Abstract
Secretin-enhanced MRCP (S-MRCP) provides multiple advantages compared with standard MRCP for imaging the pancreaticobiliary tree. By using secretin to induce fluid production from the pancreas, and leveraging fluid-sensitive MRCP sequences, S-MRCP increases visualization of ductal anatomy and provides insight into pancreatic function, allowing radiologists to offer additional insight for a range of pancreatic-related conditions. This narrative review provides detailed information on the practical implementation of S-MRCP, including patient preparation, logistics of secretin administration, and dynamic secretin-enhanced MRCP acquisition. Considerations are given for radiologists’ interpretation and reporting of S-MRCP examinations, including assessment of dynamic compliance of the main pancreatic duct and of duodenal fluid volume. Established indications for S-MRCP are reviewed, including pancreas divisum, anomalous pancreaticobiliary junction, Santorinicele, Wirsungocele, chronic pancreatitis, main pancreatic duct stenosis, and assessment of complex postoperative anatomy. Equivocal or controversial indications are also presented, along with the authors’ approach to such indications; these include acute or recurrent acute pancreatitis, pancreatic exocrine function, sphincter of Oddi dysfunction, and pancreatic neoplasms.
Introduction
MRI has been used for noninvasive evaluation of the pancreas for several decades through a combination of T1-weighted, T2-weighted, and dynamic contrast-enhanced sequences. Combined with MRCP sequences that suppress non-fluid signal to accentuate the pancreatic and biliary ducts, MRI provides excellent visualization of the pancreatic and biliary ductal systems. These techniques have been refined over time to allow MRI with MRCP to play a vital role in characterizing pancreatic ductal abnormalities as well as cystic neoplasms of the pancreas [1,2].
Secretin-enhanced MRCP (S-MRCP) has been developed specifically for the evaluation of the pancreas and pancreatic ducts [3] and further improves the diagnostic yield and clinical utility compared with MRI with standard MRCP [1,4]. Moreover, the use of secretin with MRCP provides dynamic evaluation of pancreatic exocrine volume reserve that is not possible using standard MRCP. However, secretin increases the cost of an MRCP examination, adds at least 15 minutes to the examination time, and in some practices requires a nurse to give the IV infusion. In part related to these practical considerations, S-MRCP has failed to gain widespread usage across radiology practices.
The authors of this narrative review are experienced in the clinical implementation of S-MRCP. In this article, we describe how and why to use secretin for MRCP. Approaches for optimal imaging acquisition and technique are discussed, and recommendations for reporting findings are provided. In addition, both established and controversial uses of S-MCRP are examined.
How to Use Secretin During MRCP
MRCP Technique
Sequences designed to accentuate the fluid signal in the biliary and pancreatic ducts are the cornerstone of MRCP imaging. These rely on heavily T2-weighted techniques with echo times that are often greater than 1000 msec. Such sequences minimize the signal of soft tissue and moving fluids given that the protons of such tissues completely relax over such long intervals. Stationary or slow-moving fluids, such as fluids in the bile duct and pancreatic ducts, have a longer T2 relaxation time, allowing their signal to dominate over subsided background signal from solid organs.
Numerous MRCP imaging methods are commercially available from different vendors for acquiring images in this fashion. Many of these rely on half-Fourier pulse sequences and thick slices to substantially reduce image acquisition time, allowing for short breath holds of 2–6 seconds, which decrease motion artifact and improve diagnostic image quality [4]. Quick acquisition times allow imaging of the entire pancreatic duct using different angles. These angles are centered on the distal common bile duct, providing multiple radial thick slice slab sets of images [FIGURE 1]. Thin-slice MRCP images take more time and therefore require using respiratory triggering, single coronal projection, and often 3D acquisition [5]. We recommend obtaining any 3D images prior to secretin administration given that expected increases in small bowel fluid after secretin administration may impair visualization of the pancreaticobiliary tree. While MRCP protocols generally follows these guidelines, the specific MRI pulse sequences used vary by local practice patterns. Examples of standard MRCP sequences at 1.5T and 3T from one of our institutions are provided in Table 1 and Table 2.
Figure 1.
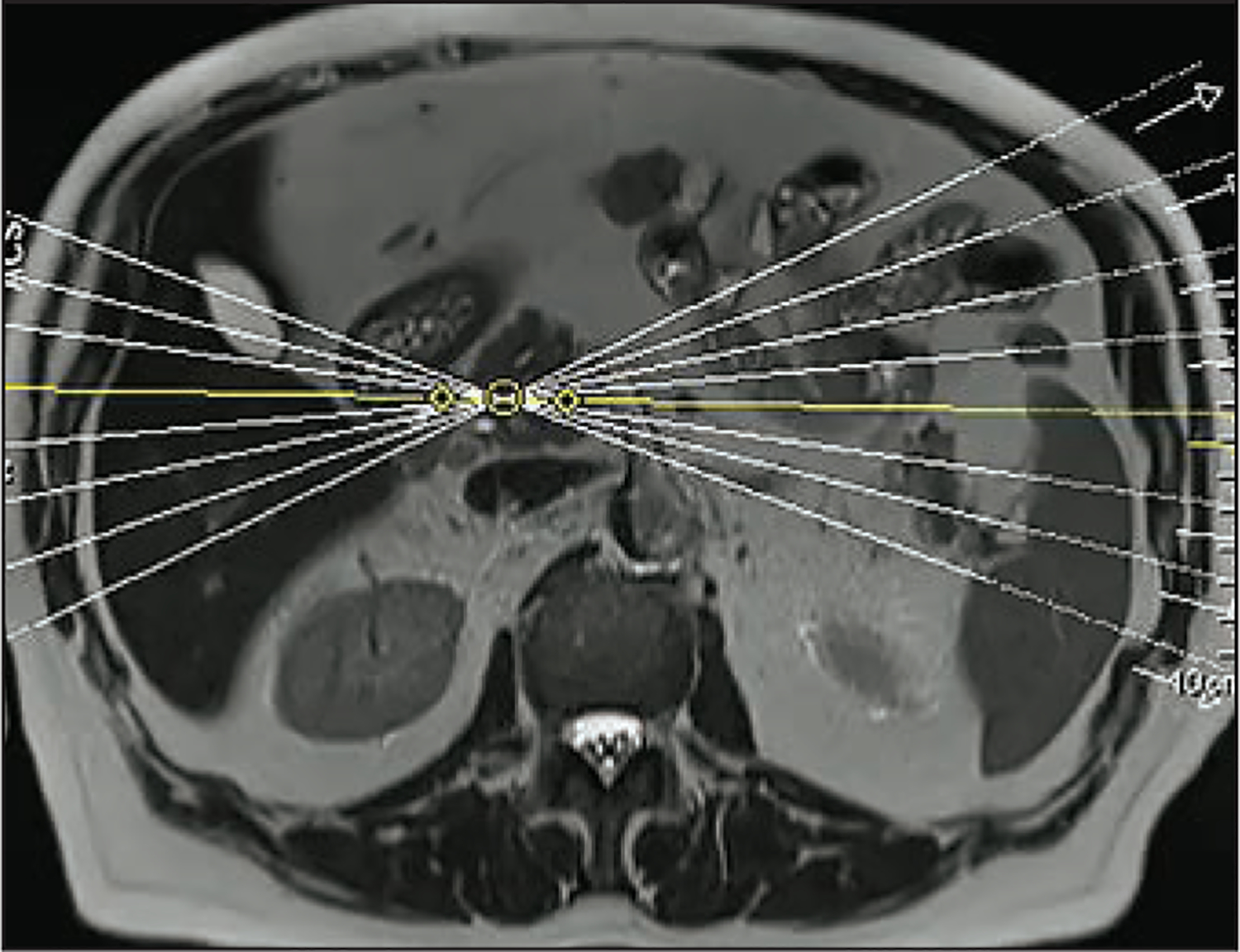
Example of setting planes for radial slab 2D MRCP sequences in a 44-year-old woman with chronic abdominal pain. Slabs can be chosen on an axial fast acquisition T2-weighted sequence. These should be centered on the distal common bile duct, each angled 5–10 degrees from one another.
Table 1.
Example of standard MRCP sequences at 1.5T
| Parameter | Radial HASTE Slab | Coronal T2-weighted 3D SPACE | Coronal HASTE slab (with secretin) |
|---|---|---|---|
| Pulse sequence | HASTE | SPACE | HASTE |
| TE (ms) | 756 | 698 | 603 |
| TR (ms) | 2000 | 2400 | 4500 |
| RBw | 300 | 352 | 352 |
| FOV (mm) | 290 | 360 | 300 |
| Slice thickness (mm) | 40 | 1 | 46 |
| Gap | 50% | 0% | 50% |
| Matrix | 256/100% | 384/98% | 384/70% |
| Saturation | Fat saturation | Fat saturation | Fat saturation |
| Flip angle | 180 | 140 | 180 |
RBw = Receive bandwidth
Table 2.
Example of standard MRCP sequences at 3T
| Parameter | Radial HASTE Slab | Coronal T2-weighted 3D SPACE | Coronal HASTE slab (with secretin) |
|---|---|---|---|
| Pulse Sequence | HASTE | SPACE | HASTE |
| TE (ms) | 622 | 699 | 749 |
| TR (ms) | 4500 | 2400 | 4500 |
| RBw | 383 | 319 | 161 |
| FOV (mm) | 300 | 380 | 300 |
| Slice thickness (mm) | 42 | 1.2 | 40 |
| Gap | 50% | 0% | 50% |
| Matrix | 384/80% | 320/82% | 384/75% |
| Saturation | Fat saturation | Fat saturation | Fat saturation |
| Flip Angle | 180 | 100 | 180 |
RBw = Receive bandwidth
Secretin and Patient Preparation
Secretin is a naturally occurring polypeptide hormone made up of 27 amino acids, originally discovered and described in 1902 [6]. Under physiologic conditions, its secretion is triggered as the duodenal mucosa senses increased acidity in the lumen, typically following emptying of gastric contents after a meal. The FDA has approved use of synthetic purified secretin peptide during ERCP via IV injection (ChiRhoStim; ChiRhoClin, Burtonsville, MD). Standard recommended dose from the manufacturer is 0.2 μg/kg of body weight, or approximately 16 μg IV administration in most adults. Pediatric use requires titrating the dose according to the child’s weight. Secretin administration is safe, with mild side effects such as nausea, abdominal pain, and flushing in only 0.5% of patients [7].
Secretin is short acting, with data from ERCP studies demonstrating increased pancreatic duct pressure within 1 minute and near complete relaxation within 5 minutes [8,9]. Bicarbonate rich fluid is released from the pancreatic ductal cells, increasing fluid signal in the pancreatic ducts that then progresses into the duodenum. To improve visualization of these small fluid-filled structures, we recommend that the patient fast for 4–6 hours prior to the examination to decrease contamination from preexisting gastrointestinal fluid signal as well as to diminish peristalsis and decrease motion artifact. The presence of ascites can also lead to substantial degradation of image quality. In these cases, it may be prudent to wait to image until the ascites has resolved. Paracentesis prior to MRCP may decrease fluid, but may not be practical.
