Abstract
Introduction:
System-level care coordination strategies can be the most effective to promote continuity of care among people with Alzheimer disease (AD); however, the evidence is lacking. The objective of this study is to determine if accountable care organizations (ACOs) are associated with lower rates of potentially preventable hospitalizations for people with AD and if hospital ACO affiliation is associated with reduced racial and ethnic disparities in preventable hospitalizations among patients with AD.
Methods:
This study employed a cross-sectional study design and used 2015 Healthcare Cost and Utilization Project inpatient claims data from 11 states and the 2015 American Hospital Association Annual Survey. Logistic regression and the Blinder–Oaxaca decomposition method were used.
Results:
African American patients with AD were less likely to be hospitalized at ACO-affiliated hospitals than White patients. Among patients with AD that were hospitalized, hospital ACO affiliation was associated with lower odds of potentially preventable hospitalizations (OR=0.86, p=0.02; OR=0.66, p<0.001 with propensity score matching) after controlling for patient characteristics, hospital characteristics, and state indicators. Hospital ACO affiliation explained 3.01% (p<0.01) of the disparity in potentially preventable hospitalizations between White and African American patients but could not explain disparities between White and Latinx patients.
Conclusions:
Evidence suggests that ACOs may be able to improve care coordination for people with AD and to reduce disparities between Whites and African Americans. Further research is needed to determine if this benefit can be attributed to ACO formation or if providers that participate in ACOs tend to provide higher-quality care.
INTRODUCTION
Adults with Alzheimer disease (AD) are a high-need, high-cost population with complex medical needs, which are often exacerbated by varying social determinants of health.1–3 Older adults with AD have significantly higher rates of chronic medical illnesses and are more likely to have multiple chronic conditions than older adults without AD, which often results in higher rates of potentially preventable hospitalizations (PPHs).2,3,5,6 Hospitalizations for many of the chronic conditions that coexist with AD, including diabetes and heart disease, can be prevented through timely, accessible, and effective care through primary care providers or other outpatient settings.7 The rate of PPHs is even higher among African American and Latinx people with AD, which may be explained by their higher rates of unmet needs being the result of a lifetime of living in a racially unjust society coupled with substantial and systemic barriers to unbiased, high-quality, timely, and well-coordinated care.8–12
Evidence suggests that patients with AD have lower rates of unmet needs when receiving coordinated care through targeted care coordination programs.13–15 As the population ages and the burden of AD grows, there is an increasing need to provide care that is proactive and well-coordinated in order to better manage medical comorbidities and reduce avoidable healthcare utilization. To motivate providers to coordinate the care they provide and shift their focus upstream to prevention, major public and private payers are utilizing novel reimbursement mechanisms, like accountable care organizations (ACOs).16
An ACO is a virtually integrated network of physician groups, hospitals, and post-acute care providers that partner together to reduce healthcare expenditures for an attributed group of patients in order to receive bonus payments.17 One effective method to reduce expenditures is through care coordination efforts among providers, facilities, and social services.18–23 Provider groups and hospitals have been found to reduce their rates of PPHs for chronic conditions after forming or affiliating with ACOs,24–26 particularly for high-need, high-cost patient populations; however, no work has yet focused on the AD population.27,28 ACOs have also been found to effectively serve the needs of minority patients: Minority-serving ACOs have been more likely to generate bonuses in Medicare’s Shared Savings ACO program.29,30 These early successes suggest that ACOs have great potential to improve healthcare quality and reduce health disparities if care is equitably disseminated.
Hence, the authors hypothesize that patients with AD affiliated with ACOs have fewer PPHs, and the difference varies by race and ethnicity.31,32 ACOs provide the flexibility that allows providers to tailor their initiatives to fit their population’s needs.23 For example, if ACOs include AD patients in their population health initiatives, they can work to: (1) provide financial incentives to providers that participate in care coordination and management for AD patients and their family caregivers, (2) promote the integration of healthcare services to provide timely access to primary care services and improve care transitions (from hospitals to home or nursing facilities), and (3) improve patient satisfaction and healthcare quality while reducing PPH and healthcare cost. As racial and ethnic minorities have better outcomes when receiving coordination, the authors expect to see fewer disparities when more minority AD patients are served in ACO affiliated hospitals.
