Abstract
Background:
The intracardiac nervous system (ICNS) is composed of neurons, in association with Schwann cells (SC) and endoneurial cardiac fibroblasts (ECF). Besides heart rhythm control, recent studies have implicated cardiac nerves in postnatal cardiac regeneration and cardiomyocyte size regulation, but cardiac SC and ECF remain understudied. During the postnatal period, the ICNS undergoes intense remodeling with nerve fasciculation and elongation throughout the myocardium, partially guided by the extracellular matrix (ECM). Here we report the origins, heterogeneity, and functions of SC and ECF that develop in proximity to neurons during postnatal ICNS maturation.
Methods and Results:
Periostin lineage (Postn+) cells include cardiac Remak SC and ECF during the postnatal period in mice. The developmental origins of cardiac SC and ECF were examined using Rosa26eGFP reporter mice bred with Wnt1Cre, expressed in Neural crest (NC)-derived lineages, or tamoxifen-inducible Tcf21MerCreMer, expressed predominantly in epicardial-derived fibroblast lineages. ICNS components are NC-derived with the exceptions of the myelinating Plp1+ SC and the Tcf21+ lineage-derived intramural ventricular ECF. In addition, Postn+ lineage GFAP- Remak SC and ECF are present around the fasciculating cardiac nerves. Whole mount studies of the NC-derived cells confirmed postnatal maturation of the complex ICNS network from P0 to P30. Sympathetic, parasympathetic, and sensory neurons fasciculate from P0 to P7 indicated by co-staining with PSA-NCAM. Ablation of Postn+ cells from P0 to P6 or loss of Periostin leads to reduced fasciculation of cardiac sympathetic nerves. In addition, collagen remodeling surrounding maturing nerves of the postnatal heart is reduced in Postn-null mice.
Conclusions:
Postn+ cells include cardiac SC and ECF during postnatal nerve maturation, and these cells have different embryonic origins. At P7, the Postn+ cells associated with cardiac nerves are mainly Remak SC and ECF. Ablation of the Postn+ cells from P0 to P6 and also loss of Postn in Postn-null mice leads to reduced fasciculation of cardiac nerves at P7.
Keywords: Cardiac Schwann Cell, Endoneurial Cardiac Fibroblast, Cardiac nerve fasciculation, Intracardiac nervous system, Postnatal cardiac nerve maturation
Graphical abstract

1. Introduction
The heart beats 2.5 billion times in a human lifetime and disturbances of this rhythmic mechanical activity are the main cause of morbidity and mortality in the world[1]. In particular, cardiac arrhythmia and sudden death cause 15–20% of deaths worldwide[2]. One of the drivers of cardiac rhythm is the intracardiac nervous system (ICNS)[3], which also contributes to murine postnatal heart regeneration[4] and cardiomyocyte size control[5]. Nevertheless, the development and maturation of the cells of the ICNS, as well as the mechanisms behind these processes, remain underexplored.
The ICNS is composed of cardiac neurons, Schwann cells (SC), endoneurial cardiac fibroblasts (ECF), endoneurial macrophages, and nerve capillaries[3, 6]. The cardiac neurons are classified as sympathetic, parasympathetic, sensory, and local circuit neurons[3]. The neuronal bodies aggregate in ganglia distributed around the heart, and their axons form the cardiac nerves, which are mostly unmyelinated, and are surrounded by SC and ECF[6]. Single cell RNAseq analyses[7] and electron microscopy[6, 8] studies have shown the presence of SC in the heart, but the different subpopulations and their functions remain unknown. In other peripheral nerves, axons are protected by myelinating SC, that express Myelin Proteolipid Protein (PLP1), and also Remak SC, that express Neurotrophin Receptor p75 (P75NTR), which provide metabolic support to the neurons and are required for nerve regeneration and repair[9–11]. During the development of the peripheral nervous system, the SC are required for processes such as axonal fasciculation and neuronal targeting[12]. Axonal fasciculation is the process of bundling axons from multiple neurons as they adhere to each other to form larger nerves that follow similar growth trajectories[13]. During embryonic development, Remak SC express the cell activation marker Periostin (Postn)[14], while in pathological conditions Postn expression has been described in the astroglia[15], and the specific glial activation marker Glial fibrillary acidic protein (GFAP) is expressed in Schwann cells[16]. The neuronal associated fibroblasts or endoneurial fibroblast populations of the peripheral nervous system are poorly understood cells and their role remains controversial[17]. In the heart, ECF have been only morphologically described by electron microscopy[6, 8]. Their assumed function is extracellular matrix (ECM) remodelling after nerve damage, but a role in neuronal development also has also been suggested[17]. SC and ECF have been identified in multiple organ systems with functions related to nerve formation and maintenance of the ECM. In the postnatal heart, nerves elongate[18], but little is known about other maturation processes including axon fasciculation. In other tissues, supporting SC are necessary for axon fasciculation, but in the heart, SC, ECF and the ECM involved are relatively uncharacterized.
Neural crest origins of the cardiac nerves of the ICNS have been reported based on studies in chick and mouse models[19]. However, the developmental origins of ICNS supporting SC and ECF have not been identified. In other organs, SC and the peripheral nerves are known to be NC derived, but this has yet to be confirmed in the heart[11, 12]. Cardiac fibroblast populations derive from different embryonic progenitors in the epicardium, endocardium or even the NC, but no information is available about the embryonic diversity or origins of the ECF[20–22]. The sympathetic innervation of the heart starts embryonically, followed by elongation of the epicardial nerves and intramural innervation after postnatal day (P)5 in mouse[18]. Maturation of the parasympathetic system is relatively delayed and occurs largely after birth[23]. For the cardiac sensory neurons, the data are limited, but these nerves are apparent by P4[24]. The mechanisms behind neuronal growth are not completely clear, but it is known that the axons elongate and fasciculate during maturation to form the adult nerves that innervate target cells[25].
A role for the ECM during peripheral nerve development and maturation in the heart has been suggested, but the functions of the different proteins in the cellular environment are still under study[26, 27]. In our previous work, we showed that the ablation of postnatal Postn-expressing cells from P0 to P7 triggers abnormalities in the sympathetic nervous system in the heart[28]. In this study, we report heterogeneous SC and ECF that express Postn and contribute to postnatal nerve maturation and remodeling. Additionally, we have found a requirement for Periostin matricellular protein expression in cardiac sympathetic nerve fasciculation.
2. Methods
All experiments with mice were performed conforming to NIH guidelines, and all protocols involving animals were approved by the Cincinnati Children’s Hospital Institutional Animal Care and Use Committee (IACUC).
2.1. Mice
Mouse lines used in this study were: C57BL/6J (WT), Wnt1-Cre2 (Wnt1Cre, Jax stock No: 022137)[29], Postn-MerCreMer (PostnMCM, Jax Stock No: 029645)[30] and Tcf21-MerCreMer (Tcf21MCM)[31]. Wnt1Cre, PostnMCM and Tcf21MCM mice were crossbred with FVB.Cg-Gt(ROSA)26Sortm1(CAG-EGFP)Glh/J(R26eGFP) to obtain Wnt1Cre;R26eGFP, PostnMCM/+;R26eGFP, and Tcf21MCM/+;R26eGFP mice. PostnMCM were crossbred with R26DTA [Gt(ROSA)26Sortm1 (DTA)Jpmb/J] (Jax Stock No:006331) plus/minus B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo /J (Jax Stock No: 007676) to obtain PostnMCM/+;R26DTA or PostnMCM/+;R26DTA/mTmG mice. For the ablation experiments, Postn+/+;R26DTA or PostnMCM/+;R26+/mTmG littermates with tamoxifen (TAM) injections were used as controls. PostnMCM/MCM pups were used for the Postn-null studies with PostnMCM/+ littermates and age-matched Postn+/+ pups used as controls. All mice were on a C57BL/6J background and mixed litters of male and female animals were used in all experiments. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols using mice were approved by the Cincinnati Children’s Research Foundation Institutional Animal Care and Use Committee.
2.2. Animal procedures
To induce the activity of the MerCreMer protein, 3 consecutive days of TAM injections (10mg/kg) were used, and hearts were harvested one day after the final injection (P7 or P30). Up to P7, the administration of TAM was intragastric into the visible stomach, and, after P7, injections were intraperitoneal. For the cell ablation experiments, TAM was administered daily from P0 to P6, and hearts were harvested at P7. The efficiency of the ablation was confirmed at ~80% as reported previously[28].
