Abstract
Background:
Stroke rehabilitation may be improved with a better understanding of the contribution of ipsilateral motor pathways to the paretic limb and alterations in transcallosal inhibition. Few studies have evaluated these factors during dynamic, bilateral lower limb movements, and it is unclear whether they relate to functional outcomes.
Objective:
Determine if lower limb ipsilateral excitability and transcallosal inhibition after stroke depend on target limb, task, or number of limbs involved, and whether these factors are related to clinical measures.
Methods:
In 29 individuals with stroke, ipsilateral and contralateral responses to transcranial magnetic stimulation were measured in the paretic and non-paretic tibialis anterior during dynamic (unilateral or bilateral ankle dorsi/plantarflexion) and isometric (unilateral dorsiflexion) conditions. Relative ipsilateral excitability and transcallosal inhibition were assessed. Fugl Meyer, ankle movement accuracy, and walking characteristics were assessed.
Results:
Relative ipsilateral excitability was greater during dynamic than isometric conditions in the paretic limb (p≤0.02) and greater in the paretic than the non-paretic limb during dynamic conditions (p≤0.004). Transcallosal inhibition was greater in the ipsilesional than contralesional hemisphere (p=0.002) and during dynamic than isometric conditions (p=0.03). Greater ipsilesional transcallosal inhibition was correlated with better ankle movement accuracy (R2=0.18, p=0.04). Greater contralateral excitability to the non-paretic limb was correlated with improved walking symmetry (R2=0.19, p=0.03).
Conclusions:
Ipsilateral pathways have increased excitability to the paretic limb, particularly during dynamic tasks. Transcallosal inhibition is greater in the ipsilesional than contralesional hemisphere and during dynamic than isometric tasks. Ipsilateral pathways and transcallosal inhibition may influence walking asymmetry and ankle movement accuracy.
Keywords: lower extremity, neural pathways, neuroplasticity, stroke
1. Introduction
Individuals with stroke often experience persistent walking impairments (e.g. decreased walking speed) and almost 64% cannot walk independently, even after rehabilitation.1-3 These walking impairments limit safe and meaningful community engagement and reduce quality of life.4-6 Although important, standard rehabilitative strategies have had limited impact on walking for stroke survivors, and chronic impairments remain.7 The impact of standard rehabilitative strategies may be limited because we do not fully understand the neurophysiological factors that contribute to motor deficits and recovery after stroke, including: 1) the contribution of the contralesional hemisphere via ipsilateral motor pathways to the paretic limb, and 2) alterations in transcallosal inhibition (TCI) between hemispheres. An improved understanding of these neurophysiological factors and their contribution to motor deficits and recovery after stroke may reveal additional targets for rehabilitation.
There has been considerable interest into whether ipsilateral motor pathways from the contralesional hemisphere to the paretic upper limb are enhanced after stroke.8,9 Contralesional brain activation is greater during movements of the paretic than the non-paretic limb, particularly in those with greater motor impairment.10 Several transcranial magnetic stimulation (TMS) studies provide complementary evidence that ipsilateral motor pathways are upregulated; ipsilateral motor evoked potentials (MEPs) in the paretic upper limb are more common in individuals with than without stroke.11-14 There has also been interest into whether TCI is altered after stroke. In individuals without stroke, TCI of each hemisphere is balanced in the upper limb.15 After stroke, some evidence suggests that the contralesional hemisphere may exert greater inhibition of the ipsilesional hemisphere in the upper limb in individuals with chronic stroke.11,16-20 In contrast, other studies have not found alterations in TCI (particularly in non-chronic stroke), and suggest imbalance in excitability between hemispheres may reflect changes within the ipsilesional hemisphere not changes in interhemispheric inhibition.21-25
Despite interest in these neurophysiological factors, there are considerable gaps in our knowledge. First, there have been few investigations into ipsilateral motor excitability or TCI for the lower limb after stroke. These investigations are necessary to improve our understanding of motor control of the lower limb instead of generalizing from upper limb studies. Second, no studies have evaluated ipsilateral motor excitability or TCI during dynamic, bilateral lower limb movements. This is important because most functional lower limb movements like walking are inherently dynamic and bilateral, not isometric and unilateral. Third, it is inconclusive how these neurophysiological factors relate to functional outcomes.
The objective of this study was to measure ipsilateral motor excitability and TCI in the lower limb of individuals with stroke, and determine if these factors vary depending on the target limb (paretic vs. non-paretic), type of task (dynamic vs. isometric), or number of limbs involved (bilateral vs. unilateral). We hypothesized that ipsilateral excitability would be greater for the paretic than the non-paretic limb, and TCI would be greater in the ipsilesional than the contralesional hemisphere. We expected both factors to be greater during dynamic than isometric tasks and greater during bilateral than unilateral tasks. We also aimed to determine if ipsilateral excitability and TCI are related to motor impairment, walking, or ankle motor control. We hypothesized that greater ipsilateral excitability to the paretic limb and greater TCI of the ipsilesional hemisphere would be associated with greater impairment.
2. Methods
2.1. Participants
Participants had a single, mono-hemispheric stroke >6 months prior to enrollment and were 40-80 years of age (to limit the confounding effects of young or old age on corticomotor excitability). Participants were excluded if they had other neurological disorders, used anti-spasticity medications, had <5° of volitional movement in both ankles (necessary for dynamic ankle movement tasks), or had contraindications to brain stimulation, such as metal implants, skull fractures or abnormalities, history of seizures, recent concussion, pregnancy, or use of medications that alter cortical excitability. Prior to participation, participants were screened for eligibility and TMS safety. This study was approved by the institutional review board, and all participants provided written, informed consent.
