Abstract
Purpose:
The role of zoledronic acid (ZOL), a bone-targeted bisphosphonate, in the treatment of patients with breast cancer remains an active area of study. Here, we report the long-term outcomes of a randomized placebo-controlled phase II clinical trial in which ZOL treatment was added to neoadjuvant chemotherapy in women with locally advanced breast cancer.
Methods:
120 women with clinical stage II-III (≥T2 and/or ≥N1) newly diagnosed breast cancer were randomized to receive either 4 mg intravenous ZOL every 3 weeks for 1 year (17 total doses) beginning with the first dose of neoadjuvant chemotherapy, or chemotherapy alone. Clinical endpoints included time to recurrence (TTR), time to bone recurrence (TTBR), time to non-bone recurrence (TTNBR), breast cancer survival (BCS) and overall survival (OS).
Results:
With a median follow-up interval of 14.4 years, there were no significant differences in any of the clinical endpoints studied between the control and ZOL groups in the overall study population. However, ER+/HER2− patients younger than age 45 who were treated with ZOL had significantly worse TTR and TTNBR with a trend towards worse TTBR, BCS and OS (TTR: P = 0.024, HR 6.05 [1.26–29.1]; TTNBR: P = 0.026, HR 6.94 [1.26–38.1]; TTBR: P = 0.054, HR 6.01 [0.97–37.1]; BCS: P = 0.138, HR 4.43 [0.62–31.7]; OS: P = 0.138, HR 4.43 [0.62–31.7]). These differences were not seen in older ER+/HER2− patients or triple-negative patients of any age.
Conclusion:
Addition of ZOL to neoadjuvant therapy did not significantly affect clinical outcomes in the overall study population but was associated with increased extra-skeletal recurrence and a trend towards worse survival in ER+/HER2− patients younger than age 45. These findings suggest caution when using zoledronic acid in young, premenopausal women with locally advanced breast cancer and warrant further investigation.
Keywords: Breast cancer, zoledronic acid, bisphosphonate, neoadjuvant, premenopausal
Introduction
Breast cancer is the most common type of cancer in women, with over 250,000 new cases diagnosed annually in the United States [1]. Treatment of localized breast cancer is multimodal, including surgery and radiation as well as neoadjuvant and adjuvant systemic treatments based on tumor size and subtype. However, a significant number of patients develop recurrence despite these therapies. The pattern and timing of breast cancer recurrence vary substantially based on the hormone receptor status of the tumor. For example, triple-negative breast cancer (TNBC) subtype has a high rate of visceral metastasis in the first five years after diagnosis while the likelihood of recurrence after this time period is quite low [2, 3]. In contrast, the recurrence rate of estrogen receptor positive (ER+) breast cancer remains constant for up to 20 years after diagnosis [4].
The presence of disseminated tumor cells (DTCs) in the bone marrow has been associated with disease recurrence and poor clinical outcomes independent of hormone receptor status [5]. Through their interactions with the bone marrow microenvironment, these cells are protected from the effects of systemic chemotherapy and may remain dormant for years. Upon activation, they may form bone metastases through proliferation and stimulation of osteoclastic bone resorption or they may leave the bone marrow to establish metastases at other sites [6, 7]. Treatment with zoledronic acid (ZOL), a bone-targeted bisphosphonate, has been shown to decrease the proportion of patients with detectable DTCs in multiple studies [8–10]. Recently, several large clinical trials have examined the effect of ZOL treatment on clinical outcomes in early-stage breast cancer [11–13]. With follow-up intervals of 5–7 years, these trials suggest that adjuvant ZOL treatment in addition to adjuvant endocrine therapy improves disease-free survival (DFS), particularly in postmenopausal women. However, the effects of ZOL treatment vary substantially based on menopausal status, with an increase in extra-skeletal metastasis and worse overall survival noted in young premenopausal women [14].
In this study, we report the long-term outcomes of a phase II trial in which 120 women with stage II/III breast cancer were randomized to receive ZOL or no ZOL administered with standard neoadjuvant chemotherapy. A previous analysis of this trial with 5 years of follow-up suggested a possible survival benefit of ZOL in patients with ER− tumors but not those with ER+ tumors [15]. The impact of menopausal status on ZOL treatment effect was not examined in detail.
Methods
Patients
Patients with clinical stage II-III (≥T2 and/or ≥N1) newly diagnosed breast cancer, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and normal cardiac, renal, and liver function were eligible for this study. Detailed inclusion and exclusion criteria have been reported previously [8]. 120 women were enrolled in this study between March 17, 2003 and May 19, 2006, at Siteman Cancer Center, Washington University, St. Louis, MO, USA. The Institutional Review Board of Washington University approved the study and patients gave written informed consent. The Siteman Cancer Center’s quality assurance and safety monitoring committee oversaw patient safety and all aspects of the study were conducted in accordance with the Declaration of Helsinki. Patient outcomes were determined through chart review and review of the Social Security database in November 2019.
Study Design and Treatment
This study is a single-center, open-label, phase II trial to evaluate the efficacy and safety of adding ZOL to standard chemotherapy in women with stage II/III breast cancer (ClinicalTrials.gov, NCT00242203). Enrolled women were randomly assigned to receive either 4 mg intravenous ZOL every 3 weeks for 1 year (17 total doses) beginning with the first dose of neoadjuvant chemotherapy, or chemotherapy alone without ZOL. The approved dosing schedule of ZOL for bone metastasis was used [16]. The randomization process was as previously described [8].
