Abstract
Background
The Pfizer-BioNTech COVID-19 vaccine has recently received emergency approval from the US FDA. The mRNA technology was used to manufacture the Pfizer vaccine; however, as a pioneering technology that has never been used in the manufacture of vaccines, many people have concerns about the vaccine’s side effects. Thus, the current study aimed to track the short-term side effects of the vaccine.
Methods
The information in this study was gathered by a Google Form-questionnaire (online survey). The results included the responses of 455 individuals, all of whom are Saudi Arabia inhabitants. Adverse effects of the vaccine were reported after the first and the second doses.
Results
The most common symptoms were injection site pain, headaches, flu-like symptoms, fever, and tiredness. Less common side effects were a fast heartbeat, whole body aches, difficulty breathing, joint pain, chills, and drowsiness. Rare side effects include Bell’s palsy and lymph nodes swelling and tenderness. Flu-like symptoms were more common among those under 60 years of age, while injection site pain was more frequent among recipients who were 60 years and older. The study revealed a significant increase in the number of females who suffered from the vaccine side effects compared to males. Difficulty of breathing was more reported among recipients who had been previously infected with the coronavirus compared to those who had not been previously infected.
Conclusion
Most of the side effects reported in this study were consistent with Pfizer’s fact sheet for recipients and caregivers. Further studies are required to determine the long-term side effects.
Keywords: Pfizer-BioNTech COVID-19 vaccine, side effects, injection site pain, hypersensitivity, flu-like symptoms, online survey
Background
Coronavirus 2 (SARS-CoV-2), a new member of the human coronaviruses family, was identified in 2019 as the causative agent of a new disease outbreak in China associated with severe medical complications and even death in some cases.1 In March 2020, the World Health Organization (WHO) announced the novel disease outbreak as a pandemic.2
The unavailability of a vaccine or other efficacious therapeutic option has required all the nations worldwide to combat the pandemic’s spread. Several precautionary strategies, which include lockdown, social distancing, wearing facemasks, and travel limitations, have been applied to stop this pandemic. However, vaccine development is perhaps the best hope for stopping this pandemic.3
On December 11, 2020, the US Food and Drug Administration (FDA) authorized the emergency use of the Pfizer-BioNTech COVID-19 vaccine. The vaccination requires two shots given at least 21 days apart. It has been reported that the maximum efficacy, about 95%, can be reached one week following the second dose. The vaccine could be utilized for people ages 16 years and older.4 Authorization for emergency utilization has also been given to the Moderna COVID-19 vaccine for the use in people ages 18 and older with an efficacy rate of 94.1%. The Moderna vaccine also involves two shots that are administered 28 days apart.5
The technology for both the Pfizer-BioNTech and Moderna COVID-19 vaccines relies on messenger RNA (mRNA). The coronavirus possesses a spike-shaped surface structure called an S protein, and the COVID-19 mRNA vaccines give instructions on the way to produce a harmless piece of an S protein. Following vaccination, cells start making more of the protein pieces and introducing them to the coronavirus’s surfaces. The immune system will perceive that the protein does not belong there and start to create an immune response and form antibodies.6
Sometimes after a vaccination, the process of building immunity can cause adverse symptoms. The Pfizer-BioNTech COVID-19 vaccine can induce mild side effects following the first and/or the second shot, including pain, redness or swelling at the injection site, tiredness, headaches, chills, muscle and joint aches, and fever. These symptoms could be an indication that the body is developing the desired immunity for protection.7
The success of the vaccination strategies relies partly on population’s conceptions of the vaccines’ benefits and risks and on their related trust in the vaccination. Researchers believe that refusing or delaying of the vaccination is a result of a lack of knowledge about the relative benefit-to-risk ratio of the vaccination,8–10 which is a status that was defined as vaccine hesitancy by the WHO Strategic Advisory Group of Experts on Immunization in 2015.11 WHO recognized this hesitancy as one of the major global health threats in 2019.12
Pfizer-BioNTech’s COVID-19 vaccine is an actual fight against the pandemic. However, since the mRNA technique is new in vaccine manufacturing, one cannot predict its consequences. Hence, studies are essential to follow the vaccine’s side effects. Besides, understanding the Pfizer-BioNTech COVID-19 vaccine’s risk and declaring it to the public are required to raise the acceptance of the vaccination.
This study aimed to evaluate the short-term side effects after receiving the first, the second, and/or both doses of the Pfizer-BioNTech COVID-19 vaccine in a sample of 16 years and older residents in Saudi Arabia. The study intended to identify which age group is most vulnerable to the short-term side effects.
Methods
Study Design
A cross-sectional (online survey) study was conducted in a retrospective manner. The subjects participating in the study were residents in Saudi Arabia. Saudi Arabia began its campaign to vaccinate the population against the emerging coronavirus on December 17, 2020, with the Pfizer-BioNTech vaccine. The present study was conducted over the period between January 10 and 21, 2021. A questionnaire, designed on Google Forms, was written in the Arabic language (the scientific terms for the symptoms were written and explained in the public language) and delivered to participants via social media (primarily Facebook and WhatsApp). Communication between the investigators and the subjects participating in the study was established via electronic mail when required. The participants were not given any incentives to participate. Still, the importance of the research in educating the community about the vaccine’s side effects was clarified. Their participation may also prompt everyone else to receive the vaccine after comparing the acceptable side effects with the disease’s severity. The settings were adjusted to that each participant sent only one response. Still, sometimes some participants asked to be allowed to send more than one response, as they were also filling out a questionnaire about their elderly relatives who could not handle the electronic questionnaires. Responses were carefully reviewed to ensure that responses were not repeated.