METHODS
Data included the 2015 Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project’s State Inpatient Databases33 and the American Hospital Association (AHA) Annual Survey.34 State Inpatient Database data from 11 states were selected based on the availability of necessary variables, such as patients’ race/ethnicity and the linkage with the AHA annual survey: Arizona, Colorado, Florida, Kentucky, Maryland, New Jersey, North Carolina, Oregon, Rhode Island, Washington, and Wisconsin. The AHA annual survey is a database with hospital characteristics (e.g., governance, payment arrangements, services) for the majority of U.S. community hospitals.34
Study Sample
This study focused on community-dwelling adults with AD aged ≥65 years, who were either White, African American, or Latinx and had an inpatient hospitalization in 2015. Community-dwelling was defined as a discharge to home or self-care. The final sample included 10,784 White, 1,651 African American, and 1,751 Latinx older adults with AD. AD was measured using any diagnosis of AD on hospital discharge coding (ICD-9 code DX 331.0 and ICD-10 codes G30.0, G30.1, G30.8, G30.9).35
Measures
The outcome variable of interest is a PPH defined by the AHRQ Prevention Quality Indicators (PQI)36 for any of the 4 chronic conditions highly associated with AD: diabetes (PQI 1, 3, 14), hypertension (PQI 7), chronic obstructive pulmonary disease/asthma (PQI 5), and heart failure (PQI 8).2,7,37 A binary indicator was created where 1 equaled a chronic condition related PPH and 0 if otherwise. The National Quality Forum has endorsed the utilization of PQIs as a measure to evaluate the quality of care for chronic condition management and has been widely adopted to evaluate the ACO model.38,39
The key independent variable was hospital ACO affiliation, which was obtained from the 2015 AHA annual survey. A qualified member of each hospital was asked: Has your hospital or health care system established an accountable care organization (ACO)? Discharges were categorized as ACO-affiliated or unaffiliated. Literature suggests that hospital ACO affiliation is indicative of a greater care redesign effort that is taking place in the community that the hospital serves.40 ACO-affiliated hospitals have reported greater use of care coordination strategies compared to unaffiliated hospitals.18
Based on earlier work by Lin and colleagues,2 the following patient characteristics were included as controls: age, sex, admission type (emergent versus non-emergent), number of chronic conditions, expected primary payer, and mean household income of ZIP code of residence. As previous work by Colla et al.41 found that hospitals participating in ACOs were more likely to be teaching, larger, non-profit status, and located in urban areas, the following were controlled for: ownership status, bed size, teaching status, rural location, and state. An indicator was used to identify discharges during the fourth quarter of the year to account for the ICD-10 conversion.42
Statistical Analysis
First, sample characteristics, and the rates of PPHs across different racial/ethnic groups and ACO-affiliated and non-affiliated hospitals were presented. Logistic regressions were applied to estimate the variation in having a PPH by ACO affiliation and race and ethnicity, controlling for patient characteristics, hospital characteristics, and state indicators (Model 1). To improve the robustness of the finding, the study team also implemented the propensity score matching with replacement (Model 2). A “caliper” of 0.01 (i.e., requiring that propensity scores between an AD patient treated in an ACO-affiliated hospital and the closest match to an AD patient treated in a non-affiliated hospital ≤0.01) also was used.25,43,44
Then, the Blinder–Oaxaca decomposition method was used to quantify how hospital ACO affiliation may affect disparities (White versus African American; White versus Latinx) in PPH rates.45,46 For example, to decompose the difference in the probability of having a PPH among White and African American AD patients, multivariable logistic regressions for these 2 groups were estimated separately. The decomposition for these nonlinear equations were rearranged to indicate: (1) the portion of the difference due to observed population characteristics (i.e., all of the control variables) and (2) the portion of the difference due to unobserved heterogeneities, such as cultural background and discrimination. Among the observed population characteristics, disparities associated with each specific factor, such as the hospital ACO affiliation, were also quantified.
