Abstract
Background:
Hepatocellular carcinoma is a major health problem worldwide especially in Egypt. It accounts for the fifth common cancer and the second cause of death among different cancers. This study investigated the efficacy and molecular mechanism of Nisin and/or Thioridazine as anticancer treatment on human liver cancer HepG2 cell line.
Methods:
Nisin and Thioridazine were applied for 24 h on human liver cancer cell line (HepG2). 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was done to assess the cytotoxicity of Nisin and Thioridazine. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was used for the assessment of PI3K, AKT, SIRT-1, and NRF2 expression in the treated cell line. The protein level of reactive oxygen species (ROS) and vascular endothelial growth factor (VEGF) was measured in the collected media by ELISA technique. Western blot analysis was done for, tAKT, pAKT, tPI3K, and pPI3K.
Results:
Cell proliferation results showed that compared with the untreated cancer, Nisin and/or Thioridazine treated groups had decreased cell proliferation (p value< 0.0001). Nisin and/or Thioridazine decreased PI3K/AKT mRNA and protein expression in hepatocellular carcinoma cells (HCC). Also Nisin and/or Thioridazine decreased anti-oxidative SIRT1/NRF2 mRNA expression. ROS level highly increased with Nisin and/or Thioridazine treatment in contrast to VEGF protein level which was highly decreased.
Conclusion:
These results introduce Nisin and Thioridazine as new therapeutic lines in HCC.
Key Words: Hepatocellular carcinoma, Nisin, Thioridazine
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. According to death in men due to cancer, HCC represents the sixth cause in developed countries and the second cause in less developed countries. Annually, HCC leads to over 1 million deaths (1). Hepatocellular carcinoma (HCC) diagnosis is usually being at the late stage of the disease, especially in developing countries where the screening processes is absent. Although marked progress has been achieved in the cancer therapy, but the different lines of treatment including surgery and chemo- and radiation therapy cause normal cell damage and further morbidity (2,3). In recent years, many researches have been designed to improve patient survival by trying new lines of treatment.
Nisin is an antimicrobial agent produced by specific Gram-positive bacteria including Lactococcus and Streptococcus species (4,5). Over the past few decades, Nisin has been used as a natural bio preservative in food industry (5). Recently, Nisin has been participated in different biomedical fields (6). Nisin as a cytotoxic and antitumor agent was been widely investigated in last few years. Nisin mediated these effects through activation of preferential apoptosis, cell cycle arrest, and reducing cell proliferation (7).
Thioridazine (TDZ) was originally used as antipsychotic drug (8,9), and used to treat microbial resistance (10,11). Recently, many researches proved anticancer activity of Thioridazine through induction of apoptosis (12) and inhibition of angiogenesis (13) and metastasis (14). Several preclinical studies described the potent treatment effect of Thioridazine on various types of cancer cells. Studies have revealed that Thioridazine has antiproliferative activity and promote apoptosis in gastric cancer, cervical cancer, endometrial cancer, breast cancer, neuroblastoma, glioma, leukemia, and ovarian cancer (13, 15-20). These studies have demonstrated Thioridazine’s anticancer, multidrug resistance-reversing and apoptosis-inducing properties in various tumor cell lines (21-24).
Depend on the previous introduction, we thought to study anticancer effect of each of Nisin, Thioradizine and combinations of both on HepG2 cell line.
Materials and Methods
HepG-2 cell line and drugs preparation
Hepatocellular carcinoma cell line (HepG-2) was supplied from Cell Culture Department- VACSERA- EGYPT. HepG-2 cells (Hepatocellular carcinoma) were imported from the “American Type Culture Collection (ATCC)”, complete medium containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) in 5% CO2 incubator at 37 °C. Nisin (N5764-1G Nisin from Lactococcus lactis (Sigma) (lot # BCBJ3400V) and Thioridazine(1662504-Thioridazine, 10-[2-(1-Methyl-2-piperidinyl)ethyl]-2-(methylthio)-10H- phenothiazine from Merck were dissolved in dimethyl sulfoxide (DMSO), and then added to fresh culture medium.
The cell viability assay
The HepG-2 cells were cultivated in three 96-well tissue culture plates containing 103 cells/ml per well. The cells were incubated for 24 h before adding the drugs. The optimal cell number for cytotoxicity assays was determined in preliminary experiments. At the end of the incubation period, the medium was removed and 100 µg/mL of Nisin and 7.5 µM/ml of Thioridazine were added to wells for 24 h. Following 24 h, 20 µL of MTT (5 mg/mL) (TACSTM TREVIGEN1 8405 Helgerman Ct. Gaithersburg) were added to cells and incubated for a further 4 h at 37 °C. The medium was removed and 100 µL formazan was added to each well. After overnight incubation, the absorbance values were measured at a range from 490 to 630 nm using an ELISA reader (Dynatech MRX 5000; Dynex, Chantilly, VA).
