1. Introduction
Mammalian heart is considered postmitotic with limited cardiomyocyte (CM) turnover during lifetime of an individual [1]. Adult CMs are typically unable to complete the cell cycle and respond to growth signals by hypertrophy or incomplete cytokinesis resulting in binucleation and/or polyploidization, considered to be major hurdles in CM proliferation and regeneration [2]. Ensuing years have seen a tremendous effort to delineate CM cell cycle and develop strategies that promote cell cycle reentry and CM mediated regeneration of the heart following injury. Success and effectiveness of these strategies is debatable, and existence of a true CM based regenerative strategy that drives meaningful increases in CM numbers in the heart augmenting cardiac structure and function following injury remains elusive. Can the heart repair itself or in essence adult CMs can be a viable target for generating de novo, mature and functionally competent CMs after myocardial injury?
The answer may lie in how the heart regulates proliferative and regenerative responses during early postnatal cardiac stages. Fetal or neonatal CMs are known to actively proliferate and populate the heart in response cardiac tissue growth. Cellular and molecular signature of developmental CMs is unique and has provided blueprint for several strategies that have employed reactivation of developmental signaling for CM proliferation (Fig. 1). The following sections will provide a perspective on how reactivation of developmental signaling affects CM cell cycle, cardiac repair and regenerative process in the heart following injury.
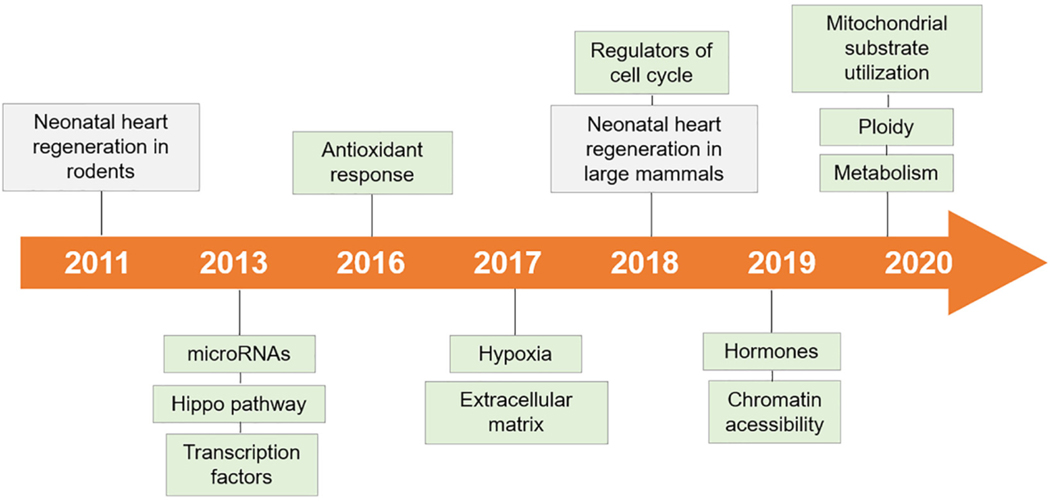
Fig. 1.
Timeline of the main strategies to enhance heart regeneration by reactivating developmental signaling. Landmark breakthroughs in neonatal heart regeneration are indicated in grey, important advances in adult heart regeneration by reintroducing developmental signaling are shown in green.
2. Transient regenerative potential of postnatal heart
Seminal studies by Porrello et al. show a transient regenerative potential in the heart during postnatal development [3]. Further have studies validated the findings showing CM proliferation in P1 and P3 murine heart ceases by P7 at which point CM become terminally differentiated. Additionally, it was shown that a final wave of DNA synthesis occurs by P14 followed by binucleation of the CMs and hypertrophy with no new myocyte formation. More recently, studies have highlighted the transient regenerative cardiac ability extends to large animal such as pigs showing complete resolution of injury following apical resection. All of these studies unequivocally show unique molecular and cellular control of CM cell cycle regulation and new myocyte formation in the heart during developmental and postnatal cardiac stages.
3. Reactivation of developmental signaling in the heart
The transition from in utero to the postnatal cardiac environment is marked by several physiological adaptations in the heart. Changes in oxygen levels, nutrient availability, and hormones have been shown to influence postnatal CM cell-cycle arrest. In parallel, distinct molecular and cellular mechanisms such as DNA damage, epigenetic factors, and metabolism have also been identified to play a critical role in CM turnover in the heart. Nevertheless, the precise role of how environment factors and interconnected molecular mechanisms influence each other and CM turnover in the cardiac tissue under physiological and pathological conditions is not well studied.
