Abstract
Background:
Erectile dysfunction (ED) has been shown to be related with inflammatory markers in humans. Chronic infusion of TNF-α caused ED in mice while TNF-α knockout mice exhibited improvement in the relaxation of the corpus cavernosum (CC).
Aim:
Since obesity triggers an inflammatory process, we aimed to investigate the hypothesis that in obesity, Toll-like receptor 9 (TLR9) activation leads to increased TNF-α levels and an impairment in CC reactivity.
Methods:
Four-week old male C57BL6 (WT) and TLR9 mutant (TLR9MUT) mice were fed a standard chow or high fat diet (HFD) for 12 weeks. Body weight and non-fasting blood glucose were analyzed. Contractile and relaxation responses of the CC were evaluated by electrical field stimulation (EFS) and concentration response curves to phenylephrine (PE) and acetylcholine (ACh). Protein expression of nNOS, TNF-α, TNF-R1, TLR9 and MyD88 were measured by western blot. Plasma levels of TNF-α were measured by ELISA.
Outcome:
In obesity, impaired cavernosal relaxation is associated with the activation of the innate immune system, by increasing the production of TNF- α through the activation of TLR9 in the macrophages.
Results:
After 12 weeks of HFD both WT and TLR9MUT mice had increased body weight and non-fasting blood glucose compared to standard chow. In the CC, ACh-induced relaxation was not changed. A trend to increased contraction to PE and KCl was seen in WT HFD only. EFS-induced relaxation of the CC was decreased in WT HFD as well as nNOS expression in the CC of WT HFD, but not in TLR9MUT HFD. In the CC, protein expression of TLR9 and MyD88 was similar in all groups. While circulating levels of TNF-α presented only a trend to increase in mice fed HFD, the CC expression of TNF-α was increased only in WT HFD mice.
Clinical Translation:
The innate immune system can be a target for the treatment of erectile complications in obesity.
Strengths and limitations:
This is the first study demonstrating that activation of TLR9 expressed in macrophages leads to impaired cavernosal relaxation. The main limitation of the study is the lack of understanding about the source/expression of the macrophages in the cavernous tissue. Further, herein, the experiments were performed only in isolated cavernous tissue (in vitro), thus the lack of knowledge on how the TLR9 modulates the in vivo response of the erectile tissue is another limitation of this study.
Conclusion:
Our findings indicate that CC dysfunction observed in obesity is at least in part mediated by the production of TNF-α upon activation of TLR9 expressed in the macrophages.
Keywords: obesity, innate immune system, macrophages, erectile dysfunction
Introduction
Erectile dysfunction (ED) has been associated with increased inflammation, independent of overt cardiovascular disease. For example, increased levels of inflammatory markers, including interleukin 1-β (IL-1β) and tumor necrosis factor alpha (TNF-α) were found in ED patients with or without coronary artery disease (CAD).(1) In addition, several studies are demonstrating that TNF-α plays an important role in the development of sexual dysfunction elicited by different etiological processes such as hormonal imbalance, neuronal damage, psychological or vasculogenic ED.(2–5) Indeed, it was earlier demonstrated that mice infused with TNF-α exhibited increased contraction and decreased relaxation of the corpus cavernosum (CC) whereas erectile function was significantly improved in TNF-α knockout mice.(6, 7) Moreover, treatment with the TNF receptor antagonist improved erectile function in aged and depressed rats.(3, 8)
Obesity is related with several cardiovascular disorders and organ damage, including ED. In this context, inflammation could be the link between obesity and ED since obesity is considered a low-grade, chronic inflammatory condition.(9) Inflammation can be triggered by activation of Toll like receptors (TLRs), which are pattern-recognition receptors of the innate immune system, by sensing pathogen- and damage-associated molecular patterns (PAMPs and DAMPS, respectively). DAMPS and PAMPs could arise as a consequence of the cellular and molecular changes in obesity and trigger the activation of TLRs. Among them, TLR9 expression seems to be increased in overweight and obese individuals with non-alcoholic steatohepatitis (NASH) and animals.(10) Liver samples of human subjects that underwent bariatric surgery, as well as liver samples from mice fed atherogenic diet, exhibited increased gene and protein expression of TLR9. In mice, this increase was accompanied by increased expression of p65 subunit of NFκB, macrophages and Kupffer cells marker (F4/80) and myeloperoxidase producing neutrophils (MPO) and these changes were, at least in part, suppressed in mouse lacking TLR9.(10) TLR9 is mainly expressed in immune system cells such as dendritic cells and macrophages, although it can be also expressed in other non-immune cells including muscle and epithelial cells. TLR9 recognizes unmethylated CpG DNA, such as fragments of mitochondrial DNA derived from cellular injury and necrosis. After activation, it will recruit the adaptor protein myeloid differentiation primary response protein 88 (MyD88) which in turn activates the mitogen-activated protein kinases (MAPK) and NFκB to increase the production of proinflammatory cytokines, including TNF-α.(11) Therefore, TLR9 has been related to cardiovascular diseases associated with inflammatory states and it has been shown to lead to vascular and non-vascular organ damage in hypertension and obesity.(12, 13) However, only a few studies have addressed whether TLRs might be involved in the physiopathology of ED. Previously, our group was the first to show that activation of TLR1/2 led to the impairment of the contraction and relaxation of the CC and this was the first evidence that TLRs activation could contribute to ED.(14) Similarly, in angiotensin II-induced hypertension in mice and rats, as well as in diabetic rats, ED was demonstrated to be associated with the activation of TLR4 and its downstream protein MyD88 overexpression.(15–17) The evidence that TLR9 may play a role in the ED was proposed by Rodrigues et al., when it was shown that the CC of rats with heart failure had increased protein expression of TLR9 and TLR9 knockout mice exhibited decreased contractile profile, which favors penile erection.(18) There are convincing data in the literature that TNF-α contributes to the impaired function of the CC and plays a role in the development of ED. However, whether TLR9 is involved in obesity-induced ED is not yet established. We hypothesize that, in obesity, activation of TLR9 of the macrophages will increase the levels of TNF-α in the CC. Hence, this work aimed to investigate the cavernosal reactivity in a TLR9 mutant mice fed HFD. In this model of TLR9 mutant mice, macrophages do not produce TNF-α upon stimulation of TLR9. The improvement of the cavernosal reactivity in TLR9 mutant mice fed HFD is proof of concept of the contribution of TLR9 for the development of ED. Therefore, TLR9 might be a potential target for the development of new therapies for the treatment of ED.
