Abstract
Cytomegalovirus (CMV) infection is linked to acceleration of solid organ transplant vascular sclerosis (TVS) and chronic rejection (CR). Donor latent CMV infection increases cardiac-resident macrophages and T cells leading to increased inflammation, promoting allograft rejection. To investigate the role of cardiac-resident passenger macrophages in CMV-mediated TVS/CR, macrophages were depleted from latently ratCMV (RCMV)-infected donor allografts prior to transplantation. Latently RCMV-infected donor F344 rats were treated with clodronate, PBS, or control liposomes 3 days prior to cardiac transplant into RCMV-naïve Lewis recipients. Clodronate treatment significantly increased graft survival from post operative day (POD)61 to POD84 and decreased TVS at rejection. To determine the kinetics of the effect of clodronate treatment’s effect, a time study revealed that clodronate treatment significantly decreased macrophage infiltration into allograft tissues as early as POD14; altered allograft cytokine/chemokine protein levels, fibrosis development, and inflammatory gene expression profiles. These findings support our hypothesis that increased graft survival due to allograft passenger macrophage depletion was in part a result of altered immune response kinetics. Depletion of donor macrophages prior to transplant is a strategy to modulate allograft rejection and reduce TVS in the setting of CMV+ donors transplanted into CMV naïve recipients.
Keywords: Cytomegalovirus, Chronic Rejection, Transplant Vascular Sclerosis, Latent Infection
Introduction
Solid organ transplantation (SOT), the only viable treatment option for organ failure, is challenged by a shortage of donor organs and the risk of both acute and chronic graft rejection. Despite improvements in surgical techniques, perioperative patient care and immunosuppressive medications, transplant vascular sclerosis (TVS), the vascular lesion associated with cardiac allograft chronic rejection (CR) remains a major cause for allograft failure. A major risk factor associated with accelerated allograft rejection is infection with human cytomegalovirus (HCMV). HCMV is linked to development of vascular disease and CR in heart, kidney and small bowel transplants1. HCMV is a nearly ubiquitous β-herpesvirus that typically results in an asymptomatic, lifelong latent infection. A complex interplay exists between the host immune system and virus that promotes a state of low-level persistence. In SOT, chronic inflammation and immunosuppression reactivate latent HCMV causing an active infection providing an ideal environment for HCMV disease. SOT recipients at highest risk of CMV disease are those without previous immunity who receive a CMV+ donor organ. Treatment of SOT patients with antivirals, such as ganciclovir, delays allograft rejection and reduces CMV disease2-5. However, ganciclovir treatment must be monitored for drug side effects and development of resistance. Thus, due to the ubiquitous nature and high association with allograft rejection, HCMV continues to play a significant role in allograft and recipient survival.
Cardiac transplantation of ratCMV (RCMV) acutely infected donor allografts into naïve recipients shares many of the hallmarks of human transplant graft vascular disease and consequent rejection5-8. Ganciclovir treatment of acutely-infected recipients increases allograft survival and decreases TVS. However, in recipients of latently-infected donor allografts (LDI), treatment of donors with ganciclovir does not impact TVS disease and CR indicating that RCMV infection predisposes donor organs to accelerated rejection5. RCMV induces the formation of clusters of macrophages and T cells that are rarely observed in uninfected hearts5. We hypothesize that donor passenger leukocytes contribute to accelerated rejection by acting as a scaffold for aggressive immune activation following transplantation5,9. Passenger macrophages and dendritic cells are considered primary leukocytes involved in SOT rejection by presenting antigen to recipient alloreactive T cells10-13. In a clinical study, macrophage graft infiltration was associated with acute renal allograft dysfunction and rejection14. Cytokines that drive immune and inflammatory activation such as IL-1β, IL-12, IL-18, TNF-α, and INF-γ are secreted by graft resident macrophages, which mediate immune and inflammatory events promoting rejection15. In addition, macrophage cytokine production in renal transplants stimulates activation of epithelial cells, generation of cytotoxic T cells, and production of CSF-1 and CCL216. Additionally, macrophage-derived growth factors contribute to intimal fibrosis and TVS17. Therefore, the ability of latent CMV to induce leuckocyte aggregate formation in the allograft prior to transplantation5 suggests virus-mediated donor-derived passenger leukocytes impact graft survival.
Depleting passenger leukocytes from donor tissues reduces acute rejection episodes through altering the graft immune environment10,18. Similarly, depletion of macrophages from mouse donor lungs prior to transplantation reduces graft damage19. In addition, coronary allograft vasculopathy elicited by Lymphochriomeningitis virus can be prevented through depletion of NK cells 20. These studies indicate a potential positive therapeutic impact on graft outcomes associated with a reduction in passenger leukocytes. While we have demonstrated accumulation of T cells and macrophages in heart grafts following RCMV infection, evidence for a direct link between RCMV-induced passenger leukocytes and accelerated TVS has been lacking. Clodronate is a bisphosphonate that when delivered in liposomes can deplete macrophages by acting as a non-hydrolyzable form of ATP. Herein, we depleted macrophages using clodronate-laden liposomes in RCMV-latently infected donors prior to cardiac transplantation into RCMV-naïve recipients. Donor macrophage depletion decreased graft TVS, prolonged graft survival and skewed allograft cytokines and chemokines profiles towards a reduced inflammatory state. These findings provide insight into the use of targeted donor treatments as a strategy to improve outcomes in recipients with CMV-mediated rejection.
Materials and Methods
Heart Transplantation
Animal experiments were performed in accordance with IACUC protocols in the OHSU/VGTI small animal facility, which is AAALAC-accredited. F344 donor rats (Charles River) were infected with 1x105 pfu RCMVMaastricht seven to nine months before organ procuremen, in order to achieve latent infectiont5. Three days prior to transplant, donor rats were treated with 3ml of phosphate buffered saline (PBS) control liposomes (10ml/kg, Liposoma), or clodronate liposomes (10ml/kg, Liposoma) by tail vein infusion. Lewis rats (Charles River) underwent heterotopic cardiac transplant with post-op cyclosporine A treatment (5mg/kg/day; Novartis) for 10 days to prevent acute rejection5-8,21. Animals were examined daily and level of graft rejection was assessed by monitoring heartbeat grade5-8,21. Blood was drawn on post-operative day (POD)14, 28, 42, and 56. Macrophage depletion was assessed in donor heart, spleen and peripheral blood collected from a subgroup of animals at the time of transplantation. Cohort1 (n=10 for each group) was harvested at CR. Cohort2 was harvested on POD7, 14, 21, 28 (n=5 per group). Heart (allograft and native), spleen, and salivary glands were harvested for immunohistochemistry (IHC); preserved in RNAlater solution (ThermoFisher); and stored at −80°C. Paraffin embedded tissues were stained with hematoxylin and eosin, elastic von Geison, or Trichrome5-8,22. The degree of TVS was calculated as neointimal index (NI=intima area/lumen+intima areax100)23,24 in ≥10 graft vessels8,24. TVS and time to rejection were compared between groups using ANOVA with post hoc Tukey test. NI index and time to CR were compared between all groups using Student’s t-test.
Flow Cytometry
Tissues collected from animals prior to transplantation were processed as previously described5. Phenotypic analysis was performed using fluorescently labeled antibodies directed against CD3 (1F4-APC, BD-Biosciences), CD4 (W3/25-APC-Cy7, Biolegend), CD8a (OX-8-PerCP, BD-Bioscience), CD45RA (OX-33-PE, BD-Biosciences), CD68 (ED1-FITC, BioRad), and CD172a (OX-41-PE, BioLegend). Cells from three hearts were pooled and stained. For blood and spleen, 2x106 cells were stained. Data was acquired using a BD-LSRII Flow Cytometer and analyzed with FlowJo software (Tree Star).
