Abstract
Self-motion perception used for locomotion and navigation requires the integration of visual, vestibular, and proprioceptive input. In the absence of vision, postural stability and locomotor tasks become more difficult. Previous research has suggested that in visually deprived children, postural stability and levels of physical activity are overall lower than in sighted controls. Here we hypothesized that visually impaired and blind children and adolescents differ from sighted controls in postural stability and gait parameters, and that physically active individuals outperform sedentary peers in postural stability and gait parameters as well as in navigation performance. Fourteen blind and visually impaired children and adolescents (8–18 years of age) and 14 matched sighted individuals took part. Assessments included postural sway, single-leg stance time, parameters of gait variability and stability, self-reported physical activity, and navigation performance. Postural sway was larger and single-leg stance time was lower in blind and visually impaired participants than in blindfolded sighted individuals. Physical activity was higher in the sighted group. No differences between the group of blind and visually impaired and blindfolded sighted participants were observed for gait parameters and navigation performance. Higher levels of physical activity were related to lower postural sway, longer single-leg stance time, higher gait stability, and superior navigation performance in blind and visually impaired participants. The present data suggest that physical activity may enhance postural stability and gait parameters, and thereby promote navigation performance in blind and visually impaired children and adolescents.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00221-021-06038-3.
Keywords: Postural control, Visual deprivation, Physical activity, Spatial cognition, Vestibular system
Introduction
To estimate self-motion used for upright standing, locomotion and navigation, the brain integrates incoming sensory signals from visual, vestibular, and proprioceptive senses (Cullen and Taube 2017). Self-motion perception becomes less precise when visual input is temporally eliminated such as when the eyes are closed (Hartmann et al. 2013). In case of permanent blindness, larger postural sway during upright standing has been reported in blind children and adults compared to sighted controls with eyes open, indicative of postural instability (Müürsepp et al. 2018). Postural control deficits have also been found in visually impaired children with amblyopia and strabismus (Zipori et al. 2018), suggesting that a moderate visual impairment is sufficient to interfere with postural stability. During walking, slower gait velocity, shorter stride length, limited ankle plantar flexion, and a prolonged duration of stance in blind children and adults compared to sighted control participants have been reported (Bennett et al. 2019; Hallemans et al. 2010, 2011; Nakamura 1997). The altered gait patterns in the absence of vision have been interpreted as a more cautious walking strategy (Hallemans et al. 2011; Nakamura 1997). Notably, when sighted individuals were tested with closed eyes, deficits in balance as well as gait variability have been described to increase to similar levels as those of the blind (Campayo-Piernas et al. 2017; da Silva et al. 2018; Duarte and Zatsiorsky 2002; Hallemans et al. 2010; Müürsepp et al. 2018; Schmid et al. 2011; Schwesig et al. 2011; Wuehr et al. 2013). These results indicate a major role of vision for motor control during upright standing and bipedal locomotion, irrespective of whether visual input had been permanently or temporally absent.
Vision plays a pivotal role in orientation and wayfinding, too. To understand the spatial properties of an environment and to continuously update one’s own position within the environment, visual (e.g., optic flow and visual landmarks), auditory, vestibular, proprioceptive, and motor signals are integrated to update mental spatial representations (Loomis et al. 1993, 2001; Medendorp and Selen 2017; Schinazi et al. 2016). Navigation and wayfinding without vision is a particular challenge because precise spatial information from distal cues are not assessable (Loomis et al. 1993; Schinazi et al. 2016). In the dark, navigation is primarily accomplished via path integration, during which the estimates of direction, distance travelled as well as velocity are derived from vestibular and proprioceptive cues (Allen et al. 2004; Cullen and Taube 2017; Medendorp and Selen 2017). In route navigation tasks, in which participants have to reproduce a previously walked and memorized route, hardly any performance differences between blind and sighted adults have been found (Loomis et al. 1993; Rieser et al. 1986; Seemungal et al. 2007; Thinus-Blanc and Gaunet 1997). By contrast, inconsistent results exist for inferential path integration, in which participants have to deduct new multi-segment routes based on previously experienced spatial relationships such as the completion of a triangle: Blind compared to sighted individuals have been described to perform better, similar, and worse in path completion tasks (Loomis et al. 1993; Rieser et al. 1986; Seemungal et al. 2007; Thinus-Blanc and Gaunet 1997; Tinti et al. 2006). Several explanations for the inconsistent results have been suggested, including the heterogeneity of blind samples with regard to age and onset of blindness, small sample sizes, and individual differences with respect to efficient navigation strategies as well as past experience with wayfinding (Schinazi et al. 2016). For example, it has been suggested that the large inter-individual differences in path integration performance in blind individuals might be related to their habitual physical activity (Seemungal et al. 2007). The authors observed that blind individuals who performed above average in path integration tasks and displayed ultra-short vestibular time constants were those who reported higher physical activity scores. Thus, more experience with locomotor tasks in physically active individuals might be related to better wayfinding and orientation skills.
There is converging evidence in sighted humans showing a beneficial role of physical activity on cognitive functions, and specifically on memory and spatial cognition (Cassilhas et al. 2016; Fernandes et al. 2017; Stimpson et al. 2018). It has been suggested that the vestibular system might play a crucial role in mediating the link between physical activity and neural changes in cortical and subcortical brain areas including the medial temporal lobe, which have been associated with memory functions and spatial cognition (for a review see Smith 2017). The vestibular system has not only been found to be crucially involved in postural control such as during balancing on unstable ground (Lucieer et al. 2018), but several studies have additionally suggested a significant vestibular contribution to spatial cognitive functions (Angelaki et al. 2009; Hitier et al. 2014; Seemungal 2015). For example, galvanic vestibular stimulation was shown to influence performance in a visual-spatial task (Lenggenhager et al. 2007). In a longitudinal study, we have recently shown that balance training was capable of improving spatial cognitive functions such as mental rotation and perspective-taking in sighted adults (Rogge et al. 2017). Moreover, in this study, we found gray matter changes in visual and vestibular cortical areas associated with self-motion perception and spatial cognitive functions (Rogge et al. 2018). These patterns are in line with results from cross-sectional studies reporting a link between balance performance and navigation skills, mental rotation abilities, and visuo-spatial working memory in sighted adults and children as young as 6 years (Dordevic et al. 2018; Frick and Möhring 2015; Jansen and Heil 2010).
Visually impaired and blind adults as well as children have been reported to more often adopt a sedentary lifestyle, compared to their sighted peers (Augestad and Jiang 2015; Houwen et al. 2009; Longmuir and Bar-Or 2000; Müürsepp et al. 2018). However, physically active blind children and adults were found to outperform blind sedentary individuals in postural tasks and displayed enhanced gait velocity (Aydoğ et al. 2006; da Silva et al. 2018; Müürsepp et al. 2018). A recent study in blind adults has shown that 12 weeks of balance training was sufficient to increase blind participants' balance performance to a level of untrained sighted adults with eyes open (Rogge et al. 2019). By contrast, cardiorespiratory fitness had not changed after training, suggesting a specific gain in postural control and self-motion perception which could not be explained by an increase in overall physical fitness.
