Abstract
Background and Objective. Epimedium koreanum Nakai is a medicinal plant known for its health beneficial effects on impotence, arrhythmia, oxidation, aging, osteoporosis, and cardiovascular diseases. However, there is no report available that shows its effects on platelet functions. Here, we elucidated antiplatelet and antithrombotic effects of ethyl acetate fraction of E. koreanum. Methodology. We analyzed the antiplatelet properties using standard in vitro and in vivo techniques, such as light transmission aggregometry, scanning electron microscopy, intracellular calcium mobilization measurement, dense granule secretion, and flow cytometry to assess integrin αIIbβ3 activation, clot retraction, and Western blot, on washed platelets. The antithrombotic effects of E. koreanum were assessed by arteriovenous- (AV-) shunt model in rats, and its effects on hemostasis were analyzed by tail bleeding assay in mice. Key Results. E. koreanum inhibited platelet aggregation in agonist-stimulated human and rat washed platelets, and it also reduced calcium mobilization, ATP secretion, and TXB2 formation. Fibrinogen binding, fibronectin adhesion, and clot retraction by attenuated integrin αIIbβ3-mediated inside-out and outside-in signaling were also decreased. Reduced phosphorylation of extracellular signal-regulated kinases (ERK), Akt, PLCγ2, and Src was observed. Moreover, the fraction inhibited thrombosis. HPLC results revealed that the fraction predominantly contained icariin. Conclusion and Implications. E. koreanum inhibited platelet aggregation and thrombus formation by attenuating calcium mobilization, ATP secretion, TXB2 formation, and integrin αIIbβ3 activation. Therefore, it may be considered as a potential candidate to treat and prevent platelet-related cardiovascular disorders.
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death in modern societies, especially in Western and other developed countries [1]. The World Health Organization stated that CVD accounted for 30% of all deaths in 2005, and in Europe, it remains the primary cause of 42% of mortalities in men and 52% in women [2]. Platelets are considered as major pathological risk factors for CVDs, such as coronary artery disease and atherosclerosis. Pathophysiologic hyper-activation of platelets leads to platelet aggregation and thrombus formation, which ultimately contribute to blood vessel stenosis, ischemia, and myocardial infarction [3]. Several drugs are available to treat and prevent platelet-related cardiovascular ailments; however, their side effects often outweigh their benefits. For example, aspirin causes gastric ulcers and clopidogrel may produce aplastic anemia and thrombocytopenic purpura [4], whereas a significant part of the population is resistant to these most commonly used anti-platelet agents [5].
Using natural products and their bioactive natural compounds, including ethnomedicinal applications for CVD treatment and prevention, has been increased [6, 7]. Similarly, several natural products, including traditional Mediterranean diet and medicinal plants, have also been reported for their cardio-protective and anti-platelet effects in primary and secondary CVD prevention [8–11]. Epimedium koreanum Nakai, commonly known as horny goat weed, is native to Korea and China, which has been extensively used as a nutraceutical in functional foods or as phyto-pharmaceutical agent for preventing and curing some serious and fatal illnesses, such as CVD and osteoporosis, and to improve neurological and sexual functions [12–14]. Zhang et al. [15] have prepared and explored the anti-oxidant activity of the several fractions of E. koreanum Nakai and found that n-butanol fraction and ethyl acetate fraction (EAF) were having most scavenging activity with maximum content of icariin in EAF.
However, no study has shown its possible anti-platelet mechanism involved in platelet-related cardiovascular disorders. Here, we evaluated the anti-platelet and anti-thrombotic effects of EAF of E. koreanum and examined its inhibitory effects on normal platelet functions.
2. Materials and Methods
2.1. Procurement and Extraction of E. koreanum Nakai
E. koreanum was purchased from Omniherb of Gyeongsan (Gyeongbuk province, Korea). The samples were pulverized to 80 mesh size using Sung Chang Machine (ACM10-INCH, Namyangju, Korea) and then fractionated, according to the organic solvent's polarity, to measure the physiologic activity. Then, 1 L of methanol was added to 100 g of the ground powder and extracted at 150 rpm for 24 hours using a shaker. After centrifugation at 4°C and 8000 rpm for 10 minutes, and filtration, the aforementioned process was repeated twice to obtain the methanol extract. Distilled water and hexane in a 1 : 1 ratio were added to the methanol extract and processed for 24 hours in a shaker; then sonication, centrifugation, and concentration were repeated twice to obtain the hexane (0.82 g) extract. By repeating the aforementioned process, chloroform (0.79 g), ethyl acetate (0.83 g), butanol (1.55 g), and water (8.61 g) extracts were obtained. The yield was measured by lyophilizing the extract and dissolving in DMSO and used for efficacy verification and then stored at −80 °C until use.
