Abstract
Purpose
Cognitive impairment is a complication that most frequently happens in patients with chronic neuropathic pain and has limited effective therapy. The aim of this study was to explore the effects of curcumin on the cognitive deficit in rats with peripheral nerve injury induced-neuropathic pain.
Methods
The neuropathic pain rat model was constructed using chronic constriction injury (CCI). The curcumin (60 mg/kg) or vehicle was intraperitoneally administered once a day, beginning at 14th day after surgery and continued for 14 consecutive days. The nociceptive threshold tests were measured by paw mechanical withdraw threshold (PMWT) and paw thermal withdrawal latency (PTWL), while the spatial memory abilities were evaluated by the Morris water maze test. The mean counts of bromodeoxyuridine (Brdu)/neuronal nuclei (NeuN) as well as Brdu/doublecortin (DCX) co-labeled cells were used to evaluate neurogenesis in the dentate gyrus of hippocampus. The ultrastructure of the synapse in hippocampal region was visualized using transmission electron microscopy (TEM).
Results
Increased PMWT and PTWL, as well as relieved memory deficits, were found in CCI rats under curcumin administration. Moreover, curcumin treatment increased the number of newly born immature (BrdU/NeuN) and newly generated mature neurons (BrdU/DCX). The TEM examination revealed increased PSD thickness and shorter active zone length as well as narrowed synaptic cleft width in the hippocampal region of CCI rats after curcumin injection.
Conclusion
Curcumin can alleviate CCI induced nociceptive behaviors and memory deficit. This effect might be associated with hippocampal neurogenesis and synaptic plasticity improvements, which indicated curcumin as a potential strategy for the cognitive impairment restoration under prolonged neuropathic pain condition.
Keywords: curcumin, peripheral nerve injury, cognitive impairment, hippocampus neurogenesis
Introduction
Patients with chronic pain frequently experienced cognitive impairment commonly characterized as memory and learning capacity deterioration, which deteriorates the quality of life and even increases patients’ financial burdens.1 Numerous evidences have revealed that memory deficits in patients with chronic pain could be attributed to the severity of the patients’ emotional disturbances, including anxiety and depression.2,3 Previous studies report the mean prevalence rates of around 20–30% for cognitive function impairment in patients with chronic neuropathic pain.4,5 Preclinical researches revealed that the neuropathic pain-related behaviors usually accompany with spatial learning as well as memory disorders in murine models with chronic peripheral nerve injury.6,7 However, although the link between chronic pain and the development of cognitive dysfunction has been extensively studied and documented, limited therapeutic options are available up to now, indicating that the molecular aspects underlying chronic pain-associated learning and memory deficits have not been well understood.
The hippocampus, a crucial component of the limbic system, is considered to play an important role during memory and mood formation in brain. Recent preclinical evidences have showed that chronic peripheral nerve injury exposure could decrease neurogenesis process in the dentate gyrus (DG) granular cell layer of adult hippocampus.1,8 The deficits in hippocampal neurogenesis might decline the generation of new immature neurons in hippocampus that promote the clearance of panic memory and maintain the ability of animal to cope with environmental challengers,9 while restoring the impaired hippocampal neurogenesis in chronic neuropathic or inflammatory pain models were beneficial for alleviation of painful syndromes.10 Additionally, it is noteworthy that the animals with a reversible neuropathic injury might persistently display cognitive impairment behaviors accompanied with constant ablation of hippocampal neurogenesis, indicating even after recovery from the physical pain of the neuropathy, its more long-term stressing consequences endure on the nervous system, and that impaired neurogenesis might be involved in the emergence of these consequences.11 Thus, enhancing the blocked hippocampal neurogenesis after peripheral nerve injury might be a key to improve cognitive function during chronic pain.
Curcumin, a phenolic compound derived from the rhizome of Curcuma longa, has been widely studied for its analgesic properties against various neuropathic pains.12–15 Recently, curcumin was demonstrated to be able to improve pain behaviors as well as cognitive impairments in cobra venom-induced trigeminal neuralgia and chronic constriction injury (CCI) rodents, which suggested the benefit effects of curcumin on neuroprotective property and improvement of learning and memory performances in chronic neuropathic pain models.7,16 However, it remains unclear whether curcumin treatment can increase hippocampal neurogenesis and improve cognitive dysfunctions in neuropathic pain elicited by peripheral nerve injury. This study was designed to report a causal evidence that curcumin plays a potential role in improving cognitive function in chronic constriction injury (CCI) pain model owing to its hippocampal neurogenesis-promoting property.