Various sensitivity analyses were implemented (Appendix). For example, the study team conducted stratified analysis by race and ethnicity (results were consistent with the decomposition finding). They also tested the model using the generalized estimating equation model to adjust clustering within hospital (results were similar, Appendix). Additionally, the sample was expanded to include AD and related dementias (AD=60%–80% of AD and related dementia cases) and the results were similar.47 Stata, version 16 MP 4 was used for all analyses.
RESULTS
Patient-level characteristics for AD inpatients by race and ethnicity are presented in Table 1. A lower proportion of African American (43% vs 51%, p<0.001) and Latinx (49% vs 51%, non-significant) patients were served by ACO-affiliated hospitals compared with White patients.
Table 1.
Sample Characteristics by Race and Ethnicity Among Patients With Alzheimer’s Disease
| White n=10,733 |
African American n=1,651 | Latinx n=1,751 |
||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | SD | N (%) | SD | P-value | N (%) | SD | P-value | |
| Potentially preventable hospitalization for selected chronic conditions | 907 (8.45) | 0.28 | 170 (10.30) | 0.30 | * | 177 (10.11) | 0.30 | * |
| Admitted to ACO affiliated hospital | 5,483 (51.09) | 0.50 | 717 (43.43) | 0.50 | *** | 860 (49.11) | 0.50 | |
| Age 65–74 years | 1,719 (16.02) | 0.37 | 331 (20.05) | 0.40 | *** | 245 (13.99) | 0.35 | * |
| Age ≥75 years | 9,014 (83.98) | 0.37 | 1,320 (79.95) | 0.40 | *** | 1,506 (86.01) | 0.35 | * |
| Female | 6,476 (60.34) | 0.49 | 1,127 (68.26) | 0.47 | *** | 1,127 (64.3) | 0.48 | *** |
| Number of chronic conditions | 7.40 | 3.15 | 7.30 | 3.15 | 6.99 | 2.96 | *** | |
| Primary payer | ||||||||
| Medicare | 9,862 (91.88) | 0.27 | 1,467 (88.86) | 0.31 | *** | 1,565 (89.38) | 0.31 | *** |
| Medicaid | 23 (0.21) | 0.05 | 23 (1.39) | 0.12 | *** | 75 (4.28) | 0.20 | *** |
| Private insurance | 745 (6.94) | 0.25 | 139 (8.42) | 0.28 | * | 75 (4.28) | 0.20 | *** |
| Other | 848 (7.90) | 0.03 | 161 (9.75) | 0.01 | * | 111 (6.34) | 0.01 | * |
| Median income of ZIP code | ||||||||
| $1–$41,999 |
3,036 (28.29) | 0.45 | 750 (45.43) | 0.50 | *** | 830 (47.40) | 0.50 | *** |
| $42,000–$51,999 | 2,775 (25.85) | 0.44 | 291 (17.63) | 0.38 | *** | 431 (24.61) | 0.43 | |
| $52,000–$67,999 | 2,415 (22.50) | 0.42 | 303 (18.35) | 0.39 | *** | 337 (19.25) | 0.39 | *** |
| ≥$68,000 | 2,507 (23.36) | 0.42 | 307 (18.59) | 0.39 | *** | 153 (8.74) | 0.28 | *** |
| Hospital characteristics | ||||||||
| For-profit | 1,122 (10.45) | 0.31 | 105 (6.36) | 0.24 | *** | 258 (14.73) | 0.35 | *** |
| Non-for-profit | 8,515 (79.33) | 0.40 | 1,312 (79.47) | 0.40 | *** | 1,282 (73.22) | 0.44 | *** |
| Government | 1,096 (10.21) | 0.30 | 234 (14.17) | 0.35 | *** | 211 (12.05) | 0.33 | *** |
| Bed size | ||||||||
| Small ≤50 | 430 (4.01) | 0.20 | 29 (1.76) | 0.13 | *** | 26 (1.48) | 0.12 | *** |
| Medium 50–200 | 3,235 (30.14) | 0.46 | 486 (29.44) | 0.46 | *** | 477 (27.24) | 0.45 | *** |
| Large >200 | 7,068 (65.85) | 0.47 | 1,136 (68.81) | 0.46 | *** | 1,248 (71.27) | 0.45 | *** |
| Rural | 1,280 (11.93) | 0.33 | 133 (8.06) | 0.27 | *** | 32 (1.83) | 0.13 | *** |
| Teaching | 1,087 (10.13) | 0.30 | 334 (20.23) | 0.40 | *** | 297 (16.96) | 0.38 | *** |
| State | ||||||||
| Arizona | 1,280 (11.93) | 0.31 | 49 (2.97) | 0.17 | *** | 229 (13.08) | 0.34 | |
| Colorado | 317 (2.95) | 0.17 | 22 (1.33) | 0.11 | *** | 39 (2.23) | 0.15 | |
| Florida | 2,384 (22.21) | 0.42 | 358 (21.68) | 0.41 | 1,085 (61.96) | 0.49 | *** | |
| Kentucky | 1,174 (10.94) | 0.32 | 53 (3.21) | 0.18 | *** | 9 (0.51) | 0.07 | *** |
| Maryland | 856 (7.98) | 0.27 | 422 (25.56) | 0.44 | *** | 33 (1.88) | 0.14 | *** |
| North Carolina | 1,251 (11.66) | 0.32 | 388 (23.50) | 0.42 | *** | 22 (1.26) | 0.11 | *** |
| New Jersey | 1,130 (10.53) | 0.31 | 240 (14.54) | 0.35 | *** | 278 (15.88) | 0.37 | *** |
| Oregon | 872 (8.12) | 0.27 | 14 (0.85) | 0.09 | *** | 19 (1.09) | 0.10 | *** |
| Rhode Island | 92 (0.86) | 0.09 | 7 (0.42) | 0.06 | 4 (0.23) | 0.05 | * | |
| Washington | 711 (6.62) | 0.25 | 31 (1.88) | 0.14 | *** | 23 (1.31) | 0.11 | *** |
| Wyoming | 666 (6.21) | 0.24 | 67 (4.06) | 0.20 | *** | 10 (0.57) | 0.08 | *** |
Notes: White is the reference group. T-tests were used to compare characteristics of the different groups.
p<0.05;
p<0.01;
p<0.001.
The measure of insurance coverage in the study: the study used the hospital discharge data for all eligible patients. And measure the primary payer as the insurance coverage. Hence, the study sample included patients who were enrolled in the Medicare Advantage, and patients who were enrolled in the Medicaid and dual eligible.
ACO, accountable care organization.
Figure 1 displays the rates of PPHs among individuals with an AD diagnosis by ACO affiliation and race and ethnicity. African American and Latinx patients had higher rates of PPHs, compared with White patients, on average. White and African American patients served by ACO-affiliated hospitals had lower rates of PPHs than their counterparts. The rates of PPHs were similar between Latinx patients receiving care at ACO-affiliated and non-affiliated hospitals.
Figure 1.
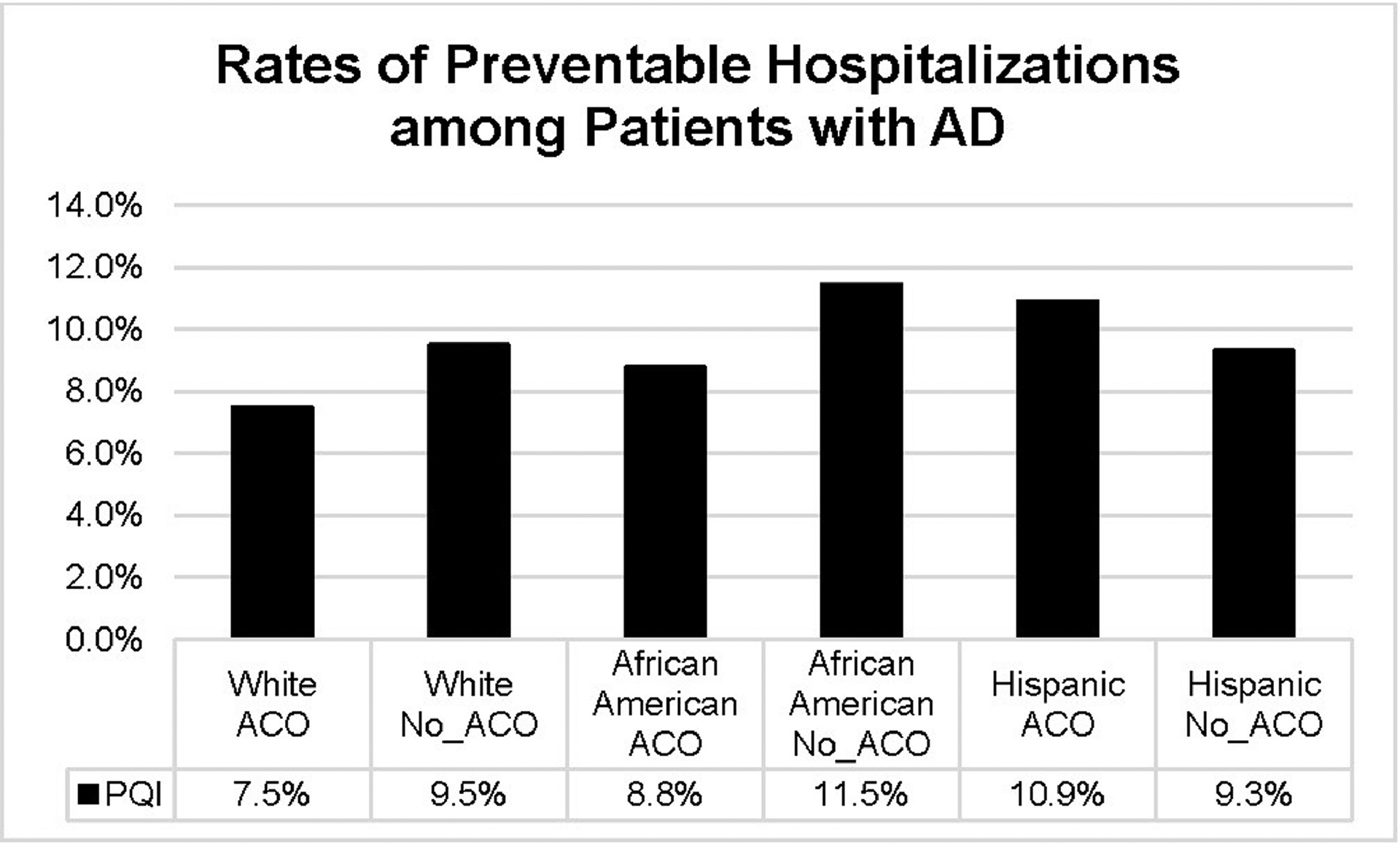
Rates of potentially preventable hospitalization among patients with Alzheimer’s disease by race/ethnicity and ACO affiliation.
