Abstract
Osteoarthritis (OA) is a highly prevalent musculoskeletal condition worldwide. More than 300 million individuals are affected by OA, and pain is the most common and challenging symptom to manage. Although many new advances have led to improved OA-related pain management, smart technology offers additional opportunities to enhance symptom management. This narrative review identifies and describes the current literature focused on smart technology for pain management in individuals with OA. In collaboration with a health sciences librarian, an interdisciplinary team of clinician-scientists searched multiple databases (e.g. PubMed, CINAHL and Embase), which generated 394 citations for review. After inclusion criteria were met, data were extracted from eight studies reporting on varied smart technologies, including mobile health, wearables and eHealth tools to measure or manage pain. Our review highlights the dearth of research in this crucial area, the implications for clinical practice and technology development, and future research needs.
Keywords: osteoarthritis, chronic pain, smart technology, mHealth, self-management, digital technology
Key messages
OA is a prevalent musculoskeletal condition that requires multimodal management.
Smart technology can enhance monitoring and management of pain in OA.
Evidence indicates the potential benefits of smart technology for pain management; more research is warranted.
Introduction
Osteoarthritis (OA) is the most common form of arthritis and a leading cause of pain and physical disability affecting an estimated 303 million people worldwide [1, 2]. The prevalence, incidence, and costs of care of OA have increased significantly over the past century [3], which has been particularly evident in high- and middle-income countries [4]. For example, in the USA, a reported US$140 billion were spent on OA-related medical costs in 2013 [5]. Despite recent medical advances and an increased focus on lifestyle approaches for self-managing OA-related symptoms [6], managing OA-related pain remains extremely challenging [7]. This is probably attributable to the dynamic and biopsychosocial nature of OA-related pain [8], which results in wide inter-individual variability in pain [9]. Furthermore, constraints in access to health-care services and the current global hesitancy in prescribing opioids for long-term pain management add to the challenge of effective pain management in OA [10–12]. Therefore, improved chronic pain management models relative to OA are needed that provide cost-efficient and accessible interventions for chronic pain management [13]. Smart technology that is tailored to the medical needs of patients might overcome the current barriers and gaps in health-care by providing remote assistance in monitoring, controlling and treating OA-related chronic pain [14]. Smart technology is defined as an Internet-connected and interconnected electronic or automated device or system that is responsive and/or reactive to real-time data input [5, 15]. Harnessing smart technology is the next step in the optimization of personalized and precision medicine in patients with chronic OA-related pain.
The past decade has yielded immense advances in the volume and innovation of digital technologies for OA management [15], including smart technology. These technologies typically include a smart design interface via artificial intelligence, machine learning and big data cloud computing and require interactive engagement with the end users (e.g. patients, providers) [15]. As outlined by Solomon & Rudin [15], smart technologies include virtual reality, mobile health (mHealth; e.g. smartphones, smartwatches, tablets or other Internet-enabled devices), wearables (e.g. smartwatches, smart clothing), digital therapeutics and voice assistants. Indeed, the US national public health agenda for OA recommends that health-care providers promote self-management through digital means, such as mHealth and wearable devices, along with traditional options, such as self-directed online programmes [16]. Smart technology empowers individuals, regardless of age, to engage actively in disease management strategies and decision-making processes that foster improved quality of life. The role of smart technology in disease self-management has been documented in chronic illness [17], gout [18], RA [19] and JIA [20]. For example, in an international study among adults aged 45–54 years, researchers reported that >50% of people with rheumatic and musculoskeletal disorders were aware of mHealth self-management applications (apps), and 42% were currently using these apps, mainly to self-monitor various health indicators and disease progression and to communicate and interact with their health-care provider directly [21]. Although there are ∼280 smart applications for chronic pain self-management, as of 2017, most are limited in terms of their functionality and do not offer comprehensive self-management capabilities [22].
The potential benefits of digital health technologies, including smart technologies, in rheumatology have been noted [15], including a significant moderate effect on pain reduction in OA [23]. Nevertheless, many challenges persist in widespread utilization of smart technologies for health and OA-related pain management. One of the most common and pervasive is the digital divide, which limits access and use by older adults, racial/ethnic minorities, and individuals who are economically disadvantaged, live in rural environments, and have low health literacy and/or cognitive and functional impairments [24]. A second major limitation is the lack of best-practice standards for digital health-care technologies [15] and promotion of technology for self-management in clinical guidelines. Despite these shortcomings, enabled technologies hold much promise for improved patient care and health outcomes through innovative treatment and education delivery platforms, advanced monitoring of symptoms and treatment responses, and real-time communication with health-care providers. To date, few reviews have assessed the available research focused on smart technology for OA pain self-management. Therefore, the purpose of this narrative review is to synthesize and understand the current literature on management of OA pain across the lifespan using smart technology to guide future research and clinical applications.
