Abstract
Background:
White matter disconnection of language-specific brain regions associates with worse aphasia recovery. Despite a loss of direct connections, many stroke survivors may maintain indirect connections between brain regions.
Objective:
To determine 1) whether preserved direct connections between language-specific brain regions relate to better post-stroke naming treatment outcomes compared to no direct connections, and 2) whether for individuals with a loss of direct connections, preserved indirect connections are associated with better treatment outcomes compared to individuals with no connections.
Methods:
We computed structural whole-brain connectomes from 69 individuals with chronic left-hemisphere stroke and aphasia who completed a three-week-long language treatment that was supplemented by either anodal transcranial direct current stimulation (A-tDCS) or sham stimulation (S-tDCS). We determined differences in naming improvement between individuals with direct, indirect, and no connections using one-way analyses of covariance and multivariable linear regressions.
Results:
Independently of tDCS modality, direct or indirect connections between the inferior frontal gyrus pars opercularis and angular gyrus were both associated with a greater increase in correct naming compared to no connections (p=0.027 and p=0.039, respectively). Participants with direct connections between the inferior frontal gyrus pars opercularis and middle temporal gyrus who received S-tDCS and participants with indirect connections who received A-tDCS significantly improved in naming accuracy.
Conclusions:
Post-stroke preservation of indirect white matter connections is associated with better treated naming improvement in aphasia even when direct connections are damaged. This mechanistic information can be used to stratify and predict treated naming recovery in individuals with aphasia.
Keywords: brain connectomics, stroke, aphasia, magnetic resonance imaging, rehabilitation, white matter
Introduction
Language processing is accomplished by complex and widespread cortical networks. Several grey matter regions located in the left hemisphere have been identified to be part of the language system and contribute in different degrees to language comprehension and production. Connections between those brain regions are essential for successful language processing as demonstrated by severe language deficits occurring after white matter lesions alone despite entirely intact grey matter regions 1.
Recently, understanding the impact of white matter damage has received much attention leading to the identification of specific white matter tracts and their particular contributions in language processing after stroke 2-10. As such, for impaired naming abilities (anomia) – a hallmark symptom of post-stroke aphasia – Baldo et al. (2013) provided evidence in a lesion symptom mapping study that naming performance is related to lesions to white matter underlying the middle und superior temporal gyri. Further, Pustina et al. (2017) found that structural pairwise white matter connectivity was one of the best predictors for naming performance among 12 different functional and structural MRI measures (correlation between true and predicted scores was r>0.7). Mirman et al. (2015) investigated subprocesses of naming and identified a frontal white matter brain area where several white matter tracts (e.g. inferior fronto-occipital fasciculus, uncinate fasciculus, anterior thalamic radiation) converge, which was particularly related to semantic errors during naming.
Importantly, white matter integrity is not only associated with language impairment after stroke, but also with aphasia recovery 11-16. Bonilha et al. (2015) showed that the macrostructural architecture in global and left temporal networks is significantly predictive of naming improvement after an intensive 2-week anomia treatment. On a meso-structural level, Meier et al. (2019) found that the integrity of the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus is significantly predictive of naming improvement after an intensive 12-week treatment. Thus, current evidence suggests that the integrity of residual white matter connections is an important determinant of treated aphasia recovery.
Nonetheless, although many stroke survivors lose subcortical tissue and direct connections between brain regions, they may still maintain several indirect connections. For instance, the axonal projections from the temporal cortex to the inferior frontal cortex may be completely damaged, but there is a possibility that the temporal cortex still communicates with the inferior frontal cortex via projections from the temporal cortex to one or more intermediary grey matter regions, which in turn connect to the inferior frontal cortex. However, it remains unknown if language improvement is mediated only by direct connections or if indirect connections can support aphasia recovery and if they do, where these indirect connections are located.
The importance of both direct and indirect structural connections in neural networks has been emphasized by research showing that brain function can be explained by direct as well as indirect connections 17,18. Catani et al. (2005) reconstructed white matter pathways between Broca’s and Wernicke’s areas and showed that besides the classical direct connection through the arcuate fasciculus, Broca’s and Wernicke’s areas are also indirectly connected by a pathway through the inferior parietal lobe. Given that aphasia presents differently across patients with disconnection syndromes, Catani et al. speculate that the direct pathway is primarily involved in phonological processing, and the indirect pathway in semantic processing. The importance of both direct and indirect connections can be traced to the economic organization of brain networks 19. The ideal network organization balances efficiency (speed of communication between nodes) and costs to maintain the network. This balance is typically achieved by small-world configurations 20-22. To save costs, small-world networks include some direct connections but do not include direct connections for every pair of nodes. Nodes that are not directly connected are indirectly connected through as few intermediary nodes as possible.
