Abstract
Background:
Polycystic ovary syndrome (PCOS) is the most common cause of ovarian dysfunction associated with infertility, Oligomenorrhea or amenorrhea, hirsutism, acne, and obesity. A large body of evidence unraveled, three major groups of genes play critical roles in underlying PCOS molecular mechanism. The aim of this study is to investigate critical exonic variant of FSHR, CYP11, and INSR and determine the functionality of these mutations in Iranian patients with PCOS.
Methods:
In this case-control study, 130 patients with PCOS who referred to the Vali-e-Asr Hospital with infertility were included. DNA extracted from three ml of peripheral blood of the participants for DNA extraction. The PCR was conducted for each gene and the PCR product was genotyped by sequencing.
Results:
The data showed that there were two polymorphisms in INSR genes which did not change the protein sequences; these alterations can also be considered as a single nucleotide polymorphism (SNP). Moreover, any exonic variant has not been detected in CYP11B1. Whereas, two missense mutation have been detected in FSHR gene including p.Ala307Thr and p.Asn680Ser. It has been shown that the polymorphisms of the FSHR gene affect the hormone response in the ovaries. Our data demonstrated that the FSHR mutations frequencies were higher in the patients with PCOS rather than control people significantly.
Conclusion:
These data showed that the polymorphisms of FSHR were significantly associated with PCOS in Iranian infertile women. Further studies with larger sample sizes are needed to be performed for explore the strength of the association.
Key Words: CYP11, FSHR, Infertile, INSR, PCOS, Polymorphisms
Introduction
Polycystic ovary syndrome (PCOS) is the most common cause of ovarian dysfunction associated with infertility, Oligomenorrhea or amenorrhea, hirsutism, acne, and obesity (1, 2). The prevalence of PCOS is about 5-15% (3). It has been shown that metabolic pathways such as steroid hormones regulatory, gonadotrophin axis, insulin signaling, and regulatory pathways of glucose and fat metabolism contributed to the development of PCOS (4). Polycystic ovary syndrome is a complex disorder, which has been demonstrated that a variety of genetic and environmental factors such as lifestyles have important roles in patients (5, 6). Furthermore, positive family history has been proposed that is a crucial predisposing factor in PCOS (7). a large body of evidence unraveled, three major groups of genes play critical roles in underlying PCOS molecular mechanism including gonadotropin, androgen, insulin secretion genes. Among the gonadotropin genes, Follicle Stimulating Hormone Receptor (FSHR) plays a major role in gonadotropin secretion (8). Its encoding gene, FSHR, is located on 2p16.3 and containing 14 exons (9, 10). In the ovary, Follicle Stimulating Hormone (FSH) binds to granulosa cell membrane receptors and impose its function through the cAMP-dependent protein kinase pathway in the cells (11). The FSH binding increases the growth of the primary follicle cells (12, 13). Among androgen secretion important genes, Cytochrome P450 family gene (CYP11), is well documented and studied. This gene has two main subunits, CYP11A1, and CYP11B1. These genes code a member of the cytochrome P450 family and contain 10 and 9 exons, respectively (14). Finally, high levels of insulin are known as a critical factor in PCOS (15). Central obesity has a significant relationship with an increase in insulin, even in the non-obese female. Different mechanisms for insulin resistance are demonstrated including tissue insulin resistance, liver dysfunction, and sensitivity (16). The level of androgens is increased by the high levels of insulin in the three ways including, the effect on insulin receptor that enhances the androgenic response of the cells to Luteinizing hormone (LH), reduces the production of Sex Hormone Binding Globulin (SHBG) in the liver and decreases the production of IGF-binding protein (17, 18). The insulin receptor is a heterothermic glycoprotein that consists of two subunits α and β which is encoded by the insulin receptor gene (INSR), is located on 19p13.2 and containing 22 exons (19). Altogether, the aim of this study is to investigate novel polymorphisms and determine the functionality of these mutations in Iranian patients with PCOS.