Negative enteric contrast agents can also be considered to decrease fluid signal in the stomach. These are usually administered 10–20 minutes before acquiring MRCP sequences [10]. This may be performed using approximately 150–200 mL of pineapple or blueberry juice. However, patient compliance can be challenging with these sweet juices. In addition, because they contain high amounts of fructose, caution is required for diabetic patients.
Previously used oral agents containing iron were expensive and are generally no longer commercially available. The use of many other oral agents has been described [11]. Negative contrast agents demonstrate varying degrees of success at fluid suppression, and we do not recommend one agent over the others. Local practice patterns and convenience may drive the decision whether to use negative contrast as well as the decision of which agent to use.
S-MRCP Technique
Secretin is administered intravenously. The effects of secretin change dynamically over time, and peak response time may depend on non-physiologic factors such as injection time. As such, we recommend using long IV tubing to avoid repositioning the patient in the scanner. To avoid losing secretin dose in the long IV tubing, a saline flush of approximately 20 mL is recommended immediately following secretin administration.
We do not recommend routine administration of a test dose of secretin prior to imaging the patient. Given a very low observed rate of adverse reactions, the FDA stopped requiring a test dose in 2017. Patients who are or have been taking nutrition by mouth are exposed to endogenous secretin at each meal, and severe side effects would usually be apparent before the examination.
Following secretin administration, images should be acquired in a plane centered on the pancreaticobiliary ductal system. We recommend 2D heavily T2-weighted coronal slab images for evaluation of secretin-enhanced images. An angled coronal plane is most effective, which can be selected based on the radial slab that gave the best view of the main pancreatic duct (FIGURE 1) on previous MRCP images. Once this angle is chosen, it should remain the same throughout the secretin-enhanced images to improve visualization of dynamic changes in the pancreatic ducts.
Sequences that can be rapidly acquired (e.g., HASTE) allow for snapshot pictures of the pancreaticobiliary system as secretin takes effect. We recommend breath hold imaging every 30-60 seconds. This time range allows for titration with the patient’s ability to perform breath holds while still providing dynamic analysis. Secretin-enhanced evaluation with ERCP is performed over at least 15 minutes, and often as long as 45 minutes. This is generally not practical for MRI given time constraints. Our experience suggests that obtaining images for 8–9 minutes is a good compromise between efficiency and completeness.
IV agents such as hyoscine butylbromide (Buscopan) or glucagon may be used to attempt to decrease motion artifact and improve MRI or MRCP image quality [12]. However, we do not recommend routine use of these agents given that they decrease peristalsis and limit physiologic filling of the duodenum with fluid after secretin administration.
S-MRCP Interpretation
Secretin-enhanced images should be interpreted in concert with standard MRI and MRCP sequences. Secretin-specific analysis relies on the coronal slab 2D images obtained over 8–9 minutes and should start with an overall assessment of image quality. Pancreatic ductal morphology should be evaluated for anatomic variants, ductal side branches, and any cystic lesions that may communicate with the ducts. Table 3 provides considerations for reporting.
Table 3.
Reporting for secretin-enhanced MRCP
| IMAGING BEFORE SECRETIN ADMINISTRATION: | DYNAMIC SECRETIN-ENHANCED IMAGING: | PANCREATIC EXOCRINE FUNCTION: |
|---|---|---|
Pancreatic parenchyma:
|
Maximum diameter of main pancreatic duct:
|
Duodenal fluid:
|
S-MRCP interpretation should also include descriptions of main pancreatic duct (MPD) size over time. Secretin-induced fluid production leads to changes in MPD caliber, and changes as small as 0.5–1 mm may be appreciated. Given that the MPD varies in size as patients age [13], the specific diameter of the MPD at any given time point in the examination is not as important as the presence of change over time. Dynamic compliance represents the ability of the MPD to dilate and relax as secretin is given (FIGURE 2) and can be altered in a variety of pathologic states, as we subsequently describe. Therefore, it is important that S-MRCP reports provide an interpretation of this ability.
Figure 2.
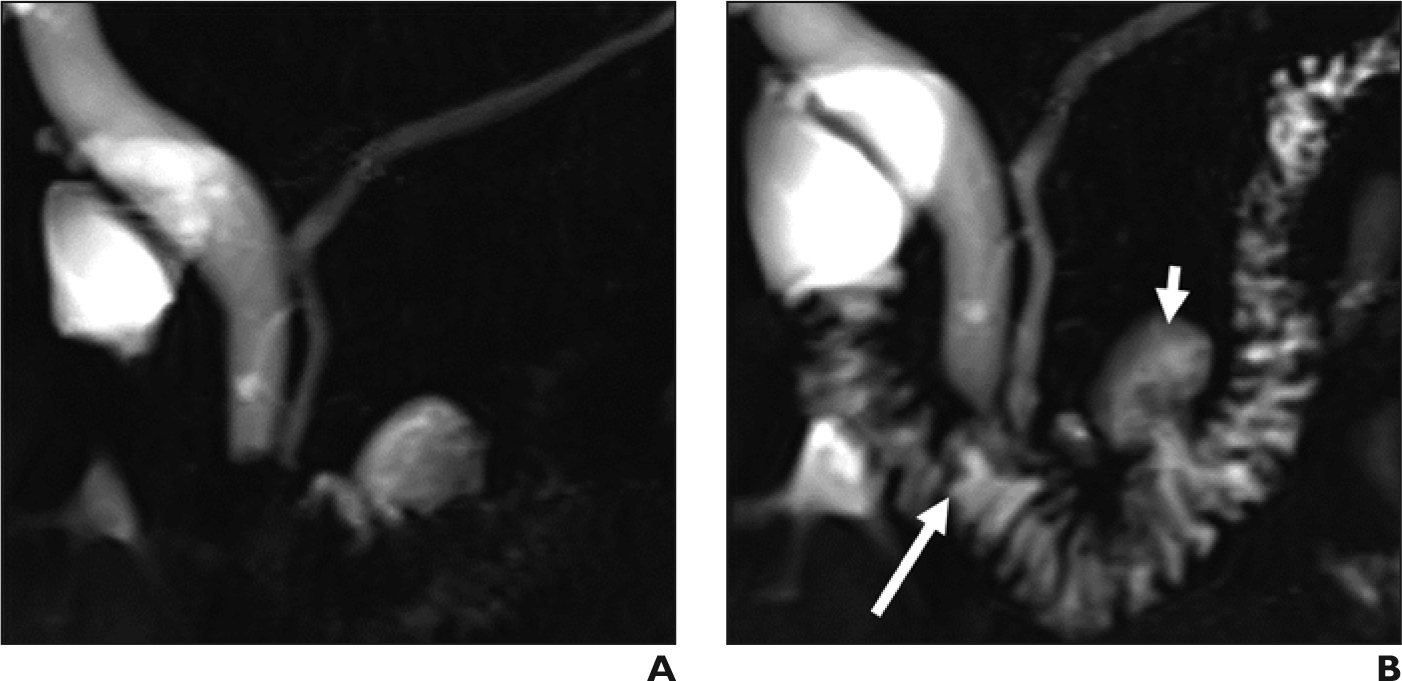
Expected dynamic compliance of the main pancreatic duct in 50-year-old woman with upper abdominal pain. (A) Coronal MRCP shows normal configuration of the main pancreatic duct with no visible dilated side branches. (B) Coronal secretin-enhanced MRCP obtained 5 minutes after secretin administration, showing expected dynamic dilation of the main pancreatic duct. Pancreatic fluid has started to fill the duodenal lumen (long arrow). Incidental note is made of a duodenal diverticulum (short arrow).
Complete reporting of S-MRCP should also include qualitative discussion of fluid volume in the duodenum as a marker of pancreas secretory reserve. Qualitative description of duodenal fluid after secretin administration can be performed using a grading scale originally described by Matos et al. [14] (FIGURE 3):
Grade 0—no increased fluid in duodenum
Grade 1—increased fluid in the duodenal bulb only
Grade 2—increased fluid to the proximal third duodenal segment
Grade 3—increased fluid in the distal third duodenal segment or beyond
While quantitative methods have been described for evaluating the volume of fluid in the duodenum after secretin administration [15], these are often impractical for normal radiology workflow. As such, we recommend subjective qualitative grading in S-MRCP reports.
Figure 3.
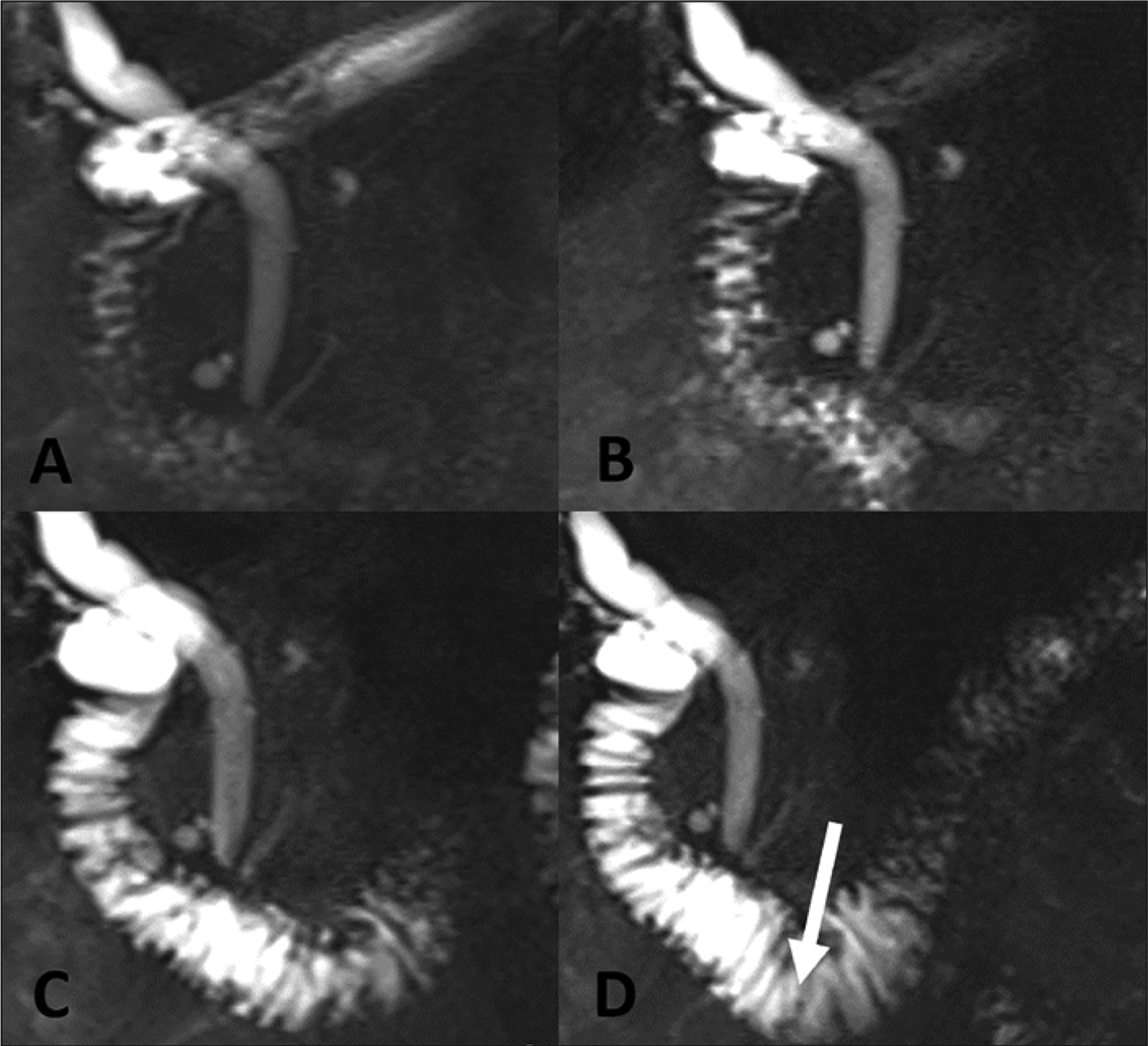
Expected filling of the duodenum with fluid after secretin administration, demonstrated in 48-year-old man with right upper quadrant pain. (A) MRCP before secretin administration shows the duodenum with minimal physiologic fluid. There is progressively increased fluid signal throughout the duodenum at 2 minutes (B) and 6 minutes (C). At 9 minutes (D), fluid is beyond the duodenal genu (arrow), consistent with grade 3 response to secretin.
