Abstract
Background
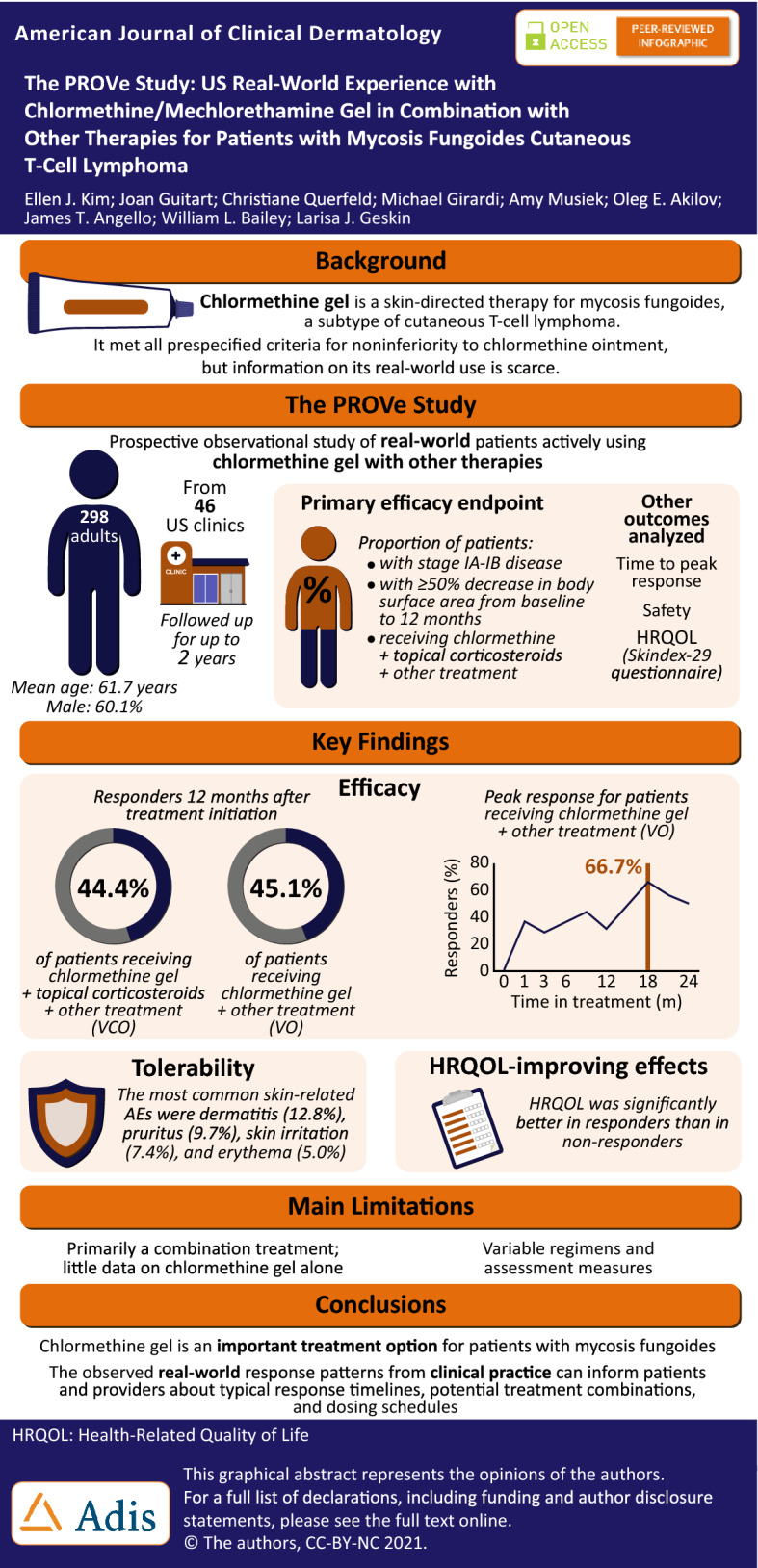
Chlormethine/mechlorethamine gel is a skin-directed therapy for patients with mycosis fungoides cutaneous T-cell lymphoma. Currently, real-world data on chlormethine gel are lacking.
Objective
Our objective was to analyze the effect of chlormethine gel in combination with other therapies on efficacy, safety, and health-related quality of life in a real-world setting.