The questionnaire comprised two categories of inquiries. The first one covers background data of the subject, such as nationality, gender, age, educational level, and previous infection with SARS-CoV-2. The second group of inquiries focused on COVID-19 vaccine-related data. The survey asks what type of COVID-19 vaccine the participant received and whether the participant received one or two doses of the vaccine. This section also asks what side effects are associated with administering the COVID-19 vaccine, in addition to the timing of the appearance of these effects (after the first or second dose). Regarding these side effects, the participants were asked to choose from symptoms including temporary face paralysis (Bell’s palsy), flu-like symptoms, pain at the site of injection, dyspnea, headache, and tachycardia and to state any other symptoms that they experienced following the vaccination. All participants were permitted to terminate the survey at any time of the study. All precautionary actions were applied to preserve the data’s confidentiality. The questionnaire was pre-tested for validity. First two experts assessed all the questions individually and minor modifications were made based on their based on their feedback. Then the questionnaire was given to three faculty members at King Abdulaziz University, and they were asked to rate each of the questions by using a 5-point Likert scale ranging from 1 to 4 (1 = very important, 2 = important, 3 = moderately important, and 4 = not important). The validity was then evaluated by calculating the Item-Content Validity Index (I-CVI). The results showed excellent content validity (I-CVI =1).
Inclusion Criteria
Inclusion criteria included the administration of at least one dose of the Pfizer-BioNTech COVID-19 vaccine.
Exclusion Criteria
Participants who were not vaccinated against COVID-19 or who had received a vaccine other than one made by the Pfizer Company were excluded.
Sample Size
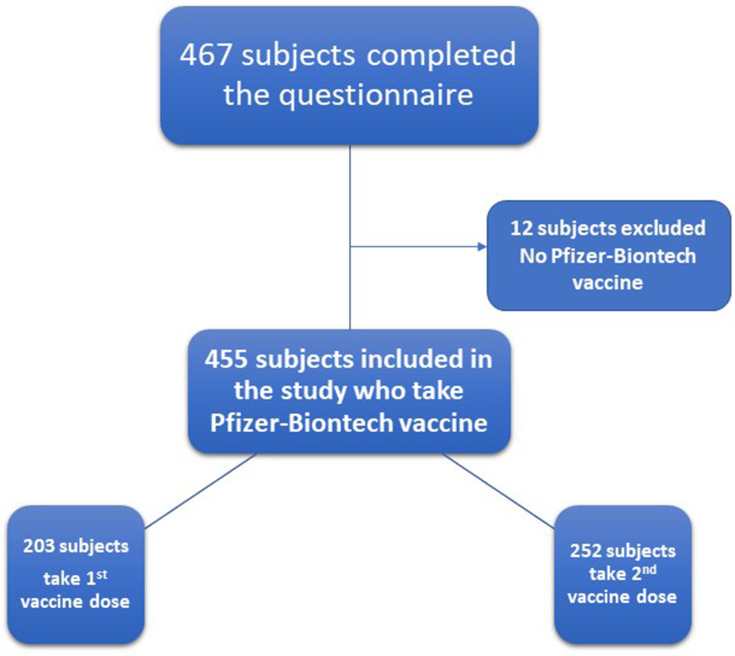
The minimum sample size for conducting this survey was 384 with a 5% margin of error and a 95% confidence level. We have hypothesized that the prevalence of the side effects in the population is 50% ± 5 (the reported side effects by FDA ranged from 14.2% to 84.1%). In this study, 467 subjects completed the COVID-19 vaccine questionnaire. The number of participants that who satisfied the inclusion and exclusion criteria was 455 (Figure 1).
Figure 1.
Flowchart for the COVID-19 vaccine questionnaire participants.
Study Variables
The age of the participants and the timing of the appearance of COVID-19 vaccine side effects were explored as independent variables. The various adverse reactions observed as side effects following a vaccination were considered as the dependent variable.
Statistical Analysis
Descriptive statistics were performed for the collected data. The responses were displayed as frequency (counts and/or percentage). A chi-squared test was employed for statistical analysis. The data were statistically analyzed using Prism® (version 8.4.0, GraphPad Software Inc., La Jolla, CA, USA). The level of significance was set at p ≤ 0.05.
Results
The Study Participants’ Demographic Characteristics, COVID-19 Past Infection, Type of Vaccine, Number of Vaccine Doses, and the Onset of Side Effects
For this study, 455 individuals, all of them of Arab descent, participated. Saudi citizens were the majority of the participants (425, 93.4%), and the minority came from other nationalities (30, 6.6%). Women were the majority of the participants (292, 64.2%), while men constituted the minority of participants (163, 35.8%). It was found that 299 (65.7%) participants were younger than 60 years of age, and 156 (34.3%) were 60 years or older. Most of the study population was made up of people with the university (207, 45.5%) and post-university (183, 40.2%) education levels, while the minority were those with pre-university (65, 14.3%) education. The study population’s past medical history revealed that, while a minority of them (19, 4.2%) had previously been infected with the SARS-CoV-2 virus, most participants (436, 95.8%) had never been infected. All study participants (455, 100%) had received their first Pfizer-BioNTech COVID-19 vaccine dose, while 252 (55.4%) had received both doses. Of the participants, 237 (52%) individuals reported the presence or absence of the side effects after receiving their first dose of the vaccine, 124 (27.3%) after receiving their second dose, and 94 (20.7%) people after receiving both doses, Table 1.
Table 1.
The Study Participants’ Demographic Characteristics, COVID-19 Past Infection, Type of Vaccine, Number of Vaccine Doses, and the Onset of Side Effects
| Characteristics | Frequency (n and %) |
|---|---|
| All Participants (n= 455) | |
| Nationality | |
| Saudi | 425 (93.4%) |
| Non-Saudi | 30 (6.6%) |
| Sex | |
| Male | 163 (35.8%) |
| Female | 292 (64.2%) |
| Age (year) | |
| < 60 | 299 (65.7%) |
| ≥ 60 | 156 (34.3%) |
| Education Level | |
| Pre-university education | 65 (14.3%) |
| University education | 207 (45.5%) |
| Post-university education | 183 (40.2%) |
| Previous Infection with SARS-CoV-2 | |
| Infected | 19 (4.2%) |
| Not infected | 436 (95.8%) |
| Type of COVID-19 Vaccine | |
| Pfizer-BioNTech vaccine | 455 (100%) |
| Inoculated Vaccine Dose | |
| 1st dose | 203 (44.6%) |
| 1st and 2nd dose | 252 (55.4%) |
| Onset of Symptoms | |
| After 1st vaccine dose | 237 (52.0%) |
| After 2nd Vaccine dose | 124 (27.3%) |
| After both 1st and 2nd dose | 94 (20.7%) |
Note: Results were offered as frequency (number (n) and percentage (%)).