Yet it is unknown to which degree balance control, gait, and physical activities are related to navigation performance in blind children and whether the level of physical activity might explain deficits in spatial skills sometimes observed in blind children. For instance, lower performance in sound localization (Cappagli and Gori 2016; Vercillo et al. 2016) and auditory distance discrimination (Cappagli et al. 2017) have been reported in blind and visually impaired children from 6 to 17 years of age compared to sighted matched controls. It has been hypothesized that lower performance in spatial tasks is related to overall developmental delays (Ochaita and Huertas 1993) or specifically to delays in the development of locomotion (for a review, see Cuturi et al. 2016). Thus, it seems plausible to hypothesize that individuals who spend more time with locomotor activities show improved balance performance and a more typical gait pattern. Based on the observed link between balance performance and spatial cognitive functions, a positive correlation with navigation skills is additionally expected. These associations are predicted to be particularly expressed during childhood and adolescence, when both balance skills and spatial cognitive functions are subject of change.
In the present study, we assessed balance control, gait parameters, habitual physical activity, and navigation performance in a group of blind and visually impaired children and adolescents as well as in age-matched sighted controls. We hypothesized that physical activity is positively correlated with balance performance and gait parameters. Moreover, we predicted that physically active blind and visually impaired children and adolescents outperform their sedentary peers.
Methods
Participants
Fourteen blind and visually impaired children (mean age: 14.26 years, range 8–18, 8 females, 9 congenitally blind and 5 late blind) and 14 sighted children matched for age and gender with normal or corrected to normal vision (mean age: 14.00 years, range 8–18, 8 female) participated in the study. All children were recruited from the local communities of either Genoa (Italy) or Hamburg (Germany).
Blindness or visual impairment was due to retinopathy of prematurity (n = 3), congenital cataract and microphtalmia (n = 2), optic nerve glioma (n = 1), optic nerve atrophy (n = 1), tuberculous meningitis (n = 1), ocular albinism (n = 1), cones dystrophy (n = 1), homocystinuria (n = 1), Leber’s amaurosis (n = 1), retinoblastoma (n = 1), or unknown reasons (n = 1).
Of the blind and visually impaired individuals, two reported having no residual vision, and five reported rudimentary light and shadow perception. Visual acuity (decimal values) of the remaining seven participants was 0.1 (n = 4), 0.05 (n = 2), and 0.01 (n = 1).
The study was approved by the local ethical boards of the University of Hamburg and Genoa (Azienda Sanitaria Locale 3 Genovese) and was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from participants or from the parents of underage participants.
Physical parameters
Postural sway
Postural sway was assessed with a force plate (Type 9260AA6, Kistler® Instrumente GmbH, Switzerland, sampling rate: 180 Hz) using the software BioWare (Kistler Instruments AG, version 4.0.1.2). Center of pressure displacement (CoP) data of the medial–lateral and anterior–posterior time series were collected during normal bipedal stance (both feet together) and during semi-tandem stance (the big toe of the dominant leg placed to the side of and against the heel of the other foot). During testing, participants were asked to direct their head straight ahead, with their hands placed on their hips. In closed eyes conditions, blind and sighted participants were blindfolded, and three trials per stance were run, each with a length of 30 s and an inter-trial rest of 30 s. Eyes open conditions were tested in sighted participants only, with three trials per stance with a length of 30 s each. The condition to start with (eyes open vs. eyes closed) was randomized for the sighted participants. To calculate the CoP sway area (in cm2) per trial, prediction ellipse areas (PEA) with 95% probability were fitted to the time series data per trial using MATLAB, version R2017b (for details on PEA see Duarte 2015; Schubert and Kirchner 2014). Technical problems during testing led to missing data of n = 1 blind and n = 2 visually impaired individuals. Data of the respective matched sighted individuals were removed for the analysis of postural sway. The mean sway area per stance and condition (eyes open/closed for the sighted) was used as dependent variable.
Single-leg stance time
Functional balance performance was assessed with barefoot single-leg stances (Springer et al. 2007) on hard ground. Participants were asked to place the hands on their hips, to lift their dominant foot, and to close their eyes if possible, with the head directed straight-ahead. Each trial had a length of 20 s, followed by 30 s of rest. Two trials were run. Trials were video-recorded, and two independent observers measured the time (in sec) the participant remained in the correct position. Trial time ended when participants touched the floor with their raised foot, rotated or moved their foot of the standing leg to maintain balance, removed their hands from the hips, or when sighted individuals opened their eyes. The mean time in sec across trials was used as dependent variable.
Gait parameters
Gait parameters were captured with a wireless inertial motion tracker (MTw sensors, Xsens Technologies B. V., Netherlands, sampling rate: 100 Hz) attached to the foot of the participants. During testing, participants walked up and down a hallway of approx. 25 m for 6 min and at their preferred walking speed wearing a blindfold. Sighted individuals were additionally tested with eyes open, the condition to start with was counterbalanced across participants. Two experimenters marked the ends of the hallway by constantly clicking their fingers. Once the participant reached the experimenter at the end of the hallway, he or she was asked to turn and walk back on the same way. Whenever a participant lost his path, he was guided back on the correct way. Before calculating the gait parameters, data of the first and the last 25 m as well as the first and the last 2.5 m of each 25 m bout were excluded. Furthermore, the kinematic time-series were visually checked. Areas with non-stationary data (e.g., when a participant stopped and was guided back on the correct way) were excluded from the following data analysis. The parameters gait velocity, stride length, stride time, minimum foot clearance and the variability of each parameter (standard deviation) were determined. The reliability of the system (inertial sensors and algorithms) has been verified (Hamacher et al. 2014). Furthermore, the largest Lyapunov exponent (LLE) as a measure of local dynamic gait stability (LDS) was calculated based on three-dimensional angular velocity data of the foot (Hamacher et al. 2015). To compute the LLE, we time-normalized the three-dimensional angular velocity data of 63 strides (minimum across participants and conditions) to 6300 samples. Using the delayed embedding approach, we chose the time delay (τ = 11) and the embedded dimension (dE = 15) based on the first minimal mutual information (Fraser and Swinney 1986) and the false nearest neighbor analysis (Kennel and Abarbanel 1992), respectively. Based on the resulting state-space, the LLE was calculated using Rosenstein and coworkers algorithm (Rosenstein et al. 1993). Higher LLE values are interpreted as lower LDS and vice versa. Gait parameters and LLE have been shown to depict reasonable construct and convergent validity (Bruijn et al. 2013). In this study, we report stride time variability (sd) and LDS, as these parameters have been shown to be related to physical activity and balance performance in healthy and fall-prone older adults (Hamacher et al. 2018; Hausdorff 2007), and have been used to characterize changes in gait due to diminished visual feedback (Hamacher et al. 2016).