Immunohistochemistry
Embedded heart tissues were cut 4μm thick in series on glass slides and stained using a 3-layer indirect immunoperoxidase technique with primary antibodies directed against CD4 (BioRad), CD8a (ThermoFisher), and CD68 (BioRad) followed by addition of peroxidase-conjugated rabbit anti-mouse IgG (DAKO A/S) and peroxidase-conjugated goat anti-rabbit IgG (ThermoFisher) using a MAB kit (Dako), and counterstained with H&E for visualization by microscopy.
qRT-PCR detection of RCMV
Total DNA was isolated from graft heart and spleen using DNAzol5. DNA (0.5μg) was analyzed by qRT-PCR using a primer and probe set recognizing the RCMV DNA polymerase sequence with a sensitivity of <100 copies8,21.
RCMV-Specific Enzyme-Linked Immunosorbent Assay (ELISA)
Corning 96-well high-binding plates were coated with RCMV-infected cell lysates (5μg/ml), washed with PBS containing 0.05% Tween (wash buffer) and blocked in 2% milk. Six serial two-fold serum dilutions were incubated for 2hrs followed by three washes. ELISA plates were incubated with horseradish peroxidase-conjugated goat anti-rat IgG (Rockland) for 1hr and then washed 3x followed by addition of chromogen OPD substrate (Life Technologies) to quantify bound antibodies using a Synergy HTX Microplate Reader (BioTek; 490nm). Antibody titers were determined using Log-to-Log transformation and the results were analyzed using GraphPad Prism-v67.
Cytokine Multiplex Assay
A rat cytokine multiplex assay (Millipore Sigma) was used to quantify 27 factors in homogenized donor heart allografts and spleens. A portion of snap frozen tissue section was homogenized in PBS with 2mm glass beads (Propper Manufacturing) using a Precellys-24 homgenizer. Samples were centrigued at 10,000 rpm (9,300xg) at 4°C for 10 minutes, resulting supernatants were collected and analyzed as per manufacturer’s instructions. Cytokines were quantified using a Luminex 200™ Detection system, and the data was analyzed using GraphPad Prism-v6.
Transcriptome Analysis
RNASeq was used to profile native and graft heart gene transcription at POD14 from recipients of latently infected allografts treated with clodronate or control liposomes. Illumina TruSeq Stranded mRNA Library Prep Kit (RS-122-2101, Illumina) was used to label 1μg of mRNA extracted using Trizol. The library was validated using Aginlent DNA 1000 kit on a bioanalyzer and quantified by RT-PCR (Kapa Biosystems) on a StepOnePlus. Samples were sequenced by the OHSU Massively Parallel Sequencing Core using an Illumina HiSeq-2500. Biostatistical analysis was performed by the ONPRC Biostatistics and Bioinformatics Core. Sequences were evaluated using FastQC combined with MultiQC, adaptor sequence trimmed and aligned to the most recent rat genome. The number of reads per gene were calculated. The basic Wald test within DESeq2 was used to identify differentially expressed genes with a FDR<0.1 and a fold change >1.5. Pathway analysis of the differentially regulated genes was performed using Qiagen’s Ingenuity Pathway Analysis (IPA).
Results
Macrophage Depletion Using Clodronate in RCMV Latently Infected Rats
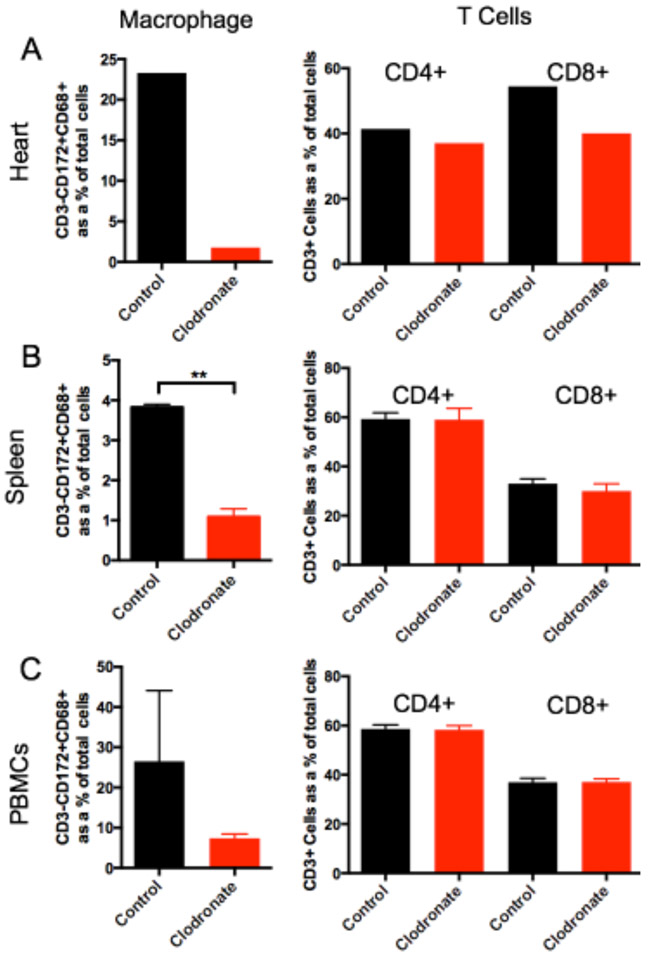
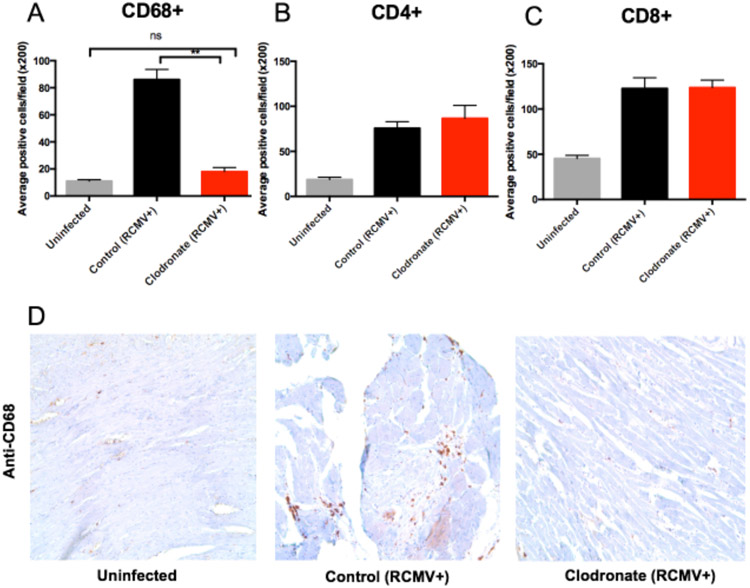
Latent donor CMV infection increases cardiac tissue resident macrophages and T cells5 that have the potential to increase allograft inflammation contributing to TVS/CR. To investigate the role of passenger macrophages in CMV-mediated TVS, macrophages were depleted from RCMV latently infected donors (LDI) at 3 days prior to transplantation by treatment with clodronate-loaded liposomes (clodronate) via intravenous infusion (Figure 1). Clodronate loaded liposomes are endocytosed by macrophages to produce a non-hydrolyzable ATP analog that initiates apoptosis25. At three days after treatment, flow cytometry and IHC were performed to analyze leukocyte populations in hearts, spleens, and blood from LDI F344 rats. Clodronate systemically depleted macrophages (CD3−CD172+CD68+) in the heart, spleen, and peripheral blood without affecting the frequency of CD4+ and CD8+ T cells (Figure 2). Macrophages were significantly decreased in CD68+ cardiac tissue cells from clodronate treated animals compared to controls (Figure 3; p=0.0001), with nearly equivalent CD68+ cell numbers in RCMV+ clodronate treated and RCMV naïve, untreated animals. These data confirm treatment-related selective macrophage depletion in hearts from RCMV-latently infected rat donors.
