Abstract
Aim.
Integrating psychosocial interventions with mobile apps may increase treatment engagement among adolescents. We examined the user experience, uptake, and clinical effects of a mobile-enhanced family-focused therapy (FFT) among adolescents at risk for mood disorders.
Method.
We created a mobile app containing 12 lesson plans corresponding to content of weekly FFT sessions, with modules concerning mood management, family communication and problem-solving. We pilot tested the app in an open trial of FFT (12 sessions in 18 weeks) for adolescents who had active depressive or hypomanic symptoms, a parent with mood disorder, and at least one parent who expressed high levels of criticism. Teens and parents made daily and weekly ratings of youths’ moods, amount of parent/offspring criticism, and practice of FFT psychoeducational, communication or problem-solving skills. Independent evaluators interviewed adolescents at baseline and every 9 weeks over 27 weeks to measure symptom trajectories.
Results.
Participants were adolescents (n=22; mean age 15.4 ± 1.8 years; 45.5% female) and their 34 parents. Completion of requested app assessment and skill practices averaged 46%–65% among adolescents and parents over 18 weeks of treatment. Adolescents showed significant improvement in clinician-rated depression scores over 27 weeks (Cohen’s d=1.58, 95% CI, 0.83 to 2.32) and reported reductions in the amount of perceived criticism expressed by parents.
Limitations.
The uncontrolled design limits inferences about whether the mobile app augmented the effects of FFT on moods or family relationships.
Conclusions.
Mobile applications may enhance users’ responses to family therapy and provide clinicians with information regarding clinical status.
Keywords: Digital mental health, mobile apps, bipolar disorder, expressed emotion, early intervention
1. Introduction
There is increasing evidence that mood disorders have onsets in childhood and early adolescence (e.g., Perlis et al., 2004). Earlier (versus later) onsets of depression and bipolar disorder are associated with higher rates of recurrence, symptom morbidity, and psychosocial impairment in adulthood (Beesdo et al., 2009; Birmaher et al., 2018; Geller et al., 2008; Hafeman et al., 2016; Klein & Depue, 1984). Researchers have begun to develop early intervention programs that aim to enhance resilience in youth at high risk for mood disorder (e.g., Garber et al., 2009; Miklowitz et al., 2020b). An example is family-focused therapy (FFT), a brief treatment consisting of weekly and biweekly parents/offspring sessions of psychoeducation about mood disorders, communication skills training, and problem-solving skills training (Miklowitz & Chung, 2016). In randomized clinical trials, FFT has been found to be an effective adjunct to pharmacotherapy in stabilizing symptoms and delaying mood recurrences among adults and adolescents with bipolar I or II disorder (Miklowitz et al., 2008; Miklowitz et al., 2014), children and adolescents with depression or hypomania who have family histories of bipolar disorder (Miklowitz et al., 2013; Miklowitz et al., 2020b), and school-aged children with major depressive disorder (Tompson et al., 2017).
Despite encouraging results, there are considerable individual differences in response to FFT. Between 50% and 60% of youth at risk for bipolar disorder who begin treatment in a depressed or hypomanic state continue to have residual symptoms and functional impairment after 4 months of treatment (Miklowitz et al., 2013; Weintraub et al., 2020). The efficacy of FFT in reducing patients’ mood symptoms depends in part on whether families are able to modulate levels of conflict and increase the expression of constructive statements and positive affect (Simoneau et al., 1999; Miklowitz et al., 2020a, 2020b). These goals are more easily achieved when families practice communication and problem-solving skills between sessions (e.g., how to listen actively).
The strategy used in this study was to encourage learning and implementation of FFT skills as well as facilitate information exchange between clinicians, teens and families in FFT using an interactive mobile app. The mobile app encouraged users (teens and parents) to practice skills related to coping with mood symptoms and communicating effectively as a family. It also enabled users to make daily and weekly ratings of moods and family relationships, providing ongoing assessment data to clinicians for treatment planning. Mobile technologies have been shown to boost the efficacy of psychosocial treatments among individuals with mood and anxiety disorders (Lindhiem et al., 2015), but have not been tested in the context of family interventions.
In this article, we describe the development of a mobile app and report results of an open trial of technology-enhanced FFT in adolescents at risk for mood disorders. Adolescents had three attributes that increased their risk for episodes of mood disorders: active mood symptoms and evidence of mood instability (e.g., Hafeman et al., 2016; Marwaha et al., 2014), high levels of parent/offspring criticism and conflict (e.g., Peris and Miklowitz, 2015), and a biological parent with a lifetime history of major depression or bipolar disorder (Birmaher et al., 2018; Loechner et al., 2020; Luby & Navsaria, 2010). We hypothesized that at-risk teens and parents would be able to engage with weekly symptom tracking and skill practice tasks on the mobile app, and that adolescents would show significant mood improvement in technology-enhanced FFT over 6 months. Secondarily, we aimed to show that adolescents who perceived less criticism from their parents over time (based on mobile app ratings) would show greater mood improvement during the trial.
2. Methods
2.1. Study overview
We conducted a 6-month open trial of technology-enhanced FFT in adolescents at risk for mood disorders. All participants received 12 sessions of FFT (8 weekly, 4 biweekly) over 4 months, interspersed with research follow-up interviews every 9 weeks for 27 weeks. Outcomes included user experiences of and adherence to the mobile app, changes in adolescents’ symptom severity, and changes in perceived criticism over 6 study months.
2.2. What is family-focused therapy?
FFT for high-risk adolescents is described in detail elsewhere (Miklowitz et al., 2013, 2020a). In the first of three modules, psychoeducation, clinicians acquaint teens and family members with the nature and course of mood symptoms, the contribution of stress to symptom aggravation, and coping strategies (e.g., keeping regular sleep cycles), with the end goal of developing a personalized mood management plan. In the second module, communication enhancement training, youth and family members learn through role-play exercises and between-session practice to express positive feelings, listen actively, make positive requests for change in each other’s behavior, communicate clearly, and constructively express negative feelings. In the third module, problem--solving, families learn to break down large problems (e.g., “we don’t get along”) into smaller ones (“we need to use lower tones of voice”), generate and evaluate pros/cons of various solutions, and choose specific solutions to implement between sessions (e.g., alert each other to aggressive voice tones). (The clinicians’ manual for FFT is available at https://www.semel.ucla.edu/champ/downloads-clinicians).