2.2. Analysis of Phenolic Compounds Using HPLC
Analysis of the major phenolic compounds in E. koreanum ethyl acetate fraction was conducted using HPLC (Alliance, Waters, USA). An Eclipse plus C18 column (Agilent Technologies Ltd.; 4.6 × 250 mm; particle size, 5 μm) was used for analysis of phenolic acid and flavonoid content. The mobile phases were 0.1% formic acid (v/v) in 10% acetonitrile (solvent A) and 0.1% formic acid (v/v) in 90% acetonitrile (solvent B). All reagents used were of HPLC grade. The gradient followed the following order: 0–6 min 0% B, 6–31 min 10%–90% B, 31–41 min 20%–80%, 41–45 min 50%–50% B, and 45–50 min 0% B. The separated phenolic compounds were detected using a photodiode array detector at 280 nm.
2.3. Preparation of Washed Human and Rat Platelets
Human platelet-rich plasma (PRP) collected from healthy volunteers, who provided informed consent, was obtained from the Korean Red Cross Blood Center (KRBC, Changwon, Korea), and its experimental use was approved by KRBC and the Korea National Institute for Bioethics Policy Public Institutional Review Board (PIRB17-1019-03). The washed human and rat platelets were prepared as previously described [16].
2.4. Platelet Aggregation Assay and Scanning Electron Microscope (SEM) Analyses
The extent of platelet aggregation was assessed by following the standard procedure of light transmission aggregometry using a Chrono-log aggregometer (Havertown, PA, USA), as previously described [17]. Briefly, the washed platelets (3 × 108 cells/mL) were pre-incubated with vehicle (DMSO, maintained at <0.1%) or different concentrations of EAF for 1 min in the presence of 1 mM calcium chloride (CaCl2), followed by stimulation with various agonists (collagen, ADP, or thrombin) for 5 min with continuous stirring at 37°C.
A field emission SEM was used to assess platelet shape change and aggregation by obtaining ultrastructure images as previously described [17,18].
2.5. Arteriovenous Shunt Model
The anti-thrombotic activity of the E. koreanum fraction was assessed in a rat extracorporeal shunt model as previously described [9,17]. Briefly, rats were orally administered with the saline (control), EAF (100–300 mg/kg), or ASA (50 mg/kg) once daily for 3 days. Then, 2 h after the last administration, rats were anesthetized and a shunt was placed for 15 min after initiating extracorporeal circulation. Subsequently, blood flow was stopped and the formed thrombus was weighed.
2.6. In Vivo Bleeding Assay
Male mice were divided into three treatment groups (n = 5) and intraperitoneally administered with saline (control), ASA (50 mg/kg), or EAF (300 mg/kg) once daily for 3 days. One hour after the last administration, mice were anesthetized, and tail bleeding assay was performed as previously described [19].
2.7. Statistical Analysis
Data were analyzed by one-way analysis of variance, followed by measuring statistically significant differences using Dunnett's post hoc test (SAS Institute Inc., Cary, NC, USA). All data were presented as the mean ± standard deviation (SD). A p value of ≤0.05 was considered statistically significant.
3. Results
3.1. E. koreanum Inhibits Agonist-Stimulated Platelet Aggregation
The fraction was tested against several agonists, that is, thrombin-, ADP-, and collagen-stimulated rat platelet aggregation, and significant and dose-dependent inhibitory effects were found compared with vehicle-treated (control) platelets, whereas it has more effectively inhibited collagen-stimulated platelet aggregation (Figure 1(a)). A similar trend has been also seen in collagen-stimulated human platelet aggregation with significant and dose-dependent inhibition, in which the fraction was more potent compared with rat platelets (Figure 1(b)). The fraction also potently inhibited ADP-induced aggregation measured in rat PRP, in a dose-dependent manner (Figure 1(c) and 1(e)). Fraction-treated rat platelets were also analyzed under a SEM, which showed the clear dose-dependent inhibition of platelet aggregation, with increased E. koreanum concentration, whereas the platelets treated with only the vehicle and agonist (collagen) caused full platelet activation and aggregation, leading to fibrin meshwork formation (Figure 1(d)).
Figure 1.