2.3. Histology and Immunofluorescence
Freshly harvested hearts were washed in PBS and fixed in 4% Paraformaldehyde at 4°C overnight. The tissue was then washed, dehydrated in ethanol (70%/95%/100%x3) and incubated in Xylene 3 X 5 minutes. The hearts were then embedded in paraffin, and 5μm sections were obtained with a microtome (Leica, Germany). For histological analysis, the paraffin sections were melted 30 minutes at 60°C and then deparaffinized and re-hydrated. Antigen retrieval was performed by boiling in 1x citrate buffer-based antigen retrieval solution (H-3300, Vector laboratories, USA) or 1x TRIS buffered-based antigen retrieval solution (ab93684, Abcam, UK) in a pressure cooker for 3 minutes. Blocking was performed using 6% Donkey serum (D9663, Sigma-Aldrich, USA) in PBS at room temperature (RT) for 1 hour. All the primary antibody incubations were performed at 4°C overnight. Alexa conjugated (488, 568, 633) donkey secondary antibodies from Abcam (UK) were incubated at 1:400 concentration for 1 hour at RT. 4′,6-diamidino-2-phenylindole (DAPI) (D1306, Thermo Fisher, USA) was used to visualize nuclei at 1:10000 concentration in PBS for 15 minutes. Specific information on antibodies and dilutions is included in Table S1 of the Supplementary Information.
Samples were mounted with Vectashield (H-1400, Vector labs, USA). The immunofluorescence was imaged using a Nikon Eclipse Ti Fluorescence microscope with NIS elements software (Tokyo, Japan). Quantification was performed in at least 6 randomly selected 40X images of the left ventricular regions per tissue section using an automated analysis program created on NIS elements software for CFs[28]. Fiji (ImageJ) image analysis software was used to manually draw the TH-defined circumference of sympathetic nerves to calculate cross sectional area (μm2). Images were captured and data collected by multiple investigators blinded to the genotype.
2.4. Collagen hybridizing peptide
Collagen Hybridizing Peptide (CHP; FLU60, 3HELIX, USA) working solution (20μM) was freshly prepared by heating to 80°C then cooling in ice for 10–15 seconds. Tissue samples were blocked in 6% Donkey serum (D9663, Sigma-Aldrich, USA) for an hour at RT and then incubated in CHP working solution (with the primary antibody for TH at the concentration described in Table S1) overnight at 4°C. The secondary antibody was incubated at the concentration described above for an hour at RT. CHP conjugated to 5-Carboxyfluorescein (5-FAM) was visualized using a Nikon Eclipse Ti Fluorescence microscope with NIS elements software (Tokyo, Japan). Total area per image and the CHP-stained area was measured using an analysis program created on NIS elements software. The ratio of CHP-stained area to total measured area per image was calculated to obtain the percentage of remodeling collagen per area.
2.5. Whole mount GFP immunofluorescence.
Hearts from Wnt1Cre;R26eGFP mice were harvested at P7 and P30, washed in PBS, and fixed in 4% PFA overnight. The clearing process was performed using the Passive Clarity protocol as described previously[32]. After clearing, the hearts were blocked in 6% Donkey serum (D9663, Sigma-Aldrich, USA) at 4°C overnight and then incubated with anti-GFP primary antibody (1:100, AB6673, Abcam) at 4°C 72h. An Alexa 488 anti-rabbit (1:400) secondary antibody was used at 4°C for 48h. Hearts were incubated with 1:10000 DAPI (D1306, Thermo Fisher, USA) overnight at 4°C followed by overnight incubation in in Refractive Index Matching Solution (RIMS). Imaging was performed using a 4x air objective on a Nikon Fn1 upright microscope with a A1R HD confocal scanhead. Images were denoised using NIS-elements denoise.ai software.
2.6. Graphical representation and statistics
Statistical analysis and graph preparation were done using GraphPad Prism 8 software. Graphs show mean + standard deviation. Statistical significance was determined by the Mann-Whitney test for two independent groups and, for more than two groups, the Kruskall-Wallis ANOVA with Dunn’s correction for pairwise comparisons. p<0.05 (*) was deemed statically significant, with p<0.01 (**), p<0.001 (***), and p<0.0001 (****).
3. Results
3.1. At P7, the ICNS support cells are mainly Remak SC and ECF that express the fibroblast activation marker Postn, but do not express the glial activation marker GFAP.
Different populations of SC and endoneurial fibroblasts support peripheral neurons in various tissues including the skin and the gut[17, 33]. The presence of heterogeneous SC and ECF has not been studied in the heart, but recently specialized populations of CF, including a subset of Postn-expressing CF required for neuronal maturation, have been reported at P7[28]. Here we examined the diversity of postnatal SC and ECF cell types using immunostaining and confocal microscopy for cell-type specific markers. At P7, the non-myelinating Remak SC and ECF (P75NTR+ cells) are present in proximity to cardiac nerves, while the number of Plp1+ myelinating SC is extremely low (Fig1A and B’). We have recently described CF activation and proliferation from P0-P7 and determined that CFs expressing Postn contribute to cardiac neuron maturation[28]. Postn+ lineage cells in postnatal SC and ECF populations were examined in PostnMCM;R26eGFP mice with Tamoxifen (TAM) administration from P4 to P6. ECF and Remak SC were identified as P75NTR+ cells and were 32% of the total Postn+ cells (Fig 1B”). To interrogate the percentage of Remak SC compared to the ECF, the SC nuclear marker Sox10[34] was used (Fig1B). Among the Postn+ supporting cells, 31% were Remak SC (P75NTR+/GFP+/Sox10+) and 69% were ECF (P75NTR+/GFP+/Sox10-) (Fig 1B”‘). In agreement with previous morphological studies performed by electron microscopy[6, 8], ECF were present in proximity to large and small nerves, while SC were located preferentially in the thicker nerves on the epicardial surface and atrio-ventricular canal. Finally, no expression of the pathological glial activation marker GFAP[16] was detected in P7 hearts (Fig1A). These results demonstrate that Postn+ lineage cells are constituents of Remak SC and ECF populations, but are not prevalent in myelinating SC. Moreover, our results confirm that the differentiation of myelinating SC and the myelinization process occur postnatally and also points to the presence of Postn+ lineage Remak SC and ECF in the maturing P7 heart.
Figure 1. Postn+ lineage cells in the ICNS include myelinating SC, Remak SC and ECF, but do not express the glial activation marker GFAP in P7 hearts.

(A) Representative immunofluorescence images from PostnMCM;R26eGFP mice show GFP co-expression in myelinating SC (PLP1+ cells) and Remak SC/ECF (P75NTR) detected in the outflow tract (top 2 rows) and the thicker epicardial nerves (bottom row) with no expression of the glia marker GFAP in P7 hearts. Pink arrows point to GFP+ cells. In top row, blue arrows point to either PLP1+/GFP- cells and in the bottom row point to P75NTR+/GFP- cells. White stars mark PLP1+/GFP+ cells in the top row and P75NTR+/GFP+ cells in the bottom row. DAPI was used to stain nuclei. SB=30 μm. (B) Representative immunofluorescence images from PostnMCM;R26eGFP mouse hearts (atrio-ventricular junction) co-stained with the general glial marker Sox10 and the Remak SC/ECF marker P75-NTR at P7. Insets show: (1) Remak SC (Sox10+/P75-NTR+/GFP-), (2) activated Remak SC (Sox10+/P75NTR+/GFP+), (3) activated ECF (Sox10-/P75NTR+/GFP+), and (4) ECF (Sox10-/P75NTR+/GFP-) SB=15 μm. Quantifications are shown for (B’) % of Remak and Myelinating SC in the ICNS at P7, (B”) % of Postn+ cells that co-express P75NTR (marker of SC and ECF) at P7, (B”‘) % of Postn+/P75NTR+ relative to expression of Sox10 (glial marker) at P7 (N=5).