Materials and Methods
Subjects
In this case-control study, 130 patients with PCOS who referred to the Vali-e-Asr hospital, with infertility were included. One hundred people without any infertility complication were included as controls (Code of ethics: IR.TUMS.REC.1395.2715). The available clinical data of the patients is presented in Table 1. In addition, the patient's complete condition was recorded and a consent form was received from each participant in the study. Three ml of the peripheral blood was taken from the participants for DNA extraction in Ethylenediaminetetraacetic acid (EDTA) tubes.
Table 1.
Clinical data of the patients.
| Parameters | Control | PCOS | p value | OR (CI) |
|---|---|---|---|---|
| Age | 30.86±7.34 | 27.96±5.02 | 0.009 | |
| BMI | 24.83±4.30 | 27.21±4.41 | 0.003 | |
| Hirsutism | Number (%) | Number (%) | 0 | 6.60 (2.44-17.82) |
| No | 51 (91.1%) | 68(60.7%) | ||
| Yes | 5(8.9%) | 44(39.3%) | ||
| Obesity | ||||
| No | 55(98.2%) | 93(83%) | 0.004 | 11.23 (1.46-86.27) |
| Yes | 1(1.8%) | 19(17%) |
OR: Odd ratios, CI: Confidence interval
DNA extraction and PCR and genotyping
The Qiagen DNA extraction kit was used for DNA extraction from the blood. The DNA concentration was read by Nanodrop and was diluted appropriately. The DNA was stored at -20 °C. In the next step, for each gene (FSHR, CYP11B1, and INSR), specific primers were designed. The primers list is presented in Table 2.
Table 2.
The list of the primers.
| Gene | Exon | Forward primer | Reverse primer | product length | TM |
|---|---|---|---|---|---|
| FSHR | 10 | CTTTCCCATCTTTGGCATCAGC | TCAGCTTCCTAATGTATCACATGG | 760 | 60 |
| INSR | 17 | TTTTCGAGGGGGTTTGGGTG | AGGGGTGGGTAGGAGGTTAC | 453 | 59 |
| CYP11B1 | 3 | ATGGCACTCAGGGCAAAGG | TTAGTTGATGGCTCTGAAGGTGA | 1512 | 59 |
Primer3 online tool was conducted for designing primer. By using the NCBI primer blast tool specificity of the primer was evaluated. The PCR was carried out in a final volume of 25 μl containing 1 μl template DNA (100 ng), 12 μl of Ampliqon master mix, 1 μl of forward primer and 1 μl of reverse primer (10 pmol). The PCR program involved an activation step for 5 minutes at 95 °C followed by 30 cycles, including a denaturation step for 30 seconds at 95 °C and a primer annealing step for 30 seconds at 60 °C and followed by a one final cycle for 30 seconds at 72 °C. Ten μL of PCR products were loaded to 2% agarose gel for evaluating the PCR product length. Sanger sequencing technique was used to determine the genotypes of each case and control PCR products. The sequencing chromatograms were analyzed with Finch TV software. ClastalW software was used to analyze the protein alteration.
Statistical analysis and results
The polymorphism and allele frequencies were calculated in different groups. Data were analyzed using SPSS software. In order to compare the frequency distribution of different genotypes in PCOS samples, the odds ratio of the genotypes were calculated by Chi-Square Pearson test. The p< 0.05 was considered significant.
Results
INSR gene sequencing data
Our data showed that the INSR gene contains two novel mutations in 130 cases and 100 controls in exon 17 (c.T3128C and c.C3174T). c.T3128C mutation encodes cysteine (Cys), occurs in nucleotide 3128 (c.T3128C), which changes the T nucleotide to C, but no change in the protein sequence. The c.C3174T mutation encodes Histidine (His). The nucleotide change in nucleotide 3174 (c.C3174T), which changes from C to T, but no change in the protein sequence. The PCR results of the INSR gene from four samples were shown on the 2% agarose gel in Figure 1. Allele frequency of the mutations is presented in Tables 3 and 4.