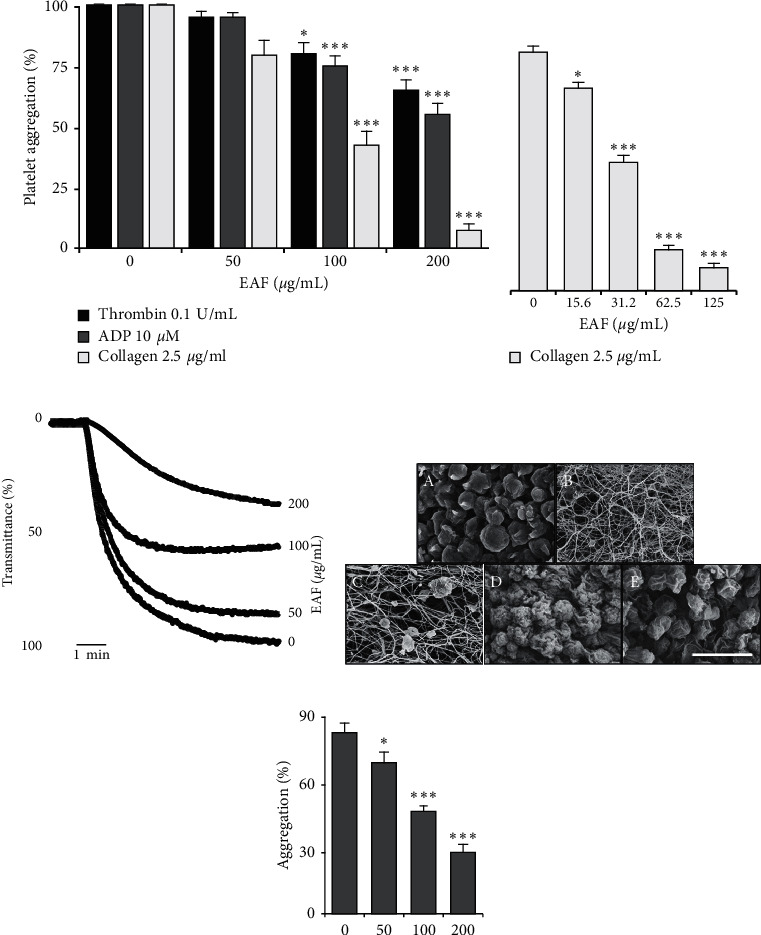
E. koreanum inhibits agonist-induced human and rat platelet aggregation. Collagen-, ADP-, or thrombin-stimulated washed platelets ((a) and (d) from rats (3 × 108 cells/mL); (b) from humans (5 × 108 cells/mL)) pretreated with vehicle or various concentrations of E. koreanum ethyl acetate fraction (EAF) in the presence of 1 mM CaCl2. (c, e) Rat PRP (5 × 108 cells/mL) was incubated with vehicle or various concentrations of extract in the presence of 10-mM CaCl2 for 1 min and then stimulated with ADP (25 μM) for 5 min. (d) Representative SEM images (5000×) of collagen (2.5 μg/mL)-stimulated rat platelets pre-treated with vehicle or various concentrations of EAF ((A) resting, (B) vehicle, (C) 50 μg/mL, (D) 100 μg/mL, and (E) 200 μg/mL). The scale bar represents 5 µm. The graphs show mean ± SD values from at least four independent experiments. ∗p < 0.05 and ∗∗∗p < 0.001 versus control.
3.2. E. koreanum Reduced Calcium Mobilization, Dense Granule Secretion, and Thromboxane-B2 Production
Intracellular calcium mobilization ([Ca2+]i) was observed to be decreased in a significant and dose-dependent manner in E. koreanum-pre-treated platelets, thereby inhibiting platelet activation (Figure 2(a)). It also dose-dependently reduced ATP secretion and thromboxane-B2 release compared with vehicle-treated rat platelets (Figures 2(b) and 2(c)).
Figure 2.
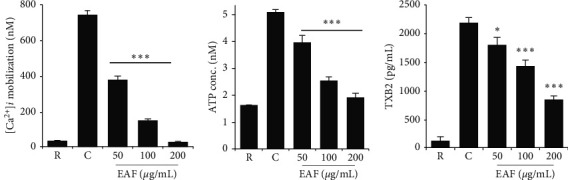
E. koreanum inhibits (Ca2+)i mobilization and reduces ATP release and TxB2 production. (a) Fura 2/AM-loaded rat platelets (3 × 108 cells/mL) pre-treated with vehicle or different concentration of EAF and stimulated with collagen (2.5 μg/mL) for 3 min. Assessment of ATP concentration (b) and thromboxane-B2 production (c) was done in a supernatant of stimulated washed platelets (3 × 108 cells/mL) suspension pre-treated with vehicle or various EAF concentrations and stimulated with collagen for 5 min on a luminometer or TxB2 ELISA kit, respectively. Results are represented as mean ± SD values from at least four independent experiments. ∗p < 0.05 and ∗∗∗p < 0.001 versus control. R: resting; C: control.
3.3. Inhibitory Effects of E. koreanum on Fibrinogen Binding, Fibronectin Adhesion, and Clot Retraction Kinetics
Taking further insight to its anti-platelet mechanism, we evaluated the effects of the fraction on integrin αIIbβ3 activation. We found that E. koreanum effectively inhibited fibrinogen binding to integrin αIIbβ3, compared with the washed vehicle-treated rat platelets (Figures 3(a) and 3(b)). Similarly, the fraction significantly inhibited platelet spreading on adhesive ligands, that is, fibronectin (Figure 3(c)). Next, we analyzed the inhibitory effects of the fraction on thrombin-stimulated clot retraction in rat PRP. Our results showed that E. koreanum dose-dependently and time-dependently inhibited clot retraction compared with the vehicle-treated control (Figures 3(d)–3(f)).
Figure 3.
