning. New Microbes New Infect. 2019;27:64–68. doi: 10.1016/j.nmni.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbye M., Sobti B., Al Nuami M.K., Al Shamsi Y., Al Khateri L., Al Saedi R., Saeed M., Ramachandran T., Hamed F., Kamal-Eldin A. Physicochemical properties, sensory quality, and coagulation behavior of camel versus bovine milk soft unripened cheeses. NFS J. 2020;20:28–36. doi: 10.1016/j.nfs.2020.06.003. [DOI] [Google Scholar]
- 21.Belkheir B., Zadi-Karam H., Karam N.E., Carballo J., Centeno J.A. Effects of selected mesophilic Lactobacillus strains obtained from camel milk on the volatile and sensory profiles of a model short-ripened pressed cows’ milk cheese. Int. Dairy J. 2020;109:104738. doi: 10.1016/j.idairyj.2020.104738. [DOI] [Google Scholar]
- 22.El Hatmi H., Jrad Z., Mkadem W., Chahbani A., Oussaief O., Ben Zid M., Nouha M., Zaidi S., Khorchani S., Belguith K., et al. Fortification of soft cheese made from ultrafiltered dromedary milk with Allium roseum powder: Effects on textural, radical scavenging, phenolic profile and sensory characteristics. LWT. 2020;132:109885. doi: 10.1016/j.lwt.2020.109885. [DOI] [Google Scholar]
- 23.Perusko M., Ghnimi S., Simovic A., Stevanovic N., Radomirovic M., Gharsallaoui A., Smiljanic K., Van Haute S., Stanic-Vucinic D., Velickovic T.C. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT. 2021;143:111091. doi: 10.1016/j.lwt.2021.111091. [DOI] [Google Scholar]
- 24.Ho T.M., Chan S., Yago A.J.E., Shravya R., Bhandari B.R., Bansal N. Changes in physicochemical properties of spray-dried camel milk powder over accelerated storage. Food Chem. 2019;295:224–233. doi: 10.1016/j.foodchem.2019.05.122. [DOI] [PubMed] [Google Scholar]
- 25.Deshwal D.K., Kumar Singh A., Kumar D., Sharma H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT. 2020;134:110117. doi: 10.1016/j.lwt.2020.110117. [DOI] [Google Scholar]
- 26.Zouari A., Briard-Bion V., Schuck P., Gaucheron F., Delaplace G., Attia H., Ayadi M.A. Changes in physical and biochemical properties of spray dried camel and bovine milk powders. LWT. 2020;128:109437. doi: 10.1016/j.lwt.2020.109437. [DOI] [Google Scholar]
- 27.Zouari A., Schuck P., Gaucheron F., Triki M., Delaplace G., Gauzelin-Gaiani C., Lopez C., Attia H., Ayadi M.A. Microstructure and chemical composition of camel and cow milk powders’ surface. LWT. 2020;117:108693. doi: 10.1016/j.lwt.2019.108693. [DOI] [Google Scholar]
- 28.Kamal-Eldin A., Alhammadi A., Gharsallaoui A., Hamed F., Ghnimi S. Physicochemical, rheological, and micro-structural properties of yogurts produced from mixtures of camel and bovine milks. NFS J. 2020;19:26–33. doi: 10.1016/j.nfs.2020.05.001. [DOI] [Google Scholar]
- 29.Buchilina A., Aryana K. Physicochemical and microbiological characteristics of camel milk yogurt as influenced by monk fruit sweetener. J. Dairy Sci. 2021;104:1484–1493. doi: 10.3168/jds.2020-18842. [DOI] [PubMed] [Google Scholar]
- 30.Atwaa E.H., Hassan M.A.A., Ramadan M.F. Production of probiotic stirred yoghurt from camel milk and oat milk. J. Food Dairy Sci. Mansoura Univ. 2020;11:259–264. doi: 10.21608/jfds.2020.118366. [DOI] [Google Scholar]
- 31.Chen C., Wang P., Zhang N., Zhang W., Ren F. Improving the textural properties of camel milk acid gel by treatment with trisodium citrate and transglutaminase. LWT. 2019;103:53–59. doi: 10.1016/j.lwt.2018.12.063. [DOI] [Google Scholar]
- 32.Kashaninejad M., Razavi S.M.A. Influence of thermosonication treatment on the average size of fat globules, emulsion stability, rheological properties and color of camel milk cream. LWT. 2020;132:109852. doi: 10.1016/j.lwt.2020.109852. [DOI] [Google Scholar]
- 33.Rahman M.S., Al-Hakmani H., Al-Alawi A., Al-Marhub I. Thermal characteristics of freeze-dried camel milk and its major components. Thermochim. Acta. 2012;549:116–123. doi: 10.1016/j.tca.2012.09.005. [DOI] [Google Scholar]
- 34.Alhaj O.A., Taufik E., Handa Y., Fukuda K., Saito T., Urashima T. Chemical characterization of oligosaccharides in commercially pasteurized dromedary camel (Camelus dromerarius) milk. Int. Dairy J. 2013;28:70–75. doi: 10.1016/j.idairyj.2012.08.008. [DOI] [Google Scholar]
- 35.Hassan R.A., El Zubeir I.E.M., Babiker S.A. Effect of pasteurization of raw camel milk and storage temperature on the chemical composition of fermented camel milk. Int. J. Dairy Sci. 2007;2:166–171. doi: 10.3923/ijds.2007.166.171. [DOI] [Google Scholar]
- 36.El Zubeir I.E.M., Marowa I. Effect of pasteurization of milk on the keeping quality of fermented camel milk (Gariss) in Sudan. Livest. Res. Rural Dev. 2009;21:1–8. [Google Scholar]