3.2. Cardiac SC and ECF populations express Transcription Factor 21 (Tcf21) at P7.
Tcf21 is expressed in epicardium-derived progenitors of CF during development, and Tcf21+ lineage quiescent fibroblasts are present in adult heart[22, 31]. During the postnatal period, the cardiac nerves elongate on the epicardium from the base of the ventricles towards the apex. To interrogate if some of the ICNS supporting cells express Tcf21 and hence are possibly epicardium-derived, we used Tcf21MCM;R26eGFP mice with Cre induction by TAM from P4 to P6 followed by heart tissue harvest at P7. Initially, we confirmed the presence of Tcf21+ lineage cells in proximity to sympathetic nerves stained with TH (Fig 2A). Consistent with Fig 1A, no GFAP signal was detected in P7 hearts and, in this case, no co-expression of Plp1 in Tcf21+ lineage cells was found (Fig 2B). This confirms the lack of pathological glia cell activation and suggests that the small number of myelinating SC found in the heart at P7 do not express Tcf21. The expression of P75NTR by Tcf21+ cells was also assessed and, interestingly, in the areas where the cardiac nerves are thicker, such as the atria and the proximal part of the ventricles, Tcf21+/P75NTR+ cells were found surrounding sympathetic nerves (TH+, Fig 2C, top row) and represented 24% of the total Tcf21+ cells (Fig 2D’). In contrast, the intramural Tcf21+ lineage cells adjacent to TH+ nerves in the cardiac wall and the apex of the heart, did not co-express P75NTR (Fig 2C). The presence of Tcf21+ Remak SC was detected by Sox10 (Fig 2D). Interestingly, 26% of the Tcf21+/P75NGFR+ cells also express the Sox10+ (Fig 2D”) marker for SC. In our previous work, we found some overlap between the postnatal Tcf21+ and the Postn+ linages, so it is possible that some of the Tcf21+ ECF and SC also express Postn. Our findings here confirm that the ECF adjacent to the thicker nerves are both Tcf21+ and P75NTR+ and hence neural crest-derived (as shown in Fig 3), while the intramural ECF that locate around the thinner nerves are Tcf21+, but P75NTR-negative, and are most likely epicardium-derived. Together, these results are evidence for diverse embryonic origins of the ECF.
Figure 2. Tcf21+ lineage cells contribute to ECF and Remak SC, but not myelinating SC, in the heart at P7.

(A) Representative images of Tcf21+ (Tcf21MCM;R26eGFP) ECF at P7 and P30. Immunofluorescence showed that Tcf21+GFP+ lineage traced cells are adjacent to intramural sympathetic nerves (TH+ areas) of the left ventricle (LV). P7 and P30 pictures were taken in the central area of the intramural region of the left ventricle. SB=15 μm. (B) Representative left ventricular immunofluorescence shows Tcf21GFP+ cells co-expressing P75NTR (Remak SC marker) but not PLP1 (non-myelinating SC marker) or GFAP (glial activation marker). Blue arrows point to PLP1+/GFP- cells in the top row and P75NTR+/GFP- cells in the bottom row. Pink arrows point to GFP+ cells. In the bottom row, white stars indicate GFP+/P75NTR+ cells. SB=15 μm. (C) Representative immunofluorescence images of Tcf21+/P75NTR+ ECF around epicardial sympathetic nerves (top row) and Tcf21+/P75NTR- ECF in the ventricular wall (bottom row). The ECF differentially express P75NTR with more expression in the epicardial, atria and atrio-ventricular space sympathetic nerves and minimal expression in the intra-mural sympathetic nerves. (N=5) SB=15 μm. (D) (Top row) Representative immunofluorescence images of Tcf21+ Remak SC in the left ventricle (LV) epicardium at P7. GFP+ lineage trace cells using a Tcf21MCM;R26eGFP co-express P75NTR (Remak SC/ECF marker) and Sox10 (glial marker), indicating that Tcf21 is not only expressed in ECF, but also in Remak SC at P7. Insets show Tcf21+GFP+ cells expressing Remak SC. (Bottom row) No co-expression of Sox10 and GFP was found in P30 Tcf21MCM;R26eGFP hearts. Yellow arrow points to Tcf21+/P75NTR-/Sox10- CF. Blue arrow points to Tcf21-/P75NTR+/Sox10+ Remak SC. (N=4). SB=15 μm. Quantifications are shown for (D’) % of Tcf21+ cells that co-express P75NTR (marker of SC and ECF) at P7 and (D”) % of Tcf21+/P75NTR+ relative to expression of Sox10 (glial marker) at P7 (N=5).
Figure 3. Wnt1Cre+ NC lineage cells contribute to sympathetic, parasympathetic and sensory cardiac neurons, along with Remak SC, but not Plp1+ myelinating SC.
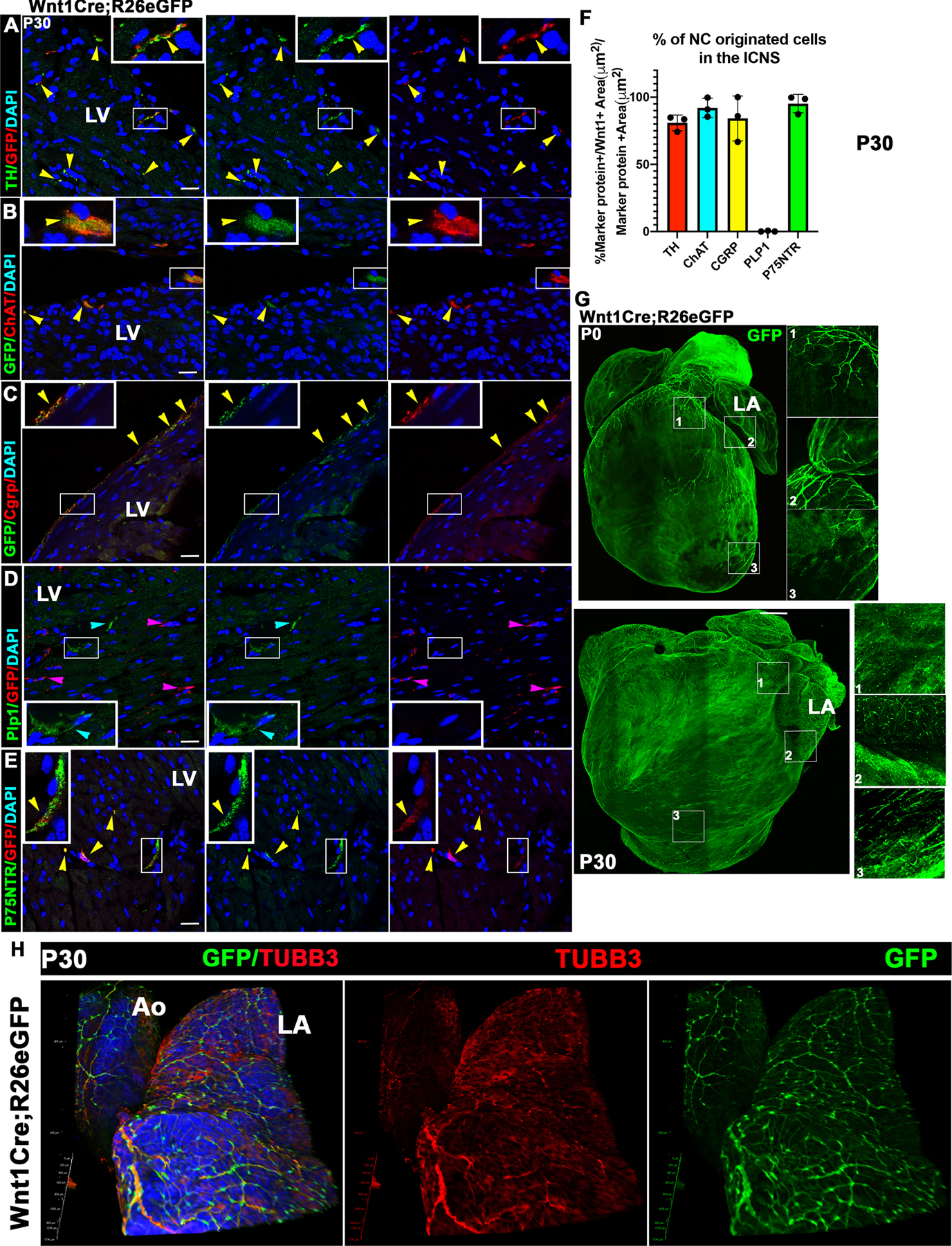
(A) Immunofluorescence of Wnt1Cre;R26eGFP mouse hearts shows co-expression of TH and GFP in sympathetic nerves at P30. SB=15 μm. (B) Immunofluorescence of Wnt1Cre;R26eGFP hearts shows co-expression of ChAT and GFP in parasympathetic nerves at P30. SB=15 μm. (C) Immunofluorescence of Wnt1Cre;R26eGFP hearts shows co-expression of Cgrp and GFP in sensory nerves at P30. SB=15 μm. (D) Immunofluorescence of Wnt1Cre;R26eGFP hearts shows Plp1 and GFP expression at P30. Cardiac myelinating SC do not co-express Wnt1GFP and hence are not NC derived. SB=15 μm. (E) Immunofluorescence of Wnt1Cre;R26eGFP hearts shows co-expression of P75-NTR and GFP in Remak SC and ECF at P30. Yellow arrows point to co-expressing areas of GFP and the cell type marker of interest. Pink arrows point to Plp1-/GFP+ cells, blue arrows point to Plp1+/GFP- cells. DAPI was used to stain nuclei. LV=Left Ventricle. SB=15 μm. (F) Quantification of the Wnt1GFP co-localizing area with the different ICNS cell markers used at P30. All markers except Plp1+ colocalize with Wnt1GFP, suggesting an embryonic origin different from the neural crest for the myelinating SC. Images shown are representative of n=3 comparable individuals. (G) Whole mount images of the NC-derived cells in the heart at P7 and P30. Increased innervation can be observed from P0 to P30 suggesting postnatal maturation of cardiac nerves. P0 SB= 500 μm, P30 SB=1000 μm. (H) Whole mount images of innervation of the Aorta (Ao) and Left atrium (LA) by ICNS from P7 Wnt1Cre;R26eGFP mice co-stained with the specific neuronal tubulin TUBB3, GFP, and DAPI for nuclear localization. The neuronal TUBB3 signal co-localizes with GFP, which also stains for NC-derived SC and ECF (TUBB3-/GFP+/DAPI+).
