Abstract
We determined the phenotypic profile of multidrug-resistant (MDR) Escherichia coli isolated from 698 samples (390 and 308 from poultry and domestic pigs, respectively). In total, 562 Enterobacteria were isolated. About 80.5% of the isolates were E. coli. Occurrence of E. coli was significantly higher among domestic pigs (73.1%) than in poultry (60.5%) (p = 0.000). In both poultry and domestic pigs, E. coli isolates were highly resistant to tetracycline (63.5%), nalidixic acid (53.7%), ampicillin (52.3%), and trimethoprim/sulfamethoxazole (50.9%). About 51.6%, 65.3%, and 53.7% of E. coli were MDR, extended-spectrum beta lactamase-producing enterobacteriaceae (ESBL-PE), and quinolone-resistant, respectively. A total of 68% of the extended-spectrum beta lactamase (ESBL) producers were also resistant to quinolones. For all tested antibiotics, resistance was significantly higher in ESBL-producing and quinolone-resistant isolates than the non-ESBL producers and non-quinolone-resistant E. coli. Eight isolates were resistant to eight classes of antimicrobials. We compared phenotypic with genotypic results of 20 MDR E. coli isolates, ESBL producers, and quinolone-resistant strains and found 80% harbored blaCTX-M, 15% aac(6)-lb-cr, 10% qnrB, and 5% qepA. None harbored TEM, SHV, qnrA, qnrS, qnrC, or qnrD. The observed pattern and level of resistance render this portfolio of antibiotics ineffective for their intended use.
Keywords: poultry, domestic pigs, antibiotics, antimicrobial resistance, Msimbazi basin, farmers, Enterobacteriaceae
1. Introduction
Antimicrobial resistance (AMR) is a complex global matter, which requires concerted efforts to curb its serious consequences [1]. Globally, it is estimated that 10 million people will die annually by 2050 if no appropriate measures are taken [2]. It is projected, by that time, that AMR will be the leading cause of death worldwide [3]. This has prompted research on natural products such as ascidians that have shown broad antimicrobial (antibacterial, antifungal, and antiviral) activity [4]. The emergence and spread of AMR genes have been largely associated with human activities such as inappropriate use of antimicrobials in human healthcare, crop and animal farming, and weak infection prevention and control practices/biosecurity in both human and animal healthcare facilities [5]. Globally, in 2014, the consumption of antibiotics for human use was estimated to be 70 billion standard units/year, while for livestock, the amount was approximately 63,151 tons/year [6]. It is predicted that by 2025, this amount will increase by 30% and 67% for humans and animals, respectively [7]. Global trends in sales of antimicrobials for animal production show an 11.5% rise by 2030, mainly due to increased demand in animal proteins [8]. Other studies have estimated that 73% of all antimicrobials sold in the world are used in animal production [9].
AMR in humans has been associated with high morbidity and mortality rates, especially in low-income countries, due to an inability to detect resistance and limited treatment options [10,11,12]. In Tanzania, infections with AMR and multidrug-resistant (MDR) bacteria, especially extended-spectrum beta lactamase (ESBL) and carbapenemase producers, have been associated with increased morbidity and mortality [13,14,15]. Some of the studies conducted in the country have associated AMR with inappropriate prescriptions and self-medication [16,17], and often antibiotics are dispensed without a prescription [18]. In animals, AMR, which is largely due to intensive farming associated with over-use of antimicrobial agents, has been shown to contribute to reduced productivity and economic crisis, as well as spreading resistance organisms to humans and the environment and causing infections that are hard to treat [19,20].
In Tanzania, the demand for short-cycle animal stocks is expected to increase sharply within a short period. For example, pork consumption has been projected to increase from 42.7 thousand to 170 thousand metric tons from 2017 to 2030 [21]. Likewise, estimates show that annual chicken meat production will increase from 22,000 tons in 2017 to 37,200 tons in 2022 [21]. This increased demand has led to intensified farming systems and increased use of antimicrobials, including antibiotics [22,23]. In a recent study conducted among poultry and pig farmers in the Msimbazi River basin, we found high usage of veterinary antimicrobials mainly for prophylaxis (87.6%) compared to therapy (80.5%) [24]. Unfortunately, regulation of antibiotic use in animal food production in Tanzania faces several challenges such as weak regulation in the use of antimicrobials, weak surveillance systems, the tendency for animal owners to stock drugs, engaging unskilled people to treat animals, and a high degree of drug abuse by livestock keepers [25,26,27]. Not surprisingly, antibiotic-resistant bacteria have been reported in about three quarters of food animals, mainly in rural and suburban areas [28,29,30,31]. Food animals carrying AMR organisms can affect human health [32] and eventually contaminate the environment [33], and therefore monitoring the magnitude and pattern of resistance in them is essential in curbing the spread of resistant genes [34].
We deliberately conducted this study in the Msimbazi River basin, a unique ecosystem and the most densely populated area in Tanzania, which supplies most of the poultry, eggs, and domestic pigs for the city of Dar es Salaam [35,36,37]. The aim was to determine phenotypic AMR profiles of MDR Escherichia coli in domestic pigs and poultry. E. coli harbor mobile genetic elements such as plasmids and transposons which facilitates the rapid spread of resistance genes from animals to humans via the environment [38]. Our focus was on genes encoding for ESBL production and quinolone resistance, responsible for resisting the most frequently used antibiotics in animal production and in the treatment of human infections in Tanzania and many African countries [19,24,39].
2. Results
2.1. Detection of Enterobacteriaceae Isolates, Prevalence of Resistance, and Comparative Analysis of Antibiotic-Resistant Profiles from Poultry and Domestic Pig Samples
As shown in Table 1, a total of 562 Enterobacteriaceae isolates were obtained from 698 samples (390 and 308 from poultry and domestic pigs, respectively). About 80.5% of the isolates were E. coli. Isolation of E. coli was higher in domestic pigs (73.1%) than in poultry (60.5%) (p = 0.000), while poultry harbored more K. pneumoniae (2.3%) compared to domestic pigs (1.9%). Other Enterobacteriaceae detected were Klebsiella oxytoca, Pantoea species, Leclercia adercarboxylate, Citrobacter species, Erwinia species, Serratia odorifera, and Salmonella enterica.
Table 1.
Frequency of Enterobacteriaceae isolates from poultry and domestic pigs.
| Organism | Isolates Recovered from Poultry (n = 310) | Isolates Recovered from Domestic Pigs (n = 252) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Escherichia coli | 236 | 60.5 | 225 | 73.1 |
| Klebsiella pneumoniae | 9 | 2.3 | 6 | 1.9 |
| Klebsiella oxytoca | 5 | 1.3 | 2 | 0.6 |
| Pantoea spp. | 32 | 8.2 | 2 | 0.6 |
| Leclercia adercarboxylate | 5 | 1.3 | 1 | 0.3 |
| Citrobacter spp. | 5 | 1.3 | 1 | 0.3 |
| Kluyvera spp. | 4 | 1.0 | 4 | 1.3 |
| Erwinia spp. | 8 | 2.1 | - | - |
| Serratia odorifera | 3 | 0.8 | 5 | 1.6 |
| Salmonella enterica | 2 | 0.5 | 1 | 0.3 |
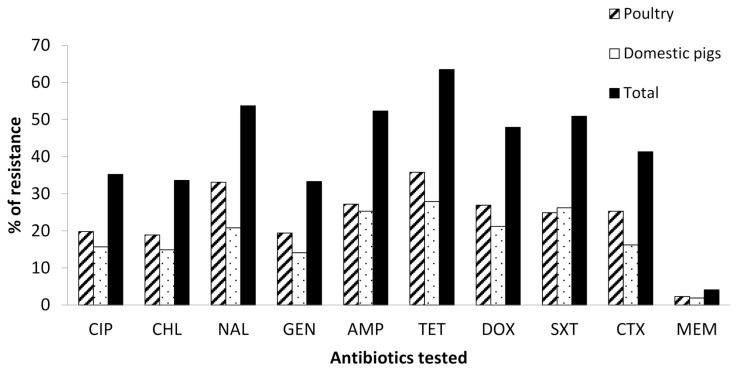
Overall, the highest percentage of resistance in both poultry and domestic pigs was for tetracycline (63.5%), followed by nalidixic acid (53.7%), ampicillin (52.3%), and trimethoprim/sulfamethoxazole (50.9%). As shown in Figure 1 and Table 2, poultry harbored more resistant isolates (55.2%) to almost all tested antibiotics compared to domestic pigs (44.8%). The resistances against nalidixic acid (p = 0.006), trimethoprim/sulfamethoxazole (p = 0.005), and cefotaxime (p = 0.016) were significantly different between poultry and domestic pigs.
