Abstract
Chemsex, a new risky sexual behavior involving participation in sexual relations under the influence of drugs, has shown a significantly increased prevalence in recent years. This fact entails a serious public health issue, especially when Chemsex is practiced by individuals with an HIV (Human Immunodeficiency Virus) diagnosis. Hence, analyzing the characteristics of Chemsex practices, associated sexual practices and the health outcomes of individuals who participate in Chemsex, is extremely important. The main aim of the present study is to analyze the prevalence and characteristics of the practice of Chemsex in a sample of 101 men with HIV who have sex with men who attended the Department of Infectious Diseases of the General University Hospital of Alicante (Spain). Furthermore, the association between Chemsex and Health-Related Quality of Life (HRQoL) was also assessed. Chemsex and sexual practices were evaluated by employing a questionnaire applied on an ad hoc basis. HRQoL was assessed by employing the Medical Outcomes Study HIV Health Survey (MOS-HIV). In total, 40.6% of the participants had practiced Chemsex during the last year. When sexual practices were compared between those individuals who practiced Chemsex and those who did not, the former presented a higher level of risky sexual behaviors, especially with occasional and multiple sexual partners. Regarding HRQoL, those individuals who practiced Chemsex exhibited a poorer HRQoL in the majority of domains, especially those participants who practiced it with a higher intensity. The present study points out the high prevalence of Chemsex practice between men with HIV who have sex with men in Spain. Moreover, this study highlights the negative effects of Chemsex on HRQoL, probably due to the mixed effects of higher levels of risky sexual practices and the consequences of drug consumption.
Keywords: Chemsex, HIV, men who have sex with men, health-related quality of life
1. Introduction
Prevalence of HIV infection is a global public health issue—it is estimated that 1.7 million people were infected in 2018 alone [1]. In Spain, according to the annual report on the epidemiology of HIV, issued by the Ministry of Health, during 2018, 3244 new HIV diagnoses were reported, of which 85.3% were men. Of that percentage, 56.4% corresponded to men who have sex with men (MSM), an epidemiological term used to describe men who have sex with other men regardless of their sexual orientation or gender identity [2]. This trend in the data remains consistent among other Western countries [3] and represents a 19-fold increase in the probability of HIV infection [4].
These data confirm that HIV infection continues to be a highly prevalent public health concern, especially in the MSM population [5]. Although new strategies such as pre-exposure prophylaxis (PReP) have emerged [6,7], prevention through condoms remains the most recommended method due to its accessibility and effectiveness [8,9]. However, the lack of condom use as a prophylactic method against HIV has contributed to an increase in the transmission of HIV and other sexually transmitted diseases (STDs), such as syphilis, gonorrhea, hepatitis, urethritis, genital warts and chlamydia in this population [10].
Beyond the classical prophylactic methods, the availability of effective antiretroviral treatments has allowed people with HIV that have achieved and maintained an undetectable viral load not to sexually transmit the virus to others [11,12]. This fact, although it has allowed for great advances and a reduction in HIV transmission, has developed a false sense of security in people living with HIV (PLWHIV), leading to a significant decrease in the employment of prophylactic measures in their sexual relationships, resulting in increased risky sexual behaviors and transmission of other STDs [13,14,15].
Although it is known that the previously explained phenomena could be the basis for the risk of transmission of HIV and other STDs in PLWHIV, in recent years, it has become clear that there is a need to identify other risky sexual behaviors that lead to a high probability of transmission of the virus. However, most studies focused on promoting the use of condoms and identifying the factors promoting their use, as shown in the review conducted by Evans [16]. Nevertheless, there is little research on newer sexual phenomena such as Chemsex. This term refers to the development of risky sexual relations under the effects of drug use, with different purposes, such as increasing pleasure or prolonging the duration of sexual intercourse [17,18,19]. This type of risky sexual behavior, which is highly prevalent among MSM, in which substance use is mixed with the absence of prophylactic measures, carries a high risk of contagion, and therefore is extremely serious, not only because of the risk it poses to the individual himself, but to public health in general [20]. Drugs frequently used to increase sexual experiences include methamphetamine, crystal meth, mephedrone, and gamma-hydroxybutyrate (GHB) [20,21,22,23]. Although the prevalence of Chemsex is difficult to estimate because few individuals report the practice of such behaviors [23,24], previous studies estimate that about 30–45% of MSM have practiced Chemsex on at least one occasion [18,22,25]. This is why the identification of explanatory variables of these types of phenomena and their relationship with both the physical and psychological health of people is a challenge that should be taken up urgently by the scientific community. In spite of this, information on this subject is scarce, partly due to the novelty of the phenomenon and the difficulty in evaluating it.
Beyond the consequences of the practice of Chemsex increasing the risk of transmission of HIV and other STDs, it is important to know the consequences of this risky sexual practice on the health of the individuals who practice it. To the best of our knowledge, no previous studies have identified the effects of Chemsex on specific health markers, such as Health-Related Quality of Life (HRQoL). HRQoL is defined as a subjective outcome measure that assesses the influence of health status and physical, mental, and social functioning in relation to an individual’s goals [26,27,28] and is considered a measure of health outcomes and treatment adherence among people with HIV [29]. Although, as has been indicated, no previous studies have analyzed the relationship between Chemsex and HRQoL in men with HIV who have sex with men, it has been demonstrated that there is a negative association between some risky sexual behaviors, such as unprotected anal penetration and HRQoL in this population [30,31,32]. There are some emerging mechanisms in the recent literature that could help to explain the possible negative effects of Chemsex on the health of this population. In this sense, the practice of Chemsex is associated with a decreased treatment adherence in PLWHIV, limiting the effects of such treatments [33,34]. On the other hand, a review conducted by Degroote, Vogelaers, and Vandijck [35] associated drug use with poorer physical and mental health, finding that 25% of MSM with HIV who consume drugs reported negative effects on their lives [36], a decrease in their autonomy in activities of daily living [37], and a negative impact on their social and work relationships [38,39]. Mental health is also negatively affected among HIV-positive MSM who practice Chemsex, with 15% of MSM experiencing a negative impact on their mental health [40,41,42,43]. It is likely that the mixed effects of higher risky sexual practices and drug consumption consequences could be the basis of the health deterioration of this population. However, more studies are needed to characterize Chemsex practices and their consequences for HRQOL in men with HIV who have sex with men.
With all this in mind, the main aim of the present study was to characterize the phenomenon of Chemsex among a sample of Spanish men with HIV infection who have sex with men, and to analyze the relationship between Chemsex and HRQoL in this population.
2. Materials and Methods
2.1. Study Design and Participants
In this cross-sectional observational study, we included 101 men with HIV infection who have sex with men. The majority of them had undertaken advanced studies, were single, employed, with a mean economic income above 1500 euros per month, and self-identified as homosexuals. The characteristics of the participants are summarized in Table 1.
Table 1.
Sociodemographic and serological status of the participants.
| n = 101 | ||
|---|---|---|
| Age | 43.62 ± 11.41 | |
| Educational level | ||
| Primary | 11 (10.8%) | |
| Secondary | 16 (15.8%) | |
| Advanced | 38 (37.7%) | |
| University | 36 (35.7%) | |
| Marital status | ||
| Single | 46 (45.5%) | |
| In a relationship | 40 (39.6%) | |
| Married | 10 (9.9%) | |
| Divorced | 4 (4%) | |
| Widowed | 1 (1%) | |
| Employment status | ||
| Student | 3 (3%) | |
| Employed | 76 (75.2%) | |
| Unemployed | 14 (13.8%) | |
| Pension | 8 (8%) | |
| Income level | ||
| <EUR 1000 | 19 (18.8%) | |
| EUR 1001–1500 | 20 (19.8%) | |
| EUR 1501–2000 | 23 (22.8%) | |
| EUR 2001–2500 | 16 (15.8%) | |
| >EUR 2500 | 23 (22.8%) | |
| Sexual orientation | ||
| Homosexual | 92 (91.1%) | |
| Bisexual | 6 (5.9%) | |
| Others | 3 (3%) | |
| HIV-related variables | ||
| Time since HIV diagnosis (years) | 9.37 ± 6.13 | |
| Current CD4+ lymphocytes (cells/µL.) | 812.22 ± 307.33 | |
| Nadir CD4+ lymphocyte (cells/µL.) | 469.28 ± 233.62 | |
| Viral load | ||
| ≤50 cop. ARN/mL | 93 (92.1%) | |
| >50 cop. ARN/mL | 8 (7.9%) |
2.2. Variables
Chemsex practices were evaluated through an ad hoc questionnaire developed for this study. The questionnaire included questions regarding the participants’ Chemsex practices and their frequency during the last year, individuals with whom the participants engaged in Chemsex (stable sexual partner, occasional sexual partner and/or both), the type and the frequency of drugs consumed, and the time at which sex developed into Chemsex (e.g., whether drugs were taken before sex, during sex or before and during sex). The assessment of other sexual practices included questions regarding the frequency of specific sexual practices (double receptive anal penetration, double insertive anal penetration, receptive anal penetration, insertive anal penetration, fist penetration (fisting), anilingus, oral sex (fellatio) or mutual masturbation), and condom use (frequency of condom use, condom use during the last anal penetration or condomless anal intercourse at least once). These questions were answered by participants separately regarding stable and occasional sexual partners.