Why We Use Secretin
Established Indications for Secretin
Pancreas Divisum—
Failure of fusion of the ventral and dorsal pancreatic ducts during embryonal development leads to pancreas divisum. Pancreas divisum is the most common ductal congenital variant and has a widely variable reported prevalence of between 3 and 22% [16–17]. The connection between pancreas divisum and both acute and chronic pancreatitis (CP), as well as between divisum and recurrent acute pancreatitis (RAP), remains controversial [18], with studies yielding conflicting results. Only 5% of patients with divisum develop CP, RAP, or chronic abdominal pain [19]. A recent study demonstrated that younger age and alcohol use significantly increased the risk of developing pancreatitis in the setting of divisum [20].
Flow of most of the pancreatic secretions through the minor papilla may place patients at higher risk for pancreas-associated pain due to increased upstream endoluminal pressure that eventually induces acute pancreatitis [4]. In addition, sphincterotomy and stent placement in patients with divisum have shown a response in symptoms in up to 76% of patients [21]. Association of pancreas divisum with RAP is currently being investigated in a multi-institutional clinical trial [22].
MRCP has been used to non-invasively evaluate ductal variants and can readily demonstrate direct communication between the dorsal pancreatic duct and the MPD (FIGURE 4). S-MRCP, however, has been shown to have better diagnostic performance in identifying pancreas divisum given that secretin-induced increased fluid excretion through the minor papilla may accentuate fluid signal in an otherwise small dorsal duct. A recent meta-analysis demonstrated sensitivity and specificity of up to 86% and 97% respectively, which were significantly better than those of standard MRCP (52% and 97%, respectively) [23]. Given this improved sensitivity, S-MRCP is well-suited for cases in which the diagnosis of pancreas divisum is unclear based on previous imaging or diagnostic workup.
Figure 4.
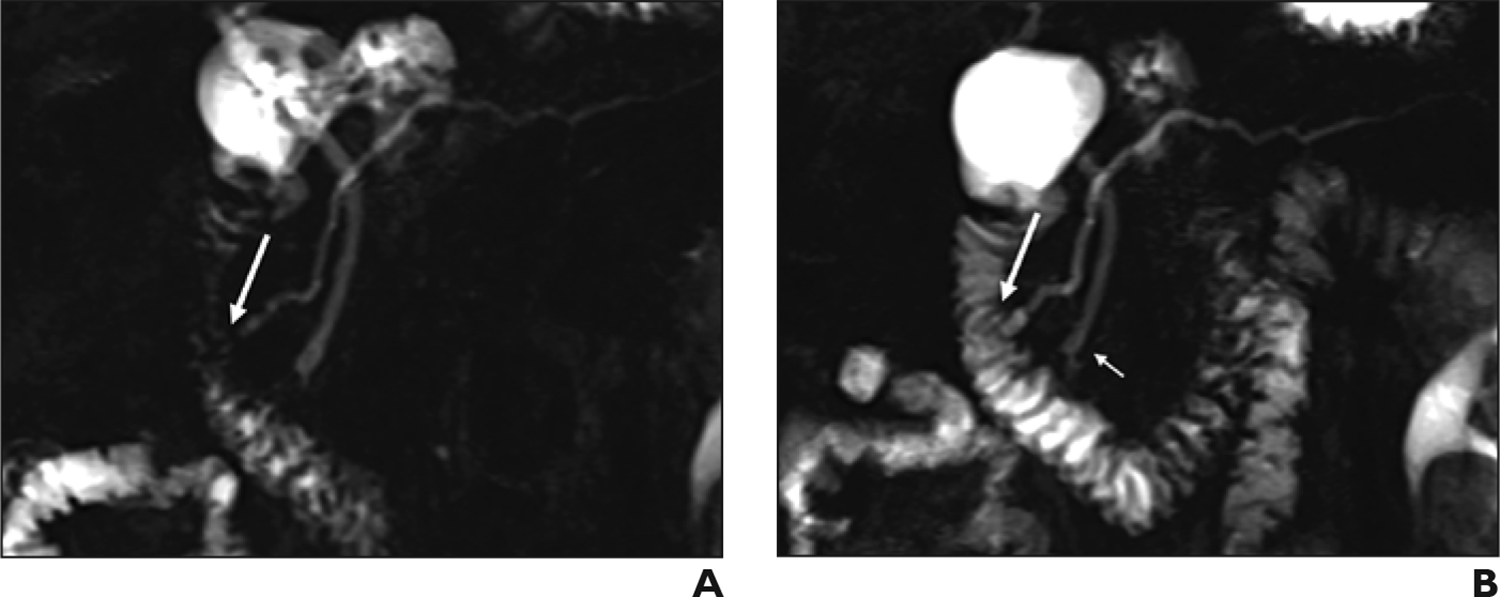
Pancreas divisum and Santorinicele, demonstrated in 76-year-old woman with chronic abdominal pain. (A) Coronal MRCP shows complete pancreas divisum. Common bile duct drains into the duodenum at the major papilla, while the dorsal pancreatic duct (duct of Santorini) drains entirely through the minor papilla (arrow). Ventral pancreatic duct is not visible. (B) Coronal secretin-enhanced MRCP shows saccular dilatation of the terminal portion of the dorsal duct, termed Santorinicele (long arrow). The ventral pancreatic duct becomes faintly visible (short arrow). Both findings were not visible before secretin administration.
Anomalous Pancreaticobiliary Junction—
Congenital malunion of the biliary and pancreatic ducts outside of the duodenal wall is the pathognomonic finding in anomalous pancreaticobiliary junction (ABPJ). In this rare condition, S-MRCP demonstrates the pancreatic and biliary ducts combining to form a long common channel that extends 15 mm or more in length [24] (FIGURE 5). This common channel allows for the possibility of bidirectional flow of pancreatic secretions, and S-MRCP has been shown to have a sensitivity of 85.7% and specificity of 68% for diagnosing reflux into the common bile duct [25]. APBJ can be seen in conjunction with pathologic dilation of the common bile duct, specifically type I choledochal cysts, but not all patients with APBJ have associated cystic dilation.
Figure 5.
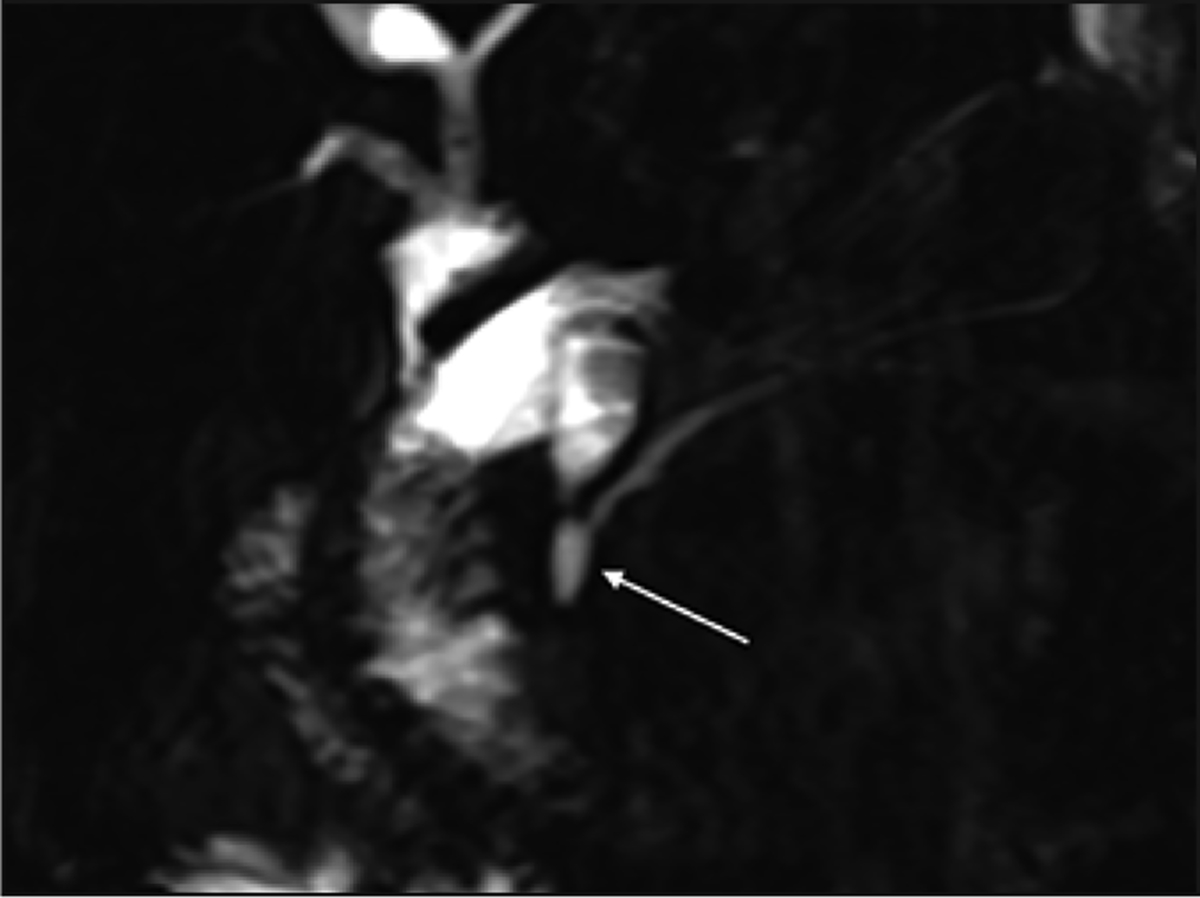
Anomalous pancreaticobiliary junction on secretin-enhanced MRCP, demonstrated in 22-year-old woman who is status post choledochal cyst resection and Roux-en-Y hepaticojejunostomy. Patient also has a long common channel secondary to anomalous junction of the common bile duct with the pancreatic duct (arrow).
