Abstract
BACKGROUND
Intensive systolic blood pressure (SBP) treatment prevents cardiovascular disease (CVD) events in patients with high CVD risk on average, though benefits likely vary among patients.
OBJECTIVES
The aim of this study was to predict the magnitude of benefit (reduced CVD and all-cause mortality risk) along with adverse event (AE) risk from intensive versus standard SBP treatment.
METHODS
This was a secondary analysis of SPRINT (Systolic Blood Pressure Intervention Trial). Separate benefit outcomes were the first occurrence of: 1) a CVD composite of acute myocardial infarction or other acute coronary syndrome, stroke, heart failure, or CVD death; and 2) all-cause mortality. Treatment-related AEs of interest included hypotension, syncope, bradycardia, electrolyte abnormalities, injurious falls, and acute kidney injury. Modified elastic net Cox regression was used to predict absolute risk for each outcome and absolute risk differences on the basis of 36 baseline variables available at the point of care with intensive versus standard treatment.
RESULTS
Among 8,828 SPRINT participants (mean age 67.9 years, 35% women), 600 CVD composite events, 363 all-cause deaths, and 481 treatment-related AEs occurred over a median follow-up period of 3.26 years. Individual participant risks were predicted for the CVD composite (C index = 0.71), all-cause mortality (C index = 0.75), and treatment-related AEs (C index = 0.69). Higher baseline CVD risk was associated with greater benefit (i.e., larger absolute CVD risk reduction). Predicted CVD benefit and predicted increased treatment-related AE risk were correlated (Spearman correlation coefficient = −0.72), and 95% of participants who fell into the highest tertile of predicted benefit also had high or moderate predicted increases in treatment-related AE risk. Few were predicted as high benefit with low AE risk (1.8%) or low benefit with high AE risk (1.5%). Similar results were obtained for all-cause mortality.
CONCLUSIONS
SPRINT participants with higher baseline predicted CVD risk gained greater absolute benefit from intensive treatment. Participants with high predicted benefit were also most likely to experience treatment-related AEs, but AEs were generally mild and transient. Patients should be prioritized for intensive SBP treatment on the basis of higher predicted benefit. (Systolic Blood Pressure Intervention Trial [SPRINT]; NCT01206062)
Keywords: blood pressure, cardiovascular disease, clinical decision making, hypertension, predictive modeling, prevention
In SPRINT (Systolic Blood Pressure Intervention Trial), intensive systolic blood pressure (BP) treatment (goal <120 mm Hg) versus standard treatment (goal <140 mm Hg) reduced the risk for cardiovascular disease (CVD) events and all-cause mortality without increasing risk for overall adverse events (AEs) (1).
Clinicians may hesitate to implement intensive systolic BP treatment because of the perception of a trade-off between a patient’s potential CVD prevention benefits and increased risk for AEs. Because intensive systolic BP treatment requires more resources (clinician time, office and laboratory visits, and medications), the highest value approach may be to prioritize intensive systolic BP treatment to “optimal” patients (i.e., those with high predicted CVD benefit and low AE risk) (2).
Ideally, treatment prioritization should be based on pre-treatment characteristics available to clinical decision makers at the point of care. In practice, identifying such heterogeneity in treatment effect has proved difficult (3–6). Typically, 2 broad classes of statistical approaches may be used to model the heterogeneity of treatment effects in randomized trials: effect modeling and risk modeling (6). Effect modeling incorporates interaction terms between the randomized treatment and baseline covariates to distinguish subgroups with varying treatment effects, usually on a risk ratio or hazard ratio scale. Using effect-modeling approaches in SPRINT, post hoc analyses by others have reported that age, race/ethnicity, estimated glomerular filtration rate (eGFR), diastolic BP, smoking status, predicted 10-year CVD risk, and triglyceride level were potentially associated with differential relative benefit from intensive versus standard systolic BP treatment (7–14). These analyses have value for hypothesis generation, but their post hoc nature means that they naturally suffer from a multiplicity of treatment comparisons and low statistical power and, as such, are not appropriate for clinical application (3–5,15).
The risk-modeling approach examines variability in absolute benefit of treatment as a function of baseline risk. Because baseline risk usually varies substantially within a trial population, the risk-modeling approach investigates how the absolute benefit of treatment (i.e., the absolute risk reduction), the most clinically relevant effect measure, grows as baseline risk increases: “risk magnification” of the treatment effect. We used a risk-modeling approach to develop multivariate prediction models for 1) SPRINT intensive systolic BP treatment benefit (i.e., one model each for the CVD composite outcome and all-cause mortality); and 2) SPRINT intensive systolic BP treatment-related AEs of interest.
METHODS
STUDY OVERSIGHT AND APPROVAL.
This analysis of the extended SPRINT individual participant dataset was approved by the Institutional Review Boards of the University of Utah and Columbia University. We used the complete SPRINT individual participant data and drew on the experience and expertise of the SPRINT Research Group, Coordinating Center, and Steering Committee. SPRINT was conducted with oversight by each study site’s Institutional Review Board and monitoring by an independent Data and Safety Monitoring Board, and written informed consent was obtained from every participant. The design of the present study and reporting was informed by the Predictive Approaches to Treatment Effect Heterogeneity Statement (6).
SPRINT DESIGN.
The rationale, study design, and results of SPRINT have been published (1,16). Briefly, participants were 50 years of age or older, at increased risk for CVD, and had systolic BP 130 to 180 mm Hg depending on the number of antihypertensive medications being taken. The main exclusion criteria included diabetes, a history of stroke, clinical heart failure, standing systolic BP <110 mm Hg, dementia, and eGFR <20 ml/min/1.73 m2. More details on the SPRINT inclusion and exclusion criteria are provided in the protocol (17).
STUDY POPULATION.