Reported COVID-19 Vaccine Side Effects and Their Correlation with the First and Second Doses
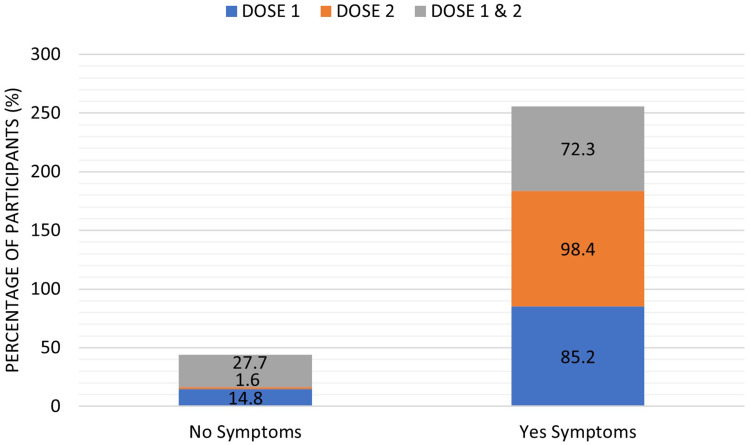
This study results showed a significant increase (p < 0.001) in the number of subjects who were suffering side effects after receiving the second dose of the vaccine (122, 98.4%) compared to those who reported side effects after the first dose (202, 85.2%) or after both doses (68, 72.3%), Table 2 and Figure 2.
Table 2.
The Reported COVID-19 Vaccine Side Effects and Their Correlation with the Vaccine’s First, Second, and Both Doses
| Frequency (n and %) | ||||
|---|---|---|---|---|
| 1st Dose (n = 237) | 2nd Dose (n = 124) | 1st & 2nd Doses (n=94) | Chi-Square p value | |
| Presence of Symptoms | ||||
| Presence | 202 (85.2%) | 122 (98.4%) | 68 (72.3%) | < 0.0001*** |
| Absence | 35 (14.8%) | 2 (1.6%) | 26 (27.7%) | |
| Local Symptoms | 167 (70.5%) | 100 (80.6%) | 60 (63.8%) | 0.0139* |
| Arm pain | 4 (1.7%) | 0 (0%) | 0 (0%) | 0.1563 |
| Injection site pain | 161 (67.9%) | 100 (80.6%) | 60 (63.8%) | 0.0116* |
| Injection site swelling and redness | 2 (0.8%) | 0 (0%) | 0 (0%) | 0.3907 |
| Hypersensitivity Symptoms | 19 (8.0%) | 18 (14.5%) | 3 (3.2%) | 0.0099** |
| A fast heartbeat | 7 (3.0%) | 11 (8.9%) | 3 (3.2%) | 0.0299* |
| Difficulty breathing | 9 (3.8%) | 5 (4.0%) | 0 (0%) | 0.1514 |
| Bad rash all over the body | 0 (0%) | 1 (0.8%) | 0 (0%) | 0.2625 |
| Severe body allergy | 1 (0.4%) | 0 (0%) | 0 (0%) | 0.6307 |
| Burning sensation in the eye | 2 (0.8%) | 1 (0.8%) | 0 (0%) | 0.6743 |
| Bone and Muscle Symptoms | 9 (3.8%) | 22 (17.7%) | 7 (7.4%) | < 0.0001*** |
| Whole body pain | 2 (0.8%) | 8 (6.5%) | 5 (5.3%) | 0.0084** |
| Muscle pain | 2 (0.8%) | 1 (0.8%) | 1 (1.1%) | 0.9764 |
| Muscle relaxation | 0 (0%) | 1 (0.8%) | 0 (0%) | 0.2625 |
| Bone pain | 3 (1.3%) | 2 (1.6%) | 0 (0%) | 0.4949 |
| Joint pain | 0 (0.0%) | 5 (4.0%) | 1 (1.1%) | 0.0060** |
| Back pain | 2 (0.8%) | 3 (2.4%) | 0 (0%) | 0.2044 |
| Total Flu-Like Symptoms | 77 (32.5%) | 112 (90.3%) | 29 (30.9%) | < 0.0001*** |
| Flu symptoms | 26 (11.0%) | 36 (29.0%) | 7 (7.4%) | < 0.0001*** |
| Headache | 43 (18.1%) | 48 (38.7%) | 17 (18.1%) | < 0.0001*** |
| Fever | 3 (1.3%) | 23 (18.5%) | 4 (4.3%) | < 0.0001*** |
| Chills | 2 (0.8%) | 5 (4.0%) | 1 (1.1%) | 0.0772 |
| Sore throat | 2 (0.8%) | 0 (0%) | 0 (0%) | 0.3907 |
| Chest infection | 1 (0.4%) | 0 (0%) | 0 (0%) | 0.6307 |
| Fatigue Symptoms | 20 (8.4%) | 25 (20.1%) | 3 (3.2%) | < 0.0001*** |
| Tiredness | 15 (6.3%) | 19 (15.3%) | 3 (3.2%) | 0.2171 |
| Dizziness and giddiness | 4 (1.7%) | 2 (1.6%) | 0 (0%) | 0.4523 |
| Desire to sleep | 1 (0.4%) | 4 (3.2%) | 0 (0%) | 0.0273* |
| GIT Symptoms | 14 (5.9%) | 2 (1.6%) | 1 (1.1%) | 0.0383* |
| Nausea and vomiting | 4 (1.7%) | 1 (0.8%) | 1 (1.1%) | 0.7615 |
| Gastroesophagitis | 2 (0.8%) | 0 (0%) | 0 (0%) | 0.3907 |
| Diarrhea | 7 (3.0%) | 1 (0.8%) | 0 (0%) | 0.1169 |
| Loss of appetite | 1 (0.4%) | 0 (0%) | 0 (0%) | 0.6307 |
| Nerve Inflammation Symptoms | 4 (1.7%) | 0 (0%) | 0 (0%) | 0.1563 |
| Bell’s palsy | 3 (1.3%) | 0 (0%) | 0 (0%) | 0.2493 |
| Tingling | 1 (0.4%) | 0 (0%) | 0 (0%) | 0.6307 |
| Miscellaneous Symptoms | 1 (0.4%) | 4 (3.2%) | 0 (0%) | 0.0273* |
| Lymph nodes tenderness and swelling | 0 (0%) | 2 (1.6%) | 0 (0%) | 0.0685 |
| Elevated blood pressure | 0 (0%) | 1 (0.8%) | 0 (0%) | 0.2625 |
| Blurred vision | 0 (0%) | 1 (0.8%) | 0 (0%) | 0.2625 |
| Burning sensation when urinating | 1 (0.4%) | 0 (0%) | 0 (0%) | 0.6307 |
Notes: Results were offered as frequency (number (n) and percent (%)). Correlation between variables was evaluated using the Chi-square test. *Significant difference at p ≤ 0.05, **significant difference at p ≤ 0.01, and ***significant difference at p ≤ 0.001.