Sexually transmitted diseases (STDs). A single question was included to evaluate if participants were diagnosed with any of the following STDs at least once: genital warts, genital ulcers, urethritis, proctitis, syphilis, chlamydia, candidiasis or gonorrhea.
Health-Related Quality of Life (HRQoL). For the evaluation of HRQoL, we employed the Spanish version of the Medical Outcome Study-HIV Health Survey [44]. This questionnaire includes 11 subscales of HRQoL: General Health Perceptions (5 items), Pain (2 items), Physical Functioning (6 items), Role Functioning (2 items), Social Functioning (1 item), Mental Health (5 items), Energy/Fatigue (4 items), Cognitive Functioning (4 items), Health Distress (4 items), Quality of Life (1 item) and Health Transition (1 item). The scores obtained for these subscales can be quantified through the calculation of two general indexes: Physical Health Summary (PHS) and Mental Health Summary (MHS). Questions refer to the last two weeks and are rated on 2, 3, 5, and 6-point scales with a final score reflected on a scale from 0 to 100. Higher scores indicate greater health [45]. A recent reliability generalization meta-analysis pointed out that this instrument is highly reliable for the evaluation of HRQoL, with an average α coefficient for the total score of MOS-HIV of 0.91 and above 0.80 for all of the subscales, except for Role Functioning [46].
2.3. Procedure
The research was conducted in the Infectious Diseases Unit of the General University Hospital of Alicante in Spain. All patients in usual care between February 2020 and December 2020 who met the inclusion criteria were invited to participate in the study by filling in the indicated self-reported questionnaires. The confidentiality and anonymity of the obtained results was assured to participants throughout the whole study. Hence, to protect the confidentiality and anonymity of the data, codes were assigned to identify the participants. Furthermore, the research was conducted following the guidelines of the Declaration of Helsinki and the European Union Good Clinical Practice Standards, and the study was approved (26 February 2020) by the Ethics Committee of the General University Hospital of Alicante (PI2019/083). Inclusion criteria were: (1) HIV infection diagnosis, (2) ≥18 years-old, (3) being men who have sex with men, (4) being a patient receiving antiretroviral therapy and (5) having signed the informed consent to participate in the study. Exclusion criteria included: (1) the presence of comorbidities identified in medical records, dementia or other central nervous system diseases, mental health condition(s) diagnosis, viral chronic hepatitis, active cancer or infection, diabetes mellitus, high blood pressure, cardiovascular disease, hypothyroidism, malnutrition and other severe health conditions; (2) mental or physical impairments that could hinder participants’ ability to complete or understand the study questionnaires. After potential participants signed the informed consent, researchers exhaustively revised their medical records in order to identify any of the previously indicated exclusion criteria. Compliance with any of these exclusion criteria by patients led to exclusion from participation in the study. Participants were retained in the final sample only if they responded to all the questions involving the dependent variables.
2.4. Data Analysis
Descriptive analyses of the sociodemographic characteristics of the sample were carried out. The frequencies of characteristics of Chemsex and other sexual practices were calculated. Differences between participants who practiced Chemsex and those who did not in terms of STD diagnosis and condom use were analyzed by employing the chi-square statistic. Differences in the frequency of sexual practices and HRQoL between individuals who practice and do not practice Chemsex were identified through T-test analyses. Moreover, specific differences between Chemsex practitioners, depending on the time of the practice (e.g., whether drugs were taken before sex, during sex or before and during sex), were evaluated by employing non-parametric analyses in the form of the Kruskal–Wallis test. p < 0.05 was considered significant in all cases. All statistical analyses were conducted using SPSS version 24.0 (Armonk, NY, USA).
3. Results
3.1. Characteristics of Chemsex Practice in the Sample
Forty-one (40.6%) participants indicated that they participated in Chemsex during the last year and 60 (59.4%) indicated that they did not. Of these 41 participants, 8 (19.5%) had a stable partner, 19 (46.3%) had occasional sexual partners and 14 (34.2%) had both. With regard to the frequency of Chemsex, 20 (48.8%) participated in this sexual practice rarely, 15 (36.6%) sometimes, 5 (12.2%) very often and 1 (2.4%) always or almost always. Concerning the time at which participants consumed substances related to sexual practices, 10 (24.4%) consumed them before sex, 13 (31.6%) during sex and 18 (44%) before and during sexual practices. Table 2 and Table 3 include information regarding the time at which participants consumed each type of substance (never, before, during or before and during sexual practices) and the frequency of consumption of each type of substance.
Table 2.
Type of drug consumed and time of drug consumption in relation to sex.
| Time of Consumption | ||||
|---|---|---|---|---|
| Drug | Never | Yes, Before Sex | Yes, during Sex | Yes, Before and during Sex |
| LSD (Lysergic acid diethylamide) | 41 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hallucinogenic Fungus | 41 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anabolic steroid | 41 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cannabis | 25 (60.9%) | 7 (17%) | 3 (7.4%) | 6 (14.7%) |
| Cocaine | 26 (63.5%) | 4 (9.7%) | 6 (14.7%) | 5 (12.1%) |
| Crack | 41 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Codeine | 40 (97.6%) | 0 (0%) | 0 (0%) | 1 (2.4%) |
| Methamphetamine | 35 (85.3%) | 0 (0%) | 0 (0%) | 6 (14.7%) |
| MDMA (Methylene dioxymethamphetamine) |
32 (78%) | 3 (7.4%) | 1 (2.4%) | 5 (12.2%) |
| GHB (Gamma hydroxybutyrate)/GBL (Gamma butyrolactone) | 30 (73.1%) | 2 (4.9%) | 5 (12.2%) | 4 (9.8%) |
| Heroin | 41 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ketamine | 39 (95.1%) | 0 (0%) | 0 (0%) | 2 (4.9%) |
| Khat | 40 (97.6%) | 1 (2.4%) | 0 (0%) | 0 (0%) |
| Mephedrone | 35 (85.3%) | 1 (2.4%) | 2 (5%) | 3 (7.3%) |
| Morphine | 41 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Opium | 40 (97.6%) | 0 (0%) | 0 (0%) | 1 (2.4%) |
| Poppers | 23 (56.1%) | 2 (4.9%) | 12 (29.3%) | 4 (9.7%) |
| Amphetamine | 29 (70.7%) | 6 (14.7%) | 2 (4.9%) | 4 (9.7%) |
| Sildenafil | 22 (53.7%) | 14 (34.2%) | 1 (2.4%) | 4 (9.7%) |
Table 3.