Figure 1.
Percentage of antibiotic resistance from the poultry and domestic pig isolates. Key: CIP, ciprofloxacin; CHL, chloramphenicol; NAL, nalidixic acid; GEN, gentamycin; AMP, ampicillin; TET, tetracycline; DOX, doxycycline; SXT, trimethoprim/sulfamethoxazole; CTX, cefotaxime; MEM, meropenem.
Table 2.
Comparative analysis of antibiotic resistance of isolates from poultry versus domestic pigs.
| Antibiotic | % of Resistance in Poultry (n = 236) | % of Resistance in Domestic Pigs (n = 225) | Chi-Square | p-Value |
|---|---|---|---|---|
| CIP (n = 199) | 28.5 | 28.6 | 0.360 | 0.835 |
| CHL (n =190) | 27.2 | 27.3 | 0.360 | 0.835 |
| NAL (n = 303) | 47.3 | 38.0 | 10.090 | 0.006 |
| GEN (n = 188) | 27.7 | 26.0 | 0.845 | 0.655 |
| AMP (n = 295) | 39.0 | 46.4 | 3.997 | 0.136 |
| TET (n = 358) | 51.3 | 51.3 | 0.447 | 0.800 |
| DOX (n 270) | 38.5 | 39.0 | 0.346 | 0.841 |
| SXT (n = 278) | 35.9 | 47.7 | 10.566 | 0.005 |
| CTX (n = 233) | 36.4 | 29.5 | 8.265 | 0.016 |
| MEM (n = 24) | 3.3 | 3.6 | 0.168 | 0.919 |
Key: CIP, ciprofloxacin; CHL, chloramphenicol; NAL, nalidixic acid; GEN, gentamycin; AMP, ampicillin; TET, tetracycline; DOX, doxycycline; SXT, trimethoprim/sulfamethoxazole; CTX, cefotaxime; MEM, meropenem.
As shown in Table 3, isolates from poultry showed no significant variations in resistance by location against all of the tested antibiotics except for gentamycin (p = 0.046), ampicillin (p = 0.026), and doxycycline (p = 0.018). For domestic pigs, there were significant variations by location in resistance against all the tested drugs except for doxycycline (p = 0.101) (Table 4).
Table 3.
Prevalence of antibiotic-resistant E. coli from poultry by ward.
| No. of E. coli per Ward | % of Resistance to the Tested Antibiotic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CIP | CHL | NAL | GEN | AMP | TET | DOX | SXT | CTX | MEM | |
| Ukonga (30) | 56.7 | 43.3 | 73.3 | 22.4 | 70.0 | 61.2 | 59.7 | 49.3 | 63.3 | 3.0 |
| Kipawa (67) | 32.8 | 29.9 | 64.2 | 30.0 | 52.2 | 80.0 | 66.7 | 56.7 | 43.3 | 10.0 |
| Gongolamboto (22) | 50.0 | 36.4 | 72.7 | 31.8 | 36.4 | 81.8 | 63.6 | 54.5 | 68.2 | 0.0 |
| Buguruni (15) | 33.3 | 20.0 | 46.7 | 40.0 | 40 | 60.0 | 20.0 | 26.7 | 33.3 | 6.7 |
| Kinyerezi (53) | 39.6 | 39.6 | 56.6 | 50.9 | 50.9 | 64.2 | 43.4 | 41.5 | 39.6 | 5.7 |
| Segerea (47) | 34.0 | 31.9 | 63.8 | 34.0 | 53.2 | 72.3 | 46.8 | 55.3 | 61.7 | 4.3 |
| Overall resistance | 39.3 | 34.2 | 63.2 | 34.2 | 52.1 | 68.4 | 52.1 | 48.7 | 50.4 | 4.7 |
| p-value | 0.237 | 0.569 | 0.419 | 0.046 | 0.223 | 0.254 | 0.018 | 0.315 | 0.026 | 0.599 |
Key: CIP, ciprofloxacin; CHL, chloramphenicol; NAL, nalidixic acid; GEN, gentamycin; AMP, ampicillin; TET, tetracycline; DOX, doxycycline; SXT, trimethoprim/sulfamethoxazole; CTX, cefotaxime; MEM, meropenem.
Table 4.
Prevalence of antibiotic-resistant E. coli from domestic pigs by ward.
| No. of E. coli per Ward | % of Resistance to the Tested Antibiotic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CIP | CHL | NAL | GEN | AMP | TET | DOX | SXT | CTX | MEM | |
| Ukonga (42) | 23.8 | 21.4 | 33.3 | 19.0 | 27.8 | 50.0 | 27.8 | 33.3 | 42.9 | 5.6 |
| Kipawa (18) | 11.1 | 5.6 | 33.3 | 11.1 | 54.8 | 64.3 | 54.2 | 61.9 | 55.6 | 7.1 |
| Kinyerezi (35) | 34.3 | 25.7 | 37.1 | 25.7 | 54.3 | 51.4 | 37.1 | 62.9 | 20.0 | 0.0 |
| Segerea (58) | 24.1 | 22.4 | 43.1 | 22.4 | 48.3 | 53.4 | 41.4 | 41.4 | 27.6 | 1.7 |
| Kisarawe (51) | 60.8 | 62.7 | 64.7 | 47.1 | 76.5 | 80.4 | 60.8 | 80.4 | 47.1 | 11.8 |
| Pugu station (18) | 38.9 | 38.9 | 44.4 | 44.4 | 72.2 | 66.7 | 50.0 | 50.0 | 38.9 | 0.0 |
| Overall resistance | 34.2 | 32.0 | 44.6 | 28.8 | 57.2 | 62.2 | 46.8 | 57.7 | 36.7 | 5.0 |
| p-value | 0.000 | 0.000 | 0.031 | 0.006 | 0.003 | 0.033 | 0.101 | 0.000 | 0.034 | 0.022 |
Key: CIP, ciprofloxacin; CHL, chloramphenicol; NAL, nalidixic acid; GEN, gentamycin; AMP, ampicillin; TET, tetracycline; DOX, doxycycline; SXT, trimethoprim/sulfamethoxazole; CTX, cefotaxime; MEM, meropenem.
2.2. Multidrug Resistance of E. coli Isolates in Cloacal and Rectal Swabs from Poultry and Domestic Pigs
Table 5 shows that out of 461 E. coli isolates, 51.6% (n = 238) were MDR against the tested drugs. The most common resistance patterns observed were QNL/PHE/AMN/PEN/TET/SUL/CEP (62 isolates), QNL/PHE/AMN/PEN/TET/SUL (20 isolates), QNL/PHE/PEN/TET/SUL (16 isolates), QNL/PEN/TET/SUL (15 isolates), and PEN/TET/SUL (14 isolates). Eight isolates were resistant to eight classes of antimicrobials.
Table 5.
Multidrug resistance patterns among 431 E. coli isolated from poultry and domestic pigs.
