Abstract
Patient-generated health data (PGHD), such as patient-reported outcomes and mobile health data, have been increasingly used to improve health care delivery and outcomes. Integrating PGHD into electronic health records (EHRs) further expands the capacities to monitor patients’ health status without requiring office visits or hospitalizations. By reviewing and discussing PGHD with patients remotely, clinicians could address the clinical issues efficiently outside of clinical settings. However, EHR-integrated PGHD may create a burden for clinicians, leading to burnout. This study aims to investigate how interactions with EHR-integrated PGHD may result in clinician burnout. We identify the potential contributing factors to clinician burnout using a modified FITT (Fit between Individuals, Task and Technology) framework. We found that technostress, time pressure, and workflow-related issues need to be addressed to accelerate the integration of PGHD into clinical care. The roles of artificial intelligence, algorithm-based clinical decision support, visualization format, human-computer interaction mechanism, workflow optimization, and financial reimbursement in reducing burnout are highlighted.
Keywords: patient-generated health data, patient-reported outcomes, mobile health, clinician burnout, human-computer interaction, electronic health record
INTRODUCTION
In recent years, health-related data are increasingly collected by technologies such as portable devices with embedded sensors,1,2 remote monitoring devices, wearable devices,3 and smartphone apps.4 These data can be collected continuously outside of the clinical settings and be shared with healthcare providers (HCPs). Health-related data that are generated, recorded, or gathered by patients outside of the clinical setting without the assistance of HCPs are termed patient-generated health data (PGHD).5 Coupled with deployed electronic health records (EHRs), patient portals, and secure messaging, these new types of data enable patients to actively engage in the health care process, further improving the connection with their HCPs. In this way, the breadth, depth, and continuity of traditional health-related data are expanded, thus contributing to improved treatment adherence, health outcomes,6 healthcare quality, and patient safety.
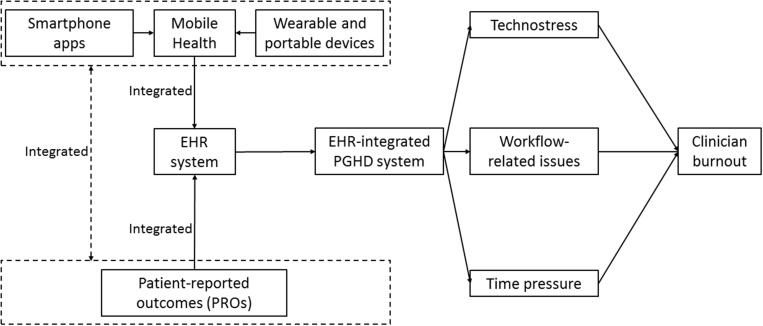
This study examines how interactions with EHR-integrated PGHD may result in clinician burnout and identifies potential contributing factors, including technostress, time pressure, and workflow-related issues. Figure 1 demonstrates the relationship between EHR-integrated PGHD and contributors to clinician burnout.
Figure 1.
Conceptual model of the integration of electronic health record (EHR) and patient-generated health data (PGHD) with the impact on clinician burnout. PRO: patient-reported outcome.
PGHD AND EHR INTEGRATION
There are 2 main differences between PGHD and traditional health-related data generated within clinical settings5:
The data are gathered and recorded by patients outside the physicians’ offices and no medical encounters are needed.
It is the patients who decide and take actions to share or distribute the data to HCPs or other stakeholders.
In this article, we focus on 2 main elements of PGHD: patient-reported outcomes (PROs) and mobile health (eg, mobile apps, wearable or portable devices). Table 1 illustrates the 2 elements and their association with burnout.
Table 1.