3.1. Oxygen tension
The embryonic cardiac tissue consists of proliferating CMs that support organ growth and maturation in a hypoxic environment. Immediately after birth, exposure to oxygen-rich ambient environment drives CM cell cycle arrest and maturation together with increases in nucleation and polyploidization [4]. Subsequent studies have identified a pool of hypoxic CMs in the adult heart responsive to proliferative signals [5] further highlighting the role of oxygen tension within the heart as a mediator of CM proliferation and cell cycle. Interestingly, adult mice exposed to chronic hypoxia show a fetal-like metabolism, enhanced CM proliferation and functional recovery following myocardial infarction [5] (Table 1). The big question is whether hypoxia can drive cell cycle activity in the human heart. Recently, Ye and colleagues conducted an elegant study showing increased cell cycle activity CMs from human heart samples isolated from patients with cyanotic heart diseases [6]. Authors collected 30 ventricular outflow myocardial tissue specimens from Tetralogy of Fallot patients and categorized them as mild hypoxia, moderate hypoxia and severe hypoxia based on blood oxygen saturation. They found that moderate hypoxia reduced mitochondrial DNA damage and increased CM proliferation. A similar result was obtained using human iPS derived CMs showing benefits of moderate hypoxia for promoting CM cell cycle activity. Nevertheless, hypoxia-based therapy is difficult to achieve in a clinical setting due to wide variety of clinical features associated with heart failure in patients parallel to chronic injury to the myocardium further complicating the problem. Moreover, there is no consideration of hypoxia sensing as a readout for hypoxia in any of the studies reported. Lower than normal oxygen levels in blood may not necessarily lead to hypoxia.
Table 1.
– Strategies reactivating developmental signaling in adult hearts.
| developmental signaling factors | Neonatal heart | Adult heart | Main altered pathways |
|---|---|---|---|
| Environmental factors | |||
| Oxygen tension | Low Oxygen saturation | Highly oxygenated | ↓ OxPHOS ↓ ROS / DNA damage |
| Thyroid hormone | Decreased | Increased | ↓ Ploidy ↓ OxPHOS ↑ Cell cycle regulators ↑ G2/M checkpoints ↑ E2F targets ↓ Muscle contraction |
| Mitochondria substrate utilization | Glucose | Fatty acids | ↑ Pyruvate oxidation ↓ ROS / DNA damage ↑ Cell cycle regulators (Ccnd1, Cdc6, and E2f1) ↓ CDK inhibitors (Cdk1a, Cdk1c) |
| Cellular and molecular factors | |||
| Meis1 | Downregulated | Upregulated | ↓ CDK inhibitors (p15, p16 and p21) ↑ Cell cycle regulators (MCM3, Chek1 and Ccnd2) |
| YAP (YAP5SA) | Upregulated | Downregulated | Chromatin remodeling to a fetal-like state ↑ Cell cycle regulators (Ccnd1, Ccna2, Ccnb2, and Ccnb1) ↓ Sarcomeric proteins (MYH6 and TNNT1) ↑ Mononucleate and diploid CMs ↑ IGF-1 and Akt signaling |
| Cell cycle regulators (CDK1, CDK4, cyclins B1 and D1) | Upregulated | Downregulated | ↑ G2/M phase progression ↓ DNA damage |
| Pkm2 | Upregulated | Downregulated | ↑ G6pd and PPP pathway ↓ ROS / DNA damage ↑ Cyclin D1 and c-Myc |
| Lamin B2 | Upregulated | Downregulated | ↓ Ploidy ↑ Karyokinesis |
| miR-15 | Downregulated | Upregulated | ↓ Sarcomere organization ↑ Cell cycle genes |
| miR-294 | Upregulated | Downregulated | ↑ Glycolysis and OxPHOS ↑ Cell cycle regulators (Cyclins B1, D1, E1 and A2, CDK1, E2F1, and E2F3) ↓ Negative cell cycle regulators (Wee1) |
| miR17–92 cluster | Upregulated | Downregulated | ↓ PTEN |
OxPhos – Oxidative phosphorylation; ROS – Reactive oxygen species; CMs – Cardiomyocytes; PPP - Pentose phosphate pathway.