Real time PCR of all studied genes
The HepG-2 cells were cultivated in 50 cm3 flask containing 106 cells/ml per flask. Before adding the drugs, the cells were incubated in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin liquid for 24 h. The HepG-2 cells were divided into four groups as following:
1. HepG-2 cell lines as an untreated cell
2. HepG-2 cell lines treated with Nisin of concentration 100 µg/mL (25) for 24 hour
3. HepG-2 cell lines treated with Thioridazine of concentration 7.5 µM/ml (26) for 24 hour
4. HepG-2 cell lines treated with combination of Nisin and Thioridazine for 24 hour.
Total RNA was extracted from cultivated cells of all studied groups with RNA easy Mini Kit (Qiagen) according to the manufacturer's instructions. Quantitation and purity assessment for RNA samples were done using the Nano Drop® (ND)-1000 spectrophotometer (Nano Drop Technologies, Inc. Wilmington, USA). The extracted RNA then stored at −80 °C until use.
SensiFAST™ SYBR® Hi-ROX One-Step Kit, catalog no. PI-50217 V had been formulated for highly reproducible first-strand cDNA synthesis and subsequent real-time PCR in a single tube in a 48-well plate using the Step One instrument (Applied Biosystem, USA). Normalization for variation in the expression of each target gene was performed referring to the mean critical threshold (CT) values of glyceraldehyde 3-phosphate dehydrogenase (β-actin) housekeeping gene expression by the ΔΔCt method. Primers sequence for all studied genes was listed as following in Table 1.
Table 1.
Primers used for RT-qPCR.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| PI3K | GAATCCAATGGGAACTGT | GGGAGGGTAATAATAAGGT |
| AKT | TGCAGCATCGCTTCTTTG | TCTGGGCCGTGAACTCCT |
| SIRT-1 | CAGGAAGTACAAAAGCCATC | AATCTCAAATGACATGCAGTG |
| Nrf2 | ATGGATTTGATTGACATACTTT | ACTGAGCCTGATTAGTAGCAAT |
| GAPDH | CAAGGTCATCCATGACAA | GTCCACCACCCTGTTGCTG |
Assessment of ROS and VEGF by ELISA
The level of reactive oxygen species (ROS) and vascular endothelial growth factor (VEGF) was measured in the collected media using ROS ELISA kit amsbio (AMS.E01R0021) and VEGF ELISA kit mybiosource (Catalog No.: MBS355343) The spectrophotometric absorbance was assessed at 450 nm in accordance with the manufacturer’s instructions. The results were expressed as IU/mL.
Western blot analysis for tAKT, pAKT, tPI3K and pPI3K
After drug exposure for 24 hours, RIPA buffer (Bio BASIC INC., Markham, Ontario, Canada) was used to extract all proteins from collected cells.
Bradford Protein Assay Kit (SK3041) for quantitative protein analysis (BIO BASIC INC., Markham, Ontario, Canada. A Bradford assay was performed according to manufacture instructions.
Total proteins were separated by 10% SDS-PAGE then electro transferred to polyvinylidene fluoride (PVDF) membranes.
The membranes were blocked for 2 hours with 5% non-fat dry milk then incubated with primary antibodies at dilution (1:200) at 4 °C overnight. After being washed twice with PBS, the membranes were incubated for 1 hour using HRP-conjugated secondary antibodies at dilution (1:500). Finally, an enhanced luminol-based chemiluminescent (ECL) kit was used to detect the target proteins.
Image analysis software was used to read the band intensity of the target proteins against control sample by total protein normalization on the ChemiDoc MP imager.
Statistical methods
Data were coded and entered using Graph Pad Prism 7. Data was summarized using mean and standard deviation. Comparisons between groups were done using analysis of variance (ANOVA) Tukey's multiple comparisons test when comparing more than 2 groups (27).
Results
Microscopic pictures of different cultured cancer cells
Showing effect of Nisin and/or Thioridazine treatment on highly proliferative viable cancer cells which became less proliferative and less viable cancer cells (Fig. 1).
Fig. 1.