Figure 2.
The percentage absence (no symptoms) and presence (yes symptoms) of COVID-19 side effects reported by the study participants following the vaccine’s first, second, and both doses. Results were offered as frequency (percentage (%)).
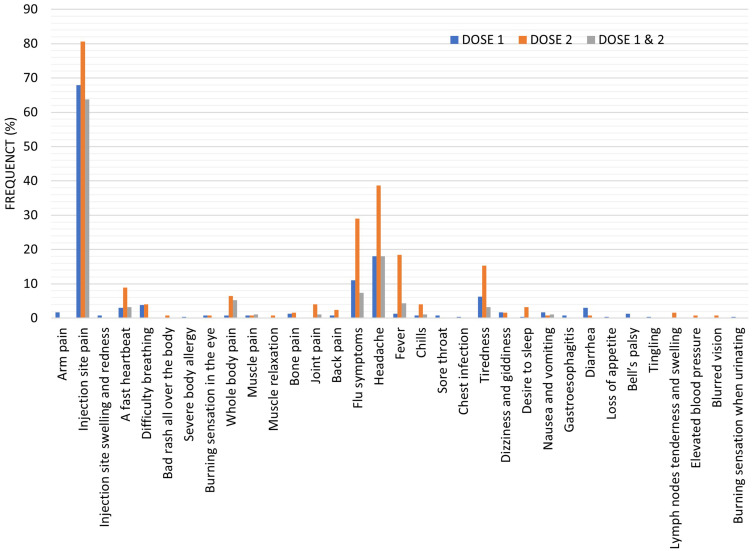
The results also showed a significant increase (p < 0.05) in the number of participants who reported injection site side effects after receiving the second dose of the vaccine (100, 80.6%) compared to the number of participants who reported local side effects of the vaccine after receiving the first dose (167, 70.5%) or both doses (60, 63.8%). After the first vaccine dose, 4 (1.7%) participants reported arm and shoulder pain, and 2 (0.8%) participants reported swelling and redness at the injection site. However, no one reported these symptoms after the second or both doses of the vaccine. There was a significant increase (p < 0.05) in the number of participants who experienced injection site pain after receiving the second dose of the vaccine (100, 80.6%) compared to those who received their first dose (161, 67.9%) or who experienced this symptom after both doses (60, 63.8%), Table 2 and Figure 3.
Figure 3.
The reported COVID-19 vaccine side effects after the vaccine’s first, second, and both doses. Results were offered as frequency (percent (%)).
These results showed a significant increase (p < 0.01) in the number of individuals who reported hypersensitivity symptoms after receiving the second dose of the vaccine (18, 14.5%) compared to the number of individuals who reported hypersensitivity symptoms after receiving the first dose (19, 8.0%) or both doses (3, 3.2%). There was a significant increase (p < 0.05) in the number of individuals who reported a fast heartbeat after receiving the second dose of the vaccine (11, 8.9%) compared to the number of individuals who reported a fast heartbeat after receiving the first dose (7, 3.0%) or both doses (3, 3.2%). Nine (3.8%) individuals reported shortness of breath after the first dose of the vaccine, and 5 (4.0%) individuals after the second dose. One individual (0.8%) reported a bad rash all over the body after the second vaccine dose, while one other individual (0.4%) also reported a severe body allergy after the first vaccine dose. A burning sensation in the eye was reported by 2 (0.8%) individuals after the first dose of the vaccine while only one individual (0.8%) after the second dose of the vaccine, Table 2 and Figure 3.
The results showed a significant increase (p < 0.001) in the number of persons who reported bone and muscle pain after receiving the second dose of the vaccine (22, 17.7%) compared to the number of persons who reported bone and muscle pain after receiving the first dose (9, 3.8%) or both doses (7, 7.4%). There was a significant increase (p < 0.01) in the number of persons who reported whole body pain and joint pain after receiving the second dose of the vaccine, 8 (6.5%) and 5 (4.0%) compared to the number of persons who reported whole body pain and joint pain after receiving the first dose, 2 (0.8%) and 0 (0.0% or both doses, 5 (5.3%) and 1 (1.1%). After the first vaccine dose, 2 (0.8%) persons reported muscle pain, 3 (1.3%) reported bone pain, and 2 (0.8%) reported back pain. After the second vaccine dose, 1 (0.8%) person reported muscle pain, 1 (0.8%) reported muscle relaxation, 2 (1.6%) reported bone pain and 3 (2.4%) reported back pain. One person (1.1%) reported muscle pain after his first and second vaccine doses, Table 2 and Figure 3.