Fig. 1.
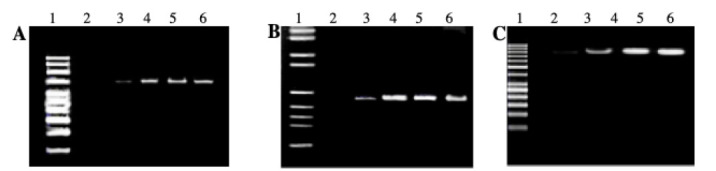
Agarose gels (2%) electrophoresis. A) FSHR (760 bp). B) INSR (453bp). C) CYP11B1 (1512bp) genes. 1: DNA ladder (100 bp). 2: PCR negative control. 3: Control Sample 1. 4: Control Sample 2. 5: Case Sample 1. 6: Case Sample 2.
Table 3.
The allele frequency of INSR c.C3174T mutation.
| Genotype | Number of participants (%) | p-value | OR (95% CI) | |
|---|---|---|---|---|
| control | case | |||
| Number (%) | Number (%) | |||
| CC | 57(95) | 73(56.2) | - | 1.0 |
| CT | 2(3.3) | 56(43.0) | 0.00 | 21.863 (5.116-93.425) |
| TT | 1(2.7) | 1(0.8) | 0.862 | 0.781 (0.048- 12.756) |
| CT+TT | 0.00 | 14.836 (4.417- 49.829) | ||
Table 4.
The allele frequency of INSR c.T3128C mutation.
| Genotype | Number of participants (%) | p-value | OR (95% CI) | |
|---|---|---|---|---|
| control | case | |||
| Number (%) | Number (%) | |||
| TT | 58(96.7) | 1(0.8) | - | 1.0 |
| TC | 1(1.7) | 1(0.8) | 0.568 | 0.453 (0.028- 7.371) |
| CC | 1(1.7) | 2(1.6) | 0.568 | 0.453 (0.028- 7.371) |
| TC+CC | 2(3.4) | 1(0.8) | 0.423 | 0.453 (0.062- 3.296) |
FSHR gene sequencing data
Sequencing data showed two novel mutations in exon 10 of the FSHR gene. According to NM_000145.3 reference sequence, one mutation in codon 307, encoding cysteine (Cys), occurs in nucleotide 919 (c. G919A), which changes the G nucleotide to A, and in the protein sequence, the amino acid of alanine converts to the threonine (p.Ala307Thr). Another mutation in codon 680, encoding Asparagine (Asn), occurs in nucleotide 2039 (c. A2039G), which changes the A nucleotide to G, and in the protein sequence, the amino acid of arginine converts to the serine (p. Asn680Ser). The PCR results of the FSHR gene from four samples were shown on the 2% agarose gel in Figure 1. Allele frequency of the mutations is presented in Tables 5 and 6. The sequencing chromatograms are presented in Figure 2.
Table 5.
The allele frequency of FSHR c.A2039G mutation.
| Genotype | Number of participants (%) | p-value | OR (95% CI) | |
|---|---|---|---|---|
| control | case | |||
| Number (%) | Number (%) | |||
| AA | 53(88.3) | 68(56.7) | - | 1.0 |
| AG | 6(10) | 43(35.8) | 0.000 | 5.586 (2.211-14.109) |
| GG | 1(1.7) | 9(7.5) | 0.037 | 7.015 (0.862-57.110) |
| AG+GG | 7(11.7) | 52(43.3) | 0.000 | 5.790 (2.433- 13.778) |
Table 6.
The allele frequency of FSHR c. G919A mutation.
| Genotype | Number of participants (%) | p-value | OR (95% CI) | |
|---|---|---|---|---|
| control | case | |||
| Number (%) | Number (%) | |||
| GG | 50(83.3) | 52(41.9) | - | 1.0 |
| GA | 8(13.3) | 28(22.6) | 0.005 | 3.365 (1.401-8.084) |
| AA | 2(3.3) | 44(35.5) | 0.000 | 21.154 (4.867- 91.935) |
| GA+GG | 10(5.4) | 72(39.1) | 0.000 | 6.923 (3.215- 14.907) |
Fig. 2.