Methods
Study design
The aim of this narrative review was to describe the use of smart technology for pain management in patients with OA. The guiding question for this narrative review was: how has smart technology been used for self-management of OA-related pain?
Search strategy
For this narrative review, a systematic search strategy was developed by a health science librarian (M.E.), in collaboration with the research team, to search the literature comprehensively. We defined smart technology as intelligent (and intuitive) and connected digital technologies that actively used interactive, adaptive and responsive self-monitoring analysis and reporting technology. This eliminated technologies that are static (e.g. pre-programmed Web-based self-management programmes, electronic health records). The search strategies developed used a combination of keywords, MeSH terms and controlled vocabulary related to the core concepts of OA, smart technologies, pain, pain management or self-management. Searched databases included PubMed, Embase, CINAHL, PsycINFO and Web of Science, with no date limits (through July 2020) and an English language filter; full search strategies are available upon request. Search results (n = 394) were uploaded into Covidence software (Melbourne, Victoria, Australia) for de-duplication and review.
Study selection
The review process was completed in Covidence and conducted in two steps: review of the title and abstract, and full-text review. Each article was assessed for eligibility and inclusion by two reviewers. All reviewers were assigned an equal number of titles and articles to review at both steps. Articles were selected based on the following inclusion criteria: (a) original quantitative and qualitative studies on OA; (b) uses smart technology for pain and symptom management; and (c) any age group. Articles were excluded if they were study protocols, literature reviews, focused on total joint replacement or did not included pain as a primary or secondary outcome. Discrepancies in study inclusion were resolved after agreement was reached through team discussion. Once discrepancies from the title and abstract review were resolved, a full-text review was performed in the same manner. Final verification and quality control were performed by the first and last authors.
Data extraction
Data were extracted from included articles by members of the research team and added to a data extraction table. The data extraction table included authors, study design, participant age group, type of smart technology used, results and reported limitations. Data abstraction was limited to descriptive data; therefore, there were no discrepancies during the data abstraction process because no analysis of the extracted data was performed.
Results
Our initial search yielded a total of 394 potential articles. After removal of 62 duplicates, 332 articles were screened, with 259 articles removed after the title and abstract screening. The full-text review was conducted on the remaining 73 articles, of which 65 did not meet our inclusion criteria. Studies were excluded for the following reasons: (a) incorrect patient population (e.g. RA, JIA, general musculoskeletal pain conditions not specific to OA); (2) did not use smart technology (e.g. static online self-management programmes with no smart technology interface); (c) wrong study design (e.g. reviews, study protocols); (d) published abstracts without corresponding full-text manuscript; and (e) non-English language.
Eight studies met the inclusion criteria for full-text review (Table 1). Of the four quantitative studies included, two were randomized controlled trials (RCTs) [25, 26] and two were pilot studies [27, 28], which examined the effects and feasibility of different mHealth smart technology applications for pain and symptom self-management among individuals with OA. Each of the quantitative studies relied on technology-assisted assessments of symptoms and intervention delivery, using applications downloaded to smart phones or tablets. Four qualitative studies were included in this review and focused on the perspectives of physicians and patients with regard to the use of smart technology to assist with pain self-management [29–32]. Participants in all studies were adults ≥18 years of age. Although we aimed to include studies with younger samples (e.g. adolescents), literature specific to OA in children and adolescents was not found. This is not surprising given the relatively low occurrence rate of OA in younger populations. Males and females were both represented in the studies. Two pilot studies [27, 28] and three qualitative studies [29, 31, 32] included a larger percentage of females than males; otherwise, study samples were balanced across sexes. The reported sample sizes ranged from 9 to 162 participants. Included studies were conducted in the USA [25, 27, 28], England [30, 32], Australia [29], Canada [31] and The Netherlands [26].
Table 1.