While both ERCP and MRCP can identify ABPJ, MRCP has the advantage of being noninvasive. However, in cases where the common channel is less than 9 mm in length, ERCP may be needed to confirm the presence of ABPJ [26]. Identifying this variant is of clinical importance given that patients with ABPJ have an increased risk of up to 10.6% of developing biliary malignancy [27]. S-MRCP has the ability to evaluate APBJ in enough detail to demonstrate reflux of fluid signal into the common bile duct after secretin administration [28].
Santorinicele and Wirsungocele—
Saccular dilations of the dorsal or ventral pancreatic ducts at their insertions to the duodenal wall have been termed Santoriniceles or Wirsungoceles, respectively. Given expected age-related variability of MPD diameter, there is no specific measurement cutoff for these entities, with diagnosis based on focal saccular morphology and relative dilation. While these changes may be seen with standard MRCP, S-MRCP may be more sensitive to Santorinicele. One study demonstrated Santorinicele in 7/81 (8.6%) patients with standard MRCP and 20/81 (25%) patients with S-MRCP [29]. Detecting these entities is important given that sphincterotomy may be performed as an attempt to control symptoms in patients who have abdominal pain. S-MRCP may also be used to evaluate changes after minor-sphincterotomy, when decreases in Santorinicele size and dorsal duct diameter may be seen [29].
While Wirsungoceles are more commonly thought to be an incidental finding, a recent study suggested that Wirsungocele was significantly more likely to be present in patients with RAP than those without RAP [30]. Further evidence indicates that patients who have both pancreas divisum and a Santorinicele are at increased risk for RAP, possibly related to transient obstruction of the minor papilla [31] (FIGURE 4b).
Chronic Pancreatitis—
CP represents a progressive inflammatory condition of the pancreas, leading to fibrosis, parenchymal morphology changes, and distortion of expected pancreatic ductal morphology. Despite being a well-known condition, the mechanisms by which CP leads to fibrosis remain poorly understood, and the clinical course, symptoms, and imaging findings are variable. As such, there is ongoing work to define the evolution of CP [32,33], as well as to improve standardization of imaging technique and reporting [34].
In the early phase of inflammation associated with CP, pancreatic duct side branches may become enlarged and rounded, which allows for visualization by ERCP. This phenomenon led to development of the Cambridge criteria as a tool for characterizing disease severity [35]. These criteria were eventually modified for use with MRCP [36]. The modified Cambridge criteria for MRCP use the presence of 3 or more prominent side branches as a marker of mild CP, and then use abnormalities of the MPD to stratify moderate and severe CP [37]. However, even prominent ductal side branches may be difficult to visualize with standard MRCP given their small size. The ability of secretin to induce fluid signal in the pancreatic ducts may increase conspicuity of these side branches, improving visualization compared to standard MRCP [38,39]. A multi-institutional study demonstrated good agreement among subspecialized radiologists for assignment of Cambridge grade using S-MRCP (weighted kappa, 0.68) [40].
Early CP represents a diagnostic challenge given that the clinical findings and imaging findings with MRCP and endoscopic ultrasound may be heterogeneous and at times contradictory [41,42]. To this end, S-MRCP may play an important role in improving diagnostic certainty in early CP, such as in the setting of a normal MRCP with continued clinical suspicion for CP [43]. S-MRCP findings correspond with ERCP scoring for mild CP [44,45] and may delineate differences in duct morphology between mild, moderate, and severe CP [46].
S-MRCP has also shown a strong association with histopathologic pancreatic fibrosis score. A study demonstrated that the presence of two or more S-MRCP features (MPD irregularity, MPD dilation, MPD stenosis, or presence of ectatic side branches) had sensitivity of 65% and specificity of 89% for identifying non-fibrotic versus fibrotic glands and sensitivity of 88% and specificity 78% of for identifying severe fibrosis [47]. Evidence-based guidelines recommend the use of S-MRCP to increase the diagnostic utility of MRI in known or suspected CP [48].
As CP progresses to moderate or severe forms, it may lead to dilation, irregularity, and stricture of the MPD. This may lead to a loss of dynamic compliance of the MPD, which may be delineated with S-MRCP by diminished dilation of the MPD after secretin administration and delayed return to normal duct caliber [4].
Main Pancreatic Duct Stenosis—
Stenosis of the MPD may have malignant (e.g. pancreatic adenocarcinoma or neuroendocrine tumors) or benign (e.g. abdominal trauma, CP, autoimmune pancreatitis) causes. Inflammation associated with benign disease may lead to enlargement or distortion of the pancreatic parenchyma, and this parenchymal edema may complicate identification of the cause of MPD stenosis.
While MRI and standard MRCP may identify solid pancreatic masses, non-malignant entities may mimic solid lesions. In situations when the diagnosis is unclear, S-MRCP may help differentiate between benign and malignant causes of MPD stenosis by identifying the duct-penetrating sign (FIGURE 6). This sign refers to the presence of pancreatic duct signal within an area of mass-like enlargement of the pancreas. Malignant stenosis tends to lead to duct obstruction and a more abrupt cutoff of the MPD, and the duct-penetrating sign has been shown to be absent in up to 96% of these cases [49]. While useful, our experience suggests this sign alone is not sufficient to rule out a mass, and we recommend using S-MRCP findings in concert with other MRI and MRCP sequences to evaluate for pancreatic neoplasm.
Figure 6.
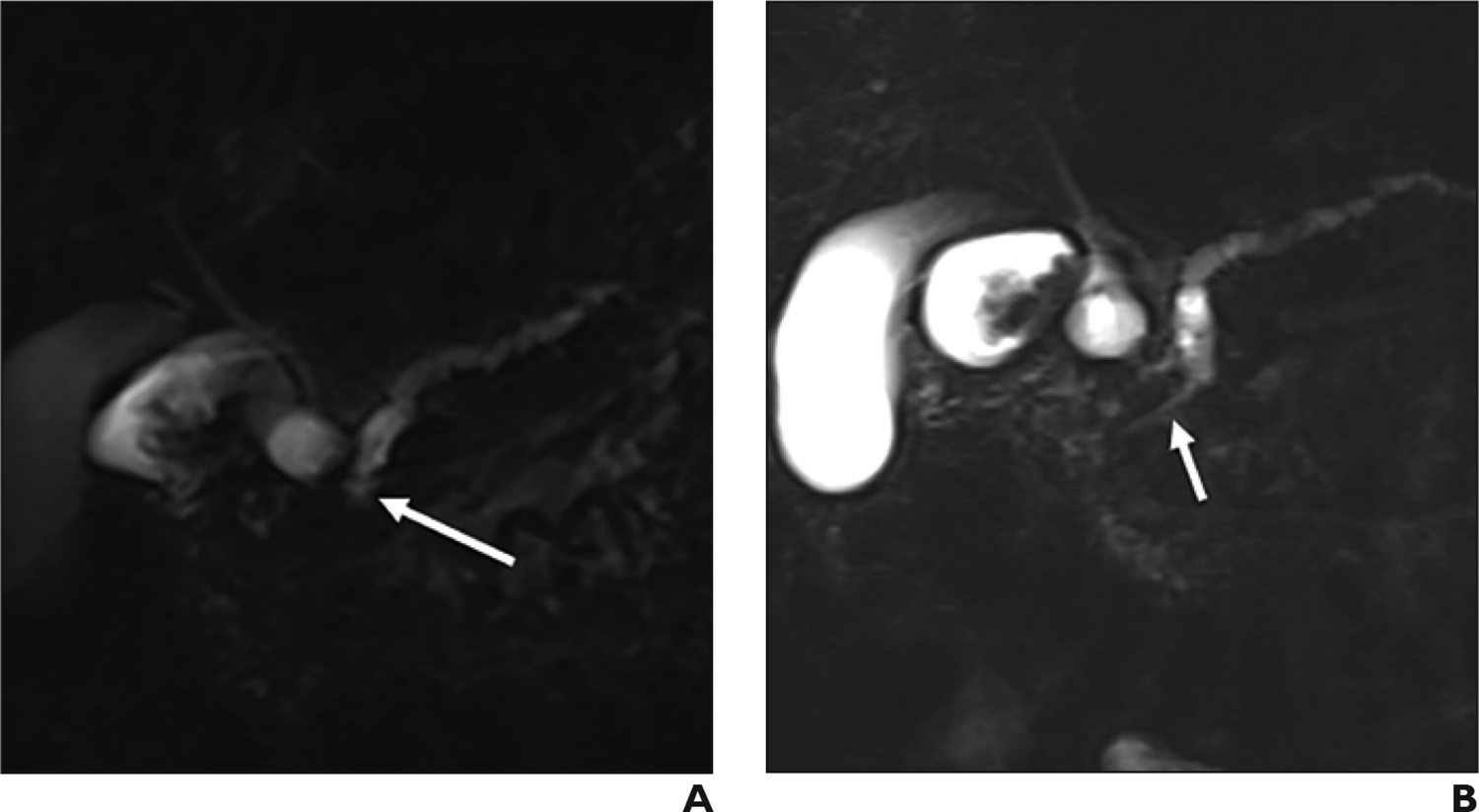
Duct penetrating sign in 58-year-old woman with history of alcohol-related pancreatitis and a dilated main pancreatic duct on prior CT. (A) Radial slab MRCP demonstrate an abrupt-appearing stricture of the main pancreatic duct (arrow). (B) Secretin-enhanced MRCP shows a narrow, but present, duct downstream to the stricture, consistent with the duct-penetrating sign. This proved to be a benign stricture related to chronic pancreatitis.
Post-Operative Evaluation of the Pancreas—
Partial pancreatectomy may be required in patients with malignant neoplasms as well as in some patients with CP for symptom management. This surgery has variable approaches but leads to changes in pancreatic ductal anatomy that often include a pancreatic-small bowel anastomosis. The changes in bowel anatomy may cause difficulty in evaluation of the pancreatic and biliary trees with ERCP, such that MRCP may be the preferred modality for follow-up in these patients [3].
Postoperatively, S-MRCP may delineate post-surgical anatomy and provide detailed visualization of anastomoses [50]. Fluid collections surrounding a postoperative pancreas may indicate a ductal leak, which can be seen on secretin-enhanced images [1] (FIGURE 7,8). In our experience, S-MRCP has limited sensitivity but excellent specificity in identifying ductal leak. While S-MRCP therefore should not be used to exclude a leak, the pancreatic ducts should be completely inspected on S-MRCP for communicating fluid collections.