All women received four cycles of intravenous neoadjuvant epirubicin (75 mg/m2) plus docetaxel (75 mg/m2) every 3 weeks, with granulocyte-colony stimulating factor support and oral dexamethasone premedication (20 mg), followed by surgery and two cycles of adjuvant epirubicin plus docetaxel administered every 3 weeks. Adjuvant radiation, endocrine, and trastuzumab therapies were administered when indicated. Patients were encouraged to take 1000 mg of calcium with 800 IU vitamin D daily. Adverse events were assessed at each follow-up. Patients were removed from the study for safety reasons, progression during chemotherapy, or recurrent disease development.
Study Assessments
Immunostaining for ER, progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) was performed at Washington University. ER and PR were considered positive if there was any detectable staining by immunohistochemistry (Ventana, Inc., Tucson, AZ, USA). HER2 was considered positive if HercepTest (Dako North America, Inc., Carpinteria, CA, USA) was 3+ or if the fluorescence in situ hybridization (FISH) score was >2.0. The FISH analysis was done for all specimens scored as 2+ by HercepTest.
Pre-treatment tumor size was defined as the largest tumor dimension documented by mammogram, breast ultrasound, or magnetic resonance imaging. Pre-treatment lymph-node status was defined as any abnormal lymph nodes on computed tomography or ultrasound imaging, or on clinical exam, or by the presence of metastatic disease from fine-needle aspiration or sentinel lymph-node biopsy. Pathologic complete response was defined as no residual invasive tumor in the breast specimen or evidence of metastasis at the time of surgery. Urinary N-telopeptide (NTx) was measured at baseline, 3 months and 12 months after starting therapy using an immunoassay at Mayo Medical Laboratories (Rochester, MN, USA). NTx was expressed as a ratio to urinary creatinine.
Clinical endpoints included time to recurrence (TTR),time tobone recurrence (TTBR), time to non-bone recurrence (TTNBR), breast cancer survival (BCS) and overall survival (OS). Breast cancer recurrence was defined as the first reappearance of breast cancer at any site and included second primary breast cancers as well as local or distant recurrences of the original breast cancer [17]. New primary malignancies which were not breast cancer were not included in this definition. Where no recurrence was recorded before a death attributed to breast cancer, it was assumed that a distant recurrence had just preceded it. TTR was defined as the time interval between surgery (the time point of all residual disease removal) and first detectable breast cancer recurrence. TTBR was defined as the time from surgery to breast cancer recurrence in bone. TTNBR was defined as the time from surgery to breast cancer recurrence outside bone. For calculation of TTR, TTBR and TTNBR, data were censored at time of death or last follow-up if recurrence had not yet occurred. BCS was defined as the time from diagnosis to death from breast cancer. OS was defined as the time from diagnosis to death from any cause. Deaths from unknown causes were included with deaths from breast cancer unless there was clear documentation that the death was not due to breast cancer. For calculation of BCS and OS, data were censored at time of last follow-up if death had not yet occurred.
Statistical Analysis
Kaplan-Meier survival curves were generated to represent TTR, TTBR, TTNBR, BCS and OS in each patient subset. Comparison between groups was performed using a log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional hazards model adjusted for age, race, ER, PR and HER2 status as appropriate. For comparison of NTx values between groups, a two-tailed Student’s t-test was used. P-values < 0.05 were considered significant. All statistical analysis was performed using IBM SPSS Statistics software, version 25.
Results
Patients and Follow-Up
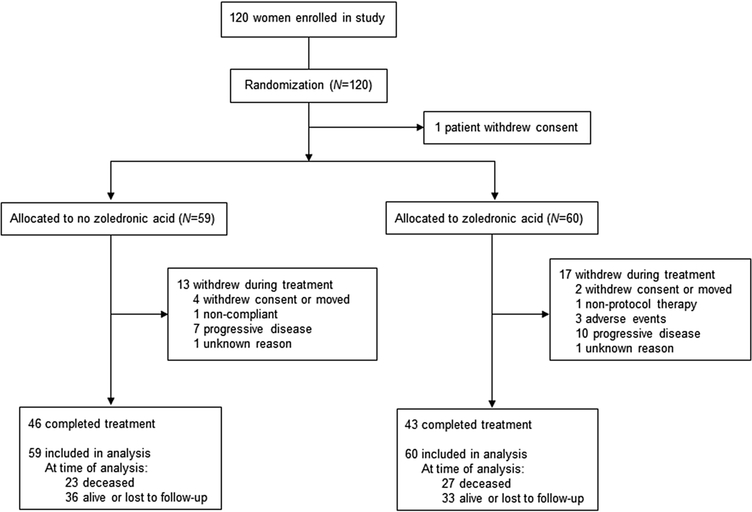
Of the 120 women enrolled in this study, one withdrew consent before receiving treatment and was not included in the final analysis (Fig. 1). All other patients were included. Of these patients, one did not have tumor biomarkers available and one did not undergo surgery for her cancer. Thirteen patients (22.0%) in the control group and 17 patients (28.3%) in the ZOL group did not complete the full course of neoadjuvant and adjuvant therapy. Patient demographics and disease characteristics are shown in Table 1. Of note, 55 patients (46.2%) had ER+/HER2− tumors and 37 patients (31.1%) had triple-negative tumors. The control and ZOL groups were similar in terms of age, race, menopausal status and tumor characteristics although the ZOL group had a slightly higher proportion of African-American patients. At the time of database review in November 2019, 23 patients (39.0%) in the control group and 27 patients (45%) in the ZOL group were known to be deceased. Of the surviving patients, median follow-up was 14.4 years and 90% had > 10 years of follow-up (Table S1).
