ABSTRACT
The placenta transports iron to the fetus to support growth and development. Mice are commonly used to study placental iron transport. The subcellular locations of transferrin receptor and ferroportin 1 in iron-transporting cells in the mouse placenta have not been directly assessed. Using immunogold electron microscopy, we determined that transferrin receptor is concentrated on the intracellular vesicles in syncytiotrophoblast I while ferroportin 1 is on the basal membrane of syncytiotrophoblast II. Fluorescent imaging of maternally injected transferrin iron in the placentas collected at 6 time points postinjection (n = 1–3 animals/time point) showed that transferrin iron was taken up and metabolized within syncytiotrophoblast I within 48 h after injection. These data indicate that the intracellular iron-trafficking mechanism is distinct in different placental cells.
Keywords: mouse, placenta, iron transport, syncytiotrophoblast, transferrin iron
This research demonstrates the mechanism of transferrin iron uptake at the subcellular level in mouse placental syncytiotrophoblasts
Introduction
Iron is an essential nutrient for human health and is especially critical during early stages of life. During pregnancy, the placenta actively transports iron from the mother to her fetus. Suboptimal iron nutrition in utero affects brain development and is associated with poor neurobehavioral and cognitive outcomes later in life (1).
The importance of iron in fetal development has stimulated interest in research on placental iron transport (2, 3). Because of the structural similarity between mouse and human placentas and the availability of transgenic strains, mice are commonly used as a model of placental iron transport and have greatly advanced the understanding of how placental iron metabolism responds to maternal and fetal signals (4, 5). However, many fundamental questions remain regarding the metabolic fate of iron across multiple placental cell layers [including syncytiotrophoblast (SCTB) and fetal endothelium] following its uptake from the maternal circulation. Unlike the human placenta, the mouse placenta has 2 SCTB layers. Whether iron remains bound to transferrin (Tf) as it traverses from SCTB I to SCTB II is unknown. In addition, the iron exporter ferroportin 1 (Fpn1) is thought to be responsible for transporting iron out of the placenta into the fetal circulation. It is unclear whether Fpn1 is expressed in SCTB II or fetal endothelium or both. Answers to these questions are essential for future research on the mechanism of placental iron transport.
Methods
Animals
Wild-type 129S6/SvEvTac mice (Taconic Biosciences) were crossed to generate female animals used in this study. Female mice were paired with males at 5–8 wk of age. Pregnancy was confirmed by the presence of a copulation plug, denoted as embryonic day (E) 0.5. Pregnant dams at E15.5 were used in all experiments. Mice were housed in the barrier facility at Boston Children's Hospital and had ad libitum access to standard feed pellets.
Immunohistochemistry
The placenta was fixed in 10% phosphate-buffered formalin and stored in 70% ethanol until paraffin embedding and sectioning. Staining was performed on formalin-fixed, paraffin-embedded 5-μm sections using the Ventana Discovery XT automated immunohistochemistry (IHC) platform (Roche). After EDTA pretreatment, slides were incubated with primary antibodies against transferrin receptor (Tfrc) and Fpn1 and anti-rabbit horseradish peroxidase secondary antibodies, followed by hematoxylin counterstaining. Images were acquired using an Olympus BX51 microscope using the 100× oil objective.
Immunogold labeling and electron microscopy
Immunogold labeling was performed using previously published procedures (6, 7). Placental tissue pieces (∼1 m3) were fixed with 4% paraformaldehyde (catalog no. 15710; EMS) and 0.01% glutaraldehyde in 0.1 M phosphate buffer at 4°C overnight. Fixed tissue was infiltrated in sucrose, cut into ultrathin frozen sections at −120°C, and transferred to formvar-carbon–coated copper grids for immunogold labeling. Briefly, sections were blocked in 1% BSA, incubated with antibodies against Tfrc (catalog no. 13-6800; Invitrogen) or Fpn1 (MTP11-A; Alpha Diagnostic International) for 30 min at room temperature, and incubated with protein A conjugated to 10-nm gold particles. Labeled grids were incubated with a mixture of methyl cellulose and a heavy metal contrasting agent, uranyl acetate, for 10 min. Grids were dried and imaged using a transmission electron microscope (JOEL 1200EX) equipped with an AMT 2k CCD camera.