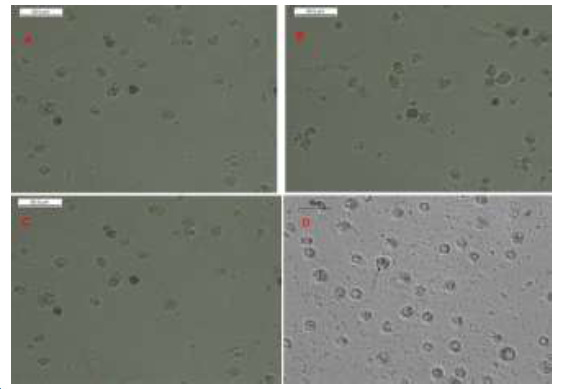
Light microscopy imaging. Cancer cells in different study groups. (A) Control cells showing highly proliferative viable control untreated cancer cells. (B) Less proliferative and less viable treated cancer cells with Nisin, (C) Less proliferative and less viable treated cancer cells with Thioridazine (D) Less proliferative and less viable treated cancer cells with Nisin and Thioridazine (black arrow).
Cancer cells proliferation using MTT assay
Cell proliferation was determined at 24 post treatment with Nisin and Thioridazine treatment. There is a significant decrease in cell proliferation in Cancer cells treated with Nisin (2 ±0.52), Cancer cells treated with Thioridazine (0.99±0.42) and Cancer cells treated with Nisin and Thioridazine (0.46±0.21) groups compared to untreated cancer group (4.9±1.2). (p value< 0.0001). There is also significant decrease between Cancer cells treated with Nisin and Thioridazine and cancer cells treated with Thioridazine against untreated cancer cells treated with Nisin (p value= 0.02 and < 0.0001 respectively) (Fig. 2).
Fig. 2.
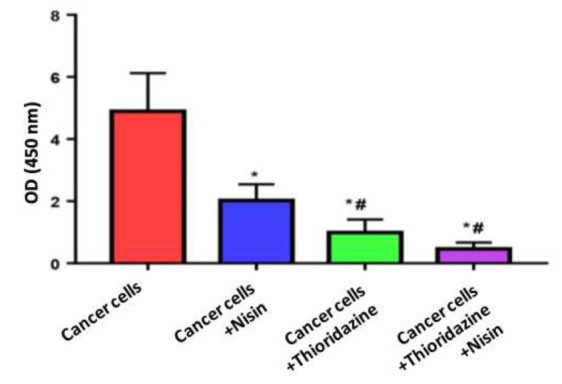
Measurement of cell proliferations in different study groups using MTT assay.
Data were expressed as Mean ± SD, p value< 0.05 was significant.
(*) Denotes significant difference versus cancer group.
(#) Denotes significant difference versus cancer+Nisin group.
Inhibition of PI3K/AKT signaling pathway in HepG-2 cell line
To examine if Nisin and/or Thioradizine affect PI3K/AKT signalling in HCC cells, after 24 hours from treatment of HepG-2 cell line with Nisin and /or Thioradizine we measure mRNA and protein expression for each of them.
PI3K and AKT mRNA expression
Figure 3Figure 3A shows that treatment with Nisin or Thioridazine decreased PIK3 mRNA expression in HepG2 cells (0.94± 0.47, o.56 ± 0.26) respectively compared with that in the untreated cancer cells (1.32 ± 0.34). This decrease was no significant with Nisin and significant with Thioridazine (p value= 0.001).
Fig. 3.
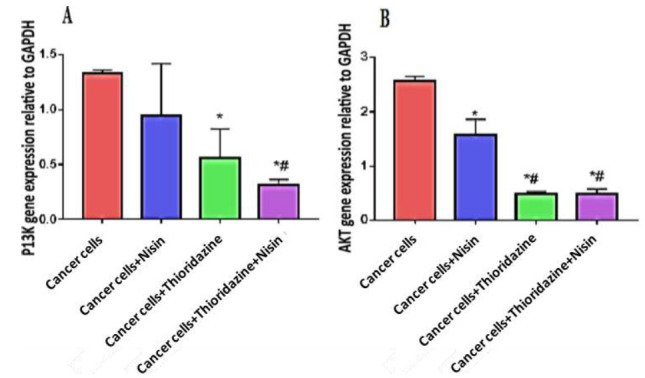
PI3K expression (A) and AKT (B) among different study groups.
Data were expressed as Mean ± SD, p value< 0.05 was significant.
(*) Denotes significant difference versus cancer group.
(#) Denotes significant difference versus cancer+Nisin group.
The treatment with Nisin and Thioridazine decreased significantly PIK3 mRNA expression (0.31 ± 0.05) compared with untreated cancer group (p value< 0.0001).
Figure 3B shows that treatment with Nisin or Thioridazine decreased AKT mRNA expression in HepG2 cells (1.57 ± 0.28, o.49 ± 0.03) respectively compared with that in the untreated cancer cells (2.5 ± 0.07) (p< 0.05).