Our study sample included 8,828 SPRINT participants (94.3% of those randomized). A total of 533 participants were omitted because they were missing at least 1 baseline predictor variable (Supplemental Figure 1). To develop prediction models for each outcome and predicted magnitude of benefit (i.e., absolute risk difference for CVD and all-cause mortality, separately) versus predicted increased risk for treatment-related AEs (i.e., absolute risk difference for AEs), we identified 36 baseline variables considered likely to be associated with overall risk for 1 or more of the benefit and AE outcomes (Table 1, Supplemental Table 1). To select the final 36 variables, we used a combination of 1) selecting variables listed by the PCORnet Common Data Model variable list version 6.0 (18) (i.e., representing structured data elements commonly available in U.S. health system electronic health record databases); 2) practical clinical review by experienced clinicians (W.S.W., R.H., A.C., W.C.C., A.E.M., and R.Y.W.); and 3) published evidence in hypertension science (see the Supplemental Appendix for more details on the variable selection process). In selecting these variables, clinicians with hypertension subject matter expertise prioritized factors likely to be available at the point of care, that is, at the time clinicians discuss decisions about intensive BP treatment with their eligible patients.
TABLE 1.
Baseline Characteristics of SPRINT Participants Included for Model Derivation
| SPRINT Participants in the Present Analysis (n = 8,828) |
||
|---|---|---|
| Intensive Treatment (n = 4,429) | Standard Treatment (n = 4,399) | |
| Demographics | ||
| Age, yrs | 67.9 ± 9.4 | 67.9 ± 9.5 |
| Female | 1,582 (35.7) | 1,531 (34.8) |
| Race/ethnicity | ||
| Non-Hispanic white | 2,883 (65.1) | 2,841 (64.6) |
| Non-Hispanic black | 1,385 (31.3) | 1,430 (32.5) |
| Hispanic | 481 (10.9) | 457 (10.4) |
| Social and behavioral | ||
| Lives with others | 3,154 (71.2) | 3,120 (70.9) |
| Has private insurance | 1,919 (43.3) | 1,843 (41.9) |
| Current smoker | 613 (13.8) | 573 (13.0) |
| Former smoker | 1,884 (42.5) | 1,876 (42.6) |
| Never smoker | 2,497 (56.4) | 2,449 (55.7) |
| Medical history | ||
| Clinical CVD | 707 (16.0) | 689 (15.7) |
| Left ventricular hypertrophy | 813 (18.4) | 837 (19.0) |
| Dizziness when standing | 186 (4.2) | 190 (4.3) |
| History of coronary revascularization | 430 (9.7) | 411 (9.3) |
| History of depression | 810 (18.3) | 822 (18.7) |
| Clinical/laboratory measurements | ||
| Systolic BP, mm Hg | 139.7 ± 15.7 | 139.7 ± 15.5 |
| Diastolic BP, mm Hg | 78.2 ± 11.9 | 78.1 ± 12.1 |
| Resting heart rate, beats/min | 66.3 ± 11.5 | 66.3 ± 11.6 |
| Serum potassium, mg/dl | 4.2 (3.9–4.5) | 4.2 (3.9–4.4) |
| Serum creatinine, mg/dl | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) |
| Albumin/creatinine ratio, mg/g | 9.6 (5.7–21.1) | 9.4 (5.6–21.7) |
| Total cholesterol, mg/dl | 187.0 (161.0–215.0) | 186.0 (160.0–214.0) |
| HDL cholesterol, mg/dl | 50.0 (43.0–60.0) | 50.0 (43.0–60.0) |
| Triglycerides, mg/dl | 107.0 (77.0–148.0) | 107.0 (78.0–152.0) |
| Body mass index, kg/m2 | 29.9 ± 5.8 | 29.8 ± 5.7 |
| Serum glucose, mg/dl | 97.0 (90.0–105.0) | 97.0 (91.0–105.0) |
| Medication use | ||
| Aspirin | 2,360 (53.3) | 2,288 (52.0) |
| Statin | 1,889 (42.7) | 1,970 (44.8) |
| NSAID | 1,744 (39.4) | 1,593 (36.2) |
| Number of nonantihypertensive medications | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) |
| Number of antihypertensive medications | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| ACE inhibitor or ARB | 2,610 (58.9) | 2,547 (57.9) |
| Calcium-channel blocker | 1,550 (35.0) | 1,592 (36.2) |
| Thiazide-type diuretic agent | 1,741 (39.3) | 1,825 (41.5) |
| Loop diuretic agent | 255 (5.8) | 253 (5.8) |
| Beta-blocker | 1,685 (38.0) | 1,570 (35.7) |
| Alpha-blocker | 446 (10.1) | 445 (10.1) |
| Other antihypertensive medication | 188 (4.2) | 164 (3.7) |
Values are mean ± SD, n (%), or median (interquartile range).
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; BP = blood pressure; CVD = cardiovascular disease; HDL = high-density lipoprotein; NSAID = nonsteroidal anti-inflammatory drug; SPRINT = Systolic Blood Pressure Intervention Trial.
TREATMENT BENEFITS VERSUS RISK FOR TREATMENT-RELATED AEs.
We examined the primary CVD composite outcome in SPRINT, defined as the adjudicated first occurrence of a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, nonfatal stroke, acute decompensated heart failure, or death due to CVD causes. We also examined all-cause mortality as an outcome reflecting treatment benefit. Treatment-related AEs of interest included time to first reported hypotension, syncope, bradycardia, electrolyte abnormality, injurious fall, or acute kidney injury or acute renal failure. The rationale for analyzing only first events is that the number of repeat events, either CVD or treatment-related AEs, was small (i.e., <2% of SPRINT participants experienced more than 1 event, either CVD or AE during follow-up).
STATISTICAL ANALYSIS.
Modified elastic net Cox models for the prediction of the CVD composite, all-cause mortality, and treatment-related AE outcomes.