Methods
This prospective, observational study enrolled adult patients actively using chlormethine gel. Patients were monitored for up to 2 years during standard-of-care clinic visits. No specific visit schedules or clinical assessments, with the exception of patient-completed questionnaires, were mandated because of the expected variability in practice patterns. The primary efficacy endpoint was the proportion of patients with stage IA–IB disease receiving chlormethine + topical corticosteroids + other with ≥ 50% decrease in body surface area from baseline to 12 months. Response was assessed at each visit using by-time analysis, which investigates the trend to treatment response and allows assessment of response over time. Health-related quality of life was assessed with the Skindex-29 questionnaire.
Results
In total, 298 patients were monitored. At 12 months post-treatment initiation, 44.4% (chlormethine + topical corticosteroids + other) and 45.1% (patients receiving chlormethine + other treatment) of efficacy-evaluable patients were responders. By-time analysis demonstrated that peak response occurred (chlormethine + other; 66.7%) at 18 months. There was a significant correlation between responder status and lower post-baseline Skindex-29 scores.
Conclusions
This real-world study confirmed that chlormethine gel is an important therapeutic option for patients with mycosis fungoides and contributes to reducing the severity of skin lesions and improving health-related quality of life.
Infographic
Key Points
| Treatment with chlormethine gel in combination with other therapies was effective and had a good tolerability profile in patients with mycosis fungoides in a real-world setting. |
| The peak response with chlormethine gel was observed at 18 months post-baseline, and quality of life was better in patients who responded to treatment. |
| The described response patterns in real-world clinical practice can inform patients and healthcare practitioners regarding typical response timelines, potential treatment combinations, and dosing schedules when using chlormethine gel. |
Introduction
Primary cutaneous lymphomas are non-Hodgkin lymphomas that present in the skin and include a heterogeneous group of cutaneous T-cell lymphomas (CTCLs). The most common type of CTCL is mycosis fungoides (MF), which is typically characterized by patches, plaques, and tumors and has an indolent clinical course during early disease stages [1, 2].
MF is a rare disease, and studies that detail its clinical course and how patients respond to treatment are scarce. The only curative option for MF is allogenic stem cell transplantation; current therapeutic strategies focus on local treatment of lesions, preventing progression of disease, minimizing long-term toxicity from treatment, and maintaining health-related quality of life (HRQOL). While numerous treatment choices are available, not all are specifically approved for MF. Recommended treatment options depend on disease stage. For patients with early-stage disease, skin-directed therapy is the main focus, including topical steroids, other topical treatments (imiquimod and retinoids), psoralen and ultraviolet A (PUVA), narrow-band ultraviolet B (nbUVB), and topical chemotherapy agents, such as chlormethine (also known as mechlorethamine) and carmustine [3–5].
Chlormethine is an alkylating agent that induces DNA damage and has been used as skin-directed therapy for MF for decades [6–9]. Early preparations of chlormethine were aqueous or ointment based and were only available as compounded agents. A chlormethine 0.016% w/w topical gel (mechlorethamine gel), equivalent to 0.02% chlormethine HCl, was approved in the USA for treatment of MF on the basis of results of the pivotal 201 study and 202 extension study (NCT00535470) [10–12]. Pharmacokinetic analysis has confirmed there is no systemic absorption of topically applied chlormethine gel [10, 11, 13, 14], so systemic drug interactions are unlikely.
In the pivotal registration trial, chlormethine gel met all prespecified criteria for noninferiority to chlormethine ointment. The primary endpoint of the study was response by Composite Assessment of Index Lesion Severity, and the response rates for chlormethine gel were higher than those for chlormethine ointment for both the intent-to-treat (58.5 vs. 47.7%) and efficacy-evaluable populations (76.7 vs. 58.9%). For the secondary endpoint, response per modified Severity-Weighted Assessment Tool, the response rates for chlormethine gel versus chlormethine ointment were 46.9 versus 46.2% for intent-to-treat and 63.3 versus 55.8% for efficacy-evaluable populations, respectively [11]. The PROVe (a PROspective, observational study assessing outcomes, adverse events [AEs], treatment patterns, and quality of life [QOL] in patients diagnosed with MF and treated with Valchlor and other therapies) study was designed to examine the real-world use of chlormethine gel in patients with MF in routine clinical practice across the USA. We have previously published patient clinical characteristics, treatment patterns, and preliminary safety data from the PROVe study [15]. Our preliminary results showed that chlormethine gel was used primarily in early-stage disease, often in combination with other therapies (the most common concomitant treatments were topical steroids, phototherapy, and oral bexarotene), and that treatment frequency varied [15]. Herein, we present final data regarding the clinical response, safety, and effect on HRQOL of chlormethine gel over a 2-year period.
