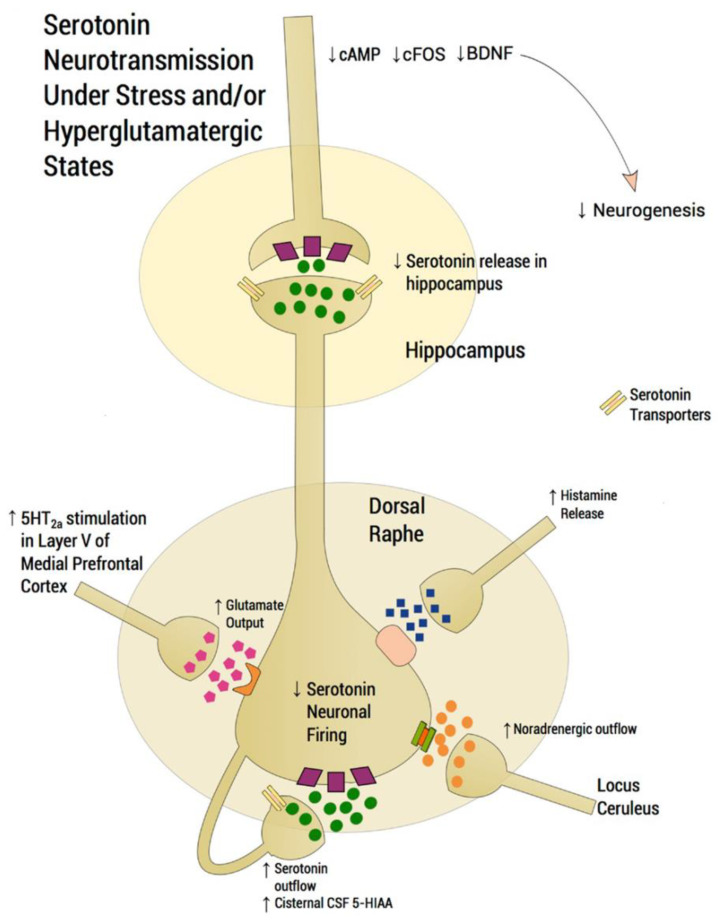
Figure 2.
Scheme depicting the functioning of a dorsal raphe nucleus (DRN) serotoninergic neuron under stress conditions that induce depression disorders (modified from [91]). In normal conditions, glutamatergic excitatory afferents from pyramidal neurons of the medial prefrontal cortex (mPFC) directly activate the DRN, thus increasing extracellular serotonin (5-HT), which is regulated by somatodendritic 5-HT1A autoreceptors, decreasing the excessive release in projection areas such as the mPFC and hippocampus. Under stress conditions, however, glutamatergic neurotransmission is hyperactivated, flooding the DRN with 5-HT (i.e., so-called “5-HT flooding”), but concomitantly attenuating 5-HT outflow. An excessive stimulation of 5-HT2ARs in the mPFC increases glutamatergic input, whereas an excessive stimulation of postsynaptic 5-HT1ARs in the hippocampus is associated with a deficit at corticolimbic projection sites, thus compromising neuroplastic processes. Based on this scheme, it is possible that SSRIs alleviate depression symptoms through two processes involving nAChRs: (1) inhibiting presynaptic nAChRs at glutamatergic afferents, which decreases 5-HT flooding to the DRN, and (2) inhibiting postsynaptic nAChRs, which attenuates DRN activity [7,92,93,94]. Since noradrenaline (NE) and histamine are also increased during stress/depression conditions, 5-HT neuron firing is inhibited by NE transmission from the locus coeruleus through α2-heteroceptors and by histamine transmission through histamine-1 receptors. Serotonin transporters are located presynaptically. CSF: cerebrospinal fluid; 5-HIAA: 5-hydroxyindoleacetic acid.