| No. of Antibiotics Classes | Resistance Pattern | No. of Isolates | Prevalence (%) |
|---|---|---|---|
| 3 | TET/SUL/CEP | 4 | 0.87 |
| QNL/PEN/SUL | 6 | 1.30 | |
| AMN/PEN/TET | 3 | 0.65 | |
| QNL/AMN/TET | 8 | 1.73 | |
| QNL/PEN/TET | 3 | 0.65 | |
| PEN/SUL/CEP | 2 | 0.43 | |
| QNL/PEN/TET | 5 | 1.08 | |
| PEN/TET/SUL | 14 | 3.04 | |
| QNL/TET/SUL | 6 | 1.30 | |
| PHE/TET/SUL | 3 | 0.65 | |
| QNL/TET/CEP | 3 | 0.65 | |
| 4 | QNL/AMN/TET/CEP | 5 | 1.08 |
| QNL/PEN/SUL/CEP | 4 | 0.87 | |
| QNL/AMN/TET/SUL | 3 | 0.65 | |
| QNL/PEN/TET/SUL | 15 | 3.25 | |
| PHE/QNL/PEN/TET | 3 | 0.65 | |
| PHE/PEN/TET/SUL | 4 | 0.87 | |
| PEN/TET/SUL/CEP | 2 | 0.43 | |
| QNL/TET/SUL/CEP | 4 | 0.87 | |
| 5 | QNL/AMN/TET/SUL/CEP | 3 | 0.65 |
| QNL/PEN/TET/SUL/CEP | 5 | 1.08 | |
| QNL/AMN/PEN/TET/SUL | 4 | 0.87 | |
| QNL/PHE/TET/SUL/CEP | 3 | 0.65 | |
| QNL/PHE/PEN/TET/SUL | 16 | 3.47 | |
| 6 | QNL/AMN/PEN/TET/SUL/CEP | 2 | 0.43 |
| QNL/PHE/AMN/PEN/TET/CEP | 2 | 0.43 | |
| QNL/PHE/PEN/TET/SUL/CEP | 3 | 0.65 | |
| QNL/PHE/AMN/PEN/TET/SUL | 20 | 4.34 | |
| 7 | QNL/PHE/PEN/TET/SUL/CEP/CAR | 11 | 2.39 |
| QNL/PHE/AMN/PEN/TET/SUL/CEP | 62 | 13.45 | |
| 8 | QNL/PHE/AMN/PEN/TET/SUL/CEP/CAR | 10 | 2.17 |
| Total | 238 | 51.6 | |
Key: QNL, quinolones; PHE, phenocols; AMN, aminoglycosides; PEN, penicillins; TET, tetracyclines; SUL, sulfonamides; CEP, cephalosporins; CAR, carbapenems.
2.3. ESBL-Producing E. coli Isolated from the Cloacal and Rectal Swabs from Poultry and Domestic Pigs
From the 461 E. coli isolates of cloacal and rectal swabs screened for ESBL production using 2 µg/mL cefotaxime, 65.3% (301/461) were positive, and of these positive isolates, all were confirmed to be extended-spectrum beta lactamase-producing Enterobacteriaceae (ESBL-PE). The ESBL isolates were significantly more resistant to tetracycline, CIP, doxycycline, trimethoprim/sulfamethoxazole, and nalidixic acid than non-ESBL producers (Table 6).
Table 6.
Comparative antibiotic resistance of extended-spectrum beta lactamase (ESBL)- and non-ESBL-producing E. coli in cloacal and rectal swabs of poultry and domestic pigs.
| Antibiotic | % of Resistant ESBL E. coli Producers (n = 301) | % of Resistant Non-ESBL E. coli Producers (n = 160) | p-Value |
|---|---|---|---|
| Ciprofloxacin | 41.2(124) | 30.6(49) | 0.000 |
| Chloramphenicol | 36.2(109) | 28.1(45) | 0.000 |
| Nalidixic acid | 59.8(180) | 45.0(72) | 0.000 |
| Gentamycin | 33.6(101) | 29.4(47) | 0.000 |
| Tetracycline | 70.1(211) | 57.5(92) | 0.000 |
| Doxycycline | 52.5(158) | 45.6(73) | 0.000 |
| Trimethoprim/ Sulfamethoxazole |
55.5(167) | 50.0(80) | 0.000 |
2.4. Quinolone-Resistant E. coli in Cloacal and Rectal Swabs from Poultry and Domestic Pigs
Out of 461 E. coli isolates tested for quinolone resistance, 37.5% (n = 173) were found to be quinolone-resistant [40] and were significantly more resistant to all the tested antibiotics compared with non-quinolone isolates (Table 7). As shown in Table 8, both ESBL producers and quinolone resistance depicted the various level of resistance, with tetracycline receiving significant resistance both in ESBL and quinolone-resistant isolates. About 68.1% of ESBL producers were resistant to quinolone. Quinolone resistance significantly predicted higher resistance to CIP, CHL, GEN, TET, and SXT compared to the ESBL phenotype.
Table 7.
Comparative antibiotic resistance of quinolone-resistant versus non-quinolone-resistant E. coli.
| Antibiotic | % Quinolone Resistance (n = 173) | % Non-Quinolone Resistance (n = 288) | Chi-Square | p-Value |
|---|---|---|---|---|
| Chloramphenicol | 70.5(122) | 11.1(32) | 171.469 | 0.000 |
| Gentamycin | 60.1(104) | 15.3(44) | 99.683 | 0.000 |
| Ampicillin | 82.1(142) | 37.2(107) | 87.830 | 0.000 |
| Tetracycline | 92.5(160) | 49.7(143) | 88.022 | 0.000 |
| Doxycycline | 80.9(140) | 31.6(91) | 105.191 | 0.000 |
| Trimethoprim/ Sulfamethoxazole |
82.7(143) | 36.1(104) | 94.152 | 0.000 |
| Cefotaxime | 52.0(90) | 40.6(117) | 5.675 | 0.017 |
| Meropenem | 11.0(19) | 1.0(3) | 24.028 | 0.000 |
Table 8.
Comparative results of ESBL producers against quinolone-resistant E. coli isolates.
| ESBL Producer Isolate | Quinolone-Resistant Isolate | ||||||
|---|---|---|---|---|---|---|---|
| R | S | p-Value | R | S | p-Value | ||
| CHL | 36.2(109) | 63.8(192) | 0.080 | CHL | 70.5(122) | 11.1(32) | 0.000 |
| GEN | 33.6(101) | 64.4(200) | 0.360 | GEN | 60.1(104) | 15.3(44) | 0.000 |
| TET | 70.1(211) | 29.9(90) | 0.007 | TET | 92.5(160) | 49.7(143) | 0.000 |
| DOX | 52.5(158) | 47.5(143) | 0.160 | DOX | 80.9(140) | 31.6(91) | 0.000 |
| SXT | 55.5(167) | 44.5(134) | 0.261 | SXT | 82.7(143) | 36.1(104) | 0.000 |
Key: CIP, ciprofloxacin; CHL, chloramphenicol; NAL, nalidixic acid; GEN, gentamycin; AMP, ampicillin; TET, tetracycline; DOX, doxycycline; SXT, trimethoprim/sulfamethoxazole; CTX, cefotaxime; MEM, meropenem.
2.5. Genotypic Identification of ESBL Resistant Genes (CTX-M, TEM, SHV) and Quinolone-Resistant Genes (qnrA, qnrB, qnrS, qnrC, qnrD, aac(6′)-lb-cr, and qepA)
Out of 20 E. coli selected from MDR isolates, the CTX-M gene was detected in 16/20 (80%), while TEM and SHV were not detected. The quinolone-resistant genes (qrnB, aac(6)-lb-cr, and qepA) were detected in 6/20 (30%) (Table 9 and Figure 2). One isolate from the poultry harbored both qepA and aac(6)-lb-cr genes. The resistant genes qnrA, qnrS, qnrC, and qnrD were not detected in any of the tested isolates.
Table 9.
Distribution of ESBL and quinolone-resistant genes from the selected multidrug-resistant (MDR) E. coli (n = 20).
| AMR Genes | E. coli No (%) | Sample Type | |
|---|---|---|---|
| Poultry | Domestic Pigs | ||
| bla CTX-M | 16/20 (80) | 7 | 9 |
| bla TEM | 0/20 (0) | 0 | 0 |
| bla SHV | 0/20 (0) | 0 | 0 |
| qrnA | 0/20 (0) | 0 | 0 |
| qrnB | 2/20 (10) | 0 | 2 |
| qnrS | 0/20 (0) | 0 | 0 |
| qnrC | 0/20 (0) | 0 | 0 |
| qnrD | 0/20 (0) | 0 | 0 |
| aac(6)-Ib-cr | 3/20 (15) | 2 | 1 |
| qepA | 1/20 (5) | 1 | 0 |
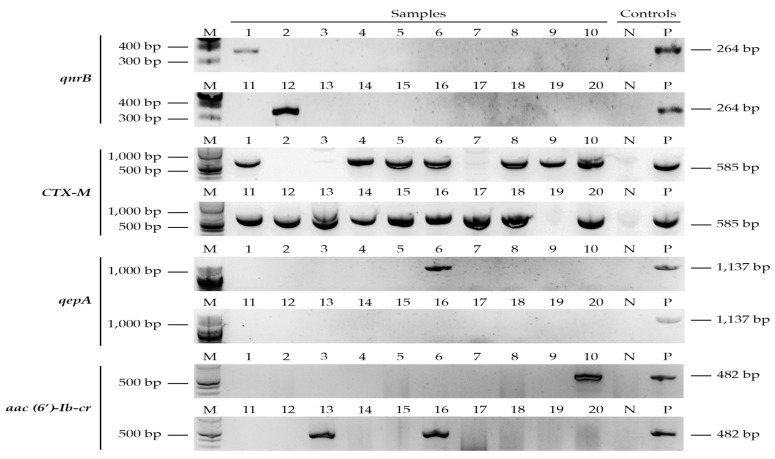
Figure 2.