3.3. The ICNS, including heterogeneous SC and ECF populations, is composed mainly of neural crest (NC)-derived cells that form a complex network from P0 to P30.
Cell lineage studies in chicken and mouse embryos demonstrate that epicardial nerves arise from neural crest-derived lineages[19]. To interrogate the embryonic origin of heterogeneous cell populations of the ICNS in the postnatal heart, we used Wnt1Cre;R26eGFP mice for tracing NC originated cells marked before they start migrating from the neural tube. At P30, the vast majority of the sympathetic (Fig 3A), parasympathetic (Fig 3B), and sensory neurons (Fig 3C), as well as P75NTR+ cells (Neurons, Remak SC and a subpopulation of ECF, (Fig 3E)), were Wnt1Cre lineage+ (Fig 3F). Contrarily, the Plp1+ myelinating SC were not NC-originated and their embryonic origin remains unclear (Fig 3D). Additionally, we compared cardiac NC-derived cells at P0 and P30 by whole mount immunostaining after tissue clearing (Fig 3G). An increased complexity of the neural crest-derived GFP-expressing cells from P0 to P30 was observed with increased intramural expansion. In all cases, increased epicardial nerve density detected by Wnt1GFP was observed with more nerves projected into the cardiac walls. Additionally, in the atria and base of the ventricles, an intense network of nerves (neuronal-specific Tubulin (TUBB3)+/GFP+), SC and ECF (GFP+/DAPI+), was observed deep in the tissue (Fig 3H). Together, these data confirm the NC origin of the ICNS and its maturation to an extremely intricate network of nerves that is supported by NC-derived SC and ECF in the atria and the cranial section of the ventricles during the postnatal period. Also, the almost complete overlap of P75NTR with the neural crest lineage Wnt1Cre-driven GFP would suggest that the Tcf21+/P75NTR+ cells in proximity to the thicker nerves are neural crest originated and express Tcf21 postnatally. On the other hand, the Tcf21+/P75NTR-ECF located around the smaller interstitial nerves are probably derived from the epicardium. These findings show distinct embryonic origins for different ECF subpopulations, confirms the NC origin of the Remak SC, and demonstrates that cardiac Plp1+ myelinating SC are not from Tcf21+ or Wnt1+ lineage origin.
3.4. The intracardiac sympathetic and sensory nerves fasciculate from P0 to P7, while the parasympathetic nerves are still fasciculating at P30.
The development and maturation of the peripheral nerves includes elongation, fasciculation of multiple axons into larger nerves, and targeting to ultimately innervated cells. To study whether the ICNS neurons fasciculate during the postnatal period, expression of the fasciculation and cell-cell adhesion marker PSA-NCAM was assessed in sympathetic, parasympathetic, and sensory nerves. PSA-NCAM has been used as general marker of maturation but it has been also been demonstrated to be necessary for normal axon fasciculation[35, 36]. Expression of PSA-NCAM in TH+ sympathetic nerves was detected from P0 to P7 (Fig 4A), while, in ChAT+ parasympathetic nerves, the expression of PSA-NCAM was maintained from P0 to P30 (Fig 4B). These results are in agreement with the observation that parasympathetic innervation in the heart develops later than the sympathetic in different mammals, including humans[23, 37]. Finally, PSA-NCAM was detected in Cgrp+ sensory nerves up to P7, but its expression was significantly decreased from P0 to P7 (Fig 4C). This points to an earlier endpoint of postnatal sensory nerve maturation, in contrast to sympathetic or parasympathetic nerves. These results are in agreement with a robust postnatal cardiac nerve fasciculation period from P0 to P7, at the same time that Postn+ SC and ECF are detected.
Figure 4. Sympathetic and sensory cardiac neurons fasciculate up to P7, while parasympathetic neurons are still maturing and fasciculating at P30.
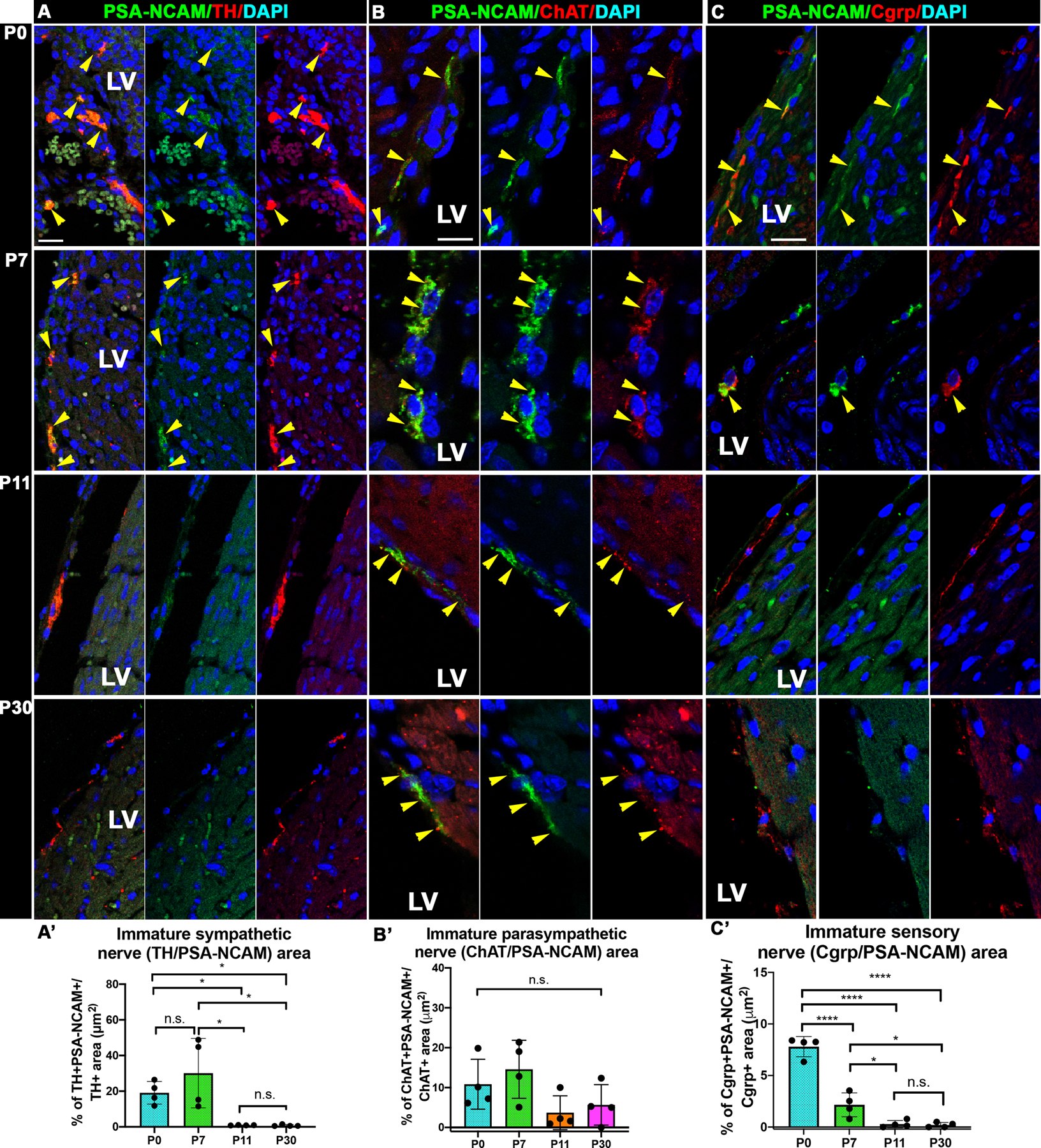
(A) Representative immunofluorescence images show cardiac sympathetic nerves (TH+) co-immunostained with the marker of axon fasciculation PSA-NCAM. SB=15 μm. (A’) Quantification of the sympathetic nerve area (TH+) co-localized with PSA-NCAM relative to the total TH+ area. Fasciculation marker PSA-NCAM expression is apparent at P0 and P7, but not at P11 or P30. N=4. (B) Representative immunofluorescence images show cardiac parasympathetic nerves (ChAT+) co-stained with PSA-NCAM. SB=10 μm. (B’) Quantification of the sympathetic nerve area (ChAT+) co-localized with PSA-NCAM relative to the total ChAT+ area. Fasciculation marker PSA-NCAM expression is apparent at P0 to P30. N=4. (C) Representative immunofluorescence images show cardiac sympathetic nerves (CGRP+) co-stained with PSA-NCAM. SB=10 μm (C’) Quantification of the sympathetic nerve area (CGRP+) co-localized with PSA-NCAM relative to the total CGRP+ area. Fasciculation marker PSA-NCAM expression is apparent at P0 and P7, but not at P11 or P30. N=4. DAPI was used to stain nuclei. Yellow arrows point to areas of fasciculation. LV= Left Ventricle. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 by Kruskall-Wallis tests.