Fig. 1.
Trial profile
Table 1.
Patient demographics and disease characteristics
| No Zoledronic Acid (N = 59) | Zoledronic Acid (N = 60) | |
|---|---|---|
| Median Age (Range) | 47 (31–68) | 49 (29–67) |
| Race, N (%): | ||
| Caucasian | 45 (76.3) | 39 (65.0) |
| African-American | 11 (18.6) | 20 (33.3) |
| Hispanic | 0 | 1 (1.7) |
| Asian | 3 (5.1) | 0 |
| Menopausal Status, N (%): | ||
| Premenopausal | 33 (55.9) | 31 (51.7) |
| Postmenopausal | 26 (44.1) | 29 (48.3) |
| Pathology, N (%): | ||
| Ductal carcinoma | 49 (83.1) | 47 (78.3) |
| Lobular carcinoma | 7 (11.8) | 7 (11.7) |
| Other | 3 (5.1) | 6 (10) |
| Mean tumor size, cm (s.d.) | 3.56 (2.41) | 3.81 (2.03) |
| Lymph-node positive, N (%) | 33 (55.9) | 38 (63.3) |
| Grade, N (%) | ||
| I | 2 (3.4) | 7 (11.7) |
| II | 28 (47.5) | 20 (33.3) |
| III | 29 (49.2) | 33 (55) |
| Receptor status, N (%) | ||
| ER+ | 34 (57.6) | 32 (53.3) |
| PR+ | 31 (52.5) | 24 (40) |
| HER2+ | 10 (16.9) | 13 (21.7) |
| ER+/HER2− | 29 (49.2) | 26 (43.3) |
| ER−/PR−/HER2− | 17 (28.8) | 20 (33.3) |
| Unknown | 1 (1.7) | - |
Recurrence and Survival
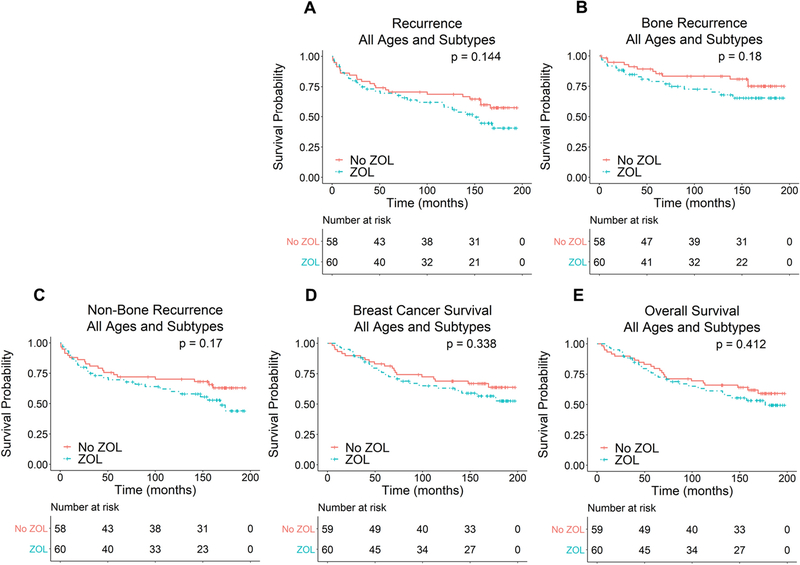
For the overall study population including all ages and breast cancer subtypes, there were no significant differences in TTR (P = 0.144), TTBR (P = 0.180), TTNBR (P = 0.170), BCS (P = 0.338) or OS ( P = 0.412) between the control and ZOL groups (Fig. 2). Twenty-three patients (39.7%) in the control group and 31 patients (51.7%) in the ZOL group had disease recurrence after surgery. The timing of these recurrences differed substantially based on the receptor status of the tumor. In patients with triple-negative tumors, 1 recurrence (11.1%) in the control group and 3 recurrences (33.3%) in the ZOL group occurred more than 5 years after surgery. In contrast, in patients with ER+/HER2− tumors, 4 recurrences (50.0%) in the control group and 7 recurrences (50.0%) in the ZOL group occurred more than 5 years after surgery.
Fig. 2.
Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in the overall study population including all ages and breast cancer subtypes
In the control group, 20 deaths were due to breast cancer and 3 deaths were due to other causes. In the ZOL group, 25 deaths were due to breast cancer and 2 deaths were due to other causes. Median BCS and OS were not reached in either the control or ZOL groups. Four patients (6.8%) in the control group and 5 patients (8.3%) in the ZOL group had a pathologic complete response to neoadjuvant chemotherapy. Of these patients, one died of an unrelated cancer (jejunal adenocarcinoma) and two had local recurrence of their breast cancer more than 10 years after surgery. The remainder are alive and disease-free. Overall, 32 patients (54.2%) in the control group and 27 patients (45.0%) in the ZOL group were alive and without recurrence at the time of last follow-up.