We fit modified elastic net Cox regression models for each outcome. The elastic net is a multivariate regression approach that reduces difficulties with multi-collinearity and overfitting by penalizing the magnitudes of regression coefficients in a way that shrinks coefficients of less informative variables either partially or entirely to zero (19). For each outcome, the standard elastic net model was extended to incorporate an interaction term between an indicator variable for treatment assignment (intensive vs. standard) and a continuous variable representing a risk score for the outcome. Inclusion of this interaction term allowed our model to account for the shape (i.e., a nonlinear functional form) of the relationship of the magnitude of the treatment effect on the predicted baseline absolute risk.
For each outcome, we used the results of the modified elastic net regression analyses to predict the overall absolute risk given the 36 baseline covariates. Absolute risk was estimated at 3.26 years, the median duration of follow-up in SPRINT. For each outcome, we evaluated the discrimination of the elastic net Cox models for overall absolute risk by computing the cross-validated C statistic between estimated absolute risk and observed survival times (20,21). We evaluated calibration through the examination of calibration plots of predicted versus observed event rates and using the modified Hosmer-Lemeshow chi-square statistic and the Greenwood-Nam-D’Agostino (GND) test (22,23). We graphically displayed the standardized regression coefficients for the pairwise association between each baseline variable and the predicted absolute risks for each outcome. We used scatterplots and histograms to show the relationships of predicted absolute risks for the CVD composite and all-cause mortality, separately, with predicted absolute risks for AEs.
Estimating the predicted magnitude of benefit and risk for AEs of intensive versus standard systolic BP treatment.
We expressed the predicted magnitude of benefit versus predicted increased risk for treatment-related AEs, separately, with intensive versus standard systolic BP treatment as the absolute risk difference at 3.26 years of follow-up. We estimated counterfactual absolute risks at 3.26 years for each participant under both intensive and standard systolic BP treatment by taking the appropriate transformation of the product of the estimated average absolute risk with terms modeling the treatment effect and its relationship with absolute risk (Supplemental Methods). For each outcome, we predicted each participant’s counterfactual risk difference by taking the difference between predicted absolute risks with intensive and standard treatment. We then used the respective predicted risk differences to summarize the predicted magnitude of benefit (reduced CVD or all-cause mortality risk) versus increased risk for AEs with intensive versus standard systolic BP treatment. For each outcome, we evaluated the adequacy of the elastic net model for estimating the risk difference by categorizing the estimated risk differences into quartiles and then using 10-fold cross-validation to compare direct Kaplan-Meier estimates of the observed risk difference to the median predicted risk difference for each quartile. For each outcome, we also evaluated the discrimination of the elastic net Cox models to predict absolute risk differences by computing the 10-fold cross-validated concordance statistic for benefit (C for benefit) (24).
We graphically displayed the modeled relationship between estimated absolute risk differences and baseline absolute risk by plotting the estimated risk difference as a function of the overall baseline predicted absolute risk for each outcome, with a 95% bootstrap pointwise confidence band. We used likelihood ratio tests to assess whether the data were consistent with directly proportional risk magnification. Analogous to the comparisons of predicted absolute risks between outcomes described previously, we used scatterplots, histograms, and bar charts based on cross-classifications of marginal tertiles to display the joint distributions of the predicted magnitude of benefit versus risk for AEs with intensive versus standard treatment. We used bootstrap resampling to compute 95% confidence intervals for the ratio of the predicted magnitude of benefit versus the likelihood of AEs and graphically displayed them in relation to the quantiles of the estimated benefit/risk for AEs ratios.
In addition, we tested a hybrid risk-modeling and effect-modeling approach by adding interaction terms between randomized treatment group and predictors representing the 6 pre-specified subgroups selected at the outset for SPRINT (i.e., previous chronic kidney disease, age <75 vs. ≥75 years, female vs. male sex, black vs. nonblack race, with and without CVD diagnosis at baseline, and tertiles of baseline systolic BP) to the elastic net Cox models described previously for each outcome. Analyses were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
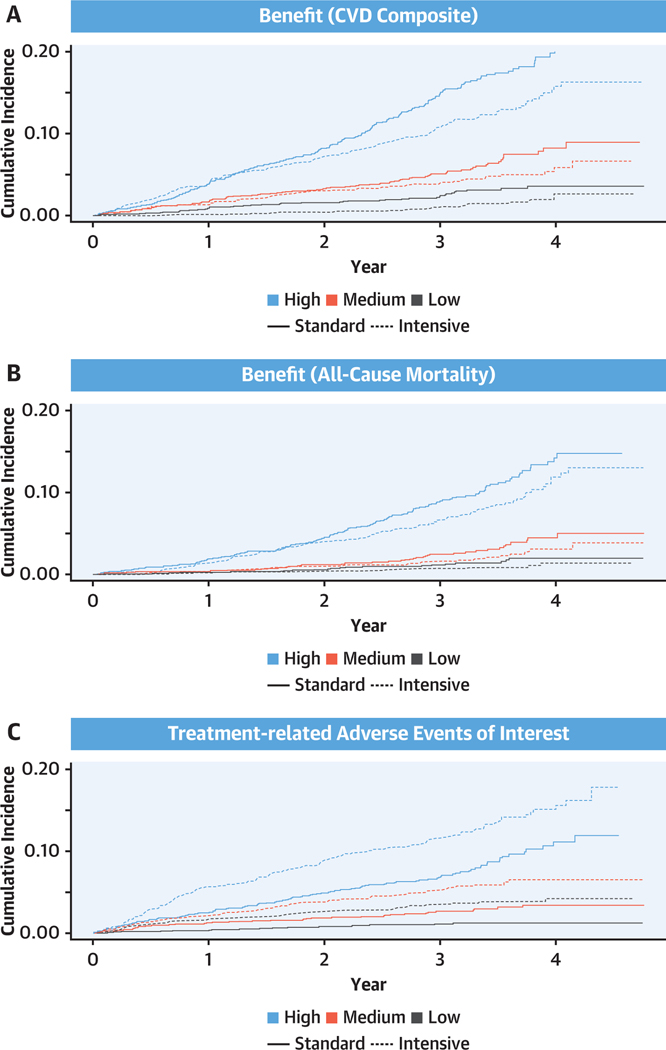
There were 4,429 and 4,399 SPRINT participants in the intensive and standard treatment groups included in the present analysis, respectively. The mean ages were 67.9 ± 9.4 and 67.9 ± 9.5 years, 35.7% and 34.8% were women, and 65.1% and 64.6% were non-Hispanic white, respectively (Table 1). Over a median follow-up duration of 3.26 years, 255 and 345 CVD composite events and 157 and 206 all-cause deaths occurred in the intensive and standard treatment groups, respectively (Figure 1, Supplemental Tables 2 and 3). A total of 312 and 169 participants experienced one of the treatment-related AEs of interest in the intensive and standard treatment groups, respectively (Supplemental Table 2).