3.5. Postn+ cells are adjacent to fasciculating sympathetic cardiac nerves at P7.
SC are required for peripheral nerve fasciculation and targeting during peripheral nerve development, but their role in development and maturation of the ICNS has not yet been described. Therefore, the relationship of Postn+ SC and ECF cells with areas of sympathetic nerve maturation and fasciculation was examined in postnatal hearts. Remak SC and ECF expressing P75NTR+/DAPI+ cells are present around the sympathetic nerve fasciculation (TH+/PSA-NCAM+) areas (Fig 5A). To corroborate the presence of Postn+ cells in proximity to nerve fasciculation, we co-stained P7 heart sections from P4-P6 TAM-injected PostnMCM;R26eGFP animals with the Remak SC and ECF marker P75NTR and the fasciculation marker PSA-NCAM. P75NTR+/Postn+ cells were located in contact with PSA-NCAM+ areas (Fig 5B), confirming the presence of Postn+ Remak and/or ECF in areas of nerve fasciculation. Moreover, Postn+ cells are localized adjacent to TH+ sympathetic nerves that express the fasciculation marker PSA-NCAM at P7 (Fig 5C). Together, these data locate postnatal Postn+ Remak SC and/or ECF in the areas of sympathetic nerve fasciculation, consistent with a possible role in postnatal cardiac nerve maturation.
Figure 5. Postn+ Remak SC and ECF are adjacent to fasciculating cardiac nerves.

(A) Representative Immunofluorescence images show cardiac sympathetic nerves (TH+) co-stained with the marker of axon fasciculation PSA-NCAM and the neuron/Remak SC/ECF marker P75NTR. Areas of complete colocalization of the three proteins can be observed in sympathetic nerves where axons are fasciculating in association with Remak SC/ECF (DAPI surrounded by P75NTR+/TH-/PSA-NCAM-) aggregated around these nerves. Yellow arrows point to Remak SC/ECF. Blue stars point to sympathetic nerve fasciculation areas. The separated channels are shown in black and white in the individual panels. SB=15 μm. (B) Representative immunofluorescence images of Postn+ ICNS supporting cells (P75NTR+/Postn+) aggregating around axon fasciculation areas (PSA-NCAM+). Yellow arrows point to Postn+ Remak SC/ECF. Blue stars indicate sympathetic axon fasciculation areas. Pink arrows point to P75NTR-/Postn+ cells. The separated channels are shown in black and white in the individual panels. SB=15 μm. (C) Representative immunofluorescence images of Postn+ cells aggregating around sympathetic axon fasciculation areas (TH+/PSA-NCAM+). Yellow arrows point to Postn+ cells. Pink arrows point to TH+/PSA-NCAM+ areas. The separated channels are shown in black and white in the individual panels. SB=15 μm. DAPI was used to stain nuclei. LA=Left Atrium.
3.6. Ablation of Postn+ cells from P0 to P7 reduces sympathetic nerve fasciculation.
Previous studies have found that ablation of Postn+ cells with TAM-mediated induction of Diphtheria Toxin antigen (DTA) in PostnMCM/+;R26DTA mice triggers abnormal maturation of the sympathetic nerves in the heart[28]. In those studies, no differences in branching or elongation were detected and the specific lesion was not identified. Here, we found differential ICNS nerve fasciculation from P0 to P7 (Fig 4) and Postn+ Remak SC localized adjacent to areas of cardiac nerve fasciculation (Fig 5). To assess the involvement of the Postn-expressing cells in ICNS fasciculation, Postn-expressing cells were ablated at P0 to P6 using TAM-inducible Diphtheria toxin antigen expression in PostnMCM/+;R26DTA mice, as previously described[28].
Initially, we confirmed a significant reduction in the number of P75NTR+/Postn+ cells in PostnMCM/+;R26DTA mice compared to DTA-negative littermate controls (Fig S1). Sympathetic nerve fasciculation was evaluated by the expression of PSA-NCAM in the TH+ nerves in P7 control and Postn+ cell ablated animals. A significant reduction in the expression of this fasciculation marker in the sympathetic cardiac nerves was found after Postn-cell ablation (Fig 6A). Additional evidence for alterations in fasciculation was obtained by examination of fasciculation and elongation protein zeta 1 (FEZ1), which is necessary for nerve elongation and fasciculation[38]. At P0, FEZ1 is expressed in the neuronal bodies and the axons (nerves) of sympathetic neurons, as well as surrounding cells (Fig S2A). At P7, FEZ1 is not expressed in TH+ cells, but its expression is maintained adjacent to the nerves in areas where Postn+ neuron-supporting cells are present (Fig S2A). Moreover, Postn+ lineage cells surrounding outflow tract nerves are in FEZ1-expressing regions at P7 (Fig S2B). Interestingly, after Postn+ cell ablation, some expression of FEZ1 was found inside the nerve or between adjacent nerves that should be merged by fasciculation (Fig 6B), suggesting a possible disruption in the normal aggregation of axons to form compacted nerves. Likewise, parallel nerve bundles, indicative of reduced fasciculation, were present in different areas of the heart including the epicardium, the coronaries or the ventricular wall (Fig 6B,C). Another marker of reduced fasciculation is the presence of thinner nerves, where the axons fail to come together properly. Consistent with our previous findings, the circumference of the sympathetic nerves was reduced in P7 Postn+ lineage-ablated hearts (Fig 6D, D’). Finally, we assessed the proximity of axons to each other inside the cardiac nerves by closest bright spot detection measurements (Fig 6E) with a trending result towards a bigger separation of axons inside the nerves of Postn+ lineage-ablated mice (Fig 6E’). In the controls, the average distance between TH-expressing neurons was an average of 0.64μm, while, after Postn+ cell ablation, the average distance was 0.72μm. During neuronal development, FEZ1 can be localized in the nucleus[39, 40], but no differences were found in the number of sympathetic neuronal nuclei expressing FEZ1, suggesting that the transcriptional role of FEZ1 might not be disrupted by Postn+ cell ablation (Fig 6F and F’). Together, these data indicate that a reduction in the number of Postn+ lineage cells results in decreased sympathetic nerve fasciculation in the postnatal heart.
Figure 6. Sympathetic nerve fasciculation is reduced after ablation of Postn+ SC and ECF from P0 to P7.