The treatment with Nisin and Thioridazine decreased significantly AKT mRNA expression (0.49 ± 0.08) compared with those in the groups treated with Nisin only and untreated cancer group (p value< 0.0001).
PI3K and AKT protein expression
The protein levels of p-PI3K and p-AKT were decreased significantly when HepG2 cells were treated with the combination of Nisin and Thioridazine (0.71 ± 0.22, 1.34 ± 0.15) respectively compared with those in the groups treated with Nisin (1.69 ± 0.15, 2.45 + 0.24) or Thioridazine (1.42 ± 0.26, 2.3 ± 0.19) only, and the untreated cancer group (2.55 ± 0.16, 3.7 ± 0.2) (p value< 0.0001) (Figs. 4 and 5).
Fig. 4.
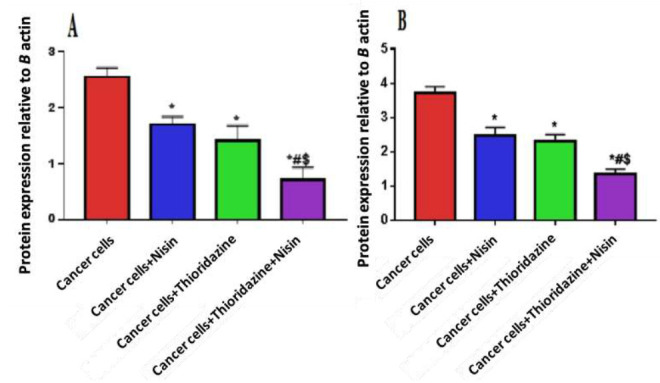
Protein level of pPI3K (A) and pAKT (B) in different study groups.
Data were expressed as Mean ± SD, p value<0.05 was significant.
(*) Denotes significant difference versus cancer group.
(#) Denotes significant difference versus cancer+Nisin group.
($) Denotes significant difference versus cancer+ Thioridazine group.
Fig. 5.
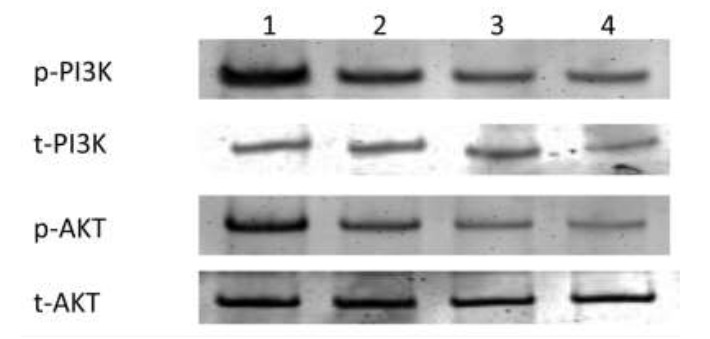
Western blot bands of PI3K and AKT proteins in different study groups.
Anti-oxidative stress effect of Nisin and Thioridazine Effect of Nisin and Thioridazine on SIRT1
The expression levels of SIRT1 were decreased significantly when HepG2 cells were treated with the combination of Nisin and Thioridazine (0.62 ± 0. 18) and treated with Thioridazine (1.6 ± 0.56) only compared to the untreated cancer group (2.5±0. 71), p value< 0.0001 and 0.02, respectively, (Fig. 6 A).
Fig. 6.
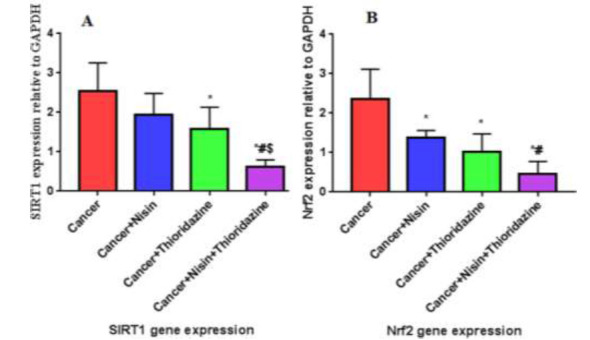
A, B: SIRT 1 and Nrf2 gene expression in different study groups.
Data were expressed as Mean ± SD, p value <0.05 was significant.
(*) Denotes significant difference versus cancer group.
(#) Denotes significant difference versus cancer+Nisin group.
($) Denotes significant difference versus cancer+Thioridazine group.
There is significant difference between the group treated with drug combination against each of group treated with Nisin (1.9 ± 0.53) and that treated with Thioridazine, p value= 0.001 and 0.02, respectively.
There is no significant difference between the groups treated with Nisin or Thioridazine (p value > 0.05).
