Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest malignancies. Present-day treatments have not shown real improvements in reducing the high mortality rate and the short survival of the disease. The average survival is less than 5% after 5 years. New innovative treatments are necessary to curtail the situation. The very dense pancreatic cancer stroma is a barrier that impedes the access of chemotherapeutic drugs and at the same time establishes a pro-proliferative symbiosis with the tumor, thus targeting the stroma has been suggested by many authors. No ideal drug or drug combination for this targeting has been found as yet. With this goal in mind, here we have explored a different complementary treatment based on abundant previous publications on repurposed drugs. The cell surface protein CD44 is the main receptor for hyaluronan binding. Many malignant tumors show over-expression/over-activity of both. This is particularly significant in pancreatic cancer. The independent inhibition of hyaluronan-producing cells, hyaluronan synthesis, and/or CD44 expression, has been found to decrease the tumor cell’s proliferation, motility, invasion, and metastatic abilities. Targeting the hyaluronan-CD44 pathway seems to have been bypassed by conventional mainstream oncological practice. There are existing drugs that decrease the activity/expression of hyaluronan and CD44: 4-methylumbelliferone and bromelain respectively. Some drugs inhibit hyaluronan-producing cells such as pirfenidone. The association of these three drugs has never been tested either in the laboratory or in the clinical setting. We present a hypothesis, sustained by hard experimental evidence, suggesting that the simultaneous use of these nontoxic drugs can achieve synergistic or added effects in reducing invasion and metastatic potential, in PDAC. A non-toxic, low-cost scheme for inhibiting this pathway may offer an additional weapon for treating pancreatic cancer.
Keywords: pancreatic ductal adenocarcinoma, pancreatic cancer stroma, bromelain, 4-methylumbelliferone, pirfenidone, desmoplastic reaction, stellate cells, hyaluronan, CD44
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC), the most aggressive type of pancreatic cancer, is characterized by its very important fibro-inflammatory stromal reaction and low vascularity [1], findings that can be considered pathognomonic for the disease. This feature, known as a desmoplastic reaction, creates a stroma that does not allow the arrival of chemotherapeutic drugs to malignant cells and is one of the fundamental reasons for treatment failures [2]. The desmoplastic reaction entails a fibroblast-rich stroma with an increased dense extracellular matrix (ECM). PDAC stroma provides “protection” to malignant epithelial cells and participates in cancer progression [3]. The intense desmoplastic reaction originates in pancreatic stellate cells (PSCs) that proliferate and produce extracellular matrix proteins, namely fibronectin and collagen creating a particularly dense environment surrounding the malignant cells [4,5]. The unique features of the ECM in pancreatic cancer go beyond “protection” modulating cancer growth through angiogenesis and growth factors [6]. Table 1 shows the evidence that sustains the pro-tumoral concept of the desmoplastic reaction.
Table 1.
Evidence of pro-tumoral effects of the desmoplastic reaction.
| Ref. | Finding |
|---|---|
| [7] | Gemcitabine resistance was promoted by collagen I through a metalloproteases (MT1-MMP) expression that in turn increases high mobility group AT 2 (HMGA2) protein which is an indirect transcription promoter. |
| [8] | Extracellular matrix proteins showed pro-proliferative activity in PDAC in vitro. |
| [9] | Pancreatic stromal fibroblasts showed the ability to promote cancer progression. |
| [10] | Matrix collagen significantly increased the production of MMPs in PDAC impacting cell migration and invasive potential. |
| [11] | Suppressing the desmoplastic process with metformin inhibited pancreatic cancer progression. |
| [12] | Desmoplasia promoted pancreatic cancer progression. |
| [13] | Extracellular matrix composition modulated PDAC and stem cells. An increased in collagen I introduced a switch in rapidly growing CSCs to a slower-growing avascular niche endothelial-type of cells, creating a supportive mechanism for tumor progression and mainly for tumor invasion. An active cross-talk between PDAC cells, CSCs, and matrix was carried out through the secretome. This process led to less proliferation but more invasion of PDAC cells into the CSCs derived vascular network. |
| [14] | The extracellular matrix of PDAC and focal adhesion kinase (FAK) were found to regulate CSCs |
| [15] | When PDAC cells were co-cultured with fibroblast, the latter acquired a myofibroblastic morphology expressing desmoplastic markers. |
| [16] | Adding stellate cells to a PDAC 3D cell culture generated a rich stroma similar to that found in vivo. |
Conclusions of Table 1: There are an intense interrelation and cross-talk between PDAC cells and fibroblasts that adopt the form of stellate cells which results in the production of a rich desmoplastic reaction that favors invasion and cancer progression.
Originally, the cancer cell was thought to be the main producer of the ECM [17], however, now it is evident that specialized cells, stellate cells, are the main ECM source. PSCs are in a quiescent stage surrounding pancreatic acini but when malignancy develops, they adopt active participation. A symbiotic relationship between malignant and stellate cells was proposed [9,18,19].
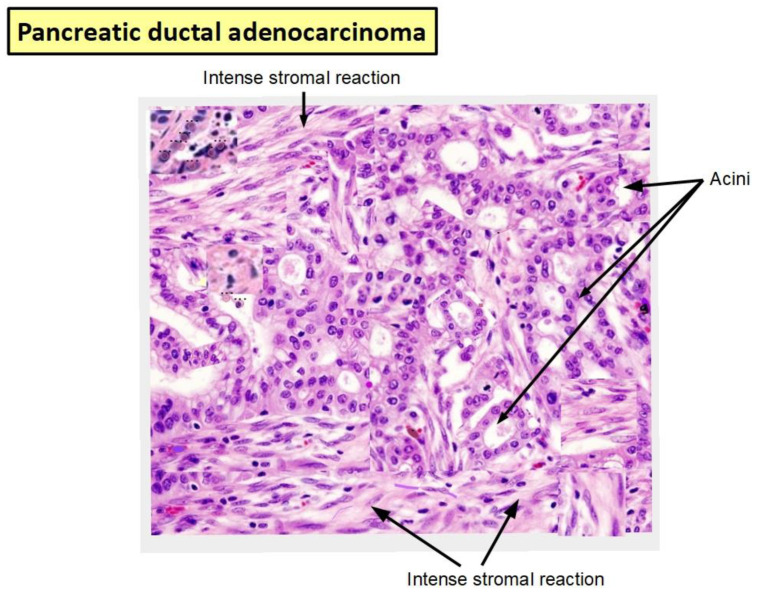
Figure 1 shows a surgically removed PDAC specimen where the relationship between tumor cells (ducts and acini) and the high levels of the intense desmoplastic stroma can be seen.
Figure 1.
Tissue specimen of a pancreatic ductal adenocarcinoma (PDAC). Intense stromal reaction surrounding tumoral ducts and acini (from the personal collection of TK). Magnification 150×.
Recently, Ogawa et al. [20] have distinguished three types of ECMs that differentially influence survival and molecular characteristics. These stromas had different fibroblast populations and transcriptome signatures. Thus, the PDAC matrix is a complex and heterogeneous issue that has direct implications in tumor progression and patient survival [21]. Furthermore, different types of fibroblasts have also been found in the normal pancreas which expands during carcinogenesis with a different prevalence according to subtypes [22].
Interestingly, there are drugs with the ability to inhibit fibroblastic proliferation and their production of collagen and pro-inflammatory cytokines. Pirfenidone is one of such drugs that have been FDA approved for the treatment of pulmonary fibrosis [23]. On the other hand, there are also non-toxic drugs that inhibit the enzymes intervening in hyaluronan production and drugs that decrease the expression of its receptor, CD44.
Targeting pancreatic extracellular environment has been investigated and proposed by many authors [24,25,26,27,28,29,30]. Many “repurposable” drugs with stromal inhibiting abilities have been identified, including metformin [31], all-trans retinoic acid [32], curcumin [33], glutamine analogs such as 6-diazo-5-oxo-l-norleucine (DON) [34], among others.
This manuscript will analyze separately the characteristics of these drugs and finally propose a unified vision of how they should act on the tumor stroma.
We strongly believe that there will be no real breakthrough in PDAC treatment unless the stroma is simultaneously targeted. Pancreatic stroma and tumor should be viewed as one pathological entity in which even if we can separate the parts, functionally they represent a non-divisible unit and so it should be treated.
2. Hyaluronan
Hyaluronan (hyaluronic acid), a glycosaminoglycan, is a major component of the extracellular matrix [35,36,37]. Its presence is also prominent in many inflammatory diseases and cancer [38]. Interestingly, when hyaluronan synthesis is pharmacologically decreased in animal models a clear benefit can be achieved in these pathological conditions [39].
Hyaluronan is consistently increased in pancreatic cancer stroma and it exerts a pro-tumoral action [40], including tumor growth [41], proliferation, invasion [42], and metastasis [43,44,45]. Hyaluronan is overexpressed in many inflammatory diseases, [46,47] and certain malignant tumors [48,49].
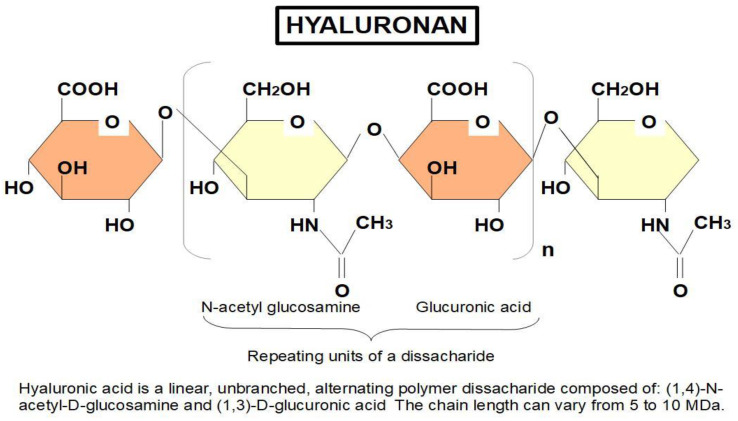
Hyaluronan consists of a large repeating disaccharide chain (Figure 2).
Figure 2.
Chemical structure of hyaluronan.
This polysaccharide has basically two functions:
-
(1)
Structural
-
(2)
Signaling
As a structural component of the extracellular matrix, it is the main molecule that provides strength and lubrication [48] playing an active role in cell adhesion [50,51] and motility [52,53,54].
As a signaling molecule, it intervenes in proliferation [55,56] and differentiation [57].
Hyaluronan’s structural functions, namely lubrication, and strength, can be fulfilled by this molecule without any receptor. However, signaling and motility require that hyaluronan binds to a specific receptor. The main binding receptor, CD44, is located on the cell surface. There is also another receptor for binding hyaluronan: the intracellular RHAMM (receptor for hyaluronan-mediated motility) While some authors have reported that RHAMM does not seem to have a direct connection with pro-tumoral activities [58], others have found that RHAMM is “an oncogene that regulates signaling through Ras and controls mitogen-activated protein kinase [extracellular signal-regulated protein kinase (ERK)] expression in embryonic murine fibroblasts” [59].