(A) Representative images from cardiac sympathetic nerves (TH+) co-immunostained with the marker of axon fasciculation PSA-NCAM. SB=15 μm. (A’) Quantification of the sympathetic nerve area (TH+) that co-localizes with PSA-NCAM relative to the total TH+ area. After ablation of Postn+ SC and ECF from P0 to P6 by injecting TAM in PostnMCM/+;R26DTA mice, reduced PSA-NCAM is observed in the sympathetic nerves. Yellow arrows point to fasciculation areas (TH+/PSA-NCAM+). N=6. (B) Representative images of sympathetic nerves in the epicardial area. After ablation of Postn+ cells from P0-P7, FEZ1+ areas can be observed inside the sympathetic nerves. After Postn+ cell ablation, parallel sympathetic nerve trajectories are observed (pink arrows). SB=15 μm. (C) Representative images of sympathetic nerves around coronary blood vessels in the LV. After ablation parallel sympathetic nerve trajectories can be observed, suggesting reduced fasciculation (pink arrows). White circles mark coronary blood vessels identified morphologically. (D) Representative pictures of sympathetic nerve cross sections. SB=15 μm (D’) Quantification of sympathetic nerve cross-section area shows a statistically significant reduction in nerve thickness after Postn+ cell ablation, which is suggestive of reduced nerve fasciculation. (E) Higher magnification of sympathetic nerve cross-section of similar size. The inset shows an example of the “closest bright point” automatic measurements performed to measure the distance between axons indicated by the TH+ signal in the immunofluorescence. (E’) Measurement of “closest bright point” performed to measure the distance between axons. After Postn+ cell ablation from P0 to P7, a trend towards bigger separation between axons was found. N=7. (F) Representative pictures of intracardiac neuronal ganglia. Some sympathetic neuronal bodies (DAPI surrounded by TH+ signal) with intranuclear expression of FEZ1 can be observed. SB=15 μm. (F’) No statistically significant differences in the number of FEZ1+ sympathetic neuron nuclei were observed. DAPI was used to stain nuclei. LA=Left Atria, LV=Left Ventricle, RV=Right Ventricle. *P < 0.05 by Mann-Whitney test.
3.7. Periostin-null mice have reduced sympathetic cardiac nerve fasciculation and collagen remodeling at P7.
The ECM has been suggested as one of the contributing factors for the normal nerve development, including fasciculation, based on its composition and biomechanical properties[27]. Periostin is known to be part of the scaffold that facilitates the maturation of the collagen triple helix and is expressed during interstitial collagen remodeling in the postnatal heart[41]. Hence, we studied the requirement for Postn protein expression in sympathetic neuron maturation/fasciculation after birth. For this, we compared P7 hearts from Postn+/+, PostnMCM/+ and PostnMCM/MCM mice since the MCM is targeted into the Postn locus and prevents Postn protein expression. Postnatal lethality, dwarfism and tooth abnormalities have been described in Postn-null mice, but the cause of lethality has not been reported[42]. In our breedings, we observed a 50% reduction of the number of expected PostnMCM/MCM pups at P7. This number was maintained at P30, suggesting that the mortality of the pups occurs during embryonic and/or neonatal development.
To assess the requirements for Postn in sympathetic nerve fasciculation, nerve cross-sectional area was measured in Postn-null and control pups at P7, the same timepoint when the Postn+ lineage DTA-ablated hearts exhibit abnormalities in this process. The Postn-null mice exhibited reduced nerve cross-section size (Fig 7A) in agreement with impaired fasciculation, which was confirmed by reduced PSA-NCAM expression (Fig 7B). However, it is not known if this is a contributing factor to the neonatal PostnMCM/MCM lethality. Interestingly, we also found reduced expression PSA-NCAM in the sympathetic nerves in the PostnMCM/+ mice, highlighting that variations in ECM proteins might trigger changes in the expression of cell-cell adhesion proteins. Postn also provides a scaffold for collagen triple helix formation and promotes collagen fiber formation and maturation. Therefore, we studied collagen remodeling in the Postn+/+, Het and null hearts using a Collagen Hybridizing Peptide (CHP) with a green fluorescent tag. While the Postn-Het mice exhibit normal collagen remodeling, Postn-null hearts have less interstitial and epicardial collagen remodeling (Fig 7C). Likewise, less CHP signal was found surrounding the Postn-null sympathetic nerves. Altogether, these data point to an important role for Postn in fasciculation and epineurial collagen remodeling in the cardiac sympathetic nerves during the postnatal period.
Figure 7. Sympathetic nerve fasciculation and collagen remodeling are impaired in Postn-null mouse hearts at P7.
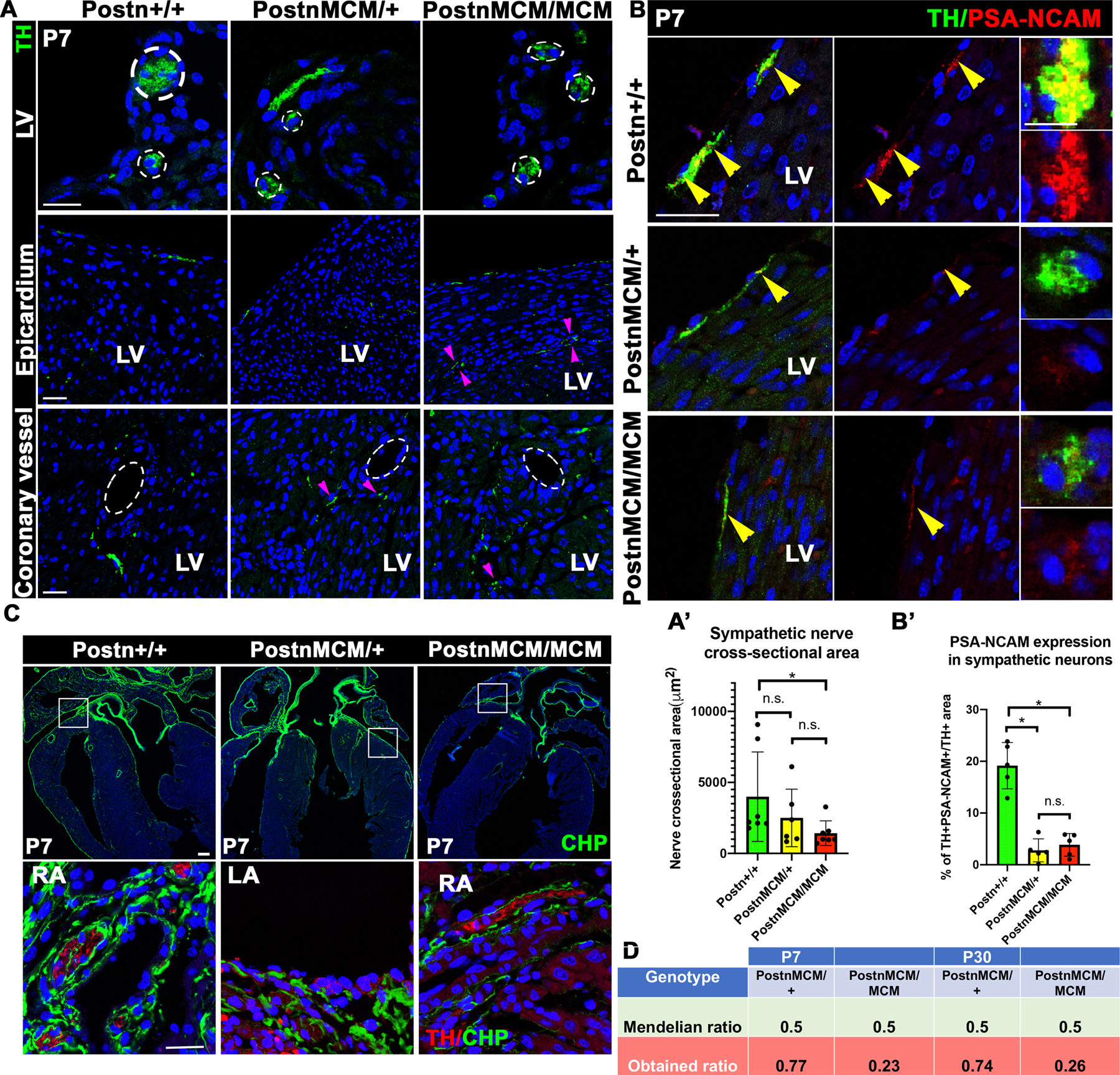
(A) Representative images of sympathetic nerves in Postn+/+ (WT), PostnMCM/+ (Het) and PostnMCM/MCM (null) mice at P7. In the top row, reduced nerve cross-section can be observed (white outlines). In the middle and bottom rows, pink arrows point to parallel nerve bundles found in different parts of the left ventricle in PostnMCM/+ and PostnMCM/MCM, which is indicative a deficient fasciculation. LV= Left ventricle. SB=15 μm. (A’) Quantification of sympathetic nerve cross-sectional area shows a statistically significant reduction in nerve thickness, suggestive of reduced of nerve fasciculation. N=7. (B) Representative pictures from cardiac sympathetic nerves (TH+) co-stained with the marker of axon fasciculation PSA-NCAM. The left and center panels show longitudinal nerves trajectories and the right panels show higher magnification cross-sectional pictures of the nerves. SB=15 μm. (B’) Quantification of the sympathetic nerve area (TH+) that co-localizes with PSA-NCAM relative to the total TH+ area. In PostnMCM/+ and PostnMCM/MCM mice, reduced PSA-NCAM is observed in the sympathetic nerves. Yellow arrows point to fasciculation areas (TH+/PSA-NCAM+). N=5. (C) Representative pictures of Collagen remodeling marked by a GFP-tagged CHP in Postn+/+, PostnMCM/+ and PostnMCM/MCM mice. In the top row, low magnification images show a reduced collagen remodeling indicated by CHP (green) in the heart overall. SB=100 μm. In the bottom row, reduced collagen remodeling around the sympathetic nerves can be observed at higher magnification. N=5. RA= Right Atria, LA= Left atria. SB=15 μm. (D) Table showing the expected ratios of Postn heterozygous and homozygous mice from PostnMCM/MCM bred with PostnMCM/+. At P7, only half of the expected PostnMCM/MCM mice were obtained, suggesting 50% Postn-null lethality up to P7. This ratio was the same at P30, suggesting that the Postn-associated lethality happens before P7.
