Abstract
Purpose
This study assessed effectiveness and safety of the novel Clareon intraocular lens (IOL; model SY60CL; Alcon Vision LLC).
Patients and Methods
This was a prospective, single-arm, unmasked clinical trial at 16 investigative clinical sites in the United States. Included were adults ≥22 years who required cataract extraction by phacoemulsification. Following phacoemulsification, 350 subjects received SY60CL IOL unilaterally; 342 completed the study. Monocular best corrected distance visual acuity (CDVA) and uncorrected distance visual acuity (UDVA) were evaluated. The primary effectiveness endpoint was the percentage of subjects with CDVA ≤0.3 logMAR at month 12. Safety was assessed by monitoring adverse events (AEs). Visual acuity and safety outcomes were compared with historical safety and performance endpoint (SPE) rates.
Results
At 12 months post-implantation, 99.7% of subjects receiving the SY60CL IOL achieved monocular CDVA ≤0.3 logMAR (primary effectiveness endpoint; 1-sided 95% upper confidence limit >SPE rate); 99.7% and 86.8% of subjects achieved monocular CDVA of ≤0.34 (20/40 Snellen or better) and ≤0.04 logMAR (20/20 Snellen or better), respectively. At 12 months, >95% of subjects achieved mean monocular UDVA ≤0.3 logMAR; 97.1% and 57.6% of subjects achieved monocular CDVA of ≤0.34 and ≤0.04 logMAR, respectively. Mean monocular CDVA and UDVA were −0.05 and 0.04 logMAR, respectively. AEs were within SPE limits. The most common nonserious ocular AE was posterior capsule opacification (5.4%). Serious AEs were <1%, and no serious ocular AEs were assessed as related to the device. There were no observations for IOL glistenings at 12 months.
Conclusion
Results of this study supported effectiveness and safety of the SY60CL IOL. Visual acuity outcomes with the SY60CL IOL exceeded the SPE rates for monocular CDVA and AEs were within the limit of historic SPE rates. (Model number SY60WF is the Clareon lens approved by the FDA.)
Keywords: glistenings, posterior capsule opacification, visual acuity, cystoid macular edema, dysphotopsia
Cataract is the leading cause of blindness worldwide; standard-of-care treatment is removal of the cloudy natural crystalline lens and implantation of an intraocular lens (IOL).1,2 In addition to the restored visual acuity (VA) provided by this treatment, advances in IOL design and materials can potentially reduce the rate of adverse events (AEs) and photic phenomena, leading to further improvements in visual function and patient satisfaction.
The Clareon® IOL (model SY60CL; Alcon Vision LLC, Fort Worth, TX, USA) is a novel monofocal aspheric hydrophobic lens composed of a flexible acrylic material (PEA/HEMA copolymer) with high refractive index (Clareon model number SY60WF will be commercially available). This ultraviolet (UV)–absorbing and blue light–filtering lens uses a different material but has the same optical design as the AcrySof® Natural IQ IOL (model SN60WF; Alcon Vision LLC). It is a foldable, single-piece IOL for implantation in the capsular bag in the posterior chamber during cataract surgery.
Different IOL materials can exhibit different lens artifacts such as microvacuoles (ie, glistenings).3,4 Both glistening and surface light scattering were reported to be more severe in hydrophobic acrylic IOLs compared with silicone and polymethyl methacrylate IOLs.4 A prospective randomized study in 273 patients reported that approximately 40% to 68% of patients developed glistenings, depending on the lens type, 2 years post-implantation.5 The SY60CL IOL material was designed to enhance clarity and has demonstrated among the lowest levels of surface haze, surface roughness, and glistenings compared with commercially available hydrophobic acrylic IOLs.6 In this in vitro study, Clareon model CNA0T0 had significantly lower microvacuole density and size compared with Tecnis model ZCB00 (Johnson & Johnson Vision, Santa Ana, CA, USA), Tecnis OptiBlue ZCB00V, or Vivinex XY1 (Hoya Surgical Optics, Inc., Chino Hills, CA, USA) lenses (P<0.001). Similar microvacuole density and size were reported for the Clareon IOL, Eternity W-60 (Santen, Inc., Osaka, Japan), and enVista MX60 (Bausch & Lomb, Rochester, NY, USA) lenses (P>0.4), which had higher water content.6 Recently, these in vitro data were corroborated by clinical studies. No glistenings or surface haze were reported in eyes with the Clareon IOL 1 year (n=110) and 9 years (n=20) post-implantation.7 A randomized controlled trial reported minimal glistenings (median grade 0 on an 8-point scale) in subjects implanted with Clareon (n=68) or Tecnis (n=68) IOLs at 1 year post-implantation, with no significant difference between the 2 groups (P=0.2).
Several studies demonstrated that IOL material and design can have a significant effect on posterior capsule opacification (PCO) and neodymium-doped yttrium aluminum garnet (Nd:YAG) capsulotomy rates.9–12 AcrySof IOLs were reported to have lower Nd:YAG capsulotomy rates compared with round-edged silicone IOLs (P=0.001).9 A meta-analysis of randomized controlled trials reported that PCO rates were significantly lower for hydrophobic acrylic versus hydrophilic acrylic IOLs at 1- and 2-year follow-up (P=0.0002 and P<0.00001, respectively). Additionally, hydrophobic acrylic IOLs were associated with significantly lower rates of Nd:YAG capsulotomy than hydrophilic IOLs (P<0.00001).10
The purpose of this study was to assess effectiveness and safety of the novel SY60CL material in a clinical trial in adults who underwent cataract surgery and to compare VA and safety outcomes of the SY60CL IOL with historical safety and performance endpoint (SPE) rates. Specifically, we evaluated the effects of the SY60CL material on glistenings, cystoid macular edema (CME), and PCO.