Gel electrophoretic bands of ESBL and quinolone-resistant genotypes (qnrB, CTX-M, qepA, and aac(6)-lb-cr). Letters: M—DNA ladder, N—negative control, and P—positive control. Positive samples are numbers 1, 10, 12, 13, and 16 (qnrB, aac(6)-lb-cr, and qepA) and samples 1, 4, 5, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, and 20 (CTX-M).
3. Discussion
In this study, 80.5% of the isolates from 698 samples of rectal and cloaca swabs from domestic pigs and poultry were recovered. The levels of resistance to the tested antibiotics were higher especially for tetracycline (65.6%), nalidixic acid (53.7%), ampicillin (52.3%), and trimethoprim/sulfamethoxazole (50.9%). Overall, 51.6%, which is close to half of all E. coli isolates from the poultry and domestic pigs, exhibited multidrug resistance against three to eight classes of antimicrobial agents tested, with the most resistant pattern being against QNL/PHE/AMN/PEN/TET/SUL/CEP. The level of resistance identified in this study compares with the findings reported in Zimbabwe, Nigeria, Ghana, and China which were attributed to extensive use of veterinary drugs in animal farming [41,42,43,44]. These levels of resistance in the current study were not surprising basing on the mode of animal farming that involved intensive use of veterinary drugs for therapeutics and disease prevention. Recent findings reported that the veterinary antimicrobial classes used most extensively in animal farming are tetracycline, penicillin, quinolones, and sulphonamides [19,24,39], thus leading to the higher resistance level. The findings on the levels of MDR are in line with those reported in Tanzania, Ghana, and Angola, of which 42%, 56.9%, and 50%, respectively, of the identified E. coli isolates from food animals were MDR [45,46,47]. However, the percentages of MDR in this study are lower than the ones reported in Ghana, Nigeria, and Zambia [43,48,49]. The possible explanation to the level of MDR in this study might certainly be due to the selection pressure and environmental contamination by a variety of wastes including plastic litter, industrial effluents, and uncontrolled disposal of human and veterinary drugs [50,51]. We found that poultry harbored isolates that were more resistant (55.2%) to almost all tested antibiotics. Previous findings from Tanzania reported that poultry farming is associated with uncontrolled use of both human and veterinary antimicrobials, mainly for growth promotion compared to therapeutics, and metaphylaxis is very commonly practiced by the majority of farmers [24,52]. The level of resistance to these tested antibiotics for isolates from poultry corresponds to previous studies [50,53,54], showing poultry farming involves intensive and extensive use of antibiotics compared to other domestic animals. Tetracycline, aminoglycosides, penicillin, quinolone, and sulphonamides are among the antibiotics reported to be commonly used in poultry in Ghana, Cameroon, and Sudan [55,56,57], leading to the development of antibiotic resistance. On the other hand, we found a low level of resistance against meropenem (less than 10%) both in poultry and domestic pigs, which is not surprising given the fact that these antibiotics are not easily accessed due to cost [58,59].
Notably, we found significant variations in antibiotic resistance by wards among isolates obtained from both poultry and domestic pigs, which may be due to variations in farming conditions and the use of antibiotics [60,61]. However, this was not investigated in this study and may therefore require further research. Nonetheless, this finding is significant, showing a lack of professional guidance on the use of antimicrobials in animal farming, supporting previous studies in the same area showing unregulated use of antibiotics that are largely obtained over the counter [24]. Farmers face several challenges that include reduced drug quality, substandard/counterfeit veterinary drugs, and uncontrolled use of drugs in healthcare, agriculture, and industrial settings, and lack of veterinary services [62,63].
In this study, the majority of the E. coli isolates (65.3%) were found to be ESBL producers, a level similar to other studies [34,64,65,66] but higher than levels reported in Zambia (20%), Nigeria (37.8%), and Ghana (29%) [49,67,68]. All ESBL producers were significantly more resistant to all the tested antimicrobials as compared to the non-ESBL producers, suggesting selective pressure due to extensive use of beta-lactam and cephalosporin in animal farming, and the existence of multiple resistance mechanisms resulting from indiscriminate use of veterinary drugs [69,70,71,72]. On the other hand, approximately half, 37.5%, of all isolates were found to be quinolone (ciprofloxacin or nalidixic acid)-resistant and these strains were more resistant to all the tested antibiotics compared to non-quinolone isolates, probably due to persistent use of antibiotics for prophylaxis, therapeutics, metaphylaxis, and growth promotion, a finding reported in other studies [42,73].
Notably, resistance to tetracycline and trimethoprim/sulfamethoxazole was higher both in ESBL producers and quinolone-resistant isolates, suggesting the presence of an association between ESBL producers and quinolone resistance, as previously reported [74,75]. The association between ESBL and quinolone resistance may highlight the presence of similar resistance mechanisms in the clinical and environmental setup, and intensive and prolonged use of beta-lactam drugs, cephalosporin, and quinolone drugs in poultry and domestic pig farming [76]. The findings are in line with other studies that reported the linkage between ESBL producers and quinolone-resistant genes due to co- transmission of resistant genes among members of the family Enterobacteriaceae, leading to dissemination of MDR organisms [70,72,74,76,77].
We compared phenotypic with genotypic results of 20 MDR E. coli isolates, ESBL producers, and quinolone-resistant strains and found that about 80% harbored blaCTX-M, 15% aac(6)-Ib-cr, and 5% qepA. None of the isolates harbored TEM, SHV, qnrA, qnrS, qnrC, or qnrD. We noted that some of the isolates that were sensitive by the phenotypic method harbored resistance genes, a phenomenon that has been observed by others [72,78]. Studies have shown that phenotypic resistance is dependent on the mode and level of gene expression [79,80]. On the other hand, some isolates that were phenotypically resistant did not harbor the screened genes, implying that other genes such as ampC, VEB, OXA, PER, oqxA, or oqxB might be responsible [74,81].
The high levels of AMR associated with poultry and domestic pig farming present a risk to human health presented by the ineffectiveness of the currently used antibiotics [62,66,82], thus causing human and animal infections that are difficult to treat [50,55,83]. This study has some limitations.
Other Enterobacteria spp. isolates were too few for detailed subanalysis; therefore, we decided to focus on E. coli. Secondly, we did not perform molecular characterization of all of the resistant isolates, which may be considered as a limitation. However, the phenotypic analysis conducted was based on the internationally recognized and standardized Clinical Laboratory Standard Institute (CLSI) 2019 protocol and guidelines [84], thus reflecting the real magnitude and pattern of AMR in the study setting. Nonetheless, we are planning to collect isolates from humans and the environment and perform genotypic studies in order to understand the flow of resistomes across the human, animal, and environment compartments. Lastly, we could not analyze samples coming from poultry and domestic pigs not treated with antibiotics as we could not find such farms due to the widespread use of antimicrobials in animal production in this community [19,26,39].
4. Methodology
4.1. Selection of Study Farms and Animals
This study was conducted in Kinondoni, Kisarawe, and Ilala districts that form part of the Msimbazi River basin. Eight wards were included, namely, Kisarawe, Pugu station, Gongolamboto, Ukonga, Kipawa, Segerea, Kinyerezi, and Buguruni. Poultry farms were selected based on having more than 100 broilers and/or layers and 50 or more improved local chickens kept for commercial purposes. For each poultry farm, 5% out of 100 flocks were sampled. Animals of less than two weeks were not sampled under the assumption that they were at the early stage of production. The selection of pig farms was based on the herd having animals that were ready to be slaughtered in which 10% of the animals were selected. Poultry and pig farms included in this study were randomly selected from a list provided by the ward livestock officers within the study area.
4.2. Sample Collection
About 1 g of fecal materials from the cloaca and rectum from poultry and domestic pigs was collected aseptically using a sterile cotton swab (Himedia, Mumbai, India). The cotton swabs were then placed into the sterile tube filled with 3 mL Cary Blair medium (Oxoid, Basingstoke, UK). All samples were transported in a cool box containing ice packs at a temperature of 2 to 8 °C and processed within 2 h of collection in the Microbiology Teaching Laboratory at the Muhimbili University and Allied Sciences (MUHAS).