3.2. Metabolism
During development, embryonic CMs rapidly proliferate and rely on glycolysis for ATP production. As the heart grows, CMs undergo metabolic changes from anaerobic glycolysis to oxygen-dependent mitochondrial oxidative phosphorylation and utilize primarily fatty-acids that coincides with cell cycle exit. This metabolic shift results in increased mitochondrial ROS production, oxidative DNA damage and activation of DNA damage response adversely impacting CM cell cycle and favoring maturation [4]. Our recent study [7] and others have shown that reactivation of developmental signaling factors in the heart leads to metabolic reprogramming of CMs that favors increased cell cycle activity and myocardial repair after injury (Table 1). Interestingly, reliance on glycolysis seems to be preferred energy generating pathway in proliferative CMs. Under hypoxic conditions, pyruvate kinase is responsible for ATP production by converting phosphoenolpyruvate (PEP) to pyruvate in the last step of the glycolysis pathway while Pkm2, an isoenzyme of the glycolytic enzyme pyruvate kinase, is expressed in CMs during development and immediately after birth but not during adulthood [8]. Pkm2 is necessary for normal cardiac development and its absence results in significantly reduced CM cell cycle, CM numbers, and myocardial size [8]. Moreover, reintroduction of Pkm2 in adult heart enhances CM proliferation, cardiac function, and long-term survival [8]. Pkm2 overexpression was found to be associated with increased glycolytic flux and boost up the biosynthetic pentose phosphate pathway which is essential for cell growth and proliferation. As a consequence, mitochondria produce ROS at a lower rate when using pyruvate relative to fatty acids as respiratory substrate reducing oxidative stress and DNA damage, involved in CM cell cycle arrest [9]. Similarly, changes in oxygen tension following birth also drive a shift in CMs metabolism from glycolysis to oxidative phosphorylation and extending anaerobic metabolism through induction of hypoxia prolongs regenerative window of the neonatal heart. Reducing fatty-acid oxidation and manipulation of pyruvate dehydrogenase kinase 4 (PDK4) has been shown to be associated with increased CM proliferation and repair after myocardial injury thereby highlighting the role played by nutrients in regulating CM cell cycle activity [9].
3.3. Nutrient Availability
Substrate availability in the embryonic heart shifts from pyruvate to fatty-acids after birth due to breast milk rich in fatty-acids. Cardoso et al., showed recently that reducing fatty-acid availability in the postnatal heart extends CM cell cycle activity beyond P7 [9]. Furthermore, increasing glucose relative to fatty-acid oxidation in adult mice decreases DNA damage, enhanced CM proliferation and cardiac function after injury [9]. Moreover, increasing fatty-acid β-oxidation was linked to increased CM hypertrophy and maturation together with cell cycle exit, thereby providing a link between substrate availability and CM proliferation in the heart (Table 1).
3.4. Hormonal Control
Beyond changes in environment, the heart is influenced by external factors and hormones secreted by other organs and tissues. However, our understanding of the crosstalk between the heart and other organs and the effect of extra-cardiac signaling on heart regeneration is limited. Recently, an elegant study by Hirose and colleagues [10] reported that thyroid hormones, major regulators of energy metabolism and thermogenesis, play a central role in the postnatal cessation of cardiac regenerative window (Table 1). Soon after birth, thyroid hormone dramatically increases in the bloodstream that induces CM cell cycle exit and increases CM binucleation. Attenuating thyroid hormone signaling in neonatal mice prevents CM binucleation leading to an increase in CM proliferation and number in the postnatal heart [10]. Furthermore, adult mouse CMs lacking thyroid hormone receptor display enhanced regenerative response following myocardial infarction, improved cardiac function and a reduced fibrotic scarring providing new insight into the hormonal regulation of cardiac regenerative capacity [10]. Interestingly, the authors observed an inverse correlation between plasma thyroid hormone levels, basal metabolic rate, and number of diploid CM. In other words, the higher the basal metabolic rate lower the percentage of diploid CM and lower regenerative potential. Nevertheless, whether metabolism can directly influence CM ploidy and nucleation status is still unknown.