2.3. Objectives of FFT app
The mobile app was co-created through a participatory process that involved the study team members (including clinicians and support staff) engaging with patients, families and other stakeholders. Because the app was integrated into a treatment study, it consisted of a system of apps, including one for families to use, one for clinicians to review clinical progress and tailor recommendations, and one for study staff to enroll and manage participants. The team used a HIPAA-compliant, web-based no-code application platform (“Chorus”; Arevian et al., 2020a, 2020b) that enables individuals without specific technical skills (e.g., knowledge of computer programming) to visually create mobile, text messaging and interactive voice apps.
The mobile app was designed to (1) enable participants to review session content and themes, practice communication and problem-solving skills between sessions, and log these practices on the app; (2) complete daily and weekly mobile assessments of mood and family functioning; and (3) use an interactive voice response system to call-in weekly and talk about how they are doing. Clinicians were expected to review participants’ assessment data and voice recordings, and assign skill-training practices between treatment sessions. Prior uses of this interactive voice system using the Chorus platform resulted in improved communication between community practitioners and patients with severe mental illnesses, and provided real-time information on patients’ clinical states (Arevian et al., 2020a).
We approached the app development task with recognition of the need to personalize psychoeducational and skill-training content. Cocreation of the app occurred in three phases: (1) needs assessment, (2) development and troubleshooting, and (3) open trial testing.
2.4. Needs assessment phase
In the initial phase, we convened three participatory workgroups of community clinicians with experience in working with teens and families, clinicians with experience in FFT, and teens and parents who had undergone FFT in the UCLA Max Gray Child and Adolescent Mood Disorders Program. The groups began with demonstration of the Chorus platform. In all three groups, the research team sought reactions to the existing user interface and proposed functions to be incorporated into the family version of the app. Both groups of clinicians saw the value in automated reminders to encourage rehearsal of communication or problem-solving skills outside of the therapy hour and the acquisition of assessment data from patients and parents. However, participants in all three groups raised the issue of “building in protections to make sure people have the necessary human contact” when using the app. Participants in the teen/family group and clinicians separately emphasized that the app could not be a substitute for face-to-face sessions.
2.5. Development of FFT apps for families and clinicians
The research team met weekly between May of 2018 and November of 2018 to develop three interrelated versions of the app. The family app (Figure 1 and home page, Fig. 2) had assessment features (e.g., daily and weekly mood assessment check-ins and weekly voice journals) and instructional features (i.e., reviews of each FFT skill with directions for logging practice of these skills between sessions).
Fig. 1.
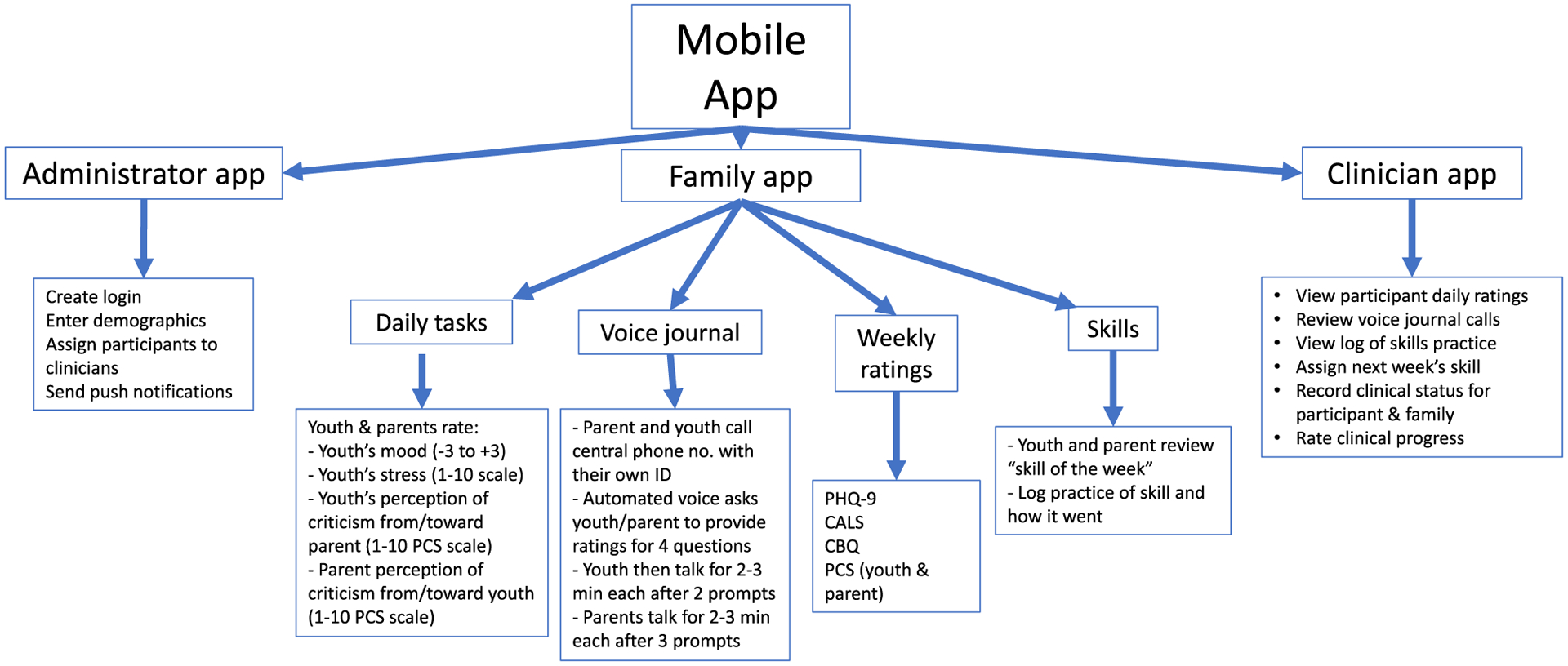
App Structure.
Fig. 2.

Family App Home Page.
After each FFT session, the therapist accessed a clinician’s version of the app and chose a “skill of the week” for the teen and family to practice. The chosen skill then appeared on the family app home page. In early sessions, clinicians emphasized weekly practices of coping strategies listed by adolescents as effective in managing stress (e.g., distraction, talking to a friend). In later sessions, clinicians emphasized skills related to communication and problem-solving, which required that the teen practice with other family members. Two buttons on the participants’ app facilitated these practices: the ‘Try It’ button (Figure 2), which directs users to a practice page; and the “Review Your Progress” button, in which users can view their mood ratings and weekly skill practices in graphic or tabular form.