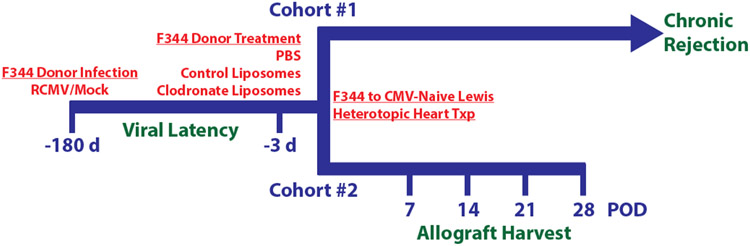
Figure 1. Experimental design.
F344 donor rats were latently infected with RCMV at least 180 days prior to transplant with 1x105 pfu/rat salivary gland-derived RCMV or mock infected with PBS. At three days prior to transplant, donor rats were treated by intravenous infusion with PBS, control liposomes, or clodronate liposomes (10mL/kg). On experimental day 0, RCMV naïve recipient Lewis rats underwent heterotopic heart transplant. Cohort #1 (n=10 per treatment group) were monitored daily for signs of rejection by heartbeat palpation and harvested at the time of chronic rejection. Blood was drawn on post-operative days (POD) 14, 28, 42, and 56. A subgroup of donor heart, spleen, and PBMC samples were collected at day −3 from Cohort #1 animals to confirm macrophage depletion by clodronate using flow cytometry. Cohort #2 recipients were harvested at POD 7, 14, 21, and 28 (n=5 per time point for each experimental group) to monitor the effect of clodronate treatment over time. At CR (Cohort #1) or predetermined time points (Cohort #2), hearts (graft and native) spleen, SMG, and blood were collected for analysis.
Figure 2. Clodronate treatment depletes macrophages but not T cells from LDI organs and blood.
To confirm macrophage depletion by clodronate liposome treatment, RCMV latently-infected donor F344 rats were treated with control liposomes (n=3, black) or clodronate liposomes (n=3, red). At three days post treatment, tissues were harvested and leukocytes were prepared from heart, spleen, and peripheral blood (PBMC). The frequency of CD4+ and CD8+ T cells and macrophages in each of these samples was quantified via flow cytometry by cell surface staining using fluorescent antibodies directed against CD172 (myeloid lineage cells), CD68 (macrophage), CD3 (T cells), CD4 (CD4+ T cells), and CD8 (CD8+ T cells). Depicted is the frequency of macrophages (CD3−CD172+CD68+) and CD4 (CD3+CD4+) and CD8 (CD3+CD8+) cells isolated from the heart (a), spleen (b), and PBMCs (c). **p≤0.01 as determined by Student’s t-test.
Figure 3. Clodronate treatment reduces allograft tissue macrophage levels to those observed in uninfected hearts.
Heart tissue sections from F344 rats that were either uninfected (gray bars), latently RCMV infected and treated with control liposomes (black bars) or latently RCMV infected and treated with clodronate liposome (red bars) were stained with antibodies directed against CD68 (macrophages) (a, d), CD4+ T cells (b) and CD8+ T cells (c). Immunohistochemical analysis was done in a blinded review and scored as follows: 0, no visible staining; 1, faint staining; 2, moderate intensity with multifocal staining; and 3, intense diffuse staining. CD4, CD8 and CD68 positively stained cells with scores 2 and 3 were counted in 10 fields from whole heart tissues at 200x magnification or in 6 fields from the coronary arteries at 400x magnification; and the mean number of positive cells per field was calculated. **p≤0.01 as determined by a One-way ANOVA with Tukey’s secondary testing.
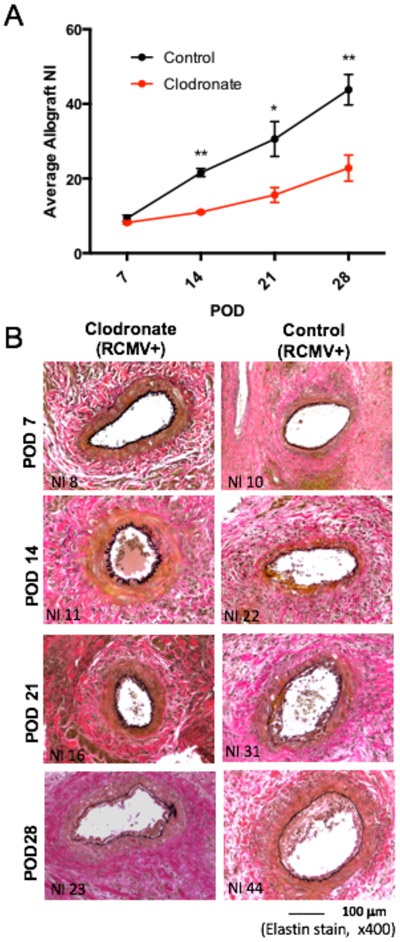
Donor Macrophage Depletion Delays CR
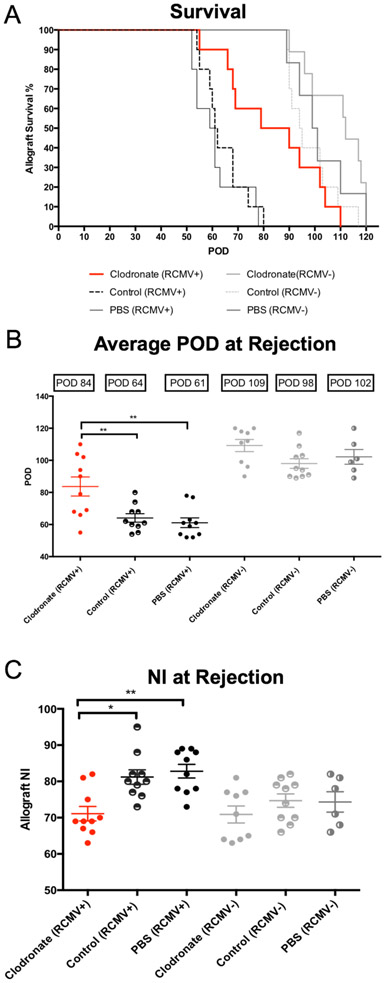
Macrophages were depleted from RCMV LDI 3 days prior to heterotopic transplantation into RCMV naïve Lewis rats. Transplanted rats were monitored daily for signs of graft failure5,6,21. At the time of CR (heartbeat grade=0-1), animals were euthanized; hearts and other tissues were collected for analysis. Clodronate treatment of LDI allografts increased the mean time to rejection (POD84) compared to recipients of LDI donor hearts treated with either control liposomes (POD64; p=0.008) or PBS (POD61; p=0.0015). This time to CR was similar to that of recipients of CMV-uninfected donor allografts treated with control liposomes (POD98), PBS (POD102), and clodronate (POD109) (Figure 4a-c). At rejection, TVS was significantly lower in LDI cardiac allografts treated with clodronate (NI=71.1) vs. controls (NI=81.2, p=0.01 and NI=82.80, p<0.001) (Figure 4c). There was no difference between the NI of clodronate treated LDI heart allografts and allografts from recipients of uninfected donor heart allografts from any treatment group (Figure 4c), further supporting the positive impact of macrophage depletion on allograft TVS and increased graft survival.
Figure 4. Clodronate liposome treatment increases the time to allograft CR and reduces TVS.