Methods
Study Design
This was a prospective, multicenter (16 sites in the United States), single-arm, unmasked clinical trial conducted from July 2017 to February 2019 (clinicaltrials.gov registration: NCT03170154). A sample size of 350 subjects was planned so that ≥300 evaluable eyes with successful implantation would be available for analysis. With 300 evaluable eyes, the probability of demonstrating that ≥90% of eyes had monocular CDVA of 0.3 logMAR or better at 12 months was 99%. Included in the study were adults aged ≥22 years who required cataract extraction by phacoemulsification in at least 1 qualifying eye and for whom postoperative emmetropia was planned (±0.5 D spherical equivalent). This clinical trial was conducted under Investigational Device Exemption G170112 approved by the FDA on May 31, 2017.
Key exclusion criteria were disease or pathology other than cataract expected to reduce potential postoperative best corrected distance visual acuity (CDVA) to worse than 0.30 logMAR, previous corneal surgery, rubella or traumatic cataract, ocular trauma or previous refractive surgery, or use of agents (eg, α1-antagonist) that would require mechanical or surgical manipulation to enlarge the pupil. Subjects were discontinued from the study during the surgery if there were intraoperative complications requiring additional procedures or leading to the surgeon’s inability to place the IOL in the capsular bag.
Subjects attended 7 study visits including a preoperative screening visit; the operative visit; and postoperative visits on day 1, week 1, month 1, month 6, and month 12. Unscheduled visits were conducted as needed.
Surgical Procedure
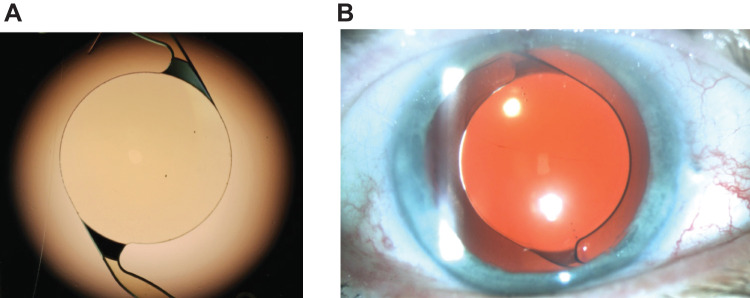
Cataracts were removed by phacoemulsification using a surgeon’s preferred methodology, and subjects received a SY60CL monofocal IOL unilaterally using a Monarch® III D cartridge and handpiece. Routine operative medications were used per standard surgical protocol at individual sites. Surgical manipulation to enlarge the pupil was not allowed. The reference provisional A-constant was 119.1. The SY60CL IOL is a 1-piece aspheric hydrophobic acrylic monofocal lens made with a UV-absorbing and blue light–filtering acrylate/methacrylate copolymer. SY60CL was the model designated for clinical investigation. Model number SY60WF is the Clareon lens that has been approved by the FDA. Other lens characteristics include Stableforce® modified-L haptics (Alcon Vision LLC), a 6.0-mm optic diameter, and 13.0-mm overall length. Representative images of the SY60CL IOL include an in vitro image taken with a Nikon Eclipse Ti-U inverted microscope at 15× magnification (Figure 1A) and a slit lamp image taken at study visit 3 (month 1) using retroillumination at 10× magnification, with a flash intensity of 75% and an aperture setting of 4 under dilated pupil conditions (Figure 1B). Toric axis markings were incorporated on the non-toric SY60CL monofocal IOL for evaluation of rotational stability and are not part of the commercially available lens.
Figure 1.
Representative SY60CL IOL images. aSY60CL IOL with the Nikon Eclipse Ti-U inverted microscope at 15× magnification (A) and slit lamp photography using retroillumination captured under pupil dilation conditions at 10× magnification, with a flash intensity of 75% and an aperture setting of 4 (B). aSY60CL was the designated lens model for clinical investigation; model number SY60WF is the Clareon lens that has been approved by the United States Food and Drug Administration.
Effectiveness and Safety Assessments
The primary effectiveness endpoint was the percentage of all-implanted subjects with CDVA ≤0.3 logMAR at month 12. Refraction was performed using a phoropter or trial frames at 4 m, and the spherical component was increased or decreased in 0.25 D steps with the aim of achieving maximum plus diopter spherical correction for maximum visual acuity. If needed, cylinder was refined using the cross-cylinder technique. One-sided exact 95% upper confidence limit was determined and study success was concluded if it was 0.3 logMAR or better compared with the SPE rate of 92.5% for the all-implanted analysis set. VA was measured using the fast Early Treatment Diabetic Retinopathy Study (ETDRS) method.13 Effectiveness was also assessed by monocular CDVA and uncorrected distance visual acuity (UDVA) at 4 m. CDVA and UDVA data were summarized using descriptive statistics.