Frequency of type of drug consumed.
| Frequency | |||||
|---|---|---|---|---|---|
| Drug | Almost Never | Rarely | Sometimes | Very Often | Always or Almost Always |
| LSD (Lysergic acid diethylamide) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hallucinogenic Fungus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anabolic steroid | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cannabis | 0 (0%) | 3 (18.8%) | 4 (25%) | 4 (25%) | 5 (31.2%) |
| Cocaine | 1 (6.7%) | 8 (53.3%) | 5 (33.3%) | 0 (0%) | 1 (6.7%) |
| Crack | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Codeine | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Methamphetamine | 0 (0%) | 5 (83.3%) | 1 (16.7%) | 0 (0%) | 0 (0%) |
| MDMA (Methylene dioxymethamphetamine) |
0 (0%) | 7 (77.8%) | 2 (22.2%) | 0 (0%) | 0 (0%) |
| GHB (Gamma hydroxybutyrate)/GBL (Gamma butyrolactone) |
0 (0%) | 5 (45.4%) | 3 (27.3%) | 3 (27.3%) | 0 (0%) |
| Heroin | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ketamine | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Khat | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mephedrone | 0 (0%) | 5 (83.3%) | 1 (16.7%) | 0 (0%) | 0 (0%) |
| Morphine | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Opium | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Poppers | 3 (16.6%) | 5 (27.7%) | 7 (38.9%) | 1 (5.55%) | 2 (11.1%) |
| Amphetamine | 1 (8.3%) | 6 (50%) | 3 (25%) | 1 (8.3%) | 1 (8.3%) |
| Sildenafil | 4 (21%) | 3 (15.9%) | 5 (26.3%) | 5 (26.3%) | 2 (10.5%) |
3.2. Sexual Practices of Participants with Stable Sexual Partners (n = 59) and Differences Based on Chemsex Practice
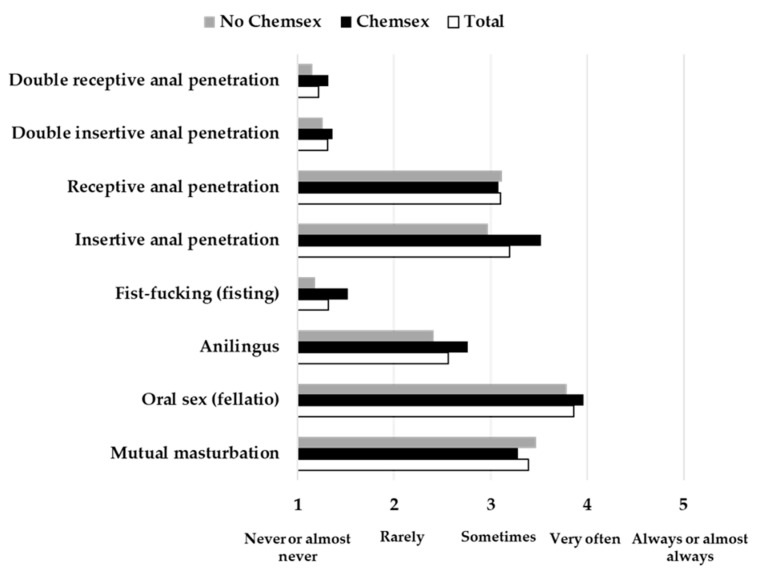
In the case of participants with stable sexual partners, 25 (42.4%) practiced Chemsex with their stable sexual partners and 34 (57.6%) did not. No differences were found between groups of Chemsex practitioners in terms of the frequency of any of the sexual practices evaluated with stable sexual partners (p > 0.05) (Figure 1).
Figure 1.
Frequency of sexual practices for all participants and for practitioners and non-practitioners of Chemsex separately, with stable sexual partners.
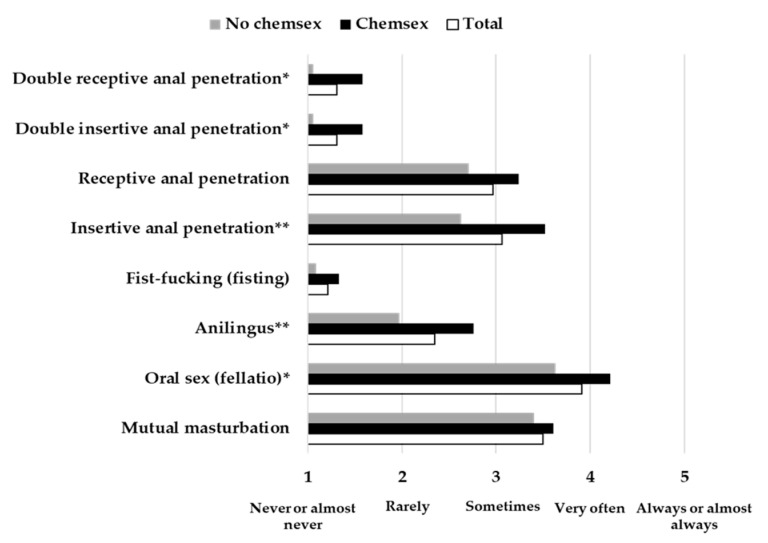
3.3. Sexual Practices of Participants with Occasional Sexual Partners (n = 68) and Differences Based on Chemsex Practice
For participants with occasional sexual partners, 33 (48.5%) practiced Chemsex with occasional sexual partners and 35 (51.5%) did not. In this case, significant differences were found for double receptive anal penetration t (36.991) = −2.445, p = 0.019, d = 0.80; double insertive anal penetration t(37.226) = −2.498, p = 0.017, d = 0.81; insertive anal penetration t(66) = −2.992, p = 0.004, d = 0.73; anilingus t(66) = −2.821, p = 0.006, d = 0.69; and oral sex t(59.053) = −2.403, p = 0.019, d = 0.62. In all cases, those participants who practiced Chemsex exhibited a higher frequency of the development of these sexual practices with occasional sexual partners (Figure 2).
Figure 2.
Frequency of sexual practices for all participants and for practitioners and non-practitioners of Chemsex separately, with occasional sexual partners. * p < 0.05, ** p < 0.01.
3.4. Differences in Condom Use between Participants Who Practiced Chemsex and Those Who Did Not
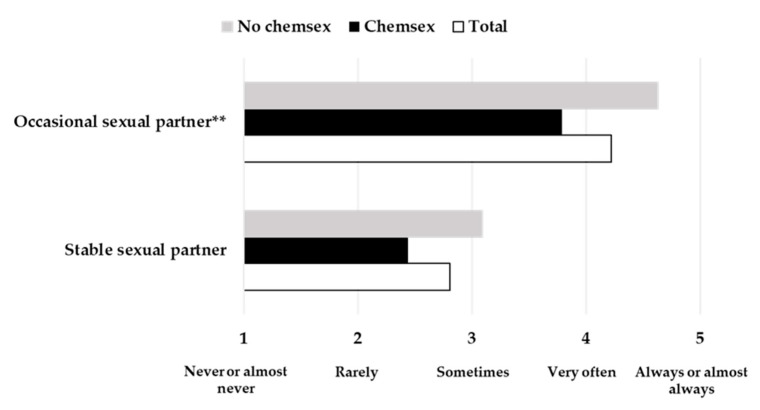
Regarding the frequency of condom use, differences were found in its employment with occasional sexual partners between participants who practiced Chemsex and those who did not (t (43.607) = 3.053, p = 0.004, d = 0.92). In this case, participants who practiced Chemsex employed condoms with a lesser frequency with occasional sexual partners in comparison to their counterparts who did not practice Chemsex. No differences were found in the case of condom use with stable sexual partners (p > 0.05) (Figure 3).
Figure 3.
Frequency of condom use for all participants and for practitioners and non-practitioners of Chemsex separately, with occasional and stable sexual partners. ** p < 0.01.
As can be observed in Table 4, regarding condom use during the last anal penetration, differences were found between groups of participants based on the practice of Chemsex, specifically regarding the last anal penetration when this occurred with occasional sexual partners. In this sense, individuals who practiced Chemsex presented a lower use of condoms in the last anal penetration when this occurred with occasional sexual partners in comparison to individuals who did not practice Chemsex. No differences were found in the case of the last anal penetration when this occurred with a stable sexual partner (Table 4).
Table 4.
Condom use during the last anal penetration with stable and occasional sexual partners.
| Condom Use during the Last Anal Penetration | |||||
|---|---|---|---|---|---|
| Stable Sexual Partner | |||||
| Total n = 59 |
Chemsex n = 25 |
No Chemsex n = 34 |
χ2 | p | |
| Yes | 24 (40.7%) | 8 (32%) | 16 (47.1%) | 1.354 | 0.245 |
| No | 35 (59.3%) | 17 (68%) | 18 (52.9%) | ||
| Occasional sexual partner | |||||
| Total n = 68 |
Chemsex n = 33 |
No Chemsex n = 35 |
χ2 | p | |
| Yes | 55 (80.9%) | 23 (69.7%) | 32 (91.4%) | 5.188 | 0.023 |
| No | 13 (19.1%) | 10 (30.3%) | 3 (8.6%) | ||
Regarding condomless anal intercourse at least once with stable and occasional sexual partners of discordant/unknown serological status, participants who practiced Chemsex exhibited a higher frequency of this practice with stable sexual partners. No differences were found regarding occasional sexual partners (Table 5).