4.3. Isolation of Bacteria
Specimens from cloacal/rectal swabs were directly streaked onto the MacConkey agar (Oxoid, Basingstoke, UK) without antibiotics and incubated at 37 °C aerobically for 24 h. A single colony from predominant morphologically similar colonies was picked from each plain MacConkey agar plate and subcultured in a nutrient agar (Hi media, Mumbai, India). Colonies on nutrient agar were identified by colonial morphology, Gram stain, catalase and oxidase production [85], and various biochemical tests (indole, methyl red, Voges–Proskauer, and citrate utilization test) and were later confirmed by API 20E following the manufacturer’s recommendations (BioMérieux, Marcyl’Etoile, France) [86]. Briefly, a single colony was emulsified into sterile saline and filled in the compartments and then incubated at 37 °C for 18 to 24 h aerobically in a wet chamber of analytical profile index (API), API 20E strips (BioMérieux, Marcy-Etoile, France). E. coli and other Enterobacteriaceae were identified at the species level.
4.4. Antibiotic Susceptibility Testing
The antimicrobial susceptibility testing was conducted using the Kirby–Bauer disc diffusion method on Mueller Hinton Agar (Becton, Dickinson and Company, MD, USA) based on the CLSI standards [76]. Antibiotics tested were doxycycline (30 µg), cefotaxime (30 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), ampicillin (10µg), tetracycline (30 µg), chloramphenicol (30 µg), gentamicin (10 µg), meropenem (30 µg), and trimethoprim/sulfamethoxazole (1.25 µg/23.75 µg). These antibiotics are considered by the World Health Organization (WHO) to be clinical and useful in animal production [87]. One to two colonies from the pure culture of the identified lactose fermenters were emulsified into 5 mL of sterile saline. The suspensions were adjusted to achieve turbidity equivalent to 0.5 McFarland standard solutions [84], emulsified using sterile cotton swabs onto Mueller Hinton Agar plate, and incubated at 37 °C for 16 to 18 h. The inhibition zone of each antimicrobial agent was measured after 16 to 18 hours’ incubation. Results were interpreted according to the CLSI standards, and E. coli strain American Type Culture Collection (ATCC) 29522 and K. pneumoniae strain ATCC 700603 were used as controls. An isolate was considered to be multidrug-resistant (MDR) if it was non-susceptible to three or more drugs from different classes of antimicrobial [88].
4.5. Screening for ESBL
The confirmed E. coli isolates (by API 20E strips) from plain MacConkey agar were inoculated onto MacConkey agar containing 2 µg/mL cefotaxime for preliminary screening of ESBL production [89]. Confirmation of ESBL production was conducted using the combination disk diffusion method, with cefotaxime (30 µg) alone and in combination with clavulanic acid (10 µg), and with ceftazidime (30 µg) alone and combination with clavulanic acid (10 µg), and a zone of inhibition of more than or equal to 5 mm confirmed ESBL production. K. pneumoniae (ATCC 700603) was used as a positive control (ESBL-positive strain) and E. coli (ATCC 25922) used as the ESBL-negative strain, and results were interpreted as per CLSI standards 2019.
4.6. Polymerase Chain Reaction (PCR)
4.6.1. DNA Extraction
ESBL-producing E. coli isolates were inoculated on nutrient agar and incubated aerobically at 37 °C for 24 h. DNA was extracted by boiling in a water bath at 100 °C for 10 min, followed by centrifugation at 1500 rpm for 3 min. The supernatant containing DNA was transferred into a sterile Eppendorf PCR tube (Eppendorf AG, Hamburg, Germany) and centrifugation and separation of supernatant were repeated three times. The concentration of DNA was determined by a Nanodrop spectrophotometer (Biochrom LTD, Cambridge, England) at 260/280 wavelength (ranging from 1.5 to 1.8). DNA was stored at −20 °C, before being used for detection of ESBL genes (CTX-M, TEM, and SHV) and PMQR genes (qnrA, qnrB, qnrS, qnrC, qnrD, qepA, and aac(6′)-Ib-cr).
The One Tag Master Mix Hot Start DNA polymerase kit (New England Biolabs, Ipswich, MA, USA) was used in detection of resistance genes. Total PCR reaction volumes were 25 µL, consisting of One Tag Master Mix 2X Standard buffer 12.5 µL, 10 µM forward primer 0.5 µL, 10 µM reverse primer 0.5 µL, nuclease-free water 9.5 µL, and DNA template 2 µL. The primers used in amplification of the respective E. coli resistance genes are listed in Table 10 below.
Table 10.
PCR primers, sequences, and protocols used.
| Gene | Primer Set and Sequence (5′-3′) | Amplicon Size | Reference |
|---|---|---|---|
| CTX-M | F: SCSATGTGCAGYACCAGTAA R: ACCAGAAYVAGCGGBGC |
585 bp | [90,91] |
| qnrA | F: TCAGCAAGAGGATTTCTCA R: GGCAGCACTATTACTCCCA |
627 bp | [92] |
| qnrB | F: GGMATHGAAATTCGCCACTG R: TTTGCYGYYCGCCAGTCGAA |
264 bp | [92] |
| qnrS | F: ATGGAAACCTACAATCATAC R: AAAAACACCTCGACTTAAGT |
467 bp | [92] |
| aac(6′)-Ib-cr | F: TTGCGATGCTCTATGAGTGGCTA R: CTCGAATGCCTGGCGTGTTT |
482 bp | [92,93] |
| TEM | F: ATGAGTATTCAACATTTCCG R: CTGACAGTTACCAATGCTTA |
868 bp | [94] |
| SHV | F: GGTTATGCGTTATATTCGCC R: TTAGCGTTGCCAGTGCTC |
867 bp | [94] |
| qnrC | F: GGGTTGTACATTTATTGAATC R: TCCACTTTACGAGGTTCT |
447 bp | [75] |
| qnrD | F: CGAGATCAATTTACGGGGAATA R: AACAAGCTGAAGCGCCTG |
582 bp | [75] |
| qepA | F: TGGTCTACGCCATGGACCTCA R: TGAATTCGGACACCGTCTCCG |
1137 bp | [75] |
4.6.2. Molecular Detection of CTX-M Genes
ESBL-producing E. coli isolates were screened for the CTX-M gene using a uniplex PCR-based technique [90]. PCR conditions involved initial denaturation at 96 °C for 5 min, followed by 35 cycles of denaturation at 96 °C for 30 s, annealing at 56 °C for 40 s, extension at 72 °C for 60 s, and final extension at 72 °C for 10 min.
4.6.3. Detection of TEM and SHV Genes
ESBL genes TEM and SHV were screened by a uniplex PCR-based assay involving initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C (TEM and SHV) for 40 s, extension at 72 °C for 1 min, and final extension at 72 °C for 10 min [94].
4.6.4. Detection of Quinolone-Resistant Genes (qnrA, qnrB, and qnrS)
The quinolone-resistant genes (qnrA, qnrB, and qnrS) were amplified and detected using multiplex PCR assay [75]. This involved initial denaturation at 94 °C for 5 min, followed by 32 cycles of denaturation at 94 °C for 45 s, annealing at 53 °C for 1 min, extension at 72 °C for 1 min, and final extension at 72 °C for 10 min [75].
4.6.5. Detection of aac(6′)-lb-cr Gene
The aac(6′)-lb-cr gene was screened by a uniplex PCR-based assay [93] using the following amplification conditions: initial denaturation at 94 °C for 5 min, followed by 34 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s, extension at 72 °C for 45 s, and final extension at 72 °C for 10 min [93].
4.6.6. Detection of PMQR Genes (qepA, qnrC, and qnrD)
The qepA gene was screened by a uniplex PCR-based assay [93] using the following amplification conditions: initial denaturation at 96 °C for 5 min, followed by 30 cycles of denaturation at 96 °C for 30 s, annealing at 56 °C for 30 s, extension at 72 °C for 60 s, and final extension at 72 °C for 5 min [75].