In the focus workgroups, parents wondered whether the teen would remember to complete the assessments or skill practices. Adolescents added that they would have little motivation to do so without rewards. We addressed these issues in two ways. First, we programmed the app to send automated text reminders to users on a daily and weekly basis. Second, we constructed a “level up” emoji reward system similar to those used in activity tracking apps (e.g., Sasaki et al., 2015) as a way to “gamify” the app. On the homepage, icons illuminated when participants completed their weekly check-in, completed a voice journal, or logged a skill practice. Once each of these tasks was completed, the participant advanced to a new level and received a celebratory emoji and .gif file.
The clinician’s app provided therapists with access to the patients’ assessment data, skill practice logs, and voice journals to inform treatment planning. A separate administrator’s app enabled the research coordinator to activate accounts for newly enrolled users, plan follow-up interviews, and track unusual response patterns.
The final version of the app included over 200 pages with text, videos, drawings, graphic representations of mood ratings, and hand-outs illustrating each of the 12 FFT skills (Figure 1). In a typical session of technology-enhanced FFT, clinicians began by reviewing the teen’s mood and stress ratings for the week, and then encouraged a family discussion about mood fluctuations, family interactions or other events that may have triggered these changes, and how the family could work with the teen to address mood swings in the coming week. Then, the clinician introduced a new skill (e.g., constructively expressing negative feelings) and played the app’s video demonstration of the skill. After each pair of family members had practiced the skill, the clinician directed the family to the section of the app in which the skill was explained further and gave instructions on how to log use of the skill between sessions.
2.6. Open trial: Inclusion and exclusion criteria
Youth met the following inclusion criteria: (1) ages 13 years, 0 months to 19 years, 11 months; (2) evidence of unstable and impairing moods, as indicated by a score ≥ 6 on the 10-item Parents’ General Behavior Inventory for Mania (Youngstrom et al., 2008) or ≥ 20 on the parent or adolescent-rated Children’s Affective Lability Scale (Gerson et al., 1996); (3) current and impairing mood symptoms (scores > 11 on the Young Mania Rating Scale (YMRS; Young et al., 1978) or > 29 on the Children’s Depression Rating Scale, Revised (CDRS-R; Poznanski and Mokros, 1995); (4) at least one parent has a lifetime history of major depressive disorder or bipolar I or II disorder by direct interview; and (5) one parent is rated ‘high’ on the 1–10 Perceived Criticism Scale (PCS; Masland and Hooley, 2015; see below). Youths were excluded if they were enrolled in another treatment program, had an active substance or alcohol use disorder in the past four months, or had an autism spectrum disorder or intellectual disability by parent report.
All family members (parents, siblings, grandparents) who lived with or were in regular contact (minimum 4 hrs/week) with the teen were invited to participate in sessions. Data analyses only considered the mobile app responses of the adolescent and “primary” parent who had the most weekly contact with him or her, which in most cases was the teen’s mother.
2.7. Intake Assessments
The study was reviewed and continuously approved by the UCLA Medical Institutional Review Board. Adolescents and families were referred to the study through pediatricians or mental health practitioners, online advertisements, or posted flyers. Study coordinators conducted a brief telephone screen with parents who called the study’s contact line regarding their offspring. If the adolescent appeared eligible and the family expressed interest, they were invited to an initial consenting and intake assessment visit. At this visit, the study was explained in full and adolescents and parents were asked to read over and sign university-approved human subject consent and assent forms (the latter for under-aged participants). Then, two trained diagnosticians administered the Mini International Neuropsychiatric Inventory, Child and Adolescent Version for DSM-5 (Sheehan, 2016) to the adolescent and separately, one parent about the teen’s symptoms and behavior in the last 2 weeks and over his/her lifetime. They also interviewed biological parents about their own psychiatric histories using the Mini for DSM-5, Adult Version (Sheehan, 2016). The diagnosticians met and made consensus diagnoses of the parents and adolescent.
At intake, youth and parents filled out brief questionnaires concerning the frequency of parent/offspring criticism (see Table 1). For eligibility, adolescents had to rate one of their parents as ‘5’ or higher on the 1–10 PCS items, “How critical do you think your (mother/father) is of you?” or “When you (mother/father) criticizes you, how upset do you get?” Scores ≥ 5 on the first of these two PCS items are associated with an increased risk of recurrence in individuals with depression (Masland and Hooley, 2015). This inclusion criterion could also be met by one parent rating themselves as ≥ 5 on the item “How critical do you think you are of your (son, daughter)?”
Table 1.
Sample characteristics (N = 22)1.
| Variable | Mean + SD (or %) |
|---|---|
| Mean Age | 14.84 ± 1.8 years |
| Sex at Birth | 10 (45.5%) female, 12 (54.5%) male |
| Race, no. (%) | |
| White | 15 (68.2%) |
| African American | 2 (9.1%) |
| Biracial | 5 (22.7%) |
| Ethnicity, no. (%) Hispanic | 5 (22.7%) |
| Single parent families | 8 (36.4%) |
| DSM-5 Diagnosis | |
| Major depressive disorder | 14 (63.6%) |
| Bipolar I disorder | 1 |
| Bipolar II disorder | 1 |
| Other specified bipolar disorder | 3 |
| Attention deficit hyperactivity disorder | 3 |
| Adolescent Longitudinal Interval Follow-up, mean 1–6 Psychiatric Status Rating (PSR) of Depression | 4.3 ± 1.2 |
| Young Mania Rating Scale, Adolescent Report | 10.9 ± 6.2 |
| Children’s Depression Rating Scale-R, Adolescent report | 42.9 ± 19.0 |
| Parents’ General Behavior Inventory, 10-item Mania Scale | 9.1 ± 6.7 |
| Children’s Affective Lability Scale, Parent report | 28.6 ± 14.0 |
| Adolescent’s Perceived Criticism from Parent (1–10 scale) | 5.7 ± 2.7 |
| Primary Parent’s Perceived Criticism Toward Child (1–10 scale) | 5.1 ± 2.3 |
| Parental DSM-5 diagnoses | |
| Biological mother with major depressive disorder | 15 (68.2%) |
| Biological mother with bipolar I or II disorder | 0 |
| Biological father with major depressive disorder | 8 (36.4%) |
| Biological father with bipolar I or II disorder | 8 (36.4%) |
| Both biological parents with mood disorder | 2 (9.1%) |
| No Pharmacotherapy, no. (%) | 8 (38.1%) |
| Pharmacotherapy, no. (%) | 13 (61.9%) |
| Antidepressant alone, no. (%) | 2 |
| Psychostimulant alone, no. (%) | 2 |
| Antipsychotic alone, no. (%) | 1 |
| Antipsychotic, antidepressant | 2 |
| Mood stabilizer, antidepressant | 2 |
| Antipsychotic, antidepressant, psychostimulant | 2 |
| Antipsychotic, mood stabilizer, antidepressant | 2 |
| Unknown | 1 |
Includes one adolescent whose family consented but withdrew before any treatment sessions were conducted.