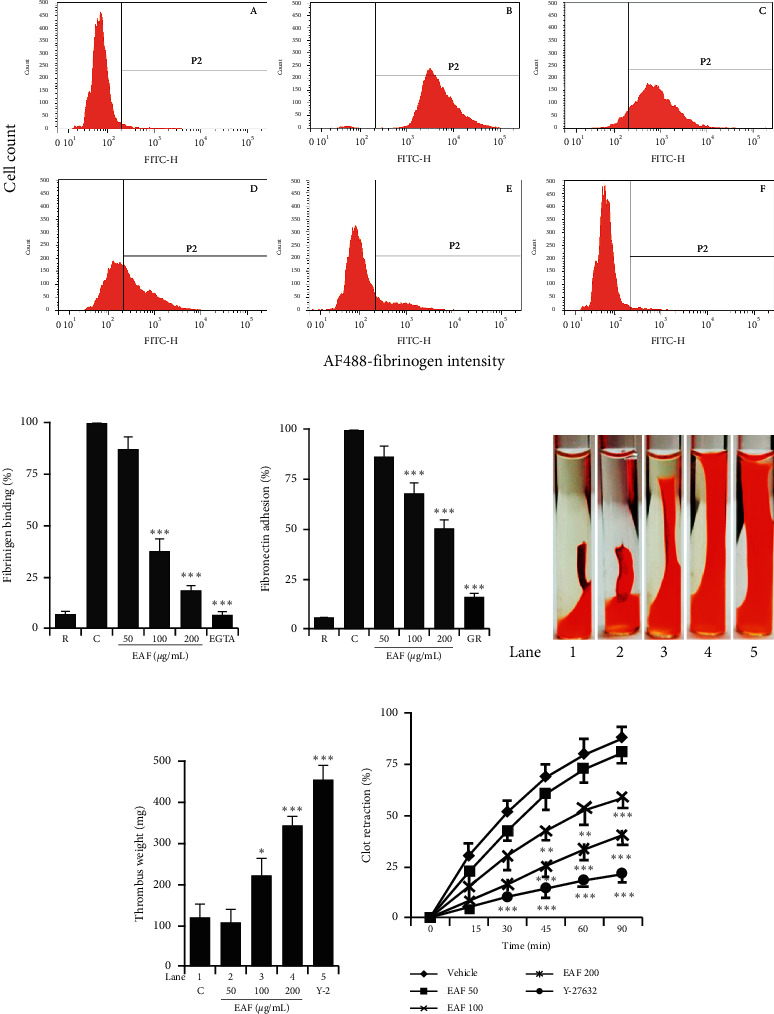
E. koreanum inhibits integrin αIIbβ3-mediated inside-out and outside-in signaling. ((a) and (b)) Flow cytometric measurements of fibrinogen binding in rat platelets (3 × 108 cells/mL) treated with vehicle or different concentrations of E. koreanum fraction (EAF), or EGTA ((A) resting, (B) vehicle, (C) 50 μg/mL, (D) 100 μg/mL, (E) 200 μg/mL, and (F) 10 μM EGTA)), and stimulated with collagen (b–f). (c) Results of fibronectin adhesion assay, which was performed using an assay kit according to the manufacturer's instructions and by following the procedure described in the methods section. (d) In vitro effect of E. koreanum on clot retraction for 2 h at room temperature after thrombin addition and photographed at 15 min intervals. Representative images of clot retraction at 90 min after thrombin addition with or without EAF. Y-27632 (ROCK inhibitor) was used as a control. Lanes 1–5 correspond to lanes 1–5 in (e). (f) Clot retraction kinetics were measured by ImageJ software, and the clot surface areas were plotted as a percentage of retraction. Bar graphs summarizing the inhibitory effect of EAF on fibrinogen binding to integrin αIIbβ3 (B), fibronectin adhesion (C), clot retraction (E), and kinetics of clot retraction (F). Results are shown as mean ± SD values from at least four independent experiments. ∗p < 0.05,∗∗p < 0.01, and ∗∗∗p < 0.001 versus control. R: resting; C: control; GR: GR155053; Y-2: Y-27632.
3.4. The Effects of E. koreanum on Phosphorylation of Src, MAPK, Akt, and PLCγ2
Further mechanistic aspects were explored by assessing the phosphorylation of key molecules involved in platelet aggregation. The mitogen-activated protein kinase (MAPK) pathway was analyzed, especially ERK and P38MAPK, along with several other molecules like Src, PLCγ2, and Akt. The results significantly reduced ERK, Src, PLCγ2, and Akt phosphorylation but not P38MAPK in washed rat platelets, showing the potential inhibitory mechanism of E. koreanum on platelet activation (Figures 4(a) and 4(b)).
Figure 4.