Materials and Methods
Patients and Study Design
This was a multicenter, prospective, observational, US-based, noninterventional study with a total of 46 participating centers. All consecutive adult (≥ 18 years) patients with a diagnosis of MF who were being treated with chlormethine gel could enroll in the study. Patients who were using chlormethine gel for less than 1 month prior to enrollment were considered to be newly initiating treatment. Patients were included regardless of disease stage or previous and concomitant therapy received before or at the time of enrollment, and no exclusion criteria were applied.
Patients were monitored during routine clinical practice and prospectively followed for a maximum of 2 years regardless of whether chlormethine gel had been discontinued. Study end was defined as patient withdrawal of consent, loss to follow-up, death, physician’s decision, study completion, or study discontinuation. Clinical characteristics, medical history, prior MF therapies, treatment patterns, concomitant therapies, clinical response, and AEs were recorded when available. No specific visit schedules or clinical assessments were mandated because of the expected variability in practice patterns, with the exception of protocol-required patient-completed HRQOL questionnaires.
The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. The appropriateness of the study protocol and all risks and benefits to participants were approved by institutional review boards. All patients provided written informed consent.
Evaluations
As specified in the statistical analysis plan, prior to conducting any analyses, four patient groups were identified on the basis that there were at least 30 patients per group who received a predominant concomitant MF therapy in combination with chlormethine gel. The numbers of patients on chlormethine gel monotherapy or chlormethine gel + topical corticosteroids only were too low to permit statistical analysis. Given this, the groups analyzed for response were as follows: chlormethine + topical corticosteroids + other (VCO; n = 185), chlormethine + phototherapy + other (VPO; n = 76), chlormethine + oral bexarotene + other (VOB; n = 47), and chlormethine + any other treatment (VO; n = 298). The term “other” encompassed multiple, overlapping therapies. This overlap exists as a result of patients frequently being treated with multiple therapies sequentially in real-world clinical practice and explains why the sum of the groups exceeds the total number of monitored patients (n = 298). The predominance of the first two concomitant treatments was the rationale for creating the VCO, VPO, and VOB subgroups for analysis, and VO was a catch-all group. The primary and other specific therapies (used in ≥ 10% of patients at enrollment) in the different groups follow. For the VCO group: chlormethine gel (100%), corticosteroids (100%), phototherapy nbUVB (42.2%), oral bexarotene (29.2%), PUVA (22.7%), local electron-beam therapy (EBT, 19.5%), topical bexarotene (14.1%), and imiquimod (10.3%). For the VPO group: chlormethine gel (100%), phototherapy nbUVB (77.6%), corticosteroids (71.1%), PUVA (32.9%), oral bexarotene (25.0%), UVB (22.4%), imiquimod (13.2%), and acitretin (13.2%). For the VOB group: chlormethine gel (100%), oral bexarotene (100%), corticosteroids (78.7%), phototherapy nbUVB (44.7%), local EBT (27.7%), PUVA (23.4%), total skin EBT (TSEBT, 21.3%), romidepsin (14.9%), imiquimod (12.8%), topical bexarotene (10.6%), and extracorporeal photopheresis (10.6%). For the VO group: chlormethine gel (100%), corticosteroids (73.8%), phototherapy nbUVB (38.6%), oral bexarotene (24.2%), PUVA (20.1%), topical bexarotene (15.8%), and local EBT (14.1%).
The primary efficacy endpoint was the proportion of patients in the VCO group with current stage IA–IB disease and evaluable clinical response data who responded to treatment, defined as a ≥ 50% reduction from baseline in the body surface area percentage (%BSA) at 12 months (365 ± 90 days). Patients who had discontinued chlormethine gel permanently were not included in the primary analysis. Secondary efficacy endpoints included %BSA response rates at 12 months in the VPO, VOB, and VO groups, and a by-time analysis of the %BSA response rates at 1, 3, 6, 9, 12, 15, 18, 21, and 24 months (± 45 days) in all patient groups. In addition, the overall response rate (ORR)-2 was determined, defined as the proportion of patients with a ≥ 50% reduction from baseline in %BSA for two consecutive visits.