2.2. TMS parameters
29 participants performed three maximal voluntary isometric contractions (MVCs) with the ankle dorsiflexors of both limbs. Muscle activity was recorded with surface electromyography (EMG; Bagnoli 8, Delsys, MA, USA; frequency: 2000 Hz, gain: 1000, band pass filter: 20-450 Hz) from the tibialis anterior (TA) using Spike2 (Cambridge Electronic Design, Cambridge, UK). The largest rectified EMG amplitude during MVCs was recorded for each limb. The optimal position for TMS (hotspot) was determined by systematically moving the coil while participants performed unilateral isometric contractions at 10% of maximal EMG.26 The optimal position was the position with the maximal, consistent MEP at the lowest stimulator intensity in the contralateral limb. Active motor threshold (AMT) was determined as the minimum stimulus intensity eliciting MEPs with a peak-to-peak amplitude of ≥0.1 mV in 5 out of 10 trials.27 Single pulse TMS (Magstim 200, Magstim Inc., MN, USA) through a double cone coil with posterior-to-anterior current flow at a maximal frequency of 0.25 Hz was used for all procedures.28,29
2.3. Experimental tasks
TMS was applied during four conditions (Fig. 1a): rest, dynamic bilateral, dynamic unilateral, and isometric unilateral. Twenty stimuli were applied to each hemisphere (Fig. 1b) for each target limb (2 hemispheres X 2 limbs) at 120% of AMT, based on the contralateral AMT for the target limb. Limb order was randomized, and order of stimulation to the ipsilateral or contralateral hemisphere was randomized for each target limb.
Fig. 1. Experimental protocol.
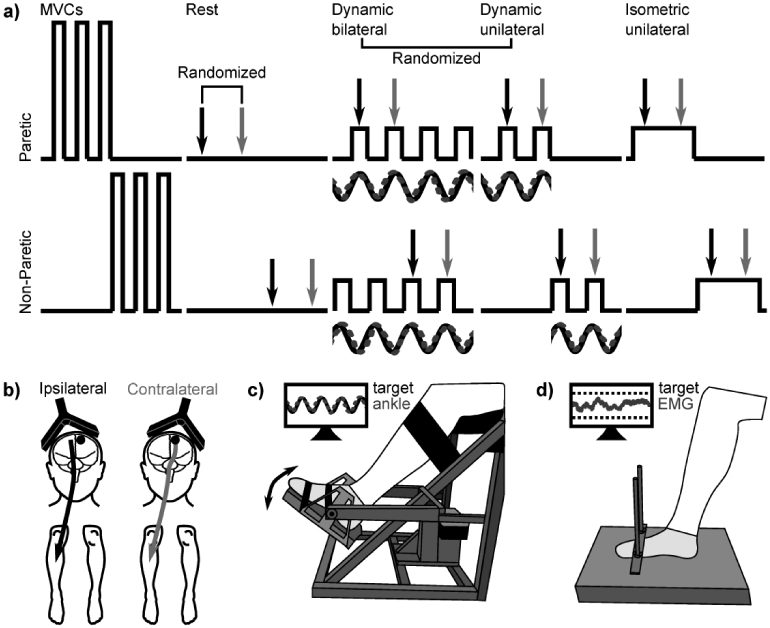
a) With both the paretic and non-paretic limb, participants performed maximal voluntary contractions (MVCs) and then four experimental conditions: rest, dynamic bilateral ankle movement, dynamic unilateral ankle movement, and isometric unilateral ankle contraction. Transcranial magnetic stimulation (TMS) was applied to the ipsilateral (black) and contralateral (light gray) hemisphere for each target limb. Limb order was randomized within each task, and order of stimulation to the ipsilateral or contralateral hemisphere was randomized for each target limb. Dynamic conditions always occurred after the resting condition and before the isometric unilateral condition. The order of dynamic conditions (bilateral or unilateral) was randomized. b) Transcranial magnetic stimulation (TMS) was applied to the ipsilateral (black) and contralateral (light gray) hemisphere for each target limb. The lesioned hemisphere is denoted with a black circle. c) During dynamic ankle movement tasks, the target limb was secured to a custom designed ankle-tracking device. Participants performed ankle dorsiflexion and plantarflexion to match the position of their ankle (dashed gray line) with the position of a computer-generated sine wave (black line). D) During isometric unilateral ankle contractions, participants performed isometric dorsiflexion with the target ankle while the other ankle remained at rest. Participants maintained an EMG level that was matched when stimulation was applied during dynamic tasks.
During dynamic bilateral and unilateral conditions, the target limb was secured to a custom designed ankle-tracking device allowing full ankle range of motion (Fig. 1c).28,30,31 A sinusoid (period: 4 seconds) customized to each participant’s ankle range of motion (ROM) was displayed on a screen, and participants were asked to perform ankle dorsiflexion and plantarflexion to match the position of the sine wave with the target limb.28,30,31 The sine wave amplitude was set at 80% of the ROM and oscillated around the midpoint between maximal dorsiflexion and plantarflexion. TMS was applied when the target ankle reached ~70% of its peak dorsiflexion, which approximately corresponds with the peak TA EMG. During the dynamic unilateral condition, participants performed the task with the target ankle, while the contralateral (non-target) limb remained at rest (visually monitored by study personnel). During the dynamic bilateral condition, the contralateral (non-target) ankle also performed dorsiflexion and plantarflexion in the opposite direction (antiphase) of the target ankle. The non-target ankle was unrestrained, no visual feedback of its performance was provided, and its relative position was not recorded. Participants were instructed to restrict movements to the ankle. Movements of proximal joints and the trunk were visually monitored by study personnel. When movements were detected, participants received verbal feedback to restrict movements to the ankle. Movements of the non-target ankle were monitored as well. During the dynamic unilateral condition, participants received verbal feedback to restrict movements to the target ankle; during the dynamic bilateral condition, verbal feedback was provided if movement of the non-target ankle ceased or became in-phase with the target ankle. Prior to TMS, participants performed three 60-second familiarization trials that were used to determine the accuracy of ankle motor control. Dynamic conditions occurred after the resting condition (to minimize experimental time) and before the isometric unilateral condition (to allow matched muscle activation intensity). The order of dynamic conditions (bilateral or unilateral) was randomized. Sufficient rest was incorporated between trials and between conditions.