We speculated that assessing only direct connections between brain regions is a limitation in comprehensively evaluating network integrity after stroke. We employed a network modeling approach accounting for communication through indirect white matter connections (connectome dynamics) 23,24. Examination of connectome dynamics allows the investigation of direct and indirect pathways in the brain connectome, which can be used to simulate the path of information transfer between brain regions, and thus the locations of indirect connections that are related to treated aphasia recovery in general or to the response to specific treatment approaches. This might be especially important in treatment approaches that rely on the underlying neurological substrate to spread or support the neuronal signaling in the language cortex, such as neuromodulation techniques. To assess the relationship between indirect connections and aphasia treatment in general and specifically neuromodulation therapy, we leveraged data from a phase 2 randomized control trial to determine the futility of further studying transcranial direct current stimulation (tDCS) for aphasia treatment 25. The results of the original clinical trial suggested that further research on the effects of anodal tDCS (A-tDCS) on aphasia treatment is not futile. A planned post-hoc preliminary superiority analysis revealed a statistically significantly better outcome for A-tDCS over sham tDCS (S-tDCS) for improving naming 26. Nevertheless, there was wide variability in the response to A-tDCS and here, we explored whether the effects of A-tDCS are related to connection profiles.
We sought to 1) investigate the impact of direct and indirect connections on treated naming recovery, 2) investigate the impact of number of steps along indirect connections on treated naming recovery, and 3) map the typical path of indirect connections. We hypothesized that 1) both direct and indirect connections between pre-selected, language-specific brain regions partly explain treatment-related naming outcomes among individuals with post-stroke aphasia; 2) fewer steps along indirect connections is associated with better outcomes; and 3) indirect connections primarily pass through neighboring regions. This information can shed light on the mechanisms of naming recovery and the anatomical pathways related to treatment related language improvement.
Materials and methods
Participants
We assessed data from individuals with chronic left hemisphere ischemic stroke who were part of a larger phase 2 clinical trial evaluating the use of transcranial direct current stimulation (tDCS) for aphasia treatment conducted at the University of South Carolina and the Medical University of South Carolina 25. We included 69 of 74 participants from the clinical trial (excluding participants with hemorrhagic stroke (N=2), no diffusion tensor imaging (N=2), no post-treatment follow-up (N=1)). As part of the clinical trial, all participants received a 3-week-long computerized language treatment focusing on lexical-semantic processing with phonological, semantic, and unrelated foils. Half of the participants were randomized to receive either A-tDCS or S-tDCS as a supplement to the computerized language training. The location of the tDCS electrode varied between participants and was identified for each individual as the left parietal or temporal coritical region that displayed the strongest naming-related activation during a previous separate fMRI session (see 25 for details of the electrode locations). All participants presented with aphasia at baseline, according to an aphasia quotient (AQ) score <93.8 on the Western Aphasia Battery-Revised (WAB) 27. Demographic and medical data are shown in Table 1 and the stroke lesion overlap from all participants in Fig. 1. Institutional Review Boards at both institutions approved the study, and all participants provided written informed consent prior to participation.
Table 1.
Demographic and medical information and performance on the Philadelphia Naming Test for all participants (N=69).
| Mean | Std. Dev. | Range | ||
|---|---|---|---|---|
| Demographic and medical information | ||||
| Age (years) | 59.54 | 10.26 | 30 – 77 | |
| Education (highest year of school completed in years) | 14.68 | 2.48 | 10 – 20 | |
| Time since stroke (months) | 41.15 | 40.00 | 6 – 202 | |
| WAB-AQ at baseline | 57.39 | 19.87 | 20.10 – 93.00 | |
| Total lesion volume (ml) | 143.99 | 92.36 | 24.02 – 472.38 | |
| PNT performance (number of items: 175) | ||||
| Correct naming at baseline | 58.34 | 43.00 | 0.00 – 139.00 | |
| Change in correct naming from baseline to 24 weeks after treatment | 6.25 | 14.53 | −32.00 – 52.50 | |
T=Philadelphia Naming Test; WAB-AQ=Aphasia Quotient of the Western Aphasia Battery
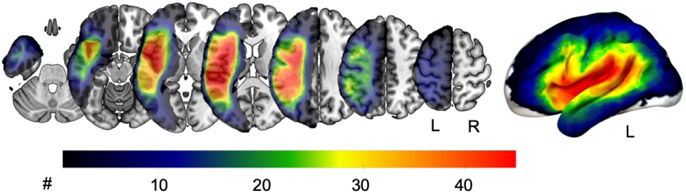
Fig. 1.
Lesion overlay of all 69 participants. The warmer the color, the more participants had a lesion in this area. L=left hemisphere, R=right hemisphere.
Outcome measures for anomia
Naming accuracy was measured twice before and twice at 24 weeks post-treatment with the Philadelphia Naming Test (PNT) 28. Given variability in day-to-day aphasic performance, the number of correct items at each baseline and at each follow-up PNT administration was averaged. The change in the number of correct responses from baseline to follow up was the behavioral outcome of our study (Table 1). To account for differences in baseline aphasia severity, we controlled for baseline WAB-AQ.