Kinetics of maternally injected Tf in the placenta
Alexa488 labeled holo-Tf (1 mg, T13342; Invitrogen) was injected through the retro-orbital venous sinus in dams under anesthesia. Mice were killed at 1, 2, 5, 30, and 120 min and 48 h post–Tf injection by carbon dioxide asphyxiation (n = 1–3 mice/time point). Three placentas were harvested from each mouse, fixed in 2% paraformaldehyde for 2 h, placed in 30% sucrose overnight, and equilibrated in a mixture of 30% sucrose and optimal cutting temperature (OCT) compound for 1 h before freezing in OCT blocks. Placental cryosections sections (10 μm) were blocked in 5% goat serum, incubated with primary antibodies [Tfrc, Fpn1, divalent metal transporter 1 (Dmt1; a gift from Dr. François Cannone-Hergaux), monocarboxylate transporter 1 (Mct1; AB1286-I; Millipore), connexin 26 (Cx26; 71-0500; Invitrogen)] overnight at 4°C. Alexa-fluorophore conjugated secondary antibodies were applied followed by counterstaining with Hoechst (H3569; ThermoFisher). Confocal imaging was performed using a Zeiss LSM710 microscope. Placentas collected at different postinjection time points were acquired using the same settings (exposure, gain, pinhole, etc.). Fibrinogen is a glycoprotein present at a similar concentration as Tf in the plasma and is not endocytosed by the placenta. As a negative control for placental uptake of maternally injected proteins, we injected Alexa594 labeled fibrinogen (F13193; Invitrogen) and Alexa488 labeled Tf at the same molar amounts in 1 pregnant mouse and harvested placentas 30 min postinjection.
Ethics statement
All animal procedures were performed under the study protocol (19-06-3946R) approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital.
Results
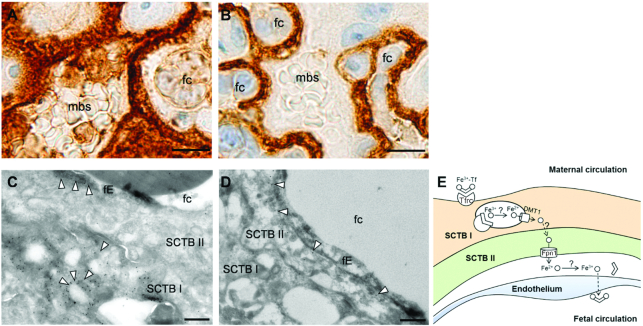
IHC staining confirmed that Tfrc was located in SCTB I adjacent to the maternal blood space (Figure 1A), whereas Fpn1 was located in SCTB II in close proximity to nucleated fetal RBCs (Figure 1B). The extremely thin fetal endothelium (<1 μm) made it nearly impossible to reliably determine whether Fpn1 staining was in SCTB II or fetal endothelium by IHC, which is known for its ability to distinguish different cell types and reveal nuanced staining patterns (8). To determine the precise subcellular locations of Tfrc and Fpn1, we performed immunogold electron microscopy (EM). Immunogold labeling localized Tfrc primarily to intracellular vesicles in SCTB I (Figure 1C). This is consistent with the endocytosis of Tfrc following iron uptake in the Tf cycle under normal conditions. It is likely that, during iron deficiency, more Tfrc will be localized to the apical surface to increase iron uptake. Tfrc was also present sparsely in the fetal endothelium. Fpn1 was found on the basal membrane of SCTB II and was absent from fetal endothelium (Figure 1D). Figure 1E is a schematic of the iron transport mechanism in the mouse placenta showing the definitive locations of Tfrc and Fpn1.
FIGURE 1.
Cellular localization of Tfrc and Fpn1 in the mouse placenta. IHC staining showing Tfrc expression in SCTB I (A) and Fpn1 expression in SCTB II (B) of E15.5 mouse placenta. Scale bar, 10 μm. High magnification immunogold EM images showing Tfrc on membranes of intracellular vesicles in SCTB and to a lesser extent on fetal endothelium (arrows) (C) and Fpn1 along the basal membrane of SCTB II (arrows) (D). Scale bar, 0.5 μm. (E) Updated mechanism of placental iron transport in the mouse placenta. DMT1, divalent metal transporter 1; E, embryonic day; EM, electron microscopy; fc, fetal capillary; fE, fetal endothelium; Fpn1, ferroportin 1; IHC, immunohistochemistry; mbs, maternal blood space; SCTB, syncytiotrophoblast; Tfrc, transferrin receptor.