Methods
Study Approval and Animal Preparation
All protocols of the current study were approved by the Animal Ethical Committee of the Third Affiliated Hospital, Sun Yat-Sen University, and conducted in accordance with the guidelines of the International Association for the Study of Pain.
Male Sprague Dawley rats (200–250 g) were obtained from the Experiment Animal Center of Guangdong Province (SCXK [Yue] 20180002) and maintained in separate cages under a 12-h light/dark cycle, with water and fed available ad libitum. The room was kept at temperature (23 ± 2°C) as well as humidity (50–60%). We made all efforts to minimize the number of animals used and their suffering.
Procedures of Pain Model Establishment
Anesthesia was administrated by intraperitoneal injection of pentobarbital sodium (50 mg/kg). Then, rats were performed either a unilateral CCI or a sham procedure as depicted by Bennett et al.17 In short, the CCI model was established by a ligation at the left sciatic nerve using four ligatures with non-absorbable 4/0 silk threads (1.0- to 1.5-mm spacing between each ligature), while only exposure of the left sciatic nerve without ligation was performed on sham rats.
Drug Treatments
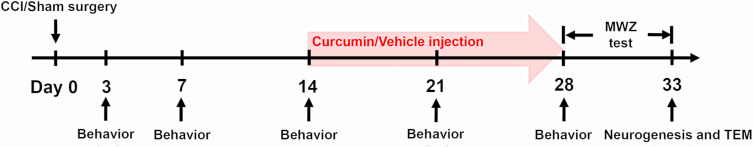
By a random table, 40 rats were randomly divided into four groups, as follows (i) sham with curcumin group (Sham + Cur, n = 10); (ii) CCI with curcumin group (CCI + Cur, n = 10), (iii) sham with vehicle group (Sham + Veh, n = 10), or (iv) CCI with vehicle group (CCI + Veh, n = 10). According to the study from Zhu et al,11 curcumin (Sigma-Aldrich, Santa Clara, CA, USA) was dissolved in 20% dimethyl sulfoxide (DMSO) with 80% normal saline solution. The curcumin (60 mg/kg) or vehicle (20% DMSO with 80% normal saline solution) was intraperitoneally administered once a day, which start at 14th day after surgery and continued for 14 consecutive days (Figure 1).
Figure 1.
Timeline of CCI or sham surgery, curcumin or vehicle (20% DMSO with 80% normal saline solution) injection, behavior and MWZ tests, neurogenesis and TEM detection.
Abbreviations: CCI, chronic constriction injury; DMSO, dimethyl sulfoxide; MWZ, Morris water maze; TEM, transmission electron microscopy.
Mechanical and Thermal Nociceptive Threshold Test
In order to determine whether the CCI model was well established, both paw mechanical withdraw threshold (PMWT) and paw thermal withdrawal latency (PTWL) were assessed 3 days before surgery and 3, 7, 14, 21 and 28 days by two research staffs who were blinded to the group (Figure 1). The behavior test was performed in a noise-free room. Each rat was placed individually on a wire mesh grid (mechanical nociceptive threshold test) or glass floored box (thermal nociceptive threshold test). At least 30 min was allowed to habituate to the surroundings.
The PMWT was assessed by the von Frey monofilaments (North Coast Medical, California, USA) weighing 1.0–15.0 g. The ascending series monofilaments were used to perpendicularly stimulate the mid-plantar surface of hind paw, and each was presented five times with an interval of 4–6 s. A positive response was considered a withdrawal, along with shaking or licking of the paw at least three times over the course of five applications. The smallest bending force of the monofilament eliciting a positive response was determined to be the PMWT.
The PTWL was measured through the thermal test apparatus (UGO BASILE 37370 Plantar Test Apparatus, Italy), which could release a focused radiant heat source from below the glass onto the mid-plantar surface of the hind paw. According to Dai et al,18 the infrared intensity was set as 45 with a cutoff time of 25 s to avoid cutaneous damage to the hind paw. This thermal nociceptive threshold test was repeated five times, and the PTWL was considered as the mean latency of withdrawal response across five tests.