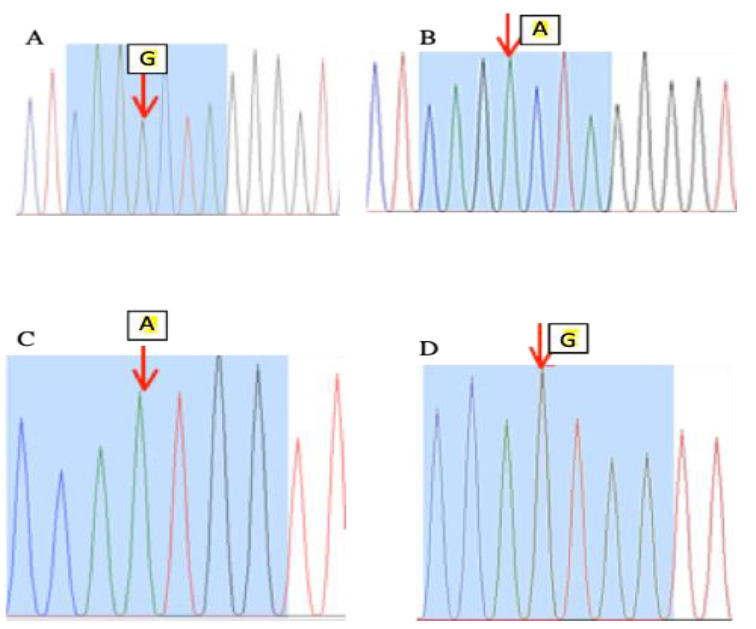
The FSHR gene (exon 10) sequencing chromatograms. A: Control. B: PCOS case. The arrow represents the point of C. G919A mutation, which changes the G nucleotide to A. C: Control. D: PCOS case. The arrow represents the point of c. A2039G mutation, which changes the A nucleotide to G.
CYP11B1 gene sequencing data
Our sequencing data did not demonstrate any mutation in the CYP11B1 gene among the patients. The PCR results of the CYP11B1 gene from four samples were shown on the 2% agarose gel in Figure 1.
Discussion
Polycystic ovary syndrome is a syndrome with endocrine, metabolic and genetic disorders which is characterized by chronic ovulation, polycystic ovary, and clinical and biochemical manifestations of hyperandrogenism (2, 20). PCOS etiology is so complicated. Recent investigations showed that the number of endocrine abnormalities increase and exacerbate each other in PCOS (21). These disorders include a defect in the function of the hypothalamus-hippophysic axis, ovarian and adrenal functions. In fact, PCOS is associated with abnormal gonadotropin secretions, LH and FSH, and increased ovarian steroid secretion as well as insulin resistance (16, 22). People with PCOS have higher levels of luteinizing hormone, testosterone, insulin, cholesterol, and triglyceride than normal ones. Also, women with PCOS have lower levels of FSH and SHBG (high hormone binding globulin) and high-density lipoproteins than healthy people (13). It is necessary to find genes which involve in infertility to understand the underlying mechanisms and treatment of infertility. Patients with polycystic ovarian syndrome are often subjected to IVF (23). Recent studies considered polymorphisms of the contributed genes in ovarian hormone response. Therefore, the evaluation of the effective gene polymorphisms in women treated with IVF (successful or unsuccessful) is important. The FSHR (follicle stimulating hormone receptor) gene is expressed in granulosa cells in the ovary and plays an important role in female reproduction, such as granulosa cell proliferation, folliculogenesis stage regulation, and oocyte maturation (24). The in FSHR codon 680 is a polymorphism that can lead to distinct genotypes, some of which may predispose individuals to infertility. According to previous reports, this polymorphism can be considered as a risk factor for unsuccessful IVF in infertile women (25). In the current study, we investigated FSHR, CYP11B1, and INSR polymorphisms in Iranian infertile women with PCOS. The data showed that there were two polymorphisms in INSR genes which did not change the protein sequences; these alterations can also be considered as an SNP. Moreover, we did not find any mutation in CYP11B1 gene. Whereas we find two missense mutation in FSHR gene including p.Ala307Thr and p. Asn680Ser. It has been shown that the polymorphisms of the FSHR gene affect the hormone response in the ovaries. Our data demonstrated that the FSHR mutations frequencies were higher in the patients