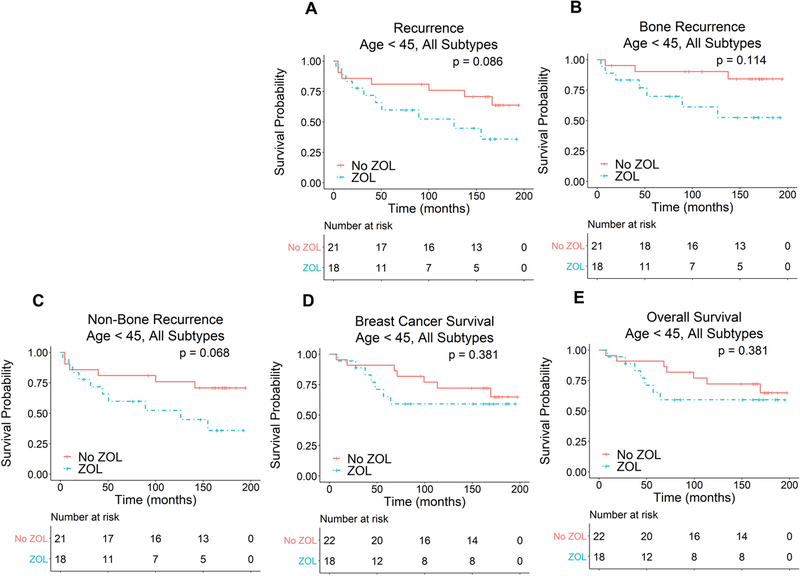
While large clinical trials have generally shown a neutral or beneficial effect of ZOL treatment, several previous studies have noted a trend towards harm, particularly in young premenopausal patients [11, 14, 18, 19]. To examine this further, we stratified our results by age with a cutoff of 45 years chosen to distinguish premenopausal patients from perimenopausal and postmenopausal patients. A subgroup analysis of perimenopausal women age 45–54 was also performed. As seen in Figure 3, there was a trend towards worse TTR, TTBR and TTNBR in patients younger than age 45 who were treated with ZOL (TTR: P = 0.086, HR 2.52 [0.88–7.23]; TTBR: P = 0.114, HR 3.08 [0.76–12.44]; TTNBR: P = 0.068, HR 2.79 [0.93–8.43]). However, there were no significant differences in BCS or OS in these patients (BCS: P = 0.381, HR 1.67 [0.53–5.23]; OS: P = 0.381, HR 1.67 [0.53–5.23]). There were also no significant differences in any of the clinical outcomes studied in patients 45 years of age and older (Fig. S1. TTR: P = 0.580; TTBR: P = 0.832; TTNBR: P = 0.738; BCS: P = 0.606; OS: P = 0.788) or in patients age 45–54 (Fig. S2. TTR: P = 0.838; TTBR: P = 0.864; TTNBR: P = 0.953; BCS: P = 0.815; OS: P = 0.927).
Fig. 3.
Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in patients younger than age 45
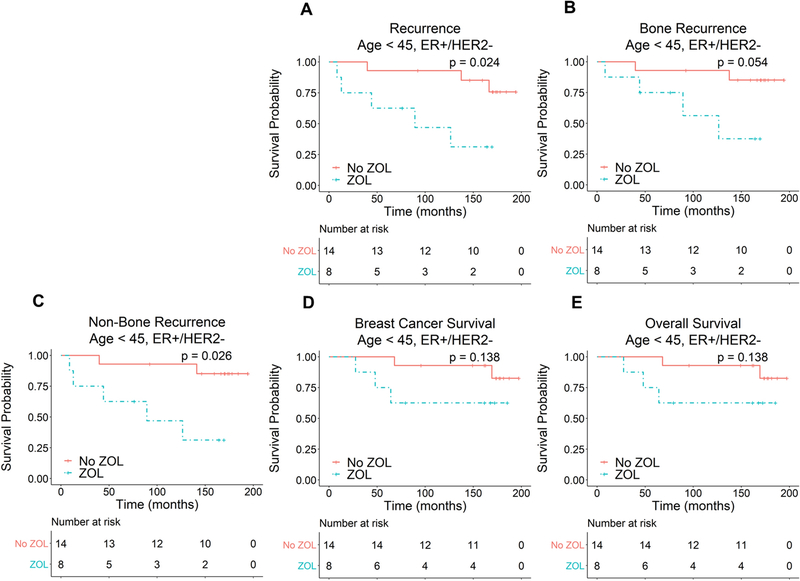
In an earlier analysis of this study, ER status was found to be an important predictor of both survival and ZOL efficacy [15]. Consequently, in this long-term follow-up study, we further stratified our patients by ER status to determine whether ZOL treatment has differential effects on patients with ER+ and ER− tumors. Due to the emergence of HER2-targeted therapy and in order to analyze more uniform subsets of breast cancer patients, we focused our analysis on patients with ER+/HER2− and triple-negative tumors. As seen in Figure 4, ER+/HER2− patients younger than age 45 who were treated with ZOL had significantly worse TTR and TTNBR with a trend towards worse TTBR, BCS and OS (TTR: P = 0.024, HR 6.05 [1.26–29.1]; TTNBR: P = 0.026, HR 6.94 [1.26–38.1]; TTBR: P = 0.054, HR 6.01 [0.97–37.1]; BCS: P = 0.138, HR 4.43 [0.62–31.7]; OS: P = 0.138, HR 4.43 [0.62–31.7]). In contrast, ZOL treatment did not significantly affect any of the clinical outcomes studied in ER+/HER2− patients 45 years of age and older (Fig. S3), age 45–54, or triple-negative patients of any age (Figs. S4 and S5). These results are summarized in Table 2.