Notes: Potentially preventable hospitalization was measured as any of the following AHRQ PQI indicators: uncontrolled diabetes (PQI 14), diabetes-related short-term and long-term complications (PQI 1, PQI 3), chronic obstructive pulmonary disease or asthma (PQI 5), hypertension (PQI 7), and heart failure (PQI 8). Total of 629 hospitals were included in11 states after merging the HCUP SID with the AHA annual survey.
ACO, accountable care organization; AD, Alzheimer disease; AHA, American Hospital Association; AHRQ, Agency for Healthcare Research and Quality; HCUP, Healthcare Cost and Utilization Project; PQI, prevention quality indicators; SID, state inpatient databases.
Table 2 presents the association between hospital ACO affiliation and PPH, controlling for patient, hospital, and state indicators. Hospitalized African American and Latinx patients with AD were more likely to have a PPH for a chronic condition (OR=1.28, p=0.01; OR=1.28, p=0.01) compared with White patients. ACO affiliation was negatively associated with PPHs (OR=0.86, p=0.02). The marginal effect of the hospital ACO association was also calculated (results not shown in table). Results showed that for 2 otherwise-average older adult patients with AD, 1 served by an ACO-affiliated hospital and the other served by an unaffiliated hospital, the probability of PPH would be 1.31 percentage points (p<0.05) lower for the patient served by an ACO-affiliated hospital.
Table 2.
Adjusted Odds of Potentially Preventable Hospitalization Among Patients With Alzheimer’s Disease
| Model 1 | Model 2 (PCM and cluster) | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Race/Ethnicity | ||||
| White | ref | ref | ||
| African American | 1.28 (1.06, 1.54) | 0.01 | 1.05 (0.87, 1.27) | 0.60 |
| Latinx | 1.28 (1.06, 1.55) | 0.01 | 0.90 (0.73, 1.10) | 0.30 |
| Admitted to ACO affiliated hospital | 0.86 (0.76, 0.98) | 0.02 | 0.66 (0.58, 0.75) | <0.001 |
| Age 65–74 years | ref | ref | ||
| Age >75 years | 1.27 (1.07, 1.51) | 0.01 | 1.22 (0.89, 1.67) | 0.21 |
| Female | 1.10 (0.98, 1.25) | 0.11 | 1.20 (0.82, 1.74) | 0.34 |
| Number of chronic conditions | 1.17 (1.15, 1.19) | <0.001 | 1.16 (1.12, 1.21) | <0.001 |
| Primary payer | ||||
| Medicare | ref | ref | ||
| Medicaid | 1.16 (0.63, 2.14) | 0.64 | 1.42 (0.65, 3.09) | 0.38 |
| Private insurance | 1.04 (0.54, 1.99) | 0.91 | 1.03 (0.40, 2.64) | 0.95 |
| Other | 0.85 (0.47, 1.54) | 0.59 | 0.60 (0.27, 1.33) | 0.21 |
| Median income of ZIP code | ||||
| $1-$41,999 | ref | ref | ||
| $42,000-$51,999 | 0.98 (0.84, 1.15) | 0.85 | 0.58 (0.34, 0.99) | 0.05 |
| $52,000-$67,999 | 0.93 (0.78, 1.10) | 0.40 | 0.54 (0.30, 0.96) | 0.04 |
| ≥$68,000 | 0.90 (0.74, 1.10) | 0.31 | 0.66 (0.33, 1.29) | 0.22 |
| Quarter 4 indicator | 0.88 (0.76, 1.01) | 0.08 | 0.69 (0.43, 1.10) | 0.12 |
| Hospital characteristics | ||||
| For-profit | ref | ref | ||
| Non-for-profit | 0.92 (0.75, 1.14) | 0.46 | 1.20 (0.65, 2.22) | 0.57 |
| Government | 0.97 (0.74, 1.27) | 0.84 | 1.13 (0.54, 2.35) | 0.75 |
| Bed size | ||||
| Small ≤50 | ref | Ref | ||
| Medium 50–200 | 0.84 (0.60, 1.17) | 0.30 | 0.78 (0.49, 1.25) | 0.30 |
| Large >200 | 0.82 (0.58, 1.16) | 0.26 | 1.12 (0.71, 1.78) | 0.62 |
| Rural | 1.16 (0.92, 1.46) | 0.22 | 0.81 (0.51, 1.29) | 0.37 |
| Teaching | 0.83 (0.68, 1.02) | 0.08 | 0.56 (0.39, 0.78) | <0.001 |
| State | ||||
| Florida | ref | Ref | ||
| Arizona | 0.69 (0.53, 0.88) | <0.001 | 0.84 (0.55, 1.29) | 0.43 |
| Colorado | 0.60 (0.38, 0.95) | 0.03 | 0.92 (0.44, 1.92) | 0.83 |
| Kentucky | 1.13 (0.88, 1.44) | 0.35 | 0.93 (0.51, 1.71) | 0.83 |
| Maryland | 0.93 (0.72, 1.20) | 0.56 | 1.31 (0.78, 2.20) | 0.31 |
| North Carolina | 0.82 (0.65, 1.04) | 0.11 | 0.96 (0.47, 1.96) | 0.92 |
| New Jersey | 1.16 (0.93, 1.45) | 0.19 | 2.06 (1.29, 3.27) | <0.001 |
| Oregon | 0.99 (0.75, 1.29) | 0.92 | 1.40 (0.83, 2.36) | 0.21 |
| Rhode Island | 1.40 (0.71, 2.78) | 0.33 | 1.58 (0.69, 3.63) | 0.28 |
| Washington | 0.79 (0.58, 1.08) | 0.14 | 0.93 (0.54, 1.62) | 0.80 |
| Wyoming | 1.07 (0.80, 1.43) | 0.66 | 1.67 (1.03, 2.71) | 0.04 |
| Constant | 0.04 (0.02, 0.06) | <0.001 | 0.05 (0.02, 0.14) | <0.001 |
Note: Model 1 is the baseline model using the logistic regression. Model 2 used propensity score matching and clustered hospital ID. The analysis also used the propensity scoring matching with replacement. And a “caliper” of 0.01 (i.e., requiring that propensity scores between an AD patients treated in an ACO affiliated hospital and the closet match to an AD patient treated in a non-ACO affiliated hospital equal to or less than 0.01) also was used. Matching variables were patient’s age, sex, race and ethnicity, number of chronic conditions, ZIP-code level income, rural and urban area, hospital teaching status, and control model. Marginal effects of the hospital ACO association were also calculated (results not shown in table). Results showed that if you had 2 otherwise-average individuals, 1 served by an ACO-affiliated and another served by an unaffiliated hospital, the probability of having PQI would be 1.11 percentage points (p<0.05) lower for those served by an affiliated hospital (Model 1); 3.29 percentage points (p<0.01) lower for those served by an affiliated hospital (Model 2). ICD-9 codes were updated to ICD-10 in 2015. ICD-9 codes were used for the discharges of quarter 1 – quarter 3 in 2015, and ICD10 for quarter 4 in 2015. A quarter indicator was controlled for to capture the possible trend shift due to the different coding systems. Boldface indicates statistical significance (*p<0.05; **p<0.01; ***p<0.001).