Safety was evaluated by recording AEs and serious AEs at every visit and determining their relation to chlormethine gel treatment. An on-study event was defined as occurring on the day of enrollment or later but prior to study discontinuation or completion. HRQOL was determined using the Skindex-29 questionnaire, which comprises results from three subscales: emotions, symptoms, and functioning (all scored 0–100) [16, 17]. Higher scores for the Skindex-29 indicate lower QOL.
Statistical Analysis
Separate analyses were conducted for patients with stage IA–IB disease, all staged patients, and all patients regardless of staging. Response over time was assessed using by-time analysis, a statistical approach where the number of patients exceeding the responder threshold of ≥ 50% reduction from baseline in %BSA score is divided by the number of patients providing clinical response data (patients with data) at each individual post-enrollment visit. As patient visits were variable, the by-visit analyses of changes from baseline were assigned to the closest time points and included data that fell within ± 45 days of each designated time point. Not all patients had data available for each time point, resulting in a variation in the number of evaluable patients for each time point and patient group. The by-time analysis of clinical response explores the treatment response trends over the course of the study. The aim of this analysis was to obtain information about response over time, which provides complementary data to the ORR and can help visualize the changing response rates during treatment.
Results
Patients
In total, 301 patients were registered, one was not enrolled and two were ineligible for the study; the remaining 298 patients were monitored. Baseline demographics and clinical characteristics are shown in Table 1. The mean age was 61.7 years, 60.1% of patients were male, and the mean MF duration was 5.5 years. At enrollment, 186 (62.4%) patients had early-stage disease (IA–IIA), 25 (8.4%) had advanced-stage disease (IIB–IV), and current staging information was not available for 87 (29.2%) patients. Most patients used chlormethine gel on a daily basis (74.5%), whereas others used chlormethine gel on a less frequent basis or used different dosing regimens over time (Table 2). The reasons for different dosing frequencies during treatment were physician decision, complete response, and AEs.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic | VCO (n = 185) | VPO (n = 76) | VOB (n = 47) | VO (n = 298) |
|---|---|---|---|---|
| Age (years) | 63.2 ± 12.6 | 62.5 ± 11.3 | 65.3 ± 12.4 | 61.7 ± 13.5 |
| Sex | ||||
| Female | 75 (40.5) | 28 (36.8) | 22 (46.8) | 119 (39.9) |
| Male | 110 (59.5) | 48 (63.2) | 25 (53.2) | 179 (60.1) |
| Duration of MF (years) | 5.2 ± 6.3 | 5.2 ± 6.0 | 4.3 ± 4.1 | 5.5 ± 6.8 |
| Months from diagnosis to enrollment, mean (minimum; maximum) | 36.6 (0.9; 210.2) | 34.2 (0.9; 140.7) | 42.6 (1.9; 210.2) | 35.8 (0.6; 210.2) |
| TNMB classification | ||||
| IA | 60 (32.4) | 21 (27.6) | 7 (14.9) | 105 (35.2) |
| IB | 49 (26.5) | 27 (35.5) | 12 (25.5) | 75 (25.2) |
| IIA | 1 (0.5) | 3 (3.9) | 2 (4.3) | 6 (2.0) |
| IIB | 11 (5.9) | 5 (6.6) | 6 (12.8) | 15 (5.0) |
| III–IV | 6 (3.2) | 3 (3.9) | 6 (12.8) | 10 (3.4) |
| Missing/unknown | 58 (31.4) | 17 (22.4) | 14 (29.8) | 87 (29.2) |
Data are presented as mean ± standard deviation or n (%) unless otherwise indicated
MF mycosis fungoides cutaneous T-cell lymphoma, TNMB tumor-node-metastasis-blood, VCO chlormethine gel + topical corticosteroids + any other treatment, VO chlormethine gel + any other treatment, VOB chlormethine + oral bexarotene + other, VPO chlormethine + phototherapy + other
Table 2.