- 37.Rankin S.A., Christiansen A., Lee W., Banavara D.S., Lopez-Hernandez A. Invited review: The application of alkaline phosphatase assays for the validation of milk product pasteurization. J. Dairy Sci. 2010;93:5538–5551. doi: 10.3168/jds.2010-3400. [DOI] [PubMed] [Google Scholar]
- 38.Elagamy E.I. Effect of heat treatment on camel milk proteins with respect to antimicrobial factors: A comparison with cows’ and buffalo milk proteins. Food Chem. 2000;68:227–232. doi: 10.1016/S0308-8146(99)00199-5. [DOI] [Google Scholar]
- 39.Loiseau G., Faye B., Serikbayeva A., Montet D. Enzymes ability to serve as markers of pasteurized camel milk; Proceedings of the Conference on New Horizons in Biotechnology; Trivandrum, India. 18–21 April 2001. [Google Scholar]
- 40.Wernery U., Johnson B., George R.M. Gamma-glutamyl transferase (GGT), a potential marker for the evaluation of heat treatment of dromedary milk. J. Camel Pract. Res. 2007;14:9. [Google Scholar]
- 41.Lorenzen P.C., Wernery R., Johnson B., Jose S., Wernery U. Evaluation of indigenous enzyme activities in raw and pasteurized camel milk. Small Rumin. Res. 2011;97:79–82. doi: 10.1016/j.smallrumres.2011.01.014. [DOI] [Google Scholar]
- 42.Tayefi-Nasrabadi H., Hoseinpour-Fayzi M.A., Mohasseli M. Effect of heat treatment on lactoperoxidase activity in camel milk: A comparison with bovine lactoperoxidase. Small Rumin. Res. 2011;99:187–190. doi: 10.1016/j.smallrumres.2011.04.007. [DOI] [Google Scholar]
- 43.Kherouatou N., Nasri M., Attia H. A study of the dromedary milk casein micelle and its changes during acidification. Braz. J. Food Technol. 2003;6:237–244. [Google Scholar]
- 44.Lund A.K., Shah A.H., Jatoi A.S., Khaskheli G.B., Khaskheli M.C., Malhi A.A., Kalwar M.A., Khanzada Q. Effect of heating on shelf life and sensory characteristics of camel milk. Pure Appl. Biol. 2020;9:74–79. doi: 10.19045/bspab.2020.90009. [DOI] [Google Scholar]
- 45.Felfoul I., Lopez C., Gaucheron F., Attia H., Ayadi M.A. Fouling behavior of camel and cow milks under different heat treatments. Food Bioprocess Technol. 2015;8:1771–1778. doi: 10.1007/s11947-015-1529-5. [DOI] [Google Scholar]
- 46.Benabdelkamel H., Masood A., Alanazi I.O., Alzahrani D.A., Alrabiah D.K., AlYahya S.A., Alfadda A.A. Proteomic profiling comparing the effects of different heat treatments on camel (Camelus dromedarius) milk whey proteins. Int. J. Mol. Sci. 2017;18:721. doi: 10.3390/ijms18040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barthe L. La composition du lait de chamelle. J. Pharm. Chim. 1905;21:386–388. [Google Scholar]
- 48.Farah Z. Effect of heat treatment on whey proteins of camel milk. Milchwissenschaft. 1986;41:763–765. [Google Scholar]
- 49.Farah Z., Atkins D. Heat coagulation of camel milk. J. Dairy Res. 1992;59:229–231. doi: 10.1017/S002202990003048X. [DOI] [Google Scholar]
- 50.Farah Z., Ruegg M.W. The size distribution of casein micelles in camel milk. Food Microstruct. 1989;52:211–216. [Google Scholar]
- 51.Attia H., Kherouatou N., Nasri M., Khorchani T. Characterization of the dromedary milk casein micelle and study of its changes during acidification. Lait. 2000;80:503–515. doi: 10.1051/lait:2000141. [DOI] [Google Scholar]
- 52.Hattem H.E., Manal A.N., Hanna S.S., Elham A.A. A study on the effect of thermal treatment on composition and some properties of camel milk. J. Brew. Distill. 2011;2:51–55. [Google Scholar]
- 53.Saleh S.K., Mira E.K.I., Marzouk N.M., Abu-Zied M.A. Effect of pasteurization on some chemical and bacteriological parameters on camel milk. Bull. Natl. Res. Cent. 2008;33:287–297. [Google Scholar]
- 54.Beg O.U., von Bahr-Lindstrom H., Zaisi Z.H., Jornvall H. Characterization of a heterogeneous camel milk whey non-casein protein. FEBS Lett. 1987;216:270–274. doi: 10.1016/0014-5793(87)80704-4. [DOI] [PubMed] [Google Scholar]
- 55.Momen S., Salami M., Alavi F., Emam-Djomeh Z., Moosavi-Movahedi A.A. The techno-functional properties of camel whey protein compared to bovine whey protein for fabrication a model high protein emulsion. LWT. 2019;101:543–551. doi: 10.1016/j.lwt.2018.11.063. [DOI] [Google Scholar]
- 56.Merin U., Bernstein S., Bloch-Damti A., Yagil R., van Creveld C., Lindner P., Gollop N. A comparative study of milk serum proteins in camel (Camelus dromedaries) and bovine colostrums. Livest. Prod. Sci. 2001;67:297–301. doi: 10.1016/S0301-6226(00)00198-6. [DOI] [Google Scholar]
- 57.Ryskaliyeva A., Henry C., Faye B., Miranda G., Konuspayeva G., Martin P. Combining different proteomic approaches to resolve complexity of the milk protein fraction of dromedary, Bactrian and hybrids from different regions of Kazakhstan. PLoS ONE. 2018;13:e0197026. doi: 10.1371/journal.pone.0197026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Genene A., Hansen E.B., Eshetu M., Hailu Y., Ipsen R. Effect of heat treatment on denaturation of whey protein and resultant rennetability of camel milk. LWT. 2019;101:404–409. doi: 10.1016/j.lwt.2018.11.047. [DOI] [Google Scholar]