AD, Alzheimer’s disease; ACO, accountable care organization.
After applying propensity score matching (Model 2), results reflected a stronger association between PPHs and hospital ACO affiliation (OR=0.66, p<0.001). The probability of PPH became 3.29 percentage points (p<0.01) lower for those served by an affiliated hospital.
Table 3 displays the results of the decomposition analysis of PPHs among White versus African American patients and White versus Latinx patients. The predicted likelihood of a hospitalization being potentially preventable was 8.4% for White, 10.3% for African American, and 10.1% for Latinx patients. The model explained 9.1% of the observed difference in the rate of PPH for chronic conditions between White and African American patients, and 9.8% of the observed difference between White and Latinx patients.
Table 3.
Decomposition Results of Racial and Ethnic Disparities in Preventable Hospitalizations Among Patients With Alzheimer’s Disease
| Variable | Whites vs African American patients | White vs Latinx patients |
|---|---|---|
| Predicted preventable hospitalization rate among AD patients | 10.3% (African American) | 10.1% (Latinx) |
| 8.4% (White) | 8.4% (White) | |
| Difference in the preventable hospitalization rate | 1.8% | 1.7% |
| Percent of the difference explained | 9.1% | 9.8% |
| Significant factors, % contributed to the difference | ||
| Hospital ACO affiliation | 3.01%** | – |
| Patient’s age ≥75 years | −5.81%** | – |
| Patient’s number of chronic conditions | −6.41%* | −52.6%*** |
| Large bed size | – | −3.56%* |
| State | 15.91%* | 28.07%* |
Notes:
p<0.05;
p<0.01;
p<0.001.
Decomposition is a regression-based analysis. Stratified analysis by race and ethnicity was conducted first. Results (Appendix) showed that marginal effect of ACO affiliation was −1.6% for white (p=0.003), 3.1% for African American (p=0.054), and 0.01% (p=0.607) for Latinx AD patients. The decomposition then rearranged the terms of these stratified analyses in pair (White vs African American, White vs. Latinx) to indicate (1) the portion of the difference due to observed population characteristics (i.e., all of the control variables, such as the hospital ACO affiliation), and (2) the portion of the difference due to unobserved heterogeneities.
AD, Alzheimer Disease; ACO, accountable care organization.
Hospital ACO affiliation explained 3.0% (p<0.01) of the total disparity in PPHs for selected chronic conditions between White and African American patients. ACO affiliation did not explain the differences between White and Latinx patients. Geographic variation contributed to 15.9% (p<0.05) and 28.7% (p<0.05) of the racial and ethnic disparities in PPHs, respectively. Age and number of chronic conditions were counter-disparity factors. Table 1 shows that compared with White patients, African American patients with AD had fewer chronic conditions and were younger; these factors were linked to lower PPH rates among African American AD patients. In other words, if African American patients were the same age and had the same number of chronic conditions as White patients, the racial disparity in PPH rates would further increase. Similarly, Latinx AD patients had significantly fewer chronic conditions (6.99 vs 7.40), which accounted for their lower PPH rates.
DISCUSSION
The study found that among hospitalized patients with AD, hospital ACO affiliation was associated with lower odds of PPH for chronic conditions (diabetes, hypertension, chronic obstructive pulmonary disease or asthma, and heart failure), and estimated marginal effect ranged from −1.1 to 3.29 percentage points by different model specifications and race (White vs African American AD patients). However modest, the finding of this study is comparable to previous studies. For example, McWilliams and colleagues48 found this association insignificant, and Barath et al.42 found the ACO association with the preventable hospitalization for people with depression had a similar magnitude to this finding for AD patients (OR=0.89, 95% CI=0.87, 0.91). The average rate of preventable hospitalization for chronic conditions among the study sample was 8.87%. Even a conservative reduction of 1.1 percentage points is equivalent to 12% of the average PPH rate, which represents a major reduction in the average PPH rate.