2.8. Outcome Assessments
The primary outcome was app engagement, operationalized as the proportion of days or weeks during the 18-week treatment in which participants completed each app-related task (mood and stress ratings, voice journals, logging of FFT skills). Secondarily, we examined depression or mania severity scores among youths over 27 study weeks (18 weeks of treatment and 9 post-treatment weeks). At the intake visit, independent evaluators interviewed the adolescent and one parent and rated the former’s symptoms during each week of the preceding 18 weeks using the Adolescent Longitudinal Interval Follow-up Evaluation Psychiatric Status Ratings (PSRs; Keller et al., 1987). The weekly PSRs are 1 (asymptomatic) to 6 (severe symptoms, meets syndromal DSM-5 criteria) point scales of symptom severity that cover depression, mania, hypomania, suicidal ideation, delusions, and hallucinations. In our recent study of youths at risk for bipolar disorder, interrater reliabilities for the 6-point PSRs averaged 0.74 (intraclass r) across independent evaluators (Miklowitz et al., 2020b). In the present study, test/retest reliability of the PSRs ranged from 0.81 (delusions) to 0.95 (depression) over a 4-week rating period.
Evaluators re-interviewed adolescents and one parent at week 9 (mid-treatment), week 18 (post-treatment), and week 27 (follow-up) using the same measures obtained at intake, but covering the 9 prior weeks. Independent evaluators also administered the YMRS (mania) and the CDRS-R (depression) scales to the teen and one parent covering the prior 1–2 week period. The evaluators considered both respondents’ reports in making consensus ratings.
At the end of each 9-week period, adolescents and parents filled out two mobile app questionnaires regarding their experiences in the treatment program. The Perceived Ease of Use Scale (Davis, 1989) consists of 6 items related to performing tasks on the mobile app, each rated on 1 (strongly agree) to 7 (strongly agree) scales (e.g., “The app is helpful in performing FFT skills”). The Satisfaction with FFT scale was adapted from the short form of the Working Alliance Inventory (Hatcher and Gillaspy, 2006). It consisted of 12 items rated on 1 (seldom) to 5 (always) scales covering the participants’ experiences of FFT sessions (e. g., “As a result of these sessions I am clearer as to how I might be able to change”). We added 6 identically scaled items concerning the participants’ satisfaction with the app.
2.9. Psychometric Attributes of App Instruments
The instruments collected weekly on the mobile app were copied from their paper-pencil format. These included the Patient Health Questionnaire-9 (PHQ-9) ratings of depression (Kroenke et al., 2010), the PCS, and the Children’s Affective Lability Scale. Based on adolescents’ self-ratings, Cronbach’s alpha for the PHQ-9 scale was 0.89 at the initial week of assessment and 0.91 at the 4th week. For adolescents’ PCS ratings of parents, alpha was 0.82 at week 1 and 0.85 at week 4; test/retest reliability for adolescents’ PCS ratings was 0.76 over 1 month. Cronbach’s alpha for the 20 items of the Children’s Affective Lability Scale was 0.96 at baseline, with test/retest reliability of 0.75 over one month.
2.10. Data Analyses
App engagement scores for the daily and weekly assessments, logging of skills, and weekly voice journals were calculated for the adolescent and primary parent. We examined the intercorrelation of adolescents’ and parents’ engagement scores for each task, using all available baseline and follow-up observations. We assessed the benefits of combining the mobile app with FFT sessions by studying the evolution in time of three symptom scales administered throughout the study. We used the PSRs of depression as the gold-standard against which to compare the other measures (e.g., youths’ weekly mobile app-based PHQ-9 ratings of depression; interview-based CDRS-R scores obtained at each 9-week interval). Changes in dimensional variables were examined using generalized linear mixed models implemented using the LME4 library of the R-statistical software (Bates et al., 2015). The models used time (e.g., weekly or 9-weekly intervals) as a fixed effect and subject as a random effect. Generalized linear mixed models account for correlations induced by repeated measurements within subjects and produce unbiased estimates of missing data as long as observations are missing at random. Correlations between app self-ratings and clinician symptom ratings were calculated using the rmcorr library in the R software to account for repeated measures within subjects (Bakdash and Marusich, 2017). Throughout the study’s analyses, we used an intent-to-treat approach. Thus, all available data were used despite participant dropout from treatment or study follow-up assessments.
3. Results
3.1. Participants
We conducted telephone screening interviews with parents of 36 adolescents who called the study’s contact line. Of these, 14 adolescents/families were excluded: 7 did not meet the study’s symptom eligibility criteria, 2 families were unable to make the time commitment, 2 adolescents and/or parents refused, and 3 did not respond to further communication after the screening call. A total of 22 adolescents (mean age 15.4 ± 1.8 years, range 13–19 years; 45.5% female; Table 1) and 34 parents (21 mothers, 13 fathers) attended an initial study visit and signed assent and consent forms. At the intake visit, adolescents were moderately or severely depressed (mean 1–6 PSR for depression scale, 4.33 ± 1.2; mean CDRS-R total score, 42.9 ± 19.0) and showed subthreshold elevations on the YMRS (mean 10.9 ± 6.2).
Of the 22 participants who consented, 1 did not complete the baseline assessments and did not attend any treatment sessions. Of the remaining 21 participants, 19 (90.5%) finished the 18 weeks of FFT and the 27-week follow-up visit.
3.2. Engagement with the family mobile app
Figure 3 gives a graphical representation of data obtained from a sample adolescent’s daily ratings of moods (−3 to + 3 mood scale). Daily app engagement among adolescents averaged 46.6% over 126 days of treatment (Table 2), whereas primary parents (19 mothers, 2 fathers) completed daily app ratings of teens in 59.6% of the treatment days. Participants were consistent in completing weekly check-in questionnaires covering moods and Perceived Criticism (51.2% to 62.3%; Table 2). Engagement with the weekly logging of FFT skills was 53.2% in youths and 55.9% in parents. Adolescents called the voice journal an average of 44.2% (vs. 55.7% for parents) of the 18 treatment weeks. Youths’ engagement scores were correlated with parents’ engagement scores for the daily (r = 0.56), weekly (r = 0.58), voice journal (r = 0.78) and skill logging (r = 0.56) tasks (for all rs, p < 0.01).