Fig. 4.
Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in ER+/HER2− patients younger than age 45
Table 2.
Summary of the effect of ZOL treatment on clinical outcomes. Data are presented as number of events / number of patients in each group followed by hazard ratio and 95% confidence interval
| Recurrence | Bone Recurrence | Non-Bone Recurrence | Breast Cancer Survival | Overall Survival | |
|---|---|---|---|---|---|
| Age < 45, ER+/HER2− |
ZOL: 5/8 No ZOL: 3/14 HR 6.05 [1.26–29.1] |
ZOL: 4/8 No ZOL: 2/14 HR 6.01 [0.97–37.1] |
ZOL: 5/8 No ZOL: 2/14 HR 6.94 [1.26–38.1] |
ZOL: 3/8 No ZOL: 2/14 HR 4.43 [0.62–31.7] |
ZOL: 3/8 No ZOL: 2/14 HR 4.43 [0.62–31.7] |
| Age >= 45, ER+/HER2− | ZOL: 9/18 No ZOL: 5/15 HR 1.95 [0.57–6.66] |
ZOL: 3/18 No ZOL: 5/15 HR 0.78 [0.16–3.83] |
ZOL: 8/18 No ZOL: 5/15 HR 1.53 [0.44–5.30] |
ZOL: 8/18 No ZOL: 3/15 HR 2.71 [0.54–13.6] |
ZOL: 9/18 No ZOL: 4/15 HR 2.13 [0.54–8.31] |
| Age 45–54, ER+/HER2− | ZOL: 5/10 No ZOL: 3/9 HR 3.12 [0.51–19.0] |
ZOL: 2/10 No ZOL: 3/9 HR 1.68 [0.15–18.9] |
ZOL: 5/10 No ZOL: 3/9 HR 2.62 [0.46–15.0] |
ZOL: 4/10 No ZOL: 1/9 HR 2.79 [0.19–40.8] |
ZOL: 5/10 No ZOL: 2/9 HR 2.10 [0.30–14.9] |
| Age < 45, Triple-Negative | ZOL: 3/7 No ZOL: 3/5 HR 0.89 [0.12–6.59] |
ZOL: 2/7 No ZOL: 1/5 HR 0.49 [0.03–7.94] |
ZOL: 3/7 No ZOL: 3/5 HR 0.89 [0.12–6.59] |
ZOL: 2/7 No ZOL: 4/6 HR 0.31 [0.03–3.11] |
ZOL: 2/7 No ZOL: 4/6 HR 0.31 [0.03–3.11] |
| Age >= 45, Triple-Negative | ZOL: 6/13 No ZOL: 6/11 HR 0.53 [0.17–1.72] |
ZOL: 2/13 No ZOL: 2/11 HR 0.54 [0.07–4.03] |
ZOL: 5/13 No ZOL: 5/11 HR 0.47 [0.13–1.72] |
ZOL: 5/13 No ZOL: 5/11 HR 0.53 [0.15–1.89] |
ZOL: 6/13 No ZOL: 6/11 HR 0.52 [0.16–1.66] |
Role of Bone Turnover
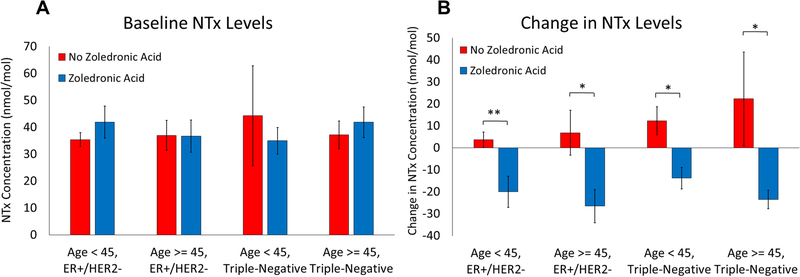
To assess whether ZOL’s effects on survival were related to its effects on bone turnover, we looked at changes in urinary N-telopeptide (NTx) levels measured at baseline and 12 months after starting treatment. We hypothesized that if ZOL’s effect on bone turnover was responsible for its effects on recurrence and survival, then patients who had the most pronounced changes in bone turnover markers would have the most dramatic differences in clinical outcomes. We again divided patients into groups based on age and ER status and compared baseline NTx levels and change in NTx levels during treatment. There was no difference between baseline NTx levels in patients treated with ZOL and patients not treated with ZOL in all groups (Fig. 5A). As expected, ZOL treatment led to a significantly negative change in NTx levels in all groups consistent with reduced bone turnover (Fig. 5B). However, there were no differences in any of the clinical outcomes studied between patients with high (greater than median) change in NTx levels and those with low (less than median) change in NTx levels in ER+/HER2− patients younger than age 45 (Table 3).
Fig. 5.