Image acquisition and processing
All participants received a structural brain MRI scan at baseline including T1- and T2-weighted images and a diffusion tensor imaging (DTI) sequence. MRI scans were performed with a 3T TIM Trio scanner (Siemens Healthcare, Erlangen, Germany) using a 12-channel head coil.
Lesion mapping
A neurologist and/or trained research specialist manually drew the chronic stroke lesions on every participants’ T2 MRI scan using MRIcron software (www.mricron.com). Lesion maps drawn on the T2-weighted images were co-registered to the T1-weighted image and normalized into standard space using SPM12 (version 7487) (Functional Imaging Laboratory, Wellcome Trust Centre for Neuroimaging Institute of Neurology, University College London; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and open source Matlab PN (Revised) scripts that were developed in-house 29. We segmented participants’ normalized T1-weighted images using the John’s Hopkins University (JHU) anatomical atlas 30 excluding all white matter, ventricular and brainstem regions of interest (ROIs).
Structural connectome
For each participant we performed whole-brain probabilistic tractography but spared the lesion for tractography. We used the same methods as described in previous publications 31,32. To account for potential spurious connections identified during the probabilistic tractography, we excluded connectivity links in the whole-brain with a weight of less than the lowest 20% in the undamaged right hemisphere 2. We normalized all connectivity weights in the whole brain by applying min/max scaling. From here on, we will refer to the weights of the pairwise connectivity links as “direct connectivity”.
Indirect connections: propagation steps
We calculated indirect pairwise connections by computing the shortest path length (i.e., the minimal number of steps) between each pair of grey matter regions. The number of steps (crossed grey matter nodes) between two regions was used as a proxy for the propagation of signals between two regions and was called propagation steps. Based on previously developed methods 23,24 validated in participants with stroke 33 we calculated propagation steps as follows: i) one grey matter ROI was seeded as the start region, ii) all neighboring regions directly connected to the start region were seeded, iii) all neighboring regions reached from the seeded regions (indirectly connected to the start-region) are seeded. These steps were repeated until all regions connected to any of the previously seeded regions by a connectivity link of > 0 were reached and seeded. The minimal number of steps it takes for a target region to be reached represents the shortest path length between the start and target region. If a region is not connected to any of the seeded regions (e.g., because the region or its connection are lesioned), then this region cannot be reached (Supplementary Fig. 1).
Selection of seeds in grey matter regions
We selected seeds in three grey matter regions based on 1) previous evidence and 2) anatomical locations. Previous evidence suggests that some links between certain grey matter areas within the language network are more important than others, i.e., are more likely to lead to language problems when damaged 31. These regions include the inferior frontal gyrus opercularis (IFGop), inferior frontal gyrus triangularis, angular gyrus (AG), supramarginal gyrus, posterior superior temporal gyrus, and middle temporal gyrus (MTG). From these 6 regions we selected three regions that are anatomically relatively far apart from each other. By focusing on anatomically distant regions, we sought to increase the chances of existing indirect connections because we assumed little importance of indirect connections between neighboring ROIs. Thus, the following ROIs were included here: IFGop, AG, and MTG.
We opted to select a small subset of pre-defined ROIs for the following reasons: 1) there is supporting literature to indicate the importance of structural connectivity related to these regions in the context of aphasia; 2) since this a connectivity study, each new region added to the model can lead to a large number of new connections, thereby drastically decreasing statistical power; 3) this study assessed indirect connections, hence the importance of surrounding regions to the chosen ROIs would still be appreciated by the analyses.
Three different combinations of ROI pairs resulted from the selected ROIs. For each of these 3 ROI pairs we calculated regional lesion load (Table 2), direct connectivity and propagation steps (Table 3). Importantly, connectome dynamics were calculated based on the whole-brain network including all possible 104 grey matter regions, though start and target seeds were limited to the 3 selected ROIs. Therefore, propagation steps reflect the integrity of the whole-brain network integrity beyond pairwise connections and regional lesions.
Table 2.
Regional lesion volume (in %) for all N=69 participants; and propagation steps (number of steps) between regions of the language network in the left hemisphere for participants with indirect connections. See supplementary Fig. 2 for visulaizations of the distributions of number of steps for each region pair
| N | Mean | Median | Std. Deviation | Range | |
|---|---|---|---|---|---|
| Regional lesion volume (in %) | |||||
| IFGop | 69 | 54.23 | 57.60 | 38.89 | 0 – 100.00 |
| AG | 69 | 41.30 | 38.67 | 34.30 | 0 – 100.00 |
| MTG | 69 | 28.19 | 25.74 | 37.77 | 0 – 100.00 |
| Propagation steps (# steps) | |||||
| IFGop – AG | 46 | 2.41 | 2 | 0.78 | 2 – 5 |
| IFGop – MTG | 46 | 2.50 | 2 | 0.91 | 2 – 6 |
| AG – MTG | 32 | 2.25 | 2 | 0.67 | 2 – 5 |
AG=angular gyrus, IFGop= inferior frontal gyrus opercularis, MTG=middle temporal gyrus, pSTG=posterior superior temporal gyrus
Table 3.