It is also worth noting that this difference may reflect the differential adoption of care system redesign efforts that are occurring in communities where providers are forming ACOs.49 Additionally, this difference may reflect a selection bias; ACO participation is currently voluntary and provider communities with a greater capacity for quality improvement or greater willingness of hospitals and physicians to work together may be more likely to form ACOs.50,51
Results substantiate early concerns that African Americans would have lower access to ACOs.50,52 Additionally, hospital ACO affiliation explained 3.0% of the higher rate of PPHs among African Americans. In other words, if African Americans with AD had the same access to ACOs as Whites, their rate of PPHs for selected chronic conditions would be 3% lower.
However, providers that are participating in ACOs are likely different than ones that do not. Encouragingly, a previous study found that minority-serving ACOs spent more on care management, health information technology, and administration, suggesting that minority-serving ACOs are willing to commit to the care redesign efforts necessary to meet performance goals and this could lead to eventual reductions in disparities.30
Hospital ACO affiliation did not explain the differences between White and Latinx patients, which might be explained by the Hispanic Paradox, the phenomenon that Latinx have better health outcomes, compared to non-Latinx White people, although they often encounter lower SES.53 Consistent with this literature, the results of this study showed that Latinx older adults with AD had fewer chronic conditions, which narrows the disparity gap. Previous studies have shown that there is no consistent pattern of physician participation in ACOs across areas with high rates of Latinx residents.50 This inconsistency, along with the high concentration of Latinx hospitalizations across a small number of hospitals may require alternative statistical techniques to explore the impact of ACO affiliation in this patient population.54
A significant variation across states has been identified. Older adults with AD had significantly higher rates of PPHs for selected chronic conditions in Florida than in Arizona and Colorado. This state variation can be attributed to differential state policies, quality improvement initiatives, or differential availability of primary care providers, dementia specialists, chronic disease management programs, or social services for community-dwelling older adults. Lastly, this variation may be attributed to state-level differences in family support, education levels, and health behaviors.55
It is worth noting that the estimation model, which controlled for comprehensive measures at patients and hospital levels, explained 9% to 10% differences. In other words, a major part of the disparities may reflect unobserved measures among racial and ethnic groups. Future studies should aim to adjust for additional factors including diverse health needs or preferences, support from formal and informal caregivers, degree of care coordination and continuity of care, and quality of healthcare services. Meanwhile, the role that unobserved factors like systemic racism and discrimination play in society and the U.S. healthcare system and how these factors influence preventable hospitalizations cannot be discounted.56
This study adds to the growing literature that ACOs may be effective at improving quality of care and preventing avoidable healthcare utilization among high-need, high-cost and minority populations as well as the effectiveness of care coordination mechanisms at reducing avoidable healthcare utilization for people with AD.13–15,58 ACOs may even have greater potential to improve health outcomes for minorities with AD than for Whites, as many ACOs have invested resources in early identification and management of unmet medical and social needs that are often greater in minority populations.9,18,23
As more evidence is generated that supports the effectiveness of ACOs in improving the quality of care, especially among patients with AD, policymakers must pay attention to potential disparities created by ACO implementation. Although recent work has found no difference in the racial composition in the patient panel of ACO-participating and non-participating physician groups, this study found African Americans with AD are less likely to be hospitalized at ACO-affiliated hospitals.28 Additionally, ACO-affiliated hospitals tend to be large and located in urban areas.41,59 Additional incentives or modifications to the ACO model should be considered to attract minority-serving and rural providers to build or join ACOs.57,60
Limitations
This study has several limitations. First, the generalizability of the findings is limited by the data restrictions. The sample only included data from 11 states. Using inpatient claims data restricted the sample to hospitalized patients, excluding AD patients who were well managed. The AD study sample might under-represent racial and ethnic minority groups given their underdiagnoses. Additionally, conditions were measured using billing data; thus, the severity and mortality risk cannot be obtained. Second, this study used a cross-sectional design, so only associations were estimated. A longitudinal analysis examining hospitals before and after ACO affiliation would be helpful to estimate the causality. However, previous studies using difference-in-differences have found reductions in preventable hospitalizations after ACO formation or affiliation.24,25 Third, the data set only contains hospital-level ACO affiliation. It does not note patient-level ACO affiliation.41 This study used hospital affiliation with an ACO as a proxy measure for patient access to ACOs or ACO-related care redesign efforts. Furthermore, the data set does not contain patient home ZIP codes. A better measure of healthcare access, such as geographic location and distance between patient’s residence to hospital, would be useful to predict the use of an ACO-affiliated or non-affiliated hospital.41,57 Lastly, this data set does not contain the type of ACO that the hospital participated in (e.g., Medicare, Medicaid, commercial). Future study should focus on specific features of ACO models, including the reimbursement approach, team members, and size.