- 59.Zhang H., Yao J., Zhao D., Liu H., Li J., Guo M. Changes in chemical composition of Alxa Bactrian camel milk during lactation. J. Dairy Sci. 2005;88:3402–3410. doi: 10.3168/jds.S0022-0302(05)73024-1. [DOI] [PubMed] [Google Scholar]
- 60.El-Hatmi H., Girardet J.M., Gaillard J.L., Yahyaoui M.H., Attia H. Characterization of whey proteins of camel (Camelus dromedaries) milk and colostrums. Small Rumin. Res. 2007;70:267–271. doi: 10.1016/j.smallrumres.2006.04.001. [DOI] [Google Scholar]
- 61.Konuspayeva G., Faye B., Loiseau G., Levieux D. Lactoferrin and Immunoglobulin content in camel milk (C. bactrianus, C. dromedarius and hybrids) from Kazakhstan. J. Dairy Sci. 2007;90:38–46. doi: 10.3168/jds.S0022-0302(07)72606-1. [DOI] [PubMed] [Google Scholar]
- 62.Levieux D., Levieux A., El-Hatmi H., Rigaudiere J.P. Immunochemical quantification of heat denaturation of camel (Camelus dromedaries) whey protein. J. Dairy Res. 2006;73:421–430. doi: 10.1017/S0022029905001391. [DOI] [PubMed] [Google Scholar]
- 63.Konuspayeva G. Camel milk composition and nutritional value. In: AlHaj O., Faye B., Agrawal R.D., editors. Health and Environmental Benefits of Camel Products. IGI Global; Delhi, PA, USA: Hershey; Delhi, PA, USA: 2020. pp. 363–378. [Google Scholar]
- 64.Ryskaliyeva A., Henry C., Miranda G., Faye B., Konuspayeva G., Martin P. The main WAP isoform usually found in camel milk arises from the usage of an improbable intron cryptic splice site in the precursor to mRNA in which a GC-AG intron occurs. BMC Genet. 2019;20:14. doi: 10.1186/s12863-018-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karray N., Lopez C., Lesieur P., Ollivon M. Dromedary milk fat, thermal and structural properties 1. Crystalline forms obtained by slow cooling. Lait. 2004;80:399–416. doi: 10.1051/lait:2004014. [DOI] [Google Scholar]
- 66.Laleye L.C., Jobe B., Wasesa A.A.H. Comparative study on heat stability and functionality of camel and bovine milk whey proteins. J. Dairy Sci. 2008;91:4527–4534. doi: 10.3168/jds.2008-1446. [DOI] [PubMed] [Google Scholar]
- 67.Mehaia M.A. Vitamin C and riboflavin content in camels’ milk, effects of heat treatments. Food Chem. 1994;50:153–155. doi: 10.1016/0308-8146(94)90113-9. [DOI] [Google Scholar]
- 68.Alhaj O.A., Metwalli A.M., Elsayed A. Heat stability of camel milk proteins after sterilisation process. J. Camel Pract. Res. 2011;18:277–282. [Google Scholar]
- 69.Mehaia M.A. Chemical composition of camel skim milk concentrated by ultrafiltration. Int. Dairy J. 1996;6:741–752. doi: 10.1016/0958-6946(95)00063-1. [DOI] [Google Scholar]
- 70.Bukovics S., Hucker A., Szafner G. Impact of microfiltration on camel milk’s microbiological and organoleptic characteristics. Sci. Pract. J. Vet. 2015;2:168–170. [Google Scholar]
- 71.Sela S., Pinto R., Merin U., Rosen B. Thermal inactivation of Escherichia coli in camel milk. J. Food Prot. 2003;66:1708–1711. doi: 10.4315/0362-028X-66.9.1708. [DOI] [PubMed] [Google Scholar]
- 72.Al-Rasheedi F.S., Altulayan B.S., Al-Amre S.S., Faye B., Konuspayeva G. Monitoring of camel milk quality in intensive dairy farm. In: Konuspayeva G., editor. Proceedings of the 4th ISOCARD Conference; Almaty, Kazakhstan. 8–12 June 2015; Almaty, Kazakhstan: Publ. ANTIGEN; 2015. pp. 234–236. Special issue Journal Veterinariya, 2. [Google Scholar]
- 73.Abusheliabi A., Al-Rumaithi H.O., Olaimat A.N., Al-Nabuls A.A., Osaili T., Shaker R., Ayyash M.M. Inhibitory effect of camel milk on Cronobacter sakazakii. J. Food Saf. 2016:37. doi: 10.1111/jfs.12343. [DOI] [Google Scholar]
- 74.Eissa O.E.H., Hamid O.I.A. Quality characteristics of camel milk during different heat treatments. J. Agric. Vet. Sci. 2017;10:1–5. doi: 10.9790/2380-1008030105. [DOI] [Google Scholar]
- 75.Mohamed I.M.A., El-Zubeir I.Y.M. Effect of heat treatment on keeping quality of camel milk. Ann. Food Sci. Technol. 2014;15:239–245. [Google Scholar]
- 76.Fguiri I., Ziadi M., Abassi M., Arroum S., Khorchani T. Suitability of camel milk to transformation in Leben by lactic starter. Afr. J. Microbiol. Res. 2012;6:7185–7192. doi: 10.5897/AJMR12.885. [DOI] [Google Scholar]
- 77.Khedid K., Faid M., Mokhtari A., Soulaumani A., Zinedine A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol. Res. 2009;164:81–91. doi: 10.1016/j.micres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 78.Kadri Z., Spitaels F., Cnockaert M., Amar M., Joossens M., Vandamme P. The bacterial diversity of raw Moroccan camel milk. Int. J. Food Microbiol. 2021;341:109050. doi: 10.1016/j.ijfoodmicro.2021.109050. [DOI] [PubMed] [Google Scholar]