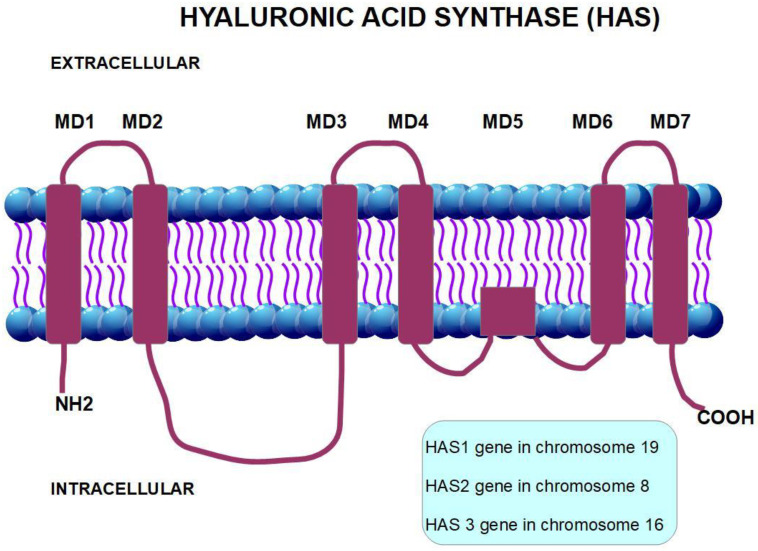
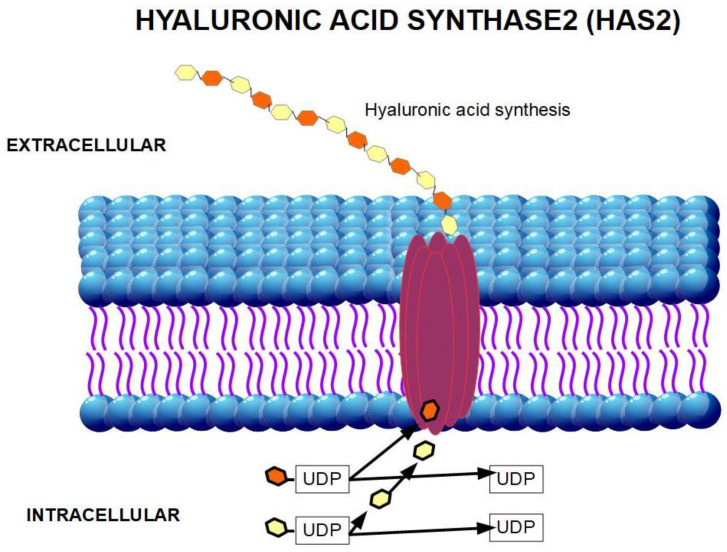
Hyaluronan is produced inside the cell membrane by the enzyme hyaluronan synthase [60]. There are three isoforms of hyaluronan synthase: HAS1, HAS2, and HAS3 (Figure 3 and Figure 4). The production and regulation of each of these enzymes are different. HAS2 produces the longest chain of hyaluronan. HAS2 over-expression produces a hyaluronan that diminishes contact inhibition and promotes cell growth. This was not found with HAS1 and HAS3 over-expression [61]. Therefore, we may assume that HAS2 is the origin of pro-tumoral hyaluronan.
Figure 3.
Structural representation of HAS2.
Figure 4.
Tri-dimensional representation of HAS2 producing hyaluronic acid (HA).
The different molecular weights of hyaluronan are important in cancer [62]: increasing molecular weight (up to 1000 kD) shows increased CD44 binding affinity [63,64].
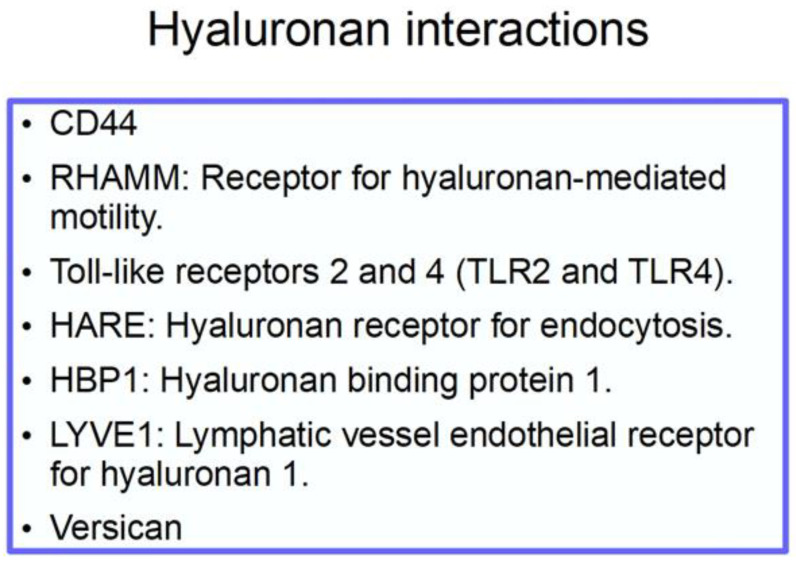
The chemical structure of hyaluronan is shown in Figure 2 [65], and its interactions in Scheme 1.
Scheme 1.
Interactions of hyaluronan. Versican is a chondroitin sulfate that with hyaluronan contributes to the extracellular matrix (ECM) density [66]. It is upregulated by TGFβ [67] and also interacts with CD44 and RHAMM.
3. The CD44 Antigen
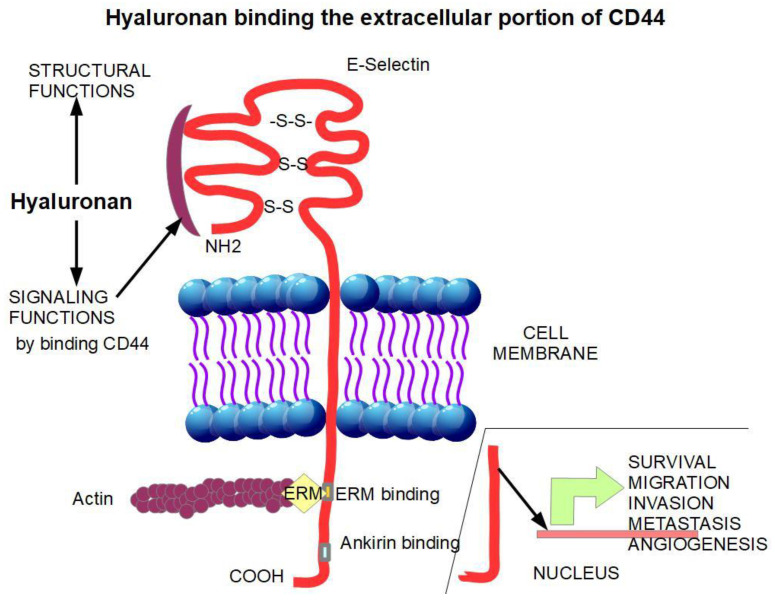
The CD44 antigen (synonym HCAM) is a glycoprotein acting as an adhesion molecule [68] on the cell surface. Cell adhesion molecules play an important role in cell migration. In fact, CD44 has been shown to be strongly related to invasion [69,70,71] and metastasis [72,73,74]. CD44 has three portions (Figure 5):
-
(1)
An extracellular amino-terminal domain to which hyaluronan binds activating signaling. The extracellular domain is also a binding site for other adhesion molecules such as E-selectin and fibronectin.
-
(2)
A transmembrane single spanning domain.
-
(3)
A short carboxy-terminal intracellular domain responsible for the signaling activity.
Figure 5.
The binding of hyaluronan to the extracellular domain of CD44 initiates pro-tumoral signaling. The intracellular portion of CD44 undergoes intra-membrane proteolysis releasing the intra-cytoplasmic domain that promotes the transcription of genes that induce survival, migration, invasion, metastasis, and angiogenesis. [75].
Importantly, there are many different isoforms produced by alternative splicing. They all have the ability to bind hyaluronan.
The hyaluronan-CD44 association represents a pro-tumoral hub. Signaling born from the intracellular portion of the HA-activated CD44 activates Ras, MAPK, PI3K [76], and RUNX2-RANKL pathways [77].
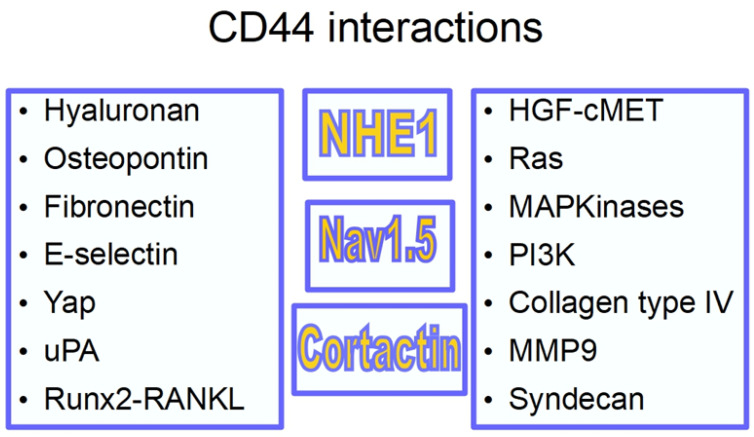
Scheme 2 shows CD44 interactions.
Scheme 2.
CD44 interactions: RunX1-RANKL responsible for bone metastasis; uPA urokinase-type plasminogen activator [78]; YAP (yes associated protein) [79] part of the Hypo signaling pathway; osteopontin [80] (a secreted integrin-binding protein whose association with cancer has not been clearly established [81], but probably having a role in tumor progression [82] and angiogenesis [83,84]); collagen type IV related with migration and invasion [85]; syndecan [86] is a proteoglycan that facilitates metastasis [87] and it is upregulated in pancreatic cancer [88]. It has a role in pancreatic cancer macropinocytosis [89]; metalloproteinase 9 transcription is regulated by the cytoplasmic tail of CD44 [90]. CD44 also has a role in MMP 9 relocation, increasing metastasis [91]. A prominent place was reserved for NHE1 because it has been shown that CD44 associates with and activates NHE1, thus participating in the pH gradient inversion, an important event in carcinogenesis and tumor progression [92,93,94,95]. The second prominent relationship is with the voltage-gated sodium channel Nav1.5 [96,97].
This triad, CD44, NHE1, Nav1.5 is present in invadopodia [98,99,100,101], the invasive structure of malignant cells, interacting and promoting invasion and metastasis [102,103].
Nav1.5 promotes invasion through the CD44-src-cortactin signaling pathway [70,104,105,106].
Importantly, CD44 is a marker of cancer stem cells (CSCs) and a regulator of stemness [107,108,109,110], integrating signals between CSCs and pre-metastatic niches [111]. There is evidence that CD44 knockdown can eradicate leukemia stem cells [112]. There is also evidence showing that CD44 positive cells play an important role in gemcitabine resistance in pancreatic cancer [113], distant metastasis, and aggressive behavior [114].
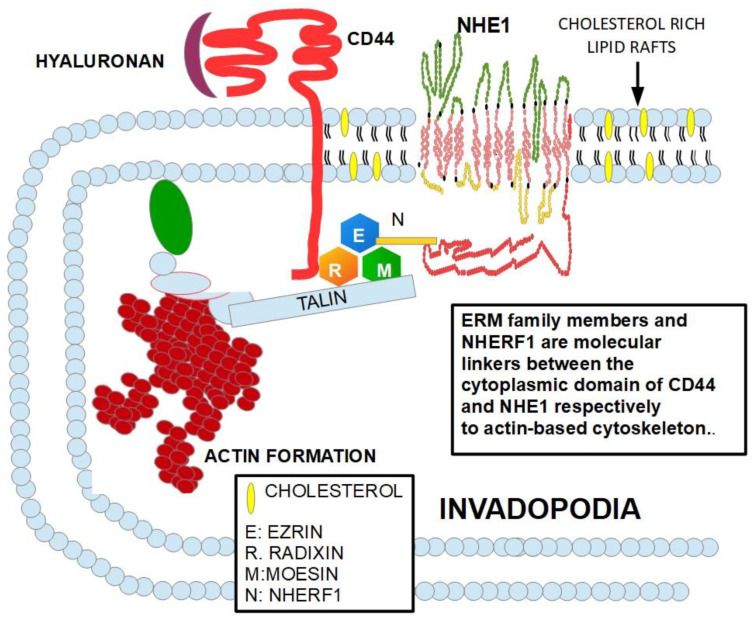
Figure 6 shows the relationship of CD44 with NHE1 and their influence on the cytoskeletal organization, which explains the reasons why CD44 is involved in cellular migration, invasion, and metastasis.
Figure 6.
CD44 interacts with the ERM family which connects CD44 to the actin synthesis at the invadopodia. The diagram is based on references [115,116,117,118,119,120,121].
These mechanisms triggering the hyaluronan-CD44 pathway and its protumoral activities can be interrupted by repurposing existing drugs.