During isometric contractions, participants performed isometric dorsiflexion with the target ankle while the other ankle remained at rest (Fig. 1d). The goal was to maintain an isometric contraction at the same percentage of maximal EMG as when stimulation was applied during the dynamic tasks. To determine this contraction amplitude, the background EMG at stimulation during dynamic conditions was determined. A target range corresponding to ±10% of the target value was displayed on a screen (Fig. 1d). TMS was delivered automatically by the Spike2 program when EMG values fell within this target range. EMG was not recorded or monitored from other muscles or the non-target leg.
2.4. Clinical outcomes
The Fugl Meyer Lower Extremity assessment (FMLE) was used to measure stroke-related motor impairment of the lower limb.32 Participants performed the 10-meter walk test (10MWT) across the GAITRite electronic mat (classic 14’ model, CIR Systems Inc., NJ, USA). Two trials were performed at self-selected comfortable speed. Time to complete each test was measured with a stopwatch, and average walking speed was calculated. Spatiotemporal characteristics of walking were assessed based on footfall characteristics calculated by the GAITRite mat and included: step length (cm), stance time, swing time, and stance/swing time (all time variables as %GC: percentage of gait cycle). All variables are presented as symmetry ratios: paretic/non-paretic.
2.5. Data analysis
The area of the rectified EMG was calculated from MEP onset to offset.29 Contralateral MEPs were quantified in the target limb when stimulation was applied to the contralateral hemisphere; ipsilateral MEPs were quantified in the target limb when stimulation was applied to the ipsilateral hemisphere. Background EMG was assessed as the area of the rectified EMG for the 50 ms prior to stimulation. We also assessed the time to MEP onset (MEP latency) for contralateral and ipsilateral MEPs. Because TMS was applied to the medial portion of the motor cortex and the double cone coil has low spatial localization, MEPs may consist of both ipsilateral and contralateral descending volleys. Thus, we used a measure of ipsilateral motor excitability to the target limb that accounts for the amplitude of ipsilateral MEPs relative to contralateral MEPs—the index of corticospinal excitability (ICE): ICE (%) = × 100%, where 100% = no ipsilateral MEP, 0% = equal ipsilateral and contralateral MEP, and - 100% = no contralateral MEP.28 For this measure, more negative values (larger ipsilateral MEPs relative to contralateral MEPs), were assumed to represent greater ipsilateral motor excitability. Our implementation of the ICE ratio differs from previous studies, which have inputted the slope of a TMS recruitment curve into the equation. Because of the number of conditions (4 conditions X 2 hemispheres X 2 limbs) and stimulations per condition (20), we did not perform recruitment curves. Instead, we inputted the area of the rectified MEP into the ICE equation.
TCI was assessed as the duration of the ipsilateral silent period (iSP), identified by the period following the MEP when EMG dropped below 25% of the background EMG until EMG returned back above this threshold.33-35 TCI of the contralesional hemisphere was assessed by the iSP in the non-paretic limb; TCI of the ipsilesional hemisphere was assessed by the iSP in the paretic limb. As a measure of intracortical inhibition, we determined the contralateral silent period (cSP) in the target limb following stimulation of the contralateral hemisphere 36. Ankle tracking accuracy of both limbs was assessed during unilateral movement as the root mean squared error value between the sine wave and the ankle position of the target limb, normalized to the individual ROM and converted to a percentage (maximum accuracy is 100%).28,30,31
2.6. Statistics
The primary outcome measures were ICE (relative ipsilateral motor excitability), iSP (TCI), and cSP (intracortical inhibition) For these measures, we performed repeated measures ANOVAs with within subject factors of limb (2 levels: paretic and non-paretic) and condition (3 levels: dynamic bilateral, dynamic unilateral, and isometric). For MEP latency, there was an additional within subject factor of stimulation side (2 levels: ipsilateral and contralateral). If sphericity was violated (Mauchly’s Test of Sphericity), the Greenhouse-Geisser correction was used. When a significant effect was detected, post-hoc t-tests were performed, with no correction for multiple comparisons. The rest condition was excluded from ICE analysis because only 1/3rd of participants (n=10) had MEPs in both legs at rest, meaning we were unable to calculate ipsilesional corticomotor excitability or calculate ICE values. MVC values, %MVC at stimulation, AMT, stimulation intensity, ankle tracking accuracy, and spatiotemporal characteristics of walking were compared between limbs with paired t-tests. Effect sizes are presented as partial eta squared (ηp2) for ANOVAs and Cohen’s d for t-tests. Prior to performing analyses, outliers whose values were >3SD from the mean were excluded. Pearson’s and Spearman’s correlations were performed to test relations with ICE, iSP, and cSP. No corrections for multiple comparisons were made.
3. Results
29 individuals with chronic stroke (demographics in Table 1) participated in the study. Participants had mild to moderate motor impairment based on the FMLE (Table 2). Representative TMS data are shown in Fig. 2. TMS data from one participant were excluded because they were an outlier; they had only ipsilateral MEPs in the paretic limb. TMS data were excluded from another participant who could not tolerate the stimulation. Spatiotemporal walking data were not collected from two participants because of equipment malfunction.
Table 1.
Demographics.
| Total (n=29) | |
|---|---|
| Age (years, mean±SD)) | 61.1±7.3 |
| Sex (male/female, counts) | 25/4 |
| More affected limb (left/right, counts) | 13/16 |
| Years since stroke (mean±SD (Range)) | 5.8±3.8 (0.9-15.7) |
| Type of stroke (ischemic/hemorrhagic, counts) | 21/8 |
Table 2. Clinical outcomes and ankle motor control.
%GC: percentage of gait cycle.
| Paretic | Non-Paretic | |||
|---|---|---|---|---|
| FMLE | 23.6±4.3 | |||
| (Range 13 – 30) | ||||
| Ankle tracking accuracy (%) | 72.4±15.5 | 73.7±13.5 | ||
| Speed (m/s) | 0.79±0.29 | Non-Paretic | Ratio | |
| Comfortable walking | Paretic | Non-Paretic | Ratio | |
| Step length (cm) | 53.1±15.3 | 49.5±14.5 | 1.12±0.31 | |
| Stance time (%GC) | 65.2±5.1 | 73.8±5.1 | 0.89±0.08 | |
| Swing time (%GC) | 34.8±5.1 | 26.2±5.1 | 1.37±0.34 | |
| Stance/swing time | 1.94±0.50 | 2.99±0.96 | 0.69±0.19 | |
Fig 2. Representative data.