Physical activity
The “Freiburger Questionnaire on Physical Activity” (Frey et al. 1999) was used to assess the overall self-reported weekly habitual physical activity. The questionnaire has been translated into Italian by one of the coauthors (G.C.) for usage in Italy. The questionnaire covers everyday basic physical activities such as taking the stairs and walking to school or work, leisure physical activities as well as physical exercise. For the present study, only questions on basic physical activity and physical exercise were included. The questionnaire has been answered with the help of the parents of underage participants. Activities were summarized for each category and are reported as minutes per week.
Navigation performance
Triangle completion task
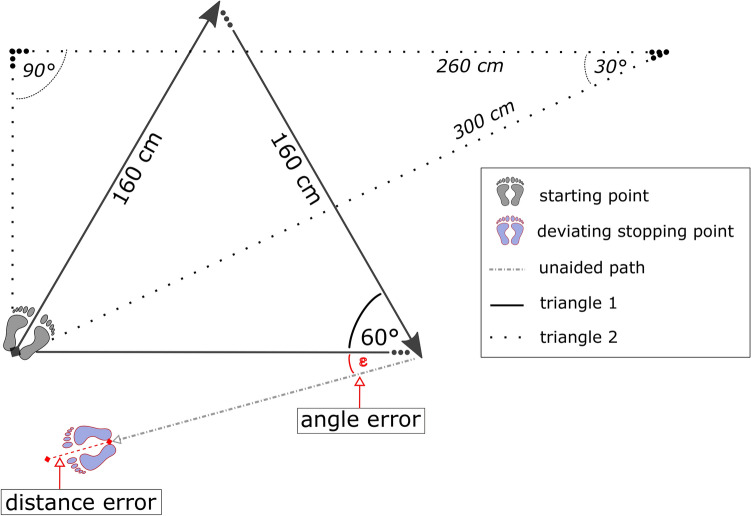
Navigation performance was assessed with a triangle completion task (Allen et al. 2004; Loomis et al. 2001). For this task, two triangles with route segment lengths between 150 and 300 cm (one equal-sided, one oblique triangle, resulting in target angles of 30°, 60° and 90°, respectively, see Fig. 1) were marked on the floor of an empty room. All participants were blindfolded and equipped with passive noise isolating headphones before entering the room. During testing, participants were guided along two segments of a triangle during which they touched the elbow of an experimenter. Upon reaching the end of the second path, the experimenter stepped aside and participants had to complete the triangle by turning and walking unaided to where they assumed the origin was. To calculate distance errors (in cm) and angle errors (in degrees), the stopping point of the participant was marked between the heels with adhesive tape on the floor after each trial. Four trials were run in a fixed order using the two triangles twice; once in clockwise and once in counterclockwise direction. The starting point was always the same. Between trials, participants were led on random walks within the room to avoid interference of previous trials. Participants did not receive feedback regarding their accuracy. Mean angle errors and mean (absolute) distance errors were used as dependent variables.
Fig. 1.
Triangles used in the path integration task (solid black lines: triangle 1, dotted black lines: triangle 2). The example depicts a deviation from the optimal path (solid lines) of triangle 1 performed in clockwise direction, with underestimated path length (red dashed line as distance error) and a turning error (red dotted line)
Data analysis
Data were analyzed and visualized in R (v.3.6.2) (R Core Team 2020) using the R packages afex (v.0.25–1) (Singmann et al. 2018), tidyverse (v.1.2.1) (Wickham et al. 2019), and emmeans (v.1.4.5) (Lenth et al. 2020).
Data of blind and visually impaired individuals did not differ and were thus analyzed together as one group. Statistical analyses were performed using mixed factorial ANOVAs. Levene’s test was used to test for homogeneity of variance between groups. Heteroscedasticity-corrected covariance matrices using White-Huber adjustments are reported, in case of variance heterogeneity. Equations to assess differences in balance skills and navigation performance between blind and sighted individuals included the between-subjects factor Group (blind/visually impaired vs. sighted), and the within-subject factor Condition (bipedal vs. semi-tandem stance for the analysis of postural sway and basic activity vs. exercise for the analysis of physical activity). Eyes open data were available for postural stability and gait parameter for sighted participants only, resulting in unequal numbers of trials for the blind and visually impaired and the sighted. Therefore, two analyses were run: The first compared the blind with the sighted using eyes closed data. The second analysis used eyes closed and eyes open data of the sighted participants only to analyze the effect of vision. Statistical comparisons utilized Type III sums of squares; effects were considered significant at p < 0.05, post hoc comparisons used estimated marginal means with Tukeys correction for multiple comparisons. Standardized effect sizes utilized Cohen’s d with the respective 95% CI of the mean difference between Group (blind/visually impaired vs. sighted) or Condition (eyes closed vs. eyes open), complemented by unstandardized effect sizes as mean differences. Data visualization utilized violin plots per Group and Condition to show the distribution and density of the data.
To assess the relationship between physical parameters and navigation performance, separate Pearson correlations (two-sided) per group (blind/visually impaired and sighted) were performed using mean data of eyes closed conditions only.
Results
Balance performance
Postural sway
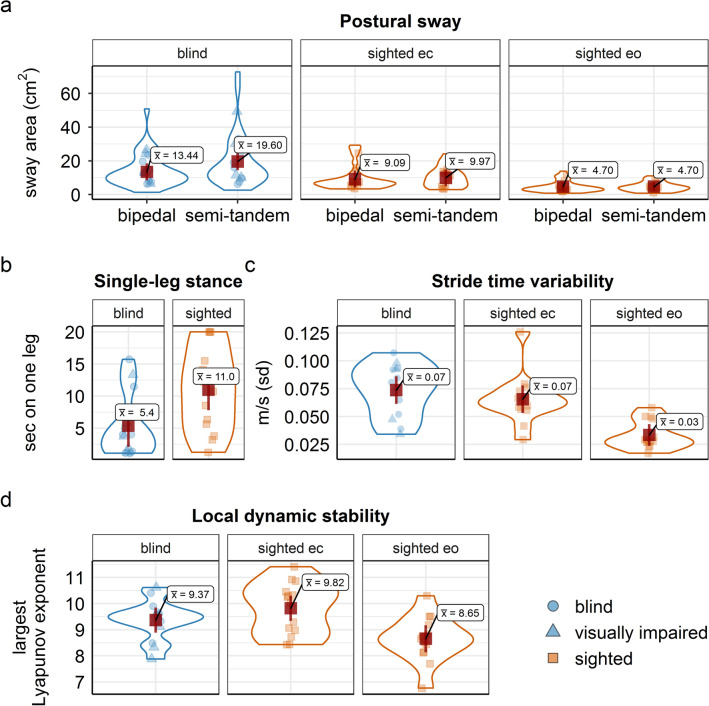
Blind vs. sighted, eyes closed. Postural sway was significantly larger for blind and visually impaired than for sighted individuals across stances when sighted were tested with closed eyes (F (1, 21) = 4.73, p = 0.041, mean difference = 6.99, d = 0.64, 95% CI [− 0.006, 1.29], see Fig. 2). Postural sway was larger in semi-tandem stance than in bipedal stance positions across groups (F (1, 21) = 7.04, p = 0.015, mean difference = 3.52, d = − 0.78, 95% CI [− 1.44, − 0.13]).