Morris Water Maze Test
When the curcumin had been administrated for 14 consecutive days, a five-day Morris water maze (MWZ) test was applied to evaluate the spatial memory abilities, according to previous study19 (Figure 1). Briefly, a circular pool (180 cm diameter and 60 cm high), which was full of 19°C to 22°C opaque water and divided into four equivalent quadrants (N, E, S, and W), was prepared for the MWZ test. A hidden escape platform was placed in one of four quadrants and submerged 0.5 cm below the water. For the first four days, rodents underwent four acquisition trials separated by 20-minute inter-trial interval per day. During the four acquisition trials, rats were placed into the water facing the pool at different predetermined positions. The swimming speed and escape latency were recorded. On fifth day, the hidden escape platform was removed, and rats were placed into the pool at the location opposite to the platform position and allowed to swim freely for 120 s. According to the previous study,20 we recorded the percentage time spent in the target quadrant and times across platform. Moreover, the time spent in the platform quadrant and the remaining three quadrants were respectively recorded for each group. Whole MWZ test was performed by two research staffs who were blinded to group allocation.
BrdU Administration
To examine the effects of curcumin on neural precursor cells proliferation in the DG of the hippocampus, rats received intraperitoneal injection of 100 mg/kg bromodeoxyuridine (BrdU) (Sigma-Aldrich, B5002) once a week studying from the day receiving CCI or sham surgery until sacrifice, which was according to the previous study.10 Two hours after the last BrdU injection, rats were anesthetized and hippocampus tissues were prepared for the following immunofluorescent and transmission electron microscopy.
Immunofluorescent
The hippocampus tissues were separated after MWZ test (Figure 1) and post-fixed at 4°C overnight, followed by the dip in a 30% sucrose solution for 48 h. Sections of 30 µm thick were prepared by a freezing microtome (VT1000S, Leica Microsystems). For all cases, slices were incubated with the mouse anti-doublecortin (DCX, 1:500, ab254133, Abcam), rabbit anti-BrdU (1:200, ab152095, Abcam), mouse anti-neuronal nuclei (NeuN, 1:400, ab104224, Abcam) antibodies. The secondary antibodies were tagged with goat anti-mouse Alexa 594 (1:500, ab150120, Abcam), goat anti-rabbit Alexa 488 (1:500, ab150081, Abcam). Then slices were stained with 4ʹ,6-diamidino-2-phenylindole (DAPI, 0.5µg/mL, 4083s, Cell Signaling Technology). After staining, slices were visualized by two experimenters blinded to group allocation with the laser scanning confocal microscopy (FV1000; Olympus). The mean counts of Brdu/NeuN as well as Brdu/DCX co-labeled cells in every fifth section for each animal was used to evaluate neurogenesis in the DG.
Transmission Electron Microscopy Examination
The collected hippocampus tissues from rats were perfused in 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium cacodylate, followed by post-fixation in 2% osmium tetroxide with 1.6% potassium ferrocyanide in 0.1 M sodium cacodylate. Subsequently, the hippocampus tissue samples were cut into small clumps of a volume of 1 mm3 and dehydrated through a graded acetone series, after which they were embedded in Eponate 812 medium (90529–77-4, Structure Probe, Inc., Pennsylvania, USA). Finally, the sections were placed on copper slot grids and stained with 2% uranyl acetate and lead citrate. Images were visualized by Hitachi HT-7700 transmission electron microscopy (Tokyo, Japan). As previously described,21,22 the morphologic features of synapse including postsynaptic dense (PSD) band, active zone and synaptic cleft were observed, and the mean values of thickness of the postsynaptic density, length of active zone as well as width of the synaptic cleft in every fifth DG region section was calculated.
Statistical Analysis
Data analysis and statistics were performed by SPSS 20.0 software (IBM CORP, New York, USA). Data were presented as mean ± standard deviation (SD), and analyzed by a two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni post hoc comparisons. Statistical significance was set as P < 0.05.
Results
Effect of Curcumin on Mechanical Allodynia and Thermal Hyperalgesia
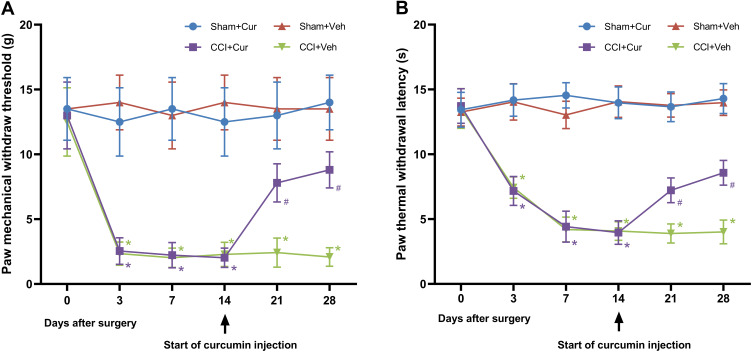
In comparison to rats in the Sham + Cur and Sham +Veh groups, those in the CCI + Veh group exhibited significant reductions of the mechanical threshold (P < 0.001; Figure 2A) and thermal latency (P < 0.001; Figure 2B) after sciatic nerve injury, which were throughout the 28-day observation period. However, curcumin treatment markedly attenuated the mechanical allodynia (P < 0.001; Figure 2A) and thermal hyperalgesia (P < 0.001; Figure 2B) in rats from CCI +Cur group. These results suggested that consecutively administrating 60 mg/kg curcumin for 14 days might alleviate both mechanical and thermal hyperalgesia resulted from peripheral nerve injury.