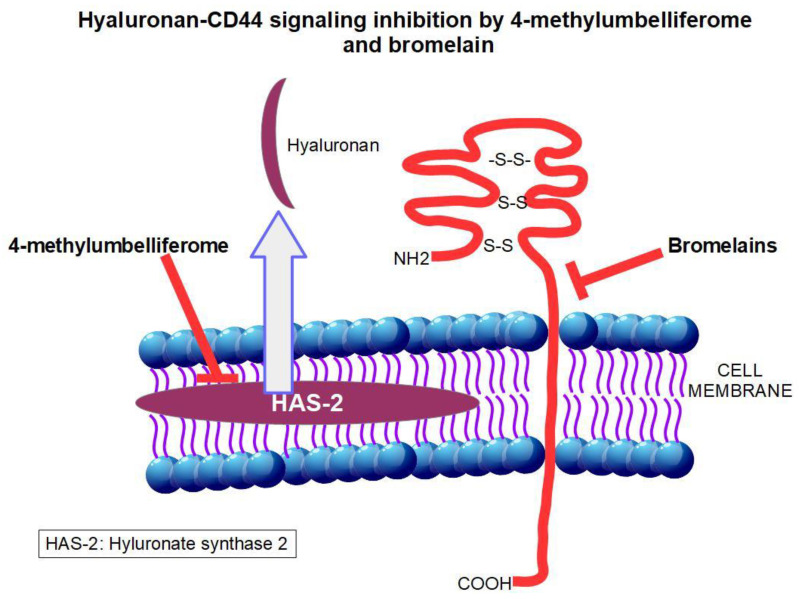
In this respect, we have selected two drugs due to their different effects on part of the pathway, and additive or synergistic effects can be expected: bromelain as an inhibitor of CD44 and hymecromone (4-methylumbelliferone) as an inhibitor of hyaluronan. Both drugs are available on the market (in Europe) but are not FDA approved.
3.1. Bromelain (BRO)
BRO is an extract from pineapple (Ananas comosus) from either the juice of the fruit or from the stem. Actually, it is present in all parts of the freshly picked plant and fruit. The Bromelain extract is a mixture of proteolytic enzymes and other substances including peroxidases.
It has been used for centuries as a folk medicine for the treatment of many different ailments. It is also a meat tenderizer and has found a place in the food industry.
In Europe, it is approved for the removal of necrotic tissues in burn lesions. For the FDA bromelain is only a dietary supplement.
The beneficial effects of bromelain are attributable to its multiple components but mainly to the proteolytic enzymes.
BRO has been used to reduce swelling, especially in ENT surgery and odontological procedures. Although used in folk medicine, the only approved use was issued in 2012 by the European Medicines Agency for removing dead tissue in skin burns. The molecular weight of purified stem bromelain determined by polyacrylamide-gel electrophoresis is 28,500 ± 1000 [122].
In 1972, Gerard was the first author to suggest an anti-cancer activity of BROs [123].
After Gerard, many publications were showed that BROs could play a role in cancer treatment (Table 2).
Table 2.
Experience with bromelain in cancer.
| Year/Ref. | Tissue/Animal | Finding |
|---|---|---|
| 1985 [124] | Lewis lung carcinoma, YC-8 lymphoma, MCA-1 ascitic tumor cells | BRO retarded tumor cell growth even when proteolytic and platelet aggregation effects were deactivated by heating when peroxidase activity was conserved. |
| 1988 [125] | Mice fed with BRO | Reduced lung metastasis of implanted Lewis lung carcinoma. |
| 1994 [126] | Human leukemia cells | BRO reduced CD44 expression on the cell surface, reducing adhesiveness to the endothelium. |
| 1994 [127] | Breast cancer, squamous carcinoma, and melanoma cells | Pre-treating lymphocytes with BRO inhibited the growth of the 3 tested cell types. BRO also had inhibitory effects when it was directly applied to the cells. |
| 1997 [128] | Mouse melanoma cells | In vitro treatment of the melanoma cells with BRO before i.v. injection into mice prevented lung colonization. |
| 2001 [129] | Glioma cells | BRO reduced migratory abilities by decreasing α3 and β1 integrin subunits and hyaluronan receptor CD44 protein levels. |
| 2007 [130] | Leukemia, sarcoma, Lewis lung carcinoma, Ehrich ascites, breast carcinoma, and melanoma cells. | These cells were injected into mice. Treatment with BRO started 24 hrs. later. With the exception of melanoma, the mice treated with BRO had significantly prolonged survival as compared with mice treated with 5FU used as controls. |
| 2009 [131] | Mouse skin tumorigenesis model | Pre-treatment with BRO reduced the total number of tumors and an average number of tumors per mouse. It inhibited COX2 expression and NF-kB activation. |
| 2010 [132] | GI-101A breast cancer cell | Dose-dependent apoptosis through the caspases pathway. |
| 2012 [133] | GI-101A breast cancer cell | Dose-dependent apoptosis through the caspases pathway. |
| 2012 [134] | Melanoma and epidermoid carcinoma cells | BRO induced apoptosis through inhibition of NF-kB nuclear translocation. BRO increased ROS by depleting intracellular glutathione. |
| 2013 [135] | Human gastrointestinal carcinoma cells | Cytotoxic effects of BRO were confirmed on these cells involving the caspases system, but also blocking Akt and attenuating Bcl2 and MUC1 oncoproteins. |
| 2013 [136] | Human umbilical vein endothelial cells (HUVEC) | BRO showed anti-angiogenic activity in vitro, inhibiting cell proliferation and tube formation. |
| 2014 [137] | Peritoneal Mesothelioma cells | Cells expressing MUC1 were treated with BRO. BRO induced cell death and increased cisplatin cytotoxicity. |
| 2015 [138] | Gastric and colorectal cancer | BRO and n-acetylcysteine showed synergistic inhibition of tumor growth in vivo. |
| 2016 [139] | Peritoneal dissemination of gastrointestinal mucin-producing cells | BRO and n-acetylcysteine reduced the dissemination and proliferation of gastrointestinal mucin-producing cancers. |
| 2016 [140] | Cholangiocarcinoma cells | BRO decreased proliferation, migration, and invasion. BRO inhibited NF-kB/AMPK signaling. |
| 2018 [141] | Lymphoma cells | BRO with peroxidases induced mitochondrial apoptosis. |
| 2019 [142] | Oral cancer cells | Viability was reduced in a dose-dependent manner. |
| 2019 [143] | Non-Hodgkin lymphoma | BRO with peroxidases reduced progression of non-Hodgkin lymphoma in vivo. |
Furthermore, BRO inhibits/blocks CD44 and reduces its expression on the cell surface but does not reduce its transcriptional output. The relation between BRO and CD44 is shown in Table 3.
Table 3.
BRO-CD44 relationship.
| Year/Ref | Tissue | Findings |
|---|---|---|
| 1992 [144] | Human T cells | BRO removed CD44 from the cell surface |
| 1994 [145] | Human peripheral blood lymphocytes | BRO reduced the CD44 mediated adhesion of human lymphocytes to human umbilical vein endothelial cells. |
| 1994 [126] | Leukemia and melanoma cells | BRO modulated CD44 expression |
| 1996 [146] | Lymphocytes | BRO modulated CD44 expression and reduced lymphocyte adhesiveness. |
| 2001 [129] | Glioma cells | BRO reduced invasiveness of glioblastoma cells by downregulating CD44 |
| 2016 [147] | Human and mouse tumor cells | BRO reduced CD44 expression from the surface of tumor cells. |
BRO was tested on MCF-7 breast cancer cells and the gene profiling showed differences in 1102 genes. The genes with more than 1.5-fold change in expression were: RNA-binding motif, single-stranded interacting protein 1 (RBMS1), ribosomal protein L29 (RPL29), glutathione S-transferase mu 2 (GSTM2), C15orf32, Akt3, B cell translocation gene 1 (BTG1), C6orf62, C7orf60, kinesin-associated protein 3 (KIFAP3), FBXO11, AT-rich interactive domain 4A (ARID4A), COPS2, TBPL1|SLC2A12, TMEM59, SNORD46, glioma tumor suppressor candidate region gene 2 (GLTSCR2), and LRRFIP [148].
At the cellular level, BRO has shown clear anti-tumoral effects and inhibitory activity on CD44. The exact nature of this inhibition is not fully known, but it is not at the transcriptional level. Probably BRO’s proteolytic activity is involved in the mechanism [149].
However, BRO seems to reduce both the CD4+ and CD8+ T- lymphocyte populations and the ratio [150]. Furthermore, BRO also removed CD44 expression from the surface of dendritic cells [151], suggesting that there might be incompatibilities with immunological checkpoint inhibitors. This anti-immune feature may be compensated by BRO’s ability to increase CD2 activation of T-cells [152] in mice. Further research is necessary to find out the exact nature of BRO’s effects on the immune system in humans.
An important question that remains to be answered is: can orally administered BRO achieve therapeutic concentrations at the tumor level?
Bioavailability
Oral BRO has been tested in rats [153]. One hour after administration the plasma concentration reached a maximum of 270 mg/mL, which represented 0.003% of the administered dose. In human volunteers, the administration of high doses of BRO resulted in an average plasma concentration of 10 picograms after 48 h [154].
There is clinical evidence that oral BROs have measurable clinical effects.
Heinicke et al. [155] administered oral BRO to 20 volunteers and it decreased platelet aggregation in 17 of them. Another experiment with orally administered BRO in rats showed decreased thrombus formation [156].
The anti-edematous and anti-fibrinolytic activity of BRO was tested in rats that received the medication through an esophageal tube. The antiphlogistic response was proportional to the dose [157].
While the evidence on achieving concentrations necessary for blocking CD44 with orally administered bromelain remains inconclusive, it has been shown that BRO can be absorbed by the human intestine without losing its biological activity [154].
In European markets, BRO formulations between 40 and 750 mg are found. The necessary dose to achieve clinical effects is around 1500 to 2000 mg. These doses are not toxic, and actually, a dose of 12 g/day has been found to lack toxicity [154,158].
3.2. 4-Methylumbelliferone (Hymecromone)
Hyaluronan is known to
increase the adhesion and motility of metastatic cells in melanoma [159];
increase the motility of pancreatic cancer cell [42] and prostate cancer cells [53];
increase proliferation [163].
Increased hyaluronan expression in tumor stroma is a sign of poor prognosis [164,165,166,167] and there is also evidence of hyaluronan building a pre-niche for driving metastasis [168].
4-methylumbelliferone (4MU) is a hydroxycoumarin that is umbelliferone replaced by a methyl group at position 4. It is an inhibitor of hyaluronan synthase and decreases the production of hyaluronan. Table 4 lists studies showing MU’s anti-tumoral properties.
Table 4.
Evidence of 4MU’s anti-tumoral effects.
| Year/Ref. | Tissue | Findings |
|---|---|---|
| 2006 [169] | Pancreatic cancer cells | 4MU increased the cytotoxicity of gemcitabine. |
| 2010 [170] | Prostate cancer cells | 4MU inhibited motility, invasion, and proliferation, inducing apoptosis of four different prostate cancer cell lines. It also inhibited NF-kB activity. |
| 2011 [171] | Hepatocarcinoma | In an orthotopic model of hepatocarcinoma, 4MU reduced proliferation and induced apoptosis. |
| 2011 [172] | Osteosarcoma | 4MU suppressed hyaluronan production and matrix formation in osteosarcoma cells, with reduction of proliferation in vitro and lung metastasis in vivo. |
| 2012 [173] | Breast cancer | 4MU inhibited tumorigenicity in vitro. It reduced tumor metastasis in vivo. |
| 2013 [174] | Canine mammary tumor cells | 4MU showed anti-tumoral actions in mesenchymal-like tumor cells. |
| 2013 [175] | Anti-angiogenic effects in vitro and in vivo | |
| 2014 [176] | Anti-angiogenic effects in vitro and in vivo | |
| 2014 [177] | Ovarian cancer cells | 4MU inhibited ovarian cancer cell proliferation in vitro, without affecting migration and invasion. In a peritoneal carcinomatosis model, 4MU inhibited tumor growth and prolonged survival. The authors suggest that thymidine phosphorylase inhibition is the reason for this anti-cancer activity. |
| 2015 [178] | Melanoma | 4MU reduced liver metastasis in a melanoma in vivo model. |
| 2016 [179] | Pancreatic cancer cells | 4MU derivatives were studied, showing a more powerful inhibition on the hyaluronan inhibition. |
| 2016 [180] | Pancreatic cancer cells | 4MU reduced hyaluronan production and suppressed cell proliferation in vitro and in vivo. |
| 2016 [181] | Non-malignant endometriotic lesions | 4MU reduced angiogenesis. |
| 2017 [182] | Implanted pancreatic cancer cells | SCID mice with cancer cells implanted in the peritoneum and treated with 4MU showed decreased progression, migration, and invasion. |
| 2018 [183] | Pancreatic cancer cells | 4MU decreased hyaluronan production and increased cytotoxicity of 5-fluorouracil. |
| 2018 [184] | Pancreatic cancer cells | 4MU decreased motility of pancreatic cancer cells. |
| 2019 [185] | Breast cancer cells | 4MU showed higher anti-tumoral activity in ERα negative tumors. |
| 2019 [186] | Ovarian cancer | 4MU decreased chemoresistance and inhibited cancer stem cell activation. |
| 2019 [187] | Fibrosarcoma cells | 4MU increased radiosensitivity by decreasing IL6 and IL8. |
| 2019 [188] | Colon cancer | Hyaluronan is a pro-tumoral molecule that also binds to TLR4 increasing proliferation and blocking apoptosis. Blockade of hyaluronan binding by any method, including decreased production with 4MU decreased proliferation. |
| 2020 [189] | Human normal endometrial cells | Reduced attachment, invasion, and invasion of endometrial epithelial and stromal cells. |
| 2021 [190] | Glioblastoma cells | 4MU diminished proliferation, cell migration, and metalloproteases inducing apoptosis. |
From Table 4 we can conclude that 4MU has clear anti-tumoral effects by decreasing hyaluronan synthesis.