Figure 7.
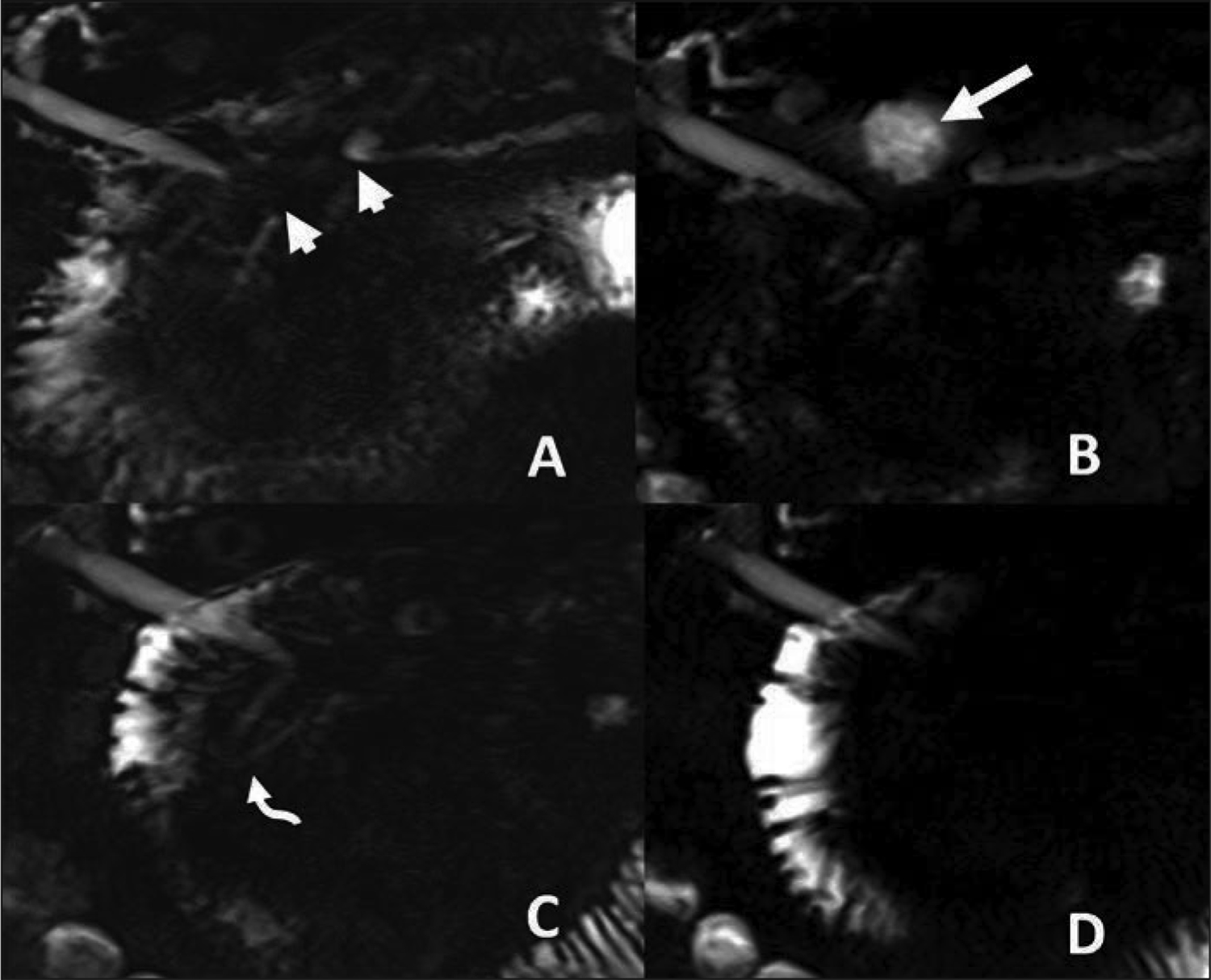
Main pancreatic duct leak 53-year-old man with multiple bouts of acute pancreatitis. A stricture of the main pancreatic duct (short arrows) is seen on MRCP before secretin administration (A). At 1 minute after secretin administration (B), fluid accumulates in the stomach, consistent with a pancreaticogastric fistula. Fluid progresses into the duodenum at 2 minutes (C) and 5 minutes (D). The downstream main pancreatic duct does not dilate (curved arrow) given flow of pancreatic fluid through the fistula.
Figure 8.
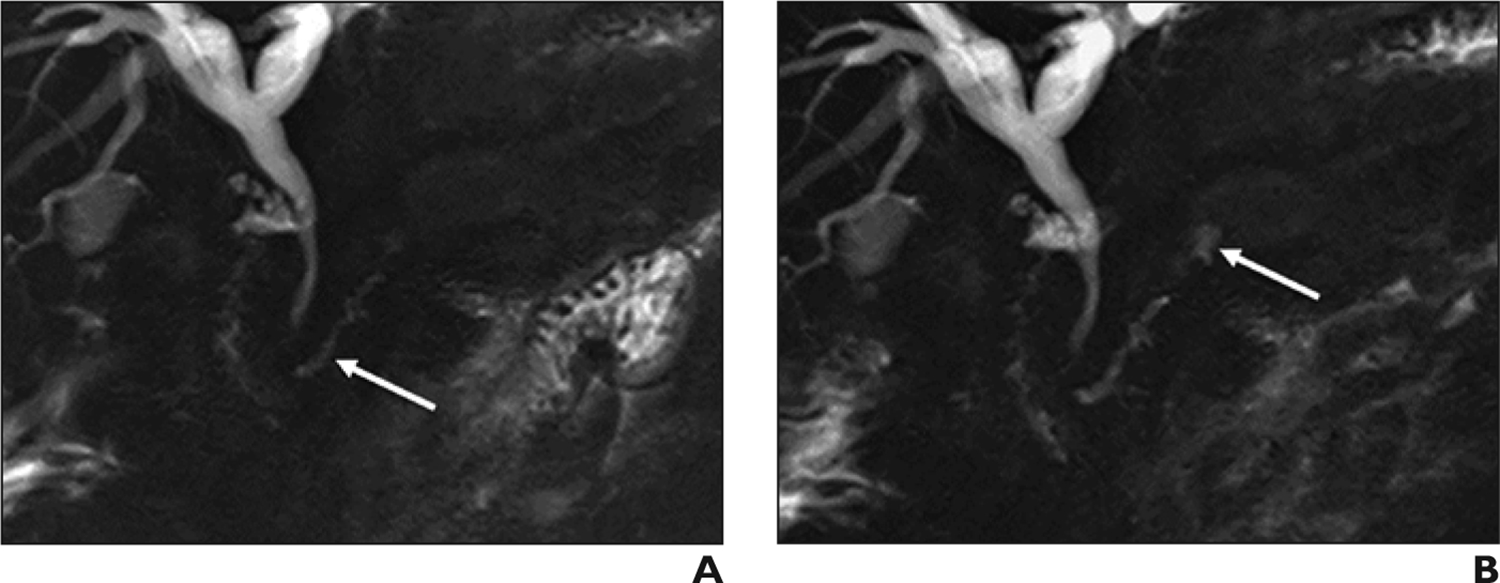
Postoperative pancreatic duct leak in 30-year-old man with history of distal pancreatectomy, splenectomy, and gastrojejunostomy. (A) Coronal MRCP before secretin administration shows remaining pancreatic duct (arrow). (B) Coronal secretin-enhanced MRCP shows a new fluid collection upstream to the remaining pancreatic duct, indicating a postoperative leak (arrow).
Visualization of the postsurgical environment by S-MRCP may establish a new baseline, which may be useful given that up to 29% of patients who undergo surgery for CP may need reoperation for recurrent abdominal pain [51]. Progressive dilation of the MPD and decreased excretion into the jejunum are common findings in anastomotic stenosis.
Asymptomatic Pancreatic Hyperenzymemia—
Patients who demonstrate prolonged elevations in serum levels of amylase and lipase without associated abdominal symptoms or known pancreatic disease are considered to have chronic asymptomatic pancreatic hyperenzymemia. Imaging in these patients may assist in identifying or ruling out pancreatic disease.
Imaging with S-MRCP identifies abnormalities in these patients more often than in healthy control patients [52] and depicts more underlying pancreatic disease than does standard MRCP [53]. The most common changes are dilated side branches, MPD dilation, and delayed emptying of the MPD. However, these changes may overlap with those found in CP, highlighting that patients may require further workup after imaging analysis.
Equivocal Indications for Use of Secretin
Other situations where S-MRCP may be performed have less robust or conflicting evidence to support their use. This section discusses some of these indications and provides the panel’s opinions to help guide practice.
Pancreatic Exocrine Function—
Normal digestion depends on adequate pancreatic secretion of fluid and enzymes. Pancreatic enzyme insufficiency is a known complication of CP, with up to half of all patients with CP developing exocrine dysfunction within 12 years of disease onset [54]. Exocrine insufficiency may be interrogated with evaluation of patient fecal fat content or through fecal elastase testing as a marker of the pancreas’s ability to secrete enzymes. While these methods may be clinically useful, they have limitations, including false-positive rates of up to 10% [55] and weaker association with mild and moderate CP [56].
Pancreatic exocrine function may also be assessed using shortened endoscopic pancreatic function testing in which pancreatic fluid is directly collected under endoscopy. This study is performed over 15–45 minutes to measure the entire pancreatic fluid reserve, and the exocrine function is determined by measuring the bicarbonate level in the collected fluid [57]. While this may be a more direct method of evaluation, it is invasive and requires specialized expertise to perform.
Secretin stimulation of the pancreas leads to increased fluid signal not only in the pancreatic ductal system, but also in the duodenal lumen as fluid is secreted through the papilla.
Semiquantitative grading and more thorough quantitative methods of pancreatic response to secretin are available [14, 58]. Early work on S-MRCP demonstrated that patients with severe CP had diminished fluid signal in the duodenum [39], and subsequent work showed a correlation of S-MRCP findings with fecal elastase and fecal fat measurements [59,60], including a correlation (r) of 0.79 with fecal elastase in one study [61]. Further, patients with abnormal endoscopic pancreatic function testing demonstrate significant decreases in pancreatic duct caliber change and duodenal filling after secretin administration [62]. In addition, quantitative changes in the ducts on S-MRCP are associated with abnormal endoscopic pancreatic function testing, including ductal non-compliance and side branch visualization.
Other studies have demonstrated a less clear association between S-MRCP and pancreatic exocrine function. In some cases, normal endoscopic pancreatic function testing may be present in the setting of S-MRCP findings that suggest CP [63]. One study evaluating traditional pancreatic function testing did not show any association between peak duodenal fluid bicarbonate secretion and S-MRCP duodenal filling at 10 minutes [64]. Secretin simulation may induce increased fluid from cells in the jejunum itself as well as the pancreas. Hafezi-Nejad et al. [65] demonstrated that fluid signal in the descending duodenum, however, was significantly lower in patients with CP than those without CP.