NRF2 mRNA expression
There is statistical significant decrease in Nrf2 gene expression in Cancer group treated with Nisin (1.38 ± 0.18), Cancer group treated with Thioridazine (1.02 ± 0.45) Cancer group treated with Nisin and Thioridazine (0.45 ± 0.32) groups compared to untreated cancer group (2.3 ± 0.0.76), p value< 0.0001.
There is also significant difference between Cancer group treated with Nisin and Thioridazine and Cancer group treated with Nisin (p value= 0.01) (Fig. 6 B).
Reactive oxygen species level
After 24 hours, there is Statistical significant increase in ROS level in cancer group treated with Nisin (0.51 ± 0.12) or Thioridazine (1.38 ± 0.49) or both of them (3.7 ± 0.76) compared to untreated cancer group (0.14± 0.03, p value< 0.001).
Statistical significant decrease in ROS level in Cancer treated with Nisin and Thioridazine compared to cancer treated with Nisin, p value = 0.01.
No significant difference between Cancer treated with Thioridazine and Cancer treated by both drugs, p value= 0.6, (Fig. 7 A).
Fig. 7.
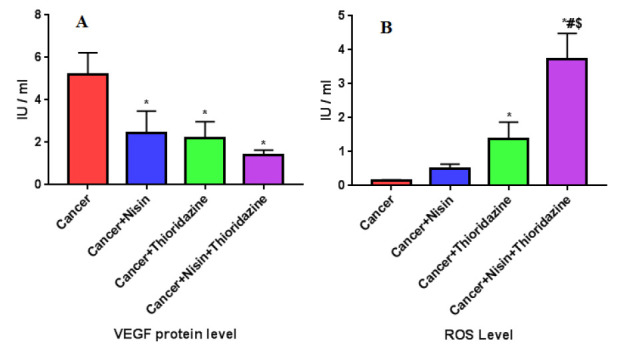
A, B: VEGF and ROS level in different study groups.
Data were expressed as Mean ± SD, p value< 0.05 was significant.
(*) Denotes significant difference versus cancer group.
(#) Denotes significant difference versus cancer+Nisin group.
($) Denotes significant difference versus cancer+Thioridazine group.
Anti –Angiogenic effect of Nisin and Thioridazine
To examine effect of Nisin and/or Thioridazine on angiogenesis in HEPG2 cells, after 24 hours from treatment of HepG-2 cell line with Nisin and /or Thioridazine we measure VEGF protein level. The protein levels of VEGF were decreased significantly when HepG2 cells were treated with the combination of Nisin and Thioridazine (1.4 ± 0.22) compared with those in the groups treated with Nisin (2.4 ± 1.05) or Thioridazine (2.2 ± 0.75) only, and the untreated cancer group (5.2 ± 1.01) (p value= 0.0002), (Fig. 7 B).
No significant difference between the groups treated with Nisin or/ and Thioridazine (p value> 0.05).
Discussion
Hepatocellular carcinoma (HCC) is a major health problem all over the world and the number of discovered cases is more than 700,000 annually. However, there are few safe and effective therapeutic lines of treatment against HCC (28-30). The accumulating studies in cancer field supposed that combination therapies can give best result than single agents (31) Here, our study was revealed that Nisin and Thioridazine can be used as therapeutic agents against HCC alone but combination of them had better results.
Cell proliferation assessment showed that both of Nisin and Thioradizaine has anti proliferative effect on HCC although Thioridazine was superior on Nisin, but combination of them had the best effect. This reduction in cell proliferation coincides with many results as Zainodini et al. proved that Nisin had anti-proliferative effect on Human Astrocytoma Cell Line (SW1088) (25). Also, Joo and colleagues explored the cytotoxic and antitumor properties of Nisin A and discovered that it blocks head and neck squamous cell carcinoma (HNSCC) tumorigenesis (7). In addition, Preet and colleagues demonstrated that combining doxorubicin, a conventional cancer drug, with Nisin can potentiate the effectiveness of the treatment in terms of decreasing tumor severity in skin carcinogenesis (32). Jiang et al. found that Thioridazine inhibited melanoma cells proliferation (33).
This effect may be explained by inhibition PI3K/AKT signalling in HCC cells. Our results showed decrease in PI3K/AKT gene and protein expressions. This reduction was higher in Thioridazine than Nisin but still the combination of both had the best effect. Goyette MA et al proved that thoridazine decrease tumor growth through reduction in PI3K/AKT/mTOR and ERK signaling IN Triple-Negative Breast Cancer (34). To our knowledge there is no studies test effect of Nisin on PI3K/AKT signaling pathway.
Cancer cells characterized by high levels of ROS due to malignant transformation which produce genetic, metabolic, and microenvironment changes (35). Cancer cells have the ability to accommodate high levels of ROS through antioxidant pathways activation. So, new potential agents targeting the ROS signaling pathways and redox mechanisms are tried to treat cancer.