Table 5.
Condomless anal intercourse at least once with stable and occasional sexual partners of discordant/unknown serological status.
| Condomless Anal Intercourse at Least Once | |||||
|---|---|---|---|---|---|
| Stable Sexual Partner of Discordant/Unknown Serological Status | |||||
| Total n = 59 |
Chemsex n = 25 |
No Chemsex n = 34 |
χ2 | p | |
| Yes | 10 (16.9%) | 9 (36%) | 1 (2.9%) | 11.185 | 0.001 |
| No | 49 (83.1%) | 16 (64%) | 33 (97.1%) | ||
| Occasional sexual partner of discordant/unknown serological status | |||||
| Total n = 68 |
Chemsex n = 33 |
No Chemsex n = 35 |
χ2 | p | |
| Yes | 18 (26.5%) | 11 (33.3%) | 7 (20%) | 1.551 | 0.213 |
| No | 50 (73.5%) | 22 (66.7%) | 28 (80%) | ||
3.5. Differences in Sexually Transmitted Diseases (STDs) between Participants Who Practiced Chemsex and Those Who Did Not
In the case of STD diagnosis in the evaluated sample, differences were found in the case of genital warts and urethritis. In both cases, participants who practiced Chemsex exhibited a higher prevalence of diagnosis of these diseases compared to their counterparts who did not practice Chemsex (Table 6).
Table 6.
Prevalence of STD diagnosis for all participants and for practitioners and non-practitioners of Chemsex.
| Total | Chemsex n = 41 |
No Chemsex n = 60 |
χ2 | p | ||
|---|---|---|---|---|---|---|
| Genital warts | Yes | 27 (%) | 16 (39%) | 11 (18.3%) | 5.324 | 0.021 |
| No | 74 (%) | 25 (61%) | 49 (81.7%) | |||
| Genital ulcers | Yes | 1 (%) | 1 (2.4%) | 0 (0%) | 1.478 | 0.224 |
| No | 100 (%) | 40 (97.6%) | 60 (100%) | |||
| Urethritis | Yes | 6 (%) | 6 (14.6%) | 0 (0%) | 9.335 | 0.002 |
| No | 95 (%) | 35 (85.4%) | 60 (100%) | |||
| Proctitis | Yes | 0 (%) | 0 (0%) | 0 (0%) | - | - |
| No | 101 (%) | 41 (100%) | 60 (100%) | |||
| Syphilis | Yes | 41 (%) | 21 (51.2%) | 20 (33.3%) | 3.231 | 0.072 |
| No | 60 (%) | 20 (48.8%) | 40 (66.7%) | |||
| Chlamydia | Yes | 11 (%) | 6 (14.6%) | 5 (8.3%) | 0.996 | 0.318 |
| No | 90 (%) | 35 (85.4%) | 55 (91.7%) | |||
| Candidiasis | Yes | 6 (%) | 4 (9.8%) | 2 (3.3%) | 1.798 | 0.180 |
| No | 95 (%) | 37 (90.2%) | 58 (96.7%) | |||
| Gonorrhea | Yes | 25 (%) | 12 (29.3%) | 13 (21.7%) | 0.756 | 0.385 |
| No | 76 (%) | 29 (70.7%) | 47 (78.3%) |
3.6. Differences between Participants Who Practiced Chemsex and Those Who Did Not in Terms of HRQoL
Differences in HRQoL between participants based on Chemsex practice were assessed. As can be observed in Table 7, significant differences were identified in the following domains: General Health Perception, Pain, Energy/Fatigue, Mental Health, Cognitive Functioning, Physical Health Summary and Mental Health Summary. In all cases, participants who practiced Chemsex obtained lower scores in these dimensions, indicating a poor HRQoL.
Table 7.
Differences in Health-Related Quality of Life (HRQoL) between participants who practiced Chemsex and those who did not.
| Chemsex n = 41 |
No Chemsex n = 60 |
t | p | Effect Size | |
|---|---|---|---|---|---|
| General Health Perception |
56.09 ± 24.14 | 68.75 ± 20.98 | t(99) = 2.798 | 0.006 | d = 0.56 |
| Pain | 65.85 ± 24.01 | 77.96 ± 21.88 | t (99) = 2.625 | 0.010 | d = 0.52 |
| Physical Functioning | 87.60 ± 17.88 | 91.38 ± 13.54 | t (99) = 1.210 | 0.229 | d = 0.24 |
| Role Functioning | 96.34 ± 17.28 | 97.50 ± 34.96 | t (99) = 0.196 | 0.845 | d = 0.03 |
| Social Functioning | 84.87 ± 23.57 | 91.33 ± 17.41 | t (68.905) = 1.497 | 0.139 | d = 0.36 |
| Energy Fatigue | 63.53 ± 19.50 | 74 ± 15.88 | t (99) = 2.961 | 0.004 | d = 0.59 |
| Mental Health | 64 ± 19.73 | 71.73 ± 16.43 | t (99) = 2.139 | 0.035 | d = 0.42 |
| Health Distress | 78.65 ± 26.29 | 87.91 ± 16.52 | t (61.477) = 2.001 | 0.050 | d = 0.51 |
| Cognitive Functioning | 74.51 ± 21.47 | 84.25 ± 14.01 | t (99) = 2.759 | 0.007 | d = 0.55 |
| Quality of Life | 62.19 ± 21.73 | 68.75 ± 17.60 | t (99) = 1.669 | 0.098 | d = 0.33 |
| Health Transition | 59.75 ± 24.92 | 60.41 ± 17.40 | t (66.112) = 0.147 | 0.884 | d = 0.03 |
| Physical Health Summary |
68.43 ± 16.85 | 78.01 ± 13.58 | t (99) = 3.153 | 0.002 | d = 0.63 |
| Mental Health Summary |
69.47 ± 18.36 | 78.55 ± 12.34 | t (64.343) = 2.769 | 0.007 | d = 0.69 |
3.7. Differences in HRQoL Depending on the Moment of the Practice of Chemsex Regarding Sex
The Kruskal–Wallis test was conducted to examine the differences in HRQoL according to the point at which sex became Chemsex (e.g., whether drugs were taken before sex, during sex or before and during sex). Significant differences were found in terms of Energy/Fatigue, Cognitive Functioning and Mental Health Summary. Post-hoc analyses were conducted, adjusting for significance (Bonferroni adjustment). In the case of Energy/Fatigue, no significant differences were found between specific groups. For Cognitive Functioning, significant differences were found between groups of participants who took drugs before sex and those who took drugs before and during sex (p = 0.004). Similarly, significant differences were found between the same groups in terms of their Mental Health Summary (p = 0.023). In both cases, participants who took drugs before and during sex presented lower scores in these dimensions of HRQoL in comparison to those who only took drugs before sex (Table 8).
Table 8.
Differences in HRQoL between participants who took drugs before sex, during sex or before and during sex.
| Chemsex n = 41 |
|||||
|---|---|---|---|---|---|
| Before Sex n = 10 |
during Sex n = 13 |
Before and during Sex n = 18 |
|||
| Mean rank | Mean rank | Mean rank | H (df) | p | |
| General Health Perception | 25.80 | 21.96 | 17.64 | 3.125 (2) | 0.210 |
| Pain | 26.80 | 18.42 | 19.64 | 3.261 (2) | 0.196 |
| Physical Functioning | 24.70 | 20.27 | 19.47 | 1.480 (2) | 0.477 |
| Role Functioning | 22 | 18.85 | 22 | 4.415 (2) | 0.110 |
| Social Functioning | 26.95 | 20.81 | 17.83 | 5.037 (2) | 0.081 |
| Energy Fatigue | 29.20 | 17.23 | 19.17 | 6.459 (2) | 0.040 |
| Mental Health | 28.55 | 19.08 | 18.19 | 5.322 (2) | 0.070 |
| Health Distress | 25.75 | 20.77 | 18.53 | 2.448 (2) | 0.294 |
| Cognitive Functioning | 30.55 | 21.46 | 15.36 | 10.478 (2) | 0.005 |
| Quality of Life | 26.65 | 21.65 | 17.39 | 4.472 (2) | 0.107 |
| Health Transition | 22.90 | 21.77 | 19.39 | 0.756 (2) | 0.685 |
| Physical Health Summary | 28.60 | 19.73 | 17.69 | 5.548 (2) | 0.062 |
| Mental Health Summary | 29.80 | 19.46 | 17.22 | 7.412 (2) | 0.025 |
df (degrees of freedom).