Methods
Animals
All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and ethical standards of Institutional Animal Care and Use Committee of Augusta University. Male 4-week-old C57BL/6J (WT) and C57BL/6J-Tlr9M7Btlr/Mmjax mice (TLR9MUT), were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and used in all experiments. Animals were housed 3-4 per cage, in temperature-controlled facilities on a 12-hour light-dark cycle, fed ad libitum either a regular diet (carbohydrate; 70%; protein: 20%; fat; 10%) or a HFD to induce obesity (HFD: carbohydrate; 20%; protein: 20%; fat: 60%; Research Diets, New Brunswick, NJ, USA) for 12 weeks.
Functional studies
The mice were anesthetized with isoflurane (5% in 100% O2) and tail blood samples were collected for non-fasting glucose measurements using a glucometer (Freestyle Lite, Abbott, Alameda, CA, USA). The penises were surgically removed and placed in chilled physiological buffer of the following composition (mmol/L): NaCl, 130; NaHCO3, 14.9; dextrose, 5.5; KCl, 4.7; KH2PO4, 1.18; MgSO47H2O, 1.17 and CaCl22H2O, 1.6. Following removal of the glans penis and urethra, the penile tissue was cleaned from connective and adventitial tissues and the fibrous septum separating the corpora cavernosa was opened from its proximal extremity towards the penile shaft. A slit was made in the tunica albuginea along the shaft to obtain two strips (11x1x1 mm) of CC from each mouse. Each strip was mounted in a myograph for isometric force recording (Danish Myograph Technology, Aarhus, Denmark) coupled to a PowerLab 8/SP™ data acquisition system (software Chart 5.0, ADInstruments, Colorado Springs, USA). The bathing solution was maintained at 37°C and continuously aerated with 95% O2 and 5% CO2. Tissues were allowed to equilibrate for 45 min under a resting tension of 5 mN.
After equilibration, the ability of the preparations to develop contraction was assessed in 120 mmol/L K+-substituted physiological buffer. Contractile and relaxation responses were performed in separated strips. Contraction obtained by transmural electrical field stimulation (EFS) was performed in the presence of the nitric oxidase synthase (NOS) inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME; 100 μmol/L) and in the presence of the muscarinic receptor antagonist, atropine (1 μmol/L). Frequency-response curve was acquired by placing the strips between two platinum ring electrodes connected to a Grass S88 stimulator (Astro-Med Industrial Park, RI, USA). EFS was conducted at 50 V, 1 ms pulse width and trains of stimuli lasting 10 sec for each frequency at the frequencies of 1, 2, 4, 8 and 16 Hz. An interval of 2 minutes was given between stimuli. After the last stimulus, concentration response curves to phenylephrine (PE) were obtained (10 nmol/L – 30 μmol/L). For relaxation responses, strips were contracted with PE (10 μmol/L) and concentration response curves to acetylcholine (ACh 1 nmol/L – 10 μmol/L) were obtained. EFS-induced relaxation was performed using the same settings as for the contraction, in the absence of L-NAME and atropine. Contractions were analyzed as force (mN) and relaxation was taken as the percentage of the contraction induced by PE.
Determination of TNF-α serum levels
Blood collected from the abdominal aorta was used to obtain serum samples for determination of systemic TNF-α levels. After arterial blood collection without anticoagulant, samples were centrifuged (1,000g) for 10 min at 4°C. The top yellow serum layer was collected without disturbing the white buffy layer and frozen at −80°C until the experiment was performed. On the day of the experiment, serum was processed according the manufacturer’s instructions and the assays were performed in duplicates. A high-sensitivity TNF-α mouse ELISA kit (Invitrogen, Vienna, Austria) was used for this assay. A microplate covered with biotin-conjugated anti-mouse TNF-α antibody was used to bind to TNF-α from samples and standards. Streptavidin-HRP was used to bind to the biotin-conjugated anti-mouse TNF-α antibody and biotinyltyramide was used to amplify the reaction. Reaction was terminated by addition of phosphoric acid. The final reaction formed a colored product proportional to the amount of TNF-α in the sample, and absorbance was read at 450 nm.
Corpora cavernosa protein extraction and western blot analysis
The CC was obtained after excising and cleaning the penises as described above. Samples were immediately frozen at −80°C. Briefly, CC tissues were pulverized, homogenized in lysis extraction buffer (100 mmol/L Tris–HCl, 1 mmol/L EDTA and 1 mmol/L EGTA containing phenylmethylsulphonyl fluoride (PMSF), protease inhibitor and phosphatase inhibitors), and centrifuged at 10,000g for 20 min at 4°C. Supernatant was collected as cytosolic fraction, and pellet was suspended in extraction buffer containing 1% Triton X-100 to obtain the membrane fraction. Protein was estimated using a commercially available kit from Bio Rad (Hercules, CA), and 40 μg of cytosolic protein was loaded for western blot, Samples were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Proteins were subsequently transferred onto nitrocellulose membranes (BioRad, Hercules, U.S.A.). Membranes were blocked by treatment with 5% milk in Tris-buffered saline containing 0.05% tween 20, and probed with antibodies against the protein of interest as follow: TLR9 (1:1000), nNOS (1:1000), TNF-α (1:1000), TNF-R1 (1:1000), MyD88 (1:1000). HSP90 (1:1000) and β-actin (1:50,000) were used as housekeeping proteins for loading normalization. Next, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody. Immunoreactivity was detected by enhanced chemiluminescence and the protein expression was normalized to β-actin or HSP90 expression. The representative blot was shown as cropped images of the membrane bands. The full membrane of each protein is presented in the supplement file 1.