- 79.Al-Otaibi M., Al-Zoreky N.S., El-Dermerdash H.A. Camel’s milk as a natural source for probiotics. Res. J. Microbiol. 2013;8:70–80. doi: 10.3923/jm.2013.70.80. [DOI] [Google Scholar]
- 80.Abdou A.M., Hedia R.H., Omara S.T., Mahmoud M.A.E., Kandil M.M., Bakry M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World. 2018;11:227–230. doi: 10.14202/vetworld.2018.227-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yateem A., Balba M.T., Al-Surrayai T., Al-Mutairi B., Al-Daher R. Isolation of lactic acid bacteria with probiotic potential from camel milk. Int. J. Dairy Sci. 2008;3:194–199. doi: 10.3923/ijds.2008.194.199. [DOI] [Google Scholar]
- 82.Ider S., Belguesmia Y., Coucheney F., Kihal M., Drider D. Impact of seasonality and environmental conditions on yeast diversity from camel’s milk collected in Algeria. Arch. Microbiol. 2019;201:399–407. doi: 10.1007/s00203-019-01626-y. [DOI] [PubMed] [Google Scholar]
- 83.Benmechernene Z., Chentouf A.F., Yahia B., Fatima G., Quintela-Baluja M., Calo-Mata P., Barros-Velázquez J. Technological aptitude and applications of Leuconostoc mesenteroides bioactive strains isolated from Algerian raw camel milk. Biomed Res. Int. 2013;2013:1–14. doi: 10.1155/2013/418132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahmeh R., Akbar A., Kishk M., Al-Onaizi T., Al-Shatti A., Shajan A., Akbar B., Al-Mutairi S., Yateem A. Characterization of semipurified enterocin produced by Enterococcus faecium strains isolated from raw camel milk. J. Dairy Sci. 2018;101:1–9. doi: 10.3168/jds.2017-13996. [DOI] [PubMed] [Google Scholar]
- 85.Abo-Amer A.R. Inhibition of foodborn pathogens by a bacteriocin-like substance produced by a novel strain of Lactobaccilus acidophilus isolated from camel milk. Appl. Biochem. Microbiol. 2013;49:270–279. doi: 10.1134/S0003683813030174. [DOI] [Google Scholar]
- 86.Davati N., Yazdi F.T., Zibaee S., Shahidi F., Reza Edalatian M. Study of lactic acid bacteria community from raw milk of Iranian one humped camel and evaluation of their probiotic properties. Jundishapur J. Micriobiol. 2014;8:e16750. doi: 10.5812/jjm.8(5)2015.16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jans C., Meile L., Kaindi D.W.M., Kogi-Makau W., Lamuka P., Renault P., Kreikemeyer B., Lacroix C., Hattendorf J., Zinsstag J., et al. African fermented dairy products—Overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 2017;250:27–36. doi: 10.1016/j.ijfoodmicro.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Kaindi D.W.M., Njage P.K. Microbial aspect of lactic acid bacteria isolated from camel milk. In: Alhaj O.A., Faye B., Agrawal R.P., editors. Health and Environmental Benefits of Camel Products. IGI Global; Delhi, PA, USA: Hershey; Delhi, PA, USA: 2020. pp. 54–74. [Google Scholar]
- 89.Baubekova A., Akhmetsadykova S., Konuspayeva G., Akhmetsadykov N., Faye B., Loiseau G. Biodiversity study of the yeasts in fresh and fermented camel and mare milk by denaturating gradient gel electrophoresis. J. Camel Pract. Res. 2015;22:91–95. doi: 10.5958/2277-8934.2015.00014.4. [DOI] [Google Scholar]
- 90.Akhmetsadykova S., Baubekova A., Konuspayeva G., Akhmetsadykov N., Faye B., Loiseau G. Lactic acid bacteria biodiversity in raw and fermented camel milk. Afr. J. Food Sci. Technol. 2015;6:84–88. doi: 10.14303/ajfst.2015.026. [DOI] [Google Scholar]
- 91.Zhao J., Fan H., Kwok L.Y., Guo F., Ji R., Ya M., Chen Y. Analyses of phys.;icochemical properties, bacterial microbiota, and lactic acid bacteria of fresh camel milk collected in Inner Mongolia. J. Dairy Sci. 2019;103:106–116. doi: 10.3168/jds.2019-17023. [DOI] [PubMed] [Google Scholar]
- 92.El-Agamy E.I., Ruppanner R., Ismail A., Champagne C.P., Assaf R. Antibacterial and antiviral activity of camel milk protective proteins. J. Dairy Res. 1992;59:169–175. doi: 10.1017/S0022029900030417. [DOI] [PubMed] [Google Scholar]
- 93.Berhe T., Ipsen R., Seifu E., Kurtu M.Y., Eshetu M., Hansen E.B. Comparison of the acidification activities of commercial starter cultures in camel and bovine milk. LWT. 2017;89:123–127. doi: 10.1016/j.lwt.2017.10.041. [DOI] [Google Scholar]
- 94.Benyagoub E., Ayat M. Biochemical, physico-chemical and microbiological properties of camel raw milk marketed in Bechar city (South-West Algeria), hygienic and safe consumers’ approach. Microbes Health. 2015;4:14–18. doi: 10.3329/mh.v4i1.23087. [DOI] [Google Scholar]
- 95.Elhaj A.E., Somaya F.A.B., Mohamed T.T. Aerobic bacteria and fungi associated with raw camel’s milk. Online J. Anim. Feed Res. 2014;4:15–17. [Google Scholar]