PROs and mHealth and their association with burnout
| PGHD Domain | Definition | Association With Burnout |
|---|---|---|
| PROs |
|
Main barriers to integrating PROs data into EHR: 1. Work overload. Clinicians are concerned that adding PROs will make their work burdensome12; 2. Lack of actionable guidance13; 3. Lack of validity of PRO scores to sufficiently support clinical decision-making; 4. Lack of financial incentives. Clinicians have no motivation to increase their job responsibilities without improved payment models14; 5. Low level of engagement of patients in completing PRO assessment. Providers have to spend extra time to explaining the purpose and assisting patients in completing the tasks.13 |
| mHealth |
|
Main barriers to integrating mHealth data into EHR: 1. Wearable device data are too noisy to be useful before compilation and interpretation by HCPs17; 2. HCPs may experience more alert fatigues in the clinical support systems18; 3. While some health systems and vendors have begun to develop user-centered design approaches to adapt workflows and collaborate with third-party wearable devices to improve the integration of PGHD and EHR, data interoperability and visualization still impede the connection between wearable PGHD and EHRs.19 |
EHR: electronic health record; mHealth: mobile health; PGHD: patient-generated health data; PRO: patient-reported outcome.
PGHD AND CLINICIAN BURNOUT
Clinician burnout is not only a syndrome of emotional exhaustion, but also a type of cynicism about job responsibilities.20 With long-term demanding working paces, emotional intensity, and stressful environment, clinicians are becoming more and more likely to be exposed to burnout. Burnout is a reaction marked by lacking the sense of accomplishment, feeling emotionally exhausted, and experiencing depersonalization.
Burnout has a variety of negative consequences. For physical health, burnout may lead to type 2 diabetes,21 arteriosclerotic disease,22 cardiovascular diseases,23 and coronary heart disease.24 Clinicians who are often experiencing burnout may have more risks to develop musculoskeletal pain, which may even cause hospitalizations.23 Moreover, respiratory issues and gastrointestinal problems25 as well as prolonged fatigues26 have been proven to be significantly associated with burnout. For occupational health, burnout may lead to less job satisfaction27 and reduced productivity,28 thus reducing patient safety29 and healthcare quality.30 Healthcare workers who felt emotionally exhausted and depersonalized under the high demand of their responsibilities were affected by burnout.31 Owing to the emotional pressure and syndrome subdimensions of cynicism, burnout could result in long-term sickness absence.32 For mental health, as a prospective predictor of depressive symptoms,33 burnout may lead to insomnia,34 mental disorders, and other psychological diseases that may further cause hospitalizations.23,35
We identify 3 factors related to EHR-integrated PGHD that can contribute to clinician burnout.
Technostress
We categorize the causes of technostress with regard to EHR-integrated PGHD systems (Table 2). All of these factors might result in clinician burnout36,37 and would be helpful to precisely identify or evaluate the extent to which technostress is introducing burnout to clinicians.
Table 2.
Technostress in EHR-integrated PGHD systems
| Causes of Technostress | Conditions |
|---|---|
| Techno-complexity | EHR-integrated PGHD systems would add new elements and functions. For some physicians, these new functions add complexities to their jobs. More time and efforts are needed in understanding and practicing new skills. Owing to the unsolved standardization issues, various jargon and complicated operation steps make physicians more intimidated and stressed. |
| Techno-uncertainty | Physicians are not trained as engineers; they are not in favor of continuing technology upgrades and short life cycles of human-computer interaction systems. Taking care of patients is already overwhelming, and being pushed to update knowledge and relearn technical-related skills rapidly makes them unsettled. |
| Techno-overload | EHR-integrated PGHD systems incorporate more data than traditional EHRs. Physicians have to work harder and even faster because the time has not been extended. |
| Techno-insecurity | Owing to the different personal characteristics, some physicians who are experiencing technophobia38 may dislike or fear technologies such as the PGHD system. Compared with young physicians or those who can better understand and handle new technologies, these physicians may lag behind and even lose their jobs. |
| Techno-invasion | With the implementation of EHR-integrated PGHD systems, patients may set higher expectations of their providers. Physicians are supposed to be reached whenever patients want to connect with them and respond to their PGHD-related queries or concerns. Sometimes, patients may even ask technical questions far beyond physicians' responsibilities. This makes physicians expand their regular roles and extend their work hours. |
EHR: electronic health record; PGHD: patient-generated health data.
Time pressure
There are several mechanisms of health-related data sharing between patients and HCPs: patients directly report to HCPs, third party platforms perform health information exchange, EHR integration, etc.