Review of included studies (n = 8)
| Type of smart technology | Study design | Population | Findings | Major limitations | Reference |
|---|---|---|---|---|---|
| Mobile application, smartphone or tablet | RCT (interventional clinical trial) |
|
No differences in pain scores between intervention and control groups. Compared with controls, the intervention group used less opiates and more adjuvant analgesics. With continued use of the app (>14 days post-TKR), compared with the control group the active app users had faster reduction of pain score during activity and faster reduction of pain scores at night, less opiate use and more adjuvant analgesic use | Study not blinded; participants knew if they were in the control or intervention group. Small sample size; study was underpowered. Cost effectiveness of app was not investigated | Pronk et al. (2020) [26] |
| Wearable technology (bluetooth-powered exercise leg sensors), mobile application, smartphone or tablet | Two-armed RCT |
|
|
Did not investigate long-term outcomes; not all individuals reported chronic pain; did not evaluate risk factors for dropouts | Mecklenburg et al. (2018) [25] |
| Wearable technology (smartwatch), mobile application | Pilot study, focus group |
|
Evaluation of PROMPT app and smartwatch via focus groups. Themes were coded, and subthemes emerged. Most participants expressed enthusiasm for wearing the smartwatch, despite its weight and lack of other desired features |
|
Manini et al. (2019) [27] |
| Mobile application, smartphone or tablet | Qualitative study | Family physicians (n = 4) and patients with knee OA (n = 5) | Patient and physician views were very different; patients were concerned about pain and health outcomes, whereas physicians did not feel OA needed to be managed aggressively or proactively | Small sample; did not reach saturation | Barber et al. (2019) [31] |
| Mobile application, smartphone or tablet | Qualitative study |
|
The increasing integration of smartphones and apps into the sphere of chronic disease self-management, coupled with increasing willingness among older people to engage with these technologies, offers opportunities to harness the ability of these modern-day approaches in helping older people manage their pain better | Small sample size, based in Australia, with sampling bias | Bhattarai et al. (2020) [29] |
| Wearable technology (orthotics) | Qualitative feasibility |
|
Participants supported the use of feedback for rehabilitation, screening and evaluation of treatment progress/success purposes. Flexifoot use by patients was encouraged as a self-management tool that might motivate them by setting attainment goals. The data interface should be secure, concise and visually appealing. The measured parameters of Flexifoot, its duration of wear and frequency of data output would all depend on the rationale for its use. The clinicians and patients must collaborate to optimize the use of Flexifoot for long-term monitoring of disease for patient care in clinical practice |
|
Lin et al. (2019) [30] |
| Wearable technology, mobile application | Pilot study |
|
|
Self-report bias, with no control group, and smartphones were required, which might limit generalizability to a different sample | Zaslavsky et al. (2019) [28] |
| Wearable technology | Qualitative study |
|
Twenty-one patients with knee OA reported positive attitudes to wearable technology on self-management of OA |
|
Belsi et al. (2016) [32] |
app: application; RCT: randomized controlled trial; VAS: visual analog scale.
Quantitative studies include two RCTs and two pilot studies, using apps downloaded to either a smartphone or tablet and/or wearable devices/sensors. Pronk et al. [26] examined the effects of the PainCoach app on participants’ opiate use and self-reported pain after total knee replacement. The PainCoach app was downloaded to patients’ smartphone or tablet and provided patients with targeted recommendations for pain medication use, exercise and rest based on participant input. Findings indicated that the PainCoach app reduced opiate consumption and increased the pain reduction rate during activity compared with the control group [26]. In a second study, researchers examined the effects of a downloadable app for smartphones or tablets (i.e. Hinge Health digital care programme), which included sensor-guided exercise therapy, education, cognitive behavioural therapy, weight loss and psychosocial support for chronic knee pain [25]. The Hinge Health programme was shown to reduce clinical pain significantly compared with the control group at the end of the 12 week programme [25]. In a pilot study, researchers assessed the attitudes and perceptions of older adults with knee OA to a smartwatch app that was designed to collect ecological momentary assessments (EMAs) of OA symptoms, and found that the majority of participants (n = 19) assessed the technology positively [27]. Participants also indicated interest in the capabilities of the smartwatch to perform other health-tracking functions and its potential use as a communication tool with health-care providers [27]. Researchers piloting wearable technology (i.e. Fitbit Charge 2) combined with motivational interviewing among adults (n = 22) with OA found the mHealth self-management intervention to be feasible and showed initial benefits for improving pain-related symptoms in OA (i.e. sleep disturbance) [28]. Although few in number, these four studies provide initial support for the integration of smart technology for improved pain management outcomes in OA.