Percentage of participants (N=69) with no, indirect or direct connections between the 3 pairs of grey matter regions.
| Participants with no connection (%) |
Participants with indirect connection (%) |
Participants with direct connection (%) |
|
|---|---|---|---|
| IFGop and AG | 11.59 | 66.67 | 21.74 |
| IFGop and MTG | 15.94 | 66.67 | 17.39 |
| AG and MTG | 8.70 | 46.38 | 44.93 |
AG=angular gyrus, IFGop= inferior frontal gyrus opercularis, MTG=middle temporal gyrus
Table 3 shows the percentage of the 69 participants who had an existing direct connection (weight of connectivity link > 0), no direct but an indirect connection (number of propagation steps was higher than 1), and neither a direct nor indirect connection between two ROIs. For each region pair, regional lesion volume of the two ROIs was significantly associated with the connection type. Participants with no connection had significantly larger lesions to the ROIs than participants with indirect or direct connections, and participants with indirect connection had significantly larger regional lesion than participants with direct connections.
Statistical analyses
In both aims 1 and 2, we sought to assess whether associations exist between indirect connections and aphasia recovery that are independent from other factors known to affect recovery (e.g., baseline aphasia severity, age). Thus, our goal was not to build a parsimonious prediction model, but to assess indirect connections as a potential mechanisum underlying recovery. To test hypothesis 1, we conducted an analysis of covariance (ANCOVA) for each region pair to determine if connection type (no connection, indirect, direct) had an effect on change in naming (correct responses) from baseline to follow-up while controlling for age, number of years of formal education, baseline WAB-AQ, treatment type (A-tDCS vs S-tDCS). Originally, we also included number of correct responses at baseline, total lesion volume, and regional lesion volume of the pair of grey matter regions (IFGop, AG, and/or MTG); however, we included only WAB-AQ because of significant multicollinearity between these variables and baseline WAB-AQ (correct responses at baseline: Pearson correlation coefficient r=0.89, p<0.001; total lesion volume: r=−0.29, p=0.015; IFGop lesion volume: r=−0.42, p<0.001, AG lesion volume: r=−0.293, p=0.017, MTG lesion volume: r=−0.43, p<0.001). Additionallly, we sought to limit the number of independent variables in the model to a maximum of one variable for every 10 observations. Thus, we performed a two-way ANCOVA and included an interaction term between connection type and treatment type to assess whether the effects of A-tDCS depend on the connection type. If there was a significant main effect of connection type or treatment type, we performed post-hoc pairwise comparisons using Bonferroni adjusted p-values to correct for multiple comparisons. Because of unequal sample sizes across the subgroups of connection type and treatment type, we assessed the equality of variance with the Levene’s test. Levene’s tests were non-significant (p>0.05), thus, equal variances were assumed.
To test hypothesis 2, we performed multivariable linear regression modeling on participants with indirect connections to test whether the number of steps along indirect connections was predictive of change in naming from baseline to follow up. We controlled for the same variables as before.
To test hypothesis 3, we calculated the percentage of grey matter regions involved in indirect connections across all participants and mapped these pathways on a standard, generic brain model.
We performed 2-tailed statistical tests for all analyses involved and P-values < 0.05 (p<0.025 for each tail) were considered statistically significant. IBM SPSS Statistics for Windows (version 25, released 2017, IBM Corp., Armonk, N.Y., USA) was used to conduct all statistical analyses.
Results
Direct and indirect connections are related to treated naming recovery
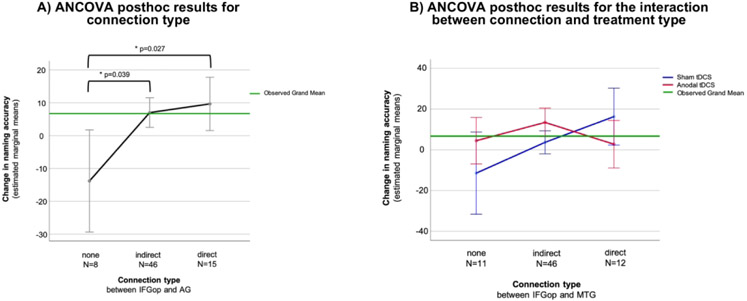
For the IFGop and AG, there were significant main effects of connection type [F(2,56)=3.70, p=0.029] and treatment type (A-tDCS / S-tDCS) [F(1,56)=5.89, p=0.019] on change in correct naming from baseline to follow-up whilst adjusting for control variables. The interaction between connection type and treatment type was not significant, nor were any other control variables. Post hoc tests showed a significant difference in naming improvement between direct and no connections (p = 0.027) and between indirect and no connections (p = 0.039). On average 23.47 more correct responses after treatment were produced by participants with a direct connection compared to participants without any connection between the IFGop and AG, and on average 20.83 more correct responses after treatment were produced by participants with an indirect connection compared to participants without any connection between the IFGop and AG. There was no significant difference in naming improvement between participants with direct versus indirect connections. Participants who received A-tDCS produced on average 14.56 more correct responses than participants with S-tDCS 6 months after treatment (post hoc pairwise comparison: p=0.019) (Fig. 2A).