Patient-directed mechanisms rely on the patients to manually send or show their data to the HCPs. This type of data sharing may be in-person or digital (eg, via email), which may cause a large burden on the HCPs, who are expected to interpret the data before or during the patient visit under strict time constraints. Linzer et al39 created a formula to measure time pressure: time pressure ratio = (time needed to provide quality care—time allotted) / time allotted. If PGHD does not go through smoothly or as planned, additional time would be needed from HCPs to provide quality care, while the allotted time is always constant, so that the ratio would be increased. Time pressure is an important source of job dissatisfaction in many domains of healthcare settings.39
Workflow-related issues
Despite the merits of PGHD-EHR integration, it may also contribute to information overload. In some cases, the PGHD systems send the patient’s data directly to a physician, likely through their mobile phones or electronic messaging systems. This mechanism, while efficient in urgent situations, may be burdensome to HCPs and can lead to fatigue if not all notifications are relevant.40 Lack of interoperability is another concern. Third-party platforms may create new systems outside of the EHR or HCPs’ working systems, leading to fragmented information sources that can be burdensome to the HCPs who have to check multiple sources of data before making a clinical decision. The interoperability issue may render the data unreadable by the HCP, which may also upset patients when their expectations are not fulfilled.
DISCUSSION
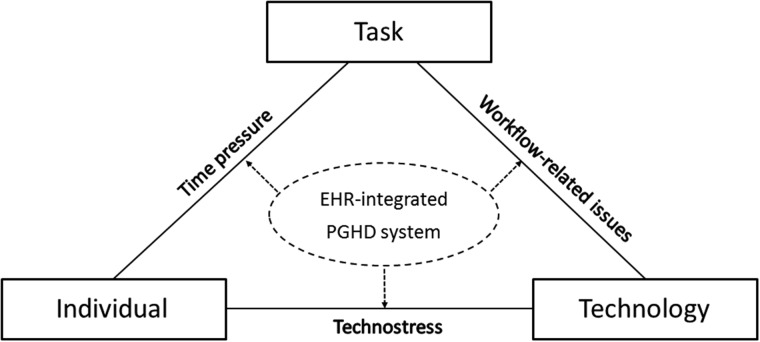
In this article, we have identified 3 factors that may contribute to clinician burnout related to PGHD-EHR integration: technostress, time pressure, and workflow-related issues. These factors can be mapped onto the modified FITT (fit between individuals, task and technology) framework (see Figure 2).41 In this section, we propose several recommendations to resolve the barriers to PGHD-EHR integration so as to reduce clinician burnout.
Figure 2.
Modified FITT (Fit between Individuals, Task and Technology) framework of electronic health record (EHR)–integrated patient-generated health data (PGHD) and factors associated with clinician burnout.
Suggestions for solving the impact of technostress on clinician burnout
To perform PGHD-EHR integration, HCPs need to have clear role assignments. That is, when they should respond to PGHD, when to interfere and take over care, what to do, and how to interfere. Best practices and protocols are needed to guide HCPs. The development and application of the FHIR (Fast Healthcare Interoperability Resources) data standard have offered opportunities to improve PGHD presentation and visualization.42
Improving the usability and usefulness of the EHR-integrated PGHD systems can help to reduce technostress. Physicians always feel frustrated and anxious about the unfamiliar interfaces and insufficient instructions. If physicians find it easy and comfortable to review and interpret PGHD, then burnouts will decrease. PGHD visualizations, such as text results alongside numeric scores, pictographs, trending lines, flag signs, and data visualized in chronological order, are useful approaches.43 HCPs and researchers should work together with other stakeholders such as health information technology vendors, wearable device and mobile health vendors, data visualization designers, cognitive scientists, and data scientists to develop and improve the frictionless user experience and usability for optimal uptake.44 For example, PGHD should be visualized as an aggregated and descriptive manner so that visualizations and any correlations could be seen at once. HCPs can recognize patterns and extrapolate meaningful findings without jumping to different screens or clicking different functional buttons or logging into other interfaces. A customized dashboard with real-time health data visualization may be helpful for HCPs to find the patients they need to care for and the most relevant information (eg, medical history from PROs).