FIGURE 1. Cumulative Incidence of the Benefit Outcomes and Risk for Adverse Events for Intensive Versus Standard Systolic Blood Pressure Treatment Within Tertiles of Predicted Benefit and Risk for Adverse Events.
Shown are the cumulative hazards for the primary outcome (a composite of myocardial infarction, acute coronary syndrome, stroke, heart failure, or death due to cardiovascular causes) (A), all-cause mortality (B), and treatment-related adverse events (C) for intensive versus standard systolic blood pressure treatment within strata of predicted benefit and tertiles of risk for adverse events. CVD = cardiovascular disease.
PREDICTED ABSOLUTE RISKS FOR EACH OUTCOME.
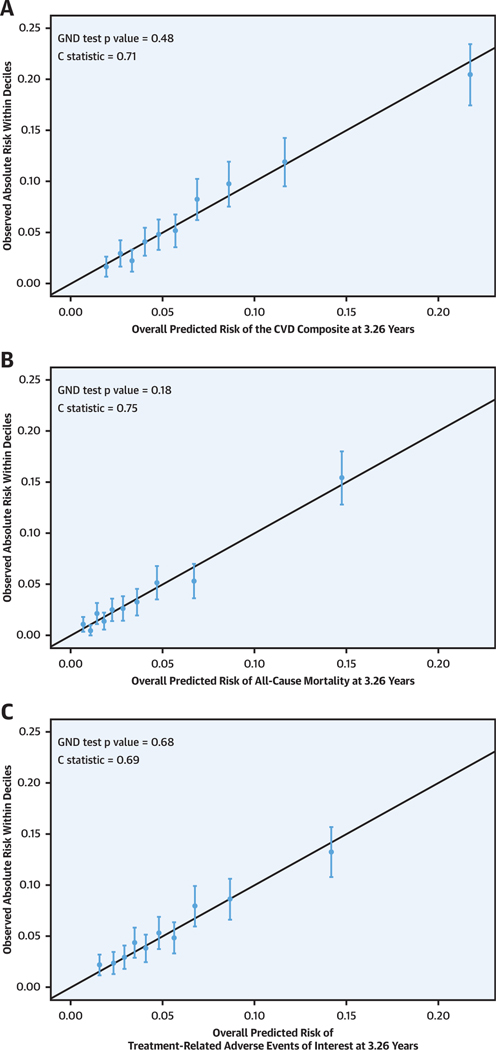
The modified elastic net Cox models predicting each outcome performed well according to C statistics and the GND test for the CVD composite (C statistic = 0.71, GND p = 0.48), all-cause mortality (C statistic = 0.75, GND p = 0.18), and treatment-related AEs (C statistic = 0.69, GND p = 0.68) (Figure 2). Supplemental Figure 2 shows the relative strength of the bivariate association of each baseline variable, with each outcome expressed as the standardized univariate hazard ratio. The baseline factors that were most strongly associated with a higher risk for the CVD composite outcome were older age, history of CVD, and impaired kidney function (lower eGFR). In general, the pattern and strengths of the bivariate associations of the 36 baseline variables were similar for the 3 outcomes. Supplemental Table 4 shows the regression coefficients for the modified elastic net Cox models for each of the 3 outcomes. There was a wide distribution of predicted absolute risk for each outcome at 3.26 years, with 10th to 90th percentiles ranging from 2.4% to 14.0% for the CVD composite, 0.80% to 8.5% for all-cause mortality, and 2.0% to 10.2% for AEs. The predicted risks for the CVD composite and treatment-related AEs were correlated (Supplemental Figures 3 and 4), with Spearman correlation coefficients of 0.72 and 0.76 for treatment-related AEs versus the CVD composite and for treatment-related AEs versus all-cause mortality, respectively.
FIGURE 2. Calibration Plots Showing the Observed Absolute Risks Within Deciles of Predicted Risk at 3.26-Year Follow-Up for the CVD Composite, All-Cause Mortality, and Adverse Events.
Calibration plots showing the relationship between elastic net Cox model–predicted Kaplan-Meier event probabilities for the cardiovascular disease (CVD) composite (A), all-cause mortality (B), and adverse events (C) versus mean observed Kaplan-Meier event probabilities for each decile of risk in SPRINT (Systolic Blood Pressure Intervention Trial) at 3.26 years. The solid black diagonal lines show a perfect expected risk versus observed risk slope of 1. Calibration assesses how well elastic net Cox model–predicted CVD event rates correspond to observed rates. A formal test of calibration was performed using the modified Hosmer-Lemeshow chi-square statistic and the Greenwood-Nam-D’Agostino (GND) test.