Four qualitative studies were included and explored the attitudes of patients and physicians to and experiences with self-management apps and wearable technology for OA-related pain [29–32]. A qualitative study using semi-structured interviews among community-dwelling adults with OA-related pain found that older adults value apps for self-management, while recognizing the need for digital technology to be aligned with patients’ preferences and clinician involvement [29]. In a focus group of 21 participants with knee OA, participants reported that wearables provided a helpful mechanism to describe more objectively and explain a subjective experience, such as pain. Many felt empowered by the opportunity to be able to control and improve their health with access to real-time feedback [32]. Another study assessing perspectives of both patients (n = 4) and physicians (n = 4) found contrasting views [31]. Patients felt that pain was as an important factor in their overall health and were open to smart technology for self-management. In contrast, physicians viewed OA-related pain as a relatively minor health problem and did not endorse its proactive management and were sceptical of technology-driven self-management [31]. In an exploratory study of the opinions of clinicians (n = 30) regarding a smart pressure-sensing insole (Flexifoot), the authors reported that all clinicians were in support of the smart technology as a self-management tool that could be used to complement existing clinical tools, providing objective feedback to promote more effective patient–provider communication, leading to improved outcomes [30]. Both physicians and patients endorsed the use of smart technology, with a few notable drawbacks. Among older adults there is a willingness to use self-management apps; however, this is coupled with the need for personalization and the fear that constant engagement will lead to ruminating thoughts about pain [29]. When paired with current methods, such as self-report, wearable technologies provide more thorough functionality data to health-care providers [30]. Although smart technology is an innovative way to include patients in their care, future developments should be mindful of the target audience and assess wearables for cost and clinical effectiveness [30].
Discussion
OA is a leading cause of chronic musculoskeletal pain and disability [33]. Self-management is a vital component of effective pain and symptom management in persons with OA [34–36] and is strongly recommended in current clinical treatment guidelines [2, 37]. Patient-centred care requires that patients become knowledgeable about their disease and its treatment, in addition to the provision of readily available tools and technologies that can assist with pain and symptom management. Using smart technologies introduces an innovative approach for management of a challenging public health concern, chronic pain. The use of smart technology to promote and support self-management in OA is promising [26, 28], with patients expressing positive views about technology for advancing self-management and improving clinician–patient interactions [32]. However, barriers to implementation remain [31], signifying a need to elicit the perspectives of end-users and to integrate such technologies into clinical practice guidelines.
In the present review, we identified eight studies that assessed the use of smart technologies for OA-related pain management. Specifically, two studies examined smartphones and tablet applications and two studies assessed wearable devices/sensors (e.g. Fitbit and Gear S3 Smartwatch). The use of the applications led to increased exercise, reduced pain levels and decreased opioid use. Of the studies using wearable sensors, one study assessed the feasibility and efficacy of an mHealth self-management intervention designed to improve sleep outcomes in older adults with OA [28], and the second study examined the attitudes and perceptions of older adults when using a smartwatch [27]. Zaslavsky et al. [28] found improvement in sleep outcomes (i.e. insomnia and acceptance of sleep difficulties) and 22 out of 24 participants who completed the study over the 19 week period. Overall, patients expressed positive views regarding the smartwatch technology and wearables that provided an opportunity to communicate their pain in a succinct and descriptive way [27, 32]. Nevertheless, authors noted several concerns and areas for improvement regarding usability (e.g. accessibility issues, notification design, and use of intuitive assessment scales) [27].
The effective implementation of mHealth applications for OA management will require the development of applications that are easy to use, accessible and meet the expectations of physicians in addition to patient needs [31]. Designers of smart technology should include easy communication between the individual and the technology [38]. The nature and content of the training might also be a barrier to use of smart technology (time required to learn to use the device, how to use, how to interpret) [39]. Furthermore, additional smart technologies for self-management exist in terms of chronic pain more generally, or specifically for musculoskeletal and/or rheumatic conditions (e.g. JIA), especially in the case of adolescents and young adults.
Implications
Gaps and opportunities for research
The present study is a narrative review focused on the current literature regarding smart technology applied to OA self-management across the lifespan. Our search revealed a dearth of knowledge regarding this important topic. We identified four quantitative and three qualitative studies addressing the use of smart technology for pain and symptom management in OA, with all studies including only adults. Although the risk for OA is substantially lower among children and adolescents, it is important to consider how this technology can be adapted effectively across the lifespan. This will be especially important as cases of OA increase owing to trauma survival and increased life expectancy.
Older adults are the fastest growing population worldwide [40]. When compared with younger adults, older adults might be less likely to adopt new technology, such as smartwatches or other wearables [39]. Inclusion of older adults in clinical trials of smart technology for OA is particularly important considering that they are predominantly affected by OA pain and disability [41]. Parker et al. [42] conducted several focus groups with older adults to identify barriers to the use of mHealth technology for pain management, and several barriers identified included product affordability and lack of familiarity with mHealth devices. The authors noted that a significant number of older adults lacked prior exposure to mHealth technology, but this barrier could be overcome with access and exposure to mHealth devices via community-based programmes. Manini et al. [27] found that for older adults, the use of a smartwatch design might be familiar enough to overcome learning barriers and promote the use of smartwatches for OA pain assessment. Prior studies included in this review highlight the positive attitude of older adults to mHealth applications [27]. Nevertheless, few intervention trials have focused on smart technology for OA symptoms among older adults. Future research is needed to determine preferences and acceptability, in addition to effectiveness, of smart technology as part of an integrative treatment strategy for OA pain and symptom management.