To avoid unrealistic expectations, patients and HCPs should be mutually aware of each other’s needs; this bidirectional relationship can be achieved by adding some communication mechanisms in the integrated system. Meanwhile, other relevant stakeholders such as technology vendors, hospital leaders, and reimbursing agencies need to reconcile differences in the expectations of using PGHD with appropriate policies and regulations.19 Keeping ownership of PGHD with patients may be another approach that may change the current landscape. Patients have discretion over whether, when, or how to share these data; this patient-driven empowerment may help to adjust the expectation of HCPs and their engagement. Meanwhile, HCPs have the privilege to set some parameters in the integrated system. For example, clinicians can adjust the frequency at which they want to receive PGHD, and patients cannot transmit any data to clinicians without an explicit order.
Technostress could also be reduced by education and training, which could be incorporated into medical boards and continuing education. Trainees include medical assistants, nurses, physicians, practice professionals, and even a broader range of participants. Training should include using PGHD to support shared decision making, patient-provider communication, etc.45
Suggestions for solving the impact of time pressure on clinician burnout
To reduce the time pressure, the EHR systems could develop standardized templates and data types for PGHD integration, so that providers can check the standardized summaries without spending time sifting through PGHD.
Financial reimbursement is important to relieve time pressure. Reimbursement models should align with the use of PGHD.46 Assigning current procedural terminology codes and the development of quality measurements associated with PGHD may facilitate reimbursement for reviewing it.47 To build a supportive infrastructure and proper incentives, it would be critical to measure the extra time spent on PGHD-related care management, home visit, or clinical consultation.45 Health systems can conduct retrospective analyses of the operationalization of work demands and resources of HCPs, which could provide more insights for the tasks that are particularly demanding or poorly designed for PGHD. The shift-work systems may be optimized to meet the personal preferences and ergonomic criteria of the HCPs, such as rescheduling of the working programs, redesigning workplace and resource allocation for PGHD, providing technical education and support, etc.48
Incorporating artificial intelligence (AI) and algorithm-based approaches49,50 into the EHR-integrated system would enable intelligent filters and customizable alerts for clinicians, providing critical and digestible information rather than large volumes of data. This automated decision support system may assist in cleaning meaningless data and generating feasible action items for clinicians. Clinical symptoms or diseases may be identified automatically by algorithms using PGHD, and the integrated system then could provide guidelines to suggest self-management strategies for patients or tailored treatment strategies for HCPs.51 All the information should be displayed on a single screen without the need for reviewing multiple windows. Incorporated with advanced clinical decision support, the integrated system can algorithmically identify which patients need attention based on the validated and robust prediction models. In addition, machine learning or deep learning algorithms are helpful to support decision making for patients such as when to interact with the clinician.52 That being said, these benefits of AI have not been validated thoroughly, and more research is needed to implement and evaluate the AI-assisted EHR-integrated PGHD system.
Suggestions for solving the impact of workflow-related issues on clinician burnout
Making workflows automatic eases the clinical operations and decreases clinicians’ workload. For example, the algorithm-embedded EHR-integrated PGHD can display a patient's medical records with understandable visualizations and send reminders to clinicians for upcoming patient-provider interaction in advance. Specifically, clinicians may want the PROs to be presented as fixed and structured templates implemented in the existing EHRs so that the review process could be streamlined; they just need to click on a few buttons, and all the necessary results would pop up. The operational process needs clear instruction. Some key elements in the workflow should be considered, including (1) entities that could be responsible for reviewing PGHD and make necessary responses—depending on the severity, they could be provider, practice, or hospital-level representatives; (2) optimal time frame and frequency of reviewing PGHD and responding; (3) modalities that HCPs can use (eg, secure message, phone call, telehealth); and (4) types of PGHD that are most valuable for self-monitoring and clinical decision support.53
Health systems should regularly evaluate the impact of the EHR-integrated PGHD on clinicians, eliminate redundancies, and simplify the workflows. Examples include building systems that allow for inter-professional sharing of relevant work, using organization-level rapid-cycle improvement approaches to do outcomes-driven workflow redesign, streamlining the process of the integration and application of PGHD and EHR, etc. The implementation, evaluation, and upgrade of EHR-integrated PGHD systems should incorporate the perspectives of the frontline end users, including clinicians and patients, for human-centered design.