PREDICTED MAGNITUDE OF BENEFIT VERSUS RISK FOR TREATMENT-RELATED AEs WITH INTENSIVE VERSUS STANDARD TREATMENT.
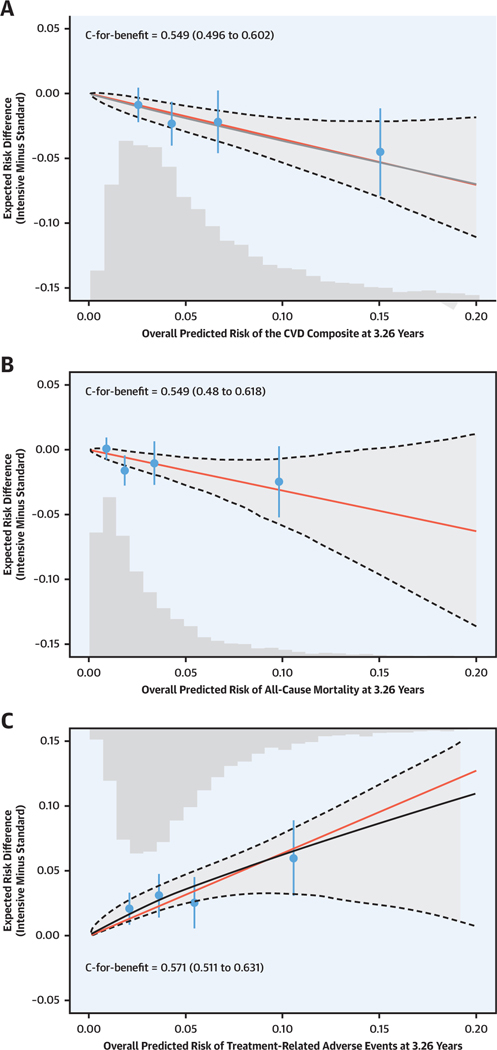
For both benefit outcomes, the risk magnification plot shows that the predicted magnitude of benefit of intensive versus standard treatment in terms of absolute risk reduction grew linearly as baseline risk increased (Figure 3, Central Illustration). However, for treatment-related AEs, the association between baseline risk and the effect of intensive versus standard systolic BP treatment appeared to be attenuated at the highest levels of baseline risk. Confidence intervals for the risk magnification curves were wide, indicating uncertainty in the shapes of the respective risk magnification relationships for each outcome. C for benefit, which is more conservative than the traditional C index and does not have established thresholds, was 0.55 and 0.55 for the benefit models for the CVD composite and all-cause mortality, respectively (Figure 3). C for benefit for the predicted increased risk for treatment-related AEs model was 0.57.
FIGURE 3. Risk Magnification Plot Showing the Predicted Magnitude of Benefit (Reduced CVD Risk and All-Cause Mortality) and Risk for Treatment-Related Adverse Events With Intensive Versus Standard Treatment Across a Range of Predicted Baseline Risk for Each Benefit Outcome and Risk for Adverse Events.
The solid red line represents direct risk magnification, where the absolute risk reduction is directly proportional to the baseline risk assuming a constant relative treatment effect. The solid black line represents the relationship between average elastic net Cox model–predicted absolute risk difference for each outcome across the full range of baseline risk. The dashed black line and shaded gray areas represent the 95% confidence bands for the predicted risk difference. The histogram represents the distribution of baseline risk for each outcome in the SPRINT population used in the present analysis. The black circles represent the average observed risk difference for each outcome within quartiles of the Cox model–predicted absolute risk difference. In contrast to the traditional C statistic, which assess a prediction model’s ability to predict outcome risk, C for benefit assesses a prediction model’s ability to predict treatment benefit by assessing the probability that among 2 randomly chosen and matched SPRINT participants on the basis of predicted benefit but with unequal observed benefit, the pair with greater observed benefit also has a higher predicted benefit (24). The bars represent 95% confidence limits of the observed risk differences. Abbreviations as in Figure 2.
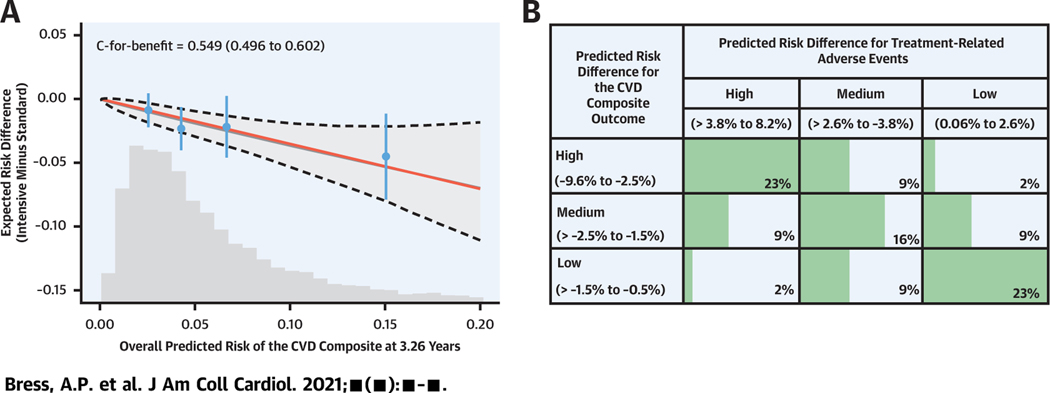
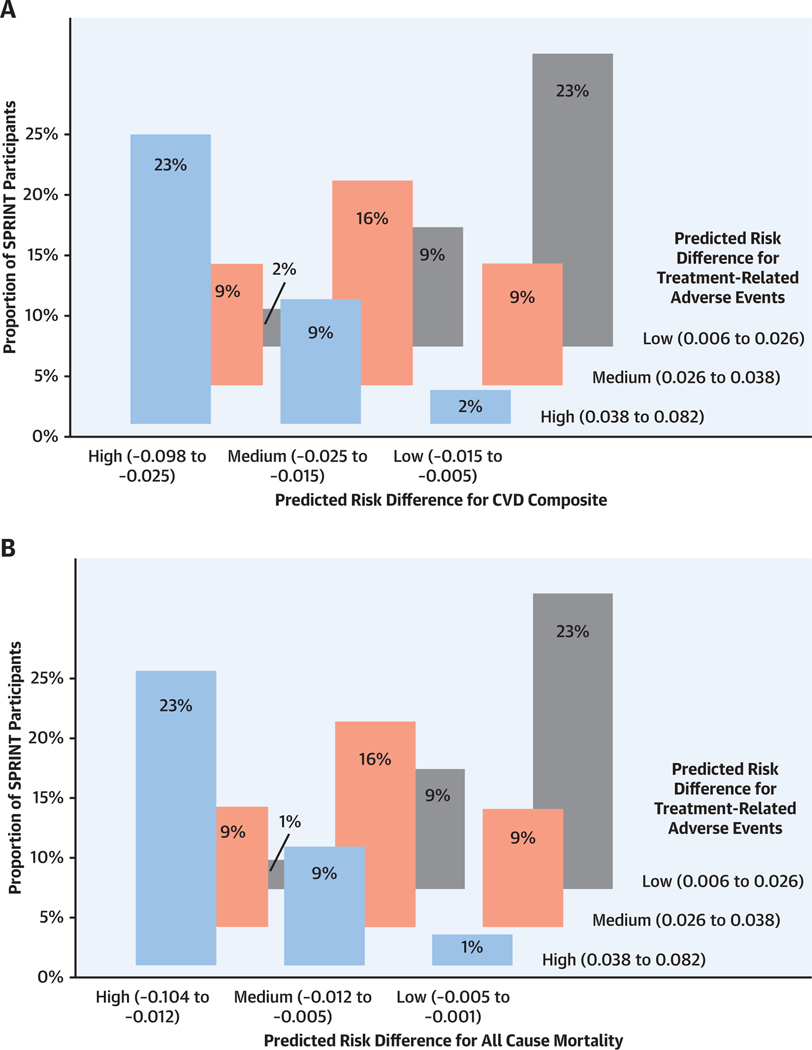
CENTRAL ILLUSTRATION. Cardiovascular Disease Benefit and Adverse Event Risk of Intensive Versus Standard Systolic Blood Pressure Treatment.