Fig. 2.
A) Results of the significant analysis of covariance (ANCOVA) post hoc tests for connection type between the inferior frontal gyrus pars opercularis (IFGop) and angular gyrus (AG). B) Results of the significant interaction between treatment type and connection type between the inferior frontal gyrus pars opercularis (IFGop) and middle temporal gyrus (MTG) on change in naming accuracy after treatment. Line graphs were chosen to visualize the interaction between treatment type and connection type. Error bars display 95%-confidence intervals. Covariates appearing in the model are evaluated at the following values: Western Aphasia Battery (Aphasia Quotient) at baseline = 58.18, Age = 60.00, highest year of school completed = 14.82.
For the IFGop and MTG we revealed a significant interaction between connection type and treatment type on change in correct naming [F(2,56)=3.45 p=0.039]. No other independent variables were significant. Two groups of participants showed significant improvement after treatment. Participants with direct connections between the IFGop and MTG who received S-tDCS significantly improved by on average 16.28 (95%-CI: 2.31 – 30.25) more correct responses. Participants with indirect connections between the IFGop and MTG who received A-tDCS significantly improved by on average 13.44 (95%-CI: 6.44 – 20.44) more correct responses. No other group of participants (no connection and A-tDCS, no connection and S-tDCS, direct connection and A-tDCS, indirect connection and S-tDCS) showed significant gains in naming accuracy.
For the AG and MTG we did not find any significant main effects or interactions.
Fewer steps along indirect connections predict better treated naming recovery
For those participants with indirect connections, fewer steps along the indirect connection between the AG and MTG significantly predicted an increase in correct naming responses after treatment (standardized beta=−0.517, p=0.012).
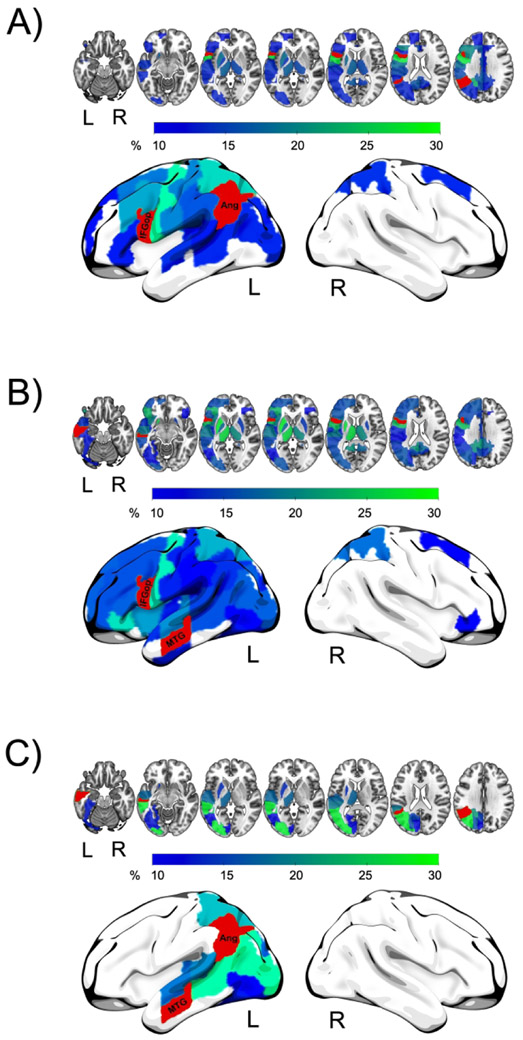
Indirect connections pass through neighboring brain regions
Fig. 3 shows the propagation of a signal for each region pair. For about one third to one fourth of participants, the indirect connection between the IFGop and AG went through the precentral gyrus, and between the IFGop and MTG through the precentral gyrus, posterior parietal regions, thalamus and putamen. In contrast, the indirect connection between the AG and MTG most often went through temporal regions.
Fig. 3.
Signal propagation between each of the three region pairs. Fig. 3A IFGop and AG, Fig. 3B: IFGop and MTG, Fog, Fig 3C: AG and MTG. Colors of regions represent the percentage of patients passing these regions on the indirect connection from one region to the other. The greener the color the more patients passed this region.
Discussion
To the best of our knowledge, this study is the first to assess indirect cortical connections to predict response to aphasia therapy. Our findings suggest that the preservation of indirect connections plays a role in treated anomia recovery.
Direct and indirect connections are related to treated naming recovery
We found significant relationships between changes in correct naming and indirect connections. Individuals with aphasia who had indirect connections between the IFGop and AG improved significantly more in naming accuracy compared to individuals with no connection between these areas. Further, individuals with direct pairwise connections between the IFGop and AG also improved significantly more in naming accuracy compared to individuals with no connection between these areas. Maintaining or increasing connectivity within the remaining language network after stroke has been previously associated with language performance and aphasia recovery. For instance, the concept of graceful degradation underlines that despite damage to parts of the language network, the remaining network can still maintain some level of functioning through connectivity of the remaining portions of the network 34. This is in line with research showing that an increase in connectivity within the language network (increase in modularity) is associated with better improvement after aphasia therapy, 35,36. Thus, aphasia treatment may strengthen the connectivity within the language network.