Participating in quality improvement programs that focus on optimizing the workflow of EHR-integrated PGHD is also an effective way to reduce clinician burnout, especially for primary care clinics.54 Through taking advantage of resources and strategies that quality improvement programs provide, health systems can redesign the workflow by conducting system-level optimization and maintain sustainable improvement. Meanwhile, the process may also enhance the level of value alignment with leadership and alleviated cynicism, which in turn may help reduce clinician burnout.
In EHR-integrated PGHD, quality control is an important task. The AI-integrated system can automatically identify and remind the clinicians of defective diagnosis and treatment items and specific reasons for the recommendation. If a certain drug is not prescribed or the dosage is insufficient, the system can remind HCPs to click the quality control prompt to complete the corresponding treatment. The AI-integrated system can also send timely feedback on the healthcare quality control report to the hospital stakeholders. There are clinical texts in the EHR, and some health systems may have patient-to-provider electronic messages or unstructured biomedical data. All those data are useful information for the AI-integrated and algorithm-enabled system. Also, social media messages could be treated as one type of PGHD, which may have a critical impact on patients’ mental health.55 Using natural language processing technology and a series of diagnosis and treatment algorithms to fully understand the details and logical relationships of the data not only can enable better integration, but also can improve the comprehensiveness and rationality of clinical decision support, which may further help to reduce clinician burnout.
FUNDING
None.
AUTHOR CONTRIBUTIONS
JY was responsible for the study design, data analysis, and interpretation of results for the work, and wrote the manuscript.
DATA AVAILABILITY
The data underlying this article are available in the article.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1. Ye J, Li N, Lu Y, et al. A portable urine analyzer based on colorimetric detection. Anal Methods 2017; 9 (16): 2464–71. [Google Scholar]
- 2. Zhang J, Fu R, Xie L, et al. A smart device for label-free and real-time detection of gene point mutations based on the high dark phase contrast of vapor condensation. Lab Chip 2015; 15 (19): 3891–6. [DOI] [PubMed] [Google Scholar]
- 3. Sim I. Mobile devices and health. N Engl J Med 2019; 381 (10): 956–68. [DOI] [PubMed] [Google Scholar]
- 4. Mosa ASM, Yoo I, Sheets L.. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak 2012; 12 (1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro M, Johnston D, Wald J, Mon D.. Patient-Generated Health Data. Research Triangle Park, NC: RTI International; 2012. [Google Scholar]
- 6. Patel RA, Klasnja P, Hartzler A, Unruh KT, Pratt A.. Probing the benefits of real-time tracking during cancer care. AMIA Annu Symp Proc 2012; 2012: 1340–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013; 346 (1): f167. [DOI] [PubMed] [Google Scholar]
- 8. Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 2004; 22 (17): 3485–90. [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Ou L, Hollis SJ.. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 2013; 13 (1): 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Detmar SB, Muller MJ, Schornagel JH, et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 2002; 288 (23): 3027–34. [DOI] [PubMed] [Google Scholar]
- 11. Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract 2014; 10 (4): e215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson EC, Eftimovska E, Lind C, Hager A, Watson JH, Lindblad S.. Patient reported outcome measures in practice. BMJ 2015; 350: g7818. [DOI] [PubMed] [Google Scholar]