Heterotopic heart allografts from LDI F344 rats or uninfected F344 donors treated three days prior to organ donation with clodronate liposomes, control liposomes, or PBS were transplanted into RCMV naïve Lewis recipients (n=10 for each treatment group). Allograft recipients were monitored daily for graft rejection. Kaplein-Meier graft survival curves are depicted in Panel A, and the average time of rejection reported as POD is shown in Panel B. At rejection, sections of allograft heart were stained with H&E and elastin to determine the degree of vascular disease in the coronary arteries. Average NI (a measure of TVS) for each group is reported along with individual allograft values (Panel C). Error bars represent standard deviation for each group. *p≤0.05, **p≤0.01 as determined by a One-way ANOVA with Tukey’s secondary testing.
To determine the timing of the effect of depleting macrophages in LDI allografts on the development of graft TVS, LDI rats were treated with clodronate or control liposomes three days before heterotopic heart transplant into RCMV-naïve recipients euthanized at POD7, 14, 21, and 28 (n=5 each). Heart allografts from clodronate-treated donors had significantly lower mean NI compared to controls (POD14 11.1 vs. 21.6, p<0.01; POD21 15.6 vs. 30.6, p=0.01; POD28 22.8 vs. 43.8, p<0.01) (Figure 5a). Representative elastin stained allograft sections (Figure 5b) demonstrate a reduced neointimal thickening in clodronate treated donor allografts compared to control allografts. This finding indicates the effects of macrophage depletion impact graft survival as early as POD14, by a reduction in TVS.
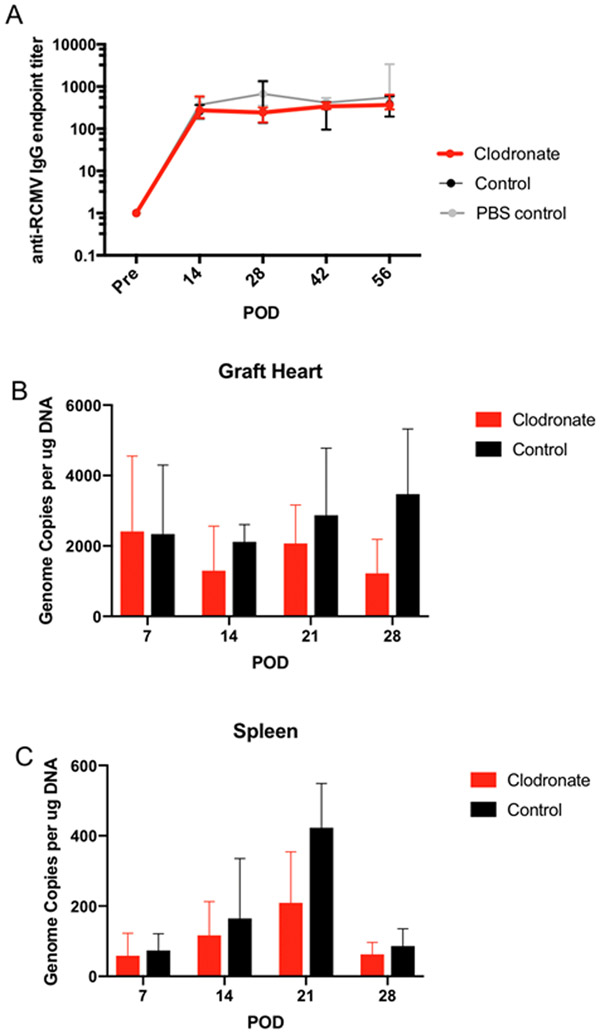
Figure 5. Macrophage depletion does not alter recipient infection status.
Allograft hearts and spleen samples were collected from Cohort#2 recipients on POD7, 14, 21, and 28 to compare RCMV levels in between clodronate and control liposome treated animals. (n=5 per group/time point). LDI F344 rats were treated with either control liposomes (black) or clodronate liposomes (red) three days prior to heterotopic heart allograft transplant into RCMV naïve Lewis recipients. Recipients (n=5 per group/time point) were euthanized on POD 7, 14, 21, and 28 at which time recipient heart allografts) and spleen were collected in DNAzol. Total DNA was prepared using the DNAzol method and viral DNA loads were determined by qRT-PCR using virus specific primers and probes. Genome copies per μg total RNA were graphed for both graft heart in Panel A and spleen in Panel B. In Panel C serum was isolated from recipient rats in Cohort #1 on POD0, 14, 28, 42, and 56. RCMV-specific antibodies were quantified by limiting dilution ELISA for serum samples using high-binding 96-well plates coated with RCMV-infected cell lysates. Serum titers for anti-RCMV IgG were compared between LDI allograft recipients in all three treatment groups (clodronate liposomes – red, control liposomes – black, and PBS control – grey) but did not differ in their peak titers or kinetics of formation.
Clodronate Reduces Tissue RCMV Loads
Macrophages represent a possible site of viral persistence in infected organs, we therefore evaluated whether clodronate treatment prevents viral reactivation from latently-infected donor hearts. Recipient RCMV-specific antibodies were quantified by virus-specific ELISA as a surrogate marker of viral reactivation in naïve recipients 26,27. All recipients of LDI allografts seroconverted by POD14 (Figure 6). No treatment-specific differences were detected in levels of anti-RCMV IgG titers or kinetics of antibody development between Cohort 1 recipients (Figure 6). Next, we quantified viral loads in tissues collected at POD7, 14, 21, and 28 (n=5). RCMV levels were reduced in clodronate-treated allografts compared to controls (Figure 6) with the largest difference at POD28. In recipient spleens, RCMV loads peaked at POD21 with a trend towards decreased levels in recipients of clodronate treated allografts (Figure 6).
Figure 6. Transplant vascular sclerosis is decreased in clodronate-liposome treated LDI allografts.
TVS was quantified in Cohort #2 recipients to compare the degree of vascular disease present in allograft vessels between clodronate and control liposomes treated animals treated animals. LDI F344 rats were treated with either control liposomes (black) or clodronate liposomes (red) three days prior to heterotopic heart allograft transplant into RCMV naïve Lewis recipients. Recipients (n=5 per group/time point) were euthanized on POD 7, 14, 21, and 28 at which time rat tissues and heart allografts were collected for analysis. Embedded allograft heart tissues were sectioned and stained with H&E and elastin to visualize vessel neointimal formation. Panel A depicts graphical representation of TVS quantification as reported as the mean allograft NI. Images shown in Panel B shows representative stained graft heart tissue sections. Clodronate liposome treated allografts have reduced TVS relative to control liposome treated allografts starting at POD14 and persisting through POD28 *p≤0.05, **p≤0.01 as determined by Student’s t- test.