with PCOS rather than control group significantly. The previous investigation demonstrated that FSHR p.Ala307Thr mutation can modulates ovarian response to gonadotropin in women with normal gonadotropin levels (26). In a recent study with 352 women with endometriosis and 510 fertile controls revealed that FSHR polymorphisms were associated with endometriosis significantly. Their finding proposed that 680Ser-Ser/GG genotype and ‘‘GG/307Ala680Ser’’ haplotype remarkably elevate the risk of endometriosis in infertile women (27). Moreover, in the study of Xianliang et al. on infertile Chinese female undergoing IVF/ICIS-ET treatment indicated a linear correlation between Ser680 of FSHR polymorphism and FSH level and oocytes retrieved. In addition, FSHR Ser680Asn polymorphism was associated with ovarian response in controlled ovarian hyperstimulation (27). While Mohiyiddeen et al. demonstrated that the FSHR p.Asn680Ser genotype frequencies were not different in IVF patient in comparison to control. They found that this polymorphism was not associated with ovarian response to gonadotropin stimulation in the IVF patients (28). Besides this, the previous study indicated that the FSHR p.Asn680Ser polymorphism did not show significant association with ovarian reserve markers including antimullerian hormone, antral follicle count, and FSH level in the controlled ovarian stimulation IVF patients (29). In a study on the female with PCOS in the Chinese population showed that The Ala307Thr and Ser680Asn polymorphisms of FSHR did not demonstrate significant association with PCOS but related to FSH and PRL levels in the patients (30). Also, Unsal et al. investigated the polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in PCOS patients. They did not find any association between the FSHR polymorphisms and PCOS (32). Whereas, in the study of Du et al. with 60 PCOS patients and 92 healthy control revealed that the FSHR thr307Ala and Asn680Ser polymorphisms were significantly associated with PCOS in Chinese women (32). Dolfin et al. showed that the heterozygote FSHR Ala307Thr polymorphism was remarkably associated to PCOS in Italian women and related to a higher responsiveness to exogenous FSH (33). However, this study met some limitations. These limitations may be guidance for future investigations to overwhelming them. The sample size of the study was quite small which could have a dramatic impact on the power of the study and the ovarian hormones and parameters were not assessed in our study. Altogether, our data showed that the Ala307Thr and Ser680Asn polymorphisms of FSHR were significantly associated with PCOS in Iranian infertile women. Further studies with larger sample sizes are needed to be performed in order to explore the strength of the association.
Acknowledgements
We acknowledge the financial supports of the research deputy of the Tehran University of Medical Sciences and our colleagues at the Vali-e-Asr Reproductive Health Research center. The authors declare no conflict of interest.