Baseline NTx levels (A) and change in NTx levels from baseline to 12 months (B). Error bars represent standard error. * - p < 0.05, ** - p < 0.01
Table 3.
Summary of clinical outcomes in ER+/HER2− patients younger than age 45 stratified by amount of change in NTx levels from baseline to 12 months. Data are presented as number of events / number of patients in each group followed by hazard ratio and 95% confidence interval
| Recurrence | Bone Recurrence | Non-Bone Recurrence | Breast Cancer Survival | Overall Survival | |
|---|---|---|---|---|---|
| Age < 45, ER+/HER2− | ΔNTx Low: 3/7 ΔNTx High: 3/7 HR 1.99 [0.30–13.4] |
ΔNTx Low: 1/7 ΔNTx High: 1/7 HR 1.48 [0.06–39.4] |
ΔNTx Low: 2/7 ΔNTx High: 2/7 HR 6.55 [0.55–78.7] |
ΔNTx Low: 3/7 ΔNTx High: 3/7 HR 1.99 [0.30–13.4] |
ΔNTx Low: 4/7 ΔNTx High: 4/7 HR 2.44 [0.44–13.6] |
Discussion
In this long-term study with a median follow-up of 14.4 years, the addition of neoadjuvant ZOL to standard therapy did not significantly affect recurrence or survival in the overall population of women with stage II/III breast cancer representing all breast cancer subtypes. However, the effect of ZOL treatment on these outcomes was notably different depending on patient age and tumor subtype. In patients with triple-negative tumors, there was a slight trend towards improved outcomes with ZOL treatment which did not reach statistical significance in patients of all ages (Table 2). This result is consistent with our previous finding at 5 years of follow-up that ZOL treatment improved DFS and OS in patients with ER− tumors [15]. In that study, ZOL treatment did not significantly affect DFS and OS in the ER+ or ER+/HER2− subgroups. With additional follow-up, however, we now find that ER+/HER2− patients younger than age 45 who were treated with ZOL had significantly worse TTR and TTNBR with a trend towards worse TTBR, BCS and OS.
Recently, several large clinical trials have studied the effects of bisphosphonate therapy on clinical outcomes in early-stage breast cancer. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-34 trial found that adjuvant use of oral clodronate improved recurrence-free interval in older patients but not in the overall study population [20]. Similarly, the Austrian Breast and Colorectal Cancer Study Group (ABCSG)-12 trial found that adjuvant use of zoledronic acid improved DFS in ER+ patients but all patients in this trial had menopause induced through the use of goserelin [13]. The Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) trial was a large multi-national study which examined the effects of adjuvant zoledronic therapy in early-stage breast cancer. With 10 years of follow-up, treatment with ZOL had no effect on the overall population but improved DFS in postmenopausal women. Interestingly, ZOL treatment led to worse outcomes in young patients, with an increase in relapse and worse OS noted [14]. Recently published results from the NEOZOTAC trial also suggest that addition of zoledronic acid to neoadjuvant chemotherapy does not improve pathological complete response and worsens overall survival in patients with HER2− stage II/III breast cancer [18, 21]. Of note, over 80% of patients in this trial were ER+ and/or PR+ and over 50% were pre- or perimenopausal. Our results are consistent with these studies.
Numerous mechanisms have been proposed to explain the differential effects of ZOL treatment in premenopausal and postmenopausal women, many of which focus on the bone microenvironment [22, 23]. Interestingly, in the AZURE study, treatment of young patients with ZOL was primarily associated with an increase in recurrence at extra-skeletal sites [11]. We, too, find that ZOL treatment significantly increases non-bone recurrence while having a less pronounced impact on skeletal recurrence (Fig. 4, Table 2). One possibility is that inhibition of bone resorption creates a more inhospitable environment for disseminated tumor cells, prompting their migration out of the bone and into other sites of recurrence. Yet, when patients were stratified by the degree to which bone turnover was impacted by therapy, there was no difference in any of the clinical outcomes studied between those who had the greatest inhibition of bone turnover and those who had the least (Table 3). This finding raises the possibility that ZOL’s impact may be independent of bone turnover and may result from effects on the immune system or direct effects on tumor cells themselves. For instance, ZOL treatment may lead to dormancy of disseminated tumor cells, thereby rendering them more resistant to chemotherapy [24]. Other biological characteristics of the tumor are also likely to contribute. For instance, the AZURE study found that premenopausal women with MAF amplification had significantly worse survival with ZOL treatment [14, 25]. These results demonstrate the need for additional studies to characterize the molecular impact of ZOL treatment on the tumor and its microenvironment.