Fig. 2.
Violin plots for balance performance and gait parameters, separately for the blind and visually impaired (in blue) and for the sighted (in orange) group. Panel a postural sway per stance condition and for eyes open (eo)/eyes closed (ec) conditions in the sighted group; b single-leg stance time; c stride time variability; d local dynamic stability. Red squares represent the respective group mean; error bars depict 95% CI of the mean. Dots represent single-subject data
Sighted, eyes open vs. eyes closed. Postural sway was significantly larger in eyes closed than in eyes open conditions (F (1, 11) = 20.97, p < 0.001, mean difference = 4.98, d = 1.32, 95% CI [0.47, 2.18], with no significant difference between stance positions (F (1, 11) = 0.47, p = 0.505, mean difference = 0.44, d = -0.20, 95% CI [− 0.83, 0.43], see Fig. 2).
Single-leg stance time
Blind vs. sighted, eyes closed. Functional balance performance assessed with the single leg stance was significantly lower for blind and visually impaired than for sighted individuals (F (1, 25) = 6.32, p = 0.019, mean difference = − 5.6, d =− 0.97, 95% CI [− 1.81, − 0.13], see Fig. 2).
Gait parameters
Blind vs. sighted, eyes closed. Blind and visually impaired and sighted participants did neither differ significantly in their stride time variability (F (1, 26) = 0.92, p = 0.345, mean difference = − 0.01, d =− 0.36, 95% CI [− 0.42, 1.15]) nor in local dynamic gait stability (F (1, 26) = 1.89, p = 0.181, mean difference = 0.45, d = 0.52, 95% CI [− 1.22, 0.27]), when sighted where tested with closed eyes.
Sighted, eyes open vs. eyes closed. Gait parameter of sighted individuals tested with eyes open differed significantly from test conditions with eyes closed: stride time variability decreased and local dynamic stability increased (lower LLE values) significantly in the eyes open condition as compared to the eyes closed condition (F (1, 13) = 27.00, p < 0.001, mean difference = − 1.17, d = 1.96, 95% CI [1.01, 2.92]) and (F (1, 13) = 39.34, p < 0.001, d = -2.37, 95% CI [1.11, 3.63]), see Fig. 2.
Physical activity
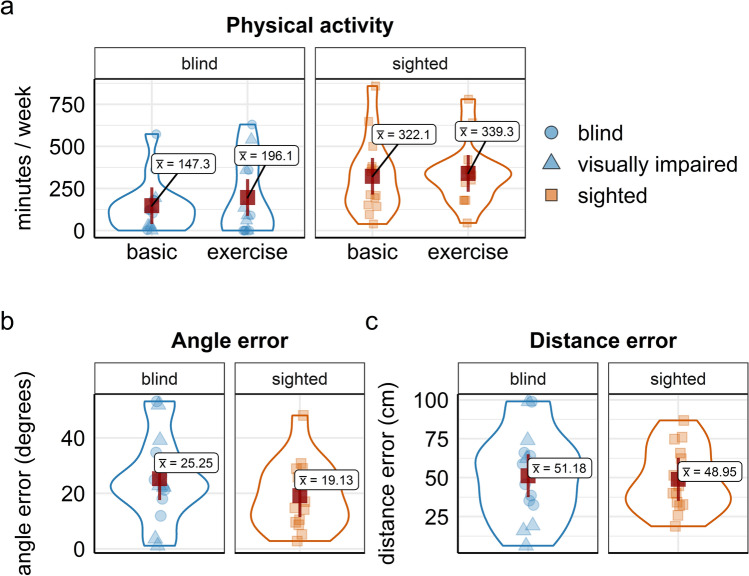
Self-reported physical activity was significantly lower in blind and visually impaired than in sighted individuals (F (1, 26) = 7.07, p = 0.013, mean difference = − 159, d =− 0.71, 95% CI [− 1.3, − 0.12]), with no significant difference between basic physical activities and physical exercise (F (1, 26) = 0.48, p = 0.494, d =− 0.18, 95% CI [− 0.72, 0.35]), see Fig. 3.
Fig. 3.
Violin plots for physical activity and navigation performance of blind and visually impaired (blue) and sighted (orange) individuals. Panel a self-reported weekly physical activity for basic everyday activities and physical exercise; b and c angle and distance errors assessed with the trial completion test. Red squares represent the respective group mean; error bars depict 95% CI of the mean, dots represent single-subject data
Navigation performance
The blind and visually impaired group and the sighted group did not differ in their path integration performance, neither in their angle errors (F (1, 26) = 1.33, p = 0.258, mean difference = 6.12, d = 0.44, 95% CI [− 0.35, 1.22]) nor in their distance errors (F (1, 26) = 0.05, p = 0.816, mean difference = 2.23, d = 0.08, 95% CI [− 0.69, 0.87]), see Fig. 3).