(A) Risk magnification plot showing the predicted magnitude of benefit (reduced risk for cardiovascular disease [CVD] composite outcome) with intensive versus standard systolic blood pressure (BP) treatment across a range of predicted baseline risk for the CVD composite outcome. The dashed black line and shaded gray areas represent the 95% confidence bands for the predicted risk difference. The bars represent 95% confidence limits of the observed risk differences. The histogram represents the distribution of baseline risk for each outcome in the SPRINT (Systolic Blood Pressure Intervention Trial) population used in the present analysis. (B) Distribution of SPRINT participants by tertile of the predicted magnitude of benefit (reduced risk for CVD composite outcome) and predicted increased risk for treatment-related adverse events with intensive versus standard systolic BP treatment.
CROSS-CLASSIFICATION AND THE RATIO OF PREDICTED MAGNITUDE OF BENEFIT TO RISK FOR TREATMENT-RELATED AEs.
Overall, the predicted magnitude of benefit with intensive versus standard treatment was strongly correlated with the predicted increased risk for treatment-related AEs, with Spearman correlation coefficients of 0.72 and 0.76 for treatment-related AEs versus the CVD composite and treatment-related AEs versus all-cause mortality, respectively (Supplemental Figures 5 and 6). For the CVD outcome, 95% of participants who fell into the highest tertile of predicted benefit also had high or moderate predicted increases in treatment-related AE risk (Central Illustration, Figure 4). Only 1.8% of all participants were categorized as high benefit with low risk for treatment-related AEs and 1.5% as low benefit with high risk for treatment-related AEs.
FIGURE 4. Distribution of SPRINT Participants by Tertile of Predicted Magnitude of Benefit (Reduced CVD Risk and All-Cause Mortality) and Predicted Increased Risk of Treatment-Related Adverse Events With Intensive Versus Standard Systolic Blood Pressure Treatment.
(A) Three-dimensional histogram showing the number of SPRINT participants within each cross-classification of tertiles of the elastic net Cox model–predicted Kaplan-Meier absolute risk differences for the CVD composite and adverse events at 3.26 years. (B) Three-dimensional histogram showing the number of SPRINT participants within each cross-classification of tertiles of the elastic net Cox model–predicted Kaplan-Meier absolute risk differences for all-cause mortality and treatment-related adverse events at 3.26 years. Abbreviations as in Figure 2.
The results of the hybrid risk- and effect-modeling analyses revealed that the C and C-for-benefit statistics were not statistically improved from the reduced model without interaction terms used in the primary analysis. These results suggest that the addition of the 6 baseline characteristic interactions with randomized treatment status did not increase the ability to predict treatment benefit or increased risk for treatment-related AEs (Supplemental Table 5).
DISCUSSION
In this predictive modeling analysis of SPRINT, higher baseline predicted CVD risk was associated with greater clinical benefit (i.e., greater absolute CVD risk reduction) with intensive versus standard systolic BP treatment. The implication of these results is that SPRINT-eligible patients at higher risk for CVD should be prioritized for intensive systolic BP treatment. Predicted CVD benefit and increased treatment-related AE risk with intensive treatment are highly correlated. Most participants with high predicted benefit also had greater predicted absolute risk increases for treatment-related AEs.