Dosing frequency and interruptions
| Characteristic | PROVe (n = 298) |
|---|---|
| Dosing frequencya | |
| Daily | 222 (74.5) |
| Five times a week | 30 (10.1) |
| Every 2 days | 112 (37.6) |
| Every 3 days | 49 (16.4) |
| Once a week | 26 (8.7) |
| Less frequent/unknown | 34 (11.4) |
| Patients with dosing interruptionb | 87 (29.2) |
| Average duration of dosing interruption, days | 9.7 (1.0–84.0) |
Data are presented as n (%) or median (range)
aPercentages exceed 100% because patients could have multiple dosing regimens over time; patients with multiple records for dosing were counted in each relevant category
bDosing interruption is defined as dosing that was stopped and restarted within 3 months
The study was completed by 188 (62.5%) patients. Reasons for early study termination were AEs (n = 9), loss to follow-up (n = 5), withdrawal of consent (n = 20), physician’s decision (n = 24), and termination of the study by the sponsor (n = 54). During the study, 77.9% of patients used other skin-directed therapies in combination with chlormethine gel, and 30.2% of patients received systemic therapies. The most common concomitant skin-directed therapy was topical corticosteroids (60.1%); the most common systemic therapy was oral bexarotene (16.1%). The median duration of treatment with chlormethine gel was 24 months for newly initiated patients and 32 months for those who had been on chlormethine gel for ≥ 3 months at time of enrollment [14]. Chlormethine gel was continued for at least 12 months by 190 (63.8%) patients and for 24 months by 134 (45.0%) patients. In total, 87 patients (29.2%) had a dosing interruption during the study, with a median duration of 9.7 days (range 1–84). The cumulative exposure to chlormethine gel and other treatment is summarized in Table 3.
Table 3.
Cumulative exposure duration to chlormethine gel and other treatments ≥5% post-enrollment (n = 298)
| Treatment received by patients | For any time | For ≥3 months | For ≥6 months | For ≥12 months | For ≥24 months |
|---|---|---|---|---|---|
| Chlormethine gel | 298 (100) | 264 (88.6) | 234 (78.5%) | 190 (63.8) | 134 (45.0) |
| Skin-directed therapy | 232 (77.9) | 216 (72.5) | 206 (69.1) | 198 (66.4) | 155 (52.0) |
| Corticosteroids, topical | 179 (60.1) | 168 (56.4) | 160 (53.7) | 156 (52.3) | 120 (40.3) |
| Other topical | 31 (10.4) | 26 (8.7) | 24 (3.0) | 24 (8.1) | 17 (5.7) |
| Phototherapy | 61 (20.5) | 54 (18.1) | 54 (18.1) | 50 (16.8) | 36 (12.1) |
| Retinoids, topical, bexarotene | 17 (5.7) | 14 (4.7) | 14 (4.7) | 12 (4.0) | 7 (2.3) |
| Systemic therapy | 90 (30.2) | 82 (27.5) | 78 (26.2) | 66 (21.8) | 48 (16.1) |
| Other systemic | 25 (8.4) | 14 (4.7) | 13 (4.4) | 11 (3.7) | 8 (2.7) |
| Retinoids, systemic, bexarotene | 48 (16.1) | 44 (14.8) | 41 (13.8) | 35 (11.7) | 25 (8.4) |
Data are presented as n (%)
Efficacy
The study design did not mandate collection of specific response scores, and modified Severity-Weighted Assessment Tool scores were not documented universally during the study. As the most common response-assessment method used during this observational study, %BSA was analyzed as a surrogate measurement of response. The %BSA does not take severity of lesions into account and can result in significant underestimation of the response rate. Limited clinical differences were observed between the different analysis groups; therefore, only data from patients with stage IA–IB disease in the VO and VCO groups are reported. In patients with stage IA–IB disease who had %BSA data recorded, the ORR at 12 months was 44.4% (24/54) in the VCO group and 45.1% (37/82) in the VO group. The ORR2 for patients with stage IA–IB disease was 43.5% (67/154) in the VO group.
A by-time analysis of the %BSA response data showed that clinical responses occurred as early as 1 month (36.7%) after treatment, and the peak response occurred at 18 months for patients with stage IA–IB disease in the VO group (66.7%) (Fig. 1).
Fig. 1.