Safety was assessed by monitoring AEs, including secondary surgical interventions. PCO and glistenings were assessed during slit-lamp examination. If present, PCO was graded as clinically non-significant (ie, early development of PCO such as fibrosis and proliferation of lens epithelial cells; no decrease in visual acuity or increase in glare), clinically significant (increased PCO with early visual acuity changes that did not require posterior capsulotomy), or clinically significant requiring YAG (PCO adversely affecting visual acuity and requiring posterior capsulotomy). For evaluation of glistenings, photographic examples were provided as a reference aid. Negative and positive dysphotopsia were evaluated during routine assessment of AEs. AEs were summarized using descriptive statistics. The one-sided exact 95% lower confidence level for incidence rates of AEs observed for the study eyes was compared with the cumulative and persistent AE SPE rates as reported in EN ISO 11979–7:2014.14
Statement of Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with Good Clinical Practice, ISO 11979–7:2014, ISO 14155:2011, and code of federal regulations. Approval was obtained from the Sterling Institutional review board (IRB). Because none of the investigators who participated were at sites with associated institutional IRBs, a central IRB was contracted to perform ethics oversight. Sterling IRB went through a rigorous vendor approval process that included routine quality assurance audits per Alcon’s procurement requirements. Subjects provided valid written informed consent before any screening or trial-related procedures.
Results
Subjects
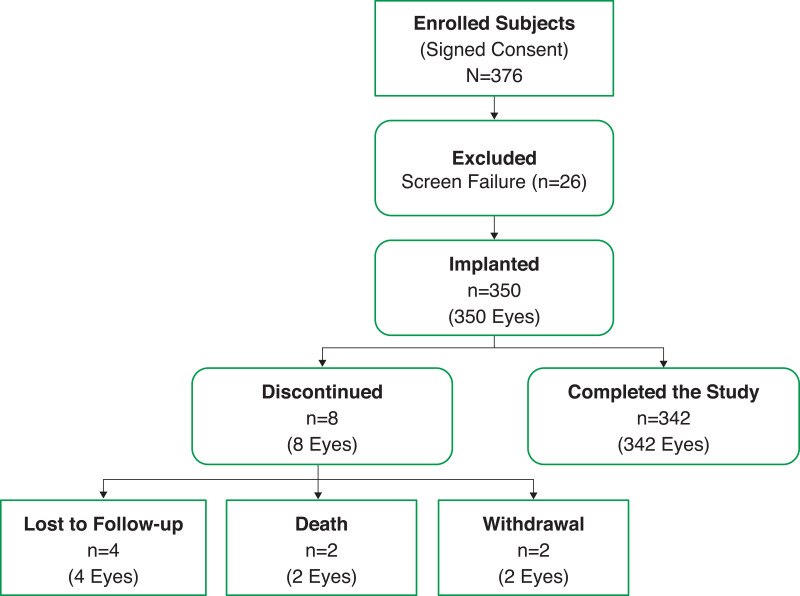
Of the 376 subjects enrolled, 26 were excluded from the study before implantation because of screen failure, and 350 received the SY60CL IOL (Figure 2). Of the 350 subjects, 342 completed the study. Eight subjects were discontinued from the study after implantation: 4 were lost to follow-up, 2 withdrew, and 2 subjects died (deaths were not related to the study device or procedure).
Figure 2.
Subject disposition.
Most subjects were white (273/350, 78.0%) and were ≥65 years old (281/350, 80.3%; Table 1), with a mean age of 69.7 years. More than half of subjects were female (213/350, 61%). Mean baseline CDVA was 0.19±0.21 logMAR, and mean baseline corneal astigmatism was 0.65±0.42 D (Table 1).
Table 1.
Subject Demographics and Baseline Characteristics (All-Implanted Analysis Set)
| Parameter | SY60CL IOL (n=350) |
|---|---|
| Age, years | |
| Mean ± SD | 69.7±6.44 |
| Range | 45–86 |
| Age group, years, n (%) | |
| <65 | 69 (20) |
| ≥65 | 281 (80) |
| Sex, n (%) | |
| Female | 213 (61) |
| Male | 137 (39) |
| Race, n (%) | |
| White | 273 (78) |
| Black or African American | 36 (10) |
| Asian | 29 (8.3) |
| Native Hawaiian or other Pacific Islander | 1 (0.3) |
| Other | 11 (3.1) |
| Monocular CDVA, logMAR | |
| Mean ± SD | 0.191±0.206 |
| Range | −0.18 to 1.70 |
| Axial length, mm | |
| Mean ± SD | 23.9±0.96 |
| Range | 21.3–27.1 |
| Axial length category, n (%) | |
| Short (<21 mm) | 0 |
| Medium (21–26 mm) | 343 (98) |
| Long (>26 mm) | 7 (2) |
| Corneal astigmatism, D | |
| Mean ± SD | 0.647±0.418 |
| Range | 0.0–3.15 |
Abbreviation: CDVA, best corrected distance visual acuity.