3.5. Nucleation and polyploidization
During embryonic development, nearly all CMs are mononucleated and diploid (MNDCMs) and able to actively proliferate. After birth, most CMs undergo one more round of DNA synthesis without cytokinesis becoming either multinucleated and/or polyploid, a feature generally associated with loss of proliferative capability in the heart. Persistence of a small population of MNDCMs in the adult heart has prompted strategies targeting activation of MNDCMs to induce myocardial regeneration following injury. Nevertheless, the functional significance and mechanisms of postnatal CM polyploidization and multinucleation has been a longstanding and unresolved question in the field. Lamin B2 has been recently identified to play a role in postnatal CM polyploidization [11]. Lamin B2 is primarily active during heart development and necessary for nuclear envelope disassembly preceding metaphase and following division [11]. Manipulation of Lamin B2 levels in neonatal mice greatly influence the formation of polyploid CM and subsequent myocardial regeneration [11] (Table 1). However, whether reintroduction of Lamin B2 in adult mouse CMs promotes regeneration after injury was not tested. Moreover, frequency of nucleation/polyploidization in the mouse and human heart is inherently different without significant consequences for endogenous regenerative capacity highlighting limitations of the mouse model system for studying nucleation.
3.6. Transcription factors
Different factors have been identified in the developing cardiac tissue, yet the transcriptional network that regulates developmental CM cell cycle proliferation and arrest is still unknown. Meis1 was one of the first transcriptional regulator reported to play a major role in neonatal heart regeneration [12]. As a transcription factor, Meis1 orchestrates an extensive network of cell cycle regulators enhancing the expression of cyclin-dependent kinase inhibitors (including p15, p16, and p21) and repressing positive regulators of cell cycle (such as CCND2, CDK4, and CHEK1) [12]. Either ablation of Meis1 [12] or overexpression of neonatal CM-enriched cell-cycle regulators (CDK1, CDK4, cyclin B1, and cyclin D1) [13] can extend the postnatal regenerative window and enhance proliferation in post-mitotic mouse [12,13], rat [13] and human CM [13] thereby enhancing cardiac function after myocardial infarction [13] (Table 1). Although the modulation of cell cycle genes and transcriptional factors may unlock the proliferative potential of CMs, mechanism governing CMs to repress the translation of these genes and whether this underlying signaling can truly be overcome is still unclear. In similar context, the Hippo pathway has been extensively described as a repressor of CM proliferation. In the postnatal heart, Yes-associated protein (Yap), a transcriptional cofactor in the Hippo signaling pathway, has been implicated in the loss of regenerative capacity [14]. Forced expression of the active form of Yap in adult hearts stimulates cardiac regeneration and improves contractility after myocardial infarction [14].
3.7. microRNAs
The post-transcriptional regulation of gene expression by microRNAs (miRNAs) poses an important aspect for cellular and molecular modulation of postnatal heart regeneration. Members of the miR-15 family and miR-17–92 cluster were the first miRNAs shown to be dysregulated after birth. Inhibition of miR-15 family or overexpression of miR-17–92 cluster from early postnatal age until adulthood enhances CM proliferation and improves cardiac function after MI. Recently, our group has identified a new role for miR-294, an embryonic stem cell cycle (ESCC) miRNA in cardiac regeneration [7] (Table 1). miR-294 is highly expressed in the heart during development and prenatal stages but lost in the neonate and adult heart. Transient overexpression of miR-294 in adult heart via targeting of the Wee1-CyclinB1/CDK1 complex recapitulates developmental signaling and phenotype promoting CM cycle reentry and enhanced cardiac function in mice after myocardial infarction [7].
3.8. Chromatin modulation
Distinct epigenetic regulating signaling pathways are known to alter in fetal, postnatal and disease CMs with consequences for CM cell cycle and turnover in the heart. Recently, Monroe and colleagues have shown reactivation of Hippo-YAP signaling pathways in adult CMs reprograms chromatin accessibility to induce expression of developmental and cell cycle genes that leads to enhancement of cardiac structure and function after injury [15] (Table 1). This is particularly interesting, since chromatin state of a differentiated CMs is considered to be stable and irreversible. Moreover, reversion to a more developmental cell state, as defined by chromatin accessibility, has been described in cancer but not in CM renewal. Nevertheless, the long-term effects of chromatin reprogramming to a developmental state on cardiac function in the adult heart still needs to be investigated.