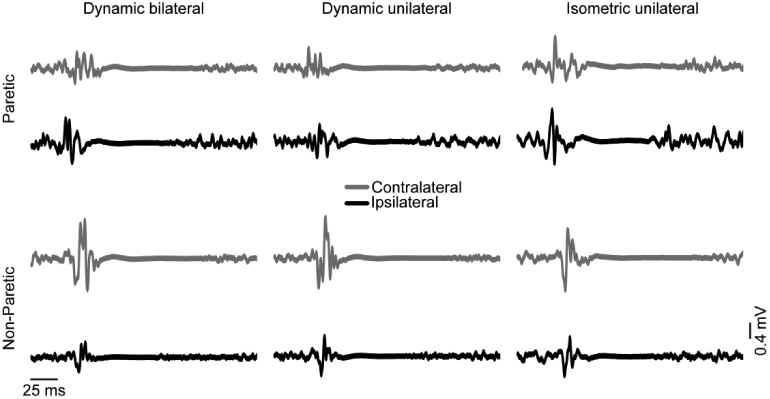
Data from one participant showing average motor evoked potentials (MEPs) in the paretic (top) and non-paretic (bottom) limb during three experimental conditions: dynamic bilateral ankle movement (left), dynamic unilateral ankle movement (middle), and isometric unilateral ankle contraction (right). Transcranial magnetic stimulation (TMS) was applied to the ipsilateral (black) and contralateral (light gray) hemisphere for each target limb during each condition. In this representative data, ipsilateral responses (relative to contralateral responses) were larger in the paretic than the non-paretic limb. Within the paretic limb, ipsilateral responses (relative to contralateral responses) were larger during dynamic conditions than during the isometric condition. iSPs were longer in the paretic than the non-paretic limb and during dynamic conditions compared to the isometric condition.
3.1. Relative ipsilateral motor excitability
For ICE (Fig. 3a), there was a limb X condition interaction (F(2,24)=3.5, p=0.04; ηp2=0.12) and a main effect of limb (F(1,25)=8.4, p=0.008; ηp2=0.25) but no main effect of condition (F(2,24)= 1.3, p=0.29). In the paretic limb, ICE was greater (more relative contralateral excitability) during the isometric than the dynamic unilateral (mean difference=7.7%, 95% CI: 1.7, 13.6; t(25)=2.6, p=0.02; Cohen’s d=0.56) and the dynamic bilateral condition (mean difference=7.8%, 95% CI: 2.5, 13.0; t(26)=2.9, p=0.007; Cohen’s d=0.50). There was no difference between the dynamic unilateral and dynamic bilateral condition (mean difference=0.1%, 95% CI: −6.0, 5.8; t(25)=0.03, p=0.98). In the non-paretic limb, ICE was not different between conditions (t(27)≤0.13, p≥0.90). ICE was smaller (more relative ipsilateral excitability) in the paretic than the non-paretic limb during the dynamic unilateral (mean difference=−18.6%, 95% CI: −30.8, −6.5; t(25)=−3.2, p=0.004; Cohen’s d=1.04) and dynamic bilateral conditions (mean difference=−18.6%, 95% CI: −31.9, −5.4; t(25)=−2.9, p=0.007; Cohen’s d=0.96). There was a trend for smaller ICE in the paretic than the non-paretic limb during the isometric condition (mean difference=−10.5%, 95% CI: −21.5, 0.4; t(25)=−1.8, p=0.08; Cohen’s d=0.56). Results were comparable with values calculated from rectified MEP area normalized to rectified background EMG.
Fig 3. Primary outcome measures.
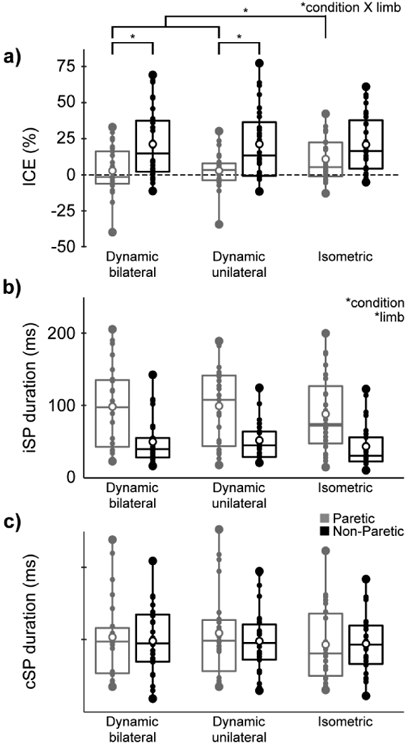
Box and whisker plots with values for the paretic (gray) and non-paretic limb (black) during dynamic bilateral ankle movement (left), dynamic unilateral ankle movement (middle), and isometric unilateral ankle contraction (right). a) Index of corticospinal excitability (ICE), b) ipsilateral silent period (iSP), c) contralateral silent period (cSP). Small dots represent individual data, while large dots represent the minimum and maximum values. Open circles represent mean values. Boxes range from the 1st to the 3rd quartile, and the middle horizontal lines represent the median values. Significant ANOVA effects are listed at the top right of each subfigure. Significant post-hoc t-tests to compare between conditions are shown in subfigure a.
3.2. Ipsilateral & contralateral silent periods
For iSP (Fig. 3b), there was an effect of limb (F(1,22)=12.5, p=0.002; ηp2=0.36) and condition (F(2,21)=4.4, p=0.03; ηp2=0.17) but no limb X condition interaction (F(2,21)=0.1, p=0.91). iSP was longer in the paretic than the non-paretic limb (mean difference=47.9 ms 95% CI: 32.8, 63.1; Cohen’s d=1.06). iSP was shorter for isometric than the dynamic unilateral (mean difference=−9.7 ms, 95% CI: −16.4, −3.0; t(49)=−2.9, p=0.006; Cohen’s d=0.20) and the dynamic bilateral condition (mean difference=−8.3 ms, 95% CI: −15.0, −1.6; t(49)=−2.5, p=0.02; Cohen’s d=0.17). There was no difference between the dynamic unilateral and dynamic bilateral condition (mean difference=0.9 ms, 95% CI: −3.5, 5.3; t(51)=0.41, p=0.68). For cSP (Fig. 3c), there was no effect of limb (F(1,22)=0.2, p=0.65) or condition (F(2,21)=2.5, p=0.10) and no limb X condition interaction (F(2,21)= 1.8, p=0.19).