Drugs, solutions and antibodies
Acetylcholine, atropine, L-NAME and PE were purchased from Millipore-Sigma (St. Louis, MO, USA). The antibodies against HSP90 (sc-515081), TNF-R1 (sc-8436) and TNF-α (sc-52746) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The antibodies against TLR9 (ab134368), and MyD88 (ab36076) were obtained from Abcam (San Francisco, CA, U.S.A.) and nNOS (07-571) from Millipore.
All other reagents used were of analytical grade. Stock solutions were prepared in deionized water and stored in aliquots at −20°C; dilutions were made immediately before use.
Data analysis
Relaxation responses are expressed as percentage of PE-induced maximum contraction. Curves were fitted to all the data using non-linear regression and halfmaximum response (pEC50) to each drug, expressed as –log molar (mol/L), was used to compare potency. All data were expressed as means ± S.E.M. of n experiments. The statistical significance of all differences between mean values was calculated using student’s t test or ANOVA followed by Bonferroni’s post hoc test as applicable. (GraphPAD Software, version 8.1.2, San Diego, USA). To reject the null hypothesis a level of p≤0.05 was considered to be statistically significant.
Results
Body weight and non-fasting blood glucose.
At the end of the study, WT mice fed HFD presented a significant higher body weight compared to WT mice fed standard chow. Body weight gain in TLR9MUT mice followed the same pattern of WT mice, according with the type of diet they were receiving. Non-fasting blood glucose at 12 weeks of study was similar in mice fed standard chow or fed HFD. These data are summarized at table 1.
Table 1.
Body weight and non-fasting blood glucose (NFBG) at the end of the study from WT mice fed standard chow (WT) or high fat diet (WT HFD) and TLR9 mutant mice fed standard chow (TLR9MUT) or high fat diet (TLR9MUT HFD) for 12 weeks. Data are mean ± S.E.M of 6 animals.
| Group | Body Weight (g) | NFBG (mg/dl) |
|---|---|---|
| WT (n=6) | 29 ± 1 | 149 ± 3.5 |
| WT HFD (n=6) | 47 ± 4* | 174 ± 11 |
| TLR9MUT (n=6) | 28 ± 2 | 157 ± 5.6 |
| TLR9MUT HFD (n=6) | 48 ± 4* | 174 ± 13 |
indicates P≤0.05 compared to the respective group fed standard chow diet.
Cavernosal reactivity
PE induced maximal contraction of the CC with a force of 1.66 ± 0.25 mN in WT mice fed standard chow (n=6), compared to 2.06 ± 0.22 mN in WT fed HFD (n=9). In TLR9MUT, maximal contraction to PE was 1.51 ± 0.26 vs 1.18 ± 0.15 mN, in standard chow vs HFD, respectively. In mice fed HFD, the maximal contraction to PE was significantly lower in TLR9MUT compared to WT. No differences in the potency of PE were observed in both standard chow (pEC50: 5.58 ± 0.05 WT vs 5.70 ± 0.05 TLR9MUT) or HFD (pEC50: 5.53 ± 0.07 WT vs 5.58 ± 0.07 TLR9MUT; Fig 1A). Electrical field stimulation in the presence of L-NAME and atropine caused contraction of the CC in a similar magnitude for all the groups studied (Fig 1B). KCl (120 mM) induced similar contraction of the CC in all groups with a force of 1.32 ± 0.25 mN in WT, 1.18 ± 0.15 mN in TLR9MUT, 1.83 ± 0.21 mN in WT HFD and 1.14 ± 0.16 mN in TLR9MUT HFD (P > 0.05; Fig 1C).
Figure 1.
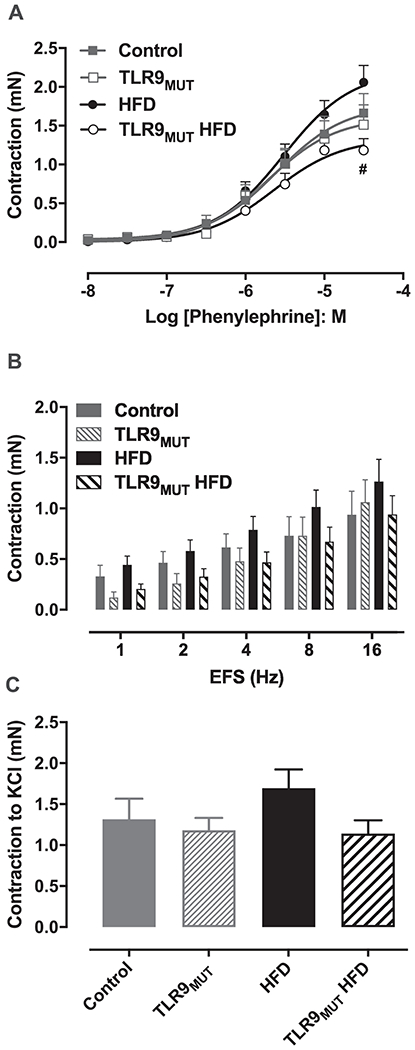
Contractile responses to phenylephrine (panel A), electrical field stimulation (EFS; panel B) and potassium chloride (KCl 120 mM; C) in the corpus cavernosum of WT and TLR9MUT mice fed standard chow or high fat diet (HFD). Data are mean ± S.E.M of 6-9 mice.