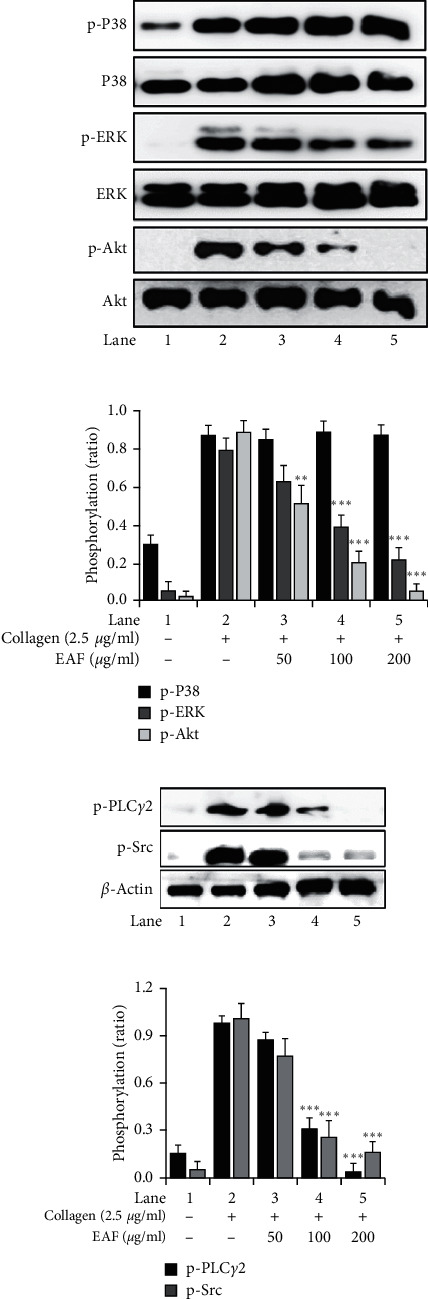
E. koreanum attenuates phosphorylation levels of ERK, Akt (a), Src, and PLCγ2 (b). Immunoblotting was conducted to analyze the phosphorylation of signaling molecules extracted from the lysates of collagen-stimulated washed rat platelets (3 × 108 cells/mL) that were pretreated with vehicle or E. koreanum fraction (EAF). Representative immunoblot images and data (mean ± SD) from at least four independent experiments are shown. Lanes 1–5 of blot images correspond to each bar graph, respectively. ∗p < 0.05 and ∗∗∗p < 0.001 versus the agonist-treated group.
3.5. E. koreanum Inhibits Thrombus Formation and Modulates Hemostasis
The AV-shunt model results revealed that E. koreanum effectively reduced thrombus weight in rats in a significant and dose-dependent manner, as compared with the control group. Similar inhibition was observed in ASA-treated rats (Figure 5(a)). Bleeding time was also increased in E. koreanum-treated and ASA-treated mice, as compared with the control group (Figure 5(b)). These results indicate that the fraction attenuated platelet activation and inhibited thrombus formation.
Figure 5.
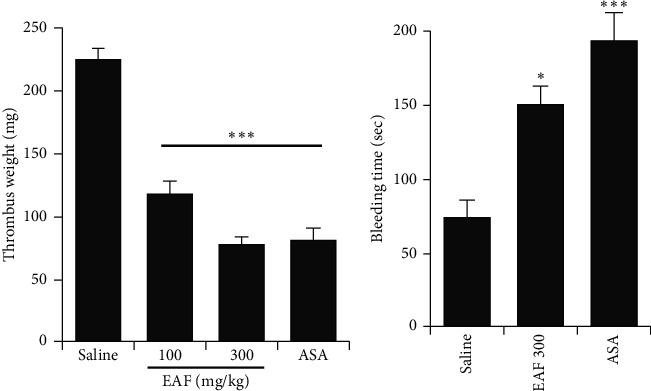
E. koreanum inhibits thrombus formation and modulates hemostasis. (a) In vivo evaluation of anti-thrombotic activity and determination of thrombus weight in AV-shunt model of rats that were orally administered with saline, ethyl acetate fraction of E. koreanum (EAF; 100–300 mg/kg), or ASA (50 mg/kg). (b) Results of tail bleeding assay for homeostasis measurement in mice administered with EAF (300 mg/kg), ASA (50 mg/kg), or saline (n = 5 in each group). Graph shows mean ± SD values from at least five independent experiments performed. ∗p < 0.05 and ∗∗∗p < 0.001 versus control.
3.6. Constituents of E. koreanum Ethyl Acetate Fraction
To analyze the phenolic compound contents in the E. koreanum ethyl acetate fraction (EAF), we compared it with standard substances (gallic acid, chlorogenic acid, caffeic acid, ellagic acid, myricetin, icariin, quercetin, and kaempferol) (Figure 6(a)). Based on the HPLC analysis, icariin was detected in the ethyl acetate fraction (tR = 17.59 min) and its contents were 58.75 mg/g (Figure 6(b)).
Figure 6.
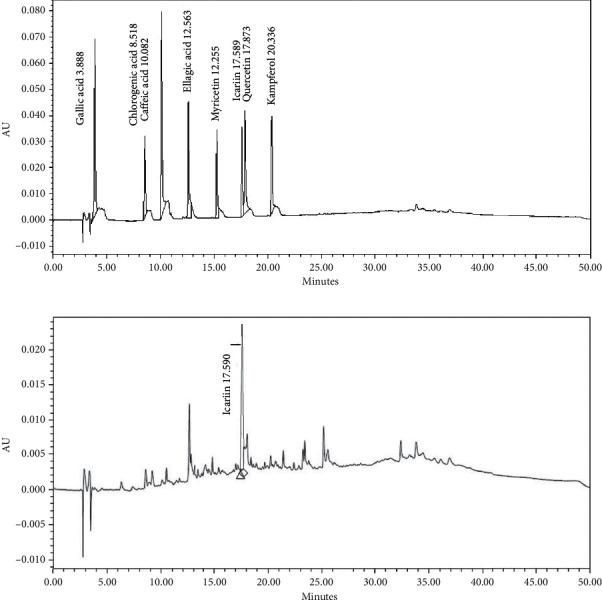
Chemical constituents of E. koreanum ethyl acetate fraction. (a) Chromatograms of the standard solution. (b) The HPLC chromatogram of ethyl acetate fraction of E. koreanum (EAF) was detected at 280 nm UV.