- 96.Jrad Z., El-Hatmi H., Fguiri I., Arroum S., Assadi M., Khorchani T. Antibacterial activity of lactic acid bacteria isolated from Tunisian camel milk. Afr. J. Microbiol. Res. 2013;7:1002–1008. doi: 10.5897/AJMR12.488. [DOI] [Google Scholar]
- 97.Konuspayeva G., Faye B. Culture des laits du Monde. Les Cahiers de l’OCHA n°15; Paris, France: 2011. Identité, vertus thérapeutiques et al.légation santé, les produits fermentés d’Asie Centrale; pp. 135–145. [Google Scholar]
- 98.Rahman N., Xiaohong C., Meiqin F., Mingsheng D. Characterization of the dominant microflora in naturally fermented camel milk shubat. World J. Microbiol. Biotechnol. 2009;25:1941–1946. doi: 10.1007/s11274-009-0092-5. [DOI] [Google Scholar]
- 99.Guo L., Xu W., Li C., Guo Y., Chagan I. Comparative study of physicochemical composition and microbial community of Khoormog, Chigee, and Airag, traditionally fermented dairy products from Xilin Gol in China. Food Sci. Nutr. 2021;9:1564–1573. doi: 10.1002/fsn3.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmed A.I., Mohamed B.E., Yousif N.M.E., Faye B., Loiseau G. Antimicrobial activity and antibiotic resistance of LAB isolated from Sudanese traditional fermented camel (Camelus dromedarius) milk gariss. Int. J. Biosci. 2012;2:129–136. [Google Scholar]
- 101.Adelgadir W.S., Ahmed T.K., Dirar H.A. The traditional fermented milk products of the Sudan. Int. J. Food Microbiol. 1998;44:1–13. doi: 10.1016/S0168-1605(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 102.Maitha M., Kaindi D.W.M., Wangoh J., Mbugua S. Microbial quality and safety of traditional fermented camel milk product suusac sampled from different regions in North Eastern, Kenya. Asian Food Sci. J. 2019;8:1–9. doi: 10.9734/afsj/2019/v8i229986. [DOI] [Google Scholar]
- 103.Algruin K., Konuspayeva G. Making Leben with Camel Milk. In: Konuspayeva G., editor. Proceedings of the 4th ISOCARD Conference; Almaty, Kazakhstan. 8–12 June 2015; Almaty, Kazakhstan: Antigen; 2015. p. 393. Special issue Journal Veterinariya, 2. [Google Scholar]
- 104.Seifu E., Abraham A., Kurtu M.Y., Yilma Z. Isolation and characterization of lactic acid bacteria from Ititu, Ethiopian traditional fermented camel milk. J. Camelid Sci. 2012;5:82–98. [Google Scholar]
- 105.Berhe T., Ipsen R., Seifu E., Kurtu M.Y., Fugl A., Hansen E.B. Metagenomic analysis of bacterial community composition in Dhanaan, Ethiopian traditional fermented camel milk. FEMS Microbiol. Lett. 2019;366:fnz128. doi: 10.1093/femsle/fnz128. [DOI] [PubMed] [Google Scholar]
- 106.Boulay S. Alimentation, Diététique et Relations Sociales au Sahara, L’exemple des Pasteurs Nomades Maures de Mauritanie. [(accessed on 23 February 2021)];2008 Available online: www.lemangeur-ocha.com.
- 107.Ismaili M.A., Hamama A., Saidi B., Zahar M., Meryem A. Chemical composition, microbial profile and identification of lactic bacteria of Moroccan fermented camel milk Lfrik. Curr. Res. Nutr. Food Sci. 2017;5:383–390. doi: 10.12944/CRNFSJ.5.3.24. [DOI] [Google Scholar]
- 108.Soleymanzadeh N., Mirdamadi S., Kianirad M. Antioxidant activity of camel and bovine milk fermented by lactic acid bacteria isolated from traditional fermented camel milk (Chal) Dairy Sci. Technol. 2016;96:443–457. doi: 10.1007/s13594-016-0278-1. [DOI] [Google Scholar]
- 109.Lore T.A., Mbugua S.K., Wangoh J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT. 2005;38:125–130. doi: 10.1016/j.lwt.2004.05.008. [DOI] [Google Scholar]
- 110.Jans C., Bugnard J., Njage P.M.K., Lacroix C., Miele L. Lactic acid bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT Food Sci. Technol. 2012;47:371–379. doi: 10.1016/j.lwt.2012.01.034. [DOI] [Google Scholar]
- 111.Adelgadir W., Nielsen D.S., Hamad S., Jakobsen M. A traditional Sudanese fermented camel’s milk product, Gariss, as a habitat of Streptococcus infantarius subsp. infantarius. Int. J. Food Microbiol. 2008;127:215–219. doi: 10.1016/j.ijfoodmicro.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 112.Holzapfel W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002;75:197–212. doi: 10.1016/S0168-1605(01)00707-3. [DOI] [PubMed] [Google Scholar]
- 113.Akhmetsadykova S., Baubekova A., Konuspayeva G., Akhmetsadykov N., Loiseau G. Microflora identification of fresh and fermented camel milk from Kazakhstan. Emir. J. Food Agric. 2014;26:327–332. doi: 10.9755/ejfa.v26i4.17641. [DOI] [Google Scholar]
- 114.Ashmaig A., Hasan A., El Gaali E. Identification of lactic acid bacteria isolated from traditional Sudanese fermented camel milk (Garris) Afr. J. Microbiol. Res. 2009;3:451–457. [Google Scholar]