Acute Pancreatitis Versus Recurrent Acute Pancreatitis—
Imaging in the setting of acute pancreatitis often focuses on patients with a severe clinical course or seeks to identify an underlying cause in those patients without known risk factors [66]. Ultrasound can assist in identifying gallstones, and CT is often used to assess severity by identifying signs of inflammation, pancreatic enhancement, and associated peripancreatic fluid collections.
MRCP has a higher sensitivity than CT for identifying pancreaticobiliary anomalies and for evaluating for choledocholithiasis [67]. In the acute setting, the addition of secretin has a more limited role and should be reserved for the evaluation of specific clinical questions. A primary such question is the potential presence of a disconnected pancreatic duct or pancreatic duct leak, which is a concern when a pancreatic fluid collection along the duct is noted in the presence of viable upstream parenchyma. S-MRCP improves visualization of the MPD and may show active leak of fluid in the setting of duct disruption in a less invasive manner than can ERCP [68].
Patients who have more than one clinical bout of acute pancreatitis are considered to have RAP, a condition for which an underlying cause cannot be found in up to 30% of cases. Identifying a cause is important given that untreated RAP may lead to CP [69]. Underlying MPD stricture may be a cause of RAP, and increased pressure in the duct in patients with pancreas divisum with Santorinicele may also cause RAP. As such, S-MRCP provides effective non-invasive evaluation in the setting of RAP given that it delineates the MPD in excellent detail.
Sphincter of Oddi Dysfunction—
Diminished flow of fluid through the sphincter of Oddi may be caused by sphincter stenosis and/or sphincter dyskinesia. Over time, these conditions have been grouped under the umbrella term of sphincter of Oddi dysfunction (SOD) [70]. Data suggest that up to 13% of patients may have SOD following cholecystectomy, although patients with a gallbladder may also be diagnosed with SOD [4]. SOD is classified into three types, all of which include right upper quadrant and/or epigastric pain. Type I also includes liver enzyme abnormalities and structural change of the bile duct, type II includes enzyme or structural change, and type III includes only pain [71].
Gold standard diagnosis of SOD relies on sphincter manometry testing, an invasive endoscopic procedure. S-MRCP may assist in non-invasive evaluation of patients suspected of having SOD. Stenosis at the sphincter may lead to changes in dynamic compliance of the MPD, such as lack of relaxation of the MPD after secretin-enhanced dilation. Increased prominence of pancreatic duct side branches and/or acinarization may also be seen [Figure 9,10] [72, 73]. Data comparing manometry with S-MRCP show that imaging is more effective in type II disease (accuracy 73%) than in type III disease (accuracy 46%) [71]. This distinction is important given that patients with type II and III disease are more likely to benefit from therapeutic sphincterotomy.
Figure 9.
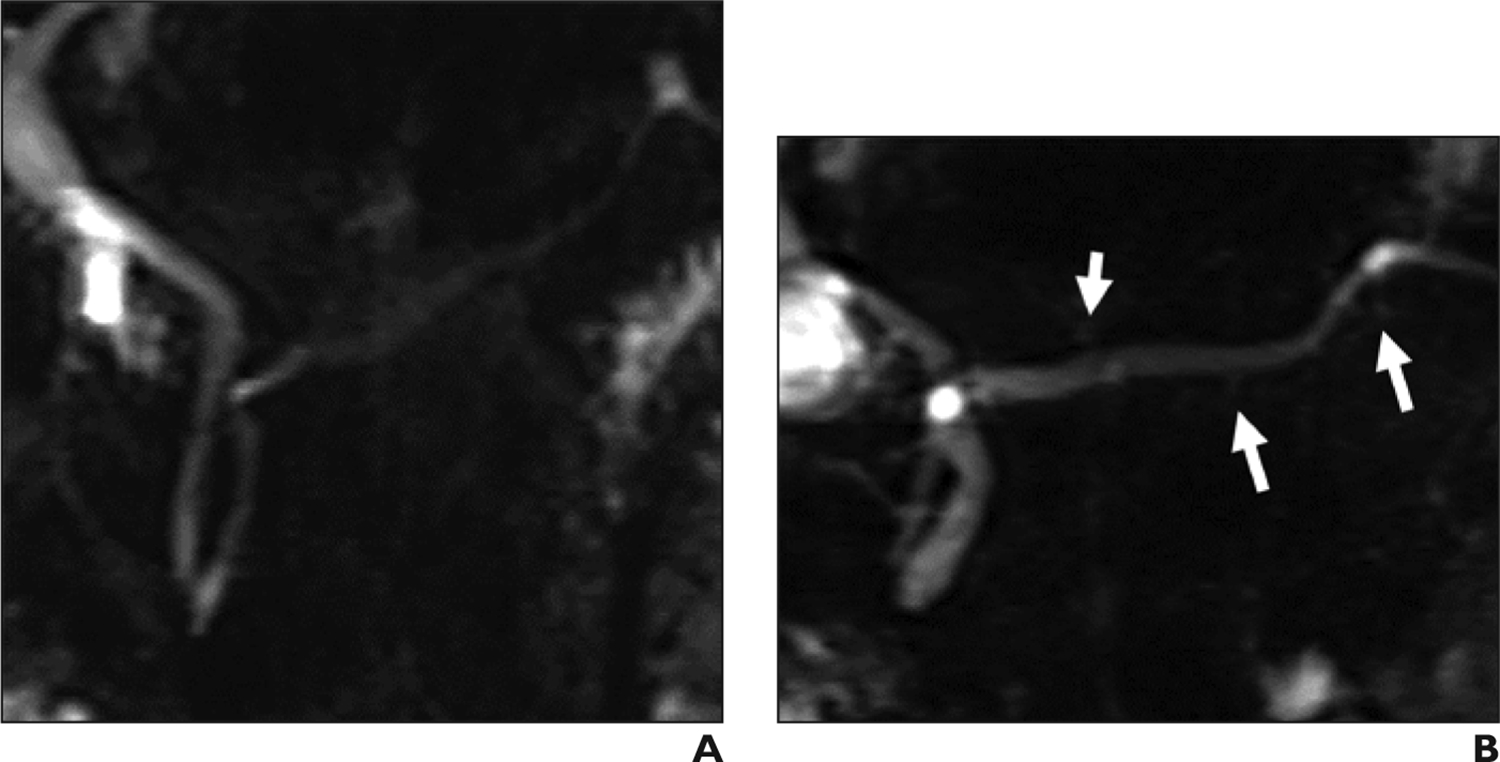
Imaging in sphincter of Oddi dysfunction in 45-year-old woman with biliary-type pain and abnormal findings on endoscopic sphincter manometry. (A) Coronal MRCP obtained before secretin administration shows nonspecific dilation of the common bile duct with smooth distal tapering. The main pancreatic duct measures up to 2 mm and does not show prominent side branches. (B) Coronal secretin-enhanced MRCP obtained 2 minutes after secretin administration shows an increase in main pancreatic duct diameter up to 5.5 mm. Multiple duct side branches show increased prominence (arrows).
Figure 10.
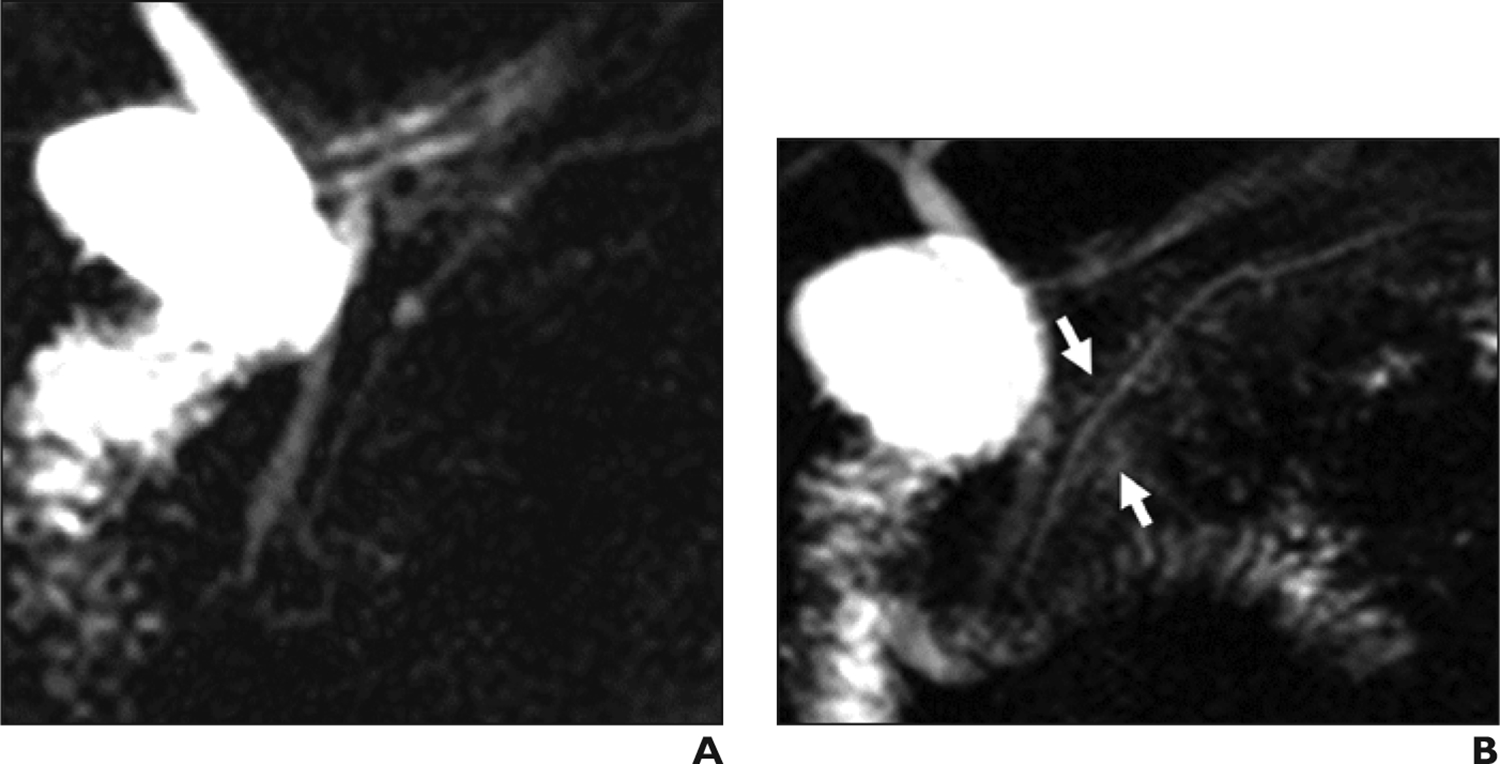
Imaging in sphincter of Oddi dysfunction (SOD) in 55-year-old man with right upper quadrant pain and clinical diagnosis of type II SOD. (A) Coronal MRCP obtained before secretin administration shows the main pancreatic duct without irregularity, dilation, or abnormal side branches. (B) Coronal MRCP obtained 3 minutes after secretin administration shows diffuse fluid signal surrounding the main pancreatic duct (arrows). This represents acinarization, or diffusely increased T2 signal surrounding the main pancreatic duct. Increased pressure in the main pancreatic duct is thought to lead to overfilling of pancreatic duct side-branches with fluid.