4. Future work and challenges
The proliferative nature of cardiac tissue during developmental stages has provided cues for understanding CM cell cycle and more importantly spawned development of strategies to promote cell cycle reentry in adult CMs (Fig. 2). Identification of therapeutic targets that drive meaningful new CM formation in the heart following injury would depend on ability of the targets to efficiently cope and adapt to various physiological and pathological changes in cardiac environment. It is well established that environmental changes during postnatal development and organism growth exert increased functional demand on CMs that leads to cell cycle exit and maturation. Following injury to the adult heart, cardiac milieu comprises of a constellation of inflammatory molecules, active immune response and severe hypoxia that can together adversely impact any CM-based regenerative strategy. True cardiac regeneration may not just depend on single factor, but rather multiple factors or ability of the therapeutic target to coordinate interaction of molecular players within CMs and their microenvironment.
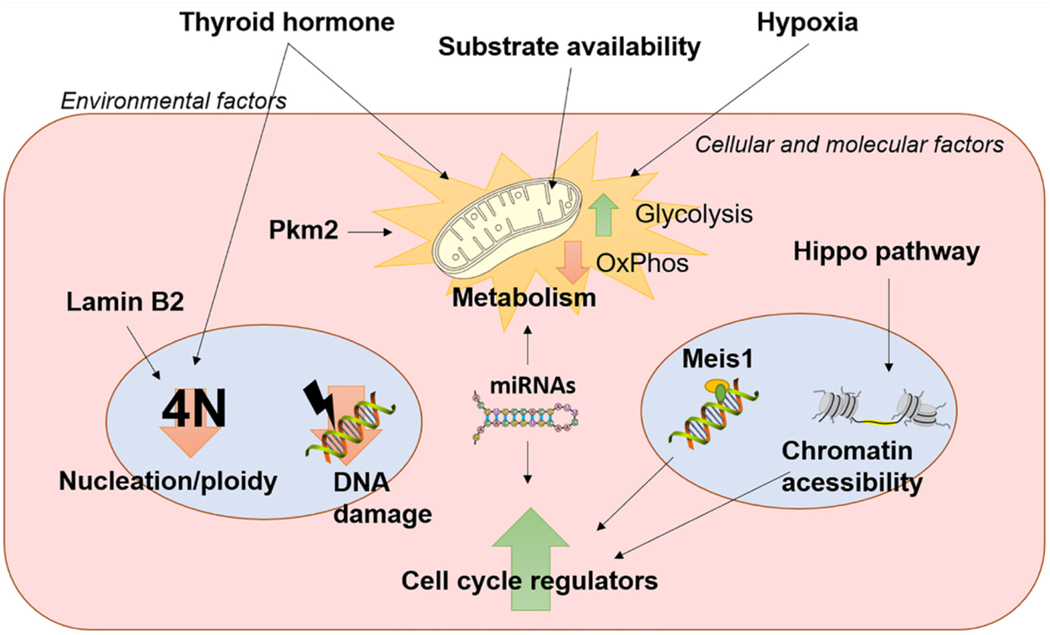
Fig. 2.
Developmental signaling factors for adult heart regeneration. Manipulation of thyroid hormone signaling, substrate availability and utilization, and oxygen levels (hypoxia) influence cardiomyocyte proliferation and heart regeneration by mainly targeting cellular metabolism. Attenuation of oxidative metabolism leads to a reduction in reactive oxygen species and subsequent oxidative DNA damage, one of the main mechanisms in postnatal cell cycle arrest. Reactivation of the active form of YAP (Hippo pathway) reprograms chromatin acessibility to enhance cell cycle regulators, while blunting Meis1 or alteration in miRNAs levels can directly upregulate cell cycle genes or downregulate cell cycle inhibitors and cardiomyocyte proliferation. Reintroduction of Lamin B2 enhances nuclear division decreasing ploidy levels, a known contributor in postnatal cell cycle arrest.
4.1. Detection benchmarks
Recent years have witnessed an explosion of new targets and strategies for activation of CM cell cycle activity and cardiac regeneration in the heart after injury. Although, these studies claim CM proliferation in the adult heart, not many have been validated in large animal models of heart failure. Whether the observed effects are limited to a particular species or widely applicable or translatable to human population remains unknown. Additionally, there have been conflicting results and interpretations largely due to discrepancies in detection methods and analysis of CM cell cycle activity and new myocyte formation in the heart after injury. Following are some of the standardization techniques that will enhance and together support the field of CM regeneration in the heart. 1) A clear distinction between activation of CM cell cycle activity with or without complete cytokinesis must be documented. 2) Combination of neonatal and adult CM cell culture systems together with human IPS-derived CMs should be prefeed. 3) A lineage tracing mouse model for CM fate mapping and new myocyte formation should be the standard to assess CM proliferation. 4) Finally, validation of mouse findings in a clinically relevant large animal model for myocardial injury.