- 115.Berzhanova R., Sartaeva A., Sagyndykov U., Mukasheva T., Shigaeva M. The studying of diversity of lactic microorganisms isolated from shubat of various areas of Kazakhstan. J. Biotechnol. 2014;185:S82. doi: 10.1016/j.jbiotec.2014.07.281. [DOI] [Google Scholar]
- 116.Njage P. Ph.D. Thesis. University of Nairobi; Nairobi, Kenya: 2010. Microbial Diversity, Safety and Role of Predominant Lactic Acid Bacteria in Raw and Spontaneously Fermented Camel Milk from Kenya and Somalia. [Google Scholar]
- 117.Baubekova A., Konuspayeva G., Akhmetsadykova S.H., Akhmetsadykov N. Пoдгoтoвка прoмышленнoгo прoизвoдства заквасoк– выделение и идентификация бактерий для кумыса и шубата. [Preparation of industrial production of starter cultures—isolation and identification of bacteria from kumis and shubat] Вестник КазНУ Серия биoлoгическая. 2014;60:178–181. [Google Scholar]
- 118.Faye B., Konuspayeva G. Innovations in camel milk processing, The new challenges for marketing camel dairy products and the consequences on genetic selection; Proceedings of the Regional Conference for Animal Genetic Resources Conservation, towards Sustainable Utilization; Muscat, Oman. 23–24 February 2016; [(accessed on 23 February 2021)]. 11p. Available online: https://oapgrc.gov.om/Documents/The%20new%20challenges%20for%20marketing%20camel%20dairy%20products%20and%20the%20consequences%20on%20genetic%20selection_Bernard%20Fye.pdf. [Google Scholar]
- 119.Abdel Rahman I.E., Dirar H.A., Osman M.A. Microbiological and biochemical changes and sensory evaluation of camel milk fermented by selected bacterial starter cultures. Afr. J. Food Sci. 2009;3:398–4059. [Google Scholar]
- 120.Ayyash M., Al-Dhaheri A.S., Al-Mahadin S., Kizhakkayil J., Abushelaibi A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic, A comparative study with fermented bovine milk. J. Dairy Sci. 2018;101:900–911. doi: 10.3168/jds.2017-13400. [DOI] [PubMed] [Google Scholar]
- 121.Dugan F.M. Fermentive microorganisms in the Prehistory of Europe, the steppes, and Indo-Iranian Asia and their contemporary use in traditional and probiotic beverages. Fungi. 2009;2:16–39. [Google Scholar]
- 122.Kappeler S.R., van den Brink H.M., Rahbek-Nielsen H., Farah Z., Puhan Z., Hansen E.B., Johansen E. Characterizations of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem. Biophys. Res. Commun. 2006;342:647–654. doi: 10.1016/j.bbrc.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 123.Konuspayeva G. Manufacture and Challenges of Camel Milk Cheese. In: Alhaj O.A., Faye B., Agrawal R.P., editors. Health and Environmental Benefits of Camel Products. IGI Global; Delhi, PA, USA: Hershey; Delhi, PA, USA: 2020. pp. 110–122. [Google Scholar]
- 124.Ramet J.P. L’aptitude fromagère du lait de dromadaire. Rev. D’elevage Et Médecine Vétérinaire Des Pays Trop. 1989;42:105–111. doi: 10.19182/remvt.8861. [DOI] [Google Scholar]
- 125.Hailu Y., Seifu E., Yilma Z. Physicochemical properties and consumer acceptability of soft unripened cheese made from camel milk using crude extract of ginger (Zingiber officinale) as coagulant. Afr. J. Food Sci. 2014;8:87–91. [Google Scholar]
- 126.Terefe M.A., Kebede A., Kebede M. Clotting Activities of Partially Purified Extracts of Moringa oleifera L. on Dromedary Camel Milk. East Afr. J. Sci. 2017;11:117–128. [Google Scholar]
- 127.Boudjenah-Haroun S., Laleye L., Moulti-Mati F., Si Ahmed S., Mahboub N., Siboukeur O.E., Mati A. Comparative study of milk clotting activity of crude gastric enzymes extracted from camels’ abomasum at different ages and commercial enzymes (rennet and pepsin) on bovine and camel milk. Emir. J. Food Agric. 2011;23:301–310. [Google Scholar]
- 128.Jones-Abeiderrhamane N. In: Camel Cheese, Seemed like a Good Idea. Jones-Abeiderrahmane N., editor. JA Publ.; Nouakchott, Mauritania: 2013. 387p [Google Scholar]
- 129.Konuspayeva G., Faye B., Baubekova A., Loiseau G. Camel gruyere cheese making. In: Johnson E.H., Maghoub O., Eljack A., Kadim I., Bobabe P.A., Tageldin M.H., Almarzooqi W.S., Eltahir Ahmed Y., editors. Proceedings of the 3rd ISOCARD Conference; Muscat, Oman. 29 January–1 February 2012; Muscat, Oman: Sultan Qaboos University; 2012. pp. 218–219. [Google Scholar]
- 130.Konuspayeva G., Camier B., Gaucheron F., Faye B. Some parameters to process camel milk into cheese. Emir. J. Food Agric. 2014;26:354–358. doi: 10.9755/ejfa.v26i4.17277. [DOI] [Google Scholar]
- 131.Konuspayeva G., Camier B., Aleilawi N., Al-Shumeimyri M., Algruin K., Alshammari F., Beaucher E., Faye B. Manufacture of dry- and brine-salted soft camel cheeses for the camel dairy industry. Int. J. Dairy Technol. 2017;70:92–101. doi: 10.1111/1471-0307.12319. [DOI] [Google Scholar]