Figure 2 shows the time course of placental uptake of maternally injected Tf. Thirty minutes after injection, Tf was fully endocytosed by SCTB I (Figure 2A), but fibrinogen injected simultaneously with Tf was not detected in SCTBs (Figure 2B). Tf fluorescence in SCTB I increased gradually, peaking at 30 min, and became undetectable 48 h post–Tf injection (Figure 2C–H). Endocytosed Tf colocalized highly with Tfrc, partially with Dmt1, and was completely polarized from Fpn1 (Figures 2I–K). Tf was absent from SCTB II at all time points.
FIGURE 2.
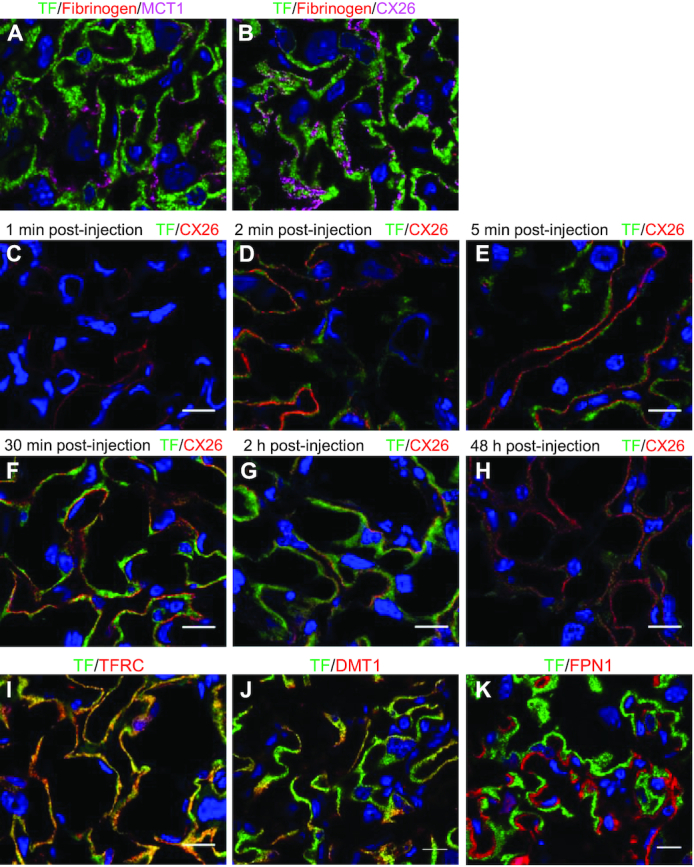
Placental transfer of maternally injected Tf. (A) In placentas collected 30 min postinjection, Tf (green) was fully endocytosed into SCTB I with no overlap with the apical membrane protein Mct1 (magenta) and (B) did not cross the border between SCTB I and SCTB II marked by the gap junction protein Cx26 (magenta). The negative endocytosis control injected simultaneously as Tf, fibrinogen (red), did not appear in SCTB I. (C–H) Accumulation and disappearance of injected Tf (green) in the mouse placenta within 48 h postinjection. Scale bar, 15 μm. Immunofluorescent staining of Tfrc (I), Dmt1 (J), and Fpn1 (K) in relation to Tf (green) in placentas 30 min post–Tf injection. Scale bar, 15 μm. Cx26/CX26, connexin 26; Dmt1/DMT1, divalent metal transporter 1; Fpn1/FPN1, ferroportin 1; Mct1/MCT1, monocarboxylate transporter 1; SCTB, syncytiotrophoblast; Tf/TF, transferrin; Tfrc/TFRC, transferrin receptor.