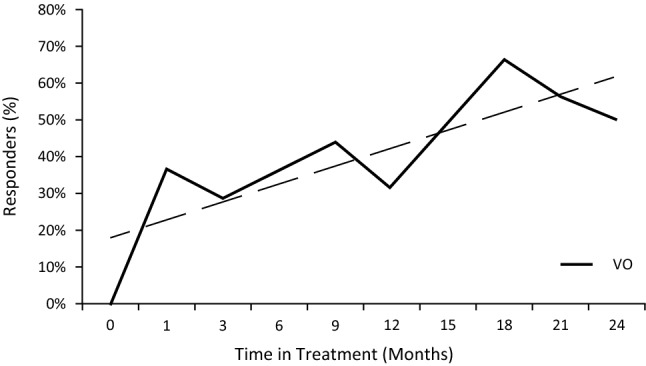
Clinical response in patients with mycosis fungoides cutaneous T-cell lymphoma. By-time analysis of the percentage body surface area response rate (proportion of patients with ≥50% reduction from baseline) of patients with stage IA–IB disease receiving chlormethine gel + any other treatment (VO) with trend line
For the Skindex-29 analysis, all patients were included (n = 298) and results were compared between responders and nonresponders at 24 months post-baseline. The mean Skindex-29 subscale scores indicated mild (symptoms and functioning) and mild to moderate (emotions) impairment of HRQOL. The post-baseline weighted mean subscale scores for responders (emotions 26.6; symptoms 25.3; functioning 13.3) showed a better HRQOL than for nonresponders (emotions 36.2; symptoms 34.4; functioning 21.2). The differences between responders and nonresponders were statistically significant (p < 0.001 for each subscale).
Safety
Overall, 125 (41.9%) patients experienced at least one AE, and 83 (27.9%) experienced treatment-related AEs. The most common skin-related AEs were dermatitis (12.8%), pruritus (9.7%), skin irritation (7.4%), and erythema (5.0%) (Table 4). No treatment-related serious AEs or deaths were reported.
Table 4.
Skin-related adverse events occurring in ≥ 3% of patients
| Adverse event | All patients (n = 298) | |
|---|---|---|
| All | Chlormethine gel related | |
| Dermatitis | 38 (12.8) | 37 (12.4) |
| Pruritus | 29 (9.7) | 22 (7.4) |
| Skin irritation | 22 (7.4) | 21 (7.0) |
| Erythema | 15 (5.0) | 12 (4.0) |
| Skin burning sensation | 11 (3.7) | 10 (3.4) |
| Rash | 10 (3.4) | 4 (1.3) |
Data are presented as n (%)
Discussion
This 2-year observational study examined the use of chlormethine 0.016% w/w gel in real-world clinical practice in the USA. The study revealed that chlormethine gel was primarily used in early-stage MF, often in combination with other topical and systemic MF therapies; treatment frequency varied from once per week to daily, with frequent treatment interruptions.
The most commonly coadministered therapies were corticosteroids, phototherapy, and oral bexarotene. The use of chlormethine gel in combination with other therapies resulted in clinical improvement as measured by a ≥ 50% reduction from baseline in %BSA scores, with an ORR in patients with stage IA–IB disease of 43.5%. In addition, the by-time analysis revealed a response rate of 36.7% after 1 month of treatment and a peak response rate of 66.7% at 18 months post-baseline. A post hoc analysis of the pivotal registration study found an ORR of 44.4% by %BSA, a response rate of 5% at 1 month, and a peak response of 55.7% at 12 months post-baseline (Helsinn Healthcare SA, data on file). The delay in peak response observed in the current study may be due to a more gradual dose increase during the PROVe study. However, no direct comparison can be made between the results from the current study and the pivotal study because of the differences in study design; the registration study was a randomized controlled trial examining chlormethine gel monotherapy, whereas PROVe was a real-world study that allowed concomitant therapy. Regardless, these data highlight the importance of continued chlormethine treatment.
While chlormethine gel is indicated for patients with stage IA–IB disease in the USA, the results of the PROVe study show that, in clinical practice, it is used across all stages. At least 31 of 298 patients enrolled in the study had stage IIA–IV MF. These patients were likely using chlormethine gel for local control of patches and plaques.
No unexpected or serious chlormethine gel-related AEs occurred during the study. The reported skin-related AEs appeared to be manageable in this real-world setting, and 63.8 and 45.0% of patients were able to continue chlormethine gel treatment for 12 and 24 months, respectively. The percentage of patients experiencing skin-related AEs of pruritus, skin irritation, and erythema were generally lower, at 9.7%, 7.4%, and 5.0% in PROVe versus 19.5%, 25%, and 17.2%, respectively, in the pivotal study; dermatitis rates were similar (12.8% in PROVe vs. 14.8% in the pivotal study) [11]. These differences may be due to coadministration of corticosteroids, the flexibility in the dosing schedule, or the fact that the majority of patients (85.2%) were using chlormethine gel for > 30 days prior to start of the study.