4.7. Ethical Considerations
The ethical clearance was provided by the Medical Research Coordinating Committee of the National Institute for Medical Research (NIMR) of Tanzania (Reference No. NIMR/HQ/R.8a/Vol. IX/3133), and Muhimbili University of Health and Allied Sciences (Permit No. DA.282/298/01.C). The permission was sought from the relevant authorities that are the municipal directors at the three districts.
4.8. Data Management
The data were entered in Excel version Office 2007 and then transferred to SPSS version 20.0 for Windows (IBM Corp, Armonk, NY, USA) software for statistical analysis. Categorical variables were described as frequencies and percentages. The chi-square test was used to determine the difference, and a p-value of less than 0.05 was considered significant.
5. Conclusions
The high levels of AMR as well as ESBL producer, quinolone-resistant, and MDR (up eight different classes) isolates associated with poultry and domestic pig farming seem to render the currently used antibiotics ineffective for their intended use, and their continued use potentially escalates the burden of antimicrobial resistance beyond these animal species.
Acknowledgments
The authors acknowledge the support provided by the SACIDS foundation for One Health for supporting the study through provision of grants. We appreciate the technical assistance provided by the local government authorities and the SACIDS staff during data collection and molecular analysis.
Author Contributions
Study design, sample collection, laboratory work and analysis, original draft preparation, Z.I.K.; organization and supervision of laboratory work, F.X.M. and G.M.; design of the outline, review of the manuscript, and supervision of the manuscript writing, M.I.N.M.; coordination of data analysis and interpretation and manuscript editing, N.M.; manuscript review and editing, S.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Government of the United Republic of Tanzania through the World Bank [WB-ACE II Grant PAD1436, IDA [Credit 5799-TZ] to the SACIDS Africa Centre of Excellence for Infectious Diseases at Sokoine University of Agriculture.
Institutional Review Board Statement
The ethical clearance was provided by the Medical Research Coordinating Committee of the National Institute for Medical Research (NIMR) of Tanzania (Reference No. NIMR/HQ/R.8a/Vol. IX/3133), and Muhimbili University of Health and Allied Sciences (Permit No. DA.282/298/01.C). The permission was sought from the relevant au-thorities that are the municipal directors at the three districts.
Data Availability Statement
The data generated is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Kraker M.E.A., Stewardson A.J., Harbarth S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016;13:e1002184. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. AMR Review; London, UK: 2016. [(accessed on 9 January 2018)]. p. 84. Available online: https://amr-review.org. [Google Scholar]
- 4.Casertano M., Menna M., Imperatore C. The ascidian-derived metabolites with antimicrobial properties. Antibiotics. 2020;9:510. doi: 10.3390/antibiotics9080510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandyopadhyay S., Samanta I. Antimicrobial Resistance in Agri-Food Chain and Companion Animals as a Re-emerging Menace in Post-COVID Epoch: Low-and Middle-Income Countries Perspective and Mitigation Strategies. Front. Vet. Sci. 2020;7:620. doi: 10.3389/fvets.2020.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A., Laxminarayan R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet. Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 7.Gelband H. The State of the World’s Antibiotics 2015. Center for Disease Dynamics, Economics & Policy; Washington, DC, USA: 2015. [Google Scholar]
- 8.Tiseo K., Huber L., Gilbert M., Robinson T.P., Van Boeckel T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics. 2020;9:918. doi: 10.3390/antibiotics9120918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Boeckel T.P., Glennon E.E., Chen D., Gilbert M., Robinson T.P., Grenfell B.T., Bonhoeffer S., Laxminarayanm R. Reducing antimicrobial use in food animals. Science. 2017;357:1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanwar J., Das S., Fatima Z., Hameed S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014;2014:541340. doi: 10.1155/2014/541340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basak S., Singh P., Rajurkar M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016;2016:1–5. doi: 10.1155/2016/4065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernabé K.J., Langendorf C., Ford N., Ronat J.B., Murphy R.A. Antimicrobial resistance in West Africa: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2017;50:629–639. doi: 10.1016/j.ijantimicag.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Blomberg B., Manji K.P., Urass W.K., Tamim B.S., Mwakagile D.S., Jureen R., Msangi V., Tellevik M.G., Holberg-Petersen M., Harthug S., et al. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: A prospective cohort study. BMC Infect. Dis. 2007;7:43. doi: 10.1186/1471-2334-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayange N., Kamugisha E., Mwizamholya D.L., Jeremiah S., Mshana S.E. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza- Tanzania. BMC Pediatr. 2010;10:39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manyahi J., Kibwana U., Mgimba E., Majigo M. Multi-drug resistant bacteria predict mortality in bloodstream infection in a tertiary setting in Tanzania. PLoS ONE. 2020;15:e0220424. doi: 10.1371/journal.pone.0220424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horumpende P.G., Said S.H., Mazuguni F.S., Antony M.L., Kumburu H.H., Sonda T.B., Mwanziva C.E., Mshana S.E., Mmbaga B.T., Kajeguka D.C., et al. Prevalence, determinants and knowledge of antibacterial self-medication: A cross sectional study in North-eastern Tanzania. PLoS ONE. 2018;13:e0206623. doi: 10.1371/journal.pone.0206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngocho J.S., Horumpende P.G., de Jonge M.I., Mmbaga B.T. Inappropriate treatment of community-acquired pneumonia among children under five years of age in Tanzania. Int. J. Infect. Dis. 2020;93:56–61. doi: 10.1016/j.ijid.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horumpende P.G., Sonda T.B., van Zwetselaar M., Antony M.L., Tenu F.F., Mwanziva C.E., Shao E.R., Mshana S.E., Mmbaga B.T., Chilongola J.O. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PLoS ONE. 2018;13:e0207465. doi: 10.1371/journal.pone.0207465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimera Z.I., Mshana S.E., Rweyemamu M.M., Mboera L.E.G., Matee M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control. 2020;9:1–12. doi: 10.1186/s13756-020-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bengtsson B., Greko C. Antibiotic resistance-consequences for animal health, welfare, and food production. Ups. J. Med. Sci. 2014;119:96–102. doi: 10.3109/03009734.2014.901445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michael S., Mbwambo N., Mruttu H., Dotto M., Ndomba C., da Silva M., Makusaro F., Nandonde S., Crispin J., Shapiro B., et al. Tanzania Livestock Master Plan. [(accessed on 23 October 2019)];2018 Available online: https://cgspace.cgiar.org/bitstream/handle/10568/92405/livestockMasterPlan.Tanzania.pdf?sequence=1.