Given that our review focused solely on OA, a condition predominantly impacting mid-life to older adults, this restricted our ability to gauge the use of smart technology for pain self-management among adolescents and young adults, thus limiting our capacity to examine use from a lifespan perspective. Arguably, understanding the implementation of these methods for pain and pain-related symptoms (e.g. sleep disturbance, disability) over the life course holds significant value for public health. Although beyond the scope of the present review, there is evidence to suggest that digital technology can serve as an effective tool among children and adolescents with chronic musculoskeletal pain [43]. Extending this review to musculoskeletal pain more broadly (e.g. inclusion of JIA) might yield more information about the utilization and efficacy of smart technology for pain self-management tools that are available and effective for individuals across generations.
Gaps and opportunities for clinical practice
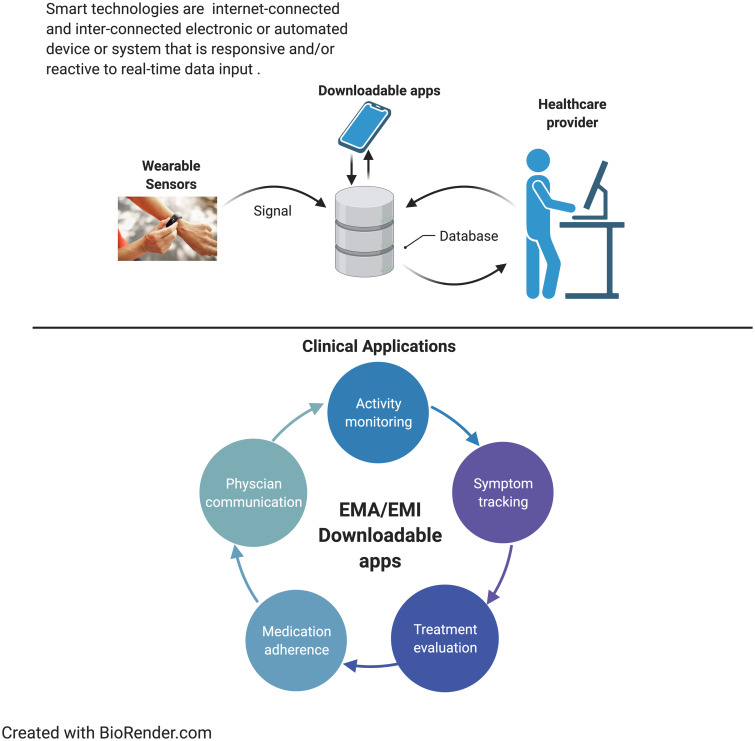
Smart technology appears to be a promising tool that might be used to improve OA-related pain outcomes (Fig. 1). Although studies provide preliminary evidence that these technologies have a positive impact on OA-related symptoms (i.e. decreased opioid use, decreased pain and improved sleep), there are several important considerations for clinical practice. First, wearable smart devices (wearable smartphones and tablets) might be cost prohibitive or simply inaccessible for some patients, which could exacerbate health-care disparities. Data from the 2012 Health Information National Trends Survey (HINTS) revealed that patients of low socioeconomic status were engaging less frequently with electronic health [44]. Second, studies investigating the long-term use of smart technology are needed to determine whether patient compliance in use and benefits are maintained. Third, it is crucial that patient preferences and clinical guidelines be addressed in the application of smart technologies for OA. Fourth, patients might need booster sessions to improve implementation and compliance in use of the smart technology over the long term, including training in the use of such technologies. Finally, health-care providers can devise ways to incorporate the output from the smart technology device to communicate ways to continue to improve the patient-related outcomes between visits. Importantly, for continuity of care and real-time assessment, it will be crucial for smart devices to be linked to electronic health records/electronic medical records in order to capture the dynamic patterns of pain and to serve as an important source of health outcome data. Optimization of smart technologies for OA will require an interdisciplinary approach, integrating the expertise of pain specialists, rheumatologists, primary care providers, patients, engineers, information technologists, data scientistis, and nurses with informatics training.
Fig. 1.
Smart technology applications for OA
EMA: Ecological Momentary Assessment; EMI: Ecological Momentary Intervention
Gaps and opportunities for technological advancement
Technological advances provide us with opportunities for self-management interventions that are cost effective for the medical community and convenient for patients, such as the expansion of wearable devices and mobile apps for smartphones. These technologies are readily available and can be accessed anywhere or anytime (untethered). The convenience of untethered apps and wearables creates opportunities for self-monitoring and reporting, including OA-related pain, physical activity and analgesic adherence. Mobile and Internet/computer-based interventions have been used successfully for the education of health issues such as weight loss [45–47], asthma [48] and diabetes [49, 50]. However, there is scant research on developing evidence-based mobile apps or wearables focused on OA and investigating their effectiveness in OA management. To fill this gap, future research is needed on incorporating artificial intelligence and adaptive nanotechnology for diagnosis, monitoring and managing pain symptoms of OA and for the evaluation of effectiveness of regenerative and precision pain treatment [51]. Specifically, research must explore how to use artificial intelligence and machine learning to leverage the responsiveness and adaptability of smart technologies to automate data-driven interventions.