Visual Acuity Outcomes
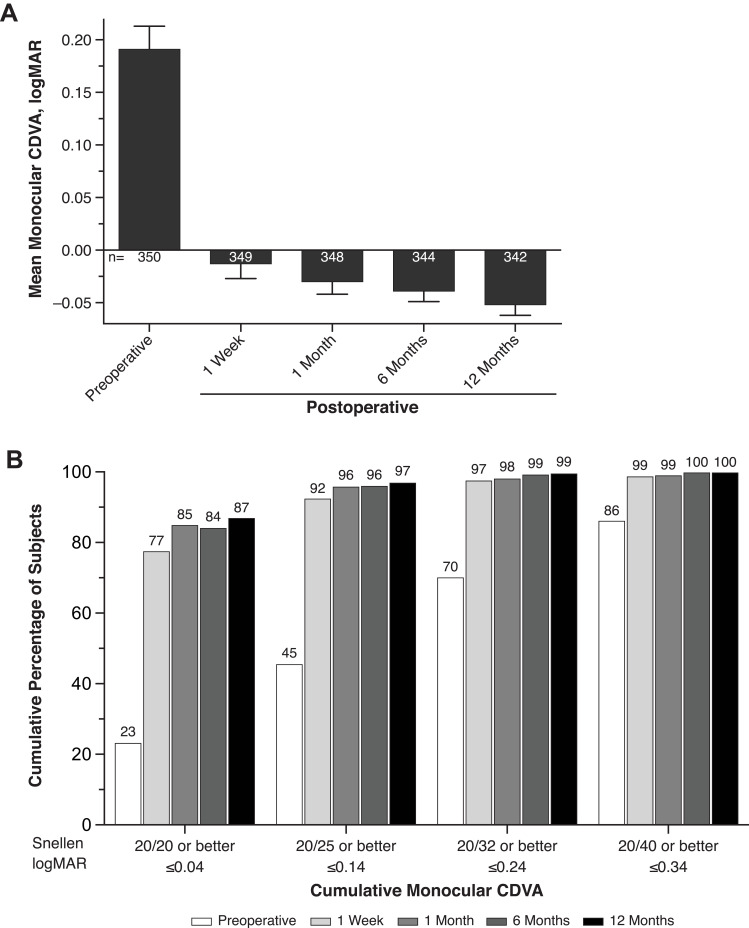
At month 12, 99.7% of subjects achieved CDVA of 0.3 logMAR or better, and the one-sided 95% upper confidence limit of 99.99% was greater than the SPE rate of 92.5%. Additionally, 81.0% of subjects achieved CDVA of 0.0 logMAR or better at month 12.
Mean monocular CDVA was −0.01 and −0.05 logMAR at week 1 and month 12, respectively (Figure 3A). The cumulative distribution of monocular CDVA in subjects was similar at most postoperative visits. Monocular CDVA ≤0.34 logMAR (20/40 Snellen or better) was achieved by 98.6% and 99.7% of subjects at week 1 and month 12, respectively (Figure 3B). Monocular CDVA ≤0.04 logMAR (20/20 Snellen or better) was achieved by 77.4% and 86.8% of subjects at week 1 and month 12, respectively (Figure 3B). One subject had CDVA of 0.44 logMAR at month 12 that was related to PCO. After Nd:YAG, the subject achieved CDVA of 0.1 logMAR (20/25 Snellen).
Figure 3.
Mean monocular CDVA at 4 m (A) and cumulative distribution of monocular CDVA (B) in subjects who received SY60CL IOL. Error bars represent 95% CIs.
Abbreviations: CDVA, best corrected distance visual acuity; IOL, intraocular lens.
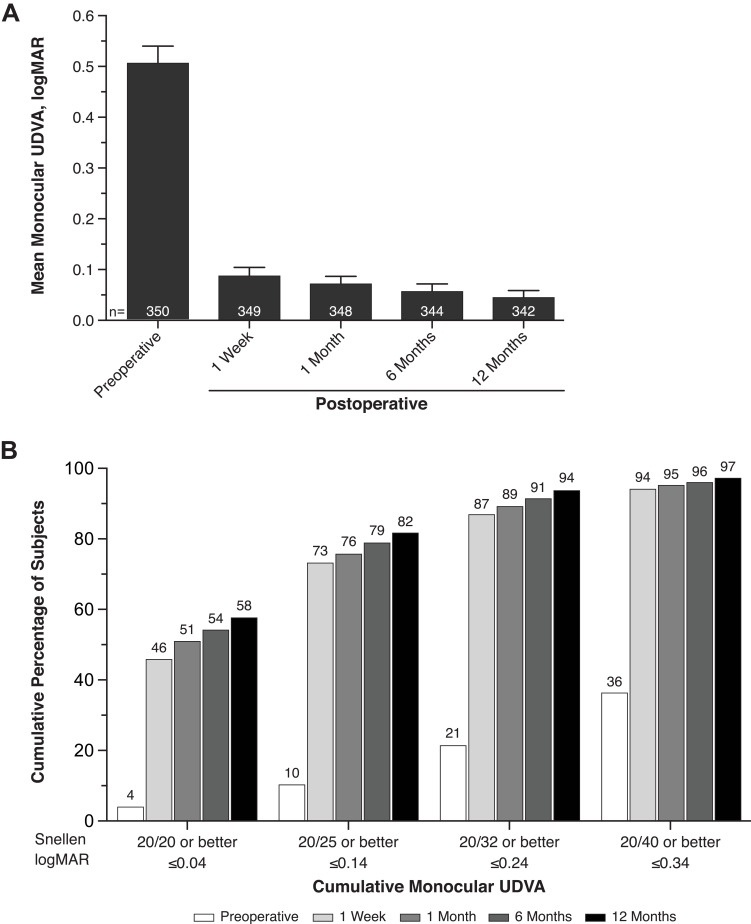
Mean monocular UDVA was 0.09 and 0.04 logMAR at week 1 and month 12, respectively (Figure 4A). More than 95% of subjects achieved monocular UDVA of 0.3 logMAR or better. Monocular UDVA ≤0.34 logMAR (20/40 Snellen or better) was achieved by 94.0% and 97.1% of subjects at week 1 and month 12, respectively (Figure 4B). Monocular UDVA ≤0.04 logMAR (20/20 Snellen or better) was achieved by 45.8% and 57.6% of subjects at week 1 and month 12, respectively (Figure 4B).