4. Discussion
In this work, we examine SC and ECF heterogeneity, maturation, lineage origins, and contributions to nerve fasciculation in the ICNS during the postnatal period. Firstly, we found dyssynchronous maturation of different neuronal types. While sensory and sympathetic neurons fasciculate up to P7, parasympathetic neurons are still fasciculating at P30. Secondarily, we report the presence of heterogeneous SC and ECF cell types that support cardiac nerve maturation. Neural crest-derived Remak SC and ECF are located around the major nerves in the atria, outflow tract and epicardium, while Tcf21 lineage non-NC derived ECF, most likely epicardium-derived, are present adjacent to the intramural nerves and axons. Additionally, we detected Postn+ lineage SC and ECF associated with nerve maturation areas and also confirmed a role for Postn in sympathetic cardiac nerve fasciculation. Together, our findings show the cellular heterogeneity and structural complexity of the ICNS, as well as demonstrate the necessity of a subpopulation of Postn+ SC and ECF, and Postn itself, for neuronal fasciculation in the heart.
The peripheral nerves and their SC are considered to be derived from NC in other systems, including the skin and the gut[11, 12], but less is known about the neuronal and supporting cell origins in the heart. Using a Wnt1Cre-mediated lineage tracing system, we demonstrate that the cardiac sympathetic, parasympathetic, sensory neurons, Remak SC, and some ECF are NC-derived. Nerve myelinization and full differentiation of myelinating SC happens later in the postnatal period[43], so, as expected, the number of myelinating SC found at P7 was low. Interestingly, the Plp1+ cells do not appear to be NC-derived. While Plp1 is considered a marker of myelinating SC, it has been shown to be expressed by some fibroblasts[44] and, in the heart, single cell RNAseq studies demonstrated expression of Plp1 in a subpopulation of cardiac fibroblasts[45]. Hence, it is possible that Plp1 is expressed in a small number of postnatal fibroblasts that are not neural crest originated, but could be of extracardiac or endocardial developmental origin[20]. On the other hand, the Remak SC and ECF that aggregate around the thicker nerves in the atria, outflow tract, and epicardium are NC-derived. We also found that this network of NC-derived Remak SC and ECF increases its complexity from P0 to P30, as can be seen in the whole mount analysis of hearts after Wnt1Cre-mediated lineage tracing. Interestingly, when the nerves narrow, to single axons in some cases, in the intramural area of the ventricles, the neuron-supporting cells are predominantly ECF that are not NC-originated and do not express P75NTR, but are Tcf21+, indicative of a possible epicardial origin. These results confirm that the majority of the cellular components of the ICNS are NC-derived, but there are also regional differences, with Tcf21+ intramural ECF likely to be of epicardial origin.
In the postnatal heart, elongation of the sympathetic nerves and their invasion of the myocardium after P5 has been previously described[18]. Interestingly, we detected sympathetic axon fasciculation up to P7, which supports the final maturation of the major sympathetic nerves on the epicardial surface of the heart in the first week after birth, followed by invasion of nerves into the myocardial wall. Very little is known about the postnatal maturation of sensory nerves in the heart, but a previous study found that the percentage of sensory neurons per cardiac area remained constant after P4[24]. Our data support the idea that the sensory nerves in the heart mature earlier than the sympathetic, as we found that sensory axon fasciculation markers decrease significantly from P0 to P7 and are not detected after P7. On the other hand, the parasympathetic innervation starts later[23], with similarly prolonged expression of fasciculation markers in these nerves continuing through the postnatal period (P0-P30). Together, these data show that the maturation of the different neuronal types in the heart after birth is not synchronized.
The information concerning SC or ECF maturation and function in the postnatal period is extremely limited. Schwann cell precursors of the sympathetic ganglia have been shown to express Postn in utero[46]. Here, we show that at P7 Postn-expressing SC and ECF aggregate around nerve maturation areas and are required for sympathetic nerve fasciculation. At this time of intense remodeling and growth of the ICNS, nerve supporting cells express Postn, which has critical functions in ECM remodeling and fibroblast activation[30, 41]. In contrast, no expression of the glial activation marker GFAP, which is expressed in pathologic remodeling enteric glia, brain astrocyte, and peripheral nerve SC, was found in the heart during postnatal maturation[16]. Postn+ lineage cells in the postnatal heart include SC and, relatively uncharacterized, ECF. RNAseq analysis of Postn+ cardiac cells at P7 demonstrated enriched expression of neuron-related ligands, including brain-derived neurotrophic factor (Bdnf) [28]. Here, we show intense fasciculation of the cardiac nerves from P0 to P7 in proximity to Postn+ SC and ECF cells. In agreement with a possible role for these Postn+ SC and ECF in fasciculation, we found that ablation of Postn+ cells from P0 to P6 impairs this process in sympathetic nerves. Abnormal fasciculation and cell targeting of peripheral nerves has been previously described in other models with impaired SC function[12]. Also, in our previous work, we observed parallel sympathetic nerves with increased total area in whole mount immunostaining after Postn+ cell ablation at P7, but the cardiac consequences were not assessed in detail[28]. Here, we show at P7 that the Postn+ ICNS supporting cells contribute to nerve postnatal remodeling in general and to axonal fasciculation in particular.
Finally, while different constituent proteins of the ECM have been reported to have a role in nerve development and fasciculation[26, 27], very little is known about the role of Postn or collagen in this process. Postn is a matricellular protein that acts as a scaffold for mature collagen fiber formation[41], is a marker of fibroblast and SC activation[14, 30], and has been reported to be necessary for cardiac fibrosis and scarring after injury[47]. Postn also is required during development, as ~50% of PostnMCM/MCM (null) mice do not survive to P7. The underlying cause of this mortality remains unclear, but abnormal sympathetic activity in neonates has been suggested to induce bradycardia and neonatal death[48]. Moreover, a trend towards slower heart rhythm has been reported in adult Postn-null mice when compared to controls[47]. In the postnatal heart, collagen remodeling by CF leads to increased stiffness of the ECM[28]. Since ECM stiffness affects axonal attraction and repulsion[27, 49], the reduction in cardiac sympathetic nerve fasciculation, together with reduced collagen remodeling, in Postn-null mice supports a role for Postn and collagen maturation in fasciculation of the cardiac nerves; but further studies will be necessary to confirm these mechanisms. Thus, Postn expression and lineage cells contribute to cardiac ECM maturation and are required for postnatal sympathetic nerve fasciculation. While the other ECM proteins have been related to neuronal development[27], this is the first report implicating Postn in axonal fasciculation in peripheral nerves.
Supplementary Material
Highlights.
The intracardiac nervous system is primarily of neural crest origin.
Cardiac nerve subtypes have dyssynchronous maturation.
At P7, Remak Schwann cells and endoneurial cardiac fibroblasts express Periostin.
Periostin+ cells are required for postnatal cardiac sympathetic nerve fasciculation.
Periostin-null mice have reduced cardiac sympathetic nerve fasciculation at P7.
Acknowledgements
We thank Drs. Peter Kohl, Callum Michael Zgierski-Johnston, Lutz Hein, Qing Richard Lu, Emmanouil Tampakakis, Marco Mongillo and Tania Zaglia for their counsel and good advice. We also thank the people from Jeffery Molkentin’s Lab as well as past and current members of the Yutzey Lab for all the valuable feedback.