#P<0.05 compared to WT HFD.
Endothelium-dependent relaxation of the CC was obtained by concentration responses curves to ACh. Maximal response to ACh was not significantly changed in the CC of the studied groups (WT: 84 ± 6%; n=6 vs WT HFD: 75 ± 2%; n=7 and TLR9MUT: 78 ± 6%; n=6 vs TLR9MUT HFD: 71 ± 6%, n=9). Likewise, no differences were observed in the potency of ACh in the CC of mice fed standard chow or HFD, in any strain (pEC50: 7.11 ± 0.11 WT; 7.06 ± 0.12 HFD; 7.03 ± 0.09 TLR9MUT; 7.00 ± 012 TLR9MUT HFD; Fig 2A). On the other hand, EFS caused relaxation of the CC of WT mice (at 16 Hz: 84 ± 5%, n=6), which was significantly reduced at all frequencies when mice were fed HFD (at 16 Hz: 59 ± 7.5%, n = 8). This reduced relaxation was not observed when TLR9MUT mice were fed HFD (at 16 Hz: 97 ± 7%, n=8; Fig 2B). In a similar fashion, nNOS expression was significantly decreased in the CC when WT mice were fed HFD. However, this reduction was not observed in the CC of TLR9MUT fed HFD (Fig 2C).
Figure 2.
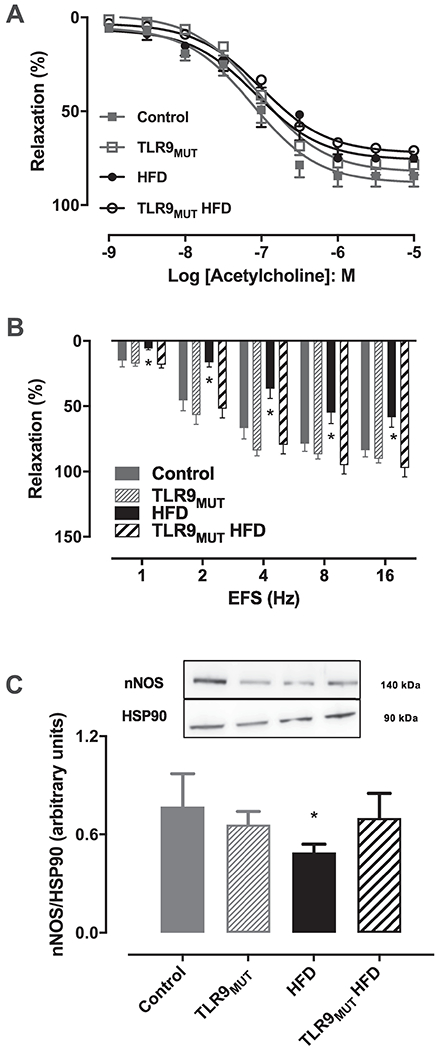
Relaxation induced by acetylcholine (panel A; n=6-9) and electrical field stimulation (EFS; panel B; n=6-9) and expression of neuronal nitric oxide synthase (nNOS; panel C; n=4) in the corpus cavernosum of WT and TLR9MUT mice fed standard chow or high fat diet (HFD). Data are mean ± S.E.M of n mice. *P<0.05 compared to WT mice. The full image of the membranes is available in the supplementary file 1.
TNF-α and TNF-R1 expression.
Next, we measured the expression of TNF-α and TNF-R1 in the CC (Fig 3A and 3B, respectively). Expression of TNF-R1 was similar in the CC of all groups, whereas a higher expression of TNF-α was observed in the CC of WT mice fed HFD (TNF-α/β-actin ratio of 1.25 ± 0.19) compared to WT fed standard chow (TNF-α/β-actin ratio of 0.62 ± 0.20). On the other hand, this increased expression of TNF-α was not seen in the CC of TLR9MUT mice fed HFD (TNF-α/β-actin ratio of 0.92 ± 0.25) compared to TLR9MUT fed standard chow (TNF-α/β-actin ratio of 0.75 ± 0.12). Plasma levels of TNF-α were not changed in any of the groups (Fig 3C).
Figure 3.