4. Discussion
Platelets play a major role in maintaining hemostasis and healing injured vessels to stop blood loss. However, aberrant platelet activation leads to thrombus formation within blood vessels, which may cause thromboembolism, eventually leading to stroke, heart attack, and deep vein thrombosis, among other morbidities. Available common anti-platelet drugs possess adverse effects, and some patients may be resistant to them [20], which necessitates the development of alternate approaches that could be best in the form of natural products. Many natural compounds have been reported to possess anti-platelet and anti-thrombotic properties with minimum or no side effects, and have also been useful in CVD treatment and prevention [21, 22]. E. koreanum Nakai has been reported for its anti-oxidant, anti-aging, and anti-atherosclerotic, as well as sexual boosting properties, and is also known for its cardio-protective effects [12–14].
In the present study, we evaluated its anti-platelet properties. Our initial screening results indicated that E. koreanum inhibited platelet aggregation against several agonists, but more potently against collagen, in both human and rat platelets. The possible reason to this could be attributed to the active ingredients contained in the ethyl acetate fraction, which may require further study and evaluation. We confirmed the platelet aggregation and shape changes by observing the ultrastructure using SEM, which clearly showed dose-dependent inhibition of aggregation in platelets pre-treated with the fraction. Agonist-stimulated elevation in cytosolic Ca2+ concentrations is essential for platelet activation in hemostasis and thrombosis [23], whereas dense granule secretion (ATP, ADP, and Ca2+) further enhances platelet adhesion, shape change, and aggregation [21]. Our results indicated that E. koreanum fraction potently inhibited [Ca2+]i mobilization and ATP secretion, thereby inhibiting platelet activation.
Conformational changes in integrin αIIbβ3 structure caused an enhanced ability to bind to fibrinogen (inside-out signaling), whereas this phenomenon further transduces signals into the cell (outside-in signaling), leading to platelet adhesion, spreading, and clot retraction [24]. Similarly, fibronectin is another adhesive ligand that stabilizes thrombus formation in blood vessels. It binds to integrins and augments platelet aggregation by developing cohesive aggregates [25]. Rho kinases (ROCKs) are downstream regulators of GTPases, which mediate RhoA-stimulated actin cytoskeletal changes via myosin light chain phosphorylation. The role of ROCKs in facilitating clot retraction has been previously described using ROCK inhibitor (Y-27632) [26]. Moreover, Src kinase and PLCγ2 involvement in clot retraction has been reported [27]. E. koreanum significantly inhibited fibrinogen-integrin αIIbβ3 binding, fibronectin adhesion, and clot retraction, indicating its potential to modulate integrin-mediated inside-out and outside-in signaling. The fraction also dose-dependently inhibited Src and PLCγ2 phosphorylation, which further confirms their involvement in inhibiting platelet functions and clot retraction.
Src is involved in early signaling of platelets and plays a key role in various downstream signaling pathways [28]. MAPK (ERK and P38MAPK) causes granule secretion and platelet aggregation [29]. Similarly, Akt has been reported to regulate platelet activation with potential consequences, such as thrombosis, and is a very important target in designing anti-thrombotic drugs [30]. The fraction's underlying inhibitory mechanisms on platelet functions were further examined by assessing the aforementioned pathways, and we found that E. koreanum inhibited Src, ERK, Akt, and PLCγ2 phosphorylation. Figure 7 summarizes the inhibitory effects of E. koreanum on intracellular platelet signaling pathways.
Figure 7.
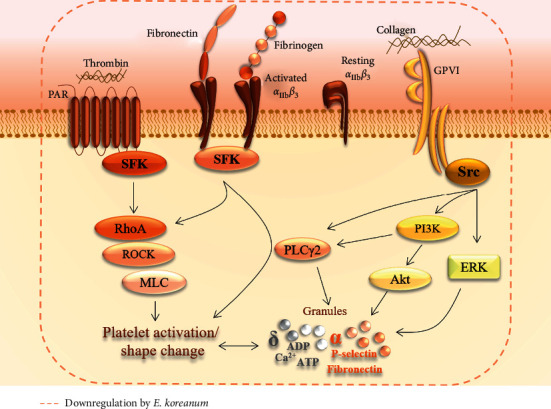
A schematic summary of the inhibitory effects of E. koreanum on intracellular platelet signaling pathway.
The AV-shunt model is commonly used to assess in vivo antithrombotic effects. The shunt was previously recognized to be made up of platelet, fibrin, and red blood cells trapped inside, and such thrombus may lead to coronary artery disease or myocardial infarction; however, this phenomenon could be reversed by antiplatelet agents [31]. In our results, E. koreanum fraction potently attenuated the thrombus formation and moderately increased bleeding time in mice, which indicated that the fraction inhibited platelet activation and aggregation.