Along with local factors, the systemic hormonal milieu has been proposed as an important determinant of ZOL’s impact on tumor growth [22, 23]. In our study, ZOL’s effect on survival specifically in young, premenopausal women with ER+ tumors suggests that estrogen signaling may play a role in the underlying mechanism. Of note, only 22.7% of ER+/HER2− patients younger than age 45 in our study received ovarian suppression therapy while the remainder were treated with either tamoxifen (50%) or an aromatase inhibitor (22.7%) alone (Table S2). This treatment approach differs from that of the ABCSG-12 trial in which all patients received ovarian suppression with goserelin in addition to either tamoxifen or anastrozole [13]. The survival benefit of ZOL treatment in premenopausal patients noted in the ABCSG-12 trial may be related to this additional ovarian suppression. Conversely, the use of aromatase inhibitors alone in some of our premenopausal patients may have led to incomplete suppression of estrogen levels which may have compounded any interaction with ZOL treatment and worsened outcomes in the premenopausal study population. ZOL treatment in perimenopausal patients age 45–54 did not appear to affect clinical outcomes in our study (Fig. S2), suggesting that these patients do not behave as premenopausal patients in this regard, possibly due to more effective suppression of estrogen activity in this population. It is also interesting to note that 72.7% of ER+/HER2− patients younger than age 45 in our study received tamoxifen while only 27.3% of ER+/HER2− patients age 45 and older received this medication. It is therefore possible that an interaction between ZOL and tamoxifen contributed to the inferior survival found in younger patients. The heterogeneity of hormonal therapies used is a limitation of our study and additional studies are needed to clarify the interactions between ZOL treatment, estrogen signaling and endocrine therapy in breast cancer patients.
Although our study is smaller than some recently published trials, it has the advantage of long-term follow-up, with a median follow-up interval of 14.4 years and 90% of patients with > 10 years of follow-up. The importance of such long-term follow-up is demonstrated particularly in our ER+/HER2− patients, where 50% of recurrences occurred more than 5 years after surgery and 31.8% of recurrences occurred more than 10 years after surgery. Our focus on triple-negative and ER+/HER2− subgroups permits analysis of more uniform subsets of patients while eliminating confounding from HER2-directed therapy. Similarly, as a single dose of ZOL is known to have antiresorptive effects in the bone for up to 3 years [26], we used 45 years of age as a threshold to identify premenopausal women who would likely remain premenopausal throughout the duration of ZOL’s effect. Nevertheless, the pharmacodynamics of ZOL as it relates to tumor development are unknown and many patients became menopausal over the course of the study, further complicating analysis. Additionally, our study was not designed or powered to draw conclusions regarding these subgroups so these results should be considered hypothesis-generating and interpreted with appropriate caution.
In summary, addition of ZOL to neoadjuvant therapy did not significantly affect recurrence or survival in the overall study population of patients with stage II/III breast cancer. However, ZOL treatment was significantly associated with inferior time to recurrence and increased extra-skeletal recurrence in ER+/HER2− patients younger than age 45. These findings, in conjunction with those of other large randomized clinical trials, suggest caution when using zoledronic acid in young, premenopausal women with locally advanced breast cancer and warrant further investigation into the mechanisms by which bisphosphonates impact tumor growth and development.
Supplementary Material
Fig. S1 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in patients 45 years of age and older
Fig. S2 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in patients age 45–54
Fig. S3 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in ER+/HER2− patients 45 years of age and older
Fig. S4 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in triple-negative patients younger than age 45
Fig. S5 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in triple-negative patients 45 years of age and older
Table S1 Length of patient follow-up
Table S2 Endocrine therapy in ER+/HER2− patients
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002344. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The Institutional Review Board of Washington University approved the study. The Siteman Cancer Center’s quality assurance and safety monitoring committee oversaw patient safety and all aspects of the study were conducted in accordance with the Declaration of Helsinki.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study.
Consent for Publication
Written informed consent was obtained from all individual participants included in the study. No identifying information about participants is included in this article.
Availability of Data and Material
Deidentified datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable
Clinical trial registration number: NCT00242203, Date of Registration: 10/17/2005
References
- 1.Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA: A Cancer Journal for Clinicians 69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115:423–428. 10.1007/s10549-008-0086-2 [DOI] [PubMed] [Google Scholar]
- 3.Reddy SM, Barcenas CH, Sinha AK, Hsu L, Moulder SL, Tripathy D, Hortobagyi GN, Valero V (2018) Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. British Journal of Cancer 118:17–23. 10.1038/bjc.2017.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard Kl, Bergh J, Dowsett M, Hayes DF (2017) 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 377:1836–1846. 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun S, Janni W, Schlimok G, Gebauer G, Oruzio D, Kundt G, Wong GYC, Pantel K (2005) A Pooled Analysis of Bone Marrow Micrometastasis in Breast Cancer. The New England Journal of Medicine 10. [DOI] [PubMed] [Google Scholar]