Future directions should also look to extend beyond direct pain management but also to use smart technology to monitor, track and manage pain-related events, such as falls and opioid-related overdoses. For example, given that a significant proportion of older adults with chronic musculoskeletal pain also report recurrent falls [52], smart technology that includes mechanisms for motion capture [53] and includes sensors to monitor gait and risk of falling in people with severe, disabling OA is a novel use of technology. Additionally, smart orthotic devices and other smart clothing technology might also support individuals with hip, knee and foot OA [54].
Limitations
Although a systematic process was used to search and review the literature, this was a narrative review; therefore, we did not perform quality assessments of the studies as typically done in systematic reviews and meta-analyses. The limited number of studies, various types of smart technology used and different outcome measurements limit the conclusions drawn and the opportunity to conduct a meta-analysis or qualitative meta-synthesis. The use of various types of smart technology in health-related topics (i.e. tracking adherence, management of health conditions and provider–patient communication) is becoming more popular among researchers and clinicians. Our inclusion criteria for pain management in OA precluded the examination of literature related to the use of smart technology for other health-related conditions. Given that this is a rapidly evolving area of research/interest, it is likely that studies have been published since our original search. Rather, at this stage in the genesis of smart technology, a narrative review of the types and ways that technology has been employed for pain management in OA is more appropriate.
Conclusion
The future of technology is now. Real-world application of smart technologies is quickly becoming an integral part of daily life, including those with chronic conditions such as OA. This review highlights the potential for smart technology to improve pain in OA. However, there are some current pitfalls that limit large scaling to various technologies. More RCTs and pragmatic clinical trials are needed to gain a better understanding of the usability, feasibility, efficacy, effectiveness and safety of smart technologies and their integration into routine care. In conclusion, self-management of chronic OA-related pain might be positively augmented by smart technology.
Funding: Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427; NIH/NIAMS K23AR076463 (S.Q.B.); NIH/NINDS K22NS102334 (E.L.T.); NIH/NHLBI K01HL153210 (K.P.-R.); NIH/NIA P30AG59297-01 (S.Q.B., K.P.-R.); and NIH/NIA T32AG049673 (S.P., K.P.-R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
No new or original data were generated or analysed in support of this research.
Contributor Information
Alisa J Johnson, Pain Research and Intervention Center of Excellence, Department of Community Dentistry and Behavioral Science, College of Dentistry.
Shreela Palit, Pain Research and Intervention Center of Excellence, Department of Community Dentistry and Behavioral Science, College of Dentistry.
Ellen L Terry, Pain Research and Intervention Center of Excellence, Department of Community Dentistry and Behavioral Science, College of Dentistry; Department of Biobehavioral Nursing Science, College of Nursing.
Osheeca J Thompson, Pain Research and Intervention Center of Excellence, Department of Community Dentistry and Behavioral Science, College of Dentistry.
Keesha Powell-Roach, Pain Research and Intervention Center of Excellence, Department of Community Dentistry and Behavioral Science, College of Dentistry; Department of Biobehavioral Nursing Science, College of Nursing.
Brenda W Dyal, Department of Biobehavioral Nursing Science, College of Nursing.
Margaret Ansell, George A. Smathers Libraries, Health Science Center Libraries, University of Florida, Gainesville, FL, USA.
Staja Q Booker, Pain Research and Intervention Center of Excellence, Department of Community Dentistry and Behavioral Science, College of Dentistry; Department of Biobehavioral Nursing Science, College of Nursing.