Interestingly, individuals with direct connections did not differ from individuals with indirect connections regarding how much they improved. These finding suggest that both indirect and direct connections between the IFGop and AG play a role in anomia treatment success. Further, this may also suggest that the type of connection – direct or indirect – does not matter as long as regions are connected – at least for general naming accuracy. This may seem contradictory to theories suggesting that direct and indirect connections between certain pairs of regions serve different functions in language processing (e.g., Catani et al. 2005). However, in our study we did not assess or compare different language functions beyond correct naming responses. Moreover, we did not assess function but recovery of language, which may play out differently with regard to the supporting neuroanatomy. Future studies are warranted that systematically test treatment-related changes for different language functions to determine if direct and indirect connections impact treated aphasia recovery differently.
Besides the significant main effect of connection type, treatment type was also independently predictive of change in correct responses. Participants who received A-tDCS produced significantly more correct responses after treatment compared to participants who received S-tDCS. However, this main effect was only observed when we controlled for connections between the IFGop and AG, but not when we controlled for connections between the IFGop and MTG, or between the AG and MTG. We speculate that the impact of A-tDCS on aphasia treatment depends on the individual’s brain physiology. Whether A-tDCS can improve outcomes of aphasia treatment is likely related to the underlying brain substrate where the A-tDCS is applied and also to the residual brain substrate where the A-tDCS current is distributed. This speculation is also supported by our findings of a significant interaction between treatment type and connection type of the IFGop and MTG. Participants who received S-tDCS made the best treatment gains if they had preserved direct connections between the IFGop and MTG. However, participants who received A-tDCS made the best treatment gains if they had indirect connections. It is plausible that A-tDCS can compensate for a lack of direct connections by using and enhancing indirect connections between certain brain areas. The electrical current from A-tDCS is usually not confined to focal areas or focal connections, but spreads through broad networks. Nevertheless, these findings require cautious interpretation because the tDCS type was not randomized according to connection type. Thus, the observed interaction may reflect group differences other than connection type and treatment type. Future studies should be devoted to assess further the impact of A-tDCS on aphasia treatment and to discern brain physiological properties that enhance its impact.
Fewer steps along indirect connections predict better treated naming recovery
In line with our hypothesis we found a significant association between the number of steps along indirect connections between the AG and MTG and treated anomia recovery. Fewer steps along indirect connections between the AG and MTG was predictive of more correct responses at follow up compared to baseline. However, we did not find this association for indirect connections between the IFGop and AG or the IFGop and MTG.
It is possible that for some regions short connections matter. For example, few steps along indirect connections likely matter for regions that work in tight and fast synchrony. For other regions it might not matter how closely they are connected. This might apply to regions that work less often or less fast together and as long as these regions are connected (indirectly or directly) recovery will be enhanced independently of how many regions are passed through by the indirect connection. Notably, there was little variability in the number of steps constituting indirect connections. Most indirect connections were based on just one region between the two critical regions. Future studies might want to include pairs of regions that are far distant from each other, for example, in different hemispheres.
Indirect connections pass through neighboring brain regions
The shortest indirect connection between the IFGop and AG and between the IFGop and MTG most often passed through the cortical sensory-motor regions of the left hemisphere. This also included subcortical structures (thalamus and putamen) for the indirect connection of the IFGop and MTG. The precentral and postcentral gyri are commonly associated with speech and language performance, especially in phonological-articulatory processing 31,37,38. The IFGop, as well as the AG and MTG are all involved in lexical-semantic as well as lexical-phonological processing during speech production and are associated with response to aphasia treatment 39-46. Using an indirect route through sensory-motor areas during language production seems an effective way to integrate earlier lexical processing stages with later articulatory planning and execution.
Further, the thalamus has been linked to speech fluency and naming abilities. Nadeau and Crosson speculated that aphasia after lesions to the thalamus is the result of damage or a disruption of the rich thalamic projections to the frontal, parietal and temporal cortices 47. Additionally, Hillis et al. speculated that the impact of thalamic lesions on language impairment stems from hypoperfusion in cortical language areas as a result of diachisis 48. Our observation that the shortest indirect pathway between the IFGop and MTG commonly went through the thalamus supports the importance of preserved connections between the thalamus and language cortex.
The preferred indirect connection between the AG and MTG went through posterior temporal regions typically grouped with association cortices. One role of the temporal association cortex is integrating auditory and visual information. Thus, if indirect connections between the AG and MTG go through the temporal association cortex, information exchange between different functional systems can be enhanced.