4. Discussion
The present study provides new findings about the Chemsex phenomenon in Spanish HIV-infected MSM. The prevalence of Chemsex practice was high between HIV-infected MSM who attended our HIV clinic, taking into account that almost half of the participants (40.6%) in the study had practiced Chemsex at least once during the last year. Engagement in this practice occurred with occasional sexual partners (46.3%), occasional and stable partners (34.2%) and, in a lesser proportion, within stable couples (19.5%). Our results are in accordance with published data from other European countries. In a review of scientific and national surveillance from the United Kingdom, the authors reported a prevalence of 17% in non-HIV MSM attending sexual health clinics and 31% in HIV-infected MSM [17]. The Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) study, which recruited HIV participants aged 18 years or older from eight HIV outpatient clinics, reported a 51% prevalence of Chemsex practices in a sample of 2248 patients [47]. A Spanish study from Madrid in HIV-positive people reported that 29.1% of the sample were Chemsex practitioners [48]. These results show a high prevalence of sexualized drug use in HIV-infected MSM, even higher than in HIV-negative people [17]. Different authors have explored several reasons for Chemsex engagement. Lafortune et al. reported that Chemsex play a role as a coping mechanism that helps individuals to deal with painful emotions or stressful events [49]. Ahmed et al. identified romantic breakups, receiving an HIV diagnosis, the death of a relative, and the accumulation of professional or domestic pressures as triggers of Chemsex practice [50]. In this regard, unpleasant or painful emotional states, such as loneliness [51,52], boredom [51], anxiety [53,54], depression [22], sleep problems [55], stigma associated with HIV-positive status [56], feelings of rejection [57,58] and negative body image [58], are more common among Chemsex users. Another reason why people engage in Chemsex is the perception of lower sexual self-efficacy and sexual pain when using drugs during sex. In fact, it has been found that sexual dysfunction and pain are more prevalent among Chemsex users than non-users [59]. As such, Chemsex practitioners use Chemsex to help themselves feel more attractive and to increase their sexual confidence, pleasure, physical sensations and/or orgasm intensity [57,58]. In this regard, suffering painful emotions, stressful events and psychological problems [60], as well as lower sexual self-efficacy and sexual pain [61], have been previously described as significant predictors of a low HRQoL in PLWHIV. Chemsex could be used as a coping mechanism to deal with distressing emotions such as anxiety, loneliness, boredom, or feelings of rejection, among others [51]. At the same time, this practice could be used to increase self-esteem, emotional closeness and feelings of attractiveness when Chemsex practitioners are struggling with sexual problems [58].
The most common Chemsex drugs used by participants in our study were cannabis, cocaine, methamphetamine, MDMA (Methylene dioxymethamphetamine), GHB (Gamma hydroxybutyrate)/GBL (Gamma butyrolactone), mephedrone, poppers, amphetamines and sildenafil. Cannabis, amphetamines and sildenafil were more frequently used before sex was initiated. This consumption pattern has been reported previously [19]. One of the most commonly used drugs is sildenafil. In general, it is employed by people without sexual problems, such as erectile dysfunction, probably due to the belief that these drugs can increase libido and improve sexual performance, helping to sustain long-lasting sexual activity and reverse the impotence-inducing effects of other substances (e.g., cocaine) or antiretroviral therapy [62]. During sex, participants used cocaine, GHB and poppers more often. GHB is a potent central nervous system depressant and, alongside poppers, can increase the libido, facilitating muscle relaxation to facilitate anal penetration and decrease pain perception [63,64]. Cocaine is probably used to compensate for the depressive symptoms caused by GHB and poppers, thereby increasing stimulation during sex [40]. Methamphetamine, cocaine, MDMA, GHB, ketamine and mephedrone were consumed both before and during sex, probably with the aim of enhancing, disinhibiting or facilitating the sexual experience [17].
Our study shows that Chemsex users have a high risk of transmission of other STDs. In this sense, it has been found that Chemsex practitioners with occasional sexual partners had more condomless sex with partners with discordant or unknown HIV serological status in comparison to non-practitioners. These results are supported by the literature, which has described the relationship between Chemsex practice and a higher number of sexual partners, a higher frequency of condomless sex, and a higher frequency of risky sexual behavior with partners of unknown or HIV-negative status while having a detectable viral load [22,58,65]. In this regard, it has been identified that the use of condoms by many is perceived as a reminder of their HIV status, interfering with their sexual pleasure, while drug use is perceived as a means to achieve a release and to escape from the burdens of HIV stigma [56]. Furthermore, Chemsex practitioners performed higher levels of risky sexual practices with occasional sexual partners, such as double receptive anal penetration, double insertive anal penetration and insertive anal penetration, which puts them at a higher risk of STD transmission [58,65,66,67]. Hence, our results point out the high incidence of STDs in HIV-positive MSM who practice Chemsex. Syphilis, gonorrhea and genital warts were the most frequent STDs in all participants, but only genital warts and urethritis of any origin were significantly more frequent in Chemsex users. In a UK study, authors found similar results in a sample of 1734 participants that used drugs during sex, reporting an increase in the incidence of new diagnoses of HIV infection, acute bacterial Sexually Transmitted Infections (STIs), rectal STIs and hepatitis C [68]. These results highlight the negative consequences of Chemsex on the health of this population.
In this sense, we analyzed the relationship between Chemsex and HRQoL in our sample. Chemsex users obtained lower scores in the domains of General Health Perception, Pain, Energy/Fatigue, Mental Health, Cognitive Functioning, Physical Health Summary and Mental Health Summary. To the best of our knowledge, this is the first study that has analyzed the specific association between Chemsex and HRQoL, employing specific evaluation instruments for the analysis of this health marker in PLWHIV. Moreover, our study points out that those who engage in more extreme forms of Chemsex, i.e., they take drugs both before and during sex, in comparison to those who only take drugs before or during sex, presented lower HRQoL scores, especially regarding Energy/Fatigue and Cognitive Functioning. This is probably related to the fact that a higher frequency of consumption and a larger quantity of drugs consumed could entail a significant deterioration of HRQoL. Taking into account that HIV infection decreases HRQoL [69,70], and also that drug use, such as methamphetamine [71] and recreational cannabis use [72] (among others [73]), affect HRQoL, it seems clear that Chemsex is an important cause of HRQoL decreases in PLWHIV. It is likely that a mixed effect of higher risky sexual practices and drug consumption could be a plausible mechanism to explain the obtained results. However, it is necessary to conduct new studies to identify how Chemsex affects HRQoL.
Although the present study advances our comprehension of Chemsex and its consequences in terms of HRQoL in men with HIV who have sex with men, some limitations should be taken into account. The design of the study only allows us to measure the association of the measures, meaning that we cannot establish any causality. Furthermore, the questionnaire was self-reported by the participants, meaning that some of them may not have been entirely truthful. Moreover, the number of HIV-positive people in the study sample was small, but we were able to confirm our results due to the fact that the participants were representative of MSM in the target population. Finally, the prevalence of depression, which has been previously related to HRQoL impairment, was not evaluated in this study.
5. Conclusions
In conclusion, our study highlights the high prevalence of Chemsex in MSM with an HIV infection who are undergoing antiretroviral treatment. MSM with HIV who practice Chemsex participate in more risky sexual practices and make less use of condoms with occasional partners, which leads to a greater risk of STD infection. In this sense, Chemsex is clearly related to a worse HRQoL. Detecting Chemsex practices could provide useful information to clinicians in order to establish prevention and intervention strategies for reducing health deterioration in this population. Future studies are necessary to analyze the specific mechanisms that explain the HRQoL deterioration in MSM who practice Chemsex, and to develop and evaluate the effectiveness of prevention and intervention programs oriented toward a reduction in Chemsex and HRQoL deterioration in MSM with an HIV infection.
Acknowledgments
We wish to thank all of the patients of the Infectious Diseases Unit of the General University Hospital of Alicante who participated in this study.