During oxidative stress, SIRT1/NRF2 signaling pathway has been proved to have principle role in regulating cellular responses (36). NRF2 is a transcription factor play important role in defense against oxidative hazards. Although, NRF2 protects healthy cells from carcinogenic agents (37), it can help cancer development by protecting cancer cells from oxidative stress through anti-oxidative stress responses (38). SIRT1 acts through the deacetylation of a number of crucial transcription factors that responsible for various biological processes, including oxidative stress (39). Our results showed that SIRT1/NRF2 decreased with different treatment especially combination between Nisin and Thioridazine suggesting that these drugs act via inhibiting anti-oxidative protective mechanisms. This inhibition leads to increase ROS level in treated groups especially in Thioridazine and drug combination. These findings coincide with Min Yong et al. (40) who found that Thioridazine produced a higher level of cellular ROS in comparison with the control group in ovarian cancer. Simultaneously, anti-oxidative stress-associated proteins were decreased, including p-Nrf2 and its downstream proteins. To our knowledge, there are no similar studies according to Nisin in cancer. But there are many studies concerned with antimicrobial effect of Nisin. Miyamoto et al. (41) examined the effect of Nisin on the Listeria monocytogenes proteome and found a higher catalase activity and amount of ferritin compared with untreated bacteria. They explained this elevated amount of antioxidant enzymes in the cells treated with Nisin to the formation of ROS. Although the activation of antioxidant mechanisms, the bacteria were not able to overcome the oxidative stress induced by antimicrobials at sublethal concentrations. Increased ROS in cancer cells may provide a good chance to kill cancer cells through elevating intracellular ROS to highly toxic levels, leading to activate various ROS-induced cell death pathways.
HCC patients have bad survival rate due to angiogenesis and metastasis (42). HCC is defined as a typical angiogenic tumor. In our study, VEGF protein level decreased with different lines of treatment especially with Nisin and Thioridazine combination compared to untreated cancer cells. There have been several studies explored the anti-angiogenic effect of Thioridazine, which have reported findings agreed with the results of our study. Thioridazine is an angiostatic agent which was found to effectively inhibit angiogenesis in a murine tumor model (43). In vitro, Thioridazine has been observed to inhibit vascular endothelial growth factor (VEGF)-induced proliferation, invasion and endothelial cell tube formation of (44).
In conclusion, the present study provided evidence that the Thioridazine and Nisin are promising effective drugs against hepatocellular carcinoma through inhibition of PI3K/AKT proliferation pathway, ROS induction and angiogenic inhibition. Thioridazine had superior effect than Nisin but both of them had synergistic effect with the best result. The potential for these drugs to be used in cancer therapy requires further investigation in clinical settings.
Acknowledgements
We would like to thank National Research Centre, Dokki, Giza, Egypt and Faculty of Medicine, Cairo University, Cairo, Egypt for all support to complete our research article.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. . CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lubelski J, Rink R, Khusainov R, Moll G N, Kuipers O P. Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic Nisin. Cell Mol Life Sci. . 2008;65(3):455–76. doi: 10.1007/s00018-007-7171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Arauz LJ, Jozala AF, Mazszola PG, et al. Nisin biotechnological production and application: a review. . Trends in Food Science & Technology. 2009;20(3-4):146–154. [Google Scholar]
- 4.Patel JD, Krilov L, Adams S, Aghajanian C, Basch E, Brose MS, et al. Clinical cancer advances 2013: Annual report on progress against cancer from the American Society of Clinical Oncology. . J Clin Oncol. 2014;32(2):129–60. doi: 10.1200/JCO.2013.53.7076. [DOI] [PubMed] [Google Scholar]