3.3. Pirfenidone
A third drug that could independently target the fibrotic process is pirfenidone.
Pirfenidone is a pyridine that has been approved by the FDA for the treatment of pulmonary fibrosis [191,192,193]. It has also been tested in other fibrogenic diseases such as diabetic nephropathy [194], liver fibrosis [195], and for the normalization of tumor microenvironment increasing chemotherapeutic drugs access to the tumor by reducing hyaluronan and collagen levels in the ECM [196].
Desmoplastic tumors, such as pancreatic cancer, show a very dense ECM that compresses blood vessels [197,198] with two direct effects:
increased hypoxia with its pro-tumoral consequences and;
inability of chemotherapeutic drugs to reach their target cells.
Importantly, it was found that pirfenidone could block the desmoplastic process in pancreatic cancer [199].
Mechanism of action: Pirfenidone achieves its anti-fibrosis effects through different mechanisms of action. Pirfenidone:
-
(a)
inhibits collagen fibrils assembly [200];
-
(b)
down-regulates the intercellular adhesion molecule-1 (ICAM1) [201];
-
(c)
decreases transformation grow factor-beta (TGFβ) [202] at the translational level [203];
-
(d)
down-regulates the pro-fibrotic hedgehog signaling pathway [204];
-
(e)
decreases fibroblast proliferation [205];
-
(f)
blocks myofibroblast differentiation [206];
-
(g)
suppresses tumor necrosis factor alpha (TNFα) [207];
-
(h)
decreases cell migration-inducing and hyaluronan-binding protein [208];
-
(i)
inhibits MUC1 [209].
In summary, pirfenidone is a small molecule that reduces fibrogenesis and shows anti-inflammatory effects, for which it has been proposed as a therapeutic drug for advanced COVID-19 infection [210].
MUC1 is highly expressed in pancreatic cancer and it was associated with invasion, metastasis, and unfavorable overall survival [211], and inducing epithelial-mesenchymal transition [212]. Many authors consider MUC1 as a valid target in pancreatic cancer treatment [213,214,215,216,217]. Therefore, the ability of pirfenidone to block MUC1 phosphorylative activation is an added benefit of this drug.
Specifically, in pancreatic cancer, pirfenidone has shown the ability to decrease the production of the dense ECM and suppressing cancer cell proliferation [218] and cancer-associated fibroblasts proliferation [219] when associated with cisplatin.
All these characteristics make pirfenidone the best choice to associate with an anti-ECM treatment.
4. Discussion
Phytochemicals have been proposed as modulators/inhibitors of the desmoplastic reaction in PDAC in many previous publications (recently reviewed by Ramakrishnan et al. [220]).
Hyaluronan, a glycosaminoglycan, is a normal component of the extracellular stroma. The structural functions of this polydissacharide do not need a receptor and are fulfilled through its physical-chemical properties. PDAC is one of the tumors that usually contains large amounts of hyaluronan [221] and it has been shown that this cancer is highly dependent on hyaluronan production and signaling [182].
Indeed, hyaluronan also shows signaling abilities with a clear pro-tumoral effect and these signaling abilities require the binding to a cell surface protein: CD44. The association of hyaluronan with CD44 represents a pro-tumoral signaling hub. Inhibiting the CD44-hyaluronan association decreases cell motility [222] and probably multidrug resistance (MDR) gene expression [223].
Fortunately, both hyaluronan and CD44 can be down-regulated by existing, non-toxic, and low-cost drugs. BRO, obtained from the pineapple, decreases CD44 on the cell surface through posttranscriptional mechanisms that are not fully known. The most probable mechanism is through BRO’s proteolytic activity on the extracellular domain of CD44. 4MU, on the other hand, acts as an inhibitor of hyaluronan synthase 2, thus reducing the production of hyaluronan.
Both drugs have shown independently anti-tumoral effects. Here, we propose the association of these two drugs to synergistically reduce hyaluronan-CD44 signaling. To the best of our knowledge, this association was never tested either in the laboratory or in the clinical setting.
Figure 7 shows the mechanism of action of the 4MU and BRO association and Table 4 shows many examples of interesting results obtained in pancreatic cancer by inhibiting the hyaluronan-CD44 pathway.
Figure 7.
Site of actions on the hyaluronan-CD44 pathway of the drugs BRO and 4MU discussed in this paper.
A third drug, pirfenidone, targets the myofibroblasts responsible for the production of the unique PDAC ECM and would complement the effects of the other two.
Here we propose a triple association of three low-toxicity drugs, namely bromelain, 4-methylumbelliferone and pirfenidone to be added to classical pancreatic cancer treatment schemes. The fundamentals behind the triple association are that the three drugs act on different levels of the desmosomal reaction, thus an additive and possibly synergistic effect can be expected from their combined use. Reducing the ECMs pressure will allow less hypoxia and better arrival of cytotoxic drugs to the tumor’s core.
5. Conclusions
The hyaluronan-CD44 axis plays two essential roles in cancer: it creates a dense ECM in association with collagen and integrins and forms part of signaling pathways that participate in cellular motion, invasion, and metastasis. Both functions of the axis deserve targeting. Attacking the matrix through the hyaluronan-CD44 axis will not only allow better drug access to the malignant cells but also will reduce invasion and metastasis.
Patients with PDAC, with its dense stroma and high invasive ability, would be particularly benefited by inhibiting collagen production and blocking CD44 activation by hyaluronan.
The hypothesis that synergistic anti-tumoral effects can be achieved by associating bromelain, 4-methylumbelliferone, and pirfenidone to classical pancreatic cancer treatments is presented here. The fundamentals and evidence gathered on these drugs were summarized. BRO and 4MU lack toxicity and pirfenidone have low toxicity, thus we presume that complementing classical treatments with this association would not add to toxicity and can increase treatment effectiveness. We believe that this non-toxic, low-cost scheme for inhibiting this pathway may offer an additional weapon for treating pancreatic cancer.
Author Contributions
T.K. wrote the original draft; S.J.R. and R.A.C. worked on editing and finalizing; T.M.A.C. worked on organizing the tables and shcemes. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 813834—pHioniC—H2020-MSCA-ITN-2018. T.M.A.C. is a doctoral fellow in that program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Provenzano P.P., Hingorani S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olive K.P., Jacobetz M.A., Davidson C.J., Gopinathan A., McIntyre D., Honess D., Madhu B., Goldgraben M.A., Caldwell M.E., Allard D., et al. Inhibi-tion of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore J., Korc M. Pancreatic Cancer Stroma: Friend or Foe? Cancer Cell. 2014;25:711–712. doi: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apte M.V., Park S., Phillips P.A., Santucci N., Goldstein D., Kumar R.K., Wilson J.S. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Apte M.V., Wilson J.S., Lugea A., Pandol S.J. A starring role for stellate cells in the pancreatic cancer microen-vironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandol S., Gukovskaya A., Edderkaoui M., Dawson D., Eibl G., Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J. Gastroenterol. Hepatol. 2012;27:127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dangi-Garimella S., Krantz S.B., Barron M.R., Shields M.A., Heiferman M.J., Grippo P.J., Bentrem D.J., Munshi H.G. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA. Cancer Res. 2011;71:1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollenhauer J., Roether I., Kern H.F. Distribution of extracellular matrix proteins in pancreatic ductal adenocar-cinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hwang R.F., Moore T., Arumugam T., Ramachandran V., Amos K.D., Rivera A., Ji B., Evans D.B., Logsdon C.D. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Procacci P., Moscheni C., Sartori P., Sommariva M., Gagliano N. Tumor–Stroma Cross-Talk in Human Pancreatic Ductal Adenocarcinoma: A Focus on the Effect of the Extracellular Matrix on Tumor Cell Phenotype and Invasive Potential. Cells. 2018;7:158. doi: 10.3390/cells7100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan W., Chen K., Jiang Z., Chen X., Sun L., Li J., Lei J., Xu Q., Ma J., Li X., et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett. 2017;385:225–233. doi: 10.1016/j.canlet.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Incio J., Liu H., Suboj P., Chin S.M., Chen I.X., Pinter M., Ng M.R., Nia H.T., Grahovac J., Kao S., et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biondani G., Zeeberg K., Greco M.R., Cannone S., Dando I., Pozza E.D., Mastrodonato M., Forciniti S., Casavola V., Palmieri M., et al. Extracellular matrix composition modulates PDAC parenchymal and stem cell plasticity and behavior through the secretome. FEBS J. 2018;285:2104–2124. doi: 10.1111/febs.14471. [DOI] [PubMed] [Google Scholar]
- 14.Begum A., Ewachiw T., Jung C., Huang A., Norberg K.J., Marchionni L., McMillan R., Penchev V., RajeshKumar N.V., Maitra A., et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS ONE. 2017;12:e0180181. doi: 10.1371/journal.pone.0180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brancato V., Comunanza V., Imparato G., Corà D., Urciuolo F., Noghero A., Netti P.A. Bioengineered tu-moral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 2017;49:152–166. doi: 10.1016/j.actbio.2016.11.072. [DOI] [PubMed] [Google Scholar]
- 16.Ware M.J., Keshishian V., Law J.J., Ho J.C., Favela C.A., Rees P., Smith B., Mohammad S., Hwang R.F., Rajapakshe K., et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials. 2016;108:129–142. doi: 10.1016/j.biomaterials.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahlbacher V., Sewing A., Elsässer H.P., Kern H.F. Hyaluronan is a secretory product of human pancreatic adenocarcinoma cells. Eur. J. Cell Biol. 1992;58:28–34. [PubMed] [Google Scholar]
- 18.Mantoni T.S., Lunardi S., Al-Assar O., Masamune A., Brunner T.B. Pancreatic stellate cells radioprotect pan-creatic cancer cells through β1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonlaufen A., Phillips P.A., Xu Z., Goldstein D., Pirola R.C., Wilson J.S., Apte M.V. Pancreatic Stellate Cells and Pancreatic Cancer Cells: An Unholy Alliance: Figure 1. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa Y., Masugi Y., Abe T., Yamazaki K., Ueno A., Fujii-Nishimura Y., Hori S., Yagi H., Abe Y., Kitago M., et al. Three Distinct Stroma Types in Human Pancreatic Cancer Identified by Image Analysis of Fibroblast Subpopulations and Collagen. Clin. Cancer Res. 2021;27:107–119. doi: 10.1158/1078-0432.CCR-20-2298. [DOI] [PubMed] [Google Scholar]
- 21.Helms E., Onate M.K., Sherman M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020;10:648–656. doi: 10.1158/2159-8290.CD-19-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia P.E., Adoumie M., Kim E.C., Zhang Y., Scales M.K., El-Tawil Y.S., Shaikh A.Z., Wen H.-J., Bednar F., Allen B.L., et al. Differential Contribution of Pancreatic Fibroblast Subsets to the Pancreatic Cancer Stroma. Cell. Mol. Gastroenterol. Hepatol. 2020;10:581–599. doi: 10.1016/j.jcmgh.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drug Approval Package: Esbriet (Pirfenidone) Capsules NDA Nº022535 U.S. Food and Drug Administration; Silver Spring, MD, USA: 1999. [Google Scholar]