Predicted CVD benefit and increased risk for AEs from intensive systolic BP treatment should not be directly compared as clinically equivalent “trade-offs.” In SPRINT, the overall incidence of any AE was similar in the intensive and standard treatment groups, and many of the AEs that were more common in the intensive treatment group were mild and transient. For example, a study of the 288 SPRINT participants with adjudicated acute kidney injury (179 in the intensive treatment group and 109 in the standard treatment group) revealed that almost 90% had only a single such event, most had Kidney Disease Improving Global Outcomes stage 1 events, and approximately 90% recovered to their baseline kidney function during trial follow-up (25). Another challenge of assessing benefit and AE trade-offs in SPRINT is the potential for bias in the ascertainment of AEs due to the unblinded nature of the study. Beneficial outcomes were assessed only at specific study visits in both treatment arms, providing an equal and unbiased opportunity to identify them nondifferentially. In contrast, AEs could be reported at any study visit, and because there were approximately 10% more study visits among those in the intensive arm versus the standard arm, it is likely that there was a disproportionate opportunity for reporting of AEs in the intensive treatment group.
In the present study, we did not find evidence of effect modification (i.e., interactions between the treatment and subgroup) on the relative hazard scale for the 6 subgroup factors pre-specified in the primary publication from the SPRINT trial. Prior post hoc analyses of publicly available SPRINT data that used effect modeling revealed heterogeneity of treatment effects on the relative hazard scale across levels of age, race/ethnicity, diastolic BP, smoking status, triglyceride levels, predicted 10-year CVD risk, and eGFR (7–14). These analyses, although intriguing, are subject to risks for false-positive and false-negative conclusions given concerns with multiple comparisons and severely limited power to detect effect modification on the relative hazard scale (3–6). The fact that we found no evidence that the addition of interaction terms between intensive treatment and the 6 pre-specified subgroups in SPRINT improved estimation of heterogeneity of treatment effects is consistent with subgroup analysis results reported in the SPRINT primary results publication, which also revealed no evidence that the hazard ratio for the CVD composite varied across the same pre-specified factors (1).
Although we found a pattern of risk magnification—the greater the baseline CVD risk, the greater the CVD benefit of intensive systolic BP treatment—limitations to the statistical power of SPRINT precluded identification of a subgroup with an unacceptable AE burden relative to the magnitude of its benefit on CVD events and all-cause mortality. This limitation prevented us from developing a clinical decision tool that discerns and balances benefit and risk considerations in a clinically meaningful way. As a result of this, and because of the relatively transient nature of the AEs observed in SPRINT, we recommend that prioritization for intensive systolic BP treatment be based on SPRINT eligibility plus higher CVD and mortality risk, irrespective of risk for treatment-related AEs.
STUDY LIMITATIONS.
Strengths include the use of the complete SPRINT extended dataset, a randomized design, and full technical support from the SPRINT Coordinating Center. Although we observed direct evidence of risk magnification—greater absolute risk reduction among participants with higher baseline predicted CVD risk—confidence intervals for absolute risk difference estimates were wide at higher levels of predicted absolute risk, and the C-for-benefit statistic suggested that the benefit model had only modest discrimination. However, unlike the traditional C statistic, which has well-accepted thresholds for good discrimination, the novel C for benefit is more conservative and does not yet have well-established benchmarks (24). We did not include a decision tool for general clinical use with our models. Limitations to the statistical power in SPRINT preclude the identification of a subgroup with an unacceptable AE burden relative to the magnitude of its benefit on CVD events and all-cause mortality, which prevented us from developing a clinical decision tool that balances benefit and risk considerations. As a result of this, and because of the relatively transient nature of the AEs observed in SPRINT, we recommend that implementation of intensive systolic BP treatment prioritize patients on the basis of their CVD risk, irrespective of their risk for treatment-related AEs. Despite the practical advantages of using an existing CVD risk prediction model such as the pooled cohort equations (PCEs) (26), we instead developed an internal risk model for two reasons. First, the PCEs did not include heart failure as an outcome. This is a limitation because heart failure was the most influential component outcome of the SPRINT combined primary endpoint (heart failure hazard ratio: 0.62; 95% confidence interval: 0.45 to 0.84; p = 0.002). Second, the PCEs were derived and, therefore, intended to be used only in those 40 to 79 years of age without pre-existing clinical CVD. The PCEs are not relevant for a SPRINT cohort composed of 31% of participants with baseline age >79 years or histories of clinical CVD. Intensive systolic BP treatment in SPRINT significantly reduced the risk for combined mild cognitive impairment or probable dementia (hazard ratio: 0.85; 95% confidence interval: 0.74 to 0.97), and therefore we believe that our estimate of predicted benefit represents a lower bound, as our composite outcome did not incorporate cognitive outcomes (27). Future work by our group will develop and validate a prediction model that quantifies SPRINT participants’ predicted cognitive benefits and net benefit, incorporating cognitive and CVD benefits and increased risk for AEs with intensive versus standard treatment.
CONCLUSIONS
SPRINT-eligible patients with the highest CVD risk gain the greatest absolute benefit from intensive systolic BP treatment but also are the most likely to experience treatment-related AEs. However, given that most SPRINT AEs were mild and transient, the present analysis supports prioritizing higher CVD risk SPRINT-eligible patients for intensive systolic BP treatment. Delivering intensive systolic BP treatment requires additional health care resources, and more than 12 million U.S. adults are eligible to initiate or intensify antihypertensive treatment under current guidelines (28). The SPRINT clinical benefit models can efficiently prioritize intensive treatment to first reach optimal benefit patients before offering intensive treatment to the remainder of SPRINT-eligible patients.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In SPRINT, intensive treatment of systolic hypertension (goal <120 mm Hg) reduced CVD events and all-cause mortality more than standard treatment (goal <140 mm Hg), generally without increasing AEs. Patients at high absolute risk for CVD events gain the most benefit, and because treatment-emergent AEs are generally mild and transient, selection of patients for intensive treatment should prioritize predicted benefit over potential toxicity.