3.3. MEP latency
For MEP latency, there was a main effect of limb (F(1,25)=22.3, p<0.001; ηp2=0.47) and stimulation side (F(1,25)=11.3, p=0.003; ηp2=0.31) but no effect of condition (F(2,24)=1.6, p=0.22) and no interactions (F(2,24)≤0.84, p≥0.44). MEP latency was longer in the paretic than the non-paretic limb (32.9±7.0 ms vs. 28.0±3.5 ms; mean difference=4.9 ms, 95% CI 3.9, 5.9; Cohen’s d=0.89) and was longer when stimulation was applied to the ipsilateral than the contralateral side (30.8±5.6 ms vs. 29.7±5.6 ms; mean difference=1.1 ms, 95% CI 0.6, 1.6; Cohen’s d=0.20).
3.4. Muscle activation & stimulation intensity
MVC was lower in the paretic than the non-paretic limb (0.10±0.07 mV vs. 0.18±0.08 mV; mean difference=−0.08 mV, 95% CI: −0.11, −0.05; t(27)=−5.9, p<0.001; Cohen’s d=0.56). However, the percentage of maximal MVC at which stimulation was applied was not different between conditions for the paretic (dynamic-43%, isometric-39%; F(1,26)=2.6, p=0.12) or the non-paretic limb (dynamic-43%, isometric-38%; F(1,26)=3.7, p=0.07). AMT was higher in the paretic than the non-paretic limb (46.9±10.0% vs. 41.5±7.7%; mean difference=5.3%, 95% CI: 2.5, 8.2; t(27)=3.8, p=0.001; Cohen’s d=0.56), and thus the stimulation intensity was higher in the paretic than the non-paretic limb (55.3±10.6% vs. 49.5±8.6%; mean difference=5.7%, 95% CI: 2.7, 8.8; t(27)=3.9, p=0.001; Cohen’s d=0.60).
3.5. Clinical outcomes & ankle motor control
Clinical outcomes and ankle motor control are shown in Table 2. Step length (mean difference=3.5 cm, 95% CI: 0.1, 6.9; t(25)=2.1, p=0.04; Cohen’s d=0.24), stance and swing time (mean difference=8.6 %GC, 95% CI: 6.1, 11.1; t(25)=7.1, p<0.001; Cohen’s d=1.69), and stance/swing time (mean difference=−1.05, 95% CI: −0.68, 1.41; t(25)=5.9, p<0.001; Cohen’s d=1.37) all differed between the paretic and non-paretic limbs. Ankle tracking accuracy measured during practice trials was not different between the paretic and the non-paretic limb (72.4±15.5% vs. 73.7±13.5%; mean difference: −1.3%; 95% CI: −5.1, 2.5; t(27)=−0.68, p=0.50).
Walking speed and FMLE were not associated with any of the TMS outcome measures. However, longer iSP (R2=0.18, p=0.04) and cSP (R2=0.19, p=0.03) in the paretic limb during isometric contractions were correlated with better paretic ankle tracking accuracy. Additionally, greater ICE (more relative contralateral excitability) in the non-paretic limb during the dynamic bilateral task was correlated with more symmetrical stance time and swing time (R2=0.19, p=0.03). See Figure 4.
Fig 4. Correlations with clinical outcomes.
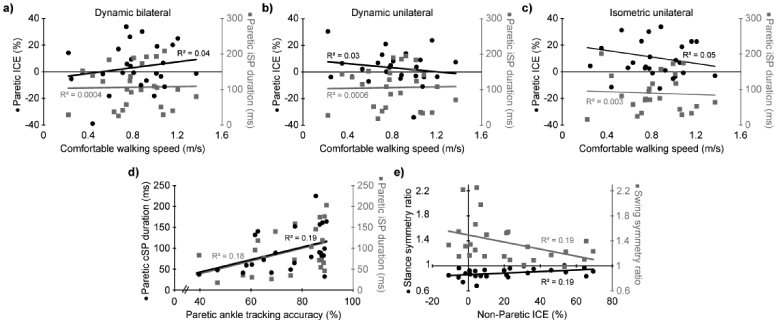
Scatter plots showing the relationships between neurophysiological and clinical outcomes. a-c) relation of comfortable walking speed with index of corticospinal excitability (ICE; black circles, left vertical axis) and ipsilateral silent period (iSP; gray squares, right vertical axis) in the paretic limb. More positive ICE values reflect greater contralateral motor excitability. Subfigure a, b, and c represent ICE values from the dynamic bilateral, dynamic unilateral, and isometric unilateral conditions, respectively. d) relation of paretic ankle track accuracy with contralateral silent period (cSP; black circles, left vertical axis) and ipsilateral silent period (iSP; gray squares, right vertical axis) in the paretic limb. e) relation of index of corticospinal excitability (ICE) in the non-paretic limb and with stance symmetry ratio (black circles, left vertical axis) and swing symmetry ratio (gray squares, right vertical axis). More positive ICE values reflect greater contralateral motor excitability. A symmetry value of 1 reflects perfect symmetry between the paretic and non-paretic limb.
4. Discussion
In this study, we measured relative ipsilateral motor excitability and TCI in the lower limb of individuals with stroke. We found that both target limb and type of task influence these measures. Relations with walking and ankle motor control suggest that relative ipsilateral motor excitability and TCI may have functional implications in the lower limb.