Figure 2.
Time-course changes of mechanical threshold (A) and thermal latency (B) in the ipsilateral hind-paw of rats.
Note: *P < 0.001 vs Sham+Veh, #P < 0.001 vs CCI+Veh.
Abbreviations: CCI, chronic constriction injury; Veh, vehicle; Cur, curcumin.
Effect of Curcumin on Spatial Memory Function
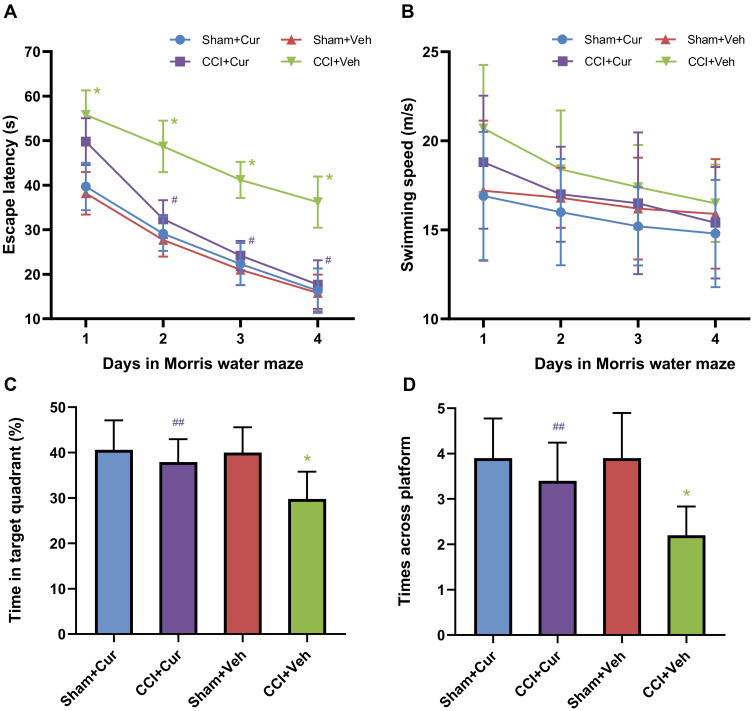
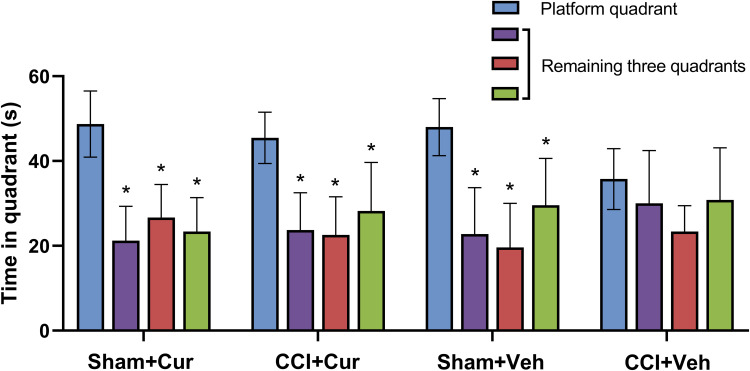
Compared to the sham rats received vehicle, the significantly longer escape latency to platform (P < 0.001, Figure 3A) for CCI rats treated with vehicle indicated that the cognitive impairment was induced by peripheral nerve injury. While the administration of curcumin restored the increased escape latency resulted from CCI (P < 0.001, Figure 3A). However, we did not observe any significant differences in swimming speed among four groups (Figure 3B). The rats in CCI-Veh group presented significant shorter swimming time in target quadrant (P < 0.001, Figure 3C) and time across platform was reduced (P < 0.001, Figure 3C) than those for the rats from sham-Veh group; on the contrary, both parameters were significantly prolonged after the injection of curcumin (P = 0.021 for time in target quadrant, P = 0.019 for time across platform, Figure 3C and D). Besides, there was no significant difference in the spent time among four quadrants in CCI rats treated with vehicle, while the CCI rats treated with curcumin spent more time in the platform quadrant than other three quadrants (P < 0.01, Figure 4).