Figure 4.
Mean monocular UDVA at 4 m (A) and cumulative distribution of monocular UDVA (B) in subjects who received SY60CL IOL. Error bars represent 95% CIs.
Abbreviations: IOL, intraocular lens; UDVA, uncorrected distance visual acuity.
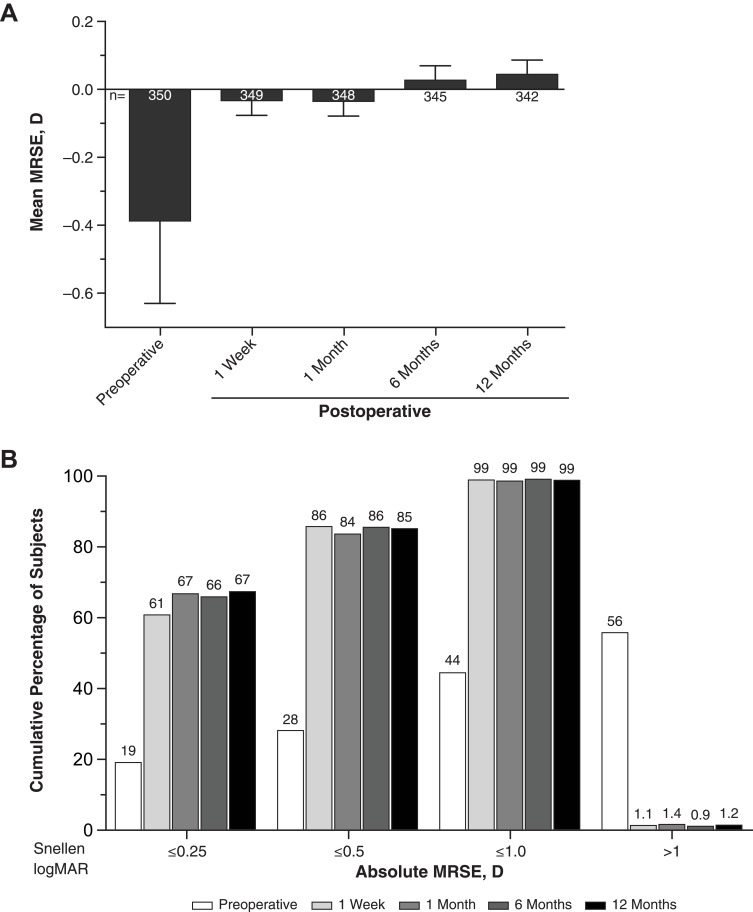
Mean Manifest Refraction Spherical Equivalent
At the preoperative visit, mean manifest refraction spherical equivalent (MRSE) was −0.39 D, and median MRSE was 0.0 D; 55.7% of subjects had MRSE >1 D. Subjects achieved mean MRSE of −0.03 and 0.04 D at week 1 and month 12, respectively (Figure 5A). Median MRSE was 0.0 D at all visits. Cumulative distribution of absolute MRSE in subjects was stable at visits between week 1 and month 12. Absolute MRSE was within 1.0 D in 98.9% (345/349) and 98.8% (338/342) of subjects at week 1 and month 12, respectively. Absolute MRSE was within 0.5 D in 85.7% (299/349) and 85.1% (291/342) of subjects at week 1 and month 12, respectively (Figure 5B).
Figure 5.
Mean MRSE (A) and cumulative distribution of absolute MRSE (B) in subjects who received SY60CL IOL. Error bars represent 95% CIs.
Abbreviations: IOL, intraocular lens; MRSE, manifest refraction spherical equivalent.
Mean target residual refractive error was −0.12 D. At week 1, mean prediction error was 0.09 D; 98.6% of subjects had absolute prediction error ≤1.0 D, and 79.1% had absolute prediction error ≤0.5 D. At month 12, mean prediction error was 0.17 D; 97.7% of subjects had absolute prediction error ≤1.0 D, and 76.3% had absolute prediction error ≤0.5 D.
Safety Outcomes
The rate of cumulative and persistent AEs was within the limit of the SPE rates based on the 1-sided exact 95% lower confidence level. The most frequently reported nonserious ocular AEs included PCO (19/350, 5.4%) and increased intraocular pressure (17/350, 4.9%). Other ocular AEs occurred at rates of ≤2.3% (Table 2). Posterior capsulotomy was required in 16 subjects (4.6%); 6 of those subjects underwent Nd:YAG at a single site (total number of subjects enrolled at that site was 20); the rate of posterior capsulotomy among the remaining sites was 10/330 (3.0%).
Table 2.
Nonserious and Serious AEs with Incidence >1.0% (Safety Analysis Set)
| AE, n (%) | SY60CL IOL (n=350) |
|---|---|
| Posterior capsule opacification | 19 (5.4) |
| Intraocular pressure increased | 17 (4.9) |
| Punctate keratitis | 8 (2.3) |
| Vitreous detachment | 8 (2.3) |
| Dry eye | 7 (2.0) |
| Conjunctival hemorrhage | 5 (1.4) |
| Corneal edema | 4 (1.1) |
| Cystoid macular edema | 4 (1.1) |
| Blepharoplasty | 4 (1.1) |
| Iritis | 4 (1.1) |
| Visual impairment | 4 (1.1) |
| Vitreous floaters | 4 (1.1) |
Abbreviations: AE, adverse event; IOL, intraocular lens.