- 132.Shahein M.R., Hassanein A.M., Zayan A.F. Evaluation of soft cheese manufactured from camel and buffalo milk. World J. Dairy Food Sci. 2014;9:213–219. [Google Scholar]
- 133.Hailu Y., Hansen E.B., Eshetu M., Ipsen R. Factors influencing the gelation and rennetability of camel milk using camel chymosin. Int. Dairy J. 2016;60:62–69. doi: 10.1016/j.idairyj.2016.01.013. [DOI] [Google Scholar]
- 134.Soltani M., Boran O.S., Hayaloglu A.A. Effect of various blends of camel chymosin and microbial rennet (Rhizomucor miehei) on microstructure and rheological properties of Iranian UF White cheese. LWT Food Sci. Technol. 2016;68:724–728. doi: 10.1016/j.lwt.2016.01.028. [DOI] [Google Scholar]
- 135.Mohammed S., Eshetu M., Tadesse Y., Hailu Y. Rheological properties and shelf life of soft cheese made from camel milk using camel chymosin. J. Dairy Vet. Sci. 2019;10:555794. doi: 10.19080/JDVS.2019.10.555794. [DOI] [Google Scholar]
- 136.Kamal M., Foukani M., Karoui R. Effects of heating and calcium and phosphate mineral supplementation on the physical properties of rennet-induced coagulation of camel and cow milk gels. J. Dairy Res. 2017;84:220–228. doi: 10.1017/S0022029917000152. [DOI] [PubMed] [Google Scholar]
- 137.Farah Z., Bachmann M.R. Rennet coagulation properties of camel milk. Milchwissenschaft. 1987;42:689–692. [Google Scholar]
- 138.Wolfschoon A. Influence of calcium chloride addition to milk on the cheese yield. Int. Dairy J. 1997;7:249–254. doi: 10.1016/S0958-6946(97)00005-8. [DOI] [Google Scholar]
- 139.Gioacchini A.M., De Santi M., Guescini M., Brandi G., Stocchi V. Characterization of the volatile organic compounds of Italian ‘Fossa’ cheese by solid-phase microextraction gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:3405–3412. doi: 10.1002/rcm.4782. [DOI] [PubMed] [Google Scholar]
- 140.El-Shafei S.M.S., Khalifa S.A. Manufacture of processed cheese spread from camel cheese based, Evaluation of cheese characteristics. Am. J. Food Sci. Nutr. Res. 2018;5:76–86. [Google Scholar]
- 141.El-Zubeir I.E.M., Jabreel S.O. Fresh cheese from camel milk coagulated with Camifloc. Int. J. Dairy Technol. 2008;61:90–95. doi: 10.1111/j.1471-0307.2008.00360.x. [DOI] [Google Scholar]
- 142.El-Gendy M.H. Impact of manufacturing processes on the industry soft cheese from camel milk. J. Biol. Chem. Environ. Sci. 2018;13:491–510. [Google Scholar]
- 143.Faye B., Jaouad J., Bhrawi K., Senoussi A., Bengoumi M. Elevage camelin en Afrique du Nord, état des lieux et perspectives. Rev. D’elevage Méd. Vétérinaire Pays. Trop. 2014;67:213–221. doi: 10.19182/remvt.20563. [DOI] [Google Scholar]
- 144.Ibrahim A.H., Khalifa S.A. Effect of freeze drying on camel’s milk nutritional properties. Int. Food Res. J. 2015;22:1438–1445. [Google Scholar]
- 145.Sulieman A.M.E., Elamin O., Elkhalifa E.A., Laleye L. Comparison of Physicochemical Properties of Spray-dried Camel’s Milk and Cow’s Milk Powder. Int. J. Food Sci. Nutr. Eng. 2014;4:15–19. doi: 10.5923/j.food.20140401.03. [DOI] [Google Scholar]
- 146.Zouari A., Marchesseau S., Chevalier-Lucia D., Raffard G., Ayadi M.A., Picart-Palmade L. Acid gelation of raw and reconstituted spray-dried dromedary milk, a dynamic approach of gel structuring. Int. Dairy J. 2018;81:95–103. doi: 10.1016/j.idairyj.2018.01.009. [DOI] [Google Scholar]
- 147.Habtegebriel H., Wawire M., Sila D. The Effect of Pretreatment (Spray Drying) on the Yield and Selected Nutritional Components of Whole Camel Milk Powder. J. Food Sci. 2018;83:2983–2991. doi: 10.1111/1750-3841.14361. [DOI] [PubMed] [Google Scholar]
- 148.Vega C., Roos Y.H. Invited review, Spray-dried dairy and dairy-like emulsions-Compositional considerations. J. Dairy Sci. 2006;89:383–401. doi: 10.3168/jds.S0022-0302(06)72103-8. [DOI] [PubMed] [Google Scholar]
- 149.Khalifa S.A., Ibrahim A.H. Influence of addition modified starches as stabilizer on physicochemical and textural properties of camel’s milk yoghurt. Zagazig J. Agric. Res. 2015;42:13–26. [Google Scholar]
- 150.Abd-Elhamid A.M., Elbayoumi M.M. Effect of heat treatment and fermentation on bioactive behavior in yoghurt made from camel milk. Am. J. Food Sci. Technol. 2017;5:109–116. doi: 10.12691/ajfst-5-3-6. [DOI] [Google Scholar]
- 151.Afolabi L., Onilude H., Afolabi M.O. Assessment of microbiological quality of yogurt sold by street vendors in Onitsha metropolis, Anambra state, Nigeria. Br. Microbiol. Res. J. 2013;3:198–200. doi: 10.9734/BMRJ/2013/1801. [DOI] [Google Scholar]
- 152.Gorelik O., Shatskikh Y., Rebezov M., Kanareikin V., Lihodeyevskaya O., Okuskhanova E. Study of Chemical and Mineral Composition of New Sour Milk Bio-product with Sapropel Powder. Annu. Res. Rev. Biol. 2017;18:1–5. doi: 10.9734/ARRB/2017/36937. [DOI] [Google Scholar]