Figure 3.
Time-course changes of escape latency (A), swimming speed (B), swimming time in target quadrant (C), time across platform (D) and spending times in four quadrants in the rats from different groups.
Note: *P < 0.001 vs Sham+Veh, #P < 0.001 vs CCI+Veh, ## P < 0.05 vs CCI+Veh.
Abbreviations: CCI, chronic constriction injury; Veh, vehicle; Cur, curcumin.
Figure 4.
Time spent in four quadrants in the rats from different groups.
Note: *P < 0.001 vs time spent in platform quadrant.
Abbreviations: CCI, chronic constriction injury; Veh, vehicle; Cur, curcumin.
Effect of Curcumin on Hippocampal Neurogenesis in DG Region
Double labeling of proliferating neurons with anti-BrdU and other marker could provide indication for the phenotype of marked cells.23 Thus, we counted the number of double immunostaining cells to identify newly born immature (BrdU/NeuN)24 and newly generated mature neurons (BrdU/DCX),23 respectively.
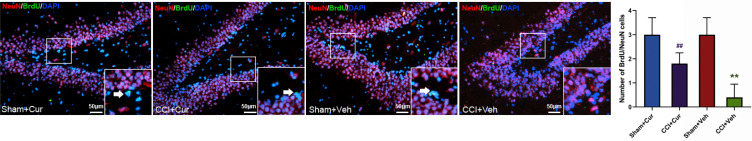
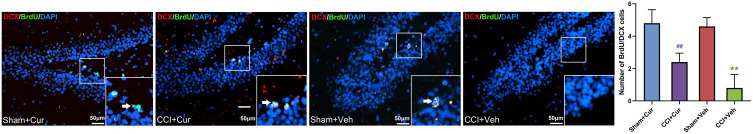
Four weeks after injury, the number of BrdU/NeuN (Figure 5) and BrdU/DCX (Figure 6) neurons was significantly lower in the dentate gyrus of the rats in CCI-Veh group compared to those in sham-Veh group Sham mice (P = 0.041 for BrdU/NeuN, P < 0.001 for BrdU/DCX). Notably, the number of BrdU/NeuN and BrdU/DCX double labeled neurons in the dentate gyrus was dramatically increased in the curcumin administered CCI rats (P = 0.014 for BrdU/NeuN, P = 0.015 for BrdU/DCX, Figures 5 and 6).
Figure 5.
Representative images and quantitative summary of hippocampal NeuN/BrdU cells in the DG regions from rats in different groups (The white arrows represent NeuN/BrdU double labeled neurons).
Note: **P < 0.05 vs Sham+Veh, ## P < 0.05 vs CCI+Veh.
Abbreviations: CCI, chronic constriction injury; Veh, vehicle; Cur, curcumin; BrdU, bromodeoxyuridine; NeuN, neuronal nuclei; DAPI, 4ʹ,6-diamidino-2-phenylindole; DG, dentate gyrus.
Figure 6.
Representative images and quantitative summary of hippocampal DCX/BrdU cells in the DG region from rats in different groups (The white arrows represent DCX/BrdU double labeled neurons).
Note: **P < 0.05 vs Sham+Veh, ## P < 0.05 vs CCI+Veh.
Abbreviations: CCI, chronic constriction injury; Veh, vehicle; Cur, curcumin; BrdU, bromodeoxyuridine; DCX, doublecortin; DAPI, 4ʹ,6-diamidino-2-phenylindole; DG, dentate gyrus.