References
- 1. Kloppenburg M, Berenbaum F.. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage 2020;28:242–8. [DOI] [PubMed] [Google Scholar]
- 2. Kolasinski SL, Neogi T, Hochberg MC. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace IJ, Worthington S, Felson DT. et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A 2017;114:9332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennan-Olsen SL, Cook S, Leech MT, Bowe SJ. et al. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization Study on global AGEing and adult health (SAGE) Wave 1. BMC Musculoskelet Disord 2017;18:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian S, Yang W, Le Grange JM. et al. Smart healthcare: making medical care more intelligent. Global Health J 2019;3:62–5. [Google Scholar]
- 6. Garver MJ, Focht BC, Taylor SJ.. Integrating lifestyle approaches into osteoarthritis care. J Multidiscip Healthc 2015;8:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malfait A-M, Schnitzer TJ.. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol 2013;9:654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain 2017;158(Suppl 1):S11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deveza LA, Melo L, Yamato TP. et al. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage 2017;25:1926–41. [DOI] [PubMed] [Google Scholar]
- 10. Choojaturo S, Sindhu S, Utriyaprasit K, Viwatwongkasem C.. Factors associated with access to health services and quality of life in knee osteoarthritis patients: a multilevel cross-sectional study. BMC Health Serv Res 2019;19:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Owens B. Opioid prescriptions down but some patients fear doctors now too strict. CMAJ 2019;191:E546–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N.. Availability and utilization of opioids for pain management: global issues. Ochsner J 2014;14:208–15. [PMC free article] [PubMed] [Google Scholar]
- 13. Peppin JF, Cheatle MD, Kirsh KL, McCarberg BH.. The complexity model: a novel approach to improve chronic pain care. Pain Med 2015;16:653–66. [DOI] [PubMed] [Google Scholar]
- 14. Papi E, Murtagh GM, McGregor AH.. Wearable technologies in osteoarthritis: a qualitative study of clinicians' preferences. BMJ Open 2016;6:e009544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solomon DH, Rudin RS.. Digital health technologies: opportunities and challenges in rheumatology. Nat Rev Rheumatol 2020;16:525–35. [DOI] [PubMed] [Google Scholar]
- 16.Osteoarthritis Action Alliance, Centers for Disease Control and Prevention, & Arthritis Foundation. A national public health agenda for osteoarthritis: 2020 update. Retrieved from cdc.gov/arthritis/docs/oaagenda2020.pdf.
- 17. Buck HG, Shadmi E, Topaz M, Sockolow PS.. An integrative review and theoretical examination of chronic illness mHealth studies using the Middle-Range Theory of Self-care of Chronic Illness. Res Nurs Health 2021;44:47–59. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen AD, Baysari MT, Kannangara DR. et al. Mobile applications to enhance self-management of gout. Int J Med Inform 2016;94:67–74. [DOI] [PubMed] [Google Scholar]
- 19. Ikeda K, Sekiguchi N, Hirai T. et al. Securely collecting multidimensional health information from patients with rheumatoid arthritis using smart device technology: beneficial effect for physicians and patients. Musculoskelet Care 2018;16:494–9. [DOI] [PubMed] [Google Scholar]
- 20. Coda A, Sculley D, Santos D. et al. Harnessing interactive technologies to improve health outcomes in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2017;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Najm A, Lempp H, Gossec L, Berenbaum F, Nikiphorou E.. Needs, experiences, and views of people with rheumatic and musculoskeletal diseases on self-management mobile health apps: mixed methods study. JMIR Mhealth Uhealth 2020;8:e14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lalloo C, Shah U, Birnie KA. et al. Commercially available smartphone apps to support postoperative pain self-management: scoping review. JMIR Mhealth Uhealth 2017;5:e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safari R, Jackson J, Sheffield D.. Digital self-management interventions for people with osteoarthritis: systematic review with meta-analysis. J Med Internet Res 2020;22:e15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brodie M, Flournoy RE, Altman DE. et al. Health information, the Internet, and the digital divide. Health Aff 2000;19:255–65. [DOI] [PubMed] [Google Scholar]
- 25. Mecklenburg G, Smittenaar P, Erhart-Hledik JC, Perez DA, Hunter S.. Effects of a 12-week digital care program for chronic knee pain on pain, mobility, and surgery risk: randomized controlled trial. J Med Internet Res 2018;20:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pronk Y, Peters MCWM, Sheombar A, Brinkman J-M.. Effectiveness of a mobile eHealth app in guiding patients in pain control and opiate use after total knee replacement: randomized controlled trial. JMIR Mhealth Uhealth 2020;8:e16415-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manini TM, Mendoza T, Battula M. et al. Perception of older adults toward smartwatch technology for assessing pain and related patient-reported outcomes: pilot study. JMIR Mhealth Uhealth 2019;7:e10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaslavsky O, Thompson HJ, McCurry SM. et al. Use of a wearable technology and motivational interviews to improve sleep in older adults with osteoarthritis and sleep disturbance: a pilot study. Res Gerontol Nurs 2019;12:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhattarai P, Newton-John TRO, Phillips JL.. Apps for pain self-management of older people's arthritic pain, one size doesn't fit all: A qualitative study. Arch Gerontol Geriatr 2020;89:104062. [DOI] [PubMed] [Google Scholar]