The results showed a significant increase (p < 0.001) in the number of individuals who reported total flu-like symptoms after receiving the second dose of the vaccine (112, 90.3%) compared to the number of individuals who reported total flu-like symptoms after receiving the first dose (77, 32.5%) or both doses (29, 30.9%). There was a significant increase (p < 0.001) in the number of individuals who reported flu symptoms after receiving the second dose of the vaccine (36, 29%) compared to the number of individuals who reported flu symptoms after receiving the first dose (26, 11.0%) or both doses (7, 7.4%). Besides, there was a significant increase (p < 0.001) in the number of individuals who reported fever after receiving the second dose of the vaccine (23, 18.5%) compared to the number of individuals who reported fever after receiving the first dose (3, 1.3%) or both doses (4, 4.3%). After the first vaccine dose, 2 (0.8%) individuals reported chills, 2 (0.8%) reported sore throat and 1 (0.4%) reported chest infection. After the second vaccine dose, 5 (4.0%) individuals reported chills. One individual (1.1%) reported chills after his first and second vaccine doses, Table 2 and Figure 3.
The results also showed a significant increase (p < 0.001) in the number of participants who reported fatigue after receiving the second dose of the vaccine (25, 20.1%) compared to the number of participants who reported fatigue after receiving the first dose (20, 8.4%) or both doses (3, 3.2%). Besides, there was a significant increase (p < 0.05) in the number of participants who reported a desire to sleep after receiving the second dose of the vaccine (4, 3.2%) compared to the number of individuals who reported a desire to sleep after receiving the first dose (1, 0.4%) or both doses (0, 0%). After the first vaccine dose, 15 (6.3%) participants reported tiredness, and 4 (1.7%) reported dizziness and giddiness. After the second vaccine dose, 19 (15.3%) participants reported tiredness, and 2 (1.6%) reported dizziness and giddiness. Three participants (3.2%) reported tiredness after their first and second vaccine doses, Table 2 and Figure 3.
The results also showed a significant increase (p < 0.05) in the number of persons who reported GIT symptoms after receiving the first dose of the vaccine (14, 5.9%) compared to the number of persons who reported GIT symptoms after receiving the second dose (2, 1.6%) or both doses (1, 1.1%). After the first vaccine dose, 4 (1.7%) persons reported nausea and vomiting, 2 (0.8%) reported gastroesophagitis, 7 (3.0%) reported diarrhea, and 1 (0.4%) reported a loss of appetite. After the second vaccine dose, 1 (0.8%) reported nausea and vomiting, and 1 (0.8%) reported diarrhea. One person (1.1%) reported nausea and vomiting after his first and second vaccine doses, Table 2 and Figure 3.
After the first vaccine dose, 3 (1.3%) individuals reported Bell’s palsy, and 1 (0.4%) reported body tingling, Table 2 and Figure 3.
The results also showed a significant increase (p < 0.05) in the number of persons who reported miscellaneous symptoms after receiving the second dose of the vaccine (4, 3.2%) compared to the number of persons who reported miscellaneous symptoms after receiving the first dose (1, 0.4%) or both doses (0, 0%). Only one (0.4%) person reported a burning sensation with urination after his first vaccine dose. After the second vaccine dose, 2 (1.6%) persons reported lymph nodes tenderness and swelling, 1 (0.8%) person reported elevated blood pressure, and 1 (0.8%) reported blurred vision, Table 2 and Figure 3.
Reported COVID-19 Vaccine Side Effects and Their Correlation with Participants’ Ages
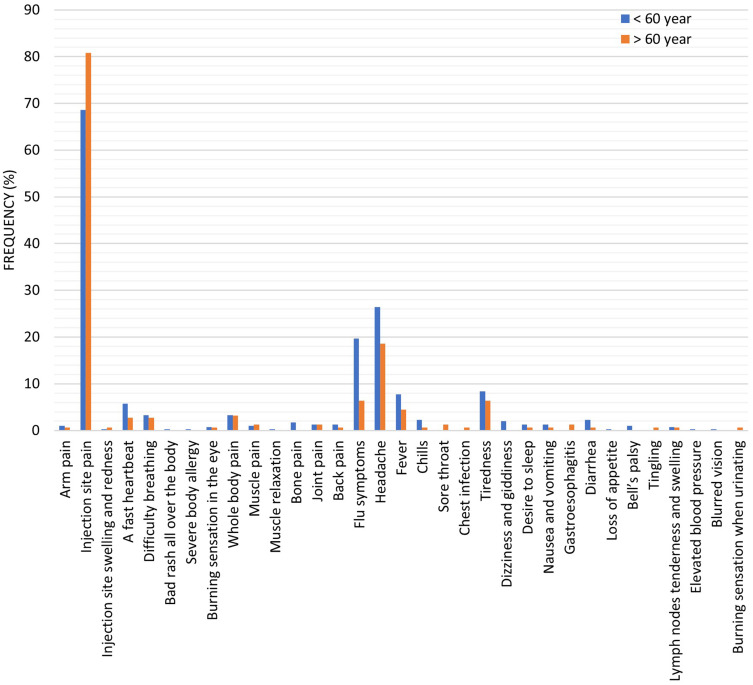
Table 3 and Figure 4 showed the reported COVID-19 vaccine side effects distribution according to the participants’ ages. The study results showed no significant difference between those who were under the age of 60 years and suffering from COVID-19 vaccine side effects and those over the age of 60. On the other hand, there was a significant increase (p < 0.01) in the number of people who reported local symptoms and injection site pain over 60 years of age, 128 (82.0%) and 126 (80.8%) compared to those under 60 years old, 209, (69.9%) and 205 (68.6%). Besides, there was a significant increase in the number of people who reported different flu-like symptoms and flu symptoms under 60 years of age, 168 (56.2%) and 59 (19.7%) compared to those over 60 years old, 49 (31.4%) and 10 (6.4%). There were no significant differences between the other side effects of the vaccine when comparing their incidence between participants according to their ages.