Effect of Curcumin on the Hippocampal DG Synapse Density
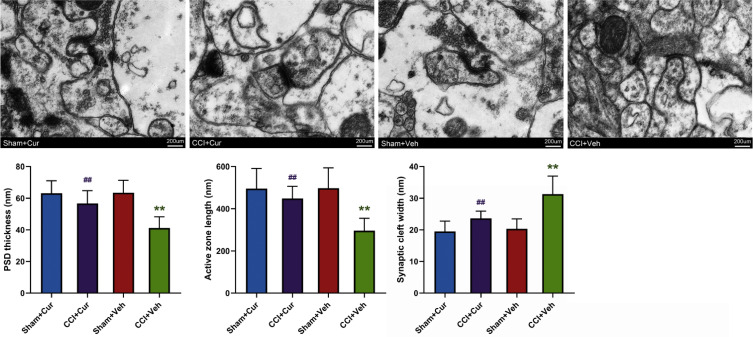
Compared with the Sham-Veh groups, a noticeably changed synapse density along with the lesser PSD thickness (P = 0.002), shorter active zone length (P = 0.006) and wider synaptic cleft width (P = 0.001) was found in the DG region of the CCI-Veh group (Figure 7). In contrast, when the CCI rats were treated with curcumin, the aforementioned abnormality of hippocampal synapse density in CCI-Veh rats appeared to return to be regular (P = 0.036 for PSD thickness, P = 0.044 for active zone length, P = 0.038 for synaptic cleft width), similar to the morphologic features of synapse in sham rats (Figure 7).
Figure 7.
Representative images and quantitative summary of synaptic ultrastructure in the hippocampal DG region as visualized by TEM.
Note: **P < 0.05 vs Sham+Veh, ## P < 0.05 vs CCI+Veh.
Abbreviations: CCI, chronic constriction injury; Veh, vehicle; Cur, curcumin; PSD, postsynaptic dense; DG, dentate gyrus; TEM, transmission electron microscopy.
Discussion
Our current results make the argument that curcumin treatment alleviated pain and improved cognition deficits in a peripheral nerve injury-induced neuropathic pain rat model. Furthermore, the protective effect of curcumin against neuropathic pain-caused cognitive impairment is partly associated with improving hippocampal neurogenesis.
Various functional and morphological alterations that related to the spatial learning and memory function impairments had been studied in neuropathic pain murine models. For example, Saffarpour S et al found that the increase in GABA concentration and decrease in the glutamate and BDNF levels in the CA1 region of the hippocampus were associated with spatial learning and memory function disorders comorbid in CCI rats.25 Our current study using the CCI model shows that a prolonged pain performance (28-day CCI) impairs spatial memory formation along with reductions in the numbers of newly born immature and newly generated mature neurons in hippocampal DG region, which were in accordance with prior studies.1,10 It was demonstrated that newborn neurons in the DG region are incorporated into the hippocampal network in existence and contributed to new cellular and behavioral capacities.26,27 Compared with their mature counterparts, newborn neurons in the DG region were appeared to be more involved in new experience-related learning capacity and memory formation.28 More specifically, mice received mitotic inhibitor displayed increased escape latency and decreased time spent in the target quadrant, accompanied by a reduced number of newborn neurons in the DG region.1 These results confirm that disruption of hippocampal neurogenesis may at least in part act as a trigger to spatial memory impairment during the development of neuropathic pain.
Considering the connection between hippocampal neurogenesis and memory formation, it was hypothesized that facilitating hippocampal neurogenesis would be helpful to improve integration of newborn neurons into hippocampal networks and alleviate pain-induced spatial memory impairment. Our results revealed that repeated administration of 60-mg/kg curcumin was able to relieve spatial memory impairment as well as nociceptive behavior in CCI rats, which were in concert with the previous studies.12–16 More specifically, such effects of curcumin on cognitive impairments were appeared to be associated with improvement of hippocampal neurogenesis in CCI rats. The earlier findings had reported that the enhancement effects of curcumin on hippocampal neurogenesis might attribute to the activation of the Extracellular Signal Regulated Kinase (ERK) and Mitogen Activated Protein (MAP) kinase pathways.29 More recently, the promotion effect of curcumin treatment on neurogenesis and memory was found to be via activation of adenosine monophosphate-activated protein kinase (AMPK)/c-Jun N-terminal kinase (JNK) signaling, which mediated both mammalian target of rapamycin (mTOR) inhibition and B-cell lymphoma-2 (Bcl-2) upregulation and in turn respectively enhanced autophagy and suppressed apoptosis in hippocampus.30,31
More importantly, production of new neurons in the hippocampus alone does not represent the complete process of neurogenesis, because the newly generated neurons must experience differentiation, maturation and then involve in formation of synapses.32 In this study, the curcumin-treatment increased not only newly born immature (BrdU/NeuN) cell number but also newly generated mature neurons (BrdU/DCX) number, simultaneously. Moreover, the PSD thickness and shorter active zone length in parallel with narrow of synaptic cleft width returned to be regular in hippocampus tissue of CCI rats along with curcumin administration. Consequently, these current results indicated the possible involvement of neuronal and synaptic plasticities in the beneficial effect of curcumin on neuropathic pain-induced cognitive impairment.