Rates of serious ocular AEs were <1% (Table 3) and included CME (3/350, 0.9%) and retinal laser coagulation, retinal tear, and vitrectomy (2/350, 0.6% each). One subject had a moderately severe herpetic lesion and received ophthalmologic treatment, including anti-viral medication and Prokera placement. All serious ocular AEs were assessed by the investigator as not related to the device. Serious AEs assessed as related to the procedure included retinal tear and retinal laser coagulation, corneal epithelial repair with amniotic membrane graft and superficial punctate keratitis, full-thickness macular hole, and CME (n=1 for each).
Table 3.
Serious AEs (Safety Analysis Set)
| AE, n (%) | SY60CL IOL (n=350) |
|---|---|
| Cystoid macular edema | 3 (0.9) |
| Retinal laser coagulation | 2 (0.6) |
| Retinal tear | 2 (0.6) |
| Vitrectomy | 2 (0.6) |
| Corneal operation | 1 (0.3) |
| Herpes virus infection | 1 (0.3) |
| Macular fibrosis | 1 (0.3) |
| Macular hole | 1 (0.3) |
| Ophthalmologic treatment | 1 (0.3) |
| Punctate keratitis | 1 (0.3) |
Abbreviations: AE, adverse event; IOL, intraocular lens.
All adverse events related to the device were mild and resolved (visual impairment including negative or positive dysphotopsia; 3/350, 0.9%). There were no observations of IOL glistenings at 12 months. Additionally, there were no reports of IOL surface haze, scratches, or cracks.
Discussion
The SY60CL IOL evaluated in this study was based on the AcrySof platform and uses a novel hydrophobic acrylic polymer lens material designed to enhance clarity.15,16 The results of this study indicate that >99% of subjects who received the SY60CL IOL achieved monocular CDVA of 0.3 logMAR or better 12 months after implantation (one-sided exact 95% upper confidence limit, 99.99%), exceeding the current performance standards for monofocal IOLs. At month 12, >95% of subjects achieved monocular UDVA of 0.3 logMAR. Additionally, 87% of subjects had a CDVA of 20/20 or better, and 97% of subjects had functional UDVA of 20/40 or better at 12 months. The cumulative and persistent AE rate was within the limits of the SPE rates, and no serious ocular AEs or secondary surgical interventions were assessed as related to the device.
The results of this study are consistent with an interim report that demonstrated improved CDVA at week 1 through month 6 and no unanticipated AEs.17 Also consistent with the 6-month interim results, there were no observations of glistenings or surface haze reported for the SY60CL IOL at 12 months.
Glistenings have been linked to IOL properties, such as IOL material, manufacturing process, and temperature fluctuations, and have been reported to be most common in hydrophobic acrylic IOLs.3,4,18 In a prospective study with 273 subjects who received 7 types of IOLs, the AcrySof IOL had a higher percentage of glistenings compared with other IOLs up to 2 years after surgery.5 Novel IOL materials, such as SY60CL, have been designed to reduce glistenings.3 In an in vitro evaluation, the SY60CL IOL had among the lowest levels of surface haze and glistenings compared with Tecnis ZCB00, Tecnis OptiBlue ZCB00V, Eternity W-60, Vivinex XY1, or enVista MX60.6 Additionally, recent clinical studies reported no or minimal glistenings in subjects who received the SY60CL IOL.7 In the current clinical study, no IOL glistenings were reported at any visit; additional long-term clinical studies are needed to confirm the low rates of glistenings in subjects receiving Clareon IOLs and to further address the effects of the material on clarity of vision.
Estimates of PCO and Nd:YAG rates vary depending on the type of lens and experimental design. A 1998 meta-analysis reported that overall pooled estimates of PCO incidence were 11.8% at year 1, 20.7% at year 3, and 28.4% at year 5 postoperatively.19 More recently, analysis of postmortem human eyes showed that the Nd:YAG capsulotomy rate ranged between 0.9% and 17.1% for modern IOLs.20 A prospective study reported that the incidence of residual capsule opacity was 44/194 (22.7%); however, only 5 cases (3.5%) were considered “visually significant” at the 6-week postoperative visit.21 A recent meta-analysis of 17,691 eyes in Finland reported that overall Nd:YAG capsulotomy incidence was 1.2% at 1 year; when stratified by lens type, the incidence ranged from 0.8% to 1.8% at year 1.22
AcrySof IOLs were often reported to have among the lowest rates of PCO and capsulotomy.20 A recent meta-analysis of randomized controlled trials found that hydrophobic acrylic AcrySof IOLs had a significantly lower hazard ratio for Nd:YAG capsulotomy compared with other types of IOLs (P<0.01 for all).23 A prospective randomized case series in 160 eyes (2 types of IOLs implanted in contralateral eyes) reported that Nd:YAG capsulotomy rate was 6% for AcrySof SN60WF compared with 16% for Eyecee One (Bausch & Lomb GmbH, Berlin, Germany) after 3 years.24 A real-world evidence study reported that AcrySof IOLs had the lowest incidence of PCO and Nd:YAG capsulotomy rates compared with other IOLs at 3 and 5 years postoperatively (PCO rates were 4.7–18.6% and Nd:YAG rates were 2.4–12.6% after 3 years; PCO rates were 7.1–22.6% and Nd:YAG capsulotomy rates were 5.8–19.3% after 5 years).11 A recent study using human capsular bag model reported similar PCO formation rates with AcrySof and Clareon IOLs.25
In this study, PCO was not assessed at the end of the cataract surgery. The rate of PCO reported in this study was 5.4% (19/350), and the rate of Nd:YAG capsulotomy was 4.6% (16/350). Although direct comparison with other IOLs has not been done, our results suggest that the rates of PCO and capsulotomy for SY60CL IOLs are somewhat higher than the ranges currently reported for AcrySof IOLs. However, 6 of 16 subjects who underwent Nd:YAG in the current study were from a single site where a total of 20 subjects were enrolled, potentially distorting the interpretation of the results. The rate of capsulotomy among the remaining sites was 3%. Additional long-term studies across multiple sites are needed to further evaluate the risks of PCO and posterior capsulotomy in subjects receiving SY60CL IOLs.