Table 3.
The Reported COVID-19 Vaccine Side Effects and Their Correlation with the Participants Ages
| Frequency (n and %) | |||
|---|---|---|---|
| Age < 60 (Year) | Age ≥ 60 (Year) | Chi-Square | |
| (n=299) | (n=156) | p value | |
| Absence of Symptoms | 39 (13%) | 24 (15.4%) | 0.5675 |
| Local Symptoms | 209 (69.9%) | 128 (82%) | 0.0049** |
| Arm pain | 3 (1.0%) | 1 (0.6%) | 1.0000 |
| Injection site pain | 205 (68.6) | 126 (80.8%) | 0.0056** |
| Injection site swelling and redness | 1 (0.3%) | 1 (0.6%) | 1.0000 |
| Hypersensitivity Symptoms | 31 (10.4%) | 9 (5.8%) | 0.1174 |
| A fast heartbeat | 17 (5.7%) | 4 (2.7%) | 0.1614 |
| Difficulty breathing | 10 (3.3%) | 4 (2.7%) | 0.7798 |
| Bad rash all over the body | 1 (0.3%) | 0 (0%) | 1.0000 |
| Severe body allergy | 1 (0.3%) | 0 (0%) | 1.0000 |
| Burning sensation in the eye | 2 (0.7%) | 1 (0.6%) | 1.0000 |
| Bone and Muscle Symptoms | 27 (9.0%) | 10 (6.4%) | 0.3714 |
| Whole body pain | 10 (3.3%) | 5 (3.2%) | 1.0000 |
| Muscle pain | 3 (1.0%) | 2 (1.3%) | 1.0000 |
| Muscle relaxation | 1 (0.3%) | 0 (0%) | 1.0000 |
| Bone pain | 5 (1.7%) | 0 (0%) | 0.1703 |
| Joint pain | 4 (1.3%) | 2 (1.3%) | 1.0000 |
| Back pain | 4 (1.3%) | 1 (0.6%) | 0.6646 |
| Flu Like Symptoms | 168 (56.2%) | 49 (31.4%) | < 0.0001*** |
| Flu symptoms | 59 (19.7%) | 10 (6.4%) | < 0.0001*** |
| Headache | 79 (26.4%) | 29 (18.6%) | 0.0644 |
| Fever | 23 (7.7%) | 7 (4.5%) | 0.2346 |
| Chills | 7 (2.3%) | 1 (0.6%) | 0.2736 |
| Sore throat | 0 (0%) | 2 (1.3%) | 0.1171 |
| Chest infection | 0 (0%) | 1 (0.6) | 0.3443 |
| Fatigue Symptoms | 35 (11.7%) | 11 (7.1%) | 0.1409 |
| Tiredness | 25 (8.4%) | 10 (6.4%) | 0.5789 |
| Dizziness and giddiness | 6 (2.0%) | 0 (0%) | 0.0988 |
| Desire to sleep | 4 (1.3%) | 1 (0.6%) | 0.6646 |
| GIT Symptoms | 12 (4.0%) | 4 (2.6%) | 0.5937 |
| Nausea and vomiting | 4 (1.3%) | 1 (0.6%) | 0.6646 |
| Gastroesophagitis | 0 (0%) | 2 (1.3%) | 0.1171 |
| Diarrhea | 7 (2.3%) | 1 (0.6%) | 0.2736 |
| Loss of appetite | 1 (0.3%) | 0 (0%) | 1.0000 |
| Nerve Inflammation Symptoms | 3 (1.0%) | 1 (0.6%) | 1.0000 |
| Bell’s palsy | 3 (1.0%) | 0 (0%) | 0.5544 |
| Tingling | 0 (0%) | 1 (0.6%) | 0.3443 |
| Miscellaneous Symptoms | 4 (1.3%) | 1 (0.6%) | 0.6646 |
| Lymph nodes tenderness and swelling | 2 (0.7%) | 0 (0.6%) | 0.5484 |
| Elevated blood pressure | 1 (0.3%) | 0 (0%) | 1.0000 |
| Blurred vision | 1 (0.3%) | 0 (0%) | 1.0000 |
| Burning sensation when urinating | 0 (0%) | 1 (0.6%) | 0.3443 |
Notes: Results were offered as frequency (number (n) and percent (%)). Correlation between variables was evaluated using the Chi-square test. **Significant difference at p ≤ 0.01, and ***significant difference at p ≤ 0.001.
Figure 4.
The reported COVID-19 vaccine side effects distribution according to the participants’ ages. Results were offered as frequency (percent (%)).
Correlation Between Presence and Absence of COVID-19 Vaccine Side Effects and the Participant’s Sex
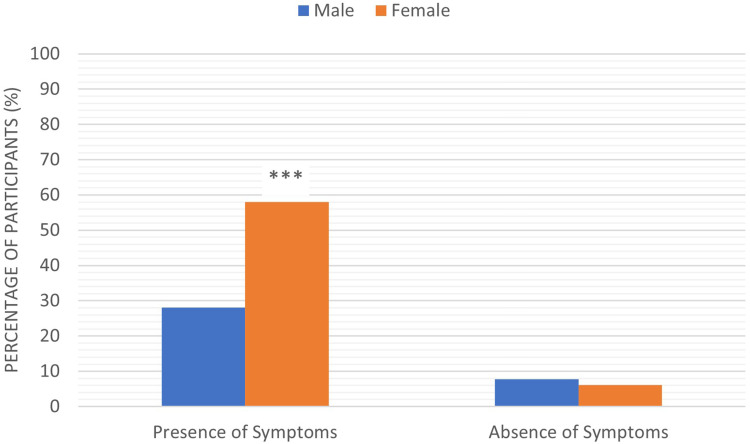
The study results showed a significant difference between the number of males and females who suffered from COVID-19 vaccine side effects. There was a significant increase (p < 0.001) in the number of female participants who reported different side effects after they receive the vaccine (264, 58%) compared to males (128, 28.1%). Furthermore, the number of females who reported no side effects (28, 6.2%) was lower than males (35, 7.7%), Figure 5.