Pancreatic Neoplasms—
The utility of S-MRCP for pancreatic neoplasms differs for cystic versus solid tumors. Cystic tumors include intraductal papillary mucinous neoplasm as well as serous and mucinous cystadenomas and cystadenocarcinomas. Identifying communication between a cystic lesion and the MPD helps differentiate between intraductal papillary mucinous neoplasm and the other mucinous neoplasms, which have a higher malignant potential. Data are mixed on the utility of S-MRCP for this indication [74,75]. Conversely, solid neoplasms of the pancreas such as pancreatic adenocarcinoma or neuroendocrine tumor do not rely on communication with the MPD for their diagnosis, relying more heavily on contrast-enhanced imaging to identify the lesions and delineate their relationship with adjacent vessels.
Consensus Statements
High-quality S-MRCP requires attention to patient preparation, logistics of secretin administration, and dynamic secretin-enhanced MRCP acquisition.
Use of a reporting template is critical for standardizing S-MRCP interpretation, including for assessment of dynamic ductal compliance. The panel cannot recommend a single MPD diameter cutoff value as abnormal given age-related variation in MPD diameter.
S-MRCP serves as an imperfect indirect test of pancreatic exocrine reserve given that pancreatic juice excreted secondary to secretin stimulation does not directly reflect pancreatic function. A highly subjective scale that is dependent on technical factors is used in clinical practice. Quantitative methods are impractical within typical radiology workflow.
S-MRCP provides limited benefit in most cases of acute pancreatitis, unless there is a specific question such as to evaluate for pancreatic ductal leak or pancreas divisum before ERCP.
S-MRCP may be useful in the setting of RAP, for example to help diagnose underlying causes. Imaging should not be performed until at least 4–6 weeks after initial presentation to allow acute inflammatory changes to decrease or subside. The time length depends on the severity of the acute episode.
S-MRCP findings in SOD are nonspecific and may overlap with other findings of CP. S-MRCP is less accurate than endoscopic sphincter manometry, but may be used when noninvasive evaluation is preferred or when endoscopic evaluation is not available or impractical.
S-MRCP provides little benefit beyond standard MRCP in the setting of cystic pancreatic neoplasm, although may help accentuate visualization of side branch IPMN communication with the MPD.
Disclosure:
Dr. Tirkes is supported by National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers 1R01DK116963 and U01DK108323 (Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All other authors have no disclosures.
Footnotes
Publisher's Disclaimer: The publication of this Accepted Manuscript is provided to give early visibility to the contents of the article, which will undergo additional copyediting, typesetting, and review before it is published in its final form. During the production process, errors may be discovered that could affect the content of the Accepted Manuscript. All legal disclaimers that apply to the journal pertain. The reader is cautioned to consult the definitive version of record before relying on the contents of this document.
References
- 1.Tirkes T, Sandrasegaran K, Sanyal R, Sherman S, Schmidt CM, Cote GA, et al. Secretin-enhanced MR Cholangiopancreatography: Spectrum of Findings. RadioGraphics 2013; 33:1889–1906 [DOI] [PubMed] [Google Scholar]
- 2.Carbognin G, Pinali L, Girardi V, Casarin A, Mansueto G, Mucelli RP. Collateral branches IPMTs: secretin-enhanced MRCP. Abdom Imaging 2007;32:374–380. [DOI] [PubMed] [Google Scholar]
- 3.Akisik MF, Sandrasegaran K, Aisen AA, Maglinte DDT, Sherman S, Lehman GA. Dynamic secretin-enhanced MR cholangiopancreatography. RadioGraphics 2006;26(3):665–677. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi R, Mucelli RP. Secretin-enhanced MR imaging of the Pancreas. Radiology 2016;279(1):29–43 [DOI] [PubMed] [Google Scholar]
- 5.Hoeffel C, Azizi L, Lewin M, Laurent V, Aubé C, Arrivé L et al. Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. RadioGraphics 2006;26(6):1603–1620. [DOI] [PubMed] [Google Scholar]
- 6.Chey WY, Chang TM. Secretin, 100 years later. J Gastroenterol 2003;38(11):1025–1035. [DOI] [PubMed] [Google Scholar]
- 7.Ng SYL, Cheng CYY, Chow BKC. Secretin. In: Kastin AJ, ed. Handbook of biologically active peptides. 2nd ed. Waltham, Mass: Academic Press/Elsevier, 2013; 924–932. [Google Scholar]
- 8.Laugier R Dynamic endoscopic manometry of the response to secretin in patients with chronic pancreatitis. Endoscopy 1994; 26(2):222–227. [DOI] [PubMed] [Google Scholar]
- 9.Geenen JE, Hogan WJ, Dodds WJ, Stewart ET, Arndorfer RC. Intraluminal pressure recording from the human sphincter of Oddi. Gastroenterology 1980;78(2):317–324. [PubMed] [Google Scholar]
- 10.Manfredi R, Costamagna G, Brizi MG, Maresca G, Vecchioli A, Colagrande C et al. Severe chronic pancreatitis versus suspected pancreatic disease: dynamic MR cholangiopancreatography after secretin stimulation. Radiology 2000;214(3): 849–855. [DOI] [PubMed] [Google Scholar]
- 11.Frish A, Walter TC, Hamm B, Denecke T. Efficacy of oral contrast agents for upper gastrointestinal signal suppression in MRCP: A systematic review of the literature. Acta Radiol Open. 2017;6(9): doi: 10.1177/2058460117727315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamokova B, Bastati N, Poetter-Lang S, Bican Y, Hodge JC et al. The clinical value of secretin-enhanced MRCP in the functional and morphological assessment of pancreatic diseases. Br J Radiol. 2018;91(1084):20170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Swensson J, Hu M, Cui E, Tirkes T, Jennings SG, Akisik F. Distribution and correlation of pancreatic gland size and duct diameters on MRCP in patients without evidence of pancreatic disease. Abdom Radiol. 2019. March;44(3):967–975 [DOI] [PubMed] [Google Scholar]
- 14.Matos C, Metens T, Devière J, Nicaise N, Braudé P, et al. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology 1997; 203: 435–41 [DOI] [PubMed] [Google Scholar]
- 15.Bali MA, Sztantics A, Metens T, Arvanitakis M, Delhaye M, et al. Quantification of pancreatic exocrine function with secretin-enhanced magnetic resonance cholangiopancreatography: normal values and short-term effects of pancreatic duct drainage procedures in chronic pancreatitis. Initial results. Eur Radiol 2005; 15: 2110–21 [DOI] [PubMed] [Google Scholar]
- 16.Kin T, Shapiro AM, Lakey JR. Pancreas divisum: a study of the cadaveric donor pancreas for islet isolation. Pancreas. 2005; 30: 325–327. [DOI] [PubMed] [Google Scholar]
- 17.Liao Z, Gao R, Wang W, Ye Z, Lai XW, Want XT et al. A systematic review on endoscopic detection rate, endotherapy, and surgery for pancreas divisum. Endoscopy. 2009; 41: 439–444. [DOI] [PubMed] [Google Scholar]
- 18.Gutta A, Fogel E, Sherman S. Identification and management of pancreas divisum. Expert Rev Gastroenterol Hepatol. 2019;13(11):1089–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferri V, Vicente E, Quijano Y, Ielpo B, Duran H, Diaz E, Fabra I, Caruso R. Diagnosis and treatment of pancreas divisum: A literature review. Hepatobiliary Pancreat Dis Int 2019;18(4):332–336. [DOI] [PubMed] [Google Scholar]
- 20.Adike A, El Kurdi BI, Gaddam S, Kosiorek HE, Fukami N, Faigel DO, Collins JM, Ramirez FC. Pancreatitis in Patients With Pancreas Divisum. Pancreas 2017;46(10) [DOI] [PubMed] [Google Scholar]
- 21.Rustagi T, Golioto M. Diagnosis and therapy of pancreas divisum by ERCP: a single center experience. J Dig Dis. 2013;14(2):93–99 [DOI] [PubMed] [Google Scholar]
- 22.Coté GA, Durkalski-Mauldin VL, Serrano J, Klintworth E, Williams AW, Cruz-Monserrate Z et al. Sphincterotomy for Acute Recurrent Pancreatitis Randomized Trial: Rationale, Methodology, and Potential Implications. Pancreas. 2019;48(8):1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustagi T, Njei B. Magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a systematic review and meta-analysis. Pancreas. 2014. August;43(6):823–8. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama M, Atomi Y, Kuroda A. Pancreatic disorders associated with anomalous pancreaticobiliary junction. Surgery 1999; 126(3):492–497. [PubMed] [Google Scholar]
- 25.Motosugi U, Ichikawa T, Araki T, Kitahara F, Sato T, Itakura J, Fujii H. Secretin-stimulating MRCP in patients with pancreatobiliary maljunction and occult pancreatobiliary reflux: direct demonstration of pancreatobiliary reflux. Eur Radiol. 2007;17(9):2262–7 [DOI] [PubMed] [Google Scholar]
- 26.Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, et al. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol. 2015. March;50(3):273–9 [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M, Takada T, Miyakawa S, Tsukada K, Nagino M, Kondo S et al. Risk factors for biliary tract and ampullary carcinomas and prophylactic surgery for these factors. J Hepatobiliary Pancreat Surg 2008;15(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosoki T, Hasuike Y, Takeda Y, Michita T, Watanabe Y et al. Visualization of pancreaticobiliary reflux in anomalous pancreaticobiliary junction by secretin-stimulated dynamic magnetic resonance cholangiopancreatography. Acta Radiol. 2004;45(4):375–382 [DOI] [PubMed] [Google Scholar]
- 29.Boninsegna E, Manfredi R, Ventriglia A, Negrelli R, Pedrinolla B et al. Santorinicele: secretin-enhanced magnetic resonance cholangiopancreatography findings before and after minor papilla sphincterotomy. Eur Radiol. 2015;25(8):2437–44 [DOI] [PubMed] [Google Scholar]
- 30.Evrimler S, Swensson JK, Soufi M, Tirkes T, Schmidt CM, Akisik F. Wirsungocele: evaluation by MRCP and clinical significance. Abdom Radiol (2020). 10.1007/s00261-020-02675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costamagna G, Ingrosso M, Tringali A, Mutignani M, Manfredi R. Santorinicele and recurrent acute pancreatitis in pancreas divisum: diagnosis with dynamic secretin stimulated magnetic resonance pancreatography and endoscopic treatment. Gastrointest Endosc 2000;52(2):262–267. [DOI] [PubMed] [Google Scholar]