Author Contributions
Conceptualization, R.F.-C., J.P. and N.R.-R.; methodology, I.P.-T., C.A.-B. and V.C.-C.; formal analysis, C.A.-B. and N.R.-R.; data curation, V.C.-C. and I.P.-T.; writing—original draft preparation, N.R-R., C.A.-B., I.P.-T.; writing—review and editing, R.F.-C. and J.P.; supervision, R.F.-C., J.P. and N.R.-R.; project administration, N.R.-R.; funding acquisition, N.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Office of the Vice President of Research and Knowledge Transfer of the University of Alicante, Grant Number: GRE-18-17B.
Institutional Review Board Statement
This research was conducted following the guidelines of the Declaration of Helsinki and the European Union Good Clinical Practice Standards, and the study was approved (26 February 2020) by the Ethics Committee of the General University Hospital of Alicante (PI2019/083).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patients regarding the publication of this paper.
Data Availability Statement
The data are not publicly available due to reasons concerning privacy of the subjects and since it belongs to an ongoing project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global HIV & AIDS Statistics—2020 Fact Sheet. [(accessed on 23 November 2020)]; Available online: https://www.unaids.org/en/resources/fact-sheet.
- 2.World Health Organization . Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. World Health Organization; Geneva, Switzerland: 2014. [PubMed] [Google Scholar]
- 3.Zuge S.S., Paula C.C., Padoin S.M.M. Eficacia de las intervenciones para la adhesión al tratamiento antirretroviral en adultos con VIH: Revisión sistemática. Rev. Esc. Enferm. USP. 2020;54 doi: 10.1590/s1980-220×2019009803627. [DOI] [PubMed] [Google Scholar]
- 4.Poteat T., Scheim A., Xavier J., Reisner S., Baral S. Global Epidemiology of HIV Infection and Related Syndemics Affecting Transgender People. J. Acquir. Immune Defic. Syndr. 1999. 2016;72:S210–S219. doi: 10.1097/QAI.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crepaz N., Marks G., Liau A., Mullins M.M., Aupont L.W., Marshall K.J., Jacobs E.D., Wolitski R.J., HIV/AIDS Prevention Research Synthesis (PRS) Team Prevalence of Unprotected Anal Intercourse among HIV-Diagnosed MSM in the United States: A Meta-Analysis. AIDS. 2009;23:1617–1629. doi: 10.1097/QAD.0b013e32832effae. [DOI] [PubMed] [Google Scholar]
- 6.Aguirrebengoa O.A., García M.V., Nuñez J.A.P., López T.P., Lotero M.G., Garcia C.E., Utrilla M.R., Pérez V.E., Del Ro-mero Guerrero J., Martín C.R. La implementación de la profilaxis preexposición podría evitar la mayoría de las nuevas infecciones por el VIH en hombres que tienen sexo con hombres y mujeres transexuales. Rev. Clin. Esp. 2019;219:360–366. doi: 10.1016/j.rce.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Mascort J., Carrillo R., Alastrue I., Zarco J., Aguado C., Rodríguez B., Fransi L., Ramon J.L. Profilaxis Pre-Exposición de La Infección Por El VIH y Atención Primaria (AP) Aten. Primaria. 2020;52:137–139. doi: 10.1016/j.aprim.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koff A., Goldberg C., Ogbuagu O. Condomless Sex and HIV Transmission among Serodifferent Couples: Current Evidence and Recommendations. Ann. Med. 2017;49:534–544. doi: 10.1080/07853890.2017.1320423. [DOI] [PubMed] [Google Scholar]
- 9.Reis R.K., Melo E.S., Fernandes N.M., Antonini M., Neves L.A. de S.; Gir, E.; Reis, R.K.; Melo, E.S.; Fernandes, N.M.; Antonini, M.; et al. Inconsistent Condom Use between Serodifferent Sexual Partnerships to the Human Immunodeficiency Virus. Rev. Lat. Am. Enferm. 2019;27 doi: 10.1590/1518-8345.3059.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Workowski K.A., Bolan G.A. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 11.Betancourt Gambino J. Adherencia al Tratamiento Antirretroviral En Pacientes Seropositivos. Rev. Cuba. Med. Gen. Integral. 2018;34:82–93. [Google Scholar]
- 12.UNAIDS Data 2020. [(accessed on 10 December 2020)]; Available online: https://www.unaids.org/en/resources/documents/2020/unaids-data.
- 13.Delavande A., Kohler H.-P. HIV/AIDS-Related Expectations and Risky Sexual Behaviour in Malawi. Rev. Econ. Stud. 2016;83:118–164. doi: 10.1093/restud/rdv028. [DOI] [Google Scholar]
- 14.George G., Beckett S., Cawood C., Khanyile D., Govender K., Kharsany A.B.M. Impact of HIV Testing and Treatment Services on Risky Sexual Behaviour in the UMgungundlovu District, KwaZulu-Natal, South Africa: A Cross-Sectional Study. AIDS Res. Ther. 2019;16:20. doi: 10.1186/s12981-019-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huerga H., Venables E., Ben-Farhat J., van Cutsem G., Ellman T., Kenyon C. Higher Risk Sexual Behaviour Is Associated with Unawareness of HIV-Positivity and Lack of Viral Suppression—Implications for Treatment as Prevention. Sci. Rep. 2017;7:16117. doi: 10.1038/s41598-017-16382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans W.D., Ulasevich A., Hatheway M., Deperthes B. Systematic Review of Peer-Reviewed Literature on Global Condom Promotion Programs. Int. J. Environ. Res. Public Health. 2020;17:2262. doi: 10.3390/ijerph17072262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmundson C., Heinsbroek E., Glass R., Hope V., Mohammed H., White M., Desai M. Sexualised Drug Use in the United Kingdom (UK): A Review of the Literature. Int. J. Drug Policy. 2018;55:131–148. doi: 10.1016/j.drugpo.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Evers Y.J., Hoebe C.J.P.A., Dukers-Muijrers N.H.T.M., Kampman C.J.G., Kuizenga-Wessel S., Shilue D., Bakker N.C.M., Schamp S.M.A.A., Van Buel H., Van Der Meijden W.C.J.P.M., et al. Sexual, Addiction and Mental Health Care Needs among Men Who Have Sex with Men Practicing Chemsex—A Cross-Sectional Study in the Netherlands. Prev. Med. Rep. 2020;18:101074. doi: 10.1016/j.pmedr.2020.101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giorgetti R., Tagliabracci A., Schifano F., Zaami S., Marinelli E., Busardò F.P. When “Chems” Meet Sex: A Rising Phenomenon Called “ChemSex”. Curr. Neuropharmacol. 2017;15 doi: 10.2174/1570159X15666161117151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Baeza A., Dolengevich-Segal H., Pérez-Valero I., Cabello A., Téllez M.J., Sanz J., Pérez-Latorre L., Bernardino J.I., Troya J., De La Fuente S., et al. Sexualized Drug Use (Chemsex) Is Associated with High-Risk Sexual Behaviors and Sexually Transmitted Infections in HIV-Positive Men Who Have Sex with Men: Data from the U-SEX GESIDA 9416 Study. AIDS Patient Care STDs. 2018;32:112–118. doi: 10.1089/apc.2017.0263. [DOI] [PubMed] [Google Scholar]
- 21.McCall H., Adams N., Mason D., Willis J. What Is Chemsex and Why Does It Matter? BMJ. 2015;351:h5790. doi: 10.1136/bmj.h5790. [DOI] [PubMed] [Google Scholar]