Figure 5.
The correlation between presence and absence of COVID-19 vaccine side effects and the participant’s sex. Results were offered as frequency (percent (%)). Correlation between variables was evaluated using the Chi-square test. ***Significant difference at p ≤ 0.001.
Reported COVID-19 Vaccine Side Effects Distribution in Participants Previously Infected with Coronavirus versus Non-Previously Infected Participants
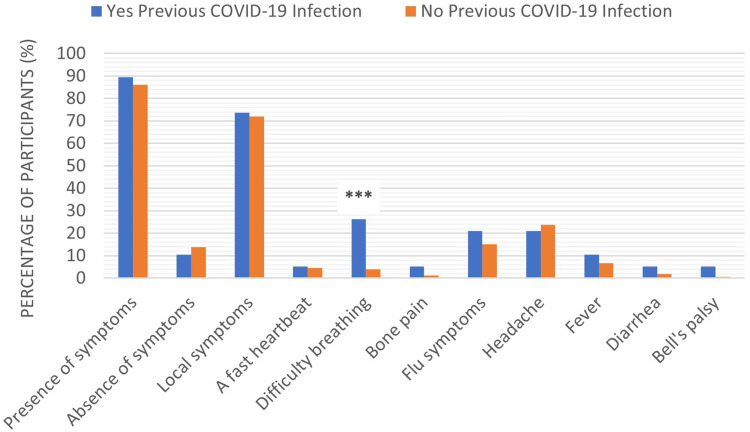
Among the study population, 89% of participants who had previously been infected with the coronavirus were suffering from some side effects of the COVID-19 vaccine, compared to 86% of those who had not previously been infected. Of the previously infected participants, 73.7% complained of local side effects, 5.3% a fast heartbeat, 26.3% difficulty breathing, 5.3% bone pain, 21.1% flu symptoms, 21.1% headaches, 10.5% fever, 5.3% diarrhea, and 5.3% Bell’s palsy. The distribution of the same side effects among participants who had not previously been infected with the coronavirus was as follows:71.9% complained of local side effects, 4.6% a fast heartbeat, 3.9% difficulty breathing, 1.1% bone pain, 15.2% flu symptoms, 23.7% headaches, 6.5% fever, 1.8% diarrhea, and 0.7% Bell’s palsy. The results also showed a significant increase (p < 0.001) in the number of participants who had previously been infected with the coronavirus and suffered from difficulty breathing after receiving the vaccine, compared to participants who had not previously been infected, Figure 6.
Figure 6.
The reported COVID-19 vaccine side effects distribution in participants previously infected with coronavirus versus non-previously infected participants. Results were offered as frequency (percent (%)). Correlation between variables was evaluated using the Chi-square test. ***Significant difference at p ≤ 0.001.
Discussion
Ever since the start of vaccine production, people have expressed worries about their administration’s hazards and risks. There is a great variation in people’s confidence in vaccines that relies on several factors, including awareness about vaccines, the possible associated risks, experiences, religious, or political aspects, in addition to social and economic status.13 Moreover, it has been revealed that people assess vaccination-associated risks compared to other risks in a different way than experts.8–10 Some side effects are improbably manifested in pre-licensure clinical studies owing to their minimal frequency, the restricted numbers of participating individuals, and other study restrictions. Therefore, post-marketing monitoring of the side effects following vaccine administration is crucial.14
In this study, the data collected from the participating individuals revealed that the adverse effects of the vaccine were reported after two doses, with the majority occurring after the second dose. The most common symptoms are injection site pain, headaches, flu symptoms, fever, and tiredness. Less common side effects are a fast heartbeat, whole body aches, difficulty breathing, joint pain, chills, and drowsiness. Rare side effects included Bell’s palsy and lymph nodes tenderness and swelling. Influenza-like symptoms were more common among those under 60 years of age, while pain at the injection site was more common among those who are 60 years and older. These results were in accordance with the FDA Fact Sheet for Recipients and Caregivers. According to the fact sheet, the most common adverse reactions, which include pain at the site of administration, tiredness, headaches, muscle and joint ache, chills, and elevated body temperature, could last for days and were more experienced following the second shot compared to the first one.15 This observation could be interpreted on the basis of the immune system’s response. The immune system could produce cytokines that could have an inflammatory effect on the blood vessels, muscles, and other tissues. It may also produce flu-like symptoms that last for days after vaccination. This also could explain the prevalence of the side effects development in people of ages below 60 years more than older people as younger people have stronger and more efficient immune systems. The possibility that the Pfizer-BioNTech COVID-19 vaccine could induce a severe allergic reaction is reported. An intense allergic response would eventually take place within a few minutes to one hour following receiving the vaccine dose. Signs of intense allergic response may include dyspnea, face and throat swelling, rapid heartbeat, body rash, dizziness and weakness. Bell’s palsy has also been reported as a rare side effect for the vaccine.16,17 The results of the present study showed a significant increase in the number of individuals who reported hypersensitivity symptoms after receiving the second dose of the vaccine. A minor percentage of individuals have also developed Bell’s palsy. These results were in agreement with the previously reported side effects for the vaccine.