- 24.Shepard H.M. Breaching the Castle Walls: Hyaluronan Depletion as a Therapeutic Approach to Cancer Therapy. Front. Oncol. 2015;5:192. doi: 10.3389/fonc.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Ma Q., Xu Q., Duan W., Lei J., Wu E. Targeting the Cancer-Stroma Interaction: A Potential Approach for Pancreatic Cancer Treatment. Curr. Pharm. Des. 2012;18:2404–2415. doi: 10.2174/13816128112092404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kota J., Hancock J., Kwon J., Korc M. Pancreatic cancer: Stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. doi: 10.1016/j.canlet.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Rucki A.A., Zheng L. Pancreatic cancer stroma: Understanding biology leads to new therapeutic strategies. World J. Gastroenterol. 2014;20:2237–2246. doi: 10.3748/wjg.v20.i9.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahrami A., Khazaei M., Bagherieh F., Ghayour-Mobarhan M., Maftouh M., Hassanian S.M., Avan A. Target-ing stroma in pancreatic cancer: Promises and failures of targeted therapies. J. Cell. Physiol. 2017;232:2931–2937. doi: 10.1002/jcp.25798. [DOI] [PubMed] [Google Scholar]
- 29.Neesse A., Krug S., Gress T.M., Tuveson D.A., Michl P. Emerging concepts in pancreatic cancer medicine: Tar-geting the tumor stroma. OncoTargets Ther. 2014;7:33. doi: 10.2147/OTT.S38111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waghray M., Yalamanchili M., Di Magliano M.P., Simeone D.M. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 2013;29:537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han H., Hou Y., Chen X., Zhang P., Kang M., Jin Q., Gao M. Metformin-induced stromal depletion to en-hance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy. J. Am. Chem. Soc. 2020;142:4944–4954. doi: 10.1021/jacs.0c00650. [DOI] [PubMed] [Google Scholar]
- 32.Kocher H.M., Basu B., Froeling F.E., Sarker D., Slater S., Carlin D., Propper D.J. Phase I clinical trial repur-posing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W., Sun L., Lei J., Wu Z., Ma Q., Wang Z. Curcumin inhibits pancreatic cancer cell invasion and EMT by interfering with tumor stromal crosstalk under hypoxic conditions via the IL 6/ERK/NF κB axis. Oncol. Rep. 2020;44:382–392. doi: 10.3892/or.2020.7600. [DOI] [PubMed] [Google Scholar]
- 34.Sharma N.S., Gupta V.K., Garrido V.T., Hadad R., Durden B.C., Kesh K., Giri B., Ferrantella A., Dudeja V., Saluja A., et al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J. Clin. Investig. 2019;130:451–465. doi: 10.1172/JCI127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudson C.B., Knudson W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 36.Toole B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 37.Blundell C.D., Seyfried N.T., Day A.J. Structural and Functional Diversity of Hyaluronan-Binding Proteins. Elsevier BV; Amsterdam, The Netherlands: 2004. pp. 189–204. [Google Scholar]
- 38.McCarthy J.B., El-Ashry D., Turley E.A. Hyaluronan, cancer-associated fibroblasts and the tumor microenvi-ronment in malignant progression. Front. Cell Dev. Biol. 2018;6:48. doi: 10.3389/fcell.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy N., Kuipers H.F., Frymoyer A.R., Ishak H.D., Bollyky J.B., Wight T.N., Bollyky P.L. 4-Methylumbelliferone Treatment and Hyaluronan Inhibition as a Therapeutic Strategy in Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2015;6:123. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N., Kohi S., Hirata K., Goggins M. Role of hyaluronan in pancreatic cancer biology and therapy: Once again in the spotlight. Cancer Sci. 2016;107:569–575. doi: 10.1111/cas.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toole B.P., Hascall V.C. Hyaluronan and Tumor Growth. Am. J. Pathol. 2002;161:745–747. doi: 10.1016/S0002-9440(10)64232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng X.-B., Kohi S., Koga A., Hirata K., Sato N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget. 2015;7:4829–4840. doi: 10.18632/oncotarget.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sironen R.K., Tammi M., Tammi R., Auvinen P.K., Anttila M., Kosma V.M. Hyaluronan in human malignan-cies. Exp. Cell Res. 2011;317:383–391. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Underhill C.B., Chen L. Hyaluronan on the surface of tumor cells is correlated with metastatic behav-ior. Cancer Res. 1995;55:428–433. [PubMed] [Google Scholar]
- 45.Toole B.P. Hyaluronan promotes the malignant phenotype. Glycobiology. 2002;12:37R–42R. doi: 10.1093/glycob/12.3.37R. [DOI] [PubMed] [Google Scholar]
- 46.Jiang D., Liang J., Noble P.W. Hyaluronan in Tissue Injury and Repair. Annu. Rev. Cell Dev. Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 47.McKee C.M., Penno M.B., Cowman M., Burdick M.D., Strieter R.M., Bao C., Noble P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laurent T.C., Laurent U.B., E. Fraser J.R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996;74:a1–a7. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 49.Schwertfeger K.L., Cowman M.K., Telmer P.G., Turley E.A., McCarthy J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015;6:236. doi: 10.3389/fimmu.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall C.L., Wang C., Lange L., Turley E. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J. Cell Biol. 1994;126:575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCourt P., Ek B., Forsberg N., Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J. Biol. Chem. 1994;269:30081–30084. doi: 10.1016/S0021-9258(18)43775-1. [DOI] [PubMed] [Google Scholar]
- 52.Hardwick C., Hoare K., Owens R., Hohn H.P., Hook M., Moore D., Cripps V., Austen L., Nance D.M., Turley E. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricciardelli C., Russell D.L., Ween M.P., Mayne K., Suwiwat S., Byers S., Horsfall D.J. Formation of hyalu-ronan-and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J. Biol. Chem. 2007;282:10814–10825. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 54.Collis L., Hall C., Lange L., Ziebell M., Prestwich R., Turley E. Rapid hyaluronan uptake is associated with enhanced motility: Implications for an intracellular mode of action. FEBS Lett. 1998;440:444–449. doi: 10.1016/S0014-5793(98)01505-1. [DOI] [PubMed] [Google Scholar]
- 55.Meran S., Thomas D.W., Stephens P., Enoch S., Martin J., Steadman R., Phillips A.O. Hyaluronan Facilitates Transforming Growth Factor-β1-mediated Fibroblast Proliferation. J. Biol. Chem. 2008;283:6530–6545. doi: 10.1074/jbc.M704819200. [DOI] [PubMed] [Google Scholar]
- 56.Slevin M., Krupinski J., Kumar S., Gaffney J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab. Investig. 1998;78:987–1003. [PubMed] [Google Scholar]
- 57.Toole B.P. Cell Biology of Extracellular Matrix. Springer; Boston, MA, USA: 1991. Proteoglycans and Hyaluronan in Morphogenesis and Differentiation; pp. 305–341. [Google Scholar]
- 58.Assmann V., Marshall J.F., Fieber C., Hofmann M., Hart I.R. The human hyaluronan receptor RHAMM is ex-pressed as an intracellular protein in breast cancer cells. J. Cell Sci. 1998;111:1685–1694. doi: 10.1242/jcs.111.12.1685. [DOI] [PubMed] [Google Scholar]
- 59.Wang C., Thor A.D., Moore D.H., Zhao Y., Kerschmann R., Stern R., Turley E.A. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated pro-tein kinase and is a significant parameter in breast cancer progression. Clin. Cancer Res. 1998;4:567–576. [PubMed] [Google Scholar]
- 60.Weigel P.H., Hascall V.C., Tammi M. Hyaluronan Synthases. J. Biol. Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 61.Itano N., Kimata K. Mammalian Hyaluronan Synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 62.Price Z.K., Lokman N.A., Ricciardelli C. Differing Roles of Hyaluronan Molecular Weight on Cancer Cell Behavior and Chemotherapy Resistance. Cancers. 2018;10:482. doi: 10.3390/cancers10120482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang L., Liu G., Liu H., Han J., Liu Z., Ma H. Molecular weight impact on the mechanical forces between hya-luronan and its receptor. Carbohydr. Polym. 2018;197:326–336. doi: 10.1016/j.carbpol.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Wolny P.M., Banerji S., Gounou C., Brisson A.R., Day A.J., Jackson D.G., Richter R.P. Analysis of CD44-hyaluronan interactions in an artificial membrane system insights into the distinct binding properties of high and low molecu-lar weight hyaluronan. J. Biol. Chem. 2010;285:30170–30180. doi: 10.1074/jbc.M110.137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garg H.G., Hales C.A., editors. Methods for Analysis of Hyaluronan and Its Fragments. Chapter Elsevier; Amsterdam, The Netherlands: 2004. [Google Scholar]
- 66.Mauri P., Scarpa A., Nascimbeni A.C., Benazzi L., Parmagnani E., Mafficini A., Della Peruta M., Bassi C., Miyazaki K., Sorio C. Identification of proteins released by pancreatic cancer cells by multidimensional protein identification technology: A strategy for identification of novel cancer markers. FASEB J. 2005;19:1125–1127. doi: 10.1096/fj.04-3000fje. [DOI] [PubMed] [Google Scholar]
- 67.Yeung T.L., Leung C.S., Wong K.K., Samimi G., Thompson M.S., Liu J., Mok S.C. TGF-β modulates ovari-an cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013;73:5016–5028. doi: 10.1158/0008-5472.CAN-13-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang B., He G., Xu G., Wen J., Yu X. miRNA-34a inhibits cell adhesion by targeting CD44 in human renal epi-thelial cells: Implications for renal stone disease. Urolithiasis. 2019;48:1–8. doi: 10.1007/s00240-019-01155-9. [DOI] [PubMed] [Google Scholar]
- 69.Merzak A., Koocheckpour S., Pilkington G.J. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994;54:3988–3992. [PubMed] [Google Scholar]
- 70.Hill A., McFarlane S., Mulligan K., Gillespie H., Draffin J., Trimble A., Ouhtit A., Johnston P.G., Harkin D.P., McCormick D., et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- 71.Dohadwala M., Luo J., Zhu L., Lin Y., Dougherty G.J., Sharma S., Huang M., Põld M., Batra R.K., Dubinett S.M. Non-small Cell Lung Cancer Cyclooxygenase-2-dependent Invasion Is Mediated by CD44. J. Biol. Chem. 2001;276:20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jothy S. CD44 and its partners in metastasis. Clin. Exp. Metastasis. 2003;20:195–201. doi: 10.1023/A:1022931016285. [DOI] [PubMed] [Google Scholar]
- 73.Rutnam Z.J., Yang B.B. The non-coding 3′ UTR of CD44 induces metastasis by regulating extracellular matrix functions. J. Cell Sci. 2012;125:2075–2085. doi: 10.1242/jcs.100818. [DOI] [PubMed] [Google Scholar]
- 74.Troness B., Spartz A., Sharma U., Miller P., Saenz K.M., Lippman M., El-Ashry D. CD44 facilitates metasta-sis by promoting co-clustering of breast cancer cells and cancer associated fibroblasts. Cancer Res. 2019 doi: 10.1158/1538-7445.AM2019-2044. [DOI] [PubMed] [Google Scholar]