Fig. 3.
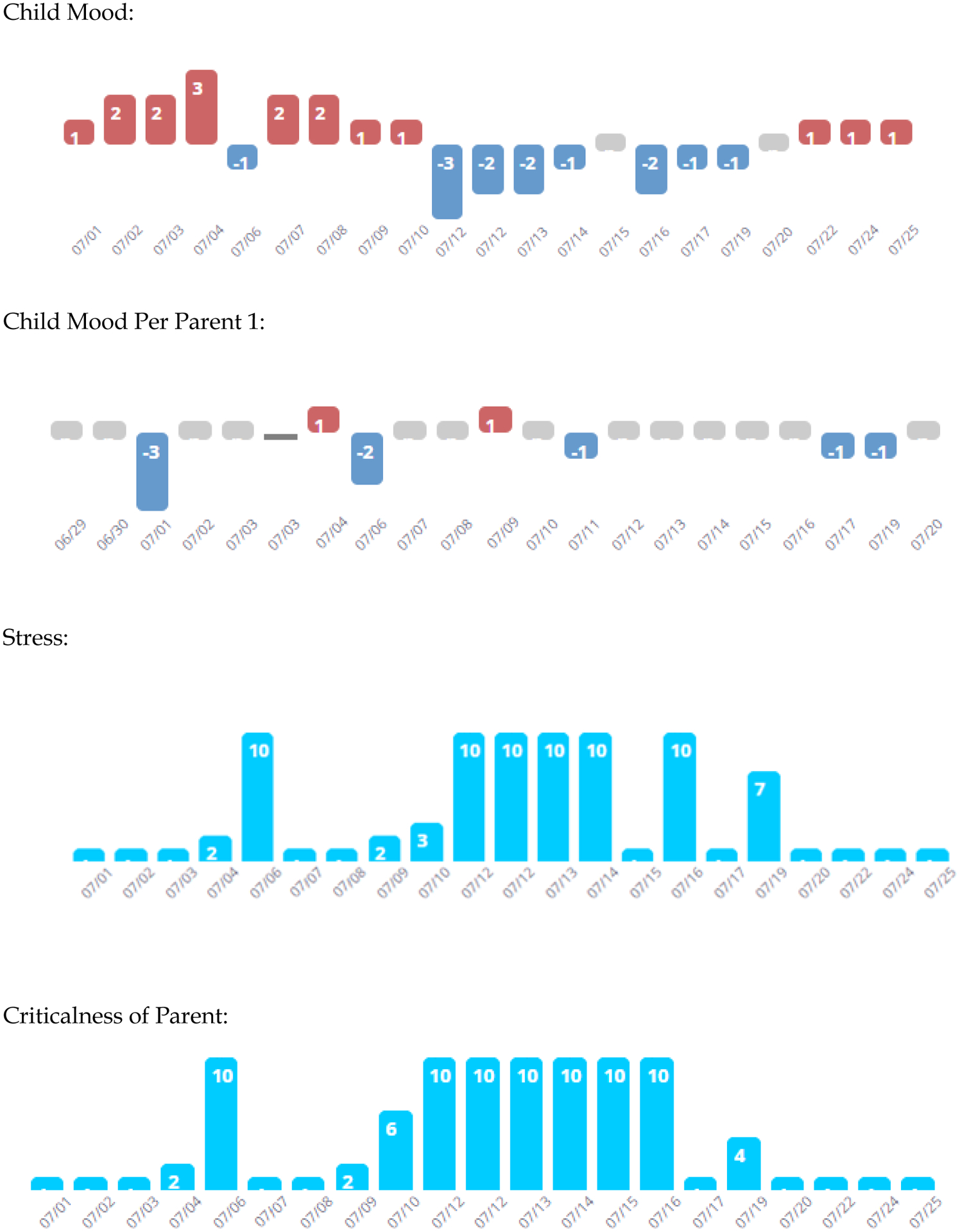
Example of Daily Mood, Stress and Perceived Criticism Ratings.
In this example, the adolescent reported more day-to-day mood variability than was observed by the parent. The adolescent rated the days with more extreme moods as those in which he or she perceived the primary parent to be more critical.
Table 2.
Engagement with Mobile App During 18-Week Family-Focused Treatment.
| Voice Journals | Daily Check ins | Weekly Check ins | FFT Skill Log | |
|---|---|---|---|---|
| Adolescent (n = 21) | ||||
| Mean Completion % | 44.2% | 46.6% | 51.2% | 53.2% |
| SD | 38% | 34% | 31% | 41% |
| Range – min | 0% – 100% | 3% – 100% | 6% – 100% | 0% – 100% |
| Parent (n = 21) | ||||
| Mean Compliance % | 55.7% | 59.6% | 62.3% | 55.9% |
| SD | 39% | 31% | 33% | 41% |
| Range – min | 6% – 100% | 8% – 100% | 9% – 100% | 6% – 100% |
Notes: Adherence with the daily ratings is calculated as the proportion of days during treatment in which mood ratings were completed. For tasks required weekly, adherence was calculated as the proportion of weeks during treatment in which the respondent completed each task (e.g., calling the interactive voice recorder and leaving a speech sample).
3.3. Subjective Usability Ratings
Perceived Ease of Use ratings for the MCC app were completed by 16 of the 19 adolescents and parents who finished 18 weeks of treatment. Using a 1 (strongly disagree) to 7 (strongly agree) scale, participants endorsed the item “The app was overall easy to use” (adolescents, M = 5.25 ± 1.84; parents, M = 6.0 ± 1.0) and “The app was helpful with performing FFT skills” (adolescents, M = 5.06 ± 1.88; parents, M = 5.70 ± 0.90). Total Satisfaction with Treatment scores, summed across 12 scale items (rated on a 1 (seldom satisfied) to 5 (always satisfied) scale) averaged 42.9 ± 13.9 (average 3.6 per item) for adolescents (n = 16) and 49.9 ± 10.6 (4.2 per item) for parents (n = 16). Summary scores for the 6 items concerning satisfaction with the app (scaled from 1 to 5) were in the ‘neutral’ zone (teen, M = 17.4 ± 7.7, or 2.9/item; parents, M = 18.3 ± 6.3, or 3.1/item). Satisfaction ratings for FFT sessions and for use of the mobile app were highly correlated among adolescents (Spearman r(16) = 0.89, p < .001) and among parents (r(16) = 0.57, p = 0.02).