Relationships between physical activity, balance, gait, and navigation performance
Physical activity with balance, gait parameters, and navigation performance
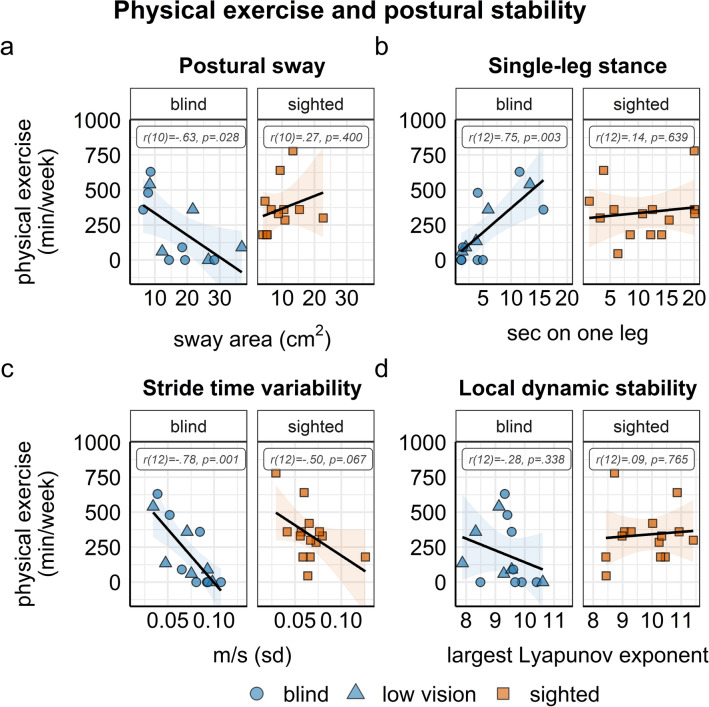
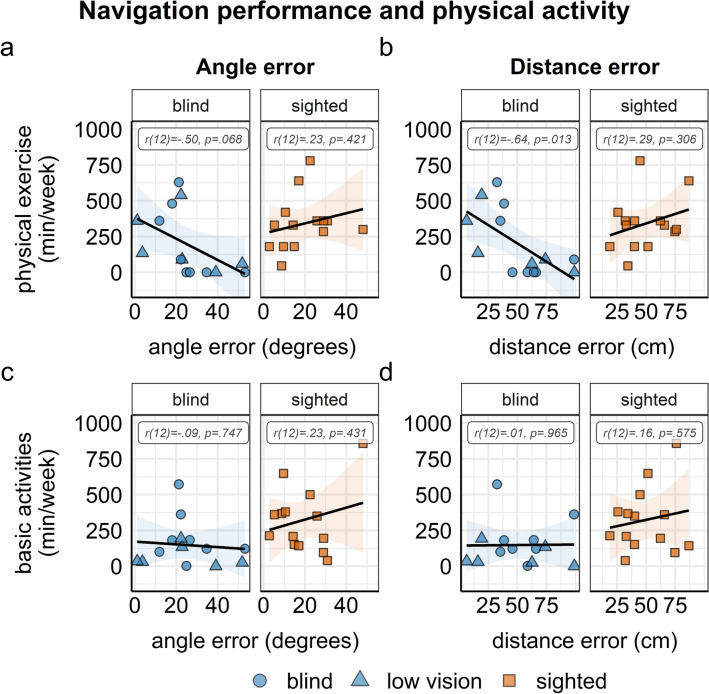
Correlational analyses revealed significant relationships between self-reported physical exercise and balance control, gait parameters, and navigation performance in the group of blind and visually impaired individuals. Higher levels of physical exercise were related to less postural sway (r (10) = − 0.63, 95% CI [− 0.88, − 0.09], p = 0.028) and longer single-leg stance time (r (12) = 0.75, 95% CI [0.33, 0.92], p = 0.003) in the blind and visually impaired group, but not in the sighted group. Moreover, more physical exercise correlated significantly with lower stride time variability within the blind and visually impaired (r (12) = − 0.78, 95% CI [− 0.93, − 0.4191], p = 0.001), but not within the sighted individuals (r (12) = − 0.50, 95% CI [− 0.82, 0.40], p = 0.067, see Fig. 4). There were no significant correlations between basic everyday physical activity and balance and gait parameters, respectively (all r < 0.47, all p > 0.093, see supplementary material, Figure S3).
Fig. 4.
Correlations of physical exercise with balance performance (a and b) and gait parameters (c and d) within the blind (blue circles) and visually impaired (blue triangles) group and the sighted (orange rectangles) group. Error bands depict 95% CI, dots represent single-subject data
Navigation performance was related to physical exercise such that higher levels of physical exercise were significantly correlated with smaller distance errors (r (12) = − 0.64, 95% CI [− 0.87, − 0.17], p = 0.013) and, marginally, with smaller angle errors in the triangle completion task (r (12) = − 0.50, 95% CI [− 0.81, 0.04], p = 0.068) in the blind and visually impaired group, but not in the sighted group. There were no significant correlations between basic physical activity and navigation performance (all r < 0.23, all p > . 431), see Fig. 5.
Fig. 5.
Correlations of navigation performance with physical exercise (a and b) and basic physical activities (c and d) within the blind (blue circles) and visually impaired (blue triangles) group and the sighted (orange rectangles) group. Error bands depict 95% CI, dots represent single-subject data
Navigation performance with balance control and gait parameters
Correlations between navigation performance, balance control and gait parameters yielded the following pattern: Smaller angle errors were significantly related to less postural sway in the sighted group (r (10) = 0.83, 95% CI [0.48, 0.95], p < 0.001). In the blind group, the relationship between smaller distance errors and less postural sway as well as longer single-leg stance time, respectively, failed to reach significance (r (10) = 0.52, 95% CI [− 0.07, 0.84], p = 0.081, and r (12) = − 0.54, 95% CI [− 0.84, 0.01], p = 0.056, see supplementary material, Figure S1). Navigation performance was linked to gait such that smaller distance errors were significantly correlated with less stride time variability (r (12) = 0.54, 95% CI [0.01, 0.81], p = 0.046) as well as with higher local dynamic stability (lower LLE values), r (12) = 0.63, 95% CI [0.14, 0.86], p = 0.017 in the blind and visually impaired group, but not in the sighted group (see supplementary material, Figure S2).
Discussion
The present study investigated postural stability, gait parameters, physical activity, and navigation performance in blind and visually impaired as compared to sighted children and adolescents (8–18 years of age), and tested whether higher levels of physical activity are related to superior balance, gait, and navigation performance in blind participants. Postural sway was larger and single-leg stance time as well as physical activity were lower in blind and visually impaired participants than in blindfolded sighted participants. Blind and visually impaired individuals did not statistically differ from sighted individuals in stride time variability and local dynamic gait stability as well as navigation performance when the sighted were tested with closed eyes. Postural stability and gait parameters of sighted participants were significantly improved when visual input was available. Higher levels of physical exercise were related to enhanced balance performance, lower stride time variability, improved local dynamic gait stability, and superior navigation performance in blind and visually impaired children and adolescents.
Postural stability and gait parameters. The present results on balance performance of blind and visually impaired individuals are in line with studies reporting lower postural stability for blind or visually impaired than for sighted children (Müürsepp et al. 2018; Zipori et al. 2018) and sighted adults (Aydoğ et al. 2006; Campayo-Piernas et al. 2017; Giagazoglou et al. 2009; Ozdemir et al. 2013; Rogge et al. 2019; Schmid et al. 2007; Schwesig et al. 2011; Sobry et al. 2014), suggesting that postural stability is affected by the absence of visual cues, irrespective of the age of the individuals. The results have been interpreted as the absence of compensatory mechanisms in the blind, that is, no enhanced or superior use of non-visual input for balance control (Campayo-Piernas et al. 2017; Ozdemir et al. 2013; Schmid et al. 2007). Signs of superior non-visual self-motion perception in blind adults have been described, such as a faster decrease of postural sway when a light finger touch was allowed for postural stabilization (Schieppati et al. 2014), superior ankle proprioception, and enhanced vestibular roll tilt discrimination, compared to blindfolded adults (Moser et al. 2015; Ozdemir et al. 2013). Yet, these improvements did not result in enhanced balance performance of blind individuals compared to blindfolded sighted individuals. It has been suggested that behavioral compensation requires extensive practice of certain skills to show improvements (Kupers and Ptito 2014; Singh et al. 2018) and that blind individuals may have lacked practice due to overall lower engagement in physical exercise (Schmid et al. 2007). In line with this assumption, blind children as well as adults have been reported to adopt a more sedentary lifestyle (Augestad and Jiang 2015; Houwen et al. 2009; Longmuir and Bar-Or 2000; Müürsepp et al. 2018). In fact, earlier studies in blind and visually impaired individuals found that higher levels of habitual physical activity predicted superior balance performance as well as gait velocity (Aydoğ et al. 2006; da Silva et al. 2018; Müürsepp et al. 2018). Thus, it is reasonable to assume that physical exercise may foster balance skills and typical gait patterns. The data of the present study are in line with this hypothesis: Blind and visually impaired children and adolescents were less physically active than age-matched sighted controls, but those blind participants reporting more weekly physical exercise performed better in balance tasks than sedentary peers and moreover showed a lower stride time variability. While no causal relationship can be derived from a cross-sectional study design, we recently demonstrated in a longitudinal training study that balance training of no more than 12 weeks significantly improved balance performance of blind adults (Rogge et al. 2019), indicating that balance skills of blind individuals can be increased by specific practice. In the present study, balance performance of blind and visually impaired individuals was related to self-reported physical exercise, but not to everyday activities such as taking the stairs or walking to school. Thus, the observed correlations between physical exercise and balance control might result from demanding physical training including balance tasks.