4.1. Increased relative ipsilateral excitability to the paretic limb
In this study, we found that relative ipsilateral excitability was greater in the paretic than the non-paretic TA. This finding is consistent with evidence from the upper limb, where ipsilateral MEPs are more common in individuals with than without stroke.11-14 In the lower limb, ipsilateral MEPs in the paretic limb have increased size relative to the non-paretic limb.28,37,38 These findings suggest that ipsilateral motor pathways may have an increased contribution to the paretic limb after stroke, which may compensate for damage to the crossed lateral corticospinal tract (CST).39 Potential ipsilateral motor pathways include the uncrossed lateral CST and cortico-subcortico-spinal pathways such as the corticoreticulospinal tract.
For insight into the ipsilateral pathway, we measured MEP onset latency. We found that MEP onset latency was ~1.1 ms longer for ipsilateral than contralateral MEPs. The difference between ipsilateral and contralateral latency is smaller than previously reported in the upper limb,40 but long enough to account for an additional synapse compared to contralateral MEPs.41 Consequently, this longer latency may also mean that ipsilateral MEPs may result from: 1) current spread to the contralateral hemisphere that activates the crossed lateral CST, 2) activation of cortico-subcortico-spinal pathways from the ipsilateral hemisphere, or 3) activation of the uncrossed lateral CST from the ipsilateral hemisphere. We have little evidence to distinguish between cortico-subcortico-spinal pathways and the uncrossed lateral CST. Anecdotally (Fig. 2), ipsilateral and contralateral MEP shapes were similar, suggesting similar innervation patterns, perhaps through the uncrossed and crossed lateral CST, respectively.
Several lines of evidence suggest that ipsilateral responses are not from current spread to the contralateral hemisphere. First, in 9 participants, the stimulation intensity for the paretic limb was equal to or lesser than that used for the non-paretic limb. If ipsilateral MEPs resulted from current spread, we would expect similar ICE for the paretic and non-paretic limb in these cases. To the contrary, ICE was lower in the paretic than the non-paretic limb in this subset of participants (−2.4±31.7% vs. 22.8±16%; mean difference=−25.2%, 95% CI: −38.8, −11.4; t=−3.8, p=0.001; Cohen’s d=1.01), and one individual, whose data we excluded, had only ipsilateral MEPs in the paretic limb (see supplemental figure). Second, as discussed below, ICE differed between isometric and dynamic conditions for the paretic but not the non-paretic limb. If ipsilateral MEPs resulted from current spread, we would expect ICE to be similarly affected by condition in the paretic and non-paretic limb.
4.2. Greater relative ipsilateral excitability during dynamic than isometric tasks
In the current study, relative ipsilateral excitability was greater during dynamic than isometric tasks in the paretic but not in the non-paretic limb. There was no difference in relative ipsilateral excitability to the paretic limb between dynamic bilateral and dynamic unilateral tasks. These findings suggest that the type of task (dynamic vs. isometric) but not the number of limbs involved (unilateral vs. bilateral) affects relative ipsilateral excitability to the paretic limb. Differences between dynamic and isometric tasks may reflect decreased inhibition of the contralesional hemisphere by the ipsilesional hemisphere during paretic movements, allowing increased ipsilateral excitability of the contralesional hemisphere to the paretic limb. Such a change in TCI has been suggested to contribute to mirror movements in the non-paretic limb and contralesional brain activation during unilateral movements of the paretic limb.42 Alternatively, increased relative ipsilateral excitability to the paretic limb may reflect greater use of subcortical pathways, which may also lead to mirror movements in the non-paretic limb.43 It is unclear why either of these causes would be more prominent during dynamic than isometric tasks. Perhaps these task-dependent differences reflect the greater difficulty or increased sensory feedback associated with dynamic movements. However, the dynamic bilateral task is more difficult and induces more sensory feedback than the dynamic unilateral task. We may not have seen differences between these conditions because descending input to the paretic limb was saturated during the dynamic unilateral task, disallowing an increase in ipsilateral input during the dynamic bilateral task. Another possibility is that dynamic tasks may evoke greater changes in corticomotor excitability than isometric tasks, possibly reflecting differences between discrete and rhythmic movements.44 Several studies have found that dynamic tasks of the hand produce a greater fMRI response than static tasks.45,46 Greater changes in corticomotor excitability may accentuate differences in excitability between the hemispheres ipsilateral and contralateral to the paretic limb.
4.3. Limb- and task-related differences in iSP but not cSP
During paretic limb contractions, stimulation of the contralesional hemisphere is thought to elicit TCI of the ipsilesional hemisphere. Because the ipsilesional hemisphere is primarily responsible for the contraction in the paretic limb, TCI elicits an iSP.47,48 iSPs are dependent on the integrity of the corpus callosum,33,47 so they are considered an index of TCI and not of ipsilaterally descending pathways. In this study, we found that iSPs were longer in the paretic than in the non-paretic limb. Other studies have found longer iSPs in the paretic hand,11,19 and paired-pulse paradigms in the paretic hand indicate greater interhemispheric inhibition of the ipsilesional hemisphere.16,20 These findings suggest that TCI of the ipsilesional hemisphere is greater than TCI of the contralesional hemisphere, consistent with the interhemispheric competition model.49 We also found that iSPs were longer during dynamic than isometric tasks in both limbs. There may be a generalized increase in TCI during dynamic than isometric conditions that promotes interlimb coordination during bilateral antiphase movements and inhibits mirror movements during unilateral movements.50,51 It is important to note that other studies in the upper limb have failed to demonstrate evidence of imbalanced TCI after stroke22-25,52 and cast doubt on whether the interhemispheric competition model extends to populations beyond individuals with chronic stroke and mild to moderate impairment.21 Additionally, imbalance in hemispheric excitability may result from changes in the ipsilesional hemisphere not changes in interhemispheric inhibition.23