- 5.Miller DK, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 6.Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Biomedical Applications of Nisin. . J Appl Microbiol. 2016;120(6):1449–65. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. . Cancer Med. 2012;1(3):295–305. doi: 10.1002/cam4.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohman R, Axelsson R. Relationship between prolactin response and antipsychotic effect of Thioridazine in psychiatric-patients. Eur J Clin Pharmacol. 1978;14(2):111–116. doi: 10.1007/BF00607441. [DOI] [PubMed] [Google Scholar]
- 9.Realmuto GM, Erickson WD, Yellin AM, Hopwood JH, Greenberg LM. Clinical comparison of thiothixene and Thioridazine in schizophrenic adolescents. . Am J Psychiatry. 1984;141(3):440–2. doi: 10.1176/ajp.141.3.440. [DOI] [PubMed] [Google Scholar]
- 10.van Soolingen D, Hernandez-Pando R, Orozco H, Aguilar D, Magis-Escurra C, Amaral L, et al. The antipsychotic Thioridazine shows promising therapeutic activity in a mouse model of multidrug-resistant tuberculosis. . PLoS One. . 2010;5(9):e12640. doi: 10.1371/journal.pone.0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorsing M, Klitgaard JK, Atilano ML, Skov MN, Kolmos HJ, Filipe SR, et al. Thioridazine induces major changes in global gene expression and cell wall composition in methicillin-resistant Staphylococcus aureus USA300. PLoS One. 2013;8(5):e64518. doi: 10.1371/journal.pone.0064518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil-Ad I, Shtaif B, Levkovitz Y, Nordenberg J, Taler M, Korov I, et al. Phenothiazines induce apoptosis in a B16 mouse melanoma cell line and attenuate in vivo melanoma tumor growth. . Oncol Rep. 2006;15(1):107–112. [PubMed] [Google Scholar]
- 13.Park MS, Dong SM, Kim BR, Seo SH, Kang S, Lee EJ, et al. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. . 2014;5(13):4929–34. doi: 10.18632/oncotarget.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Li J, Luo Z, Zhang S, Xue S, Wang K, et al. Roles of dopamine receptors and their antagonist Thioridazine in hepatoma metastasis. . Onco Targets Ther. . 2015;8:1543–1552. doi: 10.2147/OTT.S77373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byun HJ, Lee JH, Kim BR, Kang S, Dong SM, Park MS, et al. Anti-angiogenic effects of Thioridazine involving the FAK-mTOR pathway. . Microvasc Res. 2012;84(3):227–34. doi: 10.1016/j.mvr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Kang S, Dong SM, Kim BR, Park MS, Trink B, Byun HJ, et al. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012;17(9):989–97. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu J, Xu H, Yang Y, Huang W, Xiao J, Li M, et al. Thioridazine, an antipsychotic drug, elicits potent antitumor effects in gastric cancer. Oncol Rep. 2014;31(5):2107–14. doi: 10.3892/or.2014.3068. [DOI] [PubMed] [Google Scholar]
- 18.Strobl JS, Kirkwood KL, Lantz TK, Lewine MA, Peterson VA, 3rd Worley JF. Inhibition of human breast cancer cell proliferation in tissue culture by the neuroleptic agents pimozide and Thioridazine. Cancer Res. 1990;50(17):5399–405. [PubMed] [Google Scholar]
- 19.Rho SB, Kim BR, Kang S. A gene signature-based approach identifies Thioridazine as an inhibitor of phosphatidylinositol-3'-kinase (PI3K)/AKT pathway in ovarian cancer cells. . Gynecol Oncol. 2011;120(1):121–7. doi: 10.1016/j.ygyno.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Spengler G, Csonka A, Molnar J, Amaral L. The anticancer activity of the old neuroleptic phenothiazine-type drug Thioridazine. Anticancer Res. 2016;36(11):5701–5706. doi: 10.21873/anticanres.11153. [DOI] [PubMed] [Google Scholar]
- 21.Meng Q, Sun X, Wang J, Wang Y, Wang L. The important application of Thioridazine in the endometrial cancer. . Am J Transl Res. 2016;8(6):2767–2775. [PMC free article] [PubMed] [Google Scholar]
- 22.Yue H, Huang D, Qin L, Zheng Z, Hua L, Wang G, et al. Targeting lung cancer stem cells with antipsychological drug Thioridazine. . Biomed Res Int. . 2016;2016:6709828;2016:6709828. doi: 10.1155/2016/6709828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J, Ma B, Zhang X, Sun X, Han J, Wang Y, et al. Thioridazine has potent antitumor effects on lung cancer stem-like cells. . Oncol Lett. 2017;13(3):1563–1568. doi: 10.3892/ol.2017.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Gong P, Liu P, Zhou N, Zhou Y, Wang Y. Thioridazine elicits potent antitumor effects in colorectal cancer stem cells. . Oncol Rep. . 2017;37(2):1168–1174. doi: 10.3892/or.2016.5313. [DOI] [PubMed] [Google Scholar]