Cystoid macular edema is a common postoperative cause of visual disturbance, particularly in patients with additional risk factors such as diabetes mellitus and diabetic retinopathy.26,27 Analysis of 1659 consecutive cataract surgeries showed that the overall incidence of postoperative CME was 2.4%.28 A retrospective database study of electronic medical records (81,984 eyes) reported that the incidence of CME in subjects ranged from 1.17% to 4.04% within 90 days of surgery, depending on the presence of risk factors such as diabetes.29 Other studies estimated a 0.1% to 7.0% rate of CME in subjects (including those with risk factors for CME) up to 4 months postoperatively. Additionally, assessments using fluorescein angiography and optical coherence tomography resulted in greater incidence of CME compared with CME incidence based on subjects’ visual complaints.30 A study that examined 19,980 eyes in 13,556 subjects who underwent cataract surgery in outpatient clinics in France found the 2-year incidence of CME to be 0.95%.31 In a retrospective clinical chart review that examined real-world clinical practice records, acute CME incidence 3 months after cataract surgery was 0.1%.32 The CME rate of 0.9% reported in this study is consistent with other reported CME rates.
Device-related AEs reported in this study included a low rate of mild dysphotopsia (0.9%). Dysphotopsia was reported in 32% of subjects (11% had severe symptoms) in a study that assessed prevalence of pseudophakic photic phenomena more than a year after the IOL implantation of Clariflex (AMO Inc, Santa Ana, CA, USA), AMO AR40e, and Alcon AcrySof IOLs.33 Those data suggest that the SY60CL IOL may have a lower prevalence of photic phenomena compared with other IOLs.
Limitations of this study included the lack of a control group, short time frame of the study, and a possible bias inherent to the controlled clinical trial setting. Further studies in subjects with SY60CL IOLs are needed to confirm long-term effects of the SY60CL material on CME, risks of PCO and posterior capsulotomy, visual disturbances, and photic phenomena. Additional studies will need to evaluate toric IOL design and address rotational stability.
In conclusion, the effectiveness of the SY60CL IOL exceeded the SPE rates for monocular CDVA, and reported safety was within the limits of the historic SPE rates. These results support the effectiveness and safety of the SY60CL IOL in correcting distance VA in aphakic subjects.
Acknowledgments
Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON (North Wales, PA), and was funded by Alcon.
Funding Statement
This study was funded by Alcon Research LLC. Alcon assisted with the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Data Sharing Statement
The data used to support the primary findings of this study are available at clinicaltrials.gov (NCT03170154).
Disclosure
RL, AM, and DML are Alcon consultants. AF is a researcher and a speaker for Alcon. Dr David M Lubeck reports he is a consultant for NovaEye, consultant/investigator for Glaukos, outside the submitted work. Dr Andrew Maxwell reports personal fees from Alcon, outside the submitted work. Dr Robert Lehmann reports grants from Alcon, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494. [DOI] [PubMed] [Google Scholar]
- 2.Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J Cataract Refract Surg. 2012;38(6):1086–1093. doi: 10.1016/j.jcrs.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Labuz G, Knebel D, Auffarth GU, et al. Glistening formation and light scattering in six hydrophobic-acrylic intraocular lenses. Am J Ophthalmol. 2018;196:112–120. doi: 10.1016/j.ajo.2018.08.032 [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K, Hirata A, Yoshida M, Yoshimura K, Hayashi H. Long-term effect of surface light scattering and glistenings of intraocular lenses on visual function. Am J Ophthalmol. 2012;152(2):240–251.e2. doi: 10.1016/j.ajo.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 5.Tognetto D, Toto L, Sanguinetti G, Ravalico G. Glistenings in foldable intraocular lenses. J Cataract Refract Surg. 2002;28(7):1211–1216. doi: 10.1016/S0886-3350(02)01353-6 [DOI] [PubMed] [Google Scholar]
- 6.Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45(10):1490–1497. doi: 10.1016/j.jcrs.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 7.Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethylmethacrylate. J Cataract Refract Surg. 2020;46(5):682–687. doi: 10.1097/j.jcrs.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 9.Li N, Chen X, Zhang J, et al. Effect of AcrySof versus silicone or polymethyl methacrylate intraocular lens on posterior capsule opacification. Ophthalmology. 2008;115(5):830–838. doi: 10.1016/j.ophtha.2007.06.037 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Wang J, Chen Z, Tang X. Effect of hydrophobic acrylic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: meta-analysis. PLoS One. 2013;8(11):e77864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ursell PG, Dhariwal M, O’Boyle D, Khan J, Venerus A. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: a real-world evidence study of 20,763 eyes. Eye (Lond). 2020;34(5):960–968. doi: 10.1038/s41433-019-0630-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biber JM, Sandoval HP, Trivedi RH, et al. Comparison of the incidence and visual significance of posterior capsule opacification between multifocal spherical, monofocal spherical, and monofocal aspheric intraocular lenses. J Cataract Refract Surg. 2009;35(7):1234–1238. doi: 10.1016/j.jcrs.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Camparini M, Cassinari P, Ferrigno L, Macaluso C. ETDRS-fast: implementing psychophysical adaptive methods to standardized visual acuity measurement with ETDRS charts. Invest Ophthalmol Vis Sci. 2001;42(6):1226–1231. [PubMed] [Google Scholar]