Navigation performance. We observed no differences in path integration performance assessed with a triangle completion task between the group of blind and visually impaired and the group of blindfolded sighted individuals. This finding is in accord with earlier results showing no differences in path completion skills in congenitally and late blind adults compared to blindfolded sighted controls (Loomis et al. 1993). In contrast, other studies have reported both significantly worse and significantly better performance of congenitally or late blind individuals than of blindfolded controls in path completion tasks (Seemungal et al. 2007; Tinti et al. 2006). It has been speculated that the large variability of navigation performance within groups of blind participants as well as between different studies is partly explained by mobility skills as well as habitual physical activity of the blind (Loomis et al. 1993; Seemungal et al. 2007). The results of the present study are in line with this assumption: performance in the triangle completion task was significantly related to self-reported physical exercise, to local dynamic gait stability and, marginally, to postural stability within the group of blind and visually impaired children, suggesting that those blind individuals participating in more physical exercise have superior balance skills, show a more stable gait pattern, and perform better in navigation tasks than sedentary blind individuals. Path integration in the dark depends on vestibular information about translatory and rotatory accelerations of the head as well as proprioceptive information about self-motion (Glasauer et al. 2002). The vestibular system has been suggested to play a crucial role for spatial cognitive functions such as navigation, spatial memory and spatial learning (Hitier et al. 2014; Seemungal 2015). Vestibular pathways have been proposed to modulate neuroplasticity induced by physical exercise in brain regions associated with spatial cognitive functions and memory (Smith 2017). Moreover, vestibular self-motion processing and updating of one’s own position within the environment is extensively needed for physical exercise in the dark, such as blind soccer, judo, or athletic sports in the blind. In contrast, basic everyday activities were not related to navigation performance. It might thus be speculated that promoting physical exercise in blind individuals benefits not only postural stability and a stable gait pattern, but may enhance spatial navigation and orientation skills supporting individual mobility.
Limitations. Some limitations of the present study need to be considered. We included both blind and visually impaired children with an onset of visual deprivation at birth or later, leading to a heterogeneous sample with regard to the degree and duration of visual impairments. Previous studies on balance performance in blind individuals found no differences between congenitally and late blind individuals in postural stability (Rogge et al. 2019; Schmid et al. 2007) and gait parameters (da Silva et al. 2018). In our data, no systematic differences were observed between blind and visually impaired individuals in measures of balance control, gait, and levels of physical exercise. Similarly, navigation performance did neither differ between blind and visually impaired nor between congenitally and late blind participants.
In addition, blind and visually impaired children and adolescents were recruited from two different countries, which could lead to differences in mobility trainings, schools, and opportunities for physical activities for blind and visually impaired individuals. By looking at individual data, no systematic differences were observable between children from Italy and Germany, suggesting a robust relationship between physical exercise and postural stability, gait, and navigation performance in blind and visually impaired children.
Finally, the sample size of the present study was relatively small compared to similar studies in sighted children and adolescents, lowering statistical power and precluding the use of rigid corrections for multiplicity. Despite these limitations, our data revealed medium to strong associations between levels of physical activity and postural control, gait and navigation performance within the group of blind and visually impaired children and adolescents in the predicted direction. These results, thus, provide a starting point for further investigations.
Conclusion. Collectively, our findings suggest that physical activity may foster postural stability, gait, and navigation performance in blind and visually impaired children and adolescents. We speculate that this relationship is modulated by the vestibular system important for estimating self-motion. Our results suggest that rehabilitation efforts should include and promote a physically active lifestyle including exercises addressing postural stability to improve mobility and orientation skills in visually impaired individuals.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
A-KR: Conceptualization, methodology, investigation, formal analysis, visualization, writing—original draft. DH: formal analysis, writing—review and editing. GC: investigation, writing—review and editing. LK: investigation, writing—review and editing. KH: conceptualization, writing—review and editing. AZ: resources, writing—review and editing. MG: resources, funding acquisition, writing—review and editing. BR: conceptualization, funding acquisition, supervision, writing—review and editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by a grant from the European Commission [ABBI, 611452, FP7-ICT-2013-10] to Monica Gori and Brigitte Röder, and a grant from the German Research Foundation [DFG Ro 2625/10-1] to Brigitte Röder. The first author was temporarily supported by the Max-Planck School of Cognition.