References
- 1.Prapas N, Karkanaki A, Prapas I, Kalogiannidis I, Katsikis I, Panidis D. Genetics of polycystic ovary syndrome. . Hippokratia. . 2009;13(4):216–23. [PMC free article] [PubMed] [Google Scholar]
- 2.Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. . Clinics (Sao Paulo). . 2015;70(11):765–9. doi: 10.6061/clinics/2015(11)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. . Endocr Rev. . 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaaban Z, Khoradmehr A, Jafarzadeh Shirazi MR, Tamadon A. Pathophysiological mechanisms of gonadotropins- and steroid hormones-related genes in etiology of polycystic ovary syndrome. . Iran J Basic Med Sci. . 2018;22(1):3–16. doi: 10.22038/ijbms.2018.31776.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbanek M. The genetics of the polycystic ovary syndrome. . Nat Clin Pract Endocrinol Metab. . 2007;3(2):103–11. doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 6.Mykhalchenko K, Lizneva D, Trofimova T, Walker W, Suturina L, Diamond MP, et al. Genetics of polycystic ovary syndrome. . Expert Rev Mol Diagn. 2017;17(7):723–733. doi: 10.1080/14737159.2017.1340833. [DOI] [PubMed] [Google Scholar]
- 7.Strauss JF. [Epidemiology and genetics of polycystic ovary syndrome: recent date]. . J Gynecol Obstet Biol Reprod (Paris). 2003;32(3 Pt 2):S11–6. [PubMed] [Google Scholar]
- 8.Amato P, Simpson JL. The genetics of polycystic ovary syndrome. . Best Pract Res Clin Obstet Gynaecol. . 2004;18(5):707–18. doi: 10.1016/j.bpobgyn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Simoni M, Tempfer CB, Destenaves B, Fauser BC. Functional genetic polymorphisms and female reproductive disorders: Part I: Polycystic ovary syndrome and ovarian response. . Hum Reprod Update. 2008;14(5):459–84. doi: 10.1093/humupd/dmn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhasena W, Pantasri T, Piromlertamorn W, Samchimchom S, Vutyavanich T. Follicle-stimulating hormone receptor gene polymorphism in chronic anovulatory women, with or without polycystic ovary syndrome: a cross-sectional study. . Reprod Biol Endocrinol. . 2014;12:86. doi: 10.1186/1477-7827-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti M. Specificity of the cyclic adenosine 3′, 5′-monophosphate signal in granulosa cell function. . Biol Reprod. 2002;67(6):1653–61. doi: 10.1095/biolreprod.102.004952. [DOI] [PubMed] [Google Scholar]
- 12.Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. . 2005;15(7):451–6. doi: 10.1097/01.fpc.0000167330.92786.5e. [DOI] [PubMed] [Google Scholar]
- 13.Ferk P, Teran N, Gersak K. The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum Reprod. 2007;22(4):1031–6. doi: 10.1093/humrep/del457. [DOI] [PubMed] [Google Scholar]
- 14.Franks S, McCarthy M. Genetics of ovarian disorders: polycystic ovary syndrome. . Rev Endocr Metab Disord. 2004;5(1):69–76. doi: 10.1023/B:REMD.0000016125.05878.96. [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Zhu XM, Luo Q, Qian Y, Jin F, Huang HF. A novel SNP at exon 17 of INSR is associated with decreased insulin sensitivity in Chinese women with PCOS. . Mol Hum Reprod. . 2006;12(3):151–5. doi: 10.1093/molehr/gal022. [DOI] [PubMed] [Google Scholar]
- 16.Schuring AN, Schulte N, Sonntag B, Kiesel L. Androgens and insulin--two key players in polycystic ovary syndrome. Recent concepts in the pathophysiology and genetics of polycystic ovary syndrome. . Gynakol Geburtshilfliche Rundsch. 2008;48(1):9–15. doi: 10.1159/000111465. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S, Maitra A. Molecular & genetic factors contributing to insulin resistance in polycystic ovary syndrome. Indian J Med Res. . 2010;131:743–60. [PubMed] [Google Scholar]