- 25.Zainodini N, Hassanshahi G, Hajizadeh M, Khanamani Falahati-Pour S, Mahmoodi M, Mirzaei MR, et al. Nisin Induces Cytotoxicity and Apoptosis in Human Asterocytoma Cell Line (SW1088). . Asian Pac J Cancer Prev. 2018;19(8):2217–2222. doi: 10.22034/APJCP.2018.19.8.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng HW, Liang YH, Kuo YL, Chuu CP, CY Lin, MH Lee, et al. Identification of Thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. . Cell Death Dis. 2015;6(5):e1753. doi: 10.1038/cddis.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan Y. Biostatistics102: Quantitative Data – Parametric & Non-parametric Tests. . Singapore Med J. 2003;44:391–6. [PubMed] [Google Scholar]
- 28.Dawood RM, Salum GM, Abdelhafez TH, El Shenawy R, Ibrahim NE, El Awady MK. Safety and tolerability of mice to repeated subcutaneous injections of a peptide mix as a potential vaccine against HCV infection. . Hum Antibodies. 2019;27(2):105–110. doi: 10.3233/HAB-180354. [DOI] [PubMed] [Google Scholar]
- 29.Omran MH, Fotouh BE, Youssef SS, Ibrahim NE, Nabil W, Mahdy EM, et al. Association between low molecular polypeptide 7 single nucleotide polymorphism and response to therapy in hepatitis C virus infection. World J Hepatol. 2013;5(3):97–103. doi: 10.4254/wjh.v5.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaker O, Mahfouz H, Salama A, Medhat E. Long Non-Coding HULC and miRNA-372 as Diagnostic Biomarkers in Hepatocellular Carcinoma patients. Reports of Biochemistry & Molecular Biology. . 2020;9(2):230–240. doi: 10.29252/rbmb.9.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. . J Clin Oncol. 2015;33(23):2563–77. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preet S, Bharati S, Panjeta A, Tewari R, Rishi P. Effect of Nisin and doxorubicin on DMBA-induced skin carcinogenesis - a possible adjunct therapy. . Tumor Biol. . 2015;36(11):8301–8. doi: 10.1007/s13277-015-3571-3. [DOI] [PubMed] [Google Scholar]
- 33.Jiang X, Chen Z, Shen G, Jiang Y, L W, Xue Li, et al. Psychotropic agent Thioridazine elicits potent in vitro and in vivo anti-melanoma effects. . Biomed Pharmacother. 2018;97:833–837. doi: 10.1016/j.biopha.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Goyette MA, Cusseddu R, Elkholi I, Abu-Thuraia A, El-Hachem N, Haibe-Kains B, et al. AXL knockdown gene signature reveals a drug repurposing opportunity for a class of antipsychotics to reduce growth and metastasis of triple-negative breast cancer. . Oncotarget. . 2019;10(21):2055–2067. doi: 10.18632/oncotarget.26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Kim J, Bae JS. ROS homeostasis and metabolism: a critical liaison for cancer therapy. . Exp Molec Med. 2016;48(11):e269. doi: 10.1038/emm.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding YW, Zhao GJ, Li XL, Hong GL, Li MF, Qiu QM, et al. SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int J Mol Med. 2016;37(4):1049–58. doi: 10.3892/ijmm.2016.2503. [DOI] [PubMed] [Google Scholar]
- 37.Qiao YQ, Jiang PF, Gao YZ. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. . Arch Med Sci. . 2018;14(3):617–624. doi: 10.5114/aoms.2016.59871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Wijst MG, Huisman C, Mposhi A, Roelfes G, Rots MG. Targeting Nrf2 in healthy and malignant ovarian epithelial cells: Protection versus promotion. . Mol Oncol. . 2015;9(7):1259–73. doi: 10.1016/j.molonc.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. . Int J Mol Sci. . 2013;14(2):3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong M, Yu T, Tian S, Liu S, Xu J, Hu J, Hu L. DR2 blocker Thioridazine: A promising drug for ovarian cancer therapy. . Oncol Lett. . 2017;14(6):8171–8177. doi: 10.3892/ol.2017.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto KN, Monteiro KM, da Silva Caumo K, Lorenzatto KR, Ferreira HB, Brandelli A. Comparative proteomic analysis of Listeria monocytogenes ATCC 7644 exposed to a sublethal concentration of Nisin. . J. Proteomics. 2015;119:230–237. doi: 10.1016/j.jprot.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Sampat KR, and O'Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. . Oncologist. 2013;18(4):430–8. doi: 10.1634/theoncologist.2012-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin T, He S, Shen G, Ye T, Guo F, Wang Y. Dopamine receptor antagonist Thioridazine inhibits tumor growth in a murine breast cancer model. Mol Med Rep. . 2015;12:4103–8. doi: 10.3892/mmr.2015.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byun HJ, Lee JH, Kim BR, Kang S, Dong SM, Park MS, Lee SH, Park SH, Rho SB. Anti-angiogenic effects of Thioridazine involving the FAK mTOR pathway. Microvasc Res. 2012;84(2012): 227– 234. doi: 10.1016/j.mvr.2012.09.006. [DOI] [PubMed] [Google Scholar]