This study results showed that injection site pain was recorded more often among individuals 60 years and older (80.8%) than among younger individuals (68.6%). A remarkably reduced percentage of individuals proclaimed injection-site swelling and redness. The percentage of individuals suffering local symptoms significantly increased after the second dose. Our results disagree with the results recorded by Polack et al18 who reported that injection site pain was more frequent among people younger than 55 years compared to those who are older. They also noticed that the percentages of people reporting local symptoms after the first and the second doses of the vaccine were nearly the same. However, our study and their study reported the same results concerning injection-site swelling and redness. Our results agree with Polack et al18 as the two studies reported that the vaccine-associated systemic side effects were more common among younger people and more common after the second vaccine dose. The two studies reported that headaches were the most common symptom after a vaccine injection. Also, fever was most common among young people after the second dose.
Limitations and Strengths
The current study is one of the leading studies that shed light on the side effects of the Pfizer-BioNTech COVID-19 vaccine. Few or no studies have documented the vaccine’s side effects, especially the post-marketing follow-up of the vaccine. The limitation of the current study is that it monitored the vaccine’s short-term side effects (immediately after injection). In contrast, the medium and long-term effects of the vaccine are still unknown. Furthermore, the study was conducted only on Arab participants only, thus excluding other races. The small number of the study participants who were previously infected with the coronavirus suggest the need for future studies with a suitable and compatible sample size for both previously infected and non-infected persons.
Conclusion
In general, the side effects that were identified from the Pfizer-BioNTech COVID-19 vaccine are the typical symptoms of most previous vaccines, and most of them are tolerated.19,20 Also, most of the symptoms have been reported by the manufacturing company and the FDA Facts Sheet.15 However, for some of people it is mandatory to monitor them for a short time immediately after receiving their vaccine doses.
Acknowledgments
All the authors are deeply thankful to everyone who complete the questionnaire.
Data Sharing Statement
All relevant data are available in the present manuscript. The data presented in this study are available on request from the corresponding author.
Ethics Approval and Consent to Participate
The study protocol was approved by the Unit of Biomedical Ethics Research Committee, Faculty of Medicine, King Abdulaziz University, Saudi Arabia (Reference No. 111/21). The study was also approved from the Public Health Research and Health Statistics Saudi Center for Disease Control and Prevention (SCDC), Saudi Arabia (Registration number: (202,102,221; IRB number: 111-21). Concerning the informed consent statement, the study participants were informed that as they completed the questionnaire, they were accepted to be enrolled in the study. This study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors declare no conflicts of interest.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) situation report-127. World Health Organisation (WHO); 2020:1–17. Available from: https://apps.who.int/iris/handle/10665/332232. Accessed February20, 2021. [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Draft landscape and tracker of COVID-19 candidate vaccines. World Health Organisation (WHO); 2020. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed February20, 2021. [Google Scholar]
- 4.FDA Briefing Document. Pfizer-BioNTech COVID-19 vaccine. U.S. Food and Drug Administration; 2020:1–53. Available from: https://www.fda.gov/media/144245/download. Accessed February11, 2021. [Google Scholar]
- 5.FDA Briefing Document. Moderna COVID-19 Vaccine. U.S. Food and Drug Administration; 2020:1–54. Available from: https://www.fda.gov/media/144434/download. Accessed February11, 2021. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Understanding how COVID-19 vaccines work. Vaccines. 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html. Accessed February11, 2021.
- 7.Centers for Disease Control and Prevention. What to expect at your appointment to get vaccinated for COVID-19| CDC. Vaccines. 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect.html. Accessed February11, 2021.
- 8.Davis TC, Fredrickson DD, Arnold CL, et al. Childhood vaccine risk/benefit communication in private practice office settings: a national survey. Pediatrics. 2001;107:e17–e17. doi: 10.1542/peds.107.2.e17 [DOI] [PubMed] [Google Scholar]
- 9.Gust DA, Woodruff R, Kennedy A, Brown C, Sheedy K, Hibbs B. Parental perceptions surrounding risks and benefits of immunization. Semin Pediatr Infect Dis. 2003;14:207–212. doi: 10.1016/S1045-1870(03)00035-9 [DOI] [PubMed] [Google Scholar]
- 10.Bond L, Nolan T, Pattison P, Carlin J. Vaccine preventable diseases and immunisations: a qualitative study of mothers’ perceptions of severity, susceptibility, benefits and barriers. Aust N Z J Public Health. 1998;22:441–446. doi: 10.1111/j.1467-842X.1998.tb01411.x [DOI] [PubMed] [Google Scholar]
- 11.MacDonald NE, Eskola J, Liang X, et al. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 12.Aranda S. Ten Threats to Global Health in 2019. World Health Organisation (WHO); 2019:1–18. [Google Scholar]
- 13.Larson H, Cooper L, Eskola J, Katz S, Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011;378(9790):526–535. doi: 10.1016/S0140-6736(11)60678-8 [DOI] [PubMed] [Google Scholar]
- 14.Wessely S. Emergency preparedness and response. J R Army Med Corps. 2018;166:jramc-2018-000965. doi: 10.1136/jramc-2018-000929 [DOI] [Google Scholar]
- 15.Pfizer COVID-19 vaccine EUA fact sheet for healthcare providers administering vaccine (vaccination providers). 2021:1–30. Available from: https://www.fda.gov/media/144413/download. Accessed March31, 2021.
- 16.Crist C. Track vaccine recipients for facial paralysis. Available from: https://www.webmd.com/vaccines/covid-19-vaccine/news/20201217/fda-says-vaccine-recipients-should-be-monitored-for-facial-paralysis. Accessed February20, 2021.
- 17.What we know about a link between bell’s palsy and COVID-19 vaccines. Available from: https://www.businessinsider.com/what-we-know-link-between-bells-palsy-covid-19-vaccines-2020-12. Accessed February20, 2021.
- 18.Polack F, Thomas S, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Balveren-slingerland L, Rümke H, Kant A. Reported adverse events following influenza vaccination. Ned Tijdschr Geneeskd. 2014;158:A6841. [PubMed] [Google Scholar]
- 20.Zhou W, Pool V, Iskander J, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)–United States, 1991–2001. MMWR CDC Surveill Summ. 2003;52:1–24. [PubMed] [Google Scholar]