- 32.Whitcomb DC, Shimosegawa T, Chari ST, Forsmark CE, Frulloni L, Garg P et al. International consensus statements on early chronic pancreatitis: recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, American Pancreatic Association, Japan Pancreas Society, PancreasFest Working Group and European Pancreatic Club. Pancreatology 2018. May 21. pii: S1424–3903(18)30113–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav D, Park WG, Fogel EL, Li L, Chari ST, Feng Z et al. PROspective Evaluation of Chronic Pancreatitis for EpidEmiologic and Translational StuDies: Rationale and Study Design for PROCEED From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47(10):1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirkes T, Shah ZK, Takahasi N, Grajo JR, Chang ST, Venkatesh SK et al. Reporting Standards for Chronic Pancreatitis by Using CT, MRI, and MR Cholangiopancreatography: The Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Radiology 2018;290:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarner M, Cotton PB. Classification of pancreatitis. Gut 1984;25(7):756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreyer AG, Jung M, Riemann JF, Niessen C, Pregler B, Grenacher L et al. S3 guideline for chronic pancreatitis - diagnosis, classification and therapy for the radiologist. Röfo 2014;186:1002–1008 [DOI] [PubMed] [Google Scholar]
- 37.Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43(8):1143–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manfredi R, Costamagna G, Brizi MG, Maresca G, Vecchioli A, Colagrande C et al. Severe Chronic Pancreatitis versus Suspected Pancreatic Disease: Dynamic Cholangiopancreatography after Secretin Stimulation. Radiology 2000;215:848–855 [DOI] [PubMed] [Google Scholar]
- 39.Balci NC, Alkaade S, Magas L, Momtahen AJ, Burton FR. Suspected Chronic Pancreatitis with Normal MRCP: Findings on MRI in Correlation with Secretin MRCP. J Magn Reson Imaging 2008;27:125–131 [DOI] [PubMed] [Google Scholar]
- 40.Tirkes T, Shah ZK, Takahashi N, Grajo JR, Chang ST, Wachsman AM et al. Interobserver variability of radiologists for Cambridge classification of chronic pancreatitis using CT and MRCP: results from a large multi-center study. Abdom Radiol 2020;45(5):1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahuva R, Walsh RM, Kapural L, Stevens T. Morphologic Abnormalities Are Poorly Predictive of Visceral Pain in Chronic Pancreatitis. Pancreas 2013;42:6–10 [DOI] [PubMed] [Google Scholar]
- 42.Wilcox CM, Yadav D, Ye T, Gardner TB, Gelrud A, Sandhu BS et al. Chronic Pancreatitis Pain Pattern and Severity Are Independent of Abdominal Imaging Findings. Clinical Gastroenterology and Hepatology 2015;13:552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frojaker JB, Akisik F, Farooq A, Akpinar B, Dasyam A, Drewes AM et al. Guidelines for the Diagnostic Cross Sectional Imaging and Severity Scoring of Chronic Pancreatitis. Pancreatology 2018;18:764–773 [DOI] [PubMed] [Google Scholar]
- 44.Sai JK, Suyama M, Kubokawa Y, Watanabe S. Diagnosis of mild chronic pancreatitis (Cambridge classification): Comparative study using secretin injection-magnetic resonance cholangiopancreatography and endoscopic retrograde pancreatography. World J Gastoenterol 2008;14:1218–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen TM, Nilsson M, Gram M, Frojaker JB. Morphological and functional evaluation of chronic pancreatitis with magnetic resonance imaging. World J Gastoenterol 2013;19:7241–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanyal R, Stevens T, Novak E, Veniero JC. Secretin-Enhanced MRCP: Review of Technique and Application With Proposal for Quantification of Exocrine Function. Am J Roentgenol 2012;198:124–132 [DOI] [PubMed] [Google Scholar]
- 47.Trikudanathan G, Walker SP, Munigala S, Spilseth B, Malli A et al. Diagnostic Performance of Contrast-Enhanced MRI With Secretin-Stimulated MRCP for Non-Calcific Chronic Pancreatitis: A Comparison With Histopathology. Am J Gastroenterol 2015; 110:1598–1606 [DOI] [PubMed] [Google Scholar]
- 48.Lohr JM, Dominguez-Munoz E, Rosendahl J, Besslink M, Mayerle J, Lerch MM et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterology Journal 2017;5:153–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K et al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology 2001;221:107–116. [DOI] [PubMed] [Google Scholar]
- 50.Monill J, Pernas J, Clavero J, Farré A, Morales A, González M et al. Pancreatic duct after pancreatoduodenectomy: morphologic and functional evaluation with secretin-stimulated MR pancreatography. AJR Am J Roentgenol 2004;183 (5):1267–1274. [DOI] [PubMed] [Google Scholar]
- 51.Dhar VK, Levinsky NC, Xia BT, Abbott DE, Wilson GC, Sussman JJ et al. The natural history of chronic pancreatitis after operative intervention: The need for revisional operation. Surgery 2016;160:977–986 [DOI] [PubMed] [Google Scholar]
- 52.Testoni PA, Mariani A, Curioni S, Giussani A, Masci E. Pancreatic ductal abnormalities documented by secretin-enhanced MRCP in asymptomatic subjects with chronic pancreatic hyperenzymemia. Am J Gastroenterol. 2009. July;104(7):1780–6 [DOI] [PubMed] [Google Scholar]
- 53.Amodio A, Manfredi R, Katsotourchi AM, Gabbrielli A, Benini L, Mucelli RP et al. Prospective evaluation of subjects with chronic asymptomatic pancreatic hyperenzymemia. Am J Gastroenterol 2012;107:1089–1095 [DOI] [PubMed] [Google Scholar]
- 54.Diagnosis Lundkvist B. and treatment of pancreatic exocrine insufficiency. World J Gastroenterol 2013;19:7258–7266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majumder S, Chari ST. Chronic Pancreatitis. Lancet. 2016. May 7;387(10031):1957–66 [DOI] [PubMed] [Google Scholar]
- 56.Duggan SN, Chonchubhair HM, Lawal O, O’Conner DB, Conlon KC. Chronic pancreatitis: A diagnostic dilemma. World J Gastroenterol 2016;22:2304–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hart PA, Topazian M, Raimondo M, Cruz-Monserrate Z, Fisher WE, Lesinski GB et al. Endoscopic Pancreas Fluid Collection: Methods and Relevance for Clinical Care and Translational Science. Am J Gastroenterol 2016;111(9):1258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillams AR, Lees WR. Quantitative secretin MRCP (MRCPQ): results in 215 patients with known or suspected pancreatic pathology. Eur Radiol 2007;17(11):2984–2990. [DOI] [PubMed] [Google Scholar]
- 59.Manfredi R, Perandini S, Mantovani W, Frulloni L, Faccioli N, Mucelli RP. Quantitative MRCP assessment of pancreatic exocrine reserve and its correlation with faecal elastase-1 in patients with chronic pancreatitis. Abdominal Radiology 2012; 117:282–29 [DOI] [PubMed] [Google Scholar]
- 60.Schneider AR, Hammerstingl R, Heller M, Povse N, Murzinski L, Vogl T et al. Does Secretin-stimulated MRCP Predict Exocrine Pancreatic Insufficiency? A Comparison With Noninvasive Exocrine Pancreatic Function Tests. J Clin Gastroenterol 2006;40:851–855 [DOI] [PubMed] [Google Scholar]
- 61.Bian Y, Wang L, Chen C, Lu JP, Fan JB et al. Quantification of pancreatic exocrine function of chronic pancreatitis with secretin-enhanced MRCP. World J Gastroenterol. 2013; 19(41):7177–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balci NC, Smith A, Momtahen AJ, Alkaade S, Fattahi R, Tariq S, et al. MRI and S-MRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT). J Magn Reson Imaging. 2010. March;31(3):601–6 [DOI] [PubMed] [Google Scholar]
- 63.Alkaade S, Cem Balci N, Momtahen AJ, Burton F. Normal pancreatic exocrine function does not exclude MRI/MRCP chronic pancreatitis findings. J Clin Gastroenterol. 2008. September;42(8):950–5 [DOI] [PubMed] [Google Scholar]
- 64.Sainani N, Kadiyala V, Mortele K, Lee L, Suleiman S et al. Evaluation of Qualitative Magnetic Resonance Imaging Features for Diagnosis of Chronic Pancreatitis. Pancreas 2015;44(8):1280–1289 [DOI] [PubMed] [Google Scholar]
- 65.Hafezi-Nejad N, Singh VK, Faghih M, Kamel IR, Zaheer A. Jejunal response to secretin is independent of the pancreatic response in secretin-enhanced magnetic resonance cholangiopancreatography. Eur J Radiol. 2019. March;112:7–13 [DOI] [PubMed] [Google Scholar]
- 66.Murphy KP, O’Connor OJ, Maher MM. Updated imaging nomenclature for acute pancreatitis. AJR Am J Roentgenol 2014; 203:W464–W469. [DOI] [PubMed] [Google Scholar]
- 67.Sugiyama M, Haradome H, Atomi Y. Magnetic resonance imaging for diagnosing chronic pancreatitis. J Gastroenterol 2007;42(suppl 17):108–112. [DOI] [PubMed] [Google Scholar]
- 68.Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol 2006;186 (2):499–506. [DOI] [PubMed] [Google Scholar]
- 69.Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001;96(9):2540–2555. [DOI] [PubMed] [Google Scholar]
- 70.Hall TC, Dennison AR, Garcea G. The diagnosis and management of Sphincter of Oddi dysfunction: a systematic review. Langenbecks Arch Surg 2012;397(6):889–898. [DOI] [PubMed] [Google Scholar]
- 71.Pereira SP, Gillams A, Sgouros SN, Webster GJ, Hatfield AR. Prospective comparison of secretin-stimulated magnetic resonance cholangiopancreatography with manometry in the diagnosis of sphincter of Oddi dysfunction types II and III. Gut. 2007;56(6):809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cappeliez O, Delhaye M, Deviere J, Le Moine O, Metens T et al. Chronic pancreatitis: evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology 2000;215(2):358–64 [DOI] [PubMed] [Google Scholar]
- 73.Sandrasegaran K, Bodanapally U, Cote GA, Benzinger S, Patel AA et al. Acinarization (Parenchymal Blush) Observed During Secretin-Enhanced MRCP: Clinical Implications American Journal of Roentgenology 2014. 203:3, 607–614 [DOI] [PubMed] [Google Scholar]
- 74.Sahani DV, Kadavigere R, Blake M, Fernandez-del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi–detector row CT with 2D curved reformations—correlation with MRCP. Radiology 2006;238(2):560–569. [DOI] [PubMed] [Google Scholar]
- 75.Purysko AS, Gandhi NS, Walsh RM, Obuchowski NA, Veniero JC. Does secretin stimulation add to magnetic resonance cholangiopancreatography in characterizing pancreatic cystic lesions as side-branch intraductal papillary mucinous neoplasm? Eur Radiol 2014; 24: 3134–41. [DOI] [PubMed] [Google Scholar]