- 22.Pufall E.L., Kall M., Shahmanesh M., Nardone A., Gilson R., Delpech V., Ward H. Sexualized Drug Use (‘Chemsex’) and High-Risk Sexual Behaviours in HIV-Positive Men Who Have Sex with Men. HIV Med. 2018;19:261–270. doi: 10.1111/hiv.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt A.J., Bourne A., Weatherburn P., Reid D., Marcus U., Hickson F. Illicit Drug Use among Gay and Bisexual Men in 44 Cities: Findings from the European MSM Internet Survey (EMIS) Int. J. Drug Policy. 2016;38:4–12. doi: 10.1016/j.drugpo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Frankis J., Flowers P., McDaid L., Bourne A. Low Levels of Chemsex among Men Who Have Sex with Men, but High Levels of Risk among Men Who Engage in Chemsex: Analysis of a Cross-Sectional Online Survey across Four Countries. Sex. Health. 2018;15:144–150. doi: 10.1071/SH17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawn W., Aldridge A., Xia R., Winstock A.R. Substance-Linked Sex in Heterosexual, Homosexual, and Bisexual Men and Women: An Online, Cross-Sectional “Global Drug Survey” Report. J. Sex. Med. 2019;16:721–732. doi: 10.1016/j.jsxm.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Chen W.-T., Wantland D., Reid P., Corless I.B., Eller L.S., Iipinge S., Holzemer W.L., Nokes K., Sefcik E., Rivero-Mendez M., et al. Engagement with Health Care Providers Affects Self- Efficacy, Self-Esteem, Medication Adherence and Quality of Life in People Living with HIV. J. AIDS Clin. Res. 2013;4:256. doi: 10.4172/2155-6113.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaRocca M.A., Scogin F.R. The Effect of Social Support on Quality of Life in Older Adults Receiving Cognitive Behavioral Therapy. Clin. Gerontol. 2015;38:131–148. doi: 10.1080/07317115.2014.990598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shumaker S.A. The International Assessment of Health-Related Quality of Life: A Theoretical Perspective. Int. Assess. Health-Relat. Qual. Life. 1995:3–10. [Google Scholar]
- 29.Zubaran C., Medeiros G., Foresti K., May W., Michelim L., Madi J.M., Group, the U.-U.R Quality of Life and Adherence to Antiretroviral Therapy in Southern Brazil. AIDS Care. 2014;26:619–625. doi: 10.1080/09540121.2013.841838. [DOI] [PubMed] [Google Scholar]
- 30.Bouhnik A.-D., Préau M., Schiltz M.-A., Peretti-Watel P., Obadia Y., Lert F., Spire B., Group V. Unsafe Sex with Casual Partners and Quality of Life Among HIV-Infected Gay Men: Evidence From a Large Representative Sample of Outpatients Attending French Hospitals (ANRS-EN12-VESPA) JAIDS J. Acquir. Immune Defic. Syndr. 2006;42:597–603. doi: 10.1097/01.qai.0000221674.76327.d7. [DOI] [PubMed] [Google Scholar]
- 31.Dey M., Gmel G., Studer J., Mohler-Kuo M. Health-Risk Behaviors and Quality of Life among Young Men. Qual. Life Res. 2014;23:1009–1017. doi: 10.1007/s11136-013-0524-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y., Liu J., Qu B., Hu B., Zhang Y. Relationship between Quality of Life and Unprotected Anal Intercourse among Chinese Men Who Have Sex with Men: A Cross-Sectional Study. BMC Public Health. 2016;16:382. doi: 10.1186/s12889-016-3076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garin N., Zurita B., Velasco C., Feliu A., Gutierrez M., Masip M., Mangues M.A. Prevalence and Clinical Impact of Recreational Drug Consumption in People Living with HIV on Treatment: A Cross-Sectional Study. BMJ Open. 2017;7:e014105. doi: 10.1136/bmjopen-2016-014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson M.A., Aberg J.A., Hoy J.F., Telenti A., Benson C., Cahn P., Eron J.J., Günthard H.F., Hammer S.M., Reiss P., et al. Antiretroviral Treatment of Adult HIV Infection: 2012 Recommendations of the International Antiviral Society–USA Panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 35.Degroote S., Vogelaers D., Vandijck D.M. What Determines Health-Related Quality of Life among People Living with HIV: An Updated Review of the Literature. Arch. Public Health. 2014;72:40. doi: 10.1186/2049-3258-72-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn R.W., Byrne N., O’Dea S., Shanley A., Codd M., Keenan E., Ward M., Igoe D., Clarke S. Chemsex, Risk Behaviours and Sexually Transmitted Infections among Men Who Have Sex with Men in Dublin, Ireland. Int. J. Drug Policy. 2018;52:9–15. doi: 10.1016/j.drugpo.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Closson E.F., Mitty J.A., Malone J., Mayer K.H., Mimiaga M.J. Exploring Strategies for PrEP Adherence and Dosing Preferences in the Context of Sexualized Recreational Drug Use among MSM: A Qualitative Study. AIDS Care. 2018;30:191–198. doi: 10.1080/09540121.2017.1360992. [DOI] [PubMed] [Google Scholar]
- 38.Hammoud M.A., Bourne A., Maher L., Jin F., Haire B., Lea T., Degenhardt L., Grierson J., Prestage G. Intensive Sex Partying with Gamma-Hydroxybutyrate: Factors Associated with Using Gamma-Hydroxybutyrate for Chemsex among Australian Gay and Bisexual Men—Results from the Flux Study. Sex. Health. 2017;15:123–134. doi: 10.1071/SH17146. [DOI] [PubMed] [Google Scholar]
- 39.Prestage G., Hammoud M., Jin F., Degenhardt L., Bourne A., Maher L. Mental Health, Drug Use and Sexual Risk Behavior among Gay and Bisexual Men. Int. J. Drug Policy. 2018;55:169–179. doi: 10.1016/j.drugpo.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell S., Shahmanesh M., Gafos M. Chemsex Behaviours among Men Who Have Sex with Men: A Systematic Review of the Literature. Int. J. Drug Policy. 2019;63:74–89. doi: 10.1016/j.drugpo.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 41.McCarty-Caplan D., Jantz I., Swartz J. MSM and Drug Use: A Latent Class Analysis of Drug Use and Related Sexual Risk Behaviors. AIDS Behav. 2014;18:1339–1351. doi: 10.1007/s10461-013-0622-x. [DOI] [PubMed] [Google Scholar]
- 42.Tomkins A., George R., Kliner M. Sexualised Drug Taking among Men Who Have Sex with Men: A Systematic Review. Perspect. Public Health. 2019;139:23–33. doi: 10.1177/1757913918778872. [DOI] [PubMed] [Google Scholar]
- 43.Wong N.S., Kwan T.H., Lee K.C.K., Lau J.Y.C., Lee S.S. Delineation of Chemsex Patterns of Men Who Have Sex with Men in Association with Their Sexual Networks and Linkage to HIV Prevention. Int. J. Drug Policy. 2020;75:102591. doi: 10.1016/j.drugpo.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Badía X., Podzamczer D., López-Lavid C., García M. Evidence-based medicine and the validation of quality-of-life questionnaires: The Spanish version of the MOS-HIV questionnaire for the evaluation of the quality of life in patients infected by HIV. Enferm. Infecc. Microbiol. Clin. 1999;17:103–113. [PubMed] [Google Scholar]
- 45.Wu A.W., Revicki D.A., Jacobson D., Malitz F.E. Evidence for Reliability, Validity and Usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual. Life Res. 1997;6:481–493. doi: 10.1023/A:1018451930750. [DOI] [PubMed] [Google Scholar]
- 46.Alcocer-Bruno C., Ferrer-Cascales R., Rubio-Aparicio M., Ruiz-Robledillo N. The Medical Outcome Study-HIV Health Survey: A Systematic Review and Reliability Generalization Meta-Analysis. Res. Nurs. Health. 2020;43:610–620. doi: 10.1002/nur.22070. [DOI] [PubMed] [Google Scholar]