Future studies may consider assessing whether different indirect pathways are associated with different degrees and / or different components of anomia treatment success (e.g., primary indirect pathways through sensory-motor regions may be especially associated with improvement in phonological-articulatory naming errors). Understanding the location of indirect pathways and their relationship to language function and treatment response could help identify neurobiological treatment targets. Depending on a patient’s deficits and structural loss, indirect pathways could serve as treatment targets to harness signal propagation between certain grey matter areas.
Limitations
We mapped indirect connections based on structural connectomics, thus, we cannot claim that these indirect pathways are indeed functionally used. A natural follow up of our study would be comparing structural and functional brain imaging before and after treatment using the connectome dynamics approach.
Further, we binarized lesion maps into either lesioned or not lesioned tissue. While this is a common approach in lesion symptom mapping research, there might be vital tissue within the lesion that we did not consider to provide useful information. Thus, binarizing lesion maps has the risk of failing to identify existing relationships.
Lastly, because we focused on treated naming recovery, findings of this study might not generalize to improvements in other aspects of language with aphasia treatment.
Conclusions
Our findings demonstrate that the integrity of white matter tissue outside the stroke lesion in the form of both direct and indirect connections may be an important determinant for post-stroke aphasia recovery. We suggest to include indirect connections in future lesion mapping studies in addition to traditional structural brain measures to evaluate this measure as a potential biomarker for treatment response in individuals with aphasia.
Supplementary Material
Supplementary Fig. 1. Signal propagation within the brain network. The number of steps between two grey matter regions (labeled as Seed and Target) was used as a proxy for the propagation of a signal between these regions. Each circle represents a grey matter region (node). Red-colored nodes are nodes that are part of the shortest path between the seed and target region (and vice versa). Grey colored nodes are not part of the shortest path. Fig. 1A) The seed region and target region are connected through a direct connection (green line). The number of propagation steps is 1. Fig. 1B) The direct connection between the seed target and the target region is lost. The shortest path between the two regions is an indirect connection with a number of propagation steps of 4.
Supplementary Fig. 2. Histograms visualizing the distribution of the number of steps along indirect connections for each region pair: A) inferior frontal gyrus pars opercularis (IFGop) and angular gyrus (AG), B) IFGop and middle temporal gyrus (MTG), C) AG and MTG.
Acknowledgments
Funding
This study was supported by research grants from the National Institutes of Health / National Institute on Deafness and Other Communication Disorders (NIDCD): DC014021 (PI: Bonilha), DC011739 (PI: Fridriksson), DC014664 (PI: Fridriksson), DC014435 (Trainee: Basilakos), DC05375 (PI: Hillis) and from the American Heart Association: SFDRN26030003 (PI: Bonilha). No conflicts of interests to report.
Footnotes
Competing interests
Nothing to report.
Data availability
The data used in this study are available to researchers upon qualified request to the corresponding author.
References
- 1.Fridriksson J, Bonilha L, Rorden C. Severe Broca's aphasia without Broca's area damage. Behav Neurol. 2007;18(4):237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirman D, Chen Q, Zhang Y, et al. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun. 2015;6:6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing S, Lacey EH, Skipper-Kallal LM, Zeng J, Turkeltaub PE. White Matter Correlates of Auditory Comprehension Outcomes in Chronic Post-Stroke Aphasia. Front Neurol. 2017;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pani E, Zheng X, Wang J, Norton A, Schlaug G. Right hemisphere structures predict poststroke speech fluency. Neurology. 2016;86(17):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragoy O, Akinina Y, Dronkers N. Toward a functional neuroanatomy of semantic aphasia: A history and ten new cases. Cortex. 2017;97:164–182. [DOI] [PubMed] [Google Scholar]
- 6.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldo JV, Arevalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49(3):658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing S, Mandal A, Lacey EH, Skipper-Kallal LM, Zeng J, Turkeltaub PE. Behavioral Effects of Chronic Gray and White Matter Stroke Lesions in a Functionally Defined Connectome for Naming. Neurorehabil Neural Repair. 2018;32(6-7):613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pustina D, Coslett HB, Ungar L, et al. Enhanced estimations of post-stroke aphasia severity using stacked multimodal predictions. Hum Brain Mapp. 2017;38(11):5603–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45(4):988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of Anomia Treatment in Aphasia Is Associated With Preserved Architecture of Global and Left Temporal Lobe Structural Networks. Neurorehabil Neural Repair. 2015;30(3):266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain. 2014;137(Pt 7):2027–2039. [DOI] [PubMed] [Google Scholar]
- 13.Meier EL, Johnson JP, Pan Y, Kiran S. The utility of lesion classification in predicting language and treatment outcomes in chronic stroke-induced aphasia. Brain Imaging Behav. 2019;13(6):1510–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinnon ET, Fridriksson J, Glenn GR, et al. Structural plasticity of the ventral stream and aphasia recovery. Ann Neurol. 2017;82(1):147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009;1169:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil Neural Repair. 2014;28(4):325–334. [DOI] [PubMed] [Google Scholar]