- 18.Fica S, Pirouzi A, Albu A, Constantin M, Dobri GA. Insulin resistance and fertility in polycystic ovary syndrome. J Med Life. 2008;1(4):415–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Ramezani Tehrani F, Daneshpour M, Hashemi S, Zarkesh M, Azizi F. Relationship between polymorphism of insulin receptor gene, and adiponectin gene with PCOS. Iran J Reprod Med. . 2013;11(3):185–94. [PMC free article] [PubMed] [Google Scholar]
- 20.Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. . Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30(1):19–26. doi: 10.1385/ENDO:30:1:19. [DOI] [PubMed] [Google Scholar]
- 22.Chapman JC, Min SH, Freeh SM, Michael SD. The estrogen-injected female mouse: new insight into the etiology of PCOS. Reprod Biol Endocrinol. . 2009;18:7–47. doi: 10.1186/1477-7827-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santana LF, Ferriani RA, Sa MF, Reis RM. Treatment of infertility in women with polycystic ovary syndrome. Rev Bras Ginecol Obstet. . 2008;30(4):201–9. doi: 10.1590/s0100-72032008000400008. [DOI] [PubMed] [Google Scholar]
- 24.Zhao MX, Zhou GY, Zhu JY, Gong B, Hou JX, Zhou T, et al. Fluoride Exposure, Follicle Stimulating Hormone Receptor Gene Polymorphism and Hypothalamus-pituitary-ovarian Axis Hormones in Chinese Women. . Biomed Environ Sci. . 2015;28(9):696–700. doi: 10.3967/bes2015.099. [DOI] [PubMed] [Google Scholar]
- 25.Andre GM, Martins Trevisan C, Pedruzzi IN, Fernandes RFM, Oliveira R, Christofolini DM, et al. The Impact of FSHR Gene Polymorphisms Ala307Thr and Asn680Ser in the Endometriosis Development. . DNA Cell Biol. 2018;37(6):584–591. doi: 10.1089/dna.2017.4093. [DOI] [PubMed] [Google Scholar]
- 26.Rod A, Jarzabek K, Wolczynski S, Benhaim A, Reznik Y, Denoual-Ziad C. ESR1 and FSHR gene polymorphisms influence ovarian response to FSH in poor responder women with normal FSH levels. . Endocrinol Metab Synd. 2014;3:1–5. [Google Scholar]
- 27.Huang X, Li L, Hong L, Zhou W, Shi H, Zhang H, et al. The Ser680Asn polymorphism in the follicle-stimulating hormone receptor gene is associated with the ovarian response in controlled ovarian hyperstimulation. Clin Endocrinol (Oxf). . 2015;82(4):577–83. doi: 10.1111/cen.12573. [DOI] [PubMed] [Google Scholar]
- 28.Mohiyiddeen L, Newman WG, Cerra C, McBurney H, Mulugeta B, Roberts SA, et al. A common Asn680Ser polymorphism in the follicle-stimulating hormone receptor gene is not associated with ovarian response to gonadotropin stimulation in patients undergoing in vitro fertilization. . Fertil Steril. . 2013;99(1):149–155. doi: 10.1016/j.fertnstert.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Mohiyiddeen L, Newman WG, McBurney H, Mulugeta B, Roberts SA, Nardo LG. Follicle-stimulating hormone receptor gene polymorphisms are not associated with ovarian reserve markers. Fertil Steril. . 2012;97(3):677–81. doi: 10.1016/j.fertnstert.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 30.Wu X-q, Xu S-m, Liu J-f, Bi X-y, Wu Y-x, Liu DJ. Association between FSHR polymorphisms and polycystic ovary syndrome among Chinese women in north China. . J Assist Reprod Genet. 2014;31(3):371–7. doi: 10.1007/s10815-013-0166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unsal T, Konac E, Yesilkaya E, Yilmaz A, Bideci A, Onen HI, et al. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. . J Assist Reprod Genet. . 2009;26(4):205–16. doi: 10.1007/s10815-009-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, Zhang W, Guo L, Zhang Z, Shi H, Wang J, et al. Two FSHR variants, haplotypes and meta-analysis in Chinese women with premature ovarian failure and polycystic ovary syndrome. . Mol Genet Metab. . 2010;100(3):292–5. doi: 10.1016/j.ymgme.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Dolfin E, Guani B, Lussiana C, Mari C, Restagno G, Revelli A. FSH-receptor Ala307Thr polymorphism is associated to polycystic ovary syndrome and to a higher responsiveness to exogenous FSH in Italian women. J Assist Reprod Genet. 2011;28(10):925–30. doi: 10.1007/s10815-011-9619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