Acknowledgements
We thank all members of the Khan laboratory for their valuable discussions. This work was supported by National Institute of Health grant HL135177 and H1801 WW Smith Charitable Trust to M. Khan.
Footnotes
Disclosures
None.
References
- [1].Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J, Dynamics of cell generation and turnover in the human heart, Cell 161 (7) (2015) 1566–1575. [DOI] [PubMed] [Google Scholar]
- [2].Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ, Cardiomyocyte DNA synthesis and binucleation during murine development, Am. J. Phys. 271 (5 Pt 2) (1996) H2183–H2189. [DOI] [PubMed] [Google Scholar]
- [3].Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA, Transient regenerative potential of the neonatal mouse heart, Science 331 (6020) (2011) 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA, The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response, Cell 157 (3) (2014) 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA, Hypoxia induces heart regeneration in adult mice, Nature 541 (7636) (2017) 222–227. [DOI] [PubMed] [Google Scholar]
- [6].Ye L, Qiu L, Feng B, Jiang C, Huang Y, Zhang H, Zhang H, Hong H, Liu J, Role of blood oxygen saturation during post-Natal human cardiomyocyte cell cycle activities, JACC Basic Transl Sci 5 (5) (2020) 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borden A, Kurian J, Nickoloff E, Yang Y, Troupes CD, Ibetti J, Lucchese AM, Gao E, Mohsin S, Koch WJ, Houser SR, Kishore R, Khan M, Transient introduction of miR-294 in the heart promotes cardiomyocyte cell cycle Reentry after injury, Circ. Res. 125 (1) (2019) 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Magadum A, Singh N, Kurian AA, Munir I, Mehmood T, Brown K, Sharkar MTK, Chepurko E, Sassi Y, Oh JG, Lee P, Santos CXC, Gaziel-Sovran A, Zhang G, Cai CL, Kho C, Mayr M, Shah AM, Hajjar RJ, Zangi L, Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration, Circulation 141 (15) (2020) 1249–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cardoso AC, Lam NT, Savla JJ, Nakada Y, Pereira AHM, Elnwasany A, Menendez-Montes I, Ensley EL, Petric UB, Sharma G, Sherry AD, Malloy CR, Khemtong C, Kinter MT, Tan WLW, Anene-Nzelu CG, Foo RS, Nguyen NUN, Li S, Ahmed MS, Elhelaly WM, Abdisalaam S, Asaithamby A, Xing C, Kanchwala M, Vale G, Eckert KM, Mitsche MA, McDonald JG, Hill JA, Huang L, Shaul PW, Szweda LI, Sadek HA, Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression, Nat Metab 2 (2) (2020) 167–178. [PMC free article] [PubMed] [Google Scholar]
- [10].Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, Smith M, Gillett E, Muroy SE, Schmid T, Wilson E, Field KA, Reeder DM, Maden M, Yartsev MM, Wolfgang MJ, Grutzner F, Scanlan TS, Szweda LI, Buffenstein R, Hu G, Flamant F, Olgin JE, Huang GN, Evidence for hormonal control of heart regenerative capacity during endothermy acquisition, Science 364 (6436) (2019) 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Han L, Choudhury S, Mich-Basso JD, Ammanamanchi N, Ganapathy B, Suresh S, Khaladkar M, Singh J, Maehr R, Zuppo DA, Kim J, Eberwine JH, Wyman SK, Wu YL, Kuhn B, Lamin B2 Levels Regulate Polyploidization of Cardiomyocyte Nuclei and Myocardial Regeneration, Dev Cell 53(1) (2020) 42–59 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA, Meis1 regulates postnatal cardiomyocyte cell cycle arrest, Nature 497 (7448) (2013) 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D, Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration, Cell 173 (1) (2018) 104–116, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN, Hippo pathway effector yap promotes cardiac regeneration, Proc. Natl. Acad. Sci. U. S. A. 110 (34) (2013) 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Monroe TO, Hill MC, Morikawa Y, Leach JP, Heallen T, Cao S, Krijger PHL, de Laat W, Wehrens XHT, Rodney GG, Martin JF, YAP Partially Reprograms Chromatin Accessibility to Directly Induce Adult Cardiogenesis In Vivo, Dev Cell 48 (6) (2019) 765–779, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]