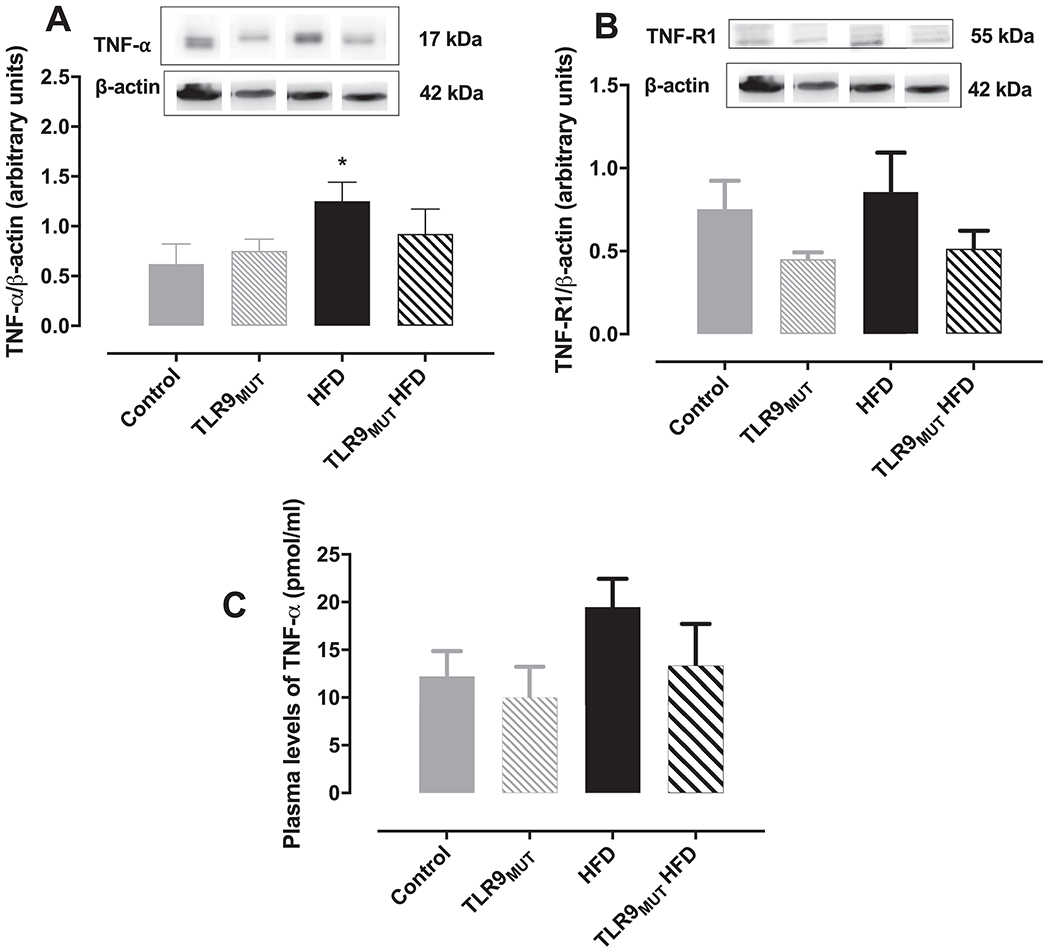
Expression of TNF-α (panel A; n=4) and TNF receptor 1 (TNF-R1; panel B; n=8) in the corpus cavernosum of WT and TLR9MUT mice fed standard chow or high fat diet (HFD). Graph C represents the circulating levels of TNF-α (n=5-6). Data are mean ± S.E.M of n mice.
*P<0.05 compared to WT mice. The full image of the membranes is available in the supplementary file 1.
TLR9 signaling in the CC
Western blot analysis revealed that TLR9 as well as its downstream adaptor protein MyD88 are expressed in the CC of mice (Fig 4A and 4B, respectively). Both proteins expression was not changed by HFD or in TLR9MUT mice. This suggests that TLR9 activation in macrophages, and not in the CC, is responsible to cause the increase in the levels of TNF-α in the CC.
Figure 4.
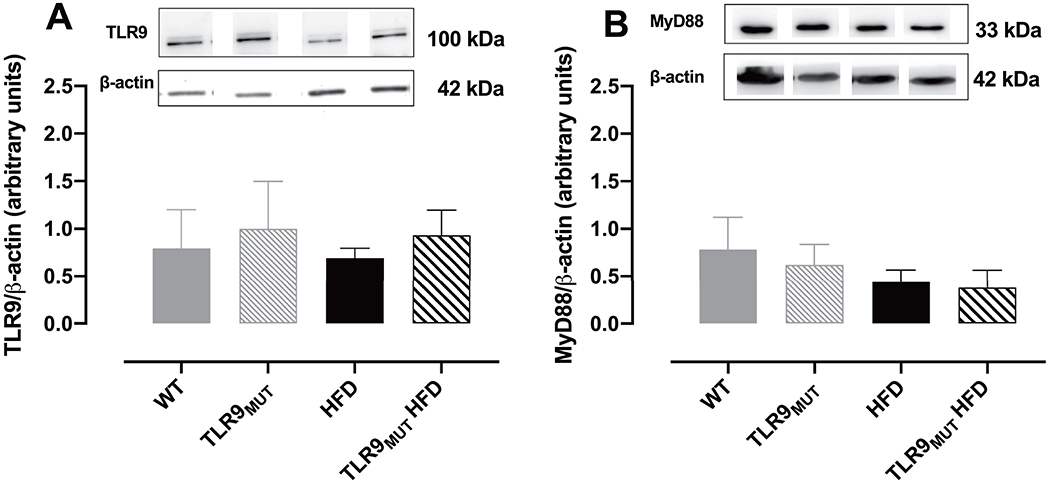
Figures A and B are the representative blot and graph of the expression of TLR9 (panel A; n=4) and its downstream adaptor protein MyD88 (panel B; n=6) in the corpus cavernosum of WT and TLR9MUT mice fed standard chow or high fat diet (HFD).
Data are mean ± S.E.M of n mice. The full image of the membranes is available in the supplementary file 1.