CONCLUSIONS
As the U.S. population ages, it will become increasingly diverse, as will the population with AD. It is crucial to identify cost-saving strategies that effectively promote coordination and quality care for this high-need group, particularly among minorities. This study is the first to provide evidence that ACOs may be effective at preventing PPHs for chronic conditions for people with AD and could reduce racial disparities. Results suggest that future research should explore the impacts of other value-based payment models on quality of care for people with AD and risk factors as well as the ability of these payment models to reduce disparities.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH National Institute of Aging (1R56AG062315-01) and National Institute on Minority Health and Health Disparities (R01MD011523) grants awarded to Dr. Jie Chen. The Robert Wood Johnson Foundation’s Health Policy Research Scholars program supports both Andrew Anderson and Deanna Barath. None of the funding bodies played a role in the design of the study, data collection, analysis, interpretation, manuscript writing, or the decision to submit this manuscript for publication. No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Beach SR, Schulz R, Friedman EM, Rodakowski J, Martsolf RG, James AE. Adverse consequences of unmet needs for care in high-need/high-cost older adults. J Gerontol B Psychol Sci Soc Sci. 2020;75(2):459–470. 10.1093/geronb/gby021. [DOI] [PubMed] [Google Scholar]
- 2.Lin PJ, Fillit HM, Cohen JT, Neumann PJ. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 2013;9(1):30–38. 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Davydow DS, Zivin K, Katon WJ, et al. Neuropsychiatric disorders and potentially preventable hospitalizations in a prospective cohort study of older Americans. J Gen Intern Med. 2014;29(10):1362–1371. 10.1007/s11606-014-2916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–194. 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367–429. 10.1016/j.jalz.2018.02.001. [DOI] [Google Scholar]
- 6.Desai U, Kirson NY, Ye W, Mehta NR, Wen J, Andrews JS. Trends in health service use and potentially avoidable hospitalizations before Alzheimer’s disease diagnosis: a matched, retrospective study of US Medicare beneficiaries. Alzheimers Dement (Amst). 2019;11(1):125–135. 10.1016/j.dadm.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moy E, Chang E, Barrett M, Centers for Disease Control and Prevention. Potentially preventable hospitalizations—United States, 2001–2009. MMWR Suppl. 2013;62(3):139–143. [PubMed] [Google Scholar]
- 8.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. 10.2105/ajph.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black BS, Johnston D, Rabins PV, Morrison A, Lyketsos C, Samus QM. Unmet needs of community-residing persons with dementia and their informal caregivers: findings from the maximizing independence at home study. J Am Geriatr Soc. 2013;61(12):2087–2095. 10.1111/jgs.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lines L Racial and Ethnic Disparities Among Individuals with Alzheimer’s Disease in the United States: A Literature Review. RTI Press. RTI Press Publication No. RR-0024–1412. Published 2014. 10.3768/rtipress.2014.rr.0024.1412. [DOI] [Google Scholar]
- 11.Dementias, including Alzheimer’s disease. Healthy People 2020. HHS. https://www.healthypeople.gov/2020/topics-objectives/topic/dementias-including-alzheimers-disease. Updated 2019. Accessed January 14, 2021. [Google Scholar]
- 12.Downer B, Al Snih S, Raji M, et al. Healthcare utilization of Mexican-American Medicare beneficiaries with and without Alzheimer’s disease and related dementias. PLoS One. 2020;15(1):e0227681. 10.1371/journal.pone.0227681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bass DM, Judge KS, Maslow K, et al. Impact of the care coordination program “Partners in Dementia Care” on veterans’ hospital admissions and emergency department visits. Alzheimers Dement (N Y). 2015;1(1):13–22. 10.1016/j.trci.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bass DM, Judge KS, Snow AL, et al. A controlled trial of Partners in Dementia Care: veteran outcomes after six and twelve months. Alzheimers Res Ther. 2014;6:9. 10.1186/alzrt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French DD, LaMantia MA, Livin LR, Herceg D, Alder CA, Boustani MA. Healthy Aging Brain Center improved care coordination and produced net savings. Health Aff (Millwood). 2014;33(5):613–618. 10.1377/hlthaff.2013.1221. [DOI] [PubMed] [Google Scholar]
- 16.Burwell SM. Setting value-based payment goals-HHS efforts to improve U.S. health care. New Engl J Med. 2015;372(10):897–899. 10.1056/nejmp1500445. [DOI] [PubMed] [Google Scholar]
- 17.Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. New Engl J Med. 2011;365(19):1753–1756. 10.1056/nejmp1111671. [DOI] [PubMed] [Google Scholar]
- 18.Anderson AC, Chen J. ACO affiliated hospitals increase implementation of care coordination strategies. Med Care. 2019;57(4):300–304. 10.1097/mlr.0000000000001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briggs ADM, Fraze TK, Glick AL, Beidler LB, Shortell SM, Fisher ES. How do accountable care organizations deliver preventive care services? A mixed-methods study. J Gen Intern Med. 2019;34(11):2451–2459. 10.1007/s11606-019-05271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winblad U, Mor V, McHugh JP, Rahman M. ACO-affiliated hospitals reduced rehospitalizations from skilled nursing facilities faster than other hospitals. Health Aff (Millwood). 2017;36(1):67–73. 10.1377/hlthaff.2016.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraze T, Lewis VA, Rodriguez HP, Fisher ES. Housing, transportation, and food: how ACOs seek to improve population health by addressing nonmedical needs of patients. Health Aff (Millwood). 2016;35(11):2109–2115. 10.1377/hlthaff.2016.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraze TK, Beidler LB, Briggs ADM, Colla CH. ‘Eyes in the home’: ACOs use home visits to improve care management, identify needs, and reduce hospital use. Health Aff (Millwood). 2019;38(6):1021–1027. 10.1377/hlthaff.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis VA, Schoenherr K, Fraze T, Cunningham A. Clinical coordination in accountable care organizations: a qualitative study. Health Care Manage Rev. 2019;44(2):127–136. 10.1097/hmr.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in Medicare. New Engl J Med. 2016;374(24):2357–2366. 10.1056/nejmsa1600142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chukmaitov A, Harless DW, Bazzoli GJ, Muhlestein DB. Preventable hospital admissions and 30-day all-cause readmissions: does hospital participation in accountable care organizations improve quality of care? Am J Med Qual. 2019;34(1):14–22. 10.1177/1062860618778786. [DOI] [PubMed] [Google Scholar]
- 26.Ouayogode MH, Mainor AJ, Meara E, Bynum JPW, Colla CH. Association between care management and outcomes among patients with complex needs in Medicare accountable care organizations. JAMA Netw Open. 2019;2(7):e196939. 10.1001/jamanetworkopen.2019.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association between Medicare accountable care organization implementation and spending among clinically vulnerable beneficiaries. JAMA Intern Med. 2016;176(8):1167–1175. 10.1001/jamainternmed.2016.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner RM, Kanter GP, Polsky D. Association of physician group participation in accountable care organizations with patient social and clinical characteristics. JAMA Netw Open. 2019;2(1):e187220. 10.1001/jamanetworkopen.2018.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis VA, Fisher ES, Colla CH. ACOs and disparities: the authors reply. Health Aff (Millwood). 2017;36(5):960. 10.1377/hlthaff.2017.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis VA, Fraze T, Fisher ES, Shortell SM, Colla CH. ACOs serving high proportions of racial and ethnic minorities lag in quality performance. Health Aff (Millwood). 2017;36(1):57–66. 10.1377/hlthaff.2016.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navathe AS, Bain AM, Werner RM. Do changes in post-acute care use at hospitals participating in an accountable care organization spillover to all Medicare beneficiaries? J Gen Intern Med. 2018;33(6):831–838. 10.1007/s11606-018-4368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisk M Health care as a public good. J Soc Philos. 1996;27(3):14–40. 10.1111/j.1467-9833.1996.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 33.Healthcare Cost and Utilization Project (HCUP). State Inpatient Databases (SID). Rockville, MD: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 34.2015 AHA Annual Survey. American Hospital Association. 2015. [Google Scholar]
- 35.Centers for Medicare and Medicaid Services. CCW Chronic Condition Algorithms. Chronic Conditions Data Warehouse. 2019. [Google Scholar]
- 36.Agency for Healthcare Research and Quality. Prevention Quality Indicators Technical Specifications Updates. Vol 2018.