TRANSLATIONAL OUTLOOK:
Further research is needed to match specific CVD risk criteria with optimal treatment regimens to enhance personalized management for patients with hypertension.
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff members, and the participants of SPRINT for their valuable contributions. The support and resources from the Center for High Performance Computing at the University of Utah are gratefully acknowledged.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This study was directly supported by grant R01HL139837 from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Bress is also supported by NHLBI grant K01HL133468. Dr. Moran is also supported by NHLBI grant R01 HL 130500–01A1. Drs. Bress, Greene, Yang, and Moran had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SPRINT is funded with federal funds from the National Institutes of Health, including the NHLBI, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13–002-001. SPRINT was also supported in part with resources and use of facilities through the U.S. Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing, and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the U.S. government. For a full list of contributors to SPRINT, see the supplementary acknowledgment list: https://www.sprinttrial.org/public/dspScience.cfm. The authors also acknowledge support from the following Clinical and Translational Science Awards funded by the National Center for Advancing Translational Sciences: Case Western Reserve University: UL1TR000439; The Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston University: UL1RR025771; Stanford University: UL1TR000093; Tufts University: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; University of Texas Southwestern Medical Center: 9U54TR000017–06; University of Utah: UL1TR000105–05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1 TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337 Centers of Biomedical Research Excellence Award (National Institute of General Medical Sciences). Drs. Bress and Derington have received research support to their institution from Amgen and Amarin (not related to the current project). Dr. Weintraub has received research support from Amarin; and is a consultant for Amarin and AstraZeneca. Dr. Cushman has received research support to his institution from Eli Lilly. Dr. Yeh is a consultant for AstraZeneca; and has received research grants from AstraZeneca. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AE
adverse event
- BP
blood pressure
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- GND
Greenwood-Nam-D’Agostino
- PCE
pooled cohort equation
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental methods, figures, tables, and references, please see the online version of this paper.
REFERENCES
- 1.Wright JT Jr., Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bress AP, Bellows BK, King JB, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med 2017;377:745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol 2004;57:229–36. [DOI] [PubMed] [Google Scholar]
- 4.Dahabreh IJ, Hayward R, Kent DM. Using group data to treat individuals: understanding heterogeneous treatment effects in the age of precision medicine and patient-centred evidence. Int J Epidemiol 2016;45:2184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5:1–56. [DOI] [PubMed] [Google Scholar]
- 6.Kent DM, van Klaveren D, Paulus JK, et al. The Predictive Approaches to Treatment Effect Heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med 2020;172:W1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpa J, Bruzelius E, Doupe P, Le M, Faghmous J, Baum A. Assessment of risk of harm associated with intensive blood pressure management among patients with hypertension who smoke: a secondary analysis of the systolic blood pressure intervention trial. JAMA Network Open 2019;2:e190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan N, Cohen-Stavi CJ, Tsadok MA, et al. Translating clinical trial results into personalized recommendations by considering multiple outcomes and subjective views. NPJ Digital Medicine 2019;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel KK, Arnold SV, Chan PS, et al. Personalizing the intensity of blood pressure control: modeling the heterogeneity of risks and benefits from SPRINT (Systolic Blood Pressure Intervention Trial). Circ Cardiovasc Qual Outcomes 2017;10: e003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu S, Sussman JB, Rigdon J, Steimle L, Denton BT, Hayward RA. Benefit and harm of intensive blood pressure treatment: derivation and validation of risk models using data from the SPRINT and ACCORD trials. PLoS Med 2017;14: e1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira JP, Gregson J, Duarte K, et al. Individualizing treatment choices in the systolic blood pressure intervention trial. J Hypertens 2018;36: 428–35. [DOI] [PubMed] [Google Scholar]
- 12.Duan T, Rajpurkar P, Laird D, Ng AY, Basu S. Clinical value of predicting individual treatment effects for intensive blood pressure therapy: a machine learning experiment to estimate treatment effects from randomized trial data. Circ Cardiovasc Qual Outcomes 2019;12:e005010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Powers S, Qian J, Jung K, et al. Some methods for heterogeneous treatment effect estimation in high dimensions. Stat Med 2018;37:1767–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips RA, Xu J, Peterson LE, Arnold RM, Diamond JA, Schussheim AE. Impact of cardiovascular risk on the relative benefit and harm of intensive treatment of hypertension. J Am Coll Cardiol 2018;71:1601–10. [DOI] [PubMed] [Google Scholar]
- 15.Kent DM, Paulus JK, van Klaveren D, et al. The Predictive Approaches to Treatment Effect Heterogeneity (PATH) statement. Ann Intern Med 2020;172:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Systolic Blood Pressure Intervention Trial (SPRINT) protocol version 4.0. Available at: https://www.sprinttrial.org/public/Protocol_Current.pdf. Accessed March 4, 2021.
- 18.The PCORnet Common Data Model; 2021. [Google Scholar]
- 19.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B Stat Methodol 2005;67:301–20. [Google Scholar]
- 20.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei L. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011;30:1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell F Jr. The PHGLM procedure. SUGI Supplemental Library User’s Guide. Cary, North Carolina: SAS Institute, 1986. [Google Scholar]
- 22.D’Agostino RB, Nam B-H. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Handbook Stat 2003;23:1–25. [Google Scholar]
- 23.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015;34:1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Klaveren D, Steyerberg EW, Serruys PW, Kent DM. The proposed “concordance-statistic for benefit” provided a useful metric when modeling heterogeneous treatment effects. J Clin Epidemiol 2018;94:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocco MV, Sink KM, Lovato LC, et al. Effects of intensive blood pressure treatment on acute kidney injury events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 2018; 71:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019;321:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.