- 6.Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, Olivere L, Comatas K, Magnani J, Lyerly HK, Cheng Q, McCall CM, Sipkins DA (2016) Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Science Translational Medicine 8:340ra73–340ra73. 10.1126/scitranslmed.aad4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nature Reviews Cancer 11:411–425. 10.1038/nrc3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, Zhai J, Kuo S, Shannon W, Diemer K, Herrmann V, Dietz J, AN A, Ellis M, Weiss P, Eberlein T, Ma C, Fracasso PM, Zoberi I, Taylor M, Gillanders W, Pluard T, Mortimer J, Weilbaecher K (2010) Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. The Lancet Oncology 11:421–428. 10.1016/S1470-2045(10)70054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banys M, Solomayer E-F, Gebauer G, Janni W, Krawczyk N, Lueck H-J, Becker S, Huober J, Kraemer B, Wackwitz B, Hirnle P, Wallwiener D, Fehm T (2013) Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer 13:480. https://doi.org/10-1186/1471-2407-13-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rack B, Juckstock J, Genss E-M, Schoberth A, Schindlbeck C, Strobl B, Heinrigs M, Rammel G, Zwingers T, Sommer H, Friese K, Janni W (2010) Effect of Zoledronate on Persisting Isolated Tumour Cells in Patients with Early Breast Cancer. Anticancer Res 30:1807–1813 [PubMed] [Google Scholar]
- 11.Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E, Keane M, Gil M, Burkinshaw R, Grieve R, Barrett-Lee P, Ritchie D, Liversedge V, Hinsley S, Marshall H (2014) Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. The Lancet Oncology 15:997–1006. 10.1016/S1470-2045(14)70302-X [DOI] [PubMed] [Google Scholar]
- 12.Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Annals of Oncology 24:398–405. 10.1093/annonc/mds277 [DOI] [PubMed] [Google Scholar]
- 13.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber E-P, Fesl C, Greil R (2011) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. The Lancet Oncology 12:631–641. 10.1016/S1470-2045(11)70122-X [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, Keane M, Gil M, Barrett-Lee P, Ritchie D, Bowman A, Liversedge V, De Boer RH, Passos-Coelho JL, O’Reilly S, Bertelli G, Joffe J, Brown JE, Wilson C, Tercero JC, Jean-Mairet J, Gomis R, Cameron D (2018) Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). Journal of Bone Oncology 13:123–135. 10.1016/j.jbo.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aft RL, Naughton M, Trinkaus K, Weilbaecher K (2012) Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breast cancer. British Journal of Cancer 107:7–11. 10.1038/bjc.2012.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS, Lipton A, Brown S (2003) American Society of Clinical Oncology 2003 Update on the Role of Bisphosphonates and Bone Health Issues in Women With Breast Cancer. JCO 21:4042–4057. 10.1200/JCO.2003.08.017 [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet 365:1687–1717. 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 18.de Groot S, Pijl H, Charehbili A, van de Ven S, Smit VTHBM, E Meershoek-Klein Kranenbarg, Heijns JB, van Warmerdam LJC, Kessels LW, Dercksen MW, Pepels MJAE, van Laarhoven HWM, Vriens BEPJ, Putter H, Fiocco M, Liefers G-J, van der Hoeven JJM, Nortier JWR, Kroep JR, on behalf of the Dutch Breast Cancer Research Group (2019) Addition of zoledronic acid to neoadjuvant chemotherapy is not beneficial in patients with HER2-negative stage II/III breast cancer: 5-year survival analysis of the NEOZOTAC trial (BOOG 2010–01). Breast Cancer Res 21:97. 10.1186/s13058-019-1180-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C (2004) Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncologica 43:650–656. 10.1080/02841860410032885 [DOI] [PubMed] [Google Scholar]
- 20.Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, Weir LM, Brufsky AM, Dakhil S, Lad T, Baez-Diaz L, Gralow JR, Robidoux A, Perez EA, Zheng P, Geyer CE, Swain SM, Costantino JP, Mamounas EP, Wolmark N (2012) Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. The Lancet Oncology 13:734–742. 10.1016/S1470-2045(12)70226-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charehbili A, van de Ven S, Smit V, Kranenbarg EM-K, Hamdy N, Putter H, Heijns J, van Warmerdam L, Kessels L, Dercksen M, Pepels M, Maartense E, van Laarhoven H, Vriens B, Wasser M, van Leeuwen-Stok A, Liefers G, van de Velde C, Nortier J, Kroep J (2014) Addition of zoledronic acid to neoadjuvant chemotherapy does not enhance tumor response in patients with HER2-negative stage II/III breast cancer: the NEOZOTAC trial (BOOG 2010–01). Annals of Oncology 25:998–1004. 10.1093/annonc/mdu102 [DOI] [PubMed] [Google Scholar]
- 22.Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, Eaton CL, Holen I (2014) Zoledronic Acid Has Differential Antitumor Activity in the Pre- and Postmenopausal Bone Microenvironment In Vivo. Clin Cancer Res 20:2922–2932. 10.1158/1078-0432.CCR-13-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman RA, Brufsky AM, Oesterreich S (2012) Zoledronic acid effectiveness against breast cancer metastases - a role for estrogen in the microenvironment? Breast Cancer Res 14:213. 10.1186/bcr3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers MA, McDonald MM, Croucher PI (2020) Cancer Cell Dormancy in Metastasis. Cold Spring Harb Perspect Med 10:a037556. 10.1101/cshperspect.a037556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman R, Hall A, Albanell J, Hanby A, Bell R, Cameron D, Dodwell D, Marshall H, Jean-Mairet J, Tercero J-C, Rojo F, Gregory W, Gomis RR (2017) Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. The Lancet Oncology 18:1543–1552. 10.1016/S1470-2045(17)30603-4 [DOI] [PubMed] [Google Scholar]
- 26.Silverman SL (2011) Defining Zoledronate’s Duration of Action and Optimal Dosing Interval for an Effective Therapy. Curr Osteoporos Rep 9:4–5. 10.1007/s11914-010-0044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in patients 45 years of age and older
Fig. S2 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in patients age 45–54
Fig. S3 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in ER+/HER2− patients 45 years of age and older
Fig. S4 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in triple-negative patients younger than age 45
Fig. S5 Effect of ZOL treatment on TTR (A), TTBR (B), TTNBR (C), BCS (D) and OS (E) in triple-negative patients 45 years of age and older
Table S1 Length of patient follow-up
Table S2 Endocrine therapy in ER+/HER2− patients