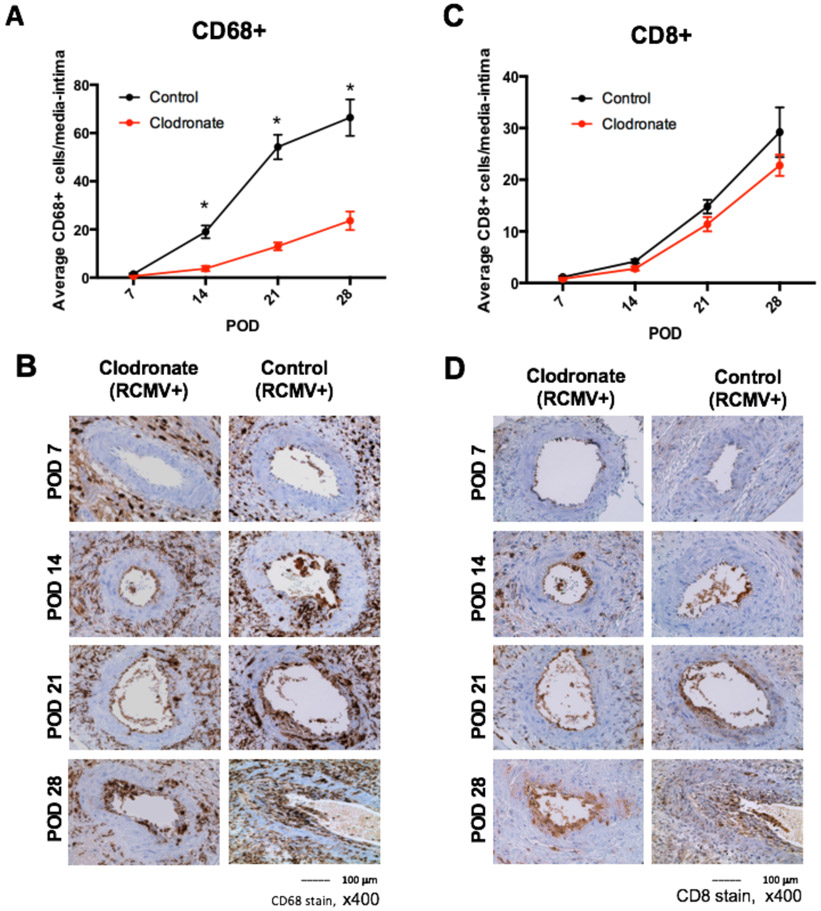
Clodronate Decreases Allograft Macrophage Infiltration
To determine whether donor clodronate treatment impacts the kinetics of immune cell graft infiltration, we evaluated graft tissue sections for macrophage and CD8+ T cell markers. Cell infiltrates were enumerated by microscopy and the average number of CD68+ or CD8+ positive cells in the allograft coronary artery tunica media and intima was calculated. There were significantly less infiltrating macrophages in allografts treated with clodronate (POD14 3.8 vs. 19, p<0.01; POD21 13 vs. 54.2, p<0.01; POD28 23.6 vs. 66.4, p<0.01) (Figure 7), which may explain the reduced levels of RCMV genomes present in allografts. In contrast, CD8+ Tcell levels did not significantly differ between clodronate and controls, and both showed increased T cell infiltration with time (Figure 7).
Figure 7. Macrophage and CD8+ T cell infiltration into the media/intima of allograft vessels is reduced in allografts derived from RCMV latently infected donors treated with clodronate.
Immunohistochemical staining for the presence of macrophages and T cells of heart allografts harvested from Cohort #2 recipients at POD7, 14, 21, and 28 (n=5 per group/time point). Embedded tissue sections were cut and stained using antibodies directed against CD68 (macrophages; panels a and b) and CD8+ T cells (panels B and C). The frequency of each cell population was measured over 10 serial tissue slides and averaged to determine the mean number of cells that infiltrated into the vessel wall media and intima. Results for clodronate liposome treated animals are depicted in red and control liposome treated animals are shown in black for panels A and C. Images are representative stained sections from each time point (panels B and D). *p≤0.05 as determined by Student’s t-test.
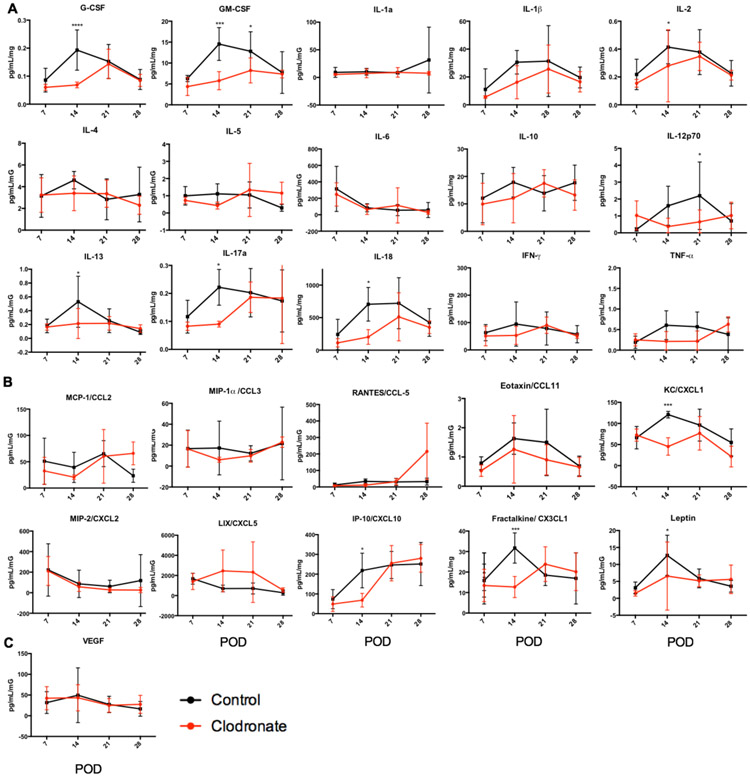
Clodronate Modulates Allograft Cytokine and Chemokine Profiles
Cytokines produced by allograft macrophages can influence downstream immune responses15,16. We determined whether LDI macrophage depletion affected the kinetics of allograft and spleen cytokine expression. Tissue homogenates were analyzed by cytokine multiplex assay. At POD7, levels of allograft cytokines were nearly equivalent between the two groups (Figure 8). POD14 is a critical time posttransplant as cytokine and chemokine levels dramatically increased but were significantly reduced in allografts from clodronate-treated LDI. These included the cytokines: G-CSF, GM-CSF, IL-1β, IL-2, IL-12, IL-13, IL-17a, IL-18, IFNγ, and TNFα; and chemokines: CCL2, CCL3, CXCL1, CXCL10, and CX3CL1 (Figure 8). By POD21 most allograft cytokine and chemokines peaked with levels decreasing at POD28. These results are in parallel with the effects of donor macrophage depletion on TVS/ time to CR (Figure 5), macrophage infiltration (Figure 7); and levels of allograft RCMV (Figure 6). Spleen cytokine levels were similar at POD7-14 for both macrophage depleted and control graft recipients, but levels for some peaked at POD28 (Supplemental Figure 1). There is a trend toward decreased levels of cytokines and chemokines in spleens from clodronate-treated LDI with signficant differences for IL-4 and CXCL10 (Supplemental Figure 1b). This finding correlates with timing of reduced RCMV levels in the spleen, supporting the potential importance of this later time point in control of RCMV infection following transplantation.
Figure 8. Macrophage depletion alters RCMV-infected allograft proinflammatory cytokine and chemokine profiles.
Rat allograft heart lysates from recipients in cohort 2 at POD 7, 14, 21, and 28 (n=5 per group/time point) were analyzed using a 27-plex cytokine magnetic bead assay to quantify the levels of cytokines (a), chemokines (b), and VEGF (c), clodronate liposome (red) and control liposome (black). *p≤0.05, **p≤0.01, ***p≤001, ****p<0.0001 as determined by Two-way ANOVA with Holm-Sidak correction for multiple comparisons.
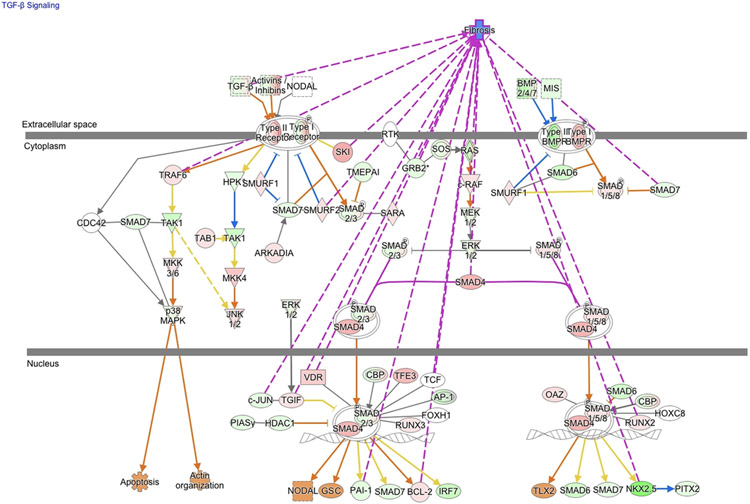
Macrophage Depletion Modifies Cardiac Allograft TGF-β Signaling Pathways and Attenuates Early Graft Fibrosis
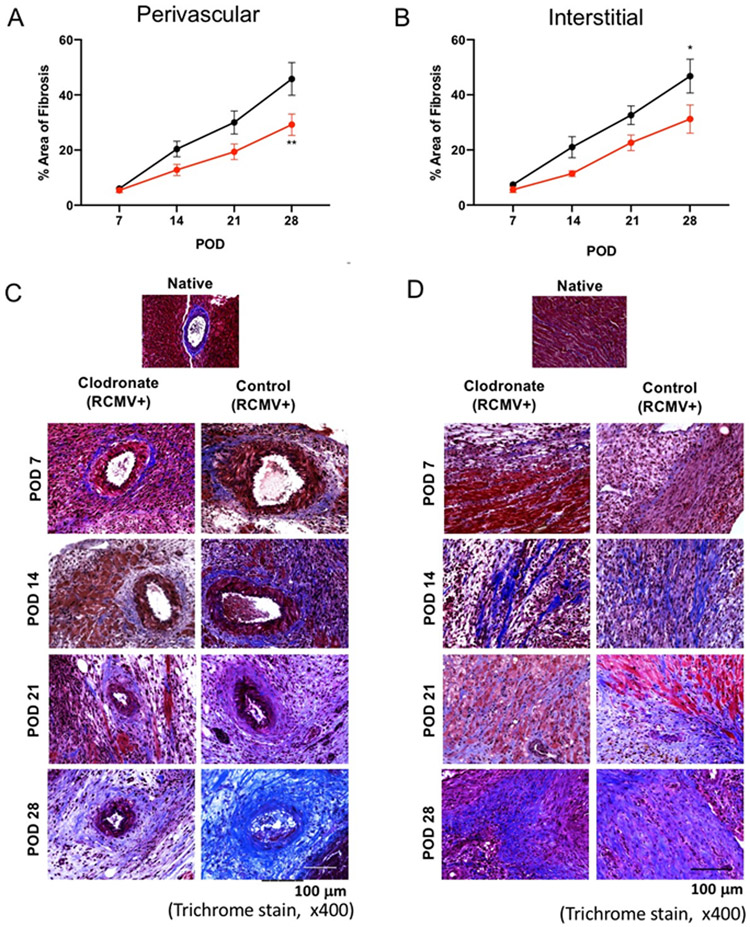
To identify the global changes elicited by donor macrophage depletion, we compared transcriptomes of allograft hearts from macrophage-depleted versus controls at POD14 using RNAseq. IPA was used to identify differentially regulated pathways, and this bioinformatic analysis identified 184 canonical pathways (p=0.05) containing differentially expressed genes (Supplemental Table 1). These pathways were broadly divided into those involved in immune response, cell signaling, tissue remodeling, and cardiovascular development. A more detailed analysis of the TGF-β pathway, a growth factor associated with CR through its chemotactic and profibrotic effects28, revealed a decrease in expression of many TGF-β pathway genes in allografts from clodronate-treated donors compared to controls (Figure 9). A hallmark of CR is interstial fibrosis and TVS29, including the accumulation of thick collagen fibers surrounding cardiomyocytes that are visible by Trichrome staining, as well as vascular intimal and medial thickening and vessel narrowing. The prolonged inflammatory response during TVS involves production of growth factors, proteolytic enzymes, angiogenic factiors, and cytokines that stimulate fibrosis. We hypothesized that the decrease in TGF-β signaling results in decreased tissue fibrosis and TVS. To determine the effects of donor macrophage depletion on the development of graft fibrosis and TVS, collagen deposition in perivascular and intersitial allograft regions was measured by trichrome staining at POD 7, 14, 21 and 28. Clodronate treatment attenuated the severity of perivascular (Figure 10a,b) and interstitial fibrosis (Figure 10c,d) starting at POD14 with significant differences by POD28. The decrease in TVS reflects a multistage process, that affects all components that promote allograft viability and adequate function. Our study revealed that depletion of passenger macrophages from LDI allografts reduces graft immune activation, tissue remodeling/fibrosis, TVS, and viral loads which leads to improved graft survival.
Figure 9. LDI donor macrophage depletion alters the fibrosis pathway normally activated at POD14.
Ingenuity pathway core analysis was used to identify downstream effects in cardiac graft samples following transplantation (Clodronate-liposomes vs. Control-liposomes). Statistically significant alterations in gene expression from RNAseq analysis of graft tissues are represented by labeled shapes depending on their molecular function. Expression and predicted activation levels determined by fold-change between Clodronate-liposomes vs. Control-liposomes are presented using the following convention on color scales with increasing saturation associated with an greater absolute fold-change value: red = increased detection in Clodronate-Liposome treated donor hearts; green = decreased detection; orange=predicted activation; pink= fibrosis associated genes. Predicted relationships are indicated by arrow-heads and blue lines to indicate predicted inhibition; yellow lines indicate inconsistent findings that make it difficult to predict outcomes of interactions.
Figure 10. LDI macrophage depletion reduces cardiac allograft fibrosis.
Heart allografts harvested from Cohort #2 recipients at POD7, 14, 21, and 28 (n=5 per group/time point) were embedded and stained with Masson’s trichrome to identify levels of cardiac tissue fibrosis (healthy areas are red and collagen-rich areas are blue). Perivascular and interstial fibrosis was measured over 6 random vields of view of 10 serial tissue slides at a magnification of x400. Values were averaged and adjusted to a tooal tissue area in the image anlayzed to determine the mean percent area with fibrosis as depicted in panels A and B. Results for clodronate liposome treated animals are depicted in red and control liposome treated animals are shown in black. Images are representative stained sections from each time pointValues are presented as the means ± standard error of the mean (SEM) of each group and were analyzed using 2way ANOVA followed by Sidak’s multiple comparison test *p≤0.05, **p≤0.01.
Discussion
CR remains a significant challenge for the long-term SOT graft survival, and transmission of donor CMV infection from donor organs leasds to accelerated rejection30. Within the allograft, CMV exacerbates both immune and fibrosis mechanisms creating a pro-TVS/CR environment by production of cytokines/chemokines, induction of adhesion molecule expression, monocyte/macrophage recruitment/activation, smooth muscle cell (SMC) proliferation, accumulation of inflammatory cells, ECM protein and lipid deposition in the intima culminating in intimal thickening, graft vascular narrowing and CR31,32. We hypothesize that latent CMV infection increases donor passenger macrophages that produce cytokines/chemokines and facilitate alloantigen presentation and fibrosis that, in aggregate, promotes accelerated graft rejection. By selectively depleting macrophages from latently infected donor hearts, the immune and inflammatory responses are dampened as is the development of TVS. Depletion of donor macrophages three days before transplantation, led to increased cardiac allograft survival, decreased TVS and a delay in CR. The concurrent reduction in RCMV levels, inflammatory response, and fibrosis improved allograft survival.
Macrophages contribute to TVS through their ability to modulate alloimmune processes including antigen presentation, immune cell stimulation, cytokine/chemokine production, and tissue repair. In our study, clodronate liposome-mediated passenger macrophage depletion from RCMV-latently infected donors three days before transplantation into RCMV-naïve recipients prolonged time to CR and decreased allograft TVS. While depletion of macrophages by clodronate liposomes from recipients after liver, kidney and corneal transplantation also increases allograft survival and/or alters the alloantigen immune response33-35, our study uniquely shows effectiveness of depletion prior to transplantation. Donor passenger macrophages can promote inflammation by releasing factors that influence immune cell functions, SMC proliferation, and fibrosis. Depletion of donor resident macrophages prior to transplantation decreased the inflammatory response within the allograft with a key time point of difference occurring at POD14, correlating with the reproducible cytokine peak in rat cardiac allografts. The cytokine peak at POD14 in control animals may be in part due to increased RCMV replication, that is decreased with clodronate treatment. At POD 28, cyctokine/chemokine levels were also significantly decreased in the spleen of recipients of allografts from clodronate treated donors compared to controls indicating a systemic anti-inflammatory effect. Importantly, during allograft rejection increasing levels of CXCL1, CXCL10 and CX3CL1 in cardiac allografts regulate recruitment of activated T cells, natural killer (NK) cells, and macrophages36-40. In humans, CX3CL1 is a marker for early renal graft rejection41 and high expression of CXCL10 correlates with CR in lung transplants42. IL-18 and IL-1β, macrophage-derived cytokines, promote proinflammatory responses through NF-κB, ultimately driving pro-rejection cytokine production43-45. In conjunction with IL-12, IL-18 helps to enhance the Th1 responses, including T cell maturation and cytotoxicity46,47. In RCMV-infected allografts, the increased immune cell recruitment caused by expression of these cytokines/chemokines at POD14 may contribute to the alloantigen immune response ultimately increasing TVS and decreasing allograft survival. Our study suggest that manipulation of the immune response in donor tissues prior to transplant is a viable option to limit early inflammatory responses and improve transplant outcomes.
In transplant allograft, the degree of fibrosis correlates with macrophage infiltration48. In mouse models of kidney injury, macrophage depletion with clodronate reduced the development of fibrosis49. We observed a similar decrease in the development of both perivascular and intersitial fibrosis in allografts from clodronate treated donors versus controls. T cell produced TGF-β activates macrophages and induces cardiac myofibroblast differentiation, while other growth factors such as FGF, PDGF, and CTGF act as highly fibrogenic factors50,51. Upstream, growth factors are, in part, controlled by the cytokines IL-6, IL-1β and TNF-α29. The importance of these cytokines and growth factors in fibrosis and allograft TVS/CR is supported by animal models of CR lacking these factors, which display improved transplant outcomes29. Gene expression analysis of cardiac allografts from donors treated with clodronate liposomes showed decreased levels of genes in the TGF- β signaling pathway and fibrosis. There was a decrease in the expression of Smad2,3,6, and 7 that are involved in TGF-β signaling in vSMC52. Activation of the Smad pathway has been described for several diseases associated with fibrosis53. TGF-β1 increases phosphorylation of Smad2/3 and trimer formation with Smad454 that promote transcription of genes involved in vascular fibrosis such as as fibronectin, type-1 collagen, and CTGF52,55,56. Overexpression of Smad6 correlates with increased TGF-β levels57. TGF-β signaling intersects with other major pathways including the MAPK pathway and small G protein signaling, with some components mediating the Smad pathway. In the current study, clodronate treatment decreased the expression of genes involved in Smad signaling and fibrosis, providing an additional potential mechanism by which clodronate treatment increases cardiac allograft survival.
Recent studies have demonstrated the impact of optimizing donors prior to organ procurement58. Our study suggests that if CMV-positive donors were treated with clodronate-laden liposomes prior to organ procurement, many of the CMV-mediated effects on TVS might be improved. In this way, we could impact recipient outcomes solely through the short-term treatment of donor organs. Future studies will focus on identifying the specific immune pathways affected by clodronate treatment and the direct effect that this intervention has on immune cell frequencies in cardiac and other SOT allografts. Ultimately, given our new age of machine perfusion59, treatment of donor organs prior to transplantation has potential to improve long-term graft durability.
Supplementary Material
Supplemental Table 1. Top canonical pathways identified by IPA at POD 14 by comparison of control liposome to clondronate liposome treated allografts.
Supplemental Figure 1. Macrophage depletion alters recipient spleen cytokine and chemokine profiles. Rat spleen lysates from recipients in cohort 2 at POD 7, 14, 21, and 28 (n=5 per group/time point) were analyzed using a 27-plex cytokine magnetic bead assay to quantify the levels of cytokines (a), chemokines (b), and VEGF (c), clodronate liposome (red) and control liposome (black). *p≤0.05, **p≤0.01, ****p<0.0001 as determined by Two-way ANOVA with Holm-Sidak correction for multiple comparisons.
Acknowledgements
This work was supported by a grant from the National Institutes of Health NIAID R01 AI116633. NH and IJ were supported by the OHSU Medical Microbiology and Immunology Host/Pathogen training grant NIH 2T32 AI007472.
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
References:
- 1.Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infection & chemotherapy. 2013;45(3):260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodson EM, Barclay PG, Craig JC, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2005(4):Cd003774. [DOI] [PubMed] [Google Scholar]
- 3.Merigan TC, Renlund DG, Keay S, et al. A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. N Engl J Med. 1992;326(18):1182–1186. [DOI] [PubMed] [Google Scholar]
- 4.Valentine VG, Weill D, Gupta MR, et al. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27(8):875–881. [DOI] [PubMed] [Google Scholar]
- 5.Orloff SL, Hwee YK, Kreklywich C, et al. Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naive recipients. Am J Transplant. 2011;11(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orloff SL, Streblow DN, Soderberg-Naucler C, et al. Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation. 2002;73(5):679–688. [DOI] [PubMed] [Google Scholar]
- 7.Streblow DN, Hwee YK, Kreklywich CN, et al. Rat Cytomegalovirus Vaccine Prevents Accelerated Chronic Rejection in CMV-Naive Recipients of Infected Donor Allograft Hearts. Am J Transplant. 2015;15(7):1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streblow DN, Kreklywich C, Yin Q, et al. Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J Virol. 2003;77(3):2182–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasr IW, Reel M, Oberbarnscheidt MH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7(5):1071–1079. [DOI] [PubMed] [Google Scholar]
- 10.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. The Journal of experimental medicine. 1982;155(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(12):3864–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung JJ, Zeevi A, Kaufman C, et al. Interactions between bronchoalveolar lymphocytes and macrophages in heart-lung transplant recipients. Human immunology. 1985;14(3):287–294. [DOI] [PubMed] [Google Scholar]
- 13.Paradis K, Sharp HL, Vallera DA, Blazar BR. Kupffer cell engraftment across the major histocompatibility barrier in mice: bone marrow origin, class II antigen expression, and antigen-presenting capacity. Journal of pediatric gastroenterology and nutrition. 1990;11(4):525–533. [DOI] [PubMed] [Google Scholar]
- 14.Girlanda R, Kleiner DE, Duan Z, et al. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8(3):600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Tian W, Sung YK, Qian J, Nicolls MR. Macrophages in solid organ transplantation. Vasc Cell. 2014;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadban SJ, Wu H, Hughes J. Macrophages and kidney transplantation. Semin Nephrol. 2010;30(3):278–289. [DOI] [PubMed] [Google Scholar]
- 17.Kitchens WH, Chase CM, Uehara S, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7(12):2675–2682. [DOI] [PubMed] [Google Scholar]
- 18.Sekine Y, Bowen LK, Heidler KM, et al. Role of passenger leukocytes in allograft rejection: effect of depletion of donor alveolar macrophages on the local production of TNF-alpha, T helper 1/T helper 2 cytokines, IgG subclasses, and pathology in a rat model of lung transplantation. J Immunol. 1997;159(8):4084–4093. [PubMed] [Google Scholar]
- 19.Tsushima Y, Jang JH, Yamada Y, et al. The depletion of donor macrophages reduces ischaemia-reperfusion injury after mouse lung transplantation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2014;45(4):703–709. [DOI] [PubMed] [Google Scholar]
- 20.Graham JA, Wilkinson RA, Hirohashi T, et al. Viral infection induces de novo lesions of coronary allograft vasculopathy through a natural killer cell-dependent pathway. Am J Transplant. 2009;9(11):2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streblow DN, Kreklywich CN, Smith P, et al. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am J Transplant. 2005;5(3):436–442. [DOI] [PubMed] [Google Scholar]
- 22.Streblow DN, Kreklywich CN, Andoh T, et al. The role of angiogenic and wound repair factors during CMV-accelerated transplant vascular sclerosis in rat cardiac transplants. Am J Transplant. 2008;8(2):277–287. [DOI] [PubMed] [Google Scholar]
- 23.Streblow DN, Kreklywich C, Yin Q, et al. Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. Journal of virology. 2003;77(3):2182–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation. 1997;63(7):941–947. [DOI] [PubMed] [Google Scholar]
- 25.Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12(9):1358–1367. [DOI] [PubMed] [Google Scholar]
- 26.Dollard SC, Keyserling H, Radford K, et al. Cytomegalovirus viral and antibody correlates in young children. BMC Res Notes. 2014;7:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iglesias-Escudero M, Moro-Garcia MA, Marcos-Fernandez R, et al. Levels of anti-CMV antibodies are modulated by the frequency and intensity of virus reactivations in kidney transplant patients. PLoS One. 2018;13(4):e0194789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. [DOI] [PubMed] [Google Scholar]
- 29.Booth AJ, Bishop DK. TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection. Immunotherapy. 2010;2(4):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald JT, Gallay B, Taranto SE, et al. Pretransplant recipient cytomegalovirus seropositivity and hemodialysis are associated with decreased renal allograft and patient survival. Transplantation. 2004;77(9):1405–1411. [DOI] [PubMed] [Google Scholar]
- 31.Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS. Allograft Vasculopathy: The Achilles' Heel of Heart Transplantation. J Am Coll Cardiol. 2016;68(1):80–91. [DOI] [PubMed] [Google Scholar]
- 32.Delgado JF, Reyne AG, de Dios S, et al. Influence of cytomegalovirus infection in the development of cardiac allograft vasculopathy after heart transplantation. J Heart Lung Transplant. 2015;34(8):1112–1119. [DOI] [PubMed] [Google Scholar]
- 33.Horne PH, Zimmerer JM, Fisher MG, et al. Critical role of effector macrophages in mediating CD4-dependent alloimmune injury of transplanted liver parenchymal cells. J Immunol. 2008;181(2):1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jose MD, Le Meur Y, Atkins RC, Chadban SJ. Blockade of macrophage colony-stimulating factor reduces macrophage proliferation and accumulation in renal allograft rejection. Am J Transplant. 2003;3(3):294–300. [DOI] [PubMed] [Google Scholar]
- 35.Slegers TP, Torres PF, Broersma L, van Rooijen N, van Rij G, van der Gaag R. Effect of macrophage depletion on immune effector mechanisms during corneal allograft rejection in rats. Invest Ophthalmol Vis Sci. 2000;41(8):2239–2247. [PubMed] [Google Scholar]
- 36.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Molecular interventions. 2010;10(5):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu N, Hossain M, Liu L. Pharmacological inhibition of p38 mitogen-activated protein kinases affects KC/CXCL1-induced intraluminal crawling, transendothelial migration, and chemotaxis of neutrophils in vivo. Mediators of inflammation. 2013;2013:290565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu K, Mitchell RN. The role of chemokines in transplant graft arterial disease. Arterioscler Thromb Vasc Biol. 2008;28(11):1937–1949. [DOI] [PubMed] [Google Scholar]
- 39.Yun JJ, Fischbein MP, Laks H, et al. Early and late chemokine production correlates with cellular recruitment in cardiac allograft vasculopathy. Transplantation. 2000;69(12):2515–2524. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Chen W, Wu C, et al. Macrophage/monocyte-specific deletion of Ras homolog gene family member A (RhoA) downregulates fractalkine receptor and inhibits chronic rejection of mouse cardiac allografts. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2017;36(3):340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Liu YF, Su ZX, Shi LP, Chen YH. Serum fractalkine and interferon-gamma inducible protein-10 concentrations are early detection markers for acute renal allograft rejection. Transplant Proc. 2014;46(5):1420–1425. [DOI] [PubMed] [Google Scholar]
- 42.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. [DOI] [PubMed] [Google Scholar]
- 43.Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162(2):1096–1100. [PubMed] [Google Scholar]
- 44.Matsumoto S, Tsuji-Takayama K, Aizawa Y, et al. Interleukin-18 activates NF-kappaB in murine T helper type 1 cells. Biochemical and biophysical research communications. 1997;234(2):454–457. [DOI] [PubMed] [Google Scholar]
- 45.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101(3):711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Micallef MJ, Ohtsuki T, Kohno K, et al. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26(7):1647–1651. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimoto T, Takeda K, Tanaka T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161(7):3400–3407. [PubMed] [Google Scholar]
- 48.Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69(7):1189–1197. [DOI] [PubMed] [Google Scholar]
- 49.Kitamoto K, Machida Y, Uchida J, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci. 2009;111(3):285–292. [DOI] [PubMed] [Google Scholar]
- 50.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989–997. [DOI] [PubMed] [Google Scholar]
- 51.Pradere JP, Kluwe J, De Minicis S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58(4):1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikedo H, Tamaki K, Ueda S, et al. Smad protein and TGF-beta signaling in vascular smooth muscle cells. Int J Mol Med. 2003;11(5):645–650. [PubMed] [Google Scholar]
- 53.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74(2):196–206. [DOI] [PubMed] [Google Scholar]
- 54.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–2810. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Huang XR, Canlas E, et al. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res. 2006;98(8):1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111(19):2509–2517. [DOI] [PubMed] [Google Scholar]
- 57.Berg DT, Myers LJ, Richardson MA, Sandusky G, Grinnell BW. Smad6s regulates plasminogen activator inhibitor-1 through a protein kinase C-beta-dependent up-regulation of transforming growth factor-beta. J Biol Chem. 2005;280(15):14943–14947. [DOI] [PubMed] [Google Scholar]
- 58.Niemann CU, Feiner J, Swain S, et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med. 2015;373(5):405–414. [DOI] [PubMed] [Google Scholar]
- 59.Salehi S, Tran K, Grayson WL. Advances in Perfusion Systems for Solid Organ Preservation. Yale J Biol Med. 2018;91(3):301–312. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Top canonical pathways identified by IPA at POD 14 by comparison of control liposome to clondronate liposome treated allografts.
Supplemental Figure 1. Macrophage depletion alters recipient spleen cytokine and chemokine profiles. Rat spleen lysates from recipients in cohort 2 at POD 7, 14, 21, and 28 (n=5 per group/time point) were analyzed using a 27-plex cytokine magnetic bead assay to quantify the levels of cytokines (a), chemokines (b), and VEGF (c), clodronate liposome (red) and control liposome (black). *p≤0.05, **p≤0.01, ****p<0.0001 as determined by Two-way ANOVA with Holm-Sidak correction for multiple comparisons.