- 13. Rotenstein LS, Agarwal A, O’Neil K, et al. Implementing patient-reported outcome surveys as part of routine care: lessons from an academic radiation oncology department. J Am Med Inform Assoc 2017; 24 (5): 964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harle CA, Listhaus A, Covarrubias CM, et al. Overcoming barriers to implementing patient-reported outcomes in an electronic health record: a case report. J Am Med Inform Assoc 2016; 23 (1): 74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye J, Ma Q.. The effects and patterns among mobile health, social determinants, and physical activity: a nationally representative cross-sectional study. AMIA Jt Summits Transl Sci Proc 2021; 2021. [PMC free article] [PubMed] [Google Scholar]
- 16. Ye J. The role of health technology and informatics in a global public health emergency: practices and implications from the COVID-19 pandemic. JMIR Med Inform 2020; 8 (7): e19866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nangalia V, Prytherch DR, Smith GB.. Health technology assessment review: Remote monitoring of vital signs-current status and future challenges. Crit Care 2010; 14 (5): 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramirez M, Maranon R, Fu J, et al. Primary care provider adherence to an alert for intensification of diabetes blood pressure medications before and after the addition of a “chart closure” hard stop. J Am Med Inform Assoc 2018; 25 (9): 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reading MJ, Merrill JA.. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc 2018; 25 (6): 759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maslach C, Jackson SE.. The measurement of experienced burnout. J Organiz Behav 1981; 2 (2): 99–113. [Google Scholar]
- 21. Melamed S, Shirom A, Toker S, et al. Burnout and risk of type 2 diabetes: a prospective study of apparently healthy employed persons. Psychosom Med 2006; 68 (6): 863–9. [DOI] [PubMed] [Google Scholar]
- 22. Kitaoka-Higashiguchi K, Morikawa Y, Miura K, et al. Burnout and risk factors for arteriosclerotic disease: follow-up study. J Occup Health 2009; 51 (2): 123–31 [DOI] [PubMed] [Google Scholar]
- 23. Toppinen‐Tanner S, Ahola K, Koskinen A, Väänänen A.. Burnout predicts hospitalization for mental and cardiovascular disorders: 10‐year prospective results from industrial sector. Stress Health 2009; 25 (4): 287–96. [Google Scholar]
- 24. Toker S, Melamed S, Berliner S, et al. Burnout and risk of coronary heart disease: a prospective study of 8838 employees. Psychosom Med 2012; 74 (8): 840–7. [DOI] [PubMed] [Google Scholar]
- 25. Kim H, Ji J, Kao D.. Burnout and physical health among social workers: a three-year longitudinal study. Social Work 2011; 56 (3): 258–68. [DOI] [PubMed] [Google Scholar]
- 26. Leone SS, Huibers MJH, Knottnerus JA, et al. The temporal relationship between burnout and prolonged fatigue: a 4‐year prospective cohort study. Stress Health 2009; 25 (4): 365–74. [Google Scholar]
- 27. Lizano EL, Barak MM.. Job burnout and affective wellbeing: A longitudinal study of burnout and job satisfaction among public child welfare workers. Children Youth Serv Rev 2015; 55: 18–28. [Google Scholar]
- 28. Dewa CS, Loong D, Bonato S, et al. How does burnout affect physician productivity? A systematic literature review. BMC Health Serv Res 2014; 14 (1): 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panagioti M, Geraghty K, Johnson J, et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction: a systematic review and meta-analysis. JAMA Intern Med 2018; 178 (10): 1317–31. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Poghosyan L, Clarke SP, Finlayson M, et al. Nurse burnout and quality of care: Cross‐national investigation in six countries. Res Nurs Health 2010; 33 (4): 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demerouti E, Le Blanc PM, Bakker AB, et al. Present but sick: a three‐wave study on job demands, presenteeism and burnout. Career Dev Int 2009; 14 (1): 50–68. [Google Scholar]
- 32. Schaufeli WB, Bakker AB, Van Rhenen W.. How changes in job demands and resources predict burnout, work engagement, and sickness absenteeism. J Organiz Behav 2009; 30 (7): 893–917. [Google Scholar]
- 33. Ahola K, Hakanen J.. Job strain, burnout, and depressive symptoms: A prospective study among dentists. J Affect Disord 2007; 104 (1–3): 103–10. [DOI] [PubMed] [Google Scholar]