3.4. Symptomatic Outcomes
Results of a general linear mixed model revealed a significant linear reduction in adolescents’ PSRs for depression over 6 months: post-treatment (18 week) PSRs (n= 20) were on average 1.53 scale points (95% CI, 1.02 – 2.05) lower than those obtained at baseline (n = 22), and follow-up ratings (week 27, n = 19) were on average 1.70 scale points (95% CI, 1.2–2.2) lower than those at baseline (p < .0001; Cohen’s d = 1.58, 95% CI (0.83, 2.32)). When considering all 564 weekly PSRs for depression for 21 adolescents, the weekly slope of improvement was −0.07 (95% CI, −0.07 to −0.06; p < .0001). Results also indicated linear improvement in evaluator-rated CDRS-R depression scores from from baseline to 9, 18 and 27 weeks, with an estimated reduction of −20.38 points (95% CI, −26.70 to −14.07; p < .001; 76 observations). YMRS scores decreased by an estimate of 7.12 points (95% CI, −10.14 to −4.10) from baseline to follow-up (p < 0.001; 76 observations) (Table 3).
Table 3.
Generalized linear mixed model regression results for dimensional ratings of evaluator-rated symptoms from baseline to 27 weeks.
| ALIFE PSR Depression | CDRS-R Total | YMRS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimates | 95% CI | p | Estimates | 95% CI | p | Estimates | 95%CI | p | |
| Baseline (N=21) |
4.33 | Lo 3.83 Hi 4.83 |
<0.001 | 57.43 | Lo 51.58 Hi 63.27 |
<0.001 | 20.52 | Lo 17.59 Hi 23.46 |
<0.001 |
| Mid-Treatment (N=21) |
−0.71 | Lo −1.22 Hi −0.21 |
0.006 | −14.76 | Lo −20.77 Hi −8.75 |
<0.001 | −4.9 | Lo −7.78 Hi −2.03 |
0.001 |
| Post-Treatment (N=20) |
−1.53 | Lo −2.05 Hi −1.02 |
<0.001 | −20.71 | Lo −27.28 Hi −14.14 |
<0.001 | −7.36 | Lo −10.51 Hi −4.22 |
<0.001 |
| Follow-up (N=19) |
−1.7 | Lo −2.24 Hi −1.17 |
<0.001 | −20.38 | Hi −26.70 Lo −14.07 |
<0.001 | −7.12 | Lo −10.14 Hi −4.10 |
<0.001 |
Note: Mid-Treatment = 9 weeks, Post-Treatment = 18 weeks, Follow-up = 27 weeks. The p-values associated with baseline indicate the degree to which scores are higher than zero.
To determine whether participants rated themselves as having improved in depression over time, we examined all app-rated PHQ-9 depression scores (289 observations) in a single generalized linear mixed model. App-rated PHQ-9 scores improved from week 1 to week 27 (slope estimate = −0.10, 95% CI, −0.16 to −0.03; p < 0.003). The repeated measure correlation between weekly PSRs of depression and app-based PHQ-9 ratings was 0.30 (95% CI, 0.17 to 0.42, p < 0.001; n = 203 paired observations).
3.5. Perceived Criticism
Adolescents’ weekly 1–10 mobile app ratings of frequency of criticism from the primary parent dropped by an average of −0.07 (95% CI, −0.11 to −0.04) scale points each week over 27 weeks (p < 0.001, 220 observations). Adolescents also reported being less distressed by criticisms over time (weekly change −0.07, 95% CI, −0.11 to −0.03; p < 0.001). Adolescents’ weekly ratings of frequency of parental criticism were modestly correlated with their own weekly depression PHQ-9 ratings on the app (repeated measure r(220) = 0.19, p < 0.01). Weekly ratings of distress from parental criticisms were also correlated with adolescents’ contemporaneous PHQ-9 scores (r(220) = 0.31, p < 0.001).
The proportion of weeks in which adolescents used the app (engagement) was associated with greater reductions in their perceptions of frequency of parental criticisms (r = −0.51, p = 0.046). Further, reductions in adolescents’ PCS frequency ratings (slope scores) were correlated with reductions in interview-based PSRs of depression over 27 weeks (r = 0.61, p = 0.01, 95% CI, 0.16 to 0.84).
4. Discussion
Mobile health apps are increasingly being used to augment the management of chronic medical and behavioral disorders (e.g., Cole--Lewis and Kershaw, 2010; Khodyakov et al., 2014), and to engage communities in tracking health behaviors (Freifeld et al., 2010; Faurholt-Jepsen et al., 2020). In this study, we created a mobile app as a technological enhancement to FFT for adolescents at risk for mood disorders. The app enabled adolescents and their parents to regularly review and practice FFT skills, and providers to monitor these practices and review adolescents’ symptomatic progress. The strategy of augmenting in-person care approaches with mobile technology, rather than replacing treatment with a stand-alone app, is consistent with recommendations for technology-enabled services in mental health (Mohr et al., 2017).
The FFT-MCC app was co-created with input from adolescents, parents, community clinicians and clinicians. There was general agreement that an app to accompany treatment would be useful, especially if it increased the users’ practice of communication skills between sessions. Teen and parent participants found the FFT-MCC app to be easy to use, and most (19 of 21 entrants) completed the full 18-week course of treatment. Rates of engagement with weekly app assessments (44.2% – 62.3% for teens and parents, respectively) and logging of skill practices (53.2% – 55.9%) were in the acceptable range, and compare favorably to rates of engagement with intersession homework practices in cognitive-behavioral therapy for depression (Gaynor et al., 2006; Simons et al., 2012). However, we observed considerable variability in engagement in adolescents and parents (Table 2) and across tasks, with participants ranging from 0% to 100% in the proportion of study weeks with app use. Many adolescents (and some parents) used the app regularly at the beginning of treatment but discontinued use as sessions were winding down.
In developing apps to facilitate evidence-based psychotherapies, developers must consider issues such as the appropriate frequency of automated reminders, the optimal number of assessment tasks, the features required to engage younger versus older users, and the minimum level of compliance that is associated with increments in treatment efficacy. Having an attractive and easy-to-use interface is also an important consideration, as much of the world has become accustomed to the sleek designs of apps developed by technology companies.
Adolescents began this study with moderate to severe levels of depression. By the end of 27 weeks, depression PSRs decreased by an average of 1.70 (out of 6) points (Cohen’s d = 1.58). Because of the study’s uncontrolled design, we cannot determine whether these improvements are indicative of the effects of FFT, concurrent pharmacotherapy, or changes in environmental factors. Nonetheless, in a randomized trial with youth at high risk for bipolar disorder who received the same 12-session FFT protocol without an app, there were smaller pre/post-treatment changes on the PSR Depression scale over 4 months (d = 0.38, 95% 0.01–0.75; (Miklowitz et al., 2020b). Between-session use of a mobile app may increase the efficacy of family interventions by focusing adolescents and parents on the skills needed to build protective environments during a post-episode period.