- 30. Lin D, Papi E, McGregor AH.. Exploring the clinical context of adopting an instrumented insole: a qualitative study of clinicians’ preferences in England. BMJ Open 2019;9:e023656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barber T, Sharif B, Teare S. et al. Qualitative study to elicit patients' and primary care physicians' perspectives on the use of a self-management mobile health application for knee osteoarthritis. BMJ Open 2019;9:e024016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belsi A, Papi E, McGregor AH.. Impact of wearable technology on psychosocial factors of osteoarthritis management: a qualitative study. BMJ Open 2016;6:e010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James SL, Abate D, Abate KH. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albert SM, Musa D, Kwoh CK, Hanlon JT, Silverman M.. Self-care and professionally guided care in osteoarthritis: racial differences in a population-based sample. J Aging Health 2008;20:198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Booker S, Herr K, Tripp-Reimer T.. Patterns and perceptions of self-management for osteoarthritis pain in African American older adults. Pain Med 2019;20:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson AJ, Sibille KT, Cardoso J. et al. Patterns and correlates of self-management strategies for osteoarthritis related pain among older non-Hispanic Black and non-Hispanic White adults. Arthritis Care Res 2020; doi: 10.1002/acr.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bannuru R, Osani M, Vaysbrot E. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. [DOI] [PubMed] [Google Scholar]
- 38. Cabrita M, Tabak M, Vollenbroek-Hutten MM.. Older adults' attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging 2019;2:e10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee C, Coughlin JF.. PERSPECTIVE: older adults' adoption of technology: an integrated approach to identifying determinants and barriers. J Prod Innov Manag 2015;32:747–59. [Google Scholar]
- 40. Centers for Disease Control and Prevention (CDC). Trends in aging–United States and worldwide. MMWR Morbid Mortal Wkly Rep 2003;52:101–4, 106. [PubMed] [Google Scholar]
- 41. Palazzo C, Nguyen C, Lefevre-Colau M-M, Rannou F, Poiraudeau S.. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 2016;59:134–8. [DOI] [PubMed] [Google Scholar]
- 42. Parker SJ, Vasquez R, Chen EK. et al. A comparison of the Arthritis Foundation Self-Help Program across three race/ethnicity groups. Ethn Dis 2011;21:444–50. [PMC free article] [PubMed] [Google Scholar]
- 43. Palermo TM, de la Vega R, Murray C, Law E, Zhou C.. A digital health psychological intervention (WebMAP Mobile) for children and adolescents with chronic pain: results of a hybrid effectiveness-implementation stepped-wedge cluster randomized trial. Pain 2020;161:2763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kontos E, Blake KD, Chou WY, Prestin A.. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res 2014;16:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee W, Chae YM, Kim S, Ho SH, Choi I.. Evaluation of a mobile phone-based diet game for weight control. J Telemed Telecare 2010;16:270–5. [DOI] [PubMed] [Google Scholar]
- 46. Turner-McGrievy G, Tate D.. Tweets, Apps, and Pods: Results of the 6-month Mobile Pounds Off Digitally (Mobile POD) randomized weight-loss intervention among adults. J Med Internet Res 2011;13:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turner-McGrievy GM, Beets MW, Moore JB. et al. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc 2013;20:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu W-T, Huang C-D, Wang C-H. et al. A mobile telephone-based interactive self-care system improves asthma control. Eur Respir J 2011;37:310–7. [DOI] [PubMed] [Google Scholar]
- 49. Kirwan M, Vandelanotte C, Fenning A, Duncan MJ.. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res 2013;15:e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pal K, Eastwood SV, Michie S. et al. Computer-based interventions to improve self-management in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2014;37:1759–66. [DOI] [PubMed] [Google Scholar]
- 51. Mohammadinejad R, Ashrafizadeh M, Pardakhty A. et al. Nanotechnological strategies for osteoarthritis diagnosis, monitoring, clinical management, and regenerative medicine: recent advances and future opportunities. Curr Rheumatol Rep 2020;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stubbs B, Eggermont L, Patchay S, Schofield P.. Older adults with chronic musculoskeletal pain are at increased risk of recurrent falls and the brief pain inventory could help identify those most at risk. Geriatr Gerontol Int 2015;15:881–8. [DOI] [PubMed] [Google Scholar]
- 53. Banach M, Wasilewska A, Dlugosz R, Pauk J.. Novel techniques for a wireless motion capture system for the monitoring and rehabilitation of disabled persons for application in smart buildings. Technol Health Care 2018;26:671–7. [DOI] [PubMed] [Google Scholar]
- 54. Underwood S. Smart clothing and disability: wearable technology for people with arthritis. In McCann J and Bryson D, eds, Smart Clothes and Wearable Technology, 2009:371–387.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new or original data were generated or analysed in support of this research.