- 17.Liang H, Wang H. Structure-Function Network Mapping and Its Assessment via Persistent Homology. PLoS Comput Biol. 2017;13(1):e1005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. [DOI] [PubMed] [Google Scholar]
- 19.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. [DOI] [PubMed] [Google Scholar]
- 20.Bassett DS, Bullmore ET. Small-World Brain Networks Revisited. Neuroscientist. 2017;23(5):499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao X, Vasilakos AV, He Y. Small-world human brain networks: Perspectives and challenges. Neurosci Biobehav Rev. 2017;77:286–300. [DOI] [PubMed] [Google Scholar]
- 22.Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature. 1998;393(6684):440–442. [DOI] [PubMed] [Google Scholar]
- 23.Misic B, Betzel RF, Nematzadeh A, et al. Cooperative and Competitive Spreading Dynamics on the Human Connectome. Neuron. 2015;86(6):1518–1529. [DOI] [PubMed] [Google Scholar]
- 24.Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nature Reviews Neuroscience. 2017;19:17. [DOI] [PubMed] [Google Scholar]
- 25.Fridriksson J, Rorden C, Elm JJ, Sen S, George MS, Bonilha L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018;75(12):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridriksson J, Basilakos A, Stark BC, et al. Transcranial direct current stimulation to treat aphasia: Longitudinal analysis of a randomized controlled trial. Brain Stimul. 2019;12(1):190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kertesz A The Western Aphasia Battery - Revised. New York: Grune & Stratton; 2007. [Google Scholar]
- 28.Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: Scoring and rationale. Clin Aphasiol. 1996;24:121–133. [Google Scholar]
- 29.Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faria AV, Joel SE, Zhang Y, et al. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage. 2012;61(3):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridriksson J, den Ouden DB, Hillis AE, et al. Anatomy of aphasia revisited. Brain. 2018;141(3):848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilmskoetter J, Marebwa BK, Basilakos A, et al. Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain. 2019;142(10):3190–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Gaizo J, Fridriksson J, Yourganov G, et al. Mapping Language Networks Using the Structural and Dynamic Brain Connectomes. eNeuro. 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadeau SE. Neural Population Dynamics and Cognitive Function. Front Hum Neurosci. 2020;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan ES, Small SL. Increased Modularity of Resting State Networks Supports Improved Narrative Production in Aphasia Recovery. Brain Connect. 2016;6(7):524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel JS, Seitzman BA, Ramsey LE, et al. Re-emergence of modular brain networks in stroke recovery. Cortex. 2018;101:44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden DB, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci U S A. 2016;113(52):15108–15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. [DOI] [PubMed] [Google Scholar]
- 39.Indefrey P The Spatial and Temporal Signatures of Word Production Components: A Critical Update. Front Psychol. 2011;2:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. [DOI] [PubMed] [Google Scholar]
- 41.Marcotte K, Adrover-Roig D, Damien B, et al. Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia. 2012;50(8):1776–1786. [DOI] [PubMed] [Google Scholar]
- 42.Abel S, Weiller C, Huber W, Willmes K. Neural underpinnings for model-oriented therapy of aphasic word production. Neuropsychologia. 2014;57(1):154–165. [DOI] [PubMed] [Google Scholar]
- 43.Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci. 2010;30(35):11558–11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson RL, Hoffman P, Pobric G, Lambon Ralph MA. The Semantic Network at Work and Rest: Differential Connectivity of Anterior Temporal Lobe Subregions. J Neurosci. 2016;36(5):1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiran S, Meier EL, Kapse KJ, Glynn PA. Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Front Hum Neurosci. 2015;9(June). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ripamonti E, Frustaci M, Zonca G, Aggujaro S, Molteni F, Luzzatti C. Disentangling phonological and articulatory processing: A neuroanatomical study in aphasia. Neuropsychologia. 2018;121:175–185. [DOI] [PubMed] [Google Scholar]
- 47.Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997;58(3):355–402; discussion 418-323. [DOI] [PubMed] [Google Scholar]
- 48.Hillis AE, Barker PB, Wityk RJ, et al. Variability in subcortical aphasia is due to variable sites of cortical hypoperfusion. Brain Lang. 2004;89(3):524–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Signal propagation within the brain network. The number of steps between two grey matter regions (labeled as Seed and Target) was used as a proxy for the propagation of a signal between these regions. Each circle represents a grey matter region (node). Red-colored nodes are nodes that are part of the shortest path between the seed and target region (and vice versa). Grey colored nodes are not part of the shortest path. Fig. 1A) The seed region and target region are connected through a direct connection (green line). The number of propagation steps is 1. Fig. 1B) The direct connection between the seed target and the target region is lost. The shortest path between the two regions is an indirect connection with a number of propagation steps of 4.
Supplementary Fig. 2. Histograms visualizing the distribution of the number of steps along indirect connections for each region pair: A) inferior frontal gyrus pars opercularis (IFGop) and angular gyrus (AG), B) IFGop and middle temporal gyrus (MTG), C) AG and MTG.
Data Availability Statement
The data used in this study are available to researchers upon qualified request to the corresponding author.