Data availability
The datasets generated for the present study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allen GL, Kirasic KC, Rashotte MA, Haun DBM. Aging and path integration skill: kinesthetic and vestibular contributions to wayfinding. Percept Psychophys. 2004;66(1):170–179. doi: 10.3758/BF03194870. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Klier EM, Snyder LH. A vestibular sensation: probabilistic approaches to spatial perception. Neuron. 2009;64(4):448–461. doi: 10.1016/j.neuron.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augestad LB, Jiang L. Physical activity, physical fitness, and body composition among children and young adults with visual impairments: a systematic review. Br J Vis Impair. 2015;33(3):167–182. doi: 10.1177/0264619615599813. [DOI] [Google Scholar]
- Aydoğ E, Aydoğ S, Çakci A, Doral M. Dynamic postural stability in blind athletes using the biodex stability system. Int J Sports Med. 2006;27(5):415–418. doi: 10.1055/s-2005-865777. [DOI] [PubMed] [Google Scholar]
- Bennett HJ, Valenzuela KA, Fleenor K, Morrison S, Haegele JA. Walking biomechanics and energetics of individuals with a visual impairment: a preliminary report. Human Movement. 2019;20(4):8–18. doi: 10.5114/hm.2019.85094. [DOI] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, van Dieën JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. 2013;10(83):20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campayo-Piernas M, Caballero C, Barbado D, Reina R. Role of vision in sighted and blind soccer players in adapting to an unstable balance task. Exp Brain Res. 2017;235(4):1269–1279. doi: 10.1007/s00221-017-4885-8. [DOI] [PubMed] [Google Scholar]
- Cappagli G, Gori M. Auditory spatial localization: developmental delay in children with visual impairments. Res Dev Disabil. 2016;53–54:391–398. doi: 10.1016/j.ridd.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Cappagli G, Cocchi E, Gori M. Auditory and proprioceptive spatial impairments in blind children and adults. Dev Sci. 2017 doi: 10.1111/desc.12374. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci. 2016;73(5):975–983. doi: 10.1007/s00018-015-2102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Taube JS. Our sense of direction: progress, controversies and challenges. Nat Neurosci. 2017;20(11):1465–1473. doi: 10.1038/nn.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi LF, Aggius-Vella E, Campus C, Parmiggiani A, Gori M. From science to technology: orientation and mobility in blind children and adults. Neurosci Biobehav Rev. 2016;71:240–251. doi: 10.1016/j.neubiorev.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Da Silva ES, Fischer G, da Rosa RG, Schons P, Teixeira LBT, Hoogkamer W, Peyré-Tartaruga LA. Gait and functionality of individuals with visual impairment who participate in sports. Gait Posture. 2018;62:355–358. doi: 10.1016/j.gaitpost.2018.03.049. [DOI] [PubMed] [Google Scholar]
- Dordevic M, Schrader R, Taubert M, Müller P, Hökelmann A, Müller NG. Vestibulo-hippocampal function is enhanced and brain structure altered in professional ballet dancers. Front Integr Neurosci. 2018;12:170. doi: 10.3389/fnint.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M. Comments on "Ellipse area calculations and their applicability in posturography" Schubert and Kirchner, vol. 39, pages 518–522, 2014) Gait Posture. 2015;41(1):44–45. doi: 10.1016/j.gaitpost.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Duarte M, Zatsiorsky VM. Effects of body lean and visual information on the equilibrium maintenance during stance. Exp Brain Res. 2002;146(1):60–69. doi: 10.1007/s00221-002-1154-1. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Arida RM, Gomez-Pinilla F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser and Swinney Independent coordinates for strange attractors from mutual information. Phys Rev A. 1986;33(2):1134–1140. doi: 10.1103/physreva.33.1134. [DOI] [PubMed] [Google Scholar]
- Frey I, Berg A, Gratwohl D, Keul J. Freiburger Fragebogen zur körperlichen Aktivität - Entwicklung, Prüfung und Anwendung. Soz Praventivmed. 1999;44:55–64. doi: 10.1007/BF01667127. [DOI] [PubMed] [Google Scholar]
- Frick A, Möhring W. A matter of balance: motor control is related to children's spatial and proportional reasoning skills. Front Psychol. 2015;6:2049. doi: 10.3389/fpsyg.2015.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagazoglou P, Amiridis IG, Zafeiridis A, Thimara M, Kouvelioti V, Kellis E. Static balance control and lower limb strength in blind and sighted women. Eur J Appl Physiol. 2009;107(5):571–579. doi: 10.1007/s00421-009-1163-x. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim M-A, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exp Brain Res. 2002;145(4):489–497. doi: 10.1007/s00221-002-1146-1. [DOI] [PubMed] [Google Scholar]
- Hallemans A, Ortibus E, Meire F, Aerts P. Low vision affects dynamic stability of gait. Gait Posture. 2010;32(4):547–551. doi: 10.1016/j.gaitpost.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Hallemans A, Ortibus E, Truijen S, Meire F. Development of independent locomotion in children with a severe visual impairment. Res Dev Disabil. 2011;32(6):2069–2074. doi: 10.1016/j.ridd.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Taylor WR, Singh NB, Schega L. Towards clinical application: repetitive sensor position re-calibration for improved reliability of gait parameters. Gait Posture. 2014;39(4):1146–1148. doi: 10.1016/j.gaitpost.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Singh NB, Taylor WR, Schega L. Towards the assessment of local dynamic stability of level-grounded walking in an older population. Med Eng Phys. 2015;37(12):1152–1155. doi: 10.1016/j.medengphy.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Krowicki M, Schega L. Gait variability in chronic back pain sufferers with experimentally diminished visual feedback: a pilot study. J Mot Behav. 2016;48(3):205–208. doi: 10.1080/00222895.2015.1073136. [DOI] [PubMed] [Google Scholar]
- Hamacher D, Liebl D, Hödl C, Heßler V, Kniewasser CK, Thönnessen T, Zech A. Gait stability and its influencing factors in older adults. Front Physiol. 2018;9:1955. doi: 10.3389/fphys.2018.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Furrer S, Herzog MH, Merfeld DM, Mast FW. Self-motion perception training: thresholds improve in the light but not in the dark. Exp Brain Res. 2013;226(2):231–240. doi: 10.1007/s00221-013-3428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26(4):555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitier M, Besnard S, Smith PF. Vestibular pathways involved in cognition. Front Integrat Neurosci. 2014;8:59. doi: 10.3389/fnint.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwen S, Hartman E, Visscher C. Physical activity and motor skills in children with and without visual impairments. Med Sci Sports Exerc. 2009;41(1):103–109. doi: 10.1249/MSS.0b013e318183389d. [DOI] [PubMed] [Google Scholar]
- Jansen P, Heil M. The relation between motor development and mental rotation ability in 5- to 6-year-old children. Int J Dev Sci. 2010;4(1):67–75. doi: 10.3233/DEV-2010-4105. [DOI] [Google Scholar]
- Kennel, Brown and Abarbanel Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys Rev A. 1992;45(6):3403–3411. doi: 10.1103/physreva.45.3403. [DOI] [PubMed] [Google Scholar]
- Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci Biobehav Rev. 2014;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Lopez C, Blanke O. Influence of galvanic vestibular stimulation on egocentric and object-based mental transformations. Exp Brain Res. 2007;184(2):211–221. doi: 10.1007/s00221-007-1095-9. [DOI] [PubMed] [Google Scholar]
- Lenth R, Buerkner P, Herve M, Love J, Riebl, H, Singmann H (2020) emmeans: Estimated marginal means, aka least-squares means. R package version 1.5.1. Retrieved from https://CRAN.R-project.org/package=emmeans