- 153.Berhe T., Seifu E., Ipsen R., Kurtu M.Y., Hansen E.B. Processing challenges and opportunities of camel dairy products. Int. J. Food Sci. 2017;2:1–8. doi: 10.1155/2017/9061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jumah R.Y., Shaker R.R., Abu-Jdayil B. Effect of milk source on the rheological properties of yogurt during the gelation process. Int. J. Dairy Technol. 2001;54:89–93. doi: 10.1046/j.1364-727x.2001.00012.x. [DOI] [Google Scholar]
- 155.Attia H., Dhouib N. Dromedary milk lactic acid fermentation, Microbiological and rheological characteristics. Microb. Biotechnol. 2001;26:263–270. doi: 10.1038/sj.jim.7000111. [DOI] [PubMed] [Google Scholar]
- 156.Lajnaf R., Picart-Palmade L., Attia H., Marchesseau S., Ayadi M.A. Foaming and adsorption behavior of bovine and camel proteins mixed layers at the air/water interface. Colloids Surf. B Biointerfaces. 2016;151:287–294. doi: 10.1016/j.colsurfb.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 157.Hashim I.B., Khalil A.H., Habib H. Quality and acceptability of a set-type yogurt made from camel milk. J. Dairy Sci. 2009;92:857–862. doi: 10.3168/jds.2008-1408. [DOI] [PubMed] [Google Scholar]
- 158.Shegaw A., Ipsen R., Kurtu M.Y., Eshetu M., Hansen E.B., Hailu Y., Waktola A., Dashe D. Development of yogurt from camel milk using Exopolysaccharide producing lactic acid bacteria. Ethiop. J. Anim. Prod. 2020;20:29–43. [Google Scholar]
- 159.Elsahoryi N.A., Al-Sayyed H.F. Manufacture of dairy and non-dairy camel milk products. Chap. 5. In: Alhaj O.A., Faye B., Agrawal R.P., editors. Health and Environmental Benefits of Camel Products. IGI Global; Delhi, PA, USA: Hershey; Delhi, PA, USA: 2020. pp. 75–109. [Google Scholar]
- 160.Shamsia S.M. Nutritional and therapeutic properties of camel and human milks. Int. J. Genet. Mol. Biol. 2009;1:52–58. [Google Scholar]
- 161.Mohsin A., Ni H., Luo Y., Wei Y., Tian X., Guan W., Ali M., Mahmood Khan I., Niazi S., Rehman S., et al. Qualitative improvement of camel milk date yoghurt by addition of biosynthesized xanthan from orange waste. LWT. 2019;108:61–68. doi: 10.1016/j.lwt.2019.03.039. [DOI] [Google Scholar]
- 162.Galeboe O., Seifu E., Sekwati-Monang B. Production of camel milk yoghurt, Physicochemical and microbiological quality and consumer acceptability. Int. J. Food Stud. 2018;7:51–63. doi: 10.7455/ijfs/7.2.2018.a5. [DOI] [Google Scholar]
- 163.Kavas N., Kavas G. Probiotic frozen yoghurt production using camel milk (Camelus dromedarius) with improved functions by strawberry guava (Psidium littorale var. cattleianum) fortification. Br. J. Appl. Sci. Technol. 2016;14:1–12. doi: 10.9734/BJAST/2016/23683. [DOI] [Google Scholar]
- 164.Ahmed S., Haroun R., Eisa M. Banana frozen yogurt from camel milk. Pak. J. Nutr. 2010;9:955. doi: 10.3923/pjn.2010.955.956. [DOI] [Google Scholar]
- 165.Konuspayeva G., Lemarie E., Faye B., Loiseau G., Montet D. Fatty acid and cholesterol composition of camel’s (Camelus bactrianus, Camelus dromedarius and hybrids) milk in Kazakhstan. Dairy Sci. Technol. 2008;88:327–340. doi: 10.1051/dst:2008005. [DOI] [Google Scholar]
- 166.Attia H., Kherouatou N., Fakhfakh N., Khorchani T., Trigui N. Dromedary milk fat, biochemical, microscopic and rheological characteristics. J. Food Lipids. 2000;7:95–112. doi: 10.1111/j.1745-4522.2000.tb00164.x. [DOI] [Google Scholar]
- 167.Berhe T., Seifu E., Kurtu M.Y. Physicochemical properties of butter made from camel milk. Int. Dairy J. 2013;31:51–54. doi: 10.1016/j.idairyj.2013.02.008. [DOI] [Google Scholar]
- 168.Farah Z., Streiff T., Bachmann M.R. Manufacture and characterization of camel milk butter. Milchwissenschaft. 1989;44:412–414. [Google Scholar]
- 169.Parmar N.B. Master’s Thesis. SMC College of Dairy Science, Anand Agricultural University; Anand, Gujarat, India: 2013. Characterization of Ghee Prepared from Camel Milk and Evaluation of Its Shelf Life during Storage. [Google Scholar]
- 170.Ahmed A.S.M., El-Zubeir I.E.M. Microbiological and sensory properties of low-fat ice cream from camel milk using natural additives. Ann. Food Sci. Technol. 2015;16:236–244. [Google Scholar]
- 171.Hashim I. Acceptance of camel milk among elementary school students in Al-Ain City, United Arab Emirates. Emir. J. Food Agric. 2017;17:54–59. doi: 10.9755/ejfa.v14i1.4985. [DOI] [Google Scholar]
- 172.Maryniak N., Hansen E., Ballegaard A.S., Sancho A., Bogh K. Comparison of the Allergenicity and Immunogenicity of Camel and Cow’s Milk—A Study in Brown Norway Rats. Nutrients. 2018;10:1903. doi: 10.3390/nu10121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.