Besides pro-neurogenic effect, the anti-inflammatory and anti-oxidant effects of curcumin on neuropathic pain had also been demonstrated by previous studies. It was reported that the administration of curcumin can ameliorate pain-related behavior and downregulate the production of pro-inflammatory cytokines, such as IL-1β, partly by inhibiting the aggregation of NALP1 inflammasome and the activation of the JAK2-STAT3 cascade in astrocytes.33 In the oxaliplatin-induced peripheral neuropathic pain and diabetic neuropathy, curcumin could not only inhibit the activation of NF-κB and inflammatory factors, but inhibiting the level of oxidative stress as well.33,34 These potential issues of curcumin effects on neuropathic pain warrant further experiments in further research.
Conclusion
In conclusion, our findings suggest that consecutively administrating 60 mg/kg curcumin for 14 days alleviates CCI induced nociceptive behaviors and memory deficit. This effect may be associated with hippocampal neurogenesis and synaptic plasticity improvements. Thus, we infer that curcumin would serve as a potential strategy for the cognitive impairment restoration under prolonged neuropathic pain condition. However, further clinical studies will be performed to clarify this possibility.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 81701104], the Fundamental Research Funds for the Central Universities (20ykpy25) and the Sun Yat-sen University Clinical Research 5010 Program (2017016).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Xia SH, Hu SW, Ge DG, et al. Chronic Pain Impairs Memory Formation via Disruption of Neurogenesis Mediated by Mesohippocampal Brain-Derived Neurotrophic Factor Signaling. Biol Psychiatry. 2020;88(8):597–610. doi: 10.1016/j.biopsych.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Mazza S, Frot M, Rey AE. Rey AE: a comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):183–192. doi: 10.1016/j.pnpbp.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 3.McCracken LM, Iverson GL. Iverson GL: predicting complaints of impaired cognitive functioning in patients with chronic pain. J Pain Symptom Manage. 2001;21(5):392–396. doi: 10.1016/S0885-3924(01)00267-6 [DOI] [PubMed] [Google Scholar]
- 4.Povedano M, Gascon J, Galvez R, Ruiz M, Rejas J. Rejas J: cognitive function impairment in patients with neuropathic pain under standard conditions of care. J Pain Symptom Manage. 2007;33(1):78–89. doi: 10.1016/j.jpainsymman.2006.07.012 [DOI] [PubMed] [Google Scholar]
- 5.Xiong B, Zhang W, Zhang L, et al. Tian X: hippocampal glutamatergic synapses impairment mediated novel-object recognition dysfunction in rats with neuropathic pain. Pain. 2020;161(8):1824–1836. doi: 10.1097/j.pain.0000000000001878 [DOI] [PubMed] [Google Scholar]
- 6.Saffarpour S, Nasirinezhad F. The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats. Scand J Pain. 2020;21(1):135–144. doi: 10.1515/sjpain-2020-0037 [DOI] [PubMed] [Google Scholar]
- 7.Saffarpour S, Janzadeh A, Rahimi B, Ramezani F, Nasirinezhad F. Chronic nanocurcumin treatment ameliorates pain-related behavior, improves spatial memory, and reduces hippocampal levels of IL-1β and TNFα in the chronic constriction injury model of neuropathic pain. Psychopharmacology. 2021;238(3):877–886. doi: 10.1007/s00213-020-05739-x [DOI] [PubMed] [Google Scholar]
- 8.Apkarian AV, Mutso AA, Centeno MV, et al. Role of adult hippocampal neurogenesis in persistent pain. Pain. 2016;157(2):418–428. doi: 10.1097/j.pain.0000000000000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao C. Shen J: metabolic Factors and Adult Neurogenesis: impacts of Chinese Herbal Medicine on Brain Repair in Neurological Diseases. Int Rev Neurobiol. 2017;135:117–147. [DOI] [PubMed] [Google Scholar]
- 10.Dellarole A, Morton P, Brambilla R, et al. Bethea JR: neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav Immun. 2014;41:65–81. doi: 10.1016/j.bbi.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrov EL, Tsuda MC, Cameron HA, Usdin TB. Usdin TB: anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity. J Neurosci. 2014;34(37):12304–12312. doi: 10.1523/JNEUROSCI.0312-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Li Q, Zhang MT, et al. Wang YQ: curcumin ameliorates neuropathic pain by down-regulating spinal IL-1beta via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep. 2016;6:28956. doi: 10.1038/srep28956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q. Huang C: curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One. 2014;9(3):e91303. doi: 10.1371/journal.pone.0091303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Chen F, Braun C, et al. Ye DW: role of curcumin in the management of pathological pain. Phytomedicine. 2018;48:129–140. doi: 10.1016/j.phymed.2018.04.045 [DOI] [PubMed] [Google Scholar]