Adolescents reported reductions in amount of perceived criticism from parents, and also reported feeling less distressed by criticisms. Appreported reductions in perceived criticism were correlated with self- and clinician-rated improvements in depression. Improved mood states among adolescents may be associated with reductions in the salience of parental criticisms. Alternatively, decreases in adolescents’ perceptions of family conflict may mediate the relationship between psychosocial interventions and symptomatic improvement, as shown in our trial in youth at risk for bipolar disorder (Miklowitz et al., 2020a).
5. Limitations and Lessons Learned
Our conclusions are limited by the trial’s open design, small sample, and short-term follow-up. Nonetheless, we have learned a number of important lessons about developing and testing mobile technologies as adjuncts to psychotherapy for youth with mood disorders. First, app development requires continuous iterative feedback and updates, a participatory process that involves regular communication between the development team and the end-users. When we received feedback from two or more families about the app interface (either in workgroups or FFT sessions), we made appropriate modifications. For example, teens recommended an incentive system for the weekly check-ins, and we introduced an emoji reward system. To address users’ reactions to receiving daily reminders, we modified the system to send reminders at a rate and time chosen by each family.
The continuous engagement of participants depended in part on the nature of the assessment tasks. Some participants found it difficult to complete research assessments while also logging their use of treatment skills. Many teens found it awkward to call and speak openly following the prompts of a robotic voice. Yet, parents reported that the voice journals were a helpful space to openly communicate their emotions. Developing methods to sustain participants’ continued engagement with the various features of mobile apps is an important research direction.
Study FFT clinicians were required to engage with the app as one of the terms of their employment. We cannot assume that community practitioners who were provided with a treatment app would find it useful, unless the data obtained were directly relevant to their treatment goals for individual patients. The input of providers is critical to developing app features that increase the quality of care without needlessly adding to one’s workload.
6. Conclusions
The present study was conceptualized as a development project for a technology-enhanced version of FFT. We have demonstrated the feasibility of engaging at-risk teens and family members with a mobile app that encourages tracking of mood states and family relationships and regular practice of FFT skills. Adolescents receiving FFT-MCC showed pre- to- post-treatment reductions in depression and parental criticism by self-report and mood symptoms by clinician-report. In an ongoing randomized trial, we are currently examining whether instructional features of the app (e.g., demonstration videos, reminders to practice specific skills) add to the efficacy of FFT or whether simply using an app to track one’s moods and family relationships is in itself of therapeutic value.
Adjunctive mobile technologies may reduce the costs associated with in-person treatment, which may make evidence-based psychotherapies such as FFT more accessible to adolescents in the early stages of psychiatric illness. Collaborations between researchers and community clinicians will help determine whether the procedures described in this article can be adapted to public mental health care, where budgets are restricted and the adoption of mobile technologies to enhance treatment is relatively new.
Acknowledgments
We wish to thank the following individuals for providing administrative support and study treatments: Alissa Ellis, PhD, Elizabeth Horstmann, MD, Sarah Marvin, PhD, Robert Suddath, MD, Cassidy Zanko, MD, Monica Done, MS, and Gigi Laurin. This study was reviewed and continuously approved by the medical institutional review boards of the UCLA Semel Institute, David Geffen School of Medicine at UCLA, Los Angeles CA, USA.
Role of the Funding Source
This study was supported in part by grants R34 MH117200 (Drs. Miklowitz and Arevian) from the National Institute of Mental Health
(NIMH), and funds from the Carl and Roberta Deutsch Foundation, Kayne Family Foundation, Danny Alberts foundation, AIM for Youth Mental Health, and the Jewish Community Fund of Los Angeles. The funding sources had no role in the design or conduct of the study; collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Declaration of Competing Interest
Dr. Miklowitz receives research support from the National Institute for Mental Health (NIMH), the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM for Mental Health, Jewish Community Foundation of Los Angeles, and the Max Gray Fund; and book royalties from Guilford Press and John Wiley and Sons. Dr. Weintraub reports research support from AIM for Mental Health, the Shear Family Foundation, and the Friends of the UCLA Semel Institute. Drs. Walshaw receives research support from Bluebird Biotech and Second Sight. Dr. Arevian is supported by NIMH P30 MH082760-05S1 and California State Center of Excellence for Behavioral Health SB 852, and NIMH grant R34MH117200. He has a financial interest in Insight Health Systems, Inc. and Arevian Technologies Inc. The other authors report no conflicts of interest.