- 47.Daskalopoulou M., Rodger A., Phillips A.N., Sherr L., Speakman A., Collins S., Elford J., Johnson M.A., Gilson R., Fisher M., et al. Recreational Drug Use, Polydrug Use, and Sexual Behaviour in HIV-Diagnosed Men Who Have Sex with Men in the UK: Results from the Cross-Sectional ASTRA Study. Lancet HIV. 2014;1:e22–e31. doi: 10.1016/S2352-3018(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 48.Dolengevich-Segal H., Gonzalez-Baeza A., Valencia J., Valencia-Ortega E., Cabello A., Tellez-Molina M.J., Perez-Elias M.J., Serrano R., Perez-Latorre L., Martin-Carbonero L., et al. Drug-Related and Psychopathological Symptoms in HIV-Positive Men Who Have Sex with Men Who Inject Drugs during Sex (Slamsex): Data from the U-SEX GESIDA 9416 Study. PLoS ONE. 2019;14:e0220272. doi: 10.1371/journal.pone.0220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lafortune D., Blais M., Miller G., Dion L., Lalonde F., Dargis L. Psychological and Interpersonal Factors Associated with Sexualized Drug Use Among Men Who Have Sex with Men: A Mixed-Methods Systematic Review. Arch. Sex. Behav. 2020 doi: 10.1007/s10508-020-01741-8. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed A.-K., Weatherburn P., Reid D., Hickson F., Torres-Rueda S., Steinberg P., Bourne A. Social Norms Related to Combining Drugs and Sex (“Chemsex”) among Gay Men in South London. Int. J. Drug Policy. 2016;38:29–35. doi: 10.1016/j.drugpo.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Amaro R. Taking Chances for Love? Reflections on Love, Risk, and Harm Reduction in a Gay Slamming Subculture. Contemp. Drug Probl. 2016;43:216–227. doi: 10.1177/0091450916658295. [DOI] [Google Scholar]
- 52.Pollard A., Nadarzynski T., Llewellyn C. Syndemics of Stigma, Minority-Stress, Maladaptive Coping, Risk Environments and Littoral Spaces among Men Who Have Sex with Men Using Chemsex. Cult. Health Sex. 2018;20:411–427. doi: 10.1080/13691058.2017.1350751. [DOI] [PubMed] [Google Scholar]
- 53.Deimel D., Stöver H., Hößelbarth S., Dichtl A., Graf N., Gebhardt V. Drug Use and Health Behaviour among German Men Who Have Sex with Men: Results of a Qualitative, Multi-Centre Study. Harm. Reduct. J. 2016;13 doi: 10.1186/s12954-016-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knight K.R., Das M., DeMicco E., Raiford J.L., Matheson T., Shook A., Antunez E., Santos G.-M., Dadasovich R., Dilley J.W., et al. A Roadmap for Adapting an Evidence-Based HIV Prevention Intervention: Personal Cognitive Counseling (PCC) for Episodic Substance-Using Men Who Have Sex with Men. Prev. Sci. 2014;15:364–375. doi: 10.1007/s11121-013-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millar B.M., Parsons J.T., Redline S., Duncan D.T. What’s Sleep Got to Do with It? Sleep Health and Sexual Risk-Taking Among Men Who Have Sex with Men. AIDS Behav. 2019;23:572–579. doi: 10.1007/s10461-018-2288-x. [DOI] [PubMed] [Google Scholar]
- 56.McCready K.C., Halkitis P.N. HIV Serostatus Disclosure to Sexual Partners among HIV–Positive Methamphetamine-Using Gay, Bisexual, and Other Men Who Have Sex with Men. AIDS Educ. Prev. 2008;20:15–29. doi: 10.1521/aeap.2008.20.1.15. [DOI] [PubMed] [Google Scholar]
- 57.Hunter C., Strike C., Barnaby L., Busch A., Marshall C., Shepherd S., Hopkins S. Reducing Widespread Pipe Sharing and Risky Sex among Crystal Methamphetamine Smokers in Toronto: Do Safer Smoking Kits Have a Potential Role to Play? Harm. Reduct. J. 2012;9:9. doi: 10.1186/1477-7517-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weatherburn P., Hickson F., Reid D., Torres-Rueda S., Bourne A. Motivations and Values Associated with Combining Sex and Illicit Drugs (‘Chemsex’) among Gay Men in South London: Findings from a Qualitative Study. Sex. Transm. Infect. 2017;93:203–206. doi: 10.1136/sextrans-2016-052695. [DOI] [PubMed] [Google Scholar]
- 59.Hibbert M.P., Brett C.E., Porcellato L.A., Hope V.D. Psychosocial and Sexual Characteristics Associated with Sexualised Drug Use and Chemsex among Men Who Have Sex with Men (MSM) in the UK. Sex. Transm. Infect. 2019;95:342–350. doi: 10.1136/sextrans-2018-053933. [DOI] [PubMed] [Google Scholar]
- 60.Corless I.B., Voss J., Guarino A.J., Wantland D., Holzemer W., Jane Hamilton M., Sefcik E., Willard S., Kirksey K., Portillo C., et al. The Impact of Stressful Life Events, Symptom Status, and Adherence Concerns on Quality of Life in People Living With HIV. J. Assoc. Nurses AIDS Care. 2013;24:478–490. doi: 10.1016/j.jana.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amini Lari M., Faramarzi H., Shams M., Marzban M., Joulaei H. Sexual Dysfunction, Depression and Quality of Life in Patients with HIV Infection. Iran. J. Psychiatry Behav. Sci. 2013;7:61–68. [PMC free article] [PubMed] [Google Scholar]
- 62.Dybdal-Hargreaves N.F., Holder N.D., Ottoson P.E., Sweeney M.D., Williams T. Mephedrone: Public Health Risk, Mechanisms of Action, and Behavioral Effects. Eur. J. Pharmacol. 2013;714:32–40. doi: 10.1016/j.ejphar.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Kurtz S.P. Post-Circuit Blues: Motivations and Consequences of Crystal Meth Use Among Gay Men in Miami. AIDS Behav. 2005;9:63–72. doi: 10.1007/s10461-005-1682-3. [DOI] [PubMed] [Google Scholar]
- 64.Lowry T.P. Psychosexual Aspects of the Volatile Nitrites. J. Psychoact. Drugs. 1982;14:77–79. doi: 10.1080/02791072.1982.10471914. [DOI] [PubMed] [Google Scholar]
- 65.Vosburgh H.W., Mansergh G., Sullivan P.S., Purcell D.W. A Review of the Literature on Event-Level Substance Use and Sexual Risk Behavior Among Men Who Have Sex with Men. AIDS Behav. 2012;16:1394–1410. doi: 10.1007/s10461-011-0131-8. [DOI] [PubMed] [Google Scholar]
- 66.Bourne A., Reid D., Hickson F., Torres-Rueda S., Weatherburn P. Illicit Drug Use in Sexual Settings (‘Chemsex’) and HIV/STI Transmission Risk Behaviour among Gay Men in South London: Findings from a Qualitative Study. Sex. Transm. Infect. 2015;91:564–568. doi: 10.1136/sextrans-2015-052052. [DOI] [PubMed] [Google Scholar]
- 67.Hagan H., Jordan A.E., Neurer J., Cleland C.M. Incidence of Sexually Transmitted Hepatitis C Virus Infection in HIV-Positive Men Who Have Sex with Men. AIDS. 2015;29:2335–2345. doi: 10.1097/QAD.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pakianathan M., Whittaker W., Lee M., Avery J., Green S., Nathan B., Hegazi A. Chemsex and New HIV Diagnosis in Gay, Bisexual and Other Men Who Have Sex with Men Attending Sexual Health Clinics. HIV Med. 2018;19:485–490. doi: 10.1111/hiv.12629. [DOI] [PubMed] [Google Scholar]
- 69.Hays R.D., Cunningham W.E., Sherbourne C.D., Wilson I.B., Wu A.W., Cleary P.D., McCaffrey D.F., Fleishman J.A., Crystal S., Collins R., et al. Health-Related Quality of Life in Patients with Human Immunodeficiency Virus Infection in the United States: Results from the HIV Cost and Services Utilization Study. Am. J. Med. 2000;108:714–722. doi: 10.1016/S0002-9343(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 70.Miners A.H., Sabin C.A., Mocroft A., Youle M., Fisher M., Johnson M. Health-Related Quality of Life in Individuals Infected with HIV in the Era of HAART. HIV Clin. Trials. 2001;2:484–492. doi: 10.1310/48ET-TT7G-35RA-D4C3. [DOI] [PubMed] [Google Scholar]
- 71.Mukherjee A., Dye B.A., Clague J., Belin T.R., Shetty V. Methamphetamine Use and Oral Health-Related Quality of Life. Qual. Life Res. 2018;27:3179–3190. doi: 10.1007/s11136-018-1957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldenberg M., IsHak W.W., Danovitch I. Quality of Life and Recreational Cannabis Use: Quality of Life and Recreational Cannabis Use. Am. J. Addict. 2017;26:8–25. doi: 10.1111/ajad.12486. [DOI] [PubMed] [Google Scholar]
- 73.Reddy K.P., Parker R.A., Losina E., Baggett T.P., Paltiel A.D., Rigotti N.A., Weinstein M.C., Freedberg K.A., Walensky R.P. Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study. J. Infect. Dis. 2016;214:1672–1681. doi: 10.1093/infdis/jiw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to reasons concerning privacy of the subjects and since it belongs to an ongoing project.