HPLC is very helpful in identifying and characterizing the chemical profiles of natural products [32]. Our results revealed that the fraction predominantly contains icariin, a drug that has been reported for several pharmacologic functions, including antiatherosclerotic properties [33] and attenuation of the prothrombotic state of atherosclerosis [34]. The results suggest that the observed inhibitory effects of the fraction treatment on platelets could be attributed to icariin that is mainly present in E. koreanum.
5. Conclusion
The ethyl acetate fraction of E. koreanum (EAF) inhibited platelet aggregation in rat and human platelets, attenuated (Ca2+)i mobilization and ATP secretion, and modulated integrin-mediated inside-out and outside-in signaling via Src, ERK, and Akt inhibition. The fraction has also inhibited thrombus formation, indicating its possible therapeutic effects, which suggest that E. koreanum is a potential candidate to treat and prevent platelet-related cardiovascular disorders in the new era of ethnomedicine.
Acknowledgments
This research was supported by the National Research Foundation of Korea (2018R1D1A1A09083797).
Abbreviations
- ACD:
Acid-citrate-dextrose
- ADP:
Adenosine diphosphate
- ASA:
Acetylsalicylic acid; aspirin
- AV shunt:
Arteriovenous shunt
- Akt:
Protein kinase B
- DMSO:
Dimethyl sulfoxide
- EAF:
Ethyl acetate fraction of Epimedium koreanum
- ERK:
Extracellular signal-regulated kinase
- GPVI:
Glycoprotein VI
- MAPK:
Mitogen-activated protein kinase
- Rho-A:
Rho kinase A
- SEM:
Scanning electron microscope
- SFK:
Src family kinase
- TXB2:
Thromboxane-B2.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
The studies involving human participants were reviewed and approved by Korean Red Cross Blood Center and the Korea National Institute for Bioethics Policy Public Institutional Review Board (PIRB17-1019-03). The animal study was reviewed and approved by Ethics Committee of College of Veterinary Medicine, Kyungpook National University, Daegu, Korea (KNU12-0125).
Consent
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Disclosure
Muhammad Irfan and Tae-Hyung Kwon are the co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
MI and MHR designed and conceptualized the study. MI performed experiments, analyzed data, and wrote and critically revised the manuscript. T-HK, D-HL, S-BH, J-WO, and S-DK performed partial experiments and analyzed data. MHR and S-DK supervised the research work. All authors read and approved the final manuscript.
Supplementary Materials
Detailed description on chemicals and reagent sources, animals and handling, preparation of washed human and rat platelets, scanning electron microscopy (SEM), ATP secretion and TXB2 production, measurement of intracellular calcium mobilization, flow cytometry, fibronectin adhesion assay, clot retraction, immunoblotting, in vivo AV-shunt assay, and bleeding time has been included in the Supplementary Materials. We followed the methods of Irfan et al. [17].
References
- 1.Benjamin E. J., Blaha M. J., Chiuve S. E., et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/cir.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols M., Townsend N., Scarborough P., Rayner M. Cardiovascular disease in Europe: epidemiological update. European Heart Journal. 2013;34(39):3028–3034. doi: 10.1093/eurheartj/eht356. [DOI] [PubMed] [Google Scholar]
- 3.Andrews R. K., Berndt M. C. Platelet physiology and thrombosis. Thrombosis Research. 2004;114(5-6):447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Barrett N. E., Holbrook L., Jones S., et al. Future innovations in anti-platelet therapies. British Journal of Pharmacology. 2008;154(5):918–939. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson A. D., Dokainish H., Lakkis N. Aspirin and clopidogrel response variability: review of the published literature. Texas Heart Institute Journal. 2008;35(3):p. 313. [PMC free article] [PubMed] [Google Scholar]
- 6.Badimon L., Vilahur G., Padro T. Nutraceuticals and atherosclerosis: human trials. Cardiovascular Therapeutics. 2010;28(4):202–215. doi: 10.1111/j.1755-5922.2010.00189.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.-H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. Journal of Ginseng Research. 2018;42(3):264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estruch R., Ros E., Salas-Salvadó J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. New England Journal of Medicine. 2013;368(14):1279–1290. doi: 10.1056/nejmoa1200303. [DOI] [PubMed] [Google Scholar]
- 9.Irfan M., Kwon T.-H., Yun B.-S., Park N.-H., Rhee M. H. Eisenia bicyclis (brown alga) modulates platelet function and inhibits thrombus formation via impaired P 2 Y 12 receptor signaling pathway. Phytomedicine. 2018;40:79–87. doi: 10.1016/j.phymed.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Rastogi S., Pandey M. M., Rawat A. K. S. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine. 2016;23(11):1082–1089. doi: 10.1016/j.phymed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Shin J.-H., Kwon H.-W., Rhee M. H., Park H.-J. Inhibitory effects of thromboxane A2 generation by ginsenoside Ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release. Journal of Ginseng Research. 2019;43(2):236–241. doi: 10.1016/j.jgr.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S., Chen K., Li S., Zhang K. In vitro and in vivo studies of the effect of a Chinese herb medicine on osteoclastic bone resorption. The Chinese Journal of Dental Research: The Official Journal of the Scientific Section of the Chinese Stomatological Association (CSA) 1999;2(1):7–11. [PubMed] [Google Scholar]