In contrast to iSPs, cSPs are thought to represent intracortical inhibition.53 In the current study, we did not find differences in cSP between limbs or conditions. Results from previous studies have been mixed, with some finding longer cSPs (greater intracortical inhibition) in the ipsilesional vs. contralesional hemisphere and others finding no difference or shorter cSPs in the ipsilesional hemisphere.54 Variability between studies likely reflects differences in participant characteristics, including stroke location and time since stroke.23 Strokes that directly involve the motor cortex may result in absent or shorter cSPs, while strokes of other cortical or subcortical locations may result in longer cSPs.54 We were unable to collect information about stroke location in some of our participants, so we are unable to test this relationship with our dataset. In terms of time since stroke, cSP differences between limbs appear to decrease with increased stroke chronicity.19,54 Unlike many of the studies that demonstrated prolonged cSPs in the ipsilesional vs. contralesional hemisphere in acute participants, our study involved individuals who were ~6 years post-stroke on average. Thus, the lack of difference between hemispheres may reflect the chronic state of our participants and variability across the timespan of stroke chronicity.23
4.4. Walking and clinical outcomes
We also tested whether neurophysiological factors (ICE, iSP, and cSP) were related to clinical measures, walking, or ankle motor control. Contrary to our hypothesis, we did not find significant relations between TMS measures and walking speed or FMLE score. In one previous study, Fugl Meyer scores were worse and walking speed was slower in individuals with greater relative ipsilateral motor excitability.37 Discrepancies between that study and the current study may reflect different tested muscles—vastus lateralis37 vs. tibialis anterior. In the current study, we found that greater relative contralateral excitability to the non-paretic limb during dynamic bilateral movements were related to improved temporal symmetry during walking. We also found that increased intracortical inhibition and TCI of the ipsilesional hemisphere during isometric contractions were related to improved paretic ankle tracking accuracy. These findings suggest that less involvement of the ipsilesional hemisphere in the control of both the non-paretic and paretic limb may be adaptive, and there may be a functional benefit of inhibiting the output of the ipsilesional hemisphere. However, these relations had p-values near 0.05 without correction for multiple comparisons and may merely reflect Type-I error. A larger sample size may be needed to look at clinical outcomes. Other studies in the upper limb have found no clear relationship of TCI with motor impairment and function and suggested that this measure may not be causative but rather reflect recovery processes.21 Hence, abnormal TCI in chronic stroke may not be an effective target for reducing impairment and improving function. Others have suggested that the relation of TCI with impairment and function may be more complex and depend on factors such as stroke severity55 and that neuromodulatory approaches may need to be individually tailored.56
4.5. Limitations
Although our results may provide insight into ipsilateral motor contributions to the paretic lower limb, it is important to note that the excitability of a pathway assessed with TMS does not necessarily reflect that pathway’s contribution to movement.57 MEP area and silent period duration may be affected by differences in stimulation intensity. Absolute stimulation intensity was different between limbs, but relative stimulation intensity was the same. Moreover, in 9 participants where absolute stimulation intensity in the paretic limb was equal to or lesser than the non-paretic limb, ICE was lower in the paretic than the non-paretic limb. These findings suggest that differences in absolute stimulation intensity do not explain our results. Most studies have evaluated iSPs during a unilateral contraction at 50% of MVC, whereas we used a variety of tasks and contraction intensities. These methodological differences must be considered when interpreting our results. Furthermore, the notion that iSPs reflect TCI may have less validity in individuals with stroke who have poor contralateral and enhanced ipsilateral control of the paretic limb.
Several other methodological characteristics may have impacted our results. The order of conditions (rest, dynamic, isometric) was not randomized. This was done to minimize experimental time (dynamic after rest) and to allow the intensity of muscle activation to be matched across conditions (isometric after dynamic). Muscle activation in muscles besides the TA in the target limb were not monitored or recorded. Hence, proximal or trunk movements, activation of antagonists, and inappropriate activation of the non-target TA may have impacted our results. However, study personnel did visually monitor for unintended movements. Finally, potential participants were excluded if they had <5° of volitional ankle movement. Although this was exclusion was necessary to test the dynamic conditions, observations from these excluded individuals at rest or during isometric contractions may provide important insights into ipsilateral excitability and TCI.
4.6. Conclusions
Ipsilateral motor pathways may have increased relative excitability to the paretic lower limb, particularly during dynamic movements. TCI of the ipsilesional hemisphere may be greater than TCI of the contralesional hemisphere during lower limb movements, and TCI may be greater during dynamic than isometric conditions. Both relative ipsilateral motor excitability and TCI may have functional implications, although more work is needed.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health [F32HD102214 & R01HD075777].
Footnotes
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
References
- 1.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76(1):27–32. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull GI, Charteris J, Wall JC. A comparison of the range of walking speeds between normal and hemiplegic subjects. Scand J Rehabil Med. 1995;27(3):175–182. [PubMed] [Google Scholar]
- 3.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999;21(5-6):258–268. [DOI] [PubMed] [Google Scholar]
- 4.English C, Manns PJ, Tucak C, Bernhardt J. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys Ther. 2014;94(2):185–196. [DOI] [PubMed] [Google Scholar]
- 5.Desrosiers J, Malouin F, Bourbonnais D, Richards CL, Rochette A, Bravo G. Arm and leg impairments and disabilities after stroke rehabilitation: relation to handicap. Clin Rehabil. 2003;17(6):666–673. [DOI] [PubMed] [Google Scholar]
- 6.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83(8):1035–1042. [DOI] [PubMed] [Google Scholar]
- 7.Dickstein R Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22(6):649–660. [DOI] [PubMed] [Google Scholar]
- 8.Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front Hum Neurosci. 2013;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alawieh A, Tomlinson S, Adkins D, Kautz S, Feng W. Preclinical and Clinical Evidence on Ipsilateral Corticospinal Projections: Implication for Motor Recovery. Transl Stroke Res. 2017;8(6):529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126(Pt 6):1430–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120 ( Pt 9):1579–1586. [DOI] [PubMed] [Google Scholar]