References
- Arevian AC, Bone D, Malandrakis N, Martinez VR, Wells KB, Miklowitz DJ, Narayanan S, 2020a. Clinical state tracking in serious mental illness through computational analysis of speech. PLoS One 15 (1), e0225695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevian A, O’Hora J, Rosser J, Mango JD, Miklowitz DJ, Wells KB, 2020b. Patient and provider cocreation of mobile texting apps to support behavioral health: usability study. J Med Internet Res– Mhealth Uhealth 8 (7), e12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakdash JZ, Marusich LR, 2017. Repeated measures correlation. Frontiers Psychol 8, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Software 67 (1), 1–48. [Google Scholar]
- Beesdo K, Höfler M, Leibenluft E, Lieb R, Bauer M, Pfennig A, 2009. Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disord 11, 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Merranko JA, Goldstein TR, Gill MK, Goldstein BI, Hower H, Yen S, Hafeman D, Strober M, Diler RS, Axelson D, Ryan ND, Keller MB, 2018. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adolesc Psychiatry 57 (10), 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Lewis H, Kershaw T, 2010. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev 32 (1), 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FD, 1989. Perceived usefulness, perceived ease of use, and user acceptance of information technology MIS Quarterly 13(3), 319–340. [Google Scholar]
- Faurholt-Jepsen M, Christensen EM, Frost M, Bardram JE, Vinberg M, Kessing LV, 2020. Hypomania/mania by DSM-5 definition based on daily smartphone-based patient-reported assessments. J Affective Disord 264, 272–278. [DOI] [PubMed] [Google Scholar]
- Freifeld CC, Chunara R, Mekaru SR, Chan EH, Kass-Hout T, Iacucci Ayala, A, Brownstein J,S, 2010. Participatory epidemiology: use of mobile phones for community-based health reporting. PLOS Med 7 (12), e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Clarke GN, Weersing VR, Beardslee WR, Brent DA, Gladstone TR, DeBar LL, Lynch FL, D’Angelo E, Hollon SD, Shamseddeen W, Iyengar S, 2009. Prevention of depression in at-risk adolescents: a randomized controlled trial. JAMA 301 (21), 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor ST, Lawrence PS, Nelson-Gray RO, 2006. Measuring homework compliance in cognitive-behavioral therapy for adolescent depression: Review, preliminary findings, and implications for theory and practice. Behav Modif 30 (5), 647–672. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Bolhofner K, Zimerman B, 2008. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry 65 (10), 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, Denckla MB, 1996. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatr Res 65 (3), 189–198. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Merranko J, Axelson D, Goldstein BI, Goldstein T, Monk K, Hickey MB, Sakolsky D, Diler R, Iyengar S, Brent D, Kupfer D, Birmaher B, 2016. Toward the definition of a bipolar prodrome: dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry 173 (7), 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher RL, Gillaspy JA, 2006. Development and validation of a revised short version of the working alliance inventory. Psychotherapy Res 16 (1), 12–25. [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC, 1987. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 44, 540–548. [DOI] [PubMed] [Google Scholar]
- Khodyakov D, Sharif MZ, Dixon EL, Mendel P, Chung B, Linski B, Jones JB, 2014. An implementation evaluation of the community engagement and planning intervention in the CPIC Depression Care Improvement Trial. Community Mental Health J 50 (3), 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Depue RA, 1984. Continued impairment in persons at risk for bipolar disorder: Results of a 19-month follow-up. J Abnormal Psychol 93, 345–347. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Löwe B, 2010. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom scales: a systematic review. Gen Hosp Psychiatry 32 (4), 345–359. [DOI] [PubMed] [Google Scholar]
- Lindhiem O, Bennett CB, Rosen D, Silk J, 2015. Mobile technology boosts the effectiveness of psychotherapy and behavioral interventions: a meta-analysis. Behav Modif 39 (6), 785–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loechner J, Sfälea A, Starman K, Oort F, Thomsen LA, Schulte-Körne G, Platt B, 2020. Risk of depression in the offspring of parents with depression: the role of emotion regulation, cognitive style, parenting and life events. Child Psychiatry Hum Dev 51 (2), 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Navsaria N, 2010. Pediatric bipolar disorder: evidence for prodromal states and early markers. J Child Psychol Psychiatry 51 (4), 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha S, He Z, Broome M, Singh S, Scott J, Eyden J, Wolke D, 2014. How is affective instability defined and measured? A systematic review. Psychol Med 44 (9), 1793–1808. [DOI] [PubMed] [Google Scholar]
- Masland SR, Hooley JM, 2015. Perceived criticism: a research update for clinical practitioners. Clin Psychol Sci Pract 22 (3), 211–222. [Google Scholar]
- Miklowitz DJ, Axelson DA, Birmaher B, George EL, Taylor DO, Schneck CD, Beresford CA, Dickinson LM, Craighead WE, Brent DA, 2008. Family-focused treatment for adolescents with bipolar disorder: results of a 2-year randomized trial. Arch Gen Psychiatry 65 (9), 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Chung BD, 2016. Family-focused therapy for bipolar disorder: reflections on 30 years of research. Fam Process 55 (3), 483–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Merranko JA, Weintraub MJ, Walshaw PD, Singh MK, Chang KD, Schneck CD, 2020a. Effects of family-focused therapy on suicidal ideation and behavior in youth at high risk for bipolar disorder. J Affect Disord 275, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Schneck CD, George EL, Taylor DO, Sugar CA, Birmaher B, Kowatch RA, Delbello MP, Axelson DA, 2014. Pharmacotherapy and family-focused treatment for adolescents with bipolar I and II disorders: a 2-Year randomized trial. Am J Psychiatry 171 (6), 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Schneck CD, Singh MK, Taylor DO, George EL, Cosgrove VE, Howe ME, Dickinson LM, Garber J, Chang KD, 2013. Early intervention for symptomatic youth at risk for bipolar disorder: a randomized trial of family-focused therapy. J Am Acad Child Adolesc Psychiatry 52 (2), 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Schneck CD, Walshaw P, Singh M, Sullivan AE, Suddath R, Forgey Borlik M, Sugar CA, Chang KD, 2020b. Effects of family-focused therapy vs enhanced usual care for symptomatic youths at high risk for bipolar disorder: a randomized clinical trial. JAMA Psychiatry 77 (5), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Weingardt K, Reddy M, Schueller S, 2017. Three problems with current digital mental health research … and three things we can do about them. Psychiatr Serv 68 (5), 427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris T, Miklowitz DJ, 2015. Parental expressed emotion and youth psychopathology: new directions for an old construct. Child Psychiatr Hum Dev 46 (6), 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA, the STEP-BD Investigators, 2004. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 55, 875–881. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB, 1995. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Western Psychological Services, Los Angeles, CA. [Google Scholar]
- Sasaki JE, Hickey A, Mavilia M, Tedesco J, John D, Kozey Keadle S, Freedsonet PJ, 2015. Validation of the Fitbit wireless activity tracker for prediction of energy expenditure. J Phys Act Health 12 (2), 149–154. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, 2016. Mini International Neuropsychiatric Interview Version 7.02 for DSM-5 Harm Research Institute. [Google Scholar]
- Simoneau TL, Miklowitz DJ, Richards JA, Saleem R, George EL, 1999. Bipolar disorder and family communication: Effects of a psychoeducational treatment program. J Abnorm Psychol 108, 588–597. [DOI] [PubMed] [Google Scholar]
- Simons AD, Marti CN, Rohde P, Lewis CC, Curry J, March J, 2012. Does homework “matter” in cognitive behavioral therapy for adolescent depression? J Cogn Psychoth 26 (4), 390–404. [Google Scholar]
- Tompson MC, Sugar CA, Langer DA, Asarnow JR, 2017. A randomized clinical trial comparing family-focused treatment and individual supportive therapy for depression in childhood and early adolescence. J Am Acad Child Adolesc Psychiatry 56 (6), 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub MJ, Schneck CD, Axelson DA, Kowatch R, Birmaher B, Miklowitz DJ, 2020. Classifying mood symptom trajectories in adolescents with bipolar disorder J Am Acad Child Adolesc Psychiatry, 59(3), 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: Reliability, validity, and sensitivity. Br J Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL, 2008. Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. J Clin Psychiatry 69 (5), 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]