- 75.Thorne R.F., Legg J.W., Isacke C.M. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J. Cell Sci. 2019;117:373–380. doi: 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 76.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018;11:1–23. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta A., Cao W., Chellaiah M.A. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB ligand signaling axis. Mol. Cancer. 2012;11:1–17. doi: 10.1186/1476-4598-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi H., Suzuki M., Kanayama N., Nishida T., Takigawa M., Terao T. CD44 stimulation by fragmented hyaluronic acid induces upregulation of urokinase-type plasminogen activator and its receptor and subsequently facilitates invasion of human chondrosarcoma cells. Int. J. Cancer. 2002;102:379–389. doi: 10.1002/ijc.10710. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka K., Osada H., Murakami-Tonami Y., Horio Y., Hida T., Sekido Y. Statin suppresses Hippo pathway-inactivated malignant mesothelioma cells and blocks the YAP/CD44 growth stimulatory axis. Cancer Lett. 2017;385:215–224. doi: 10.1016/j.canlet.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 80.Weber G.F., Ashkar S., Glimcher M.J., Cantor H. Receptor-Ligand Interaction between CD44 and Osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 81.Furger K.A., Menon R.K., Tuck A.B., Bramwell V.H., Chambers A.F. The functional and clinical roles of oste-opontin in cancer and metastasis. Curr. Mol. Med. 2001;1:621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- 82.Rittling S.R., Chambers A.F. Role of osteopontin in tumour progression. Br. J. Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai J., Peng L., Fan K., Wang H., Wei R., Ji G., Guo Y. Osteopontin induces angiogenesis through activa-tion of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412–3422. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi F., Akutagawa S., Fukumoto H., Tsukiyama S., Ohe Y., Takahashi K., Nishio K. Osteopontin in-duces angiogenesis of murine neuroblastoma cells in mice. Int. J. Cancer. 2002;98:707–712. doi: 10.1002/ijc.10261. [DOI] [PubMed] [Google Scholar]
- 85.Knutson J.R., Iida J., Fields G.B., McCarthy J.B. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 in-tegrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol. Biol. Cell. 1996;7:383–396. doi: 10.1091/mbc.7.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jalkanen M., Elenius K., Salmivirta M. Syndecan—A cell surface proteoglycan that selectively binds extracellular effector molecules. Heparin Relat. Polysacch. 1992;313:79–85. doi: 10.1007/978-1-4899-2444-5_8. [DOI] [PubMed] [Google Scholar]
- 87.Sayyad M.R., Puchalapalli M., Vergara N.G., Wangensteen S.M., Moore M., Mu L., Edwards C., Anderson A., Kall S., Sullivan M., et al. Syndecan-1 facilitates breast cancer metastasis to the brain. Breast Cancer Res. Treat. 2019;178:35–49. doi: 10.1007/s10549-019-05347-0. [DOI] [PubMed] [Google Scholar]
- 88.Conejo J.R., Kleeff J., Koliopanos A., Matsuda K., Zhu Z.W., Goecke H., Büchler M.W. Syndecan-1 expres-sion is up-regulated in pancreatic but not in other gastrointestinal cancers. Int. J. Cancer. 2000;88:12–20. doi: 10.1002/1097-0215(20001001)88:1<12::AID-IJC3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 89.Yao W., Rose J.L., Wang W., Seth S., Jiang H., Taguchi A., Liu J., Yan L., Kapoor A., Hou P., et al. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nat. Cell Biol. 2019;568:410–414. doi: 10.1038/s41586-019-1062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miletti-González K.E., Murphy K., Kumaran M.N., Ravindranath A.K., Wernyj R.P., Kaur S., Rodríguez-Rodríguez L. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): Modulation of matrix metal-loproteinase 9 (MMP-9) transcription via novel promoter response element. J. Biol. Chem. 2012;287:18995–19007. doi: 10.1074/jbc.M111.318774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H.-S., Su C.-H., Kuo C.-C., Shaw C.-F., Peng S.-T. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int. J. Oncol. 2007;31:1119–1126. doi: 10.3892/ijo.31.5.1119. [DOI] [PubMed] [Google Scholar]
- 92.Bourguignon L.Y., Singleton P.A., Diedrich F., Stern R., Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell inva-sion. J. Biol. Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 93.Suleiman M., Abdulrahman N., Yalcin H., Mraiche F. The role of CD44, hyaluronan and NHE1 in cardiac remodeling. Life Sci. 2018;209:197–201. doi: 10.1016/j.lfs.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Chang G., Wang J., Zhang H., Zhang Y., Wang C., Xu H., Pang T. CD44 targets Na(+)/H(+) exchanger 1 to mediate MDA-MB-231 cells’ metastasis via the regulation of ERK1/2. Br. J. Cancer. 2014;110:916–927. doi: 10.1038/bjc.2013.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stock C., Cardone R.A., Busco G., Krähling H., Schwab A., Reshkin S.J. Protons extruded by NHE1: Digestive or glue? Eur. J. Cell Biol. 2008;87:591–599. doi: 10.1016/j.ejcb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Nelson M., Yang M., Millican-Slater R., Brackenbury W.J. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget. 2015;6:32914–32929. doi: 10.18632/oncotarget.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang M., James A.D., Suman R., Kasprowicz R., Nelson M., O’Toole P.J., Brackenbury W.J. Volt-age-dependent activation of Rac1 by Nav1. 5 channels promotes cell migration. J. Cell. Physiol. 2020;235:3950–3972. doi: 10.1002/jcp.29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao P., Xu Y., Wei Y., Qiu Q., Chew T.-L., Kang Y., Cheng C. The CD44s splice isoform is a central mediator for invadopodia activity. J. Cell Sci. 2016;129:1355–1365. doi: 10.1242/jcs.171959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weaver A.M. Invadopodia: Specialized Cell Structures for Cancer Invasion. Clin. Exp. Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 100.Petropoulos C., Guichet P.-O., Masliantsev K., Wager M., Karayan-Tapon L. Functional invadopodia formed in glioblastoma stem cells are important regulators of tumor angiogenesis. Oncotarget. 2018;9:20640–20657. doi: 10.18632/oncotarget.25045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gould C.M., Courtneidge S. Regulation of invadopodia by the tumor microenvironment. Cell Adhes. Migr. 2014;8:226–235. doi: 10.4161/cam.28346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brisson L., Driffort V., Benoist L., Poet M., Counillon L., Antelmi E., Roger S. NaV1. 5 Na+ channels allosteri-cally regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013;126:4835–4842. doi: 10.1242/jcs.123901. [DOI] [PubMed] [Google Scholar]
- 103.Eddy R.J., Weidmann M.D., Sharma V.P., Condeelis J.S. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017;27:595–607. doi: 10.1016/j.tcb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo Q., Wu T., Wu W., Chen G., Luo X., Jiang L., Tao H., Rong M., Kang S., Deng M. The Functional Role of Voltage-Gated Sodium Channel Nav1.5 in Metastatic Breast Cancer. Front. Pharmacol. 2020;11:1111. doi: 10.3389/fphar.2020.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grass G.D., Tolliver L.B., Bratoeva M., Toole B.P. CD147, CD44, and the Epidermal Growth Factor Receptor (EGFR) Signaling Pathway Cooperate to Regulate Breast Epithelial Cell Invasiveness. J. Biol. Chem. 2013;288:26089–26104. doi: 10.1074/jbc.M113.497685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chellaiah M. CD44-Src signaling promotes invadopodia formation in prostate cancer (PC3) cells. OA Cancer. 2013;1 doi: 10.13172/2053-3918-1-2-985. [DOI] [Google Scholar]
- 107.Wang L., Zuo X., Xie K., Wei D. Methods in Molecular Biology. Humana Press; New York, NY, USA: 2017. The Role of CD44 and Cancer Stem Cells; pp. 31–42. [DOI] [PubMed] [Google Scholar]
- 108.Yan Y., Zuo X., Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. STEM CELLS Transl. Med. 2015;4:1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zoeller M. CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Frontiers Immunol. 2015;6:235. doi: 10.3389/fimmu.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu H., Mitsuhashi N., Klein A., Barsky L.W., Weinberg K., Barr M.L., Wu G.D. The role of the hyalu-ronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 111.Williams K., Motiani K., Giridhar P.V., Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp. Biol. Med. 2013;238:324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jin L., Hope K.J., Zhai Q., Smadja-Joffe F., Dick J.E. Targeting of CD44 eradicates human acute myeloid leuke-mic stem cells. Nat. Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 113.Hong S.P., Wen J., Bang S., Park S., Song S.Y. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 114.Li X.-P., Zhang X.-W., Zheng L.-Z., Guo W.-J. Expression of CD44 in pancreatic cancer and its significance. Int. J. Clin. Exp. Pathol. 2015;8:6724–6731. [PMC free article] [PubMed] [Google Scholar]
- 115.Tsukita S., Oishi K., Sato N., Sagara J., Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsukita S., Yonemura S., Tsukita S. ERM proteins: Head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem. Sci. 1997;22:53–58. doi: 10.1016/S0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- 117.Aziz K.A., Till K.J., Zuzel M., Cawley J.C. Involvement of CD44-hyaluronan interaction in malignant cell hom-ing and fibronectin synthesis in hairy cell leukemia. Blood J. Am. Soc. Hematol. 2000;96:3161–3167. [PubMed] [Google Scholar]
- 118.Ichikawa T., Itano N., Sawai T., Kimata K., Koganehira Y., Saida T., Taniguchi S.I. Increased synthesis of hya-luronate enhances motility of human melanoma cells. J. Investig. Dermatol. 1999;113:935–939. doi: 10.1046/j.1523-1747.1999.00804.x. [DOI] [PubMed] [Google Scholar]
- 119.Favia M., Guerra L., Fanelli T., Cardone R.A., Monterisi S., Di Sole F., Casavola V. Na+/H+ exchanger regu-latory factor 1 overexpression-dependent increase of cytoskeleton organization is fundamental in the rescue of F508del cystic fibrosis transmembrane conductance regulator in human airway CFBE41o-cells. Mol. Biol. Cell. 2010;21:73–86. doi: 10.1091/mbc.e09-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Belizzi A., Greco M.R., Rubino R., Paradiso A., Forciniti S., Zeeberg K., Cardone R.A., Reshkin S.J. The scaffolding protein NHERF1 sensitizes EGFR-dependent tumor growth, motility and invadopodia function to gefitinib treatment in breast cancer cells. Int. J. Oncol. 2014;46:1214–1224. doi: 10.3892/ijo.2014.2805. [DOI] [PubMed] [Google Scholar]
- 121.Koltai T., Reshkin S.J., Harguindey S. An Innovative Approach to Understanding and Treating Cancer: Targeting ph: From Etiopathogenesis to New Therapeutic Avenues. Academic Press; Cambridge, MA, USA: Elsevier; Amsterdam, The Netherlands: 2020. Chapter 11. [Google Scholar]
- 122.Wharton C.W. The structure and mechanism of stem bromelain. Evaluation of the homogeneity of purified stem bromelain, determination of the molecular weight and kinetic analysis of the bromelain-catalysed hydrolysis of N-benzyloxycarbonyl-l-phenylalanyl-l-serine methyl ester. Biochem. J. 1974;143:575–586. doi: 10.1042/bj1430575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerard G. Anticancer treatment and bromelains. Agressol. Revue Int. Physio-Biologie Pharma-Cologie Appl. Eff. L’agression. 1972;13:261. [PubMed] [Google Scholar]
- 124.Taussig S.J., Szekerczes J., Batkin S. Inhibition of tumour growth in vitro by bromelain, an extract of the pineap-ple plant (Ananas comosus) Planta Med. 1985;51:538–539. doi: 10.1055/s-2007-969596. [DOI] [PubMed] [Google Scholar]
- 125.Batkin S., Taussig S.J., Szekerezes J. Antimetastatic effect of bromelain with or without its proteolytic and antico-agulant activity. J. Cancer Res. Clin. Oncol. 1988;114:507–508. doi: 10.1007/BF00391501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harrach T., Gebauer F., Eckert K., Kunze R., Maurer H.R. Bromelain proteinases modulate the cd44 expression on human molt-4/8 leukemia and sk-mel-28 melanoma-cells in-vitro. Int. J. Oncol. 1994;5:485–488. doi: 10.3892/ijo.5.3.485. [DOI] [PubMed] [Google Scholar]
- 127.Garbin F., Harrach T., Eckert K., Maurer H. Bromelain proteinase-f9 augments human lymphocyte-mediated growth-inhibition of various tumor-cells in-vitro. Int. J. Oncol. 1994;5:197–203. doi: 10.3892/ijo.5.2.197. [DOI] [PubMed] [Google Scholar]
- 128.Grabowska E., Eckert K., Fichtner I., Schulzeforster K., Maurer H. Bromelain proteases suppress growth, inva-sion and lung metastasis of B16F10 mouse melanoma cells. Int. J. Oncol. 1997;11:243–248. doi: 10.3892/ijo.11.2.243. [DOI] [PubMed] [Google Scholar]