- 34. Armon G, Shirom A, Shapira I, et al. On the nature of burnout–insomnia relationships: A prospective study of employed adults. J Psychosomat Res 2008; 65 (1): 5–12. [DOI] [PubMed] [Google Scholar]
- 35. Ye J. Pediatric mental and behavioral health in the period of quarantine and social distancing with COVID-19. JMIR Pediatr Parent 2020; 3 (2): e19867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ennis LA. The evolution of technostress. Comput Libr 2005; 25 (8): 10–2. [Google Scholar]
- 37. Tarafdar M, Tu Q, Ragu-Nathan BS, et al. The impact of technostress on role stress and productivity. J Manag Inf Syst 2007; 24 (1): 301–28. [Google Scholar]
- 38. Brosnan MJ. Technophobia: The Psychological Impact of Information Technology. London, United Kingdom: Routledge; 2002. [Google Scholar]
- 39. Linzer M, Konrad TR, Douglas J, et al. ; Society of General Internal Medicine (SGIM) Career Satisfaction Study Group (CSSG). Managed care, time pressure, and physician job satisfaction: results from the physician worklife study. J Gen Intern Med 2000; 15 (7): 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Graham KC, Cvach M.. Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care 2010; 19 (1): 28–34. [DOI] [PubMed] [Google Scholar]
- 41. Ammenwerth E, Iller C, Mahler C.. IT-adoption and the interaction of task, technology and individuals: a fit framework and a case study. BMC Med Inform Decis Mak 2006; 6 (1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Totterdale RL. Study case the utilization of low-code development technology to support research data collection. Issues Inf Syst 2018; 19 (2): 132–9. [Google Scholar]
- 43. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff (Millwood) 2016; 35 (4): 575–82. [DOI] [PubMed] [Google Scholar]
- 44. Gensheimer SG, Wu AW, Snyder CF; PRO-EHR Users’ Guide Steering Group. Oh, the places we’ll go: patient-reported outcomes and electronic health records. Patient 2018; 11 (6): 591–8. [DOI] [PubMed] [Google Scholar]
- 45. Jim HSL, Hoogland AI, Brownstein NC, et al. Innovations in research and clinical care using patient‐generated health data. CA Cancer J Clin 2020; 70 (3): 182–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adler-Milstein J, Nong P.. Early experiences with patient generated health data: health system and patient perspectives. J Am Med Inform Assoc 2019; 26 (10): 952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocrine Pract 2016; 22 (8): 1008–21. [DOI] [PubMed] [Google Scholar]
- 48. Demerouti E, Bakker AB, Nachreiner F, et al. A model of burnout and life satisfaction amongst nurses. J Adv Nurs 2000; 32 (2): 454–64. [DOI] [PubMed] [Google Scholar]
- 49. Dilsizian SE, Siegel EL.. Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep 2014; 16 (1): 441. [DOI] [PubMed] [Google Scholar]
- 50. Ye J, Sanchez-Pinto LN.. Three data-driven phenotypes of multiple organ dysfunction syndrome preserved from early childhood to middle adulthood. AMIA Annu Symp Proc 2020; 2020. [PMC free article] [PubMed] [Google Scholar]
- 51. Blackford AL, Wu AW, Snyder C.. Interpreting and acting on PRO results in clinical practice: lessons learned from the PatientViewpoint system and beyond. Med Care 2019; 57: S46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ye J, Yao L, Shen J, et al. Predicting mortality in critically ill patients with diabetes using machine learning and clinical notes. BMC Med Inform Decis Mak 2020; 20 (S11): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chung AE, Basch EM.. Potential and challenges of patient-generated health data for high-quality cancer care. J Oncol Pract 2015; 11 (3): 195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ye J, Zhang R, Bannon JE, et al. Identifying Practice Facilitation Delays and Barriers in Primary Care Quality Improvement . J Am Board Fam Med 2020; 33 (5): 655–64. [DOI] [PubMed] [Google Scholar]
- 55. Le Covec E, Ghanem E., Patient-generated health data (social media) are a potential source for ADR reporting. In: SAS Conference Proceedings: Pharmaceutical Users Software Exchange 2017; October 8-11, 2017; Edinburgh, United Kingdom.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.