- 14.Ophthalmic implants - intraocular lenses - part 7: clinical investigations (ISO 11979-7:2014). International Organization for Standardization; 2014. [Google Scholar]
- 15.Lane S, Collins S, Das K, et al. Evaluation of the mechanical behaviour of a new single-piece intraocular lens as compared to commercially available IOLs. Presented at: European Society of Cataract & Refractive Surgeons; 2017; Lisbon, Portugal. [Google Scholar]
- 16.Auffarth GU, Schickhardt S, Wang Q, Fang H, Hengerer FH. Laboratory evaluation of the new Clareon hydrophobic acrylic IOL material: biomaterial properties and capsular bag behavior. Presented at: European Society of Cataract & Refractive Surgeons; 2017; Lisbon, Portugal. [Google Scholar]
- 17.Nanavaty MA, Borkum S, Wedham M, et al. Clinical evaluation of visual, refractive, and adverse event outcomes of a new monofocal IOL: 6-month UK site–level results. Presented at: European Society of Cataract & Refractive Surgeons; 2019; Paris, France. [Google Scholar]
- 18.Thomes BE, Callaghan TA. Evaluation of in vitro glistening formation in hydrophobic acrylic intraocular lenses. Clin Ophthalmol. 2013;7:1529–1534. doi: 10.2147/OPTH.S44208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998;105(7):1213–1221. doi: 10.1016/S0161-6420(98)97023-3 [DOI] [PubMed] [Google Scholar]
- 20.Apple DJ, Peng Q, Visessook N, et al. Eradication of posterior capsule opacification: documentation of a marked decrease in Nd: YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology. 2001;108(3):505–518. doi: 10.1016/S0161-6420(00)00589-3 [DOI] [PubMed] [Google Scholar]
- 21.Mootha VV, Tesser R, Qualls C. Incidence of and risk factors for residual posterior capsule opacification after cataract surgery. J Cataract Refract Surg. 2004;30(11):2354–2358. doi: 10.1016/j.jcrs.2004.03.038 [DOI] [PubMed] [Google Scholar]
- 22.Lindholm JM, Laine I, Tuuminen R. Five-year cumulative incidence and risk factors of Nd:YAG capsulotomy in 10 044 hydrophobic acrylic 1-piece and 3-piece intraocular lenses. Am J Ophthalmol. 2019;200:218–223. doi: 10.1016/j.ajo.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 23.Thom H, Ender F, Samavedam S, et al. Effect of AcrySof versus other intraocular lens properties on the risk of Nd:YAG capsulotomy after cataract surgery: a systematic literature review and network meta-analysis. PLoS One. 2019;14(8):e0220498. doi: 10.1371/journal.pone.0220498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leydolt C, Schartmuller D, Schwarzenbacher L, et al. Comparison of posterior capsule opacification development with 2 single-piece intraocular lens types. J Cataract Refract Surg. 2017;43(6):774–780. doi: 10.1016/j.jcrs.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 25.Hillenmayer A, Wertheimer CM, Kassumeh S, et al. Evaluation of posterior capsule opacification of the Alcon Clareon IOL vs the Alcon Acrysof IOL using a human capsular bag model. BMC Ophthalmol. 2020;20(1):77. doi: 10.1186/s12886-020-01349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyewole K, Tsogkas F, Westcott M, Patra S. Benchmarking cataract surgery outcomes in an ethnically diverse and diabetic population: final post-operative visual acuity and rates of post-operative cystoid macular oedema. Eye (Lond). 2017;31(12):1672–1677. doi: 10.1038/eye.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634. [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33(9):1550–1558. doi: 10.1016/j.jcrs.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Chu CJ, Johnston RL, Buscombe C, et al. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016;123(2):316–323. doi: 10.1016/j.ophtha.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 30.Hollo G, Aung T, Cantor LB, Aihara M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: mechanism, diagnosis, and management. Surv Ophthalmol. 2020;65(5):496–512. doi: 10.1016/j.survophthal.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Daien V, Papinaud L, Domerg C, et al. Incidence and characteristics of cystoid macular edema after cataract surgery. Ophthalmology. 2016;123(3):663–664. doi: 10.1016/j.ophtha.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Packer M, Lowe J, Fine H. Incidence of acute postoperative cystoid macular edema in clinical practice. J Cataract Refract Surg. 2012;38(12):2108–2111. doi: 10.1016/j.jcrs.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 33.Aslam TM, Gupta M, Gilmour D, Patton N, Dhillon B. Long-term prevalence of pseudophakic photic phenomena. Am J Ophthalmol. 2007;143(3):522–524. doi: 10.1016/j.ajo.2006.10.031 [DOI] [PubMed] [Google Scholar]