- 13.Kim J.-Y., Shim S. H. Epimedium koreanum extract and its flavonoids reduced atherosclerotic risk via suppressing modification of human HDL. Nutrients. 2019;11(5):p. 1110. doi: 10.3390/nu11051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H., He X., Yang Y., Li M., Hao D., Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. Journal of Ethnopharmacology. 2011;134(3):519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W., Chen H., Wang Z., Lan G., Zhang L. Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. Journal of Food Science and Technology. 2013;50(6):1122–1129. doi: 10.1007/s13197-011-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irfan M., Jeong D., Kwon H.-W., et al. Ginsenoside-Rp3 inhibits platelet activation and thrombus formation by regulating MAPK and cyclic nucleotide signaling. Vascular Pharmacology. 2018;109:45–55. doi: 10.1016/j.vph.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Irfan M., Kwon H.-W., Lee D.-H., et al. Ulmus parvifolia modulates platelet functions and inhibits thrombus formation by regulating integrin αIIbβ3 and cAMP signaling. Frontiers in Pharmacology. 2020;11:p. 698. doi: 10.3389/fphar.2020.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White J. G. Platelets and Megakaryocytes. Berlin, Germany: Springer; 2004. Electron microscopy methods for studying platelet structure and function; pp. 47–63. [DOI] [PubMed] [Google Scholar]
- 19.Irfan M., Jeong D., Saba E., et al. Gintonin modulates platelet function and inhibits thrombus formation via impaired glycoprotein VI signaling. Platelets. 2019;30(5):589–598. doi: 10.1080/09537104.2018.1479033. [DOI] [PubMed] [Google Scholar]
- 20.Cattaneo M. Resistance to anti-platelet agents. Thrombosis Research. 2011;127:S61–S63. doi: 10.1016/s0049-3848(11)70017-2. [DOI] [PubMed] [Google Scholar]
- 21.Estevez B., Du X. New concepts and mechanisms of platelet activation signaling. Physiology. 2017;32(2):162–177. doi: 10.1152/physiol.00020.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irfan M., Kim M., Rhee M. H. Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. Journal of Ginseng Research. 2020;44(1):24–32. doi: 10.1016/j.jgr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga-Szabo D., Braun A., Nieswandt B. Calcium signaling in platelets. Journal of Thrombosis and Haemostasis. 2009;7(7):1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 24.Calderwood D. A. Integrin activation. Journal of Cell Science. 2004;117(Pt 5):657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 25.Pankov R., Yamada K. M. Fibronectin at a glance. Journal of Cell Science. 2002;115(20):p. 3861. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 26.Liao J. K., Seto M., Noma K. Rho kinase (ROCK) inhibitors. Journal of Cardiovascular Pharmacology. 2007;50(1):p. 17. doi: 10.1097/fjc.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki-Inoue K., Hughes C. E., Inoue O., et al. Involvement of Src kinases and PLCγ2 in clot retraction. Thrombosis Research. 2007;120(2):251–258. doi: 10.1016/j.thromres.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senis Y. A., Mazharian A., Mori J. Src family kinases: at the forefront of platelet activation. Blood. 2014;124(13):2013–2024. doi: 10.1182/blood-2014-01-453134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam F., Kauskot A., Rosa J.-P., Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. Journal of Thrombosis and Haemostasis. 2008;6(12):2007–2016. doi: 10.1111/j.1538-7836.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim S., Jin J., Kunapuli S. P. Akt activation in platelets depends on Gi signaling pathways. Journal of Biological Chemistry. 2004;279(6):4186–4195. doi: 10.1074/jbc.m306162200. [DOI] [PubMed] [Google Scholar]
- 31.Silvain J., Collet J.-P., Nagaswami C., et al. Composition of coronary thrombus in acute myocardial infarction. Journal of the American College of Cardiology. 2011;57(12):1359–1367. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M.-H., Tong X., Wang J.-X., Zou W., Cao H., Su W.-W. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula Shenqi Fuzheng Injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2013;74:141–155. doi: 10.1016/j.jpba.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Fang J., Zhang Y. Icariin, an anti-atherosclerotic drug from Chinese medicinal herb horny goat weed. Frontiers in Pharmacology. 2017;8:p. 734. doi: 10.3389/fphar.2017.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W.-P., Bai X.-J., Zheng X.-P., Xie X.-L., Yuan Z.-Y. Icariin attenuates the enhanced prothrombotic state in atherosclerotic rabbits independently of its lipid-lowering effects. Planta Medica. 2013;79(09):731–736. doi: 10.1055/s-0032-1328551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed description on chemicals and reagent sources, animals and handling, preparation of washed human and rat platelets, scanning electron microscopy (SEM), ATP secretion and TXB2 production, measurement of intracellular calcium mobilization, flow cytometry, fibronectin adhesion assay, clot retraction, immunoblotting, in vivo AV-shunt assay, and bleeding time has been included in the Supplementary Materials. We followed the methods of Irfan et al. [17].
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