- 22.Wilson R.T., Swai E.S. Pig Production in Tanzania: A Critical Review. Tropicultura. 2014;32:46–53. [Google Scholar]
- 23.Rugumisa B.T., Call D.R., Mwanyika G.O., Mrutu R.I., Luanda C.M., Lyimo B.M., Subbiah M., Buza J.J. Prevalence of Antibiotic-Resistant Fecal Escherichia coli Isolates from Penned Broiler and Scavenging Local Chickens in Arusha, Tanzania. J. Food Prot. 2016;79:1424–1429. doi: 10.4315/0362-028X.JFP-15-584. [DOI] [PubMed] [Google Scholar]
- 24.Kimera Z.I., Frumence G., Mboera L.E.G., Rweyemamu M.M., Mshana S.E., Matee M.I.N. Assessment of drivers of antimicrobial use and resistance in pig and poultry farming in the Msimbazi River Basin in Tanzania. Antibiotics. 2020;9:838. doi: 10.3390/antibiotics9120838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonga H.E., Mariki M., Karimuribo E.D. Antimicrobial Usage and Residue in Morogoro. Pak. J. Nutr. 2009;8:203–207. doi: 10.3923/pjn.2009.203.207. [DOI] [Google Scholar]
- 26.Caudell M.A., Quinlan M.B., Subbiah M., Call D.R., Roulette C.J., Roulette J.W., Roth A., Matthews L., Quinlan R.J. Antimicrobial use and veterinary care among agro-pastoralists in Northern Tanzania. PLoS ONE. 2017;12:e0170328. doi: 10.1371/journal.pone.0170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afema J.A., Byarugaba D.K., Shah D.H., Atukwase E., Nambi M., Sischo W.M. Potential sources and transmission of Salmonella and antimicrobial resistance in Kampala, Uganda. PLoS ONE. 2016;11:e0152130. doi: 10.1371/journal.pone.0152130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamisi Z., Tuntufye H., Shahada F. Antimicrobial resistance phenotypes of Escherichia coli isolated from tropical free range chickens. Int. J. Sci. Res. 2014;3:34–37. [Google Scholar]
- 29.Mwambete K.D., Stephen W.S. Antimicrobial resistance profiles of bacteria isolated from chicken droppings in Dar es Salaam. Int. J. Pharm. Pharm. Sci. 2015;7:268–271. [Google Scholar]
- 30.Shah S.Q.A., Colquhoun D.J., Nikuli H.L., Sørum H. Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania. Environ. Sci. Technol. 2012;46:8672–8679. doi: 10.1021/es3018607. [DOI] [PubMed] [Google Scholar]
- 31.Katakweba A., Espinosa-gongora C. Spa typing and antimicrobial resistance of Staphylococcus aureus from healthy humans, pigs and dogs in Tanzania. J. Infect. Dev. Ctries. 2016;10:143–148. doi: 10.3855/jidc.6790. [DOI] [PubMed] [Google Scholar]
- 32.Muloi D., Ward M.J., Pedersen A.B., Fèvre E.M., Woolhouse M.E.J., Van Bunnik B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog Dis. 2018;15:467–474. doi: 10.1089/fpd.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson T.P., Bu D.P., Carrique-Mas J., Fèvre E.M., Gilbert M., Grace D., Hay S.E., Jiwakanonh J., Kakkari M., Kariuki S., et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016;110:377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seni J., Falgenhauer L., Simeo N., Mirambo M.M., Imirzalioglu C., Matee M., Rweyemamu M., Chakraborty T., Mshana S.E. Multiple ESBL-producing Escherichia coli sequence types carrying quinolone and aminoglycoside resistance genes circulating in companion and domestic farm animals in Mwanza, Tanzania, harbor commonly occurring plasmids. Front. Microbiol. 2016;7:142. doi: 10.3389/fmicb.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayombo M.C., Mayo A.W. Assessment of Microbial Quality of Vegetables Irrigated with Polluted Waters in Dar es Salaam City, Tanzania. Environ. Ecol. Res. 2018;6:229–239. doi: 10.13189/eer.2018.060403. [DOI] [Google Scholar]
- 36.Mrutu A., Nkotagu H., Luilo G. Spatial distribution of heavy metals in Msimbazi River mangrove sediments in Dar es Salaam coastal zone, Tanzania. Int. J. Environ. Sci. 2013;3:1641–1655. [Google Scholar]
- 37.Mwegoha W.J.S., Leonard L.S., Kihampa C. Heavy metal pollutions and urban agriculture in Msimbazi River valley: Health risk and public awareness. Int. J. Plants Anim. Environ. Stud. 2012;2:107–118. [Google Scholar]
- 38.Vitas A.I., Naik D., Pérez-Etayo L., González D. Increased exposure to extended-spectrum β-lactamase-producing multidrug-resistant Enterobacteriaceae through the consumption of chicken and sushi products. Int. J. Food Microbiol. 2018;269:80–86. doi: 10.1016/j.ijfoodmicro.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Sindato C., Mboera L.E.G., Katale B.Z., Frumence G., Kimera S., Clark T.G., Legido-Quigley H., Mshana S.E., Rweyemamu M., Matee M.I. Knowledge, attitudes and practices regarding antimicrobial use and resistance among communities of Ilala, Kilosa and Kibaha districts of Tanzania. Antimicrob. Resist. Infect. Control. 2020;9:1–17. doi: 10.1186/s13756-020-00862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dellgren L., Claesson C., Högdahl M., Forsberg J., Hanberger H., Nilsson L.E., Hällgren A. Phenotypic screening for quinolone resistance in Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1765–1771. doi: 10.1007/s10096-019-03608-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khumalo J., Saidi B., Mbanga J. Evolution of antimicrobial resistance of Salmonella enteritidis (1972–2005) Onderstepoort J. Vet. Res. 2014;81:6–11. doi: 10.4102/ojvr.v81i1.807. [DOI] [PubMed] [Google Scholar]
- 42.Adenipekun E.O., Jackson C.R., Oluwadun A., Iwalokun B.A., Frye J.G., Barrett J.B., Hiott L.M., Woodley T.A. Prevalence and Antimicrobial Resistance in Escherichia coli from Food Animals in Lagos, Nigeria. Microb. Drug Resist. 2015;21:358–365. doi: 10.1089/mdr.2014.0222. [DOI] [PubMed] [Google Scholar]
- 43.Donkor E.S., Newman M.J., Yeboah-Manu D. Epidemiological aspects of non-human antibiotic usage and resistance: Implications for the control of antibiotic resistance in Ghana. Trop. Med. Int. Health. 2012;17:462–468. doi: 10.1111/j.1365-3156.2012.02955.x. [DOI] [PubMed] [Google Scholar]
- 44.Yassin A.K., Gong J., Kelly P., Lu G., Guardabassi L., Wei L., Han X., Qiu H., Price S., Cheng D., et al. Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS ONE. 2017;12:e0185326. doi: 10.1371/journal.pone.0185326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro T.G., Novais Â., Peixe L., Machado E. Atypical epidemiology of CTX-M-15 among Enterobacteriaceae from a high diversity of non-clinical niches in Angola. J. Antimicrob. Chemother. 2016;71:1169–1173. doi: 10.1093/jac/dkv489. [DOI] [PubMed] [Google Scholar]
- 46.Lupindu A.M., Dalsgaard A., Msoffe P.L.M., Ngowi H.A., Mtambo M.M., Olsen J.E. Transmission of antibiotic-resistant Escherichia coli between cattle, humans and the environment in peri-urban livestock keeping communities in Morogoro, Tanzania. Prev. Vet. Med. 2015;118:477–482. doi: 10.1016/j.prevetmed.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen M.M., Opintan J.A., Frimodt-Møller N., Styrishave B. Beta-lactamase producing Escherichia coli isolates in imported and locally produced chicken meat from Ghana. PLoS ONE. 2015;10:e0139706. doi: 10.1371/journal.pone.0139706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ojo O.E., Fabusoro E., Majasan A.A., Dipeolu M.A. Antimicrobials in animal production: Usage and practices among livestock farmers in Oyo and Kaduna States of Nigeria. Trop. Anim. Health Prod. 2016;48:189–197. doi: 10.1007/s11250-015-0939-8. [DOI] [PubMed] [Google Scholar]
- 49.Chishimba K., Hang’ombe B.M., Muzandu K., Mshana S.E., Matee M.I., Nakajima C., Suzuki Y. Detection of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Market-Ready Chickens in Zambia. Int. J. Microbiol. 2016;2016:5275724. doi: 10.1155/2016/5275724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso C.A., Zarazaga M., Sallem R.B., Jouini A., Slama K.B., Torres C. Antibiotic resistance in Escherichia coli in husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017;64:318–334. doi: 10.1111/lam.12724. [DOI] [PubMed] [Google Scholar]
- 51.Rasool F.N., Saavedra M.A., Pamba S., Perold V., Mmochi A.J., Maalim M., Simonsen L., Buur L., Pedersen R.H., Syberg K., et al. Isolation and characterization of human pathogenic multidrug resistant bacteria associated with plastic litter collected in Zanzibar. J. Hazard. Mater. 2021;405:124591. doi: 10.1016/j.jhazmat.2020.124591. [DOI] [PubMed] [Google Scholar]
- 52.Katakweba A.A., Muhairwa A.P., Lupindu A.M., Damborg P., Rosenkrantz J.T., Minga U.M., Mtambo M.M., Olsen J.E. First Report on a Randomized Investigation of Antimicrobial Resistance in Fecal Indicator Bacteria from Livestock, Poultry, and Humans in Tanzania. Microb. Drug Resist. 2018;24:260–268. doi: 10.1089/mdr.2016.0297. [DOI] [PubMed] [Google Scholar]