- Longmuir PE, Bar-Or O. Factors influencing the physical activity levels of youths with physical and sensory disabilities. Adapt Phys Activity Quart. 2000;17(1):40–53. doi: 10.1123/apaq.17.1.40. [DOI] [Google Scholar]
- Loomis JM, Klatzky RL, Golledge RG, Cicinelli JG, Pellegrino JW, Fry PA. Nonvisual navigation by blind and sighted: assessment of path integration ability. J Exp Psychol Gen. 1993;122(1):73–91. doi: 10.1037/0096-3445.122.1.73. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Klatzky RL, Golledge RG. Navigating without vision: basic and applied research. Optom Vis Sci (Optometry and Vision Science: Official Publication of the American Academy of Optometry) 2001;78(5):282–289. doi: 10.1097/00006324-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Lucieer F, Duijn S, van Rompaey V, Pérez Fornos A, Guinand N, Guyot JP, et al. Full spectrum of reported symptoms of bilateral vestibulopathy needs further investigation—a systematic review. Front Neurol. 2018;9:352. doi: 10.3389/fneur.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Selen LJP. Vestibular contributions to high-level sensorimotor functions. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Moser I, Grabherr L, Hartmann M, Mast FW. Self-motion direction discrimination in the visually impaired. Exp Brain Res. 2015;233(11):3221–3230. doi: 10.1007/s00221-015-4389-3. [DOI] [PubMed] [Google Scholar]
- Müürsepp I, Arjokesse R, Ereline J, Pääsuke M, Gapeyeva H. Impact of visual impairment on static and dynamic postural control and habitual physical activity in children aged 10–16 years. Br J Vis Impair. 2018;36(3):227–237. doi: 10.1177/0264619618780918. [DOI] [Google Scholar]
- Nakamura T. Quantitative analysis of gait in the visually impaired. Disabil Rehabil. 1997;19(5):194–197. doi: 10.3109/09638289709166526. [DOI] [PubMed] [Google Scholar]
- Ochaita E, Huertas JA. Spatial representation by persons who are blind: a study of the effects of learning and development. J Vis Impair Blind. 1993;87(2):37–41. doi: 10.1177/0145482X9308700201. [DOI] [Google Scholar]
- Ozdemir RA, Pourmoghaddam A, Paloski WH. Sensorimotor posture control in the blind: superior ankle proprioceptive acuity does not compensate for vision loss. Gait Posture. 2013;38(4):603–608. doi: 10.1016/j.gaitpost.2013.02.003. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from URL https://www.R-project.org/
- Rieser JJ, Guth DA, Hill EW. Sensitivity to perspective structure while walking without vision. Perception. 1986;15(2):173–188. doi: 10.1068/p150173. [DOI] [PubMed] [Google Scholar]
- Rogge A-K, Röder B, Zech A, Nagel V, Hollander K, Braumann K-M, Hötting K. Balance training improves memory and spatial cognition in healthy adults. Sci Rep. 2017;7:572. doi: 10.1038/s41598-017-06071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge A-K, Röder B, Zech A, Hötting K. Exercise-induced neuroplasticity: balance training increases cortical thickness in visual and vestibular cortical regions. NeuroImage. 2018;179:471–479. doi: 10.1016/j.neuroimage.2018.06.065. [DOI] [PubMed] [Google Scholar]
- Rogge A-K, Hötting K, Nagel V, Zech A, Hölig C, Röder B. Improved balance performance accompanied by structural plasticity in blind adults after training. Neuropsychologia. 2019;129:318–330. doi: 10.1016/j.neuropsychologia.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Rosenstein MT, Collins JJ, de Luca CJ. A practical method for calculating largest Lyapunov exponents from small data sets. Physica D. 1993;65(1–2):117–134. doi: 10.1016/0167-2789(93)90009-P. [DOI] [Google Scholar]
- Schieppati M, Schmid M, Sozzi S. Rapid processing of haptic cues for postural control in blind subjects. Clin Neurophysiol. 2014;125(7):1427–1439. doi: 10.1016/j.clinph.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Schinazi VR, Thrash T, Chebat D-R. Spatial navigation by congenitally blind individuals. Wiley Interdisciplinary Rev Cogn Sci. 2016;7(1):37–58. doi: 10.1002/wcs.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Nardone A, de Nunzio AM, Schmid M, Schieppati M. Equilibrium during static and dynamic tasks in blind subjects: no evidence of cross-modal plasticity. Brain. 2007;130(Pt 8):2097–2107. doi: 10.1093/brain/awm157. [DOI] [PubMed] [Google Scholar]
- Schmid M, Bottaro A, Sozzi S, Schieppati M. Adaptation to continuous perturbation of balance: progressive reduction of postural muscle activity with invariant or increasing oscillations of the center of mass depending on perturbation frequency and vision conditions. Hum Mov Sci. 2011;30(2):262–278. doi: 10.1016/j.humov.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Schubert P, Kirchner M. Ellipse area calculations and their applicability in posturography. Gait Posture. 2014;39(1):518–522. doi: 10.1016/j.gaitpost.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Schwesig R, Goldich Y, Hahn A, Müller A, Kohen-Raz R, Kluttig A, Morad Y. Postural control in subjects with visual impairment. Eur J Ophthalmol. 2011;21(3):303–309. doi: 10.5301/EJO.2010.5504. [DOI] [PubMed] [Google Scholar]
- Seemungal BM. The components of vestibular cognition—motion versus spatial perception. Multisens Res. 2015;28(5–6):507–524. doi: 10.1163/22134808-00002507. [DOI] [PubMed] [Google Scholar]
- Seemungal BM, Glasauer S, Gresty MA, Bronstein AM. Vestibular perception and navigation in the congenitally blind. J Neurophysiol. 2007;97(6):4341–4356. doi: 10.1152/jn.01321.2006. [DOI] [PubMed] [Google Scholar]
- Singh AK, Phillips F, Merabet LB, Sinha P. Why does the cortex reorganize after sensory loss? Trends Cogn Sci. 2018;22(7):569–582. doi: 10.1016/j.tics.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singmann H, Bolker B, Westfall J, Aust F (2018) afex: Analysis of factorial experiments.: R package version: 0.20–2. Retrieved from https://CRAN.R-project.org/package=afex
- Smith PF. Is hippocampal neurogenesis modulated by the sensation of self-motion encoded by the vestibular system? Neurosci Biobehav Rev. 2017;83:489–495. doi: 10.1016/j.neubiorev.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Sobry V, Badin P, Cernaianu S, Agnani O, Toussaint M. Do visually impaired people have a static balance as effective as sighted people? Neuro Rehabilit. 2014;35(4):851–861. doi: 10.3233/NRE-141181. [DOI] [PubMed] [Google Scholar]
- Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatric Phys Ther. 2007;30(1):8–15. doi: 10.1519/00139143-200704000-00003. [DOI] [PubMed] [Google Scholar]
- Stimpson NJ, Davison G, Javadi A-H. Joggin' the Noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci Biobehav Rev. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Thinus-Blanc C, Gaunet F. Representation of space in blind persons: Vision as a spatial sense? Psychol Bull. 1997;121(1):20–42. doi: 10.1037/0033-2909.121.1.20. [DOI] [PubMed] [Google Scholar]
- Tinti C, Adenzato M, Tamietto M, Cornoldi C. Visual experience is not necessary for efficient survey spatial cognition: evidence from blindness. Quart J Exp Psychol 2006. 2006;59(7):1306–1328. doi: 10.1080/17470210500214275. [DOI] [PubMed] [Google Scholar]
- Vercillo T, Burr D, Gori M. Early visual deprivation severely compromises the auditory sense of space in congenitally blind children. Dev Psychol. 2016;52(6):847–853. doi: 10.1037/dev0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- Wuehr M, Schniepp R, Pradhan C, Ilmberger J, Strupp M, Brandt T, Jahn K. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp Brain Res. 2013;224(2):287–294. doi: 10.1007/s00221-012-3310-6. [DOI] [PubMed] [Google Scholar]
- Zipori AB, Colpa L, Wong AMF, Cushing SL, Gordon KA. Postural stability and visual impairment: assessing balance in children with strabismus and amblyopia. PLoS ONE. 2018;13(10):e0205857. doi: 10.1371/journal.pone.0205857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for the present study are available from the corresponding author on reasonable request.
Not applicable.