Funding
Funding to support this work was provided by NIH Grant R01 HL142217 (to KEY) and a postdoctoral fellowship from the American Heart Association; the Lawrence J. and Florence A. DeGeorge Charitable Trust Grant #19POST34380004 (to LH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
References
- [1].Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015, J Am Coll Cardiol 70(1) (2017) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Srinivasan NT, Schilling RJ, Sudden Cardiac Death and Arrhythmias, Arrhythm Electrophysiol Rev 7(2) (2018) 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duraes Campos I, Pinto V, Sousa N, Pereira VH, A brain within the heart: A review on the intracardiac nervous system, J Mol Cell Cardiol 119 (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [4].Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, et al. , Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration, Dev Cell 34(4) (2015) 387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pianca N, Di Bona A, Lazzeri E, Costantini I, Franzoso M, Prando V, et al. , Cardiac sympathetic innervation network shapes the myocardium by locally controlling cardiomyocyte size through the cellular proteolytic machinery, J Physiol 597(14) (2019) 3639–3656. [DOI] [PubMed] [Google Scholar]
- [6].Pauziene N, Pauza DH, Stropus R, Morphology of human intracardiac nerves: an electron microscope study, J Anat 197 Pt 3 (2000) 437–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR, Single-Cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart, Cell Rep 22(3) (2018) 600–610. [DOI] [PubMed] [Google Scholar]
- [8].Pauziene N, Rysevaite-Kyguoliene K, Alaburda P, Pauza AG, Skukauskaite M, Masaityte A, et al. , Neuroanatomy of the Pig Cardiac Ventricles. A Stereomicroscopic, Confocal and Electron Microscope Study, Anat Rec (Hoboken) 300(10) (2017) 1756–1780. [DOI] [PubMed] [Google Scholar]
- [9].Jessen KR, Mirsky R, The Success and Failure of the Schwann Cell Response to Nerve Injury, Front Cell Neurosci 13 (2019) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, et al. , Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity, Nat Neurosci 12(7) (2009) 839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jessen KR, Mirsky R, Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves, Front Mol Neurosci 12 (2019) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jessen KR, Mirsky R, Lloyd AC, Schwann Cells: Development and Role in Nerve Repair, Cold Spring Harb Perspect Biol 7(7) (2015) a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davis O, Merrison-Hort R, Soffe SR, Borisyuk R, Studying the role of axon fasciculation during development in a computational model of the Xenopus tadpole spinal cord, Sci Rep 7(1) (2017) 13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sonnenberg-Riethmacher E, Miehe M, Riethmacher D, Promotion of periostin expression contributes to the migration of Schwann cells, J Cell Sci 128(17) (2015) 3345–55. [DOI] [PubMed] [Google Scholar]
- [15].Shih CH, Lacagnina M, Leuer-Bisciotti K, Proschel C, Astroglial-derived periostin promotes axonal regeneration after spinal cord injury, J Neurosci 34(7) (2014) 2438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang Z, Wang KK, Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker, Trends Neurosci 38(6) (2015) 364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, et al. , Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells, Development 131(22) (2004) 5599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nam J, Onitsuka I, Hatch J, Uchida Y, Ray S, Huang S, et al. , Coronary veins determine the pattern of sympathetic innervation in the developing heart, Development 140(7) (2013) 1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM, Fate of the mammalian cardiac neural crest, Development 127(8) (2000) 1607–16. [DOI] [PubMed] [Google Scholar]
- [20].Moore-Morris T, Guimaraes-Camboa N, Yutzey KE, Puceat M, Evans SM, Cardiac fibroblasts: from development to heart failure, J Mol Med (Berl) 93(8) (2015) 823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ivey MJ, Tallquist MD, Defining the Cardiac Fibroblast, Circ J 80(11) (2016) 2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tallquist MD, Molkentin JD, Redefining the identity of cardiac fibroblasts, Nat Rev Cardiol 14(8) (2017) 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fregoso SP, Hoover DB, Development of cardiac parasympathetic neurons, glial cells, and regional cholinergic innervation of the mouse heart, Neuroscience 221 (2012) 28–36. [DOI] [PubMed] [Google Scholar]
- [24].Ieda M, Kanazawa H, Ieda Y, Kimura K, Matsumura K, Tomita Y, et al. , Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts, Circulation 114(22) (2006) 2351–63. [DOI] [PubMed] [Google Scholar]
- [25].Spead O, Poulain FE, Trans-Axonal Signaling in Neural Circuit Wiring, Int J Mol Sci 21(14) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bhat KM, Post-guidance signaling by extracellular matrix-associated Slit/Slit-N maintains fasciculation and position of axon tracts in the nerve cord, PLoS Genet 13(11) (2017) e1007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Long KR, Huttner WB, How the extracellular matrix shapes neural development, Open Biol 9(1) (2019) 180216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hortells L, Valiente-Alandi I, Thomas ZM, Agnew EJ, Schnell DJ, York AJ, et al. , A specialized population of Periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation, Proc Natl Acad Sci U S A (2020). [DOI] [PMC free article] [PubMed]
- [29].Lewis AE, Vasudevan HN, O’Neill AK, Soriano P, Bush JO, The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling, Dev Biol 379(2) (2013) 229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. , Genetic lineage tracing defines myofibroblast origin and function in the injured heart, Nat Commun 7 (2016) 12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD, Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney, Genesis 49(11) (2011) 870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Muntifering M, Castranova D, Gibson GA, Meyer E, Kofron M, Watson AM, Clearing for Deep Tissue Imaging, Curr Protoc Cytom 86(1) (2018) e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grubisic V, Gulbransen BD, Enteric glia: the most alimentary of all glia, J Physiol 595(2) (2017) 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, et al. , The transcription factor Sox10 is a key regulator of peripheral glial development, Genes Dev 15(1) (2001) 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cremer H, Chazal G, Goridis C, Represa A, NCAM is essential for axonal growth and fasciculation in the hippocampus, Mol Cell Neurosci 8(5) (1997) 323–35. [DOI] [PubMed] [Google Scholar]
- [36].Wakade CG, Mehta SH, Maeda M, Webb RC, Chiu FC, Axonal fasciculation and the role of polysialic acid-neural cell adhesion molecule in rat cortical neurons, J Neurosci Res 91(11) (2013) 1408–18. [DOI] [PubMed] [Google Scholar]
- [37].Chatow U, Davidson S, Reichman BL, Akselrod S, Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations, Pediatr Res 37(3) (1995) 294–302. [DOI] [PubMed] [Google Scholar]
- [38].Maturana AD, Fujita T, Kuroda S, Functions of fasciculation and elongation protein zeta-1 (FEZ1) in the brain, ScientificWorldJournal 10 (2010) 1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bertini Teixeira M, Figueira ACM, Furlan AS, Aquino B, Alborghetti MR, Paes Leme AF, et al. , Fasciculation and elongation zeta-1 protein (FEZ1) interacts with the retinoic acid receptor and participates in transcriptional regulation of the Hoxb4 gene, FEBS Open Bio 8(1) (2018) 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Whitehouse C, Chambers J, Howe K, Cobourne M, Sharpe P, Solomon E, NBR1 interacts with fasciculation and elongation protein zeta-1 (FEZ1) and calcium and integrin binding protein (CIB) and shows developmentally restricted expression in the neural tube, Eur J Biochem 269(2) (2002) 538–45. [DOI] [PubMed] [Google Scholar]
- [41].Hortells L, Johansen AKZ, Yutzey KE, Cardiac Fibroblasts and the Extracellular Matrix in Regenerative and Nonregenerative Hearts, J Cardiovasc Dev Dis 6(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, et al. , periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype, Mol Cell Biol 25(24) (2005) 11131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Eccleston PA, Mirsky R, Jessen KR, Sommer I, Schachner M, Postnatal development of rat peripheral nerves: an immunohistochemical study of membrane lipids common to non-myelin forming Schwann cells, myelin forming Schwann cells and oligodendrocytes, Brain Res 432(2) (1987) 249–56. [DOI] [PubMed] [Google Scholar]
- [44].Regis S, Grossi S, Corsolini F, Biancheri R, Filocamo M, PLP1 gene duplication causes overexpression and alteration of the PLP/DM20 splicing balance in fibroblasts from Pelizaeus-Merzbacher disease patients, Biochim Biophys Acta 1792(6) (2009) 548–54. [DOI] [PubMed] [Google Scholar]
- [45].Tabula Muris C, Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris, Nature 562(7727) (2018) 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, et al. , Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer, Dev Biol 307(2) (2007) 340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. , Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling, Circ Res 101(3) (2007) 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].VanDusen NJ, Vincentz JW, Firulli BA, Howard MJ, Rubart M, Firulli AB, Loss of Hand2 in a population of Periostin lineage cells results in pronounced bradycardia and neonatal death, Dev Biol 388(2) (2014) 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, et al. , Mechanosensing is critical for axon growth in the developing brain, Nat Neurosci 19(12) (2016) 1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