- 53.Bernadether T.R., Douglas R.C., Gaspary O.M., Murugan S., Joram B. Comparison of the prevalence of antibiotic-resistant Escherichia coli isolates from commercial-layer and free-range chickens in Arusha district, Tanzania. Afr. J. Microbiol. Res. 2016;10:1422–1429. doi: 10.5897/AJMR2016.8251. [DOI] [Google Scholar]
- 54.Hlashwayo D.F., Sigaúque B., Bila C.G. Epidemiology and antimicrobial resistance of Campylobacter spp. in animals in Sub-Saharan Africa: A systematic review. Heliyon. 2020;6:e03537. doi: 10.1016/j.heliyon.2020.e03537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boamah V.E., Agyare C., Odoi H., Dalsgaard A. Antibiotic Practices and Factors Influencing the Use of Antibiotics in Selected Poultry Farms in Ghana. J. Antimicrob. Agents. 2016;2:2. [Google Scholar]
- 56.Kamini M.G., Keutchatang F.T., Mafo H.Y., Kansci G., Nama G.M. Antimicrobial usage in the chicken farming in yaoundé, Cameroon: A cross-sectional study. Int. J. Food Contam. 2016;3:1. [Google Scholar]
- 57.Eltayb A., Barakat S., Marrone G., Shaddad S., Sta C. Antibiotic Use and Resistance in Animal Farming: A Quantitative and Qualitative Study on Knowledge and Practices among Farmers in Khartoum, Sudan. Zoonoses Public Health. 2012;59:330–338. doi: 10.1111/j.1863-2378.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 58.Mainda G., Bessell P.B., Muma J.B., McAteer S.P., Chase-Topping M.E., Gibbons J., Stevens M.P., Gally D.L., Bronsvoort B.M.C. Prevalence and patterns of antimicrobial resistance among Escherichia coli isolated from Zambian dairy cattle across different production systems. Sci. Rep. 2015;5:12439. doi: 10.1038/srep12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van T.T.H., Yidana Z., Smooker P.M., Coloe P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020;20:170–177. doi: 10.1016/j.jgar.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Manyi-Loh C., Mamphweli S., Meyer E., Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules. 2018;23:795. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahoo K.C., Tamhankar A.J., Sahoo S., Sahu P.S., Klintz S.R., Lundborg C.S. Geographical variation in antibiotic-resistant Escherichia coli isolates from stool, cow-dung and drinking water. Int. J. Environ. Res. Public Health. 2012;9:746–759. doi: 10.3390/ijerph9030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6:1–8. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tufa T.B., Gurmu F., Beyi A.F., Hogeveen H., Beyene T.J., Ayana D., Woldemariyam F.T., Hailemariam E., Gutema F.D., Stegeman J.A. Veterinary medicinal product usage among food animal producers and its health implications in Central Ethiopia. BMC Vet. Res. 2018;14:1–7. doi: 10.1186/s12917-018-1737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montso K.P., Dlamini S.B., Kumar A., Ateba C.N. Antimicrobial Resistance Factors of Extended-Spectrum Beta-Lactamases Producing Escherichia coli and Klebsiella pneumoniae Isolated from Cattle Farms and Raw Beef in North-West Province, South Africa. Biomed. Res. Int. 2019;2019:1–13. doi: 10.1155/2019/4318306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Founou L.L., Founou R.C., Ntshobeni N., Govinden U., Bester L.A., Chenia H.Y., Djoko C.F., Essack S.Y. Emergence and spread of extended spectrum Beta-lactamase producing enterobacteriaceae (ESBL-PE) in pigs and exposed workers: A multicentre comparative study between Cameroon and South Africa. Pathogens. 2019;8:10. doi: 10.3390/pathogens8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Badri A.M., Ibrahim I.T., Mohamed S.G., Garbi M.I., Kabbashi A.S., Arbab M.H. Prevalence of Extended Spectrum Beta Lactamase (ESBL) Producing Escherichia coli and Klebsiella pneumoniae Isolated from Raw Milk Samples in Al Jazirah State, Sudan. Mol. Biol. 2017;7:1–4. doi: 10.4172/2168-9547.1000201. [DOI] [Google Scholar]
- 67.Aworh M.K., Kwaga J., Okolocha E., Mba N., Thakur S. Prevalence and risk factors for multi-drug resistant Escherichia coli among poultry workers in the Federal Capital Territory, Abuja, Nigeria. PLoS ONE. 2019;14:e0225379. doi: 10.1371/journal.pone.0225379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falgenhauer L., Imirzalioglu C., Oppong K., Akenten C.W., Hogan B., Krumkamp R., Poppert S., Levermann V., Schwengers O., Sarpong N., et al. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front. Microbiol. 2019;9:3358. doi: 10.3389/fmicb.2018.03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fortini D., Fashae K., Garcia-Fernandez A., Villa L., Carattoli A. Plasmid-mediated quinolone resistance and Beta-lactamases in Escherichia coli from healthy animals from Nigeria. J. Antimicrob. Chemother. 2011;66:1269–1272. doi: 10.1093/jac/dkr085. [DOI] [PubMed] [Google Scholar]
- 70.Teklu D.S., Negeri A.A., Legese M.H., Bedada T.L., Woldemariam H.K., Tullu K.D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control. 2019;8:1–12. doi: 10.1186/s13756-019-0488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadiq M., Syed-Hussain S., Ramanoon S., Saharee A., Ahmad N., Zin N.M., Khalid S., Naseeha D., Syahirah A., Mansor R. Knowledge, attitude and perception regarding antimicrobial resistance and usage among ruminant farmers in Selangor, Malaysia. Prev. Vet. Med. 2018;156:76–83. doi: 10.1016/j.prevetmed.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Bergšpica I., Kaprou G., Alexa E.A., Prieto M., Alvarez-Ordóñez A. Extended spectrum β-lactamase (ESBL) producing Escherichia coli in pigs and pork meat in the European Union. Antibiotics. 2020;9:678. doi: 10.3390/antibiotics9100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Founou L.L., Founou R.C., Essack S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016;7:1881. doi: 10.3389/fmicb.2016.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X., Liu H., Wang L., Peng Q., Li Y., Zhou H., Li Q. Molecular characterization of extended-spectrum Beta-lactamase-producing multidrug resistant Escherichia coli from swine in Northwest China. Front. Microbiol. 2018;9:1756. doi: 10.3389/fmicb.2018.01756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zurfluh K., Abgottspon H., Hächler H., Nuësch-Inderbinen M., Stephan R. Quinolone resistance mechanisms among Extended- Spectrum Beta-Lactamase (ESBL) producing Escherichia coli isolated from rivers and lakes in Switzerland. PLoS ONE. 2014;9:e95864. doi: 10.1371/journal.pone.0095864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alouache S., Estepa V., Messai Y., Ruiz E., Torres C., Bakour R. Characterization of ESBLs and Associated Quinolone Resistance in Escherichia coli and Klebsiella pneumoniae Isolates from an Urban Wastewater Treatment Plant in Algeria. Microb. Drug Resist. 2013;20:30–38. doi: 10.1089/mdr.2012.0264. [DOI] [PubMed] [Google Scholar]
- 77.Salah F.D., Soubeiga S.T., Ouattara A.K., Sadji A.Y., Metuor-Dabire A., Obiri-Yeboah D., Banla-Kere A., Karou S., Simpore J. Distribution of quinolone resistance gene (qnr) in ESBL-producing Escherichia coli and Klebsiella spp. in Lomé, Togo. Antimicrob. Resist. Infect. Control. 2019;8:1–8. doi: 10.1186/s13756-019-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song H.J., Moon D.C., Mechesso A.F., Kang H.Y., Kim M.H., Choi J.H., Kim S., Yoon S., Lim S. Resistance profiling and molecular characterization of extended-spectrum/plasmid-mediated Ampc β-lactamase-producing Escherichia coli isolated from healthy broiler chickens in South Korea. Microorganisms. 2020;8:1434. doi: 10.3390/microorganisms8091434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Livermore D.M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur. J. Clin. Microbiol. 1987;6:439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- 80.Liu P.Y.F., Gur D., Hall L.M.C., Livermore D.M. Survey of the prevalence of β-lactamases amongst 1000 gram-negative bacilli isolated consecutively at the royal london hospital. J. Antimicrob. Chemother. 1992;30:429–447. doi: 10.1093/jac/30.4.429. [DOI] [PubMed] [Google Scholar]
- 81.Gregova G., Kmet V. Antibiotic resistance and virulence of Escherichia coli strains isolated from animal rendering plant. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-72851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cox J.A., Vlieghe E., Mendelson M., Wertheim H., Ndegwa L., Villegas M.V., Gould I., Hara G.L. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Olowe O.A., Adewumi O., Odewale G., Ojurongbe O., Adefioye O.J. Phenotypic and Molecular Characterisation of Extended-Spectrum Beta-Lactamase Producing Escherichia coli Obtained from Animal Fecal Samples in Ado Ekiti, Nigeria. J. Envrion. Public Health. