Abstract
Pericytes apposed to the capillary endothelium are known to stabilize and promote endothelial integrity. Recent studies indicate that lung pericytes play a prominent role in lung physiology, and they are involved in the development of various lung diseases including lung injury in sepsis, pulmonary fibrosis, asthma, and pulmonary hypertension. Accordingly, human lung pericyte studies are important for understanding the mechanistic basis of lung physiology and pathophysiology; however, human lung pericytes can only be cultured for a few passages and no immortalized human lung pericyte cell line has been established so far. Thus, our study aims to establish an immortalized human lung pericyte cell line. Developed using SV40 large T antigen lentivirus, immortalized pericytes exhibit stable SV40T expression, sustained proliferation and have significantly higher telomerase activity compared to normal human lung pericytes. In addition, these cells retained pericyte characteristics, marked by similar morphology, and expression of pericyte cell surface markers such as PDGFRβ, NG2, CD44, CD146, CD90 and CD73. Furthermore, similar to that of primary pericytes, immortalized pericytes promoted endothelial cell tube formation and responded to different stimuli. Our previous data showed that friend leukemia virus integration 1 (Fli-1), a member of the ETS transcription factor family, is a key regulator that modulates inflammatory responses in mouse lung pericytes. We further demonstrated that Fli-1 regulates inflammatory responses in immortalized human lung pericytes. To summarize, we successfully established an immortalized human lung pericyte cell line, which serves as a promising tool for in vitro pericyte studies to understand human lung pericyte physiology and pathophysiology.
Keywords: human lung pericytes, immortalization, Fli-1, IL-6
Introduction
Pericytes, first characterized by Eberth and Rouget in the 1870s, are perivascular cells present at intervals along the walls of capillaries [1]. Pericytes are vascular smooth muscle lineage cells, and they wrap around microvascular endothelial cells (EC) [2]. They can be identified by the common markers platelet-derived growth factor receptorβ (PDGFRβ) and the proteoglycan neural glial antigen-2 (NG2) which is a co-receptor for PDGF [1, 3]. Other markers such as CD73, CD90, and CD146 have also been used to characterize pericytes, although CD146 is not consistently found on lung pericytes [4–7]. Pericytes can prevent vascular leakage by stabilizing endothelial cells and regulating vascular tone and perfusion pressure [8, 9]. They have been studied extensively in the blood-brain barrier (BBB) where they regulate vascular permeability and their loss contributes to BBB disruption [10, 11]. Brain pericyte abnormalities are associated with numerous diseases including Alzheimer’s disease, multiple sclerosis and stroke [12, 13]. Furthermore, LPS-induced pericyte loss in the heart has been associated with microvascular dysfunction and mortality in mice [14, 15].
In the lung, pericytes can modulate the inflammatory response and are a key player in the pathogenesis of various lung diseases including pulmonary fibrosis, asthma, pulmonary hypertension and sepsis [15, 16]. Pericyte activation and differentiation contribute to the development of pulmonary fibrosis [17]. Pericyte accumulation in airway walls may contribute to airway remodeling in chronic allergic asthma [18]. Impaired endothelial-pericyte interactions are associated with pulmonary arterial hypertension [19]. In addition, LPS-induced lung pericyte loss has been associated with microvascular dysfunction and mortality [15]. Our previous study demonstrated that lung pericyte loss is also correlated with increased lung vascular leak and mortality in cecal ligation and puncture (CLP)-induced murine sepsis [20]. Despite their importance in lung physiology and pathophysiology, the underlying mechanisms of lung pericyte function remain largely unknown. Therefore, in order to facilitate lung pericyte studies, in vitro lung pericyte models are required. Normal human pericytes divide only a limited number of times before entering a state of replicative senescence. Immortalized human pericyte lines including human fetal brain pericytes and human retinal pericytes have been successfully established [21, 22]. However, a human lung pericyte cell line has not yet been developed. Thus, our present study aims to establish and characterize a new immortalized human lung pericyte cell line.
Materials and methods
Human lung pericyte culture and stimulation
Pericytes were isolated from a failed human lung donor as previously described [7]. Briefly, tissue was minced and digested in HBSS buffer containing collagenase I (300 U/ml) and dispase (5 U/ml). The suspension was filtered (70 μm), centrifuged (350 × g, 10 min), and washed with DMEM + 10% FBS. The dissociated cells were plated on 0.2% gelatin-coated dishes in human Pericyte Medium (ScienCell Research Laboratories, Carlsbad, CA). After expansion, cells were negatively selected by CD45, CD31, and CD326 magnetic beads (Miltenyi Biotec Inc. San Diego, CA) to deplete leukocytes, endothelial cells, and epithelial cells, respectively. Pericytes were collected for labeling with PE-conjugated anti-PDGFR-β (clone REA363; Miltenyi) and anti-PE magnetic microbeads (Miltenyi) and passed through a magnetized column. Retained pericytes (PDGFR-β+) were cultured in human Pericyte Medium (ScienCell). Pericytes were stimulated with LPS (100 ng/ml), TNF-α (10 ng/ml) or thrombin (0.1 U) for 12h and total RNA was collected for further analysis. In another set of experiments, pericytes were transfected with control or Fli-1 antisense gapmer (Qiagen, Germantown, MD) for 24h and stimulated with LPS (100 ng/ml, Sigma, St. Louis, MO) for another 12 or 24h. Total RNA and supernatant were collected for further analysis.
Establishment of an immortalized human lung pericyte cell line
Human lung pericytes at passage 6 were seeded at 2 × 105 per well into a six-well plate, which resulted in 50–60% confluence after 12h. Cells were then treated with polybrene (5 μg/ml), a cation polymer used to increase the efficiency of infection in cells with retrovirus, and incubated at 37 °C for 20 min. Then 4 μl lentiviral particles (viral titer: 1.0 × 109 IU/ml; pLenti-SV40-T, Applied Biological Materials Inc., Richmond, BC, Canada) were added and the cells were incubated for 24h. Viable cells were expanded into a 75-cm2 flask and used for subsequent experiments. Non-transfected cells stopped proliferating within 10 passages.
Immunocytochemistry
HEK293 cells, normal and immortalized human lung pericytes grown on 12-well plates coated with 0.2% gelatin were fixed with 4% PFA for 15 min at room temperature and then washed with DPBS. After blocking with 1% BSA for 15 min at room temperature, cells were incubated overnight at 4°C with a rabbit monoclonal PDGFR-β antibody (clone Y92; Abcam, Cambridge MA) or a rabbit polyclonal NG2 antibody (H-300; Santa Cruz Biotechnology, Dallas, Texas). Cells were washed with DPBS and incubated for 1h at room temperature with Alexa Fluor 594 goat anti-rabbit IgG (H+L) secondary antibody (Invitrogen, Carlsbad, CA), diluted 1:200 in DPBS. After washing with DPBS, images were acquired using a Zeiss Observer.Z1 microscope with Zen 2 software.
Flow Cytometry
Immortalized human lung pericytes at passage 16 were detached with Accutase, washed and suspended in Dulbecco’s phosphate-buffered saline containing 2% BSA and 0.75 mM NaN3. After blocking CD16/CD32 with Human Fc Block (BD Biosciences, Franklin Lakes, NJ), cells were stained with the following antibodies (Biolegend, San Diego, CA): APC-anti human CD34, APC-anti human CD45, PE-anti human CD31, APC-anti human CD44, PE-anti human CD146, PE-anti human CD90, PE-anti human CD73 and PE-anti human CD140b, and isotype controls diluted according to the manufacturer’s instructions. Cells were analyzed using a CytoFLEX flow cytometer and CytExpert 2.3 software (Beckman Coulter, Brea, CA).
Telomerase activity assay
Normal human lung pericytes and immortalized human lung pericytes at passage 9 were lysed with nondenaturing lysis buffer. Telomerase activity was measured using TRAPEZE RT telomerase detection kit, according to the manufacturer’s instructions (EMD Millipore, Burlington, MA).
ELISA assay
IL-6 levels in the supernatant of cultured human lung pericytes were measured using a Human IL-6 ELISA kit according to the instructions (EMD Millipore).
In vitro Matrigel assay
Primary human umbilical vein endothelial cells (HUVECs) were purchased from ATCC (Manassas, VA), and cultured in vascular cell basal medium supplemented with endothelial cell growth kit. The cells were co-cultured with normal (P7) or immortalized human lung pericytes (P25) at a 20:1 ratio of endothelial cells (EC) to pericytes in a 24-well plate coated with Matrigel at 10 mg/ml. EC number was kept constant in all Matrigel assays (120,000/well). 289 μl Matrigel was added to each well and incubated at 37°C for 30 min prior to addition of cells. The cells were incubated at 37°C and 5% CO2 with either 21% or 1% O2 for 16h. Calcein AM fluorescent dye was added at 8 μg/mL in Hanks Balanced Salt Solution (HBSS) to visualize tube formation. After washing cells twice with DPBS, images were acquired using an Olympus fluorescent microscope.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured pericytes using the RNeasy Plus Mini kit (Qiagen). cDNA was synthesized with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA). Quantitative real-time PCR was performed using the SYBR Green PCR Kit (Qiagen) and CFX96 Real-Time PCR system (Bio-Rad, Hercules, CA). Data were analyzed with 2−ΔΔCt value calculation using GAPDH for normalization.
Western blot analysis
Lung pericytes were lysed with ice-cold RIPA lysis buffer (Cell Signaling, Danvers, MA). All lysed samples were kept on ice for 30 min and centrifuged for 10 min at 4°C at 12,000 × g. Cell lysates were subjected to 12% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 7% milk in TBST (20 mM Tris, 500 mM NaCl, and 0.1% Tween 20) for 1h. After washing with TBST twice, membranes were incubated with primary antibody overnight at 4°C. A rabbit monoclonal PDGFRβ antibody (clone Y92; Abcam), a rabbit polyclonal NG2 antibody (H-300; Santa Cruz Biotechnology) and a mouse monoclonal SV40T-antigen antibody (PAb416; Abcam) were used. Primary antibodies to β-actin and α-tubulin were obtained from Cell Signaling. Fli-1 primary antibody was provided by Dr. Xiankui Zhang (Medical University of South Carolina). The membranes were washed twice with TBST and incubated with HRP conjugated secondary antibody in blocking buffer for 1h. After washing three times with TBST, immunoreactive bands were visualized by incubation with ECL plus detection reagents (GE Healthcare, Chicago, IL). The densitometry of bands was quantified with Image J2 software.
Data analysis
Data are expressed as means ± standard error of the mean (SE). Statistical significance was determined by analysis of variance (ANOVA) with Fisher’s probable least-squares difference test or Student’s t-test using GraphPad Prism software. A value of p<0.05 was considered statistically significant.
Results
Generation of an immortalized human lung pericyte cell line
Human lung pericytes at passage 6 were immortalized using SV40 large T antigen (SV40T) lentivirus. We found that immortalized pericytes (P9) exhibited similar morphology to normal human lung pericytes (P9) by phase contrast microscopy (Fig. 1A). Immortalized pericytes at either early passage (P9) or late passage (P25) were positive for PDGFR-β and NG2, the two most common markers expressed on the cell surface of pericytes (Fig. 1B–D; Supplemental Fig. 1). In addition, SV40T expression was detected in immortalized pericytes but not in normal pericytes (Fig. 1D). Furthermore, we used quantitative RT-PCR to analyze normal and immortalized pericytes for the expression of other pericyte specific or non-specific genes [7]. Expression of genes was considered absent if Ct values were ≥35. Our results confirmed that both normal and immortalized pericytes have similar expression of pericyte-specific genes including desmin, nestin and angiopoietin 1 (ANG1) but have no expression of mesenchymal stem cell markers such as transcription factor Gli1 and the ATP-binding cassette transporter ABCG2 (Supplemental Table 1). Moreover, the immortalized pericytes retained high proliferation ability up to 28 passages; however, normal human lung pericyte proliferation usually stopped at continuous passage 10 (Fig. 2A). We detected relative telomerase activity in normal/immortalized pericytes at passage 9 using a telomerase activity assay. Consistently, immortalized pericytes showed a 19.2-fold increase in telomerase activity compared to normal pericytes (19.2 ± 1.0-fold, Fig. 2B).
Figure 1. Cell morphology and characteristics of normal and immortalized human lung pericytes.
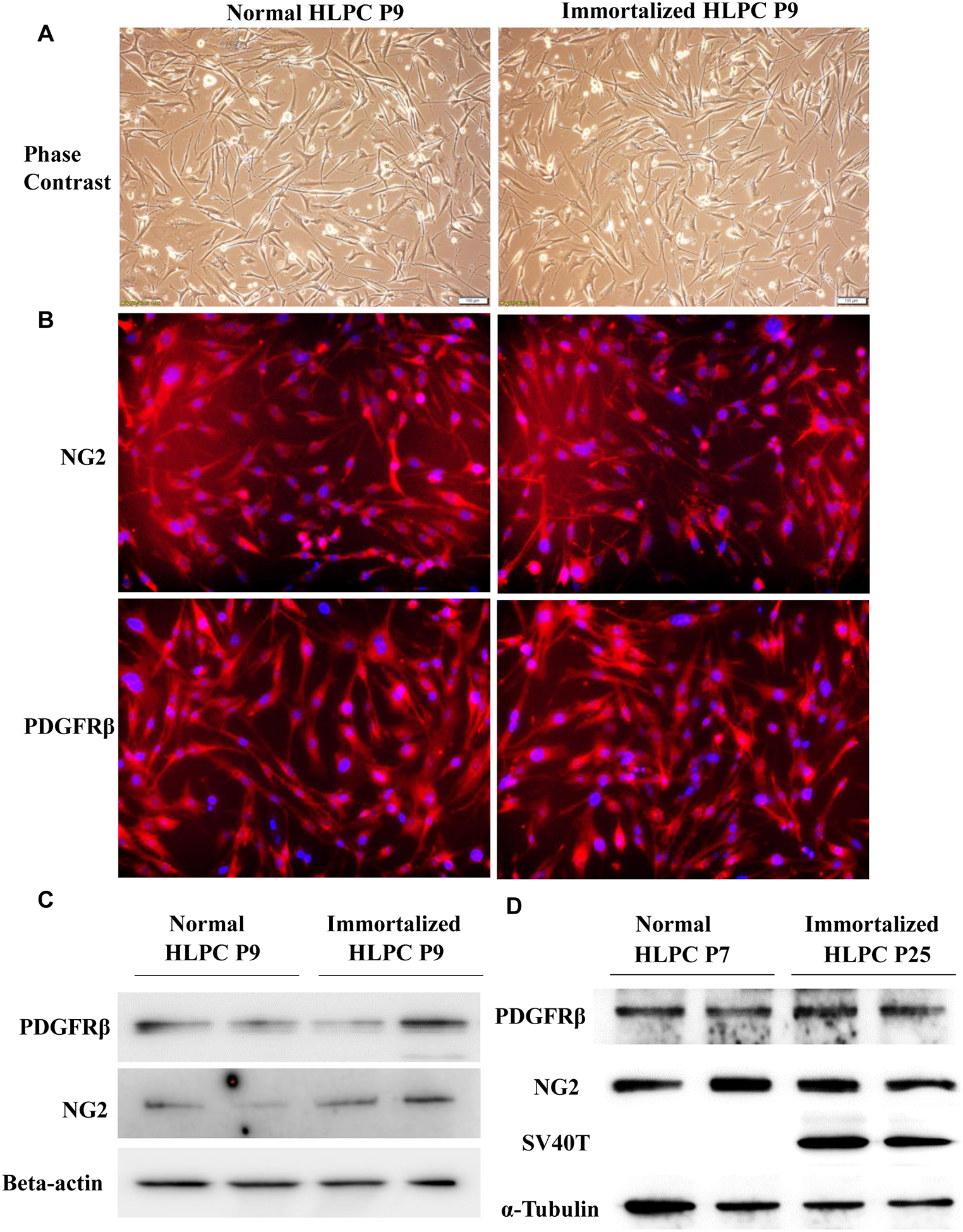
(A) Normal and immortalized human lung pericyte morphology were assessed by phase contrast light microscopy. Scale bar: 50 μm. Pericytes were also assessed by immunostaining (B) and western blot (C-D) for PDGFRβ, neural glial antigen-2 (NG2) and SV40T. Nucleus stained blue and PDGFRβ or NG2 stained red. Magnification: 100x. HLPC: human lung pericytes.
Figure 2. Relative telomerase activity of normal and immortalized human lung pericytes.
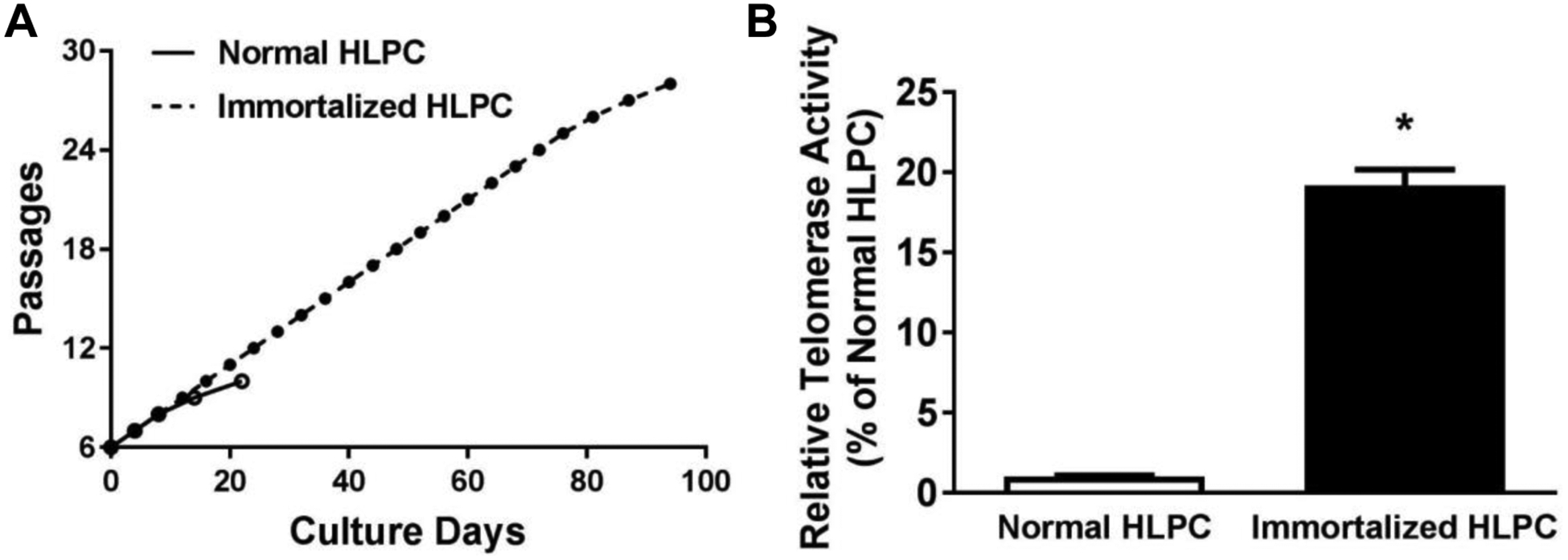
(A) Cumulative culture of normal and immortalized human lung pericytes. (B) Relative telomerase activity in normal and immortalized human lung pericytes at passage 9 were measured. N=3. *p < 0.05 compared to control human lung pericyte (HLPC) group.
Expression of cell-surface markers in immortalized human lung pericytes
To characterize the immortalized human lung pericytes, flow cytometry was used to analyze cell-surface markers. Our data demonstrated that immortalized pericytes (P16) expressed pericyte markers including CD44, CD90, CD73 and CD140b (PDGFR-β); this isolate was also positive for CD146, although this marker is not consistently detected on human lung pericytes. In addition, they did not express non-pericyte markers such as hematopoietic cell marker CD34, leukocyte marker CD45, or endothelial cell marker CD31 (Fig. 3). Taken together, the above results indicate that we successfully established an immortalized human lung pericyte cell line and the cells retained pericyte characteristics.
Figure 3. Representative histograms of cell-surface marker staining on immortalized human lung pericytes.
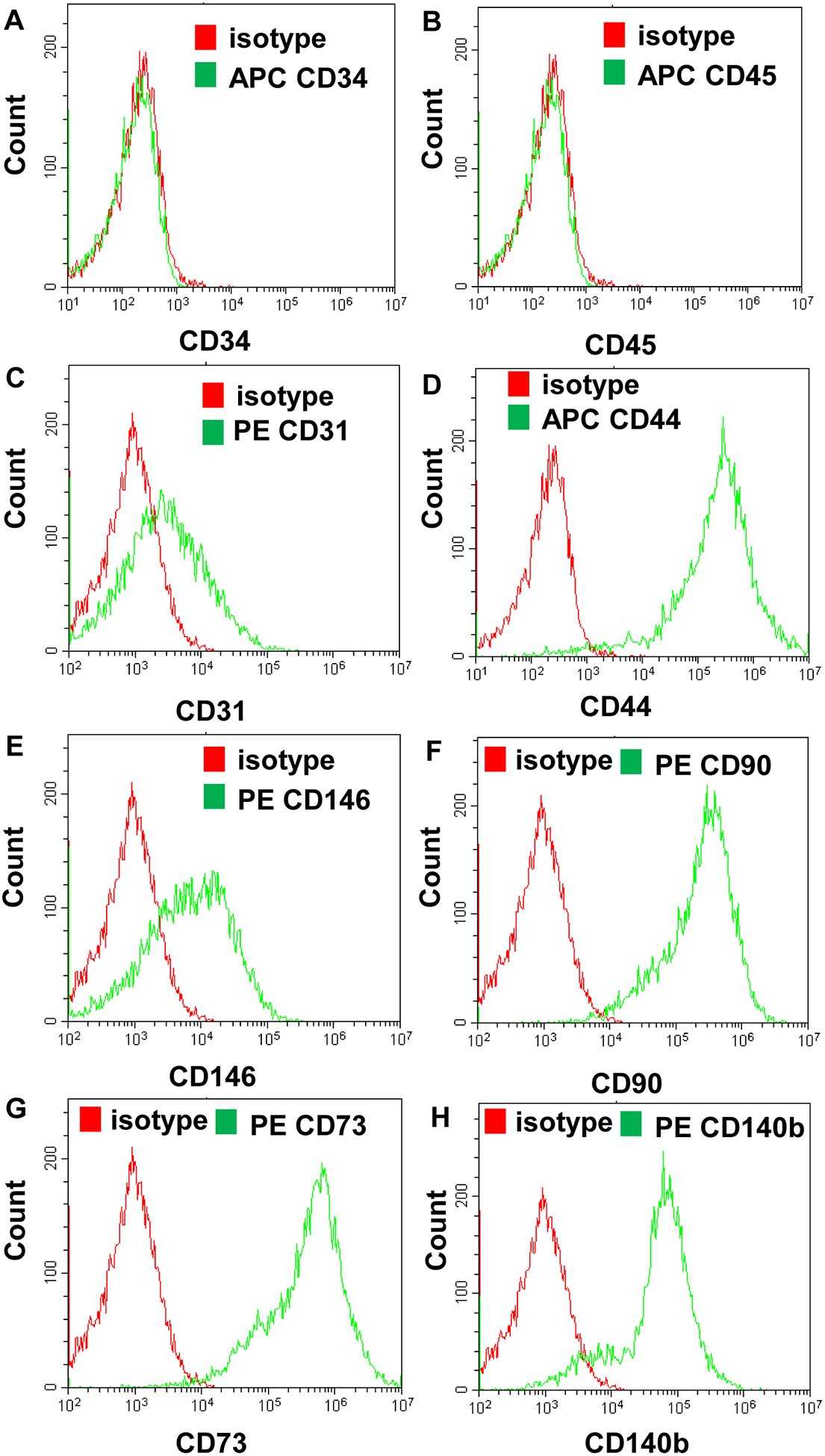
Immortalized human lung pericytes (P16) were stained for CD34 (A), CD45 (B), CD31 (C), CD44 (D), CD146 (E), CD90 (F), CD73 (G) and CD140b (H, PDGFR-β) and analyzed by flow cytometry.
Functional characterization of immortalized human lung pericytes following different forms of stimulations
To determine if pericytes retain normal function after immortalization, we determined the gene expression profile of normal and immortalized pericytes in response to different stimuli. We stimulated normal and immortalized pericytes with LPS, TNF-α or thrombin for 12h and measured related inflammatory gene expression. Our data demonstrated that immortalized pericytes exhibited a similar related gene expression pattern to that of normal pericytes (Fig. 4). In addition, the expression of inflammatory genes including HMGB1, VCAM1, ICAM1 and CX3CL1 was also similar between normal and immortalized human lung pericytes (Supplemental Fig. 2).
Figure 4. Response of normal and immortalized human lung pericytes to different stimuli.
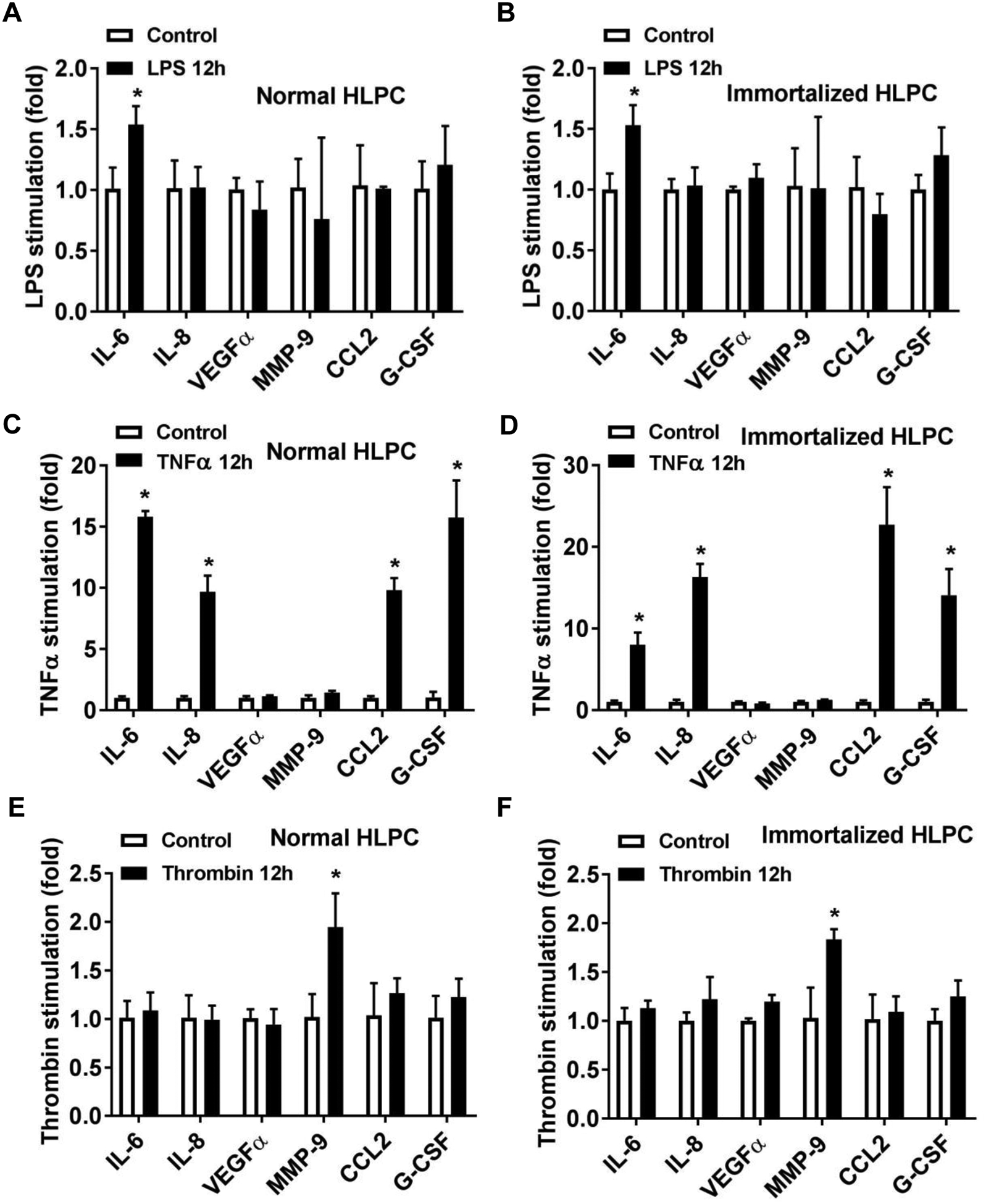
Normal and immortalized human lung pericytes (HLPC) were stimulated with LPS (100 ng/ml), TNFα (10 ng/ml) or Thrombin (0.1 U) for 12h. (A-F) Total RNA were extracted and mRNA levels of IL-6, IL-8, VEGFα, MMP-9, CCL2 and G-CSF were measured by Real-time PCR. N=3 independent experiments. *p < 0.05 compared to control group.
Immortalized human lung pericytes promote endothelial cell tube formation under hypoxic conditions
To determine if pericytes retain their ability to support angiogenesis after immortalization, we determined the effect of immortalized pericytes (P25) on endothelial cell tube formation under hypoxic conditions. HUVECs and pericytes were co-incubated in a Matrigel-coated 24-well plate and formation of endothelial cords was analyzed. HUVECs under normal culture conditions (21% oxygen) demonstrated adhesion and alignment (Fig. 5A). However, under hypoxic conditions (1% oxygen), endothelial cells appeared less elongated and tube formation was inhibited (Fig. 5A). By contrast, addition of either normal or immortalized pericytes to EC at a 1:20 ratio significantly promoted the organization of the ECs into tubes (Fig. 5). These results indicate that pericytes retain their functionality after immortalization.
Figure 5. The effect of normal and immortalized human lung pericytes on endothelial cell tube formation under hypoxic conditions.
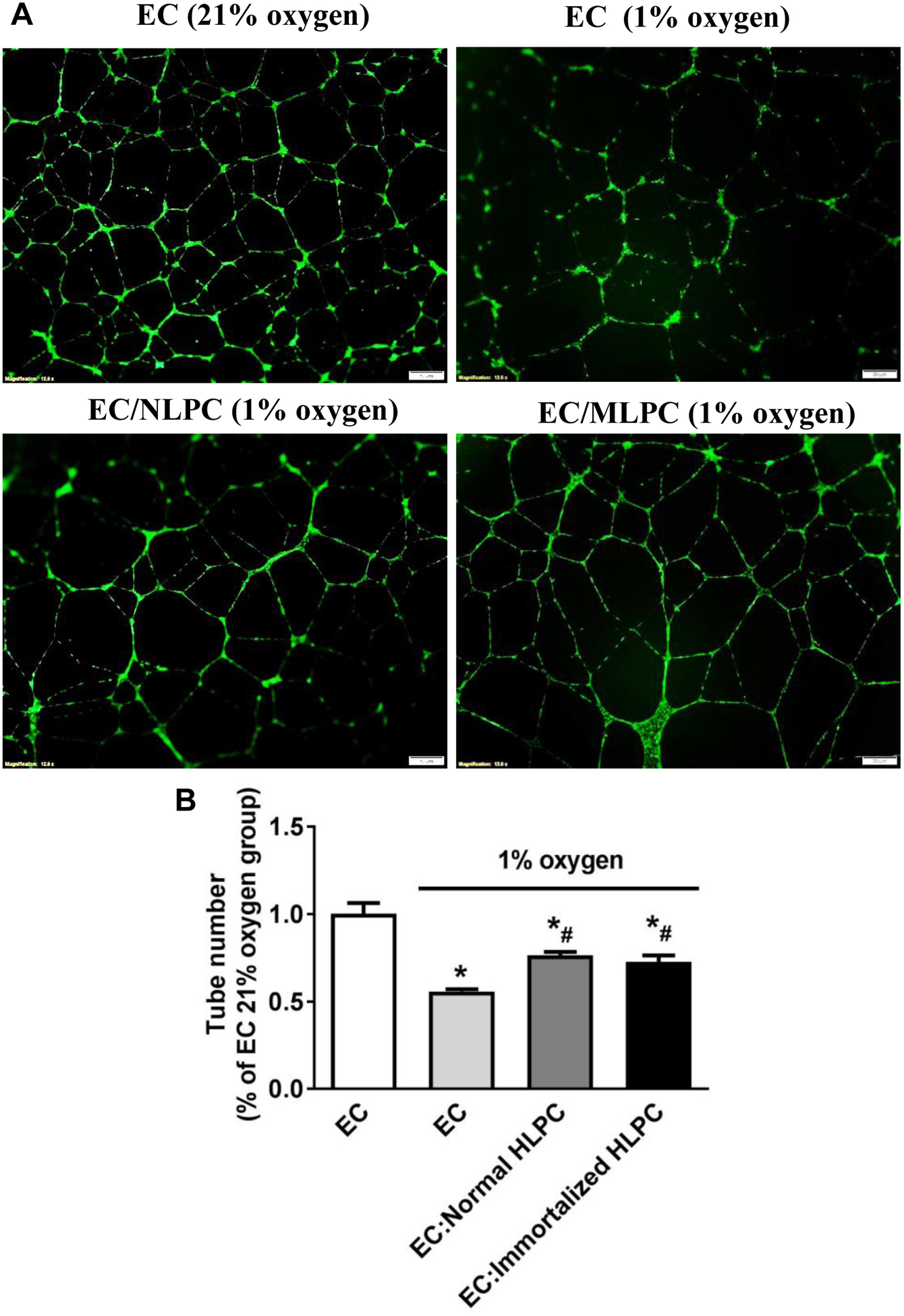
HUVECs were cultured under normal (21% oxygen) or hypoxic (1% oxygen) conditions without or with pericytes at 20:1 ratio (EC versus pericytes) in Matrigel-coated 24-well plate for 16h and then stained by Calcein AM fluorescent dye for 30 min. (A) Endothelial cell tube formation was observed and (B) the tube number was counted under a fluorescent microscope. Scale bar: 50 μm. N=3 independent experiments. *p < 0.05 compared to EC under 21% oxygen group. #p < 0.05 compared to EC under 1% oxygen group. EC: endothelial cell; NLPC: normal human lung pericytes; MLPC: immortalized human lung pericytes; HLPC: human lung pericytes.
Fli-1 regulates pericyte inflammatory response in immortalized human lung pericytes
Our previous data demonstrated that Fli-1, an ETS transcription factor, governs vascular dysfunction and mortality in cecal ligation and puncture-induced murine sepsis by regulating the pericyte inflammatory response [20]; however, the role of Fli-1 in human lung pericytes has not been studied. Knockdown of Fli-1 by a Fli-1 antisense gapmer significantly inhibited Fli-1 mRNA and protein levels (Fig. 6A), and reduced LPS-induced IL-6 mRNA and protein levels (Fig. 6B–C), indicating the important role of Fli-1 in regulating inflammatory response in human lung pericytes.
Figure 6. Fli-1 regulates inflammatory response in immortalized human lung pericytes.
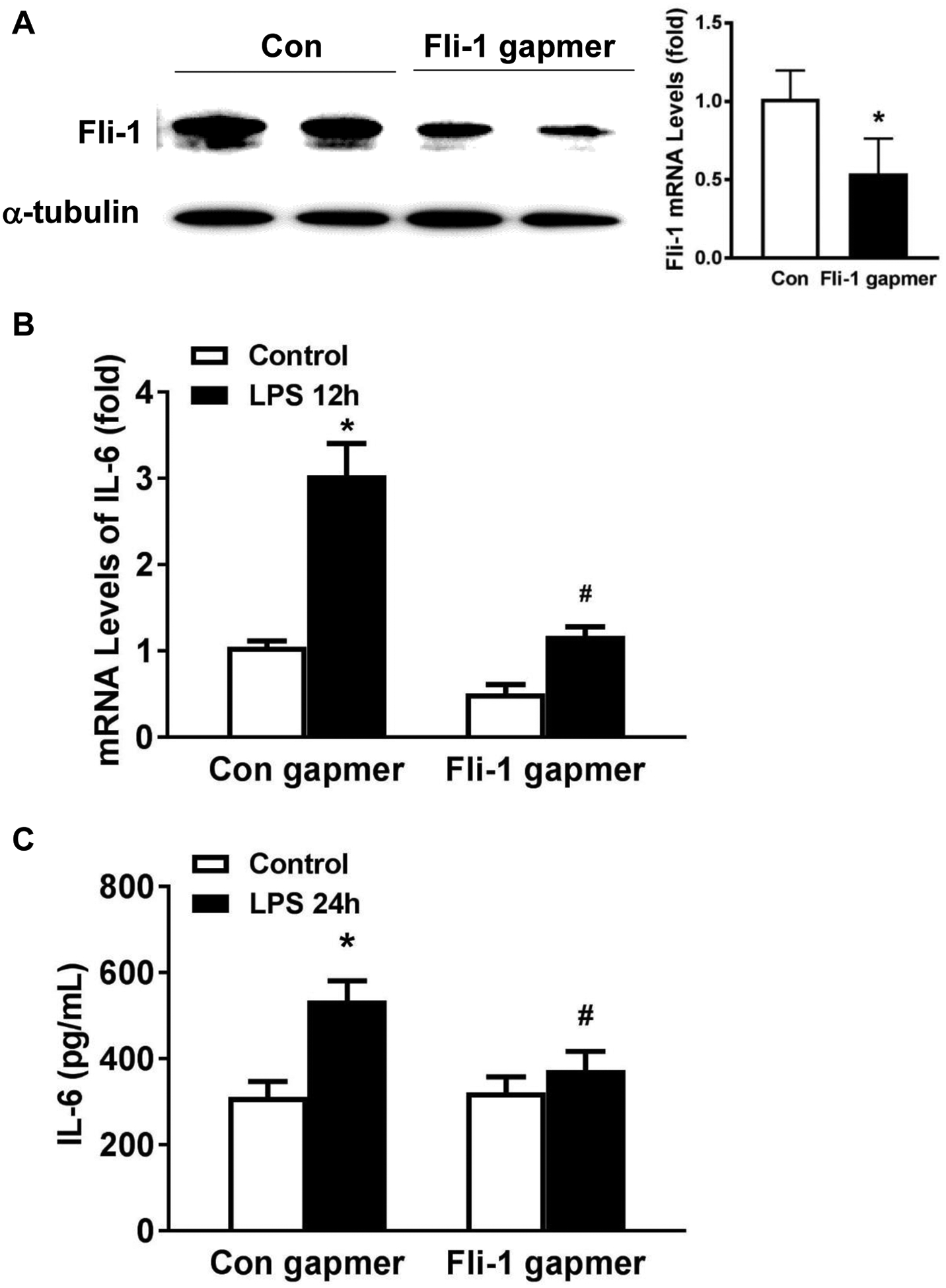
Immortalized human lung pericytes were transfected with control or specific Fli-1 antisense gapmer for 24h and further stimulated with LPS for another 12 or 24h. mRNA (A) and protein (B) levels of IL-6 were measured. N=3 independent experiments. *p < 0.05 compared to control group. #p < 0.05 compared to LPS control group.
Discussion
Pericytes play an important role in the onset and progression of various lung diseases including pulmonary fibrosis, asthma, pulmonary hypertension and sepsis [17–20]. In this study, we developed a novel immortalized human lung pericyte cell line, using SV40 large T antigen lentivirus, which will be useful in future in vitro lung pericyte models. Our novel immortalized cell line exhibits enhanced proliferation ability and telomerase activity compared to primary human lung pericytes. This immortalized cell line retains the same characteristic morphology and pericyte marker expression as primary cell cultures. We found that the responses of immortalized human lung pericytes to different inflammatory stimuli or hypoxic conditions were similar to that of primary cells, suggesting that the immortalized cell line retains pericyte identity and function. To our knowledge, this is the first report of an immortalized human lung pericyte cell line that was studied for its characteristics and function in vitro.
Replicative senescence is a barrier that cells must overcome to become immortalized [23]. Telomerase regulates the senescence of cells through telomere maintenance and there is evidence that human epithelial cell lines and brain pericyte cell lines can be immortalized or have their in vitro lifespan extended by the transfer of SV40 large T antigen [21, 24]. In this study, we introduced SV40 large T antigen lentivirus into primary human lung pericytes. This extended the replicative lifespan of human lung pericytes up to 28 passages and led to a ~19.2-fold increase in telomerase activity, whereas primary lung pericytes had a limited lifespan and spontaneously became senescent within 10 passages. This finding is important for human lung pericyte biology research, as the short lifespan of primary pericytes makes it difficult to obtain enough cells for in vitro studies.
Our analysis revealed that the immortalized lung pericyte cell line retained pericyte-specific characteristics, including normal pericyte morphology and expression of the commonly used pericyte markers, PDGFRβ and NG2. Lung pericytes have also been characterized using other markers including CD73 and CD90 [5, 7]. We further observed expression of these pericyte markers and PDGFRβ (CD140b) on the cell surface of immortalized pericytes by flow cytometry. In addition, CD44, but not CD31 (endothelial cell marker), CD45 (hematopoietic cell marker) or CD34 (leukocyte marker), was present on the cell surface of the immortalized pericytes, as vascular CD44+ multipotent stem cells have been reported to give rise to pericytes [25]. Therefore, these results clearly show that immortalized pericytes possess the typical repertoire of cell-surface markers detected on normal lung pericytes; however, a global gene expression comparison between immortalized cells and primary cells needs further study.
In addition to these characteristics, we further demonstrated that immortalized cells exhibit similar responsiveness to different inflammatory stimuli compared with normal pericytes, which is critical for in vitro pericyte studies because the inflammatory reactions of lung pericytes has emerged as an important area of lung pericyte study [20, 26–29]. For example, LPS can induce pro-inflammatory mediators such as IL-6 in lung pericytes [7, 30]. Vascular endothelial growth factor (VEGF), a potent angiogenic and vascular permeability factor, was upregulated in both rat and mouse lung pericytes when exposed to LPS [20, 31]. Inflammatory responses in murine lung-pericyte like cells contribute to lung injury [26]. Moreover, inhibition of inflammation in mouse lung pericytes was reported to attenuate lung injury and mortality during sepsis [20, 29]. Our data showed that the immortalized pericytes express similar levels of inflammatory genes such as HMGB1, VCAM1, ICAM1 and CX3CL1 to that of normal pericytes. In addition, knockdown of Fli-1 reduced LPS-induced IL-6 mRNA and protein levels in immortalized human lung pericytes, consistent with previous findings in primary mouse lung pericytes [20]. Therefore, immortalized pericytes could be a promising tool to study the role and mechanisms of inflammatory response in lung pericytes and how pericytes affect the development of various lung inflammatory diseases. Regulation of angiogenesis and capillary structure is an intrinsic role of pericytes [7]. Our results confirmed that immortalized pericytes retain this function by promoting endothelial cell tube formation. Thus, these immortalized pericytes will be a key tool for in vitro models to study the interaction between lung pericytes and endothelial cells.
In summary, we have successfully established a new human lung pericyte cell line with extended proliferation capability and the ability to respond to inflammatory stimuli and promote angiogenesis. This cell line will be useful in in vitro models to investigate the role and mechanisms of lung pericytes in health and diseases.
Supplementary Material
Acknowledgments
We thank Dr. Carol Feghali-Bostwick for providing us the human lung sample through her collaboration with Dr. Joseph Pilewski at the University of Pittsburgh.
Funding
This work was supported in part by National Institute of Health grants [1R01GM113995 (HF), 1R01GM130653 (HF), 3R01GM130653-03S1 (HF), 1K23HL135263-01A1 (AG), UL1TR001451 (PVH) and ULTR001450 (PVH)].
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016; 36:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005; 97:512–523. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011; 21:193–215. [DOI] [PubMed] [Google Scholar]
- 4.Shammout B, Johnson JR. Pericytes in Chronic Lung Disease. Adv Exp Med Biol. 2019; 1147:299–317. [DOI] [PubMed] [Google Scholar]
- 5.Bichsel CA, Hall SR, Schmid RA, Guenat TO, Geiser T. Primary Human Lung Pericytes Support and Stabilize In Vitro Perfusable Microvessels. Tissue Eng. Part A 2015; 21:2166–2176. [DOI] [PubMed] [Google Scholar]
- 6.Evdokiou A, Kanisicak O, Gierek S, Barry A, Ivey JM, Zhang X, et al. Characterization of Burn Eschar Pericytes. J Clin Med. 2020; 9:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson CL, Stephenson SE, Higuero JP, Bostwick FC, Hung FC, Schnapp ML. Characterization of human PDGFR-beta-positive pericytes from IPF and non-IPF lungs. Am J Physiol Lung Cell Mol Physiol. 2018; 315:L991–L1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottke MA, Walters TJ. Where’s the Leak in Vascular Barriers? A Review. Shock 2016; 46:20–36. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez E, Raoul W, Calippe B, Sahel AJ, Guillonneau X, Paques M, et al. Experimental Branch Retinal Vein Occlusion Induces Upstream Pericyte Loss and Vascular Destabilization. PLoS One. 2015; 10:e0132644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, et al. Disruption of Bmal1 Impairs Blood-Brain Barrier Integrity via Pericyte Dysfunction. J Neurosci. 2017; 37:10052–10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armulik A, Genove G, Mae M, Nisancioglu HM, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010; 468:557–561. [DOI] [PubMed] [Google Scholar]
- 12.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011; 12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van DH HJ, Jansen JFA, Van MJP, Buchem VAM, Muller M, Wong MS, et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging. 2016; 45:190–196. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013; 123: 3436–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng H, He X, Tuo QH, Liao FD, Zhang QG, Chen XJ. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2alpha/Notch3 pathways. Sci Rep. 2016; 6:20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung CF, Wilson CL, Schnapp LM. Pericytes in the Lung. Adv Exp Med Biol. 2019; 1122:41–58. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Sun W, Yang N, Liu J, Tang HI, Li FZ, et al. The effect of core fucosylation-mediated regulation of multiple signaling pathways on lung pericyte activation and fibrosis. Int J Biochem Cell Biol. 2019; 117:105639. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Folestad E, Rowley JE, Noll ME, Walker AS, Lloyd MC, et al. Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2015; 308:L658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan K, Shamskhou EA, Orcholski ME, Nathan A, Reddy S, Honda H, et al. Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation. 2019; 139:1710–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Zhou Y, Goodwin AJ, Cook AJ, Halushka VP, Zhang KX, et al. Fli-1 Governs Pericyte Dysfunction in a Murine Model of Sepsis. J Infect Dis. 2018; 218:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umehara K, Sun Y, Hiura S, Hamada K, Itoh M, Kitamura K, et al. A New Conditionally Immortalized Human Fetal Brain Pericyte Cell Line: Establishment and Functional Characterization as a Promising Tool for Human Brain Pericyte Studies. Mol Neurobiol. 2018; 55:5993–6006. [DOI] [PubMed] [Google Scholar]
- 22.Berrone E, Beltramo E, Buttiglieri S, Tarallo S, Rosso A, Hammes PH, et al. Establishment and characterization of a human retinal pericyte line: a novel tool for the study of diabetic retinopathy. Int J Mol Med. 2009; 23:373–378. [DOI] [PubMed] [Google Scholar]
- 23.Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000; 14:183–188. [PubMed] [Google Scholar]
- 24.Mitani A, Kobayashi T, Hayashi Y, Matsushita N, Matsushita S, Nakao S, et al. Characterization of doxycycline-dependent inducible Simian Virus 40 large T antigen immortalized human conjunctival epithelial cell line. PLoS One. 2019; 14:e0222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein D, Weisshardt P, Kleff V, Jastrow H, Jakob JH, Ergün S. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One. 2011; 6:e20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CF, Mittelsteadt KL, Brauer R, McKinney LB, Hallstrand ST, Parks CW, et al. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol. 2017; 312:L556–L567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelman DA, Jiang Y, Tyburski JG, Wilson FR, Steffes PC. Lipopolysaccharide up-regulates heat shock protein expression in rat lung pericytes. J Surg Res. 2007; 140:171–176. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Goodwin AJ, Cook JA, Halushka VP, Zhang XK, Fan HK. Fli-1 transcription factor regulates the expression of caspase-1 in lung pericytes. Mol Immunol. 2019; 108:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Li P, Goodwin AJ, Cook JA, Halushka VP, Zingarelli B, et al. miR-145a Regulates Pericyte Dysfunction in a Murine Model of Sepsis. J Infect Dis. 2020; 222:1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelman DA, Jiang Y, Tyburski JG, Wilson FR, Steffes PC. Cytokine production in lipopolysaccharide-exposed rat lung pericytes. J Trauma. 2007; 62:89–93. [DOI] [PubMed] [Google Scholar]
- 31.Kim CO, Huh AJ, Kim MS, Chin BS, Han SH, Choi SH, et al. LPS-induced vascular endothelial growth factor expression in rat lung pericytes. Shock. 2008; 30:92–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