Chlormethine gel treatment also had an impact on HRQOL. The Skindex-29 results showed a significant correlation between clinical responder status and improved HRQOL scores. Patients who responded to treatment had significantly lower mean scores post-baseline in all subscales of the Skindex-29, indicating a lower impact of skin disease compared with nonresponders.
The real-world setting of the PROVe study does carry a number of limitations. As no specific assessments were mandated, a limited number of patients had both pre-enrollment and post-baseline %BSA data available for analysis. In addition, the clinical responses reported herein likely also reflect the use of concomitant therapies. Treatment schedules and frequency of chlormethine gel use varied, and patients could also have multiple dosing regimens over time, which can complicate data analysis but is representative of chlormethine gel use in daily clinical practice.
Conclusion
The PROVe study showed that chlormethine gel is effective and has a good tolerability profile in a real-world setting, where it was coadministered with other therapies in patients with early and advanced disease. Peak response occurred at 18 months, emphasizing the importance of continued treatment. HRQOL was higher in patients who responded to treatment, as indicated by lower Skindex-29 subscale scores. The chlormethine gel formulation offers patients with MF access to an important therapeutic option that can be safely applied at home and contributes to improving HRQOL by reducing the severity of skin lesions and symptoms.
Acknowledgements
The authors acknowledge and thank the volunteers, investigators, and the study teams at the centers participating in this study. The PROVe investigators collected data and provided and cared for study patients. Expert advice was provided by Prof. Youn H. Kim from Stanford Cancer Institute, Stanford, CA, USA. Statistical programming and analysis were overseen by Michael J. Williams, PhD, and David Mink, MS, from ICON Commercialization & Outcomes, Dublin, Ireland. Editorial and medical writing assistance was provided by Judith Land, PhD, from Aptitude Health, The Hague, The Netherlands, funded by Helsinn Healthcare SA. The authors are fully responsible for all content and editorial decisions for this manuscript.
Declarations
Funding
This study was supported by Helsinn Healthcare SA, who were involved in the analysis plan and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript and the decision to submit it for publication. Writing and editorial assistance was funded by Helsinn Healthcare SA.
Conflict of Interest
E.J. Kim has received research support from and/or acted as principal investigator for Actelion, Galderma, MedImmune, and Soligenix; has received consultant, speaking, or travel support from Actelion, Galderma, Helsinn, and Soligenix; and has served on a scientific advisory board for Helsinn and Kyowa Kirin. J. Guitart has received research support from Galderma and Soligenix and served on a scientific advisory board for Helsinn, Kyowa Kirin, miRagen, and Leo Pharma. C. Querfeld has received a research grant from Celgene; acted as clinical investigator for Celgene, Trillium, miRagen, Bioniz, and Kyowa Kirin; and served on a steering committee or advisory board for Helsinn/Actelion, miRagen, Bioniz, Trillium, and Kyowa Kirin. M. Girardi has served on an advisory board for Helsinn and Mallinckrodt and received research support and/or acted as principal investigator for AbbVie and Soligenix. A. Musiek has acted as investigator for Elorac, Soligenix, miRagen, Pfizer, Menlo, and Connect and served on an advisory board for Kyowa and Helsinn. O.E. Akilov has received research support from Trillium Therapeutics, Kyowa Kirin, and Pfizer; served as a clinical investigator for Celgene, Trillium Therapeutics, Bioniz, Kyowa, Soligenix, Galderma, and Innate Pharma; and received consulting fees or honorarium from Kyowa Kirin, Bioniz, and Dr. Reddy’s. J.T. Angello and W.L. Bailey are employed by Helsinn Therapeutics (U.S.), Inc. L.J. Geskin has received a research grant from Actelion; consulting fees or honorarium from Actelion, Helsinn, Mallinckrodt, Sanofi, and Regeneron; and served on a speakers’ bureau for Helsinn.
Ethics Approval
The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. The appropriateness of the study protocol and all risks and benefits to participants were approved by institutional review boards.
Consent to Participate
All patients provided written informed consent.
Consent for Publication
Not applicable.
Availability of Data and Material
Data are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Prof. EJK and JTA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: EJK, JG, CQ, MG, AM, OEA, JTA, WLB, LJG. Analysis and interpretation of the data: EJK, JG, CQ, MG, AM, JTA, WLB, LJG. Drafting of the article: EJK, JG, CQ, MG, AM, OEA, JTA, WLB, LJG. Critical revision of the article for important intellectual content: EJK, JG, CQ, MG, AM, OEA, JTA, WLB, LJG.
Footnotes
The original online version of this article was revised due to nifographic update.
Change history
3/15/2022
A Correction to this paper has been published: 10.1007/s40257-022-00676-1
Contributor Information
Ellen J. Kim, Email: Ellen.Kim@pennmedicine.upenn.edu
Joan Guitart, Email: j-guitart@northwestern.edu.
Christiane Querfeld, Email: cquerfeld@coh.org.
Michael Girardi, Email: michael.girardi@yale.edu.
Amy Musiek, Email: amusiek@wustl.edu.
Oleg E. Akilov, Email: akilovoe@upmc.edu
James T. Angello, Email: James.Angello@helsinn.com
William L. Bailey, Email: Bill.Bailey@helsinn.com
Larisa J. Geskin, Email: ljg2145@cumc.columbia.edu
References
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92(10):1085–1102. doi: 10.1002/ajh.24876. [DOI] [PubMed] [Google Scholar]
- 3.Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for research and treatment of cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - update 2017. Eur J Cancer. 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M, ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv30–40. [DOI] [PubMed]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Primary cutaneous lymphomas. Version 1.2020. 2020. https://www.nccn.org/professionals/physician_gls/default_nojava.aspx. Accessed 25 Jun 2020.
- 6.Denis D, Beneton N, Laribi K, Maillard H. Management of mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL): focus on chlormethine gel. Cancer Manag Res. 2019;11:2241–2251. doi: 10.2147/CMAR.S138661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonderheid EC, Tan ET, Kantor AF, Shrager L, Micaily B, Van Scott EJ. Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J Am Acad Dermatol. 1989;20(3):416–428. doi: 10.1016/S0190-9622(89)70051-7. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9(5):1031–1056. doi: 10.1016/S0889-8588(18)30057-1. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Martinez G, Varghese A, Hoppe RT. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol. 2003;139(2):165–173. doi: 10.1001/archderm.139.2.165. [DOI] [PubMed] [Google Scholar]
- 10.Valchlor [prescribing information]. Iselin, NJ: Helsinn Therapeutics (US) Inc. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202317s009lbl.pdf. Accessed 25 Jun 2020.
- 11.Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149(1):25–32. [DOI] [PMC free article] [PubMed]
- 12.Kim YH, Duvic M, Guitart J, Lessin S. Efficacy and safety of mechlorethamine (MCH) 0.04% gel in mycosis fungoides (MF) after treatment with topical MCH 0.02%. J Clin Oncol. 2014;32(suppl 15): abstract 9093.
- 13.Ledaga [summary of product characteristics]. Dublin, Ireland: Helsinn Birex Pharmaceuticals Ltd. 2017. https://www.ema.europa.eu/en/documents/product-information/ledaga-epar-product-information_en.pdf. Accessed 25 Jun 2020.
- 14.Querfeld C, Geskin LJ, Kim EJ, Scarisbrick JJ, Quaglino P, Papadavid E, Angello JT, Angello JT, Ortiz-Romero PL. Lack of Systemic Absorption of Topical Mechlorethamine Gel in Patients with Mycosis Fungoides Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2021 doi: 10.1016/j.jid.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EJ, Geskin L, Guitart J, Querfeld C, Girardi M, Musiek A, et al. Real-world experience with mechlorethamine gel in patients with mycosis fungoides-cutaneous lymphoma: preliminary findings from a prospective observational study. J Am Acad Dermatol. 2020;83(3):928–930. doi: 10.1016/j.jaad.2019.12.070. [DOI] [PubMed] [Google Scholar]
- 16.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133(11):1433–1440. doi: 10.1001/archderm.1997.03890470111018. [DOI] [PubMed] [Google Scholar]
- 17.Prinsen CA, Lindeboom R, de Korte J. Interpretation of Skindex-29 scores: cutoffs for mild, moderate, and severe impairment of health-related quality of life. J Invest Dermatol. 2011;131(9):1945–1947. doi: 10.1038/jid.2011.138. [DOI] [PubMed] [Google Scholar]