- 37.Westrick E, Kogut S. Medicare ambulatory care indicators for the elderly: refinement of the access to care for the elderly project indicators: MedPAC; 2006.
- 38.Hackbarth GM, Christianson J, Miller ME. Report to the Congress: Medicare and the health care delivery system. June ed. Washington, DC: Medicare Payment Advisory Commission; 2015. [Google Scholar]
- 39.Feng Z, Silver BC, Segelman MD, et al. Developing risk-adjusted avoidable hospitalizations and emergency department visits quality measures: Medicare Payment Advisory Commission; 2019. [Google Scholar]
- 40.Niles J, Litton T, Mechanic R. An initial assessment of initiatives to improve care for high-need, high-cost individuals in accountable care organizations. Health Affairs Blog. April 11, 2019. 10.1377/hblog20190411.143015. [DOI] [Google Scholar]
- 41.Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals participating in ACOs tend to be large and urban, allowing access to capital and data. Health Aff (Millwood). 2016;35(3):431–439. 10.1377/hlthaff.2015.0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barath D, Amaize A, Chen J. Accountable care organizations and preventable hospitalizations among patients with depression. Am J Prev Med. 2020;59(1):e1–e10. 10.1016/j.amepre.2020.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical Software Components: Boston College Department of Economics; 2003. Revised February 1, 2018. [Google Scholar]
- 44.Stuart EA, Huskamp HA, Duckworth K, et al. Using propensity scores in difference-in-differences models to estimate the effects of a policy change. Health Serv Outcomes Res Methodol. 2014;14(4):166–182. 10.1007/s10742-014-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairlie R An extension of the Blinder-Oaxaca decomposition technique to logit and probit models. J Econ Soc Meas. 2005;30(4):305–316. 10.3233/jem-2005-0259. [DOI] [Google Scholar]
- 46.Oaxaca RL, Ransom MR. Identification in detailed wage decompositions. Rev Econ Stat. 1999;81(1):154–157. 10.1162/003465399767923908. [DOI] [Google Scholar]
- 47.What is Alzheimer’s Disease? Alzheimer’s Association. https://www.alz.org/alzheimers-dementia/what-is-alzheimers. Published 2020. Accessed January 14, 2021. [Google Scholar]
- 48.McWilliams JM, Chernew ME, Landon BE. Medicare ACO program savings not tied to preventable hospitalizations or concentrated among high-risk patients. Health Aff (Millwood). 2017;36(12):2085–2093. 10.1377/hlthaff.2017.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bleser WK, Muhlestein D, Saunders RS, McClellan MB. Half a decade in, Medicare accountable care organizations are generating net savings: part 2. Health Affairs Blog. September 21, 2018. 10.1377/hblog20180920.604462. [DOI] [Google Scholar]
- 50.Yasaitis LC, Pajerowski W, Polsky D, Werner RM. Physicians’ participation in ACOs is lower in places with vulnerable populations than in more affluent communities. Health Aff (Millwood). 2016;35(8):1382–1390. 10.1377/hlthaff.2015.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markovitz AA, Hollingsworth JM, Ayanian JZ, et al. Risk adjustment in Medicare ACO program deters coding increases but may lead ACOs to drop high-risk beneficiaries. Health Aff (Millwood). 2019;38(2):253–261. 10.1377/hlthaff.2018.05407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Millwood). 2014;33(1):95–102. 10.1377/hlthaff.2013.1063. [DOI] [PubMed] [Google Scholar]
- 53.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11(3):496–518. [PubMed] [Google Scholar]
- 54.Jha AK, Orav EJ, Zheng J, Epstein AM. The characteristics and performance of hospitals that care for elderly Hispanic Americans. Health Aff (Millwood). 2008;27(2):528–537. 10.1377/hlthaff.27.2.528. [DOI] [PubMed] [Google Scholar]
- 55.Falster MO, Jorm LR, Douglas KA, Blyth FM, Elliott RF, Leyland AH. Sociodemographic and health characteristics, rather than primary care supply, are major drivers of geographic variation in preventable hospitalizations in Australia. Med Care. 2015;53(5):436–445. 10.1097/mlr.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Ann Rev Sociol. 2015;41:311–330. 10.1146/annurev-soc-073014-112305. [DOI] [Google Scholar]
- 57.Zhu X, Ullrich F, Huang H, Mueller KJ. Rural Hospital Participation in Medicare Accountable Care Organizations. Rural Policy Brief: RUPRI Center for Rural Health Policy Analysis; 2020. [Google Scholar]
- 58.Sen AP, Chen LM, Wong Samson L, Epstein AM, Joynt Maddox KE. Performance in the Medicare shared savings program by accountable care organizations disproportionately serving dual and disabled populations. Med Care. 2018;56(9):805–811. 10.1097/mlr.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, DuGoff EH, Novak P, Wang MQ. Variation of hospital-based adoption of care coordination services by community-level social determinants of health. Health Care Manage Rev. 2018;45(4):332–341. 10.1097/hmr.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Malley AS, Rich EC, Sarwar R, et al. How accountable care organizations use population segmentation to care for high-need, high-cost patients. The Commonwealth Fund. https://www.commonwealthfund.org/publications/issue-briefs/2019/jan/how-acos-use-segmentation-high-need-high-cost. Published Janury 3, 2019. Accessed January 14, 2021. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