Discussion
Using immunogold EM, we demonstrated for the first time the precise subcellular locations of the 2 primary iron transporters, Tfrc and Fpn1, in the mouse placenta. In human SCTB, Tfrc is localized to the apical membrane and intracellular vesicles (9). Our immunogold data in the mouse placental SCTB I clearly show that Tfrc is concentrated on the membranes of intracellular vesicles. This, together with the colocalization of maternally injected Tf with Tfrc and Dmt1, supports the generally accepted model of Tf iron uptake by Tfrc-mediated endocytosis in the mouse placenta, which likely resembles that utilized by the human placenta. Because human placentas have only 1 SCTB while mice have 2 SCTB layers, the metabolic fate of Tf iron after apical uptake may be different between the 2 species. Nevertheless, our data clarify that, in the mouse placenta, maternal Tf does not translocate to SCTB II and thus it is highly likely that iron released from Tf is chaperoned by other proteins [such as Poly(rC) binding proteins 1 and 2] until it reaches the fetal circulation.
Immunogold EM shows that Fpn1 is localized to the basolateral membrane of SCTB II and is absent from fetal endothelium. This is the first time that Fpn1 localization has been resolved at the subcellular level in the placenta and has important implications regarding the role of fetal endothelium as a site of iron transport. We observed little Tfrc labeling in fetal endothelium, particularly when compared with that in SCTB I. This suggests that Tfrc may not be the primary mechanism of iron trafficking in fetal endothelium and that systems independent of Tfrc and Fpn1, such as those utilized by non–Tf-bound iron, may play critical roles in iron delivery across the endothelium to the fetal circulation.
In summary, in mature mouse placentas, we show the precise locations of Tfrc and Fpn1 and provide evidence that the Tf cycle is confined in SCTB I and that iron transfer in SCTB II and fetal endothelium may be independent of Tf. Last, we describe a novel approach to study placental nutrient kinetics using modern imaging techniques.
ACKNOWLEDGEMENTS
We thank Maria Ericsson (Electron Microscopy Core, Harvard Medical School) for her advice and expertise in the immunogold labeling experiment. We appreciate the skillful assistance of Monica Calicchio (Department of Pathology, Boston Children's Hospital) in the IHC experiment. We gratefully acknowledge the critical support by Dr. Neil Dani (Department of Pathology, Boston Children's Hospital) in the acquisition of confocal images. Finally, we thank Dr. François Canonne-Hergaux (Institut de Recherche en Santé Digestive, Université de Toulouse) for the generous gift of the Dmt1 antibody. The authors’ responsibilities were as follows—CC: conceived the project, conducted the experiments, and wrote the manuscript; MDF: supervised the project and edited the manuscript; and both authors: read and approved the final manuscript.
Notes
This work was supported by the National Heart, Lung, and Blood Institute grant 5T32HL110852 (CC) and Children's Hospital Pathology Foundation, Inc. (MDF).
Author disclosures: The authors have no conflicts of interest.
Abbreviations used: Dmt1, divalent metal transporter 1; E, embryonic day; EM, electron microscopy; Fpn1, ferroportin 1; IHC, immunohistochemistry; OCT, optimal cutting temperature; SCTB, syncytiotrophoblast; Tf, transferrin; Tfrc, transferrin receptor.
Contributor Information
Chang Cao, Email: chang.cao@childrens.harvard.edu, Department of Pathology, Boston Children's Hospital, Boston, MA, USA.
Mark D Fleming, Department of Pathology, Boston Children's Hospital, Boston, MA, USA.
References
- 1. Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020;223:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sangkhae V, Nemeth E. Placental iron transport: the mechanism and regulatory circuits. Free Radic Biol Med. 2019;133:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev. 2016;74(7):421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelić M, Ganz T, Nemeth E. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest. 2019;130(2):625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kämmerer L, Mohammad G, Wolna M, Robbins PA, Lakhal-Littleton S. Fetal liver hepcidin secures iron stores in utero. Blood. 2020;136(13):1549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tebar F, Sorkina T, Sorkin A, Ericsson M, Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem. 1996;271(46):28727–30. [DOI] [PubMed] [Google Scholar]
- 7. Mailly L, Xiao F, Lupberger J, Wilson GK, Aubert P, Duong FHT, Calabrese D, Leboeuf C, Fofana I, Thumann Cet al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33(5):549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison LE, Lefever MR, Behman LJ, Leibold T, Roberts EA, Horchner UB, Bauer DR. Brightfield multiplex immunohistochemistry with multispectral imaging. Lab Invest. 2020;100(8):1124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuchs R, Ellinger I. Endocytic and transcytotic processes in villous syncytiotrophoblast: role in nutrient transport to the human fetus. Traffic. 2004;5(10):725–38. [DOI] [PubMed] [Google Scholar]