- 12.Caramia MD, Iani C, Bernardi G. Cerebral plasticity after stroke as revealed by ipsilateral responses to magnetic stimulation. Neuroreport. 1996;7(11):1756–1760. [DOI] [PubMed] [Google Scholar]
- 13.Alagona G, Delvaux V, Gerard P, et al. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32(6):1304–1309. [DOI] [PubMed] [Google Scholar]
- 14.Misawa S, Kuwabara S, Matsuda S, Honma K, Ono J, Hattori T. The ipsilateral corticospinal tract is activated after hemiparetic stroke. Eur J Neurol. 2008;15(7):706–711. [DOI] [PubMed] [Google Scholar]
- 15.Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41(4):1382–1394. [DOI] [PubMed] [Google Scholar]
- 16.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. [DOI] [PubMed] [Google Scholar]
- 17.Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55(3):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236–246. [DOI] [PubMed] [Google Scholar]
- 19.Takechi U, Matsunaga K, Nakanishi R, et al. Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clin Neurophysiol. 2014;125(10):2055–2069. [DOI] [PubMed] [Google Scholar]
- 20.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28(4):940–946. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Branscheidt M, Schambra H, et al. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann Neurol. 2019;85(4):502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stinear CM, Petoe MA, Byblow WD. Primary Motor Cortex Excitability During Recovery After Stroke: Implications for Neuromodulation. Brain Stimul. 2015;8(6): 1183–1190. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017;10(4):721–734. [DOI] [PubMed] [Google Scholar]
- 24.Ludemann-Podubecka J, Bosl K, Nowak DA. Inhibition of the contralesional dorsal premotor cortex improves motor function of the affected hand following stroke. Eur J Neurol. 2016;23(4):823–830. [DOI] [PubMed] [Google Scholar]
- 25.Dimyan MA, Perez MA, Auh S, Tarula E, Wilson M, Cohen LG. Nonparetic arm force does not overinhibit the paretic arm in chronic poststroke hemiparesis. Arch Phys Med Rehabil. 2014;95(5):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivaramakrishnan A, Tahara-Eckl L, Madhavan S. Spatial localization and distribution of the TMS-related 'hotspot' of the tibialis anterior muscle representation in the healthy and post-stroke motor cortex. Neurosci Lett. 2016;627:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhavan S, Rogers LM, Stinear JW. A paradox: after stroke, the non-lesioned lower limb motor cortex may be maladaptive. Eur J Neurosci. 2010;32(6):1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madhavan S, Stinear JW. Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul. 2010;3(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madhavan S, Weber KA 2nd, Stinear JW. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res. 2011;209(1):9–17. [DOI] [PubMed] [Google Scholar]
- 31.Sriraman A, Oishi T, Madhavan S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014;1581:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 33.Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118 ( Pt 2):429–440. [DOI] [PubMed] [Google Scholar]
- 34.Fleming MK, Newham DJ. Reliability of Transcallosal Inhibition in Healthy Adults. Front Hum Neurosci. 2016;10:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson TW, Bolic M, Tremblay F. Predicting Modulation in Corticomotor Excitability and in Transcallosal Inhibition in Response to Anodal Transcranial Direct Current Stimulation. Front Hum Neurosci. 2016;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128(4):539–542. [DOI] [PubMed] [Google Scholar]
- 37.Jayaram G, Stagg CJ, Esser P, Kischka U, Stinear J, Johansen-Berg H. Relationships between functional and structural corticospinal tract integrity and walking post stroke. Clin Neurophysiol. 2012;123(12):2422–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83(2):419–426. [DOI] [PubMed] [Google Scholar]
- 39.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12(1):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziemann U, Ishii K, Borgheresi A, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518 ( Pt 3):895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz B, Miledi R. The Measurement of Synaptic Delay, and the Time Course of Acetylcholine Release at the Neuromuscular Junction. Proc R Soc Lond B Biol Sci. 1965;161:483–495. [DOI] [PubMed] [Google Scholar]
- 42.Kim YH, Jang SH, Chang Y, Byun WM, Son S, Ahn SH. Bilateral primary sensori-motor cortex activation of post-stroke mirror movements: an fMRI study. Neuroreport. 2003;14(10):1329–1332. [DOI] [PubMed] [Google Scholar]
- 43.Ejaz N, Xu J, Branscheidt M, et al. Evidence for a subcortical origin of mirror movements after stroke: a longitudinal study. Brain. 2018;141(3):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiegel P, Kurz A, Leukel C. Evidence that distinct human primary motor cortex circuits control discrete and rhythmic movements. J Physiol. 2020;598(6):1235–1251. [DOI] [PubMed] [Google Scholar]
- 45.Keisker B, Hepp-Reymond MC, Blickenstorfer A, Kollias SS. Differential representation of dynamic and static power grip force in the sensorimotor network. Eur J Neurosci. 2010;31(8):1483–1491. [DOI] [PubMed] [Google Scholar]
- 46.Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Sacco P, Mastaglia FL. Differences in functional magnetic resonance imaging of sensorimotor cortex during static and dynamic finger flexion. Exp Brain Res. 1999;126(3):431–438. [DOI] [PubMed] [Google Scholar]
- 47.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144(1-2):160–170. [DOI] [PubMed] [Google Scholar]
- 48.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boddington LJ, Reynolds JN. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017;10(2):214–222. [DOI] [PubMed] [Google Scholar]
- 50.Beaule V, Tremblay S, Theoret H. Interhemispheric control of unilateral movement. Neural Plast. 2012;2012:627816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daffertshofer A, Peper CL, Beek PJ. Stabilization of bimanual coordination due to active interhemispheric inhibition: a dynamical account. Biol Cybern. 2005;92(2):101–109. [DOI] [PubMed] [Google Scholar]
- 52.Mang CS, Borich MR, Brodie SM, et al. Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clin Neurophysiol. 2015;126(10):1959–1971. [DOI] [PubMed] [Google Scholar]
- 53.Chen R, Cros D, Curra A, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119(3):504–532. [DOI] [PubMed] [Google Scholar]
- 54.van Kuijk AA, Pasman JW, Geurts AC, Hendricks HT. How salient is the silent period? The role of the silent period in the prognosis of upper extremity motor recovery after severe stroke. J Clin Neurophysiol. 2005;22(1):10–24. [DOI] [PubMed] [Google Scholar]
- 55.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. [DOI] [PubMed] [Google Scholar]
- 56.Plow EB, Sankarasubramanian V, Cunningham DA, et al. Models to Tailor Brain Stimulation Therapies in Stroke. Neural Plast. 2016;2016:4071620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res. 2015;233(3):679–689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