- 15.Arora R, Kuhad A, Kaur IP, Chopra K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur J Pain. 2015;19(7):940–952. doi: 10.1002/ejp.620 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Ding X, Wu Z, Wang M, Tian M. Tian M: curcumin alleviates pain and improves cognitive impairment in a rat model of cobra venom-induced trigeminal neuralgia. J Pain Res. 2018;11:1095–1104. doi: 10.2147/JPR.S162668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett GJ, Xie Y-K. Xie YK: a peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6 [DOI] [PubMed] [Google Scholar]
- 18.Dai WL, Yan B, Bao YN, Fan JF. Liu JH: suppression of peripheral NGF attenuates neuropathic pain induced by chronic constriction injury through the TAK1-MAPK/NF-kappaB signaling pathways. Cell Commun Signal. 2020;18(1):66. doi: 10.1186/s12964-020-00556-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Jiao H, Ren W, Ren F. Ren F: TRESK alleviates trigeminal neuralgia induced by infraorbital nerve chronic constriction injury in rats. Mol Pain. 2019;15:1744806919882511. doi: 10.1177/1744806919882511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hossain MM, Belkadi A, Al-Haddad S, Richardson JR. Richardson JR: deltamethrin exposure inhibits adult hippocampal neurogenesis and causes deficits in learning and memory in mice. Toxicol Sci. 2020;178(2):347–357. doi: 10.1093/toxsci/kfaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Zhou X, Yang L, et al. Neonatal Exposure to Low-Dose (1.2%) Sevoflurane Increases Rats’ Hippocampal Neurogenesis and Synaptic Plasticity in Later Life. Neurotox Res. 2018;34(2):188–197. doi: 10.1007/s12640-018-9877-3 [DOI] [PubMed] [Google Scholar]
- 22.Yan Y, Cheng L, Chen X, et al. Ai J: estrogen deficiency is associated with hippocampal morphological remodeling of early postmenopausal mice. Oncotarget. 2017;8(13):21892–21902. doi: 10.18632/oncotarget.15702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojtowicz JM, Kee N. Kee N: brdU assay for neurogenesis in rodents. Nat Protoc. 2006;1(3):1399–1405. doi: 10.1038/nprot.2006.224 [DOI] [PubMed] [Google Scholar]
- 24.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Kronenberg G: milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–452. doi: 10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 25.Saffarpour S, Shaabani M, Naghdi N, Farahmandfar M, Janzadeh A, Nasirinezhad F. In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol Behav. 2017;175:97–103. doi: 10.1016/j.physbeh.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 26.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Gage FH: functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakashiba T, Cushman JD, Pelkey KA, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng W, Aimone JB, Gage FH. Gage FH: new neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, Son TG, Park HR, et al. Lee J: curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283(21):14497–14505. doi: 10.1074/jbc.M708373200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi LT, Dong SQ, Wang SS, et al. Cheng J: curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol Dis. 2020;136:104715. doi: 10.1016/j.nbd.2019.104715 [DOI] [PubMed] [Google Scholar]
- 31.Namgyal D, Ali S, Mehta R, Sarwat M. Sarwat M: the neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology. 2020;442:152542. doi: 10.1016/j.tox.2020.152542 [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Li Q, Zhang MT, et al. Wang YQ: curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep. 2016;6:28956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Guan Z, Wang X, et al. Yue X: curcumin Alleviates Oxaliplatin-Induced Peripheral Neuropathic Pain through Inhibiting Oxidative Stress-Mediated Activation of NF-κB and Mitigating Inflammation. Biol Pharm Bull. 2020;43(2):348–355. doi: 10.1248/bpb.b19-00862 [DOI] [PubMed] [Google Scholar]
- 34.Zhao WC, Zhang B, Liao MJ, et al. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014;560:81–85. doi: 10.1016/j.neulet.2013.12.019 [DOI] [PubMed] [Google Scholar]