- 129.Tysnes B.B., Maurert H.R., Porwol T., Probst B., Bjerkvig R., Hoover F. Bromelain Reversibly Inhibits Invasive Properties of Glioma Cells. Neoplasia. 2001;3:469–479. doi: 10.1038/sj.neo.7900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baez R., Lopes M.T., Salas C.E., Hernandez M. In vivo antitumoral activity of stem pineapple (Ananas como-sus) bromelain. Planta Med. 2007;73:1377–1383. doi: 10.1055/s-2007-990221. [DOI] [PubMed] [Google Scholar]
- 131.Bhui K., Prasad S., George J., Shukla Y. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009;282:167–176. doi: 10.1016/j.canlet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 132.Paroulek A.F., Jaffe M., Rathinavelu A. Ph.D. Thesis. Nova Southeastern University; Fort Lauderdale, FL, USA: 2010. The Effects of the Herbal Enzyme Bromelain against Breast Cancer Cell Line Gi-101a. [Google Scholar]
- 133.Dhandayuthapani S., Perez H.D., Paroulek A., Chinnakkannu P., Kandalam U., Jaffe M., Rathinavelu A. Bromelain-Induced Apoptosis in GI-101A Breast Cancer Cells. J. Med. Food. 2010;15:344–349. doi: 10.1089/jmf.2011.0145. [DOI] [PubMed] [Google Scholar]
- 134.Bhui K., Tyagi S., Srivastava A.K., Singh M., Roy P., Singh R., Shukla Y. Bromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G2/M arrest to apop-tosis. Mol. Carcinog. 2012;51:231–243. doi: 10.1002/mc.20769. [DOI] [PubMed] [Google Scholar]
- 135.Amini A., Ehteda A., Moghaddam S.M., Akhter J., Pillai K., Morris D.L. Cytotoxic effects of bromelain in hu-man gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21) OncoTargets Ther. 2013;6:403. doi: 10.2147/OTT.S43072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mohr T., Desser L. Plant proteolytic enzyme papain abrogates angiogenic activation of human umbilical vein en-dothelial cells (HUVEC) in vitro. BMC Complementary Altern. Med. 2013;13:231. doi: 10.1186/1472-6882-13-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pillai K., Ehteda A., Akhter J., Chua T.C., Morris D.L. Anticancer effect of bromelain with and without cispla-tin or 5-FU on malignant peritoneal mesothelioma cells. Anti-Cancer Drugs. 2014;25:150–160. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 138.Amini A., Masoumi-Moghaddam S., Ehteda A., Liauw W., Akhter J., Pilai K., Morris D.L. Abstract LB-007: Synergistic inhibition of human gastric and colorectal cancers by Bromelain and N-acetylcysteine: An in vivo study. Exp. Mol. Ther. 2015;75 doi: 10.1158/1538-7445.am2015-lb-007. [DOI] [Google Scholar]
- 139.Amini A., Masoumi-Moghaddam S., Morris D.L. Utility of Bromelain and N-acetylcysteine in Treatment of Peri-Toneal Dissemination of Gastrointestinal Mucin-Producing Malignancies. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 140.Plentz R., Müller A., Barat S., Chen X., Bui C., Bozko P., Malek N. Treatment of Cholangiocarcinoma by Bro-melain and Papain. J. Hepatol. 2016;64:S577. doi: 10.1016/S0168-8278(16)01054-0. [DOI] [Google Scholar]
- 141.Debnath R., Majumder D., Singha A.K., Ghosh D., Maiti D. Bromelain plus peroxidase from pine-apple induces apoptosis via mitochondrial dependent pathway in lymphoma cells. Int. J. Pharm. Sci. Res. 2018;9:4610–4618. [Google Scholar]
- 142.Lee J.H., Lee J.T., Park H.R., Kim J.B. The potential use of bromelain as a natural oral medicine having anticar-cinogenic activities. Food Sci. Nutr. 2019;7:1656–1667. doi: 10.1002/fsn3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Debnath R., Majumder D., Nath P., Ghosh D., Maiti D. Bromelain plus peroxidase reduces non-Hodgkin lym-phoma progression in invivo via up-regulation of antioxidant enzymes and modulating apoptotic protein expression. Nutr. Cancer. 2019;72:1–11. doi: 10.1080/01635581.2019.1670217. [DOI] [PubMed] [Google Scholar]
- 144.Hale L.P., Haynes B.F. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J. Immunol. 1992;149:3809–3816. [PubMed] [Google Scholar]
- 145.Munzig E., Eckert K., Harrach T., Graf H., Maurer H.R. Bromelain protease F9 reduces the CD44 mediated ad-hesion of human peripheral blood lymphocytes to human umbilical vein endothelial cells. FEBS Lett. 1994;351:215–218. doi: 10.1016/0014-5793(94)00860-4. [DOI] [PubMed] [Google Scholar]
- 146.Kleef R., Delohery T.M., Bovbjerg D.H. Selective modulation of cell adhesion molecules on lymphocytes by bro-melain protease. Pathobiology. 1996;64:339–346. doi: 10.1159/000164070. [DOI] [PubMed] [Google Scholar]
- 147.Rathnavelu V., Alitheen N.B., Sohila S., Kanagesan S., Ramesh R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016;5:283–288. doi: 10.3892/br.2016.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fouz N., Amid A., Hashim Y.Z.H.Y. Gene expression analysis in MCF-7 breast cancer cells treated with recom-binant bromelain. Appl. Biochem. Biotechnol. 2014;173:1618–1639. doi: 10.1007/s12010-014-0947-6. [DOI] [PubMed] [Google Scholar]
- 149.Maurer H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Secor E.R., Jr., Carson W.F., IV, Cloutier M.M., Guernsey L.A., Schramm C.M., Wu C.A., Thrall R.S. Brome-lain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell. Immunol. 2005;237:68–75. doi: 10.1016/j.cellimm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Secor E.R., Szczepanek S.M., Castater C.A., Adami A.J., Matson A.P., Rafti E.T., Guernsey L., Natarajan P., McNamara J.T., Schramm C.M., et al. Bromelain Inhibits Allergic Sensitization and Murine Asthma via Modulation of Dendritic Cells. Evidence-Based Complement. Altern. Med. 2013;2013:1–9. doi: 10.1155/2013/702196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Engwerda C.R., Andrew D., Murphy M., Mynott T.L. Bromelain Activates Murine Macrophages and Natural Killer Cells in Vitro. Cell. Immunol. 2001;210:5–10. doi: 10.1006/cimm.2001.1793. [DOI] [PubMed] [Google Scholar]
- 153.White R.R., Crawley F.E.H., Vellini M., Rovati L.A. Bioavailability of125I bromelain after oral administration to rats. Biopharm. Drug Dispos. 1988;9:397–403. doi: 10.1002/bod.2510090408. [DOI] [PubMed] [Google Scholar]
- 154.Castell J.V., Friedrich G., Kuhn C.S., Poppe G.E. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am. J. Physiol. Liver Physiol. 1997;273:G139–G146. doi: 10.1152/ajpgi.1997.273.1.G139. [DOI] [PubMed] [Google Scholar]
- 155.Heinicke R.M., Van Der Wal L., Yokoyama M. Effect of bromelain (ananase®) on human platelet aggregation. Cell. Mol. Life Sci. 1972;28:844–845. doi: 10.1007/BF01923166. [DOI] [PubMed] [Google Scholar]
- 156.Metzig C., Grabowska E., Eckert K., Rehse K., Maurer H.R. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo. 1999;13:7–12. [PubMed] [Google Scholar]
- 157.Pirotta F., de Giuli-Morghen C. Bromelain: Anti-inflammatory and serum fibronolytic activity after oral administration in the rat. Drugs Exptl. Clin. Res. 1978;4:1–20. [Google Scholar]
- 158.Pavan R., Jain S., Shraddha K.A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012;2012:1–6. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kudo D., Kon A., Yoshihara S., Kakizaki I., Sasaki M., Endo M., Takagaki K. Effect of a hyaluronan synthase suppressor, 4-methylumbelliferone, on B16F-10 melanoma cell adhesion and locomotion. Biochem. Biophys. Res. Commun. 2004;321:783–787. doi: 10.1016/j.bbrc.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 160.Jacobetz M.A., Chan D.S., Neesse A., Bapiro T.E., Cook N., Frese K.K., Feig C., Nakagawa T., Caldwell M.E., Zecchini H.I., et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Misra S., Ghatak S., Zoltan-Jones A., Toole B.P. Regulation of Multidrug Resistance in Cancer Cells by Hyaluronan. J. Biol. Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 162.Toole B.P., Slomiany M.G. Hyaluronan: A constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kultti A., Zhao C., Singha N.C., Zimmerman S., Osgood R.J., Symons R., Jacobetz M.A. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvi-ronment. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lipponen P., Aaltomaa S., Tammi R., Tammi M., Ågren U., Kosma V.M. High stromal hyaluronan level is as-sociated with poor differentiation and metastasis in prostate cancer. Eur. J. Cancer. 2001;37:849–856. doi: 10.1016/S0959-8049(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 165.Setälä L.P., Tammi M.I., Tammi R.H., Eskelinen M.J., Lipponen P.K., Ågren U.M., Kosma V.M. Hyalu-ronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br. J. Cancer. 1999;79:1133. doi: 10.1038/sj.bjc.6690180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anttila M.A., Tammi R.H., Tammi M.I., Syrjänen K.J., Saarikoski S.V., Kosma V.M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–155. [PubMed] [Google Scholar]
- 167.Bharadwaj A.G., Kovar J.L., Loughman E., Elowsky C., Oakley G.G., Simpson M.A. Spontaneous Metastasis of Prostate Cancer Is Promoted by Excess Hyaluronan Synthesis and Processing. Am. J. Pathol. 2009;174:1027–1036. doi: 10.2353/ajpath.2009.080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brichkina A., Bertero T., Loh H.M., Nguyen N.T.M., Emelyanov A., Rigade S., Ilie M., Hofman P., Gaggioli C., Bulavin D.V. p38MAPK builds a hyaluronan cancer niche to drive lung tumorigenesis. Genes Dev. 2016;30:2623–2636. doi: 10.1101/gad.290346.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakazawa H., Yoshihara S., Kudo D., Morohashi H., Kakizaki I., Kon A., Takagaki K., Sasaki M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother. Pharmacol. 2006;57:165–170. doi: 10.1007/s00280-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 170.Lokeshwar V.B., Lopez L.E., Munoz D., Chi A., Shirodkar S.P., Lokeshwar S.D., Escudero D.O., Dhir N., Altman N. Antitumor Activity of Hyaluronic Acid Synthesis Inhibitor 4-Methylumbelliferone in Prostate Cancer Cells. Cancer Res. 2010;70:2613–2623. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Piccioni F., Malvicini M., Garcia M.G., Rodriguez A., Atorrasagasti C., Kippes N., Viola M. Antitumor ef-fects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology. 2011;22:400–410. doi: 10.1093/glycob/cwr158. [DOI] [PubMed] [Google Scholar]
- 172.Arai E., Nishida Y., Wasa J., Urakawa H., Zhuo L., Kimata K., Ishiguro N. Inhibition of hyaluronan reten-tion by 4-methylumbelliferone suppresses osteosarcoma cells in vitro and lung metastasis in vivo. Br. J. Cancer. 2011;105:1839. doi: 10.1038/bjc.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Urakawa H., Nishida Y., Wasa J., Arai E., Zhuo L., Kimata K., Ishiguro N. Inhibition of hyaluronan synthe-sis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int. J. Cancer. 2012;130:454–466. doi: 10.1002/ijc.26014. [DOI] [PubMed] [Google Scholar]
- 174.Saito T., Dai T., Asano R. The hyaluronan synthesis inhibitor 4-methylumbelliferone exhibits antitumor effects against mesenchymal-like canine mammary tumor cells. Oncol. Lett. 2013;5:1068–1074. doi: 10.3892/ol.2013.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.García-Vilas J.A., Quesada A.R., Medina M. Ángel 4-Methylumbelliferone Inhibits Angiogenesis in Vitro and in Vivo. J. Agric. Food Chem. 2013;61:4063–4071. doi: 10.1021/jf303062h. [DOI] [PubMed] [Google Scholar]
- 176.Garcia-Vilas J.A., Rodriguez Quesada A., Medina Torres M.A. 4-Methylumbelliferone, an inhibitor of hya-luronic acid biosynthesis, inhibits key steps of angiogenesis. Angiogenesis. 2014;17:753–754. [Google Scholar]