Discussion
Currently, the role of the innate immune system for the development of ED has been under-investigated and in obesity it has not been addressed. The innate immune system is a host defense mechanism formed by pattern recognition receptors (PRRs) located on the endosome and cell surface that recognize specific motifs to activate the downstream signaling cascades, increasing proinflammatory cells and recruiting immune cells to eliminate the perceived danger(19). TLRs are PRRs that recognizes DAMPs and PAMPs, and their activation has been reported in several chronic diseases. Indeed, evidence supports the idea that activation of the innate immune system could play a role in the development of sexual dysfunction in males and females(20). Since obesity is considered a low grade of chronic inflammation, herein we tested the hypothesis that ED caused by a HFD could be triggered by the release of TNF-α produced by TLR9 on macrophages, since it is believed that macrophage infiltration into end organ tissues contributes to the pro-inflammatory state.(21) TNF-α can be produced upon activation of TLR9(22) and previous studies have shown that TNF-α impairs the cavernosal reactivity.(6, 7) It has also been proposed that TNF-α induces hypercontractility of the CC by stimulation of sympathetic nerves and calcium sensitization. In this study, we observed a trend of higher contraction to PE, EFS and KCl in the CC of WT mice fed HFD, but not in TLR9MUT HFD mice, which exhibited a significant smaller contraction to PE.(23) However, because these changes were not significant, we did not focus on the contractile mechanisms, although it can be speculated that a prolonged time course of HFD would favor the calcium sensitization and the contractile mechanisms related to the flacid state of the CC. Endothelium-dependent and neurogenic relaxation of the CC were performed. Contrary to previous studies investigating cavernosal/erectile dysfunction in diabetic mice(24) or mice fed HFD,(25–27) in our study it was not observed a significant impairment in the endothelium-dependent relaxation induced by ACh. Although all studies led to increased body weight, the diet composition, age of the animals and/or duration of the studies differed slightly, which could be leading to a different profile of metabolic changes and thus, a different pattern for the development of endothelial dysfunction. Indeed, a recent study showed that 12 weeks of cafeteria diet was more effective than 12 weeks of HFD to induce vascular dysfunction and changes in the perivascular adipose tissue, despite of similar body weight gain, blood glucose levels and fat pad mass.(28) This corroborates the idea that even the small differences on the diet composition can cause a different pattern for the development of endothelial dysfunction. Therefore, we believe that a prolonged intake of the HFD used herein would result in impairment of endothelial-dependent relaxation in the corpus cavernosum. However, in this study we only observed that WT mice fed HFD had impaired cavernosal relaxation induced by neuronal release of NO and it was accompanied by decreased expression of nNOS in the CC. On the other hand, the neuronal NO induced relaxation of the CC as well as expression of nNOS were not reduced in TLR9MUT mice fed HFD. Since TLR9MUT mice do not produce TNF-α upon activation of TLR9 expressed in macrophages, it suggests that lack of TNF-α had a protective role for the cavernosal function. This corroborates the previous finding that TNF-α infusion impairs the CC reactivity whereas TNF-α knockout or its antagonism led to improved CC reactivity and NOS expression in mice.(6–8) Furthermore, enhanced TNF-α causes suppression of testosterone release, decrease in nNOS expression and structural changes in the CC, leading to ED.(2) TNF-α also increases reactive oxygen species (ROS) production and leads to ED in T1DM rats, which is prevented by the treatment with the TNF-α antibody, infliximab.(29) In this study, we did not measure ROS in the CC, however, in similar fashion, enhanced production of ROS in the prostate of mice fed HFD was prevented in TLR9 mutant mice.(13) Because of the lack of changes in ACh-induced relaxation of the CC, the expression of eNOS was not evaluated herein. Indeed, increased ROS in T2DM impairs the relaxation of the CC to ACh by decreasing NO bioavailability, independent of changes in the expression of eNOS and nNOS.(30,31) However, studies demonstrate that the inhibition of TNF-α improves eNOS expression in different models of ED.(3, 8) Therefore, our data bring the novel insight that in obesity, increased TNF-α is being released from the macrophages upon activation of TLR9 and contributing for the development of ED. Hence, TNF-α and TNF-R1 expression was measured in the CC. It was found increased expression of TNF-α in the CC of WT mice fed HFD. However, in TLR9MUT mice fed HFD, TNF-α expression was similar to that observed in WT mice fed standard chow, reinforcing the evidence that the impaired cavernosal reactivity is associated with the higher levels of TNF-α produced by TLR9 stimulation in the macrophages. It is important to highlight that the expression of TLR9 and its downstream protein cascade were not changed in the CC of both strains of mice fed HFD. This reinforces the idea that the activation of TLR9 expressed in the macrophages (and not in the CC) is responsible to cause the increase in the levels of TNF-α in the CC. Contrary to our findings, expression of TLR9 in the CC had been previously described and it was demonstrated to be augmented in the CC of rats with heart failure.(18) Nonetheless, no other studies have studied TLR9 in the CC and ED, and the differences may be due to the different species and/or pathology, since heart failure may cause a more aggressive and rapid impact on the activation of the innate immune system. Obesity is also correlated with increased levels of macrophages and thus, migration of macrophages to the CC in obesity cannot be ruled out. In a traumatic model of arteriogenic ED, the decreased vascularization of the penis led to augmented deposition of fat in the cavernous smooth muscle.(32) The source of macrophages releasing TNF-α to the CC could be either from the fat infiltrated in the cavernous tissue or from the adipose cells in the surrounding area releasing TNF-α for the adjacent tissues. It has been proposed that a vicious cycle between adipocytes and macrophages aggravates the inflammatory state in obesity, through a paracrine loop that involves TNF-α and free fatty acids, where TNF-α induces the release of free fatty acids from the adipocytes, which in turn will induce inflammatory changes by stimulating TNF-α production by macrophages in the adipocytes.(33) Although the source of the TNF-α in our study is unknown, it could be suggested that it is through the activation of TLR9 since a recent study revealed that in obesity there is an increased expression of TLR9 in the macrophages extracted from the epididymal fat pad accompanied by enhanced levels of DNA fragments in the circulation and in the adipose tissue that might be activating TLR9.(34) Furthermore, HFD could be inducing macrophage polarization to the proinflammatory state in the CC or increasing the levels of TNF-α in the CC by paracrine release of TNF-α from the adjacent adipose tissue after activation of TLR9 in the infiltrated macrophages. Indeed, paracrine stimulation of TNF-α and other cytokines production has been previously proposed in an in vitro assay mimicking cell-to-cell communication.(35) Therefore, TNF-α could be derived from the adipose tissue in the surrounding area of the penis, since in obesity, an increase in the inguinal and epididymal adipose tissue is observed.(36) Additionally, deposition of lipids is observed under the tunica albuginea and in the CC, and increased lipid accumulations is correlated with ED.(37, 38) However, neither macrophage migration/polarization nor TLR9 activation in the adjacent adipose tissue were investigated in this study and it will be addressed in future studies. Another possible explanation for the prevention of CC dysfunction in this study is associated with decreased systemic inflammation in TLR9MUT mice. We have concomitantly studied benign prostatic hyperplasia in these animals, and we have shown that despite similar body weight gain, WT but not TLR9MUT HFD mice had increased epididymal deposition and higher fasting blood glucose and triglyceride levels. Further, in WT mice, a HFD enhanced circulating levels of nuclear protein HMGB1, a well-established DAMP.(13) However, in TLR9MUT HFD, levels of HMGB1 were similar to the WT standard chow.(13) This is suggestive that lack of production of TNF-α by activation of macrophage-derived TLR9 decreased the cellular damage and thus attenuated the systemic inflammation, which might have contributed to the improved CC reactivity. In agreement with this idea, a recent study showed that in mice fed HFD, lack of TLR9 decreased macrophages in the visceral adipose tissue as well as the levels of TNF-α and IL-1β produced by the macrophages and improved insulin resistance.(35) Indeed, restoration of glucose levels and improvement in insulin resistance and blood lipids are also correlated with better erectile outcomes.(25,39)
In conclusion, it was determined that the expression of TNF-α in the CC of WT mice fed HFD was increased at least in part mediated by the activation of TLR9 expressed in the macrophages and possibly associated with the increased systemic inflammation.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vlachopoulos C, Aznaouridis K, loakeimidis N, Rokkas K, Vasiliadou C, Alexopoulos N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J. 2006;27(22):2640–8. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZL, Yang LY, Chen HH, Lin HH, Tsai YT, Huang WJ. Effects of TNF-alpha on penile structure alteration in rats with hyperprolactinemia. PLoS One. 2017;12(8):e0181952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demirtas Sahin T, Yazir Y, Utkan T, Gacar G, Halbutogullari ZS, Gocmez SS. Depression induced by chronic stress leads to penile cavernosal dysfunction: protective effect of anti-TNF-alpha treatment. Can J Physiol Pharmacol. 2018;96(9):933–42. [DOI] [PubMed] [Google Scholar]
- 4.Leite LN, do Vale GT, Simplicio JA, De Martinis BS, Carneiro FS, Tirapelli CR. Ethanol-induced erectile dysfunction and increased expression of pro-inflammatory proteins in the rat cavernosal smooth muscle are mediated by NADPH oxidase-derived reactive oxygen species. Eur J Pharmacol. 2017;804:82–93. [DOI] [PubMed] [Google Scholar]
- 5.Facio FN Jr., Facio MF, Spessoto LF, Pessutti D, Reis LO, Campos SG, et al. Anti-inflammatory and anti-fibrotic effects of annexin1 on erectile function after cavernous nerve injury in rats. Int J Impot Res. 2016;28(6):221–7. [DOI] [PubMed] [Google Scholar]
- 6.Carneiro FS, Sturgis LC, Giachini FR, Carneiro ZN, Lima VV, Wynne BM, et al. TNF-alpha knockout mice have increased corpora cavernosa relaxation. J Sex Med. 2009;6(1):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carneiro FS, Zemse S, Giachini FR, Carneiro ZN, Lima VV, Webb RC, et al. TNF-alpha infusion impairs corpora cavernosa reactivity. J Sex Med. 2009;6 Suppl 3:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demirtas Sahin T, Yazir Y, Utkan T, Gacar G, Furat Rencber S, Gocmez SS. TNF-alpha antagonism with etanercept enhances penile NOS expression, cavernosal reactivity, and testosterone levels in aged rats. Can J Physiol Pharmacol. 2018;96(2):200–7. [DOI] [PubMed] [Google Scholar]
- 9.Zelechowska P, Agier J, Kozlowska E, Brzezinska-Blaszczyk E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes Rev. 2018;19(5):686–97. [DOI] [PubMed] [Google Scholar]
- 10.Mridha AR, Haczeyni F, Yeh MM, Haigh WG, loannou GN, Barn V, et al. TLR9 is up-regulated in human and murine NASH: pivotal role in inflammatory recruitment and cell survival. Clin Sci (Lond) 2017;131(16):2145–59. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Nuevo A, Zorzano A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress. 2019;3(6):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, et al. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovascular Research. 2015; 107(1): 119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calmasini FB, McCarthy CG, Wenceslau CF, Priviero FBM, Antunes E, Webb RC. Toll-like receptor 9 regulates metabolic profile and contributes to obesity-induced benign prostatic hyperplasia in mice. Pharmacological Reports. 2020;72(1): 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stallmann-Jorgensen I, Ogbi S, Szasz T, Webb RC. A Toll-Like Receptor 1/2 Agonist Augments Contractility in Rat Corpus Cavernosum. The Journal of Sexual Medicine. 2015;12(8):1722–31. [DOI] [PubMed] [Google Scholar]
- 15.Nunes KP, Bomfim GF, Toque HA, Szasz T, Clinton Webb R. Toll-like receptor 4 (TLR4) impairs nitric oxide contributing to Angiotensin II-induced cavernosal dysfunction. Life Sciences. 2017;191:219–26. [DOI] [PubMed] [Google Scholar]
- 16.Nunes KP, de Oliveira AA, Szasz T, Biancardi VC, Webb RC. Blockade of Toll-Like Receptor 4 Attenuates Erectile Dysfunction in Diabetic Rats. The Journal of Sexual Medicine. 2018;15(9): 1235–45. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Zhang S, Jia L, Li H. MyD88 overexpression deteriorates Ang-II-induced ED via upregulating MPO and COX2 and downregulating eNOS in the corpus cavernosum of rats. Journal of Cellular Biochemistry. 2018;120(5):7133–46. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues FL, Fais RS, Tostes RC, Carneiro FS. There is a link between erectile dysfunction and heart failure: it could be inflammation. Curr Drug Targets. 2015;16(5):442–50. [DOI] [PubMed] [Google Scholar]
- 19.Xu M, Liu PP, Li H. Innate Immune Signaling and Its Role in Metabolic and Cardiovascular Diseases. Physiological Reviews. 2019;99(1):893–948. [DOI] [PubMed] [Google Scholar]
- 20.Calmasini FB, Klee N, Webb RC, Priviero F. Impact of Immune System Activation and Vascular Impairment on Male and Female Sexual Dysfunction. Sexual Medicine Reviews. 2019;7(4):604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arooj M, Ali I, Kang HK, Hyun JW, Koh Y-S. Inhibitory effect of particulate matter on toll-like receptor 9 stimulated dendritic cells by downregulating mitogen-activated protein kinase and NF-κB pathway. Journal of Toxicology and Environmental Health, Part A. 2020;83(9):341–50. [DOI] [PubMed] [Google Scholar]
- 23.Carneiro FS, Webb RC, Tostes RC. Emerging Role for TNF-α in Erectile Dysfunction. The Journal of Sexual Medicine. 2010;7(12):3823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes KP, Toque HA, Caldwell RB, William Caldwell R, Clinton Webb R. Extracellular Signal-Regulated Kinase (ERK) Inhibition Decreases Arginase Activity and Improves Corpora Cavernosal Relaxation in Streptozotocin (STZ)-Induced Diabetic Mice. The Journal of Sexual Medicine. 2011. ;8(12):3335–44. [DOI] [PubMed] [Google Scholar]
- 25.Silva FH, Alexandre EC, Calmasini FB, Calixto MC, Antunes E. Treatment With Metformin Improves Erectile Dysfunction in a Murine Model of Obesity Associated With Insulin Resistance. Urology. 2015;86(2):423.e1–.e6. [DOI] [PubMed] [Google Scholar]
- 26.Silva FH, Leiria LO, Alexandre EC, Davel APC, Mónica FZ, De Nucci G, et al. Prolonged Therapy with the Soluble Guanylyl Cyclase Activator BAY 60-2770 Restores the Erectile Function in Obese Mice. The Journal of Sexual Medicine. 2014;11(11 ):2661–70. [DOI] [PubMed] [Google Scholar]
- 27.Toque HA, da Silva FH, Calixto MC, Lintomen L, Schenka AA, Saad MJ, et al. High-fat diet associated with obesity induces impairment of mouse corpus cavernosum responses. BJU International. 2011;107(10):1628–34. [DOI] [PubMed] [Google Scholar]
- 28.Lang P, Hasselwander S, Li H, Xia N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Scientific Reports 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long T, Liu G, Wang Y, Chen Y, Zhang Y, Qin D. TNF-α, Erectile Dysfunction, and NADPH Oxidase-Mediated ROS Generation in Corpus Cavernosum in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. The Journal of Sexual Medicine. 2012;9(7):1801–14. [DOI] [PubMed] [Google Scholar]
- 30.Priviero FBM, Jin L-M, Ying Z, Teixeira CE, Webb RC. Up-Regulation of the RhoA/Rho-Kinase Signaling Pathway in Corpus Cavernosum from Endothelial Nitric-Oxide Synthase (NOS), but Not Neuronal NOS, Null Mice. Journal of Pharmacology and Experimental Therapeutics. 2010;333(1):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes KP, Teixeira CE, Priviero FB, Toque HA, Webb RC. Beneficial effect of the soluble guanylyl cyclase stimulator BAY 41-2272 on impaired penile erection in db/db−/− type II diabetic and obese mice. J Pharmacol Exp Ther. 2015;353(2):330–9. [DOI] [PubMed] [Google Scholar]
- 32.El-Sakka Al, B Yen TS, Lin CS, Lue TF. Traumatic arteriogenic erectile dysfunction: a rat model. International Journal of Impotence Research. 2001;13(3):162–71. [DOI] [PubMed] [Google Scholar]
- 33.Suganami T, Nishida J, Ogawa Y. A Paracrine Loop Between Adipocytes and Macrophages Aggravates Inflammatory Changes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(10):2062–8. [DOI] [PubMed] [Google Scholar]
- 34.Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Science Advances. 2016;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue Q, Lu Y, Eisele MR, Sulistijo ES, Khan N, Fan R, et al. Analysis of single-cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation. Science Signaling. 2015;8(381):ra59–ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Souza ILL, Barros BC, de Oliveira GA, Queiroga FR, Toscano LT, Silva AS, et al. Hypercaloric Diet Establishes Erectile Dysfunction in Rat: Mechanisms Underlying the Endothelial Damage. Frontiers in Physiology. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinay J, Sarquella J, Sanchez J, Algaba F, Gallegos I, Ruiz-Castañe E, et al. Acumulación de adipocitos en el cuerpo cavernoso: primera evidencia clinica e implicaciones fisiopatológicas en la disfunción eréctil. Actas Urológicas Españolas. 2017;41(2):97–102. [DOI] [PubMed] [Google Scholar]
- 38.Alwaal A, Wang L, Zaid UB, Lin G, Lue TF. Case Series of Lipid Accumulation in the Human Corpus Cavernosum. Medicine. 2015;94(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YW, Park SY, Kim JY, Huh JY, Jeon Ws, Yoon CJ, et al. Metformin Restores the Penile Expression of Nitric Oxide Synthase in High-Fat-Fed Obese Rats. Journal of Andrology. 2007. ;28(4):555–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