- 177.Tamura R., Yokoyama Y., Yoshida H., Imaizumi T., Mizunuma H. 4-Methylumbelliferone inhibits ovarian can-cer growth by suppressing thymidine phosphorylase expression. J. Ovuarian Res. 2014;7:94. doi: 10.1186/s13048-014-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yoshihara S., Kon A., Kudo D., Nakazawa H., Kakizaki I., Sasaki M., Endo M., Takagaki K. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005;579:2722–2726. doi: 10.1016/j.febslet.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 179.Morohashi H., Kon A., Nakai M., Yamaguchi M., Kakizaki I., Yoshihara S., Sasaki M., Takagaki K. Study of hyaluronan synthase inhibitor, 4-methylumbelliferone derivatives on human pancreatic cancer cell (KP1-NL) Biochem. Biophys. Res. Commun. 2006;345:1454–1459. doi: 10.1016/j.bbrc.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 180.Yoshida E., Kudo D., Nagase H., Shimoda H., Suto S., Negishi M., Kakizaki I., Endo M., Hakamada K. Antitumor effects of the hyaluronan inhibitor 4-methylumbelliferone on pancreatic cancer. Oncol. Lett. 2016;12:2337–2344. doi: 10.3892/ol.2016.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Olivares C.N., Alaniz L.D., Menger M.D., Barañao R.I., Laschke M.W., Meresman G.F. Inhibition of hyalu-ronic acid synthesis suppresses angiogenesis in developing endometriotic lesions. PLoS ONE. 2016;11:e0152302. doi: 10.1371/journal.pone.0152302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nagase H., Kudo D., Suto A., Yoshida E., Suto S., Negishi M., Hakamada K. 4-Methylumbelliferone sup-presses hyaluronan synthesis and tumor progression in SCID mice intra-abdominally inoculated with pancreatic cancer cells. Pancreas. 2017;46:190. doi: 10.1097/MPA.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoshida E., Kudo D., Nagase H., Suto A., Shimoda H., Suto S., Hakamada K. 4-Methylumbelliferone De-creases the Hyaluronan-rich Extracellular Matrix and Increases the Effectiveness of 5-Fluorouracil. Anticancer Res. 2018;38:5799–5804. doi: 10.21873/anticanres.12919. [DOI] [PubMed] [Google Scholar]
- 184.Cheng X., Sato N., Kohi S., Koga A., Hirata K. 4-Methylumbelliferone inhibits enhanced hyaluronan synthesis and cell migration in pancreatic cancer cells in response to tumor-stromal interactions. Oncol. Lett. 2018;15:6297–6301. doi: 10.3892/ol.2018.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Karalis T.T., Heldin P., Vynios D.H., Neill T., Buraschi S., Iozzo R.V., Skandalis S.S. Tumor-suppressive functions of 4-MU on breast cancer cells of different ER status: Regulation of hyaluronan/HAS2/CD44 and specific matrix ef-fectors. Matrix Biol. 2019;78:118–138. doi: 10.1016/j.matbio.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 186.Lokman N.A., Price Z.K., Hawkins E.K., MacPherson A.M., Oehler M.K., Ricciardelli C. 4-Methylumbelliferone Inhibits Cancer Stem Cell Activation and Overcomes Chemoresistance in Ovarian Cancer. Cancers. 2019;11:1187. doi: 10.3390/cancers11081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saga R., Hasegawa K., Murata K., Chiba M., Nakamura T., Okumura K., Hosokawa Y. Regulation of radio-sensitivity by 4 methylumbelliferone via the suppression of interleukin 1 in fibrosarcoma cells. Oncol. Lett. 2019;17:3555–3561. doi: 10.3892/ol.2019.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Makkar S., Riehl T.E., Chen B., Yan Y., Alvarado D.M., Ciorba M.A., Stenson W.F. Hyaluronic Acid Binding to TLR4 Promotes Proliferation and Blocks Apoptosis in Colon Cancer. Mol. Cancer Ther. 2019;18:2446–2456. doi: 10.1158/1535-7163.MCT-18-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McLaughlin J.E., Santos M.T., Binkley P.A., Sultana M., Tekmal R.R., Schenken R.S., Knudtson J.F. Inhibi-tion of Hyaluronic Acid Synthesis Decreases Endometrial Cell Attachment, Migration, and Invasion. Reprod. Sci. 2020;27:1058–1063. doi: 10.1007/s43032-019-00100-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pibuel M.A., Díaz M., Molinari Y., Poodts D., Silvestroff L., Lompardía S.L., Hajos S.E. 4-Methylumbelliferone as a potent and selective antitumor drug on a glioblastoma model. Glycobiology. 2021;31:29–43. doi: 10.1093/glycob/cwaa046. [DOI] [PubMed] [Google Scholar]
- 191.Carter N.J. Pirfenidone. Drugs. 2011;71:1721–1732. doi: 10.2165/11207710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 192.Taniguchi H., Ebina M., Kondoh Y., Ogura T., Azuma A., Suga M., Taguchi Y., Takahashi H., Nakata K., Sato A., et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2009;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 193.Noble P.W., Albera C., Bradford W.Z., Costabel U., Glassberg M.K., Kardatzke D., King T., Lancaster L.A., Sahn S., Szwarcberg J., et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 194.Sharma K., Ix J.H., Mathew A.V., Cho M., Pflueger A., Dunn S.R., Francos B., Sharma S., Falkner B., McGowan T.A., et al. Pirfenidone for Diabetic Nephropathy. J. Am. Soc. Nephrol. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Garcıa L., Hernández I., Sandoval A., Salazar A., Garcia J., Vera J., Armendariz-Borunda J. Pirfenidone ef-fectively reverses experimental liver fibrosis. J. Hepatol. 2002;37:797–805. doi: 10.1016/S0168-8278(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 196.Polydorou C., Mpekris F., Papageorgis P., Voutouri C., Stylianopoulos T. Pirfenidone normalizes the tumor mi-croenvironment to improve chemotherapy. Oncotarget. 2017;8:24506. doi: 10.18632/oncotarget.15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stylianopoulos T., Martin J.D., Snuderl M., Mpekris F., Jain S.R., Jain R.K. Coevolution of solid stress and in-terstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res. 2013;73:3833–3841. doi: 10.1158/0008-5472.CAN-12-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jain R.K., Martin J.D., Stylianopoulos T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kozono S., Ohuchida K., Eguchi D., Ikenaga N., Fujiwara K., Cui L., Tanaka M. Pirfenidone inhibits pancre-atic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345–2356. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 200.Knüppel L., Ishikawa Y., Aichler M., Heinzelmann K., Hatz R., Behr J., Staab-Weijnitz C.A. A novel antifi-brotic mechanism of nintedanib and pirfenidone. Inhibition of collagen fibril assembly. Am. J. Respir. Cell Mol. Biol. 2017;57:77–90. doi: 10.1165/rcmb.2016-0217OC. [DOI] [PubMed] [Google Scholar]
- 201.Kaneko M., Inoue H., Nakazawa R., Azuma N., Suzuki M., Yamauchi S., Saito I. Pirfenidone induces inter-cellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin. Exp. Immunol. 1998;113:72–76. doi: 10.1046/j.1365-2249.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shihab F.S., Bennett W.M., Yi H., Andoh T.F. Pirfenidone treatment decreases transforming growth factor-β1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am. J. Transplant. 2002;2:111–119. doi: 10.1034/j.1600-6143.2002.020201.x. [DOI] [PubMed] [Google Scholar]
- 203.Nakazato H., Oku H., Yamane S., Tsuruta Y., Suzuki R. A novel anti-fibrotic agent pirfenidone suppresses tu-mor necrosis factor-α at the translational level. Eur. J. Pharmacol. 2002;446:177–185. doi: 10.1016/S0014-2999(02)01758-2. [DOI] [PubMed] [Google Scholar]
- 204.Didiasova M., Singh R., Wilhelm J., Kwapiszewska G., Wujak L., Zakrzewicz D., Schaefer L., Markart P., Seeger W., Lauth M., et al. Pirfenidone exerts antifibrotic effects through inhibition of GLI transcription factors. FASEB J. 2017;31:1916–1928. doi: 10.1096/fj.201600892RR. [DOI] [PubMed] [Google Scholar]
- 205.Conte E., Gili E., Fagone E., Fruciano M., Iemmolo M., Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur. J. Pharm. Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 206.Ruwanpura S.M., Thomas B.J., Bardin P.G. Pirfenidone: Molecular mechanisms and potential clinical applica-tions in lung disease. Am. J. Respir. Cell Mol. Biol. 2020;62:413–422. doi: 10.1165/rcmb.2019-0328TR. [DOI] [PubMed] [Google Scholar]
- 207.Oku H., Nakazato H., Horikawa T., Tsuruta Y., Suzuki R. Pirfenidone suppresses tumor necrosis factor-α, en-hances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharm. 2002;446:167–176. doi: 10.1016/S0014-2999(02)01757-0. [DOI] [PubMed] [Google Scholar]
- 208.Kwapiszewska G., Gungl A., Wilhelm J., Marsh L.M., Puthenparampil H.T., Sinn K., Wygrecka M. Tran-scriptome profiling reveals the complexity of pirfenidone effects in idiopathic pulmonary fibrosis. Eur. Respir. J. 2018;52 doi: 10.1183/13993003.00564-2018. [DOI] [PubMed] [Google Scholar]
- 209.Ballester B., Milara J., Cortijo J. Pirfenidone anti-fibrotic effects are partially mediated by the inhibition of MUC1 bioactivation. Oncotarget. 2020;11:1306–1320. doi: 10.18632/oncotarget.27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ferrara F., Granata G., Pelliccia C., La Porta R., Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2. Eur. J. Clin. Pharmacol. 2020;76:1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hinoda Y., Ikematsu Y., Horinochi M., Sato S., Yamamoto K., Nakano T., Fukui M., Suehiro Y., Hamanaka Y., Nishikawa Y., et al. Increased expression of MUC1 in advanced pancreatic cancer. J. Gastroenterol. 2003;38:1162–1166. doi: 10.1007/s00535-003-1224-6. [DOI] [PubMed] [Google Scholar]
- 212.Roy L.D., Sahraei M., Subramani D.B., Besmer D., Nath S., Tinder T.L., Mukherjee P. MUC1 enhances in-vasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449–1459. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ramanathan R.K., Lee K.M., McKolanis J., Hitbold E., Schraut W., Moser A.J., Finn O.J. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pan-creatic cancer. Cancer Immunol. Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shukla S.K., Purohit V., Mehla K., Gunda V., Chaika N.V., Vernucci E., Singh P.K. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32:71–87. doi: 10.1016/j.ccell.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tsutsumida H., Swanson B.J., Singh P.K., Caffrey T.C., Kitajima S., Goto M., Yonezawa S., Hollingsworth M.A. RNA Interference Suppression of MUC1 Reduces the Growth Rate and Metastatic Phenotype of Human Pancreatic Cancer Cells. Clin. Cancer Res. 2006;12:2976–2987. doi: 10.1158/1078-0432.CCR-05-1197. [DOI] [PubMed] [Google Scholar]
- 216.Nath S., Daneshvar K., Roy L.D., Grover P., Kidiyoor A., Mosley L., Mukherjee P. MUC1 induces drug re-sistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51. doi: 10.1038/oncsis.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chaika N.V., Gebregiworgis T., Lewallen M.E., Purohit V., Radhakrishnan P., Liu X., Zhang B., Mehla K., Brown R.B., Caffrey T., et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2012;109:13787–13792. doi: 10.1073/pnas.1203339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Usugi E., Ishii K., Hirokawa Y., Kanayama K., Matsuda C., Uchida K., Shiraishi T., Watanabe M. Antifibrotic Agent Pirfenidone Suppresses Proliferation of Human Pancreatic Cancer Cells by Inducing G0/G1 Cell Cycle Arrest. Pharmacology. 2019;103:250–256. doi: 10.1159/000496831. [DOI] [PubMed] [Google Scholar]
- 219.Mediavilla-Varela M., Boateng K., Noyes D., Antonia S.J. The anti-fibrotic agent pirfenidone synergizes with cisplatin in killing tumor cells and cancer-associated fibroblasts. BMC Cancer. 2016;16:1–9. doi: 10.1186/s12885-016-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ramakrishnan P., Loh W.M., Gopinath S.C., Bonam S.R., Fareez I.M., Mac Guad R., Sim M.S., Wu Y.S. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm. Sin. B. 2020;10:399–413. doi: 10.1016/j.apsb.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Suh H., Pillai K., Morris D.L. Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and ap-plications in diagnosis and therapy. Am. J. Cancer Res. 2017;7:1372. [PMC free article] [PubMed] [Google Scholar]
- 222.Sugahara K.N., Hirata T., Hayasaka H., Stern R., Murai T., Miyasaka M. Tumor Cells Enhance Their Own CD44 Cleavage and Motility by Generating Hyaluronan Fragments. J. Biol. Chem. 2006;281:5861–5868. doi: 10.1074/jbc.M506740200. [DOI] [PubMed] [Google Scholar]
- 223.Bourguignon L.Y., Xia W., Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates β-catenin signaling and NFκB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J. Biol. Chem. 2009;284:26. doi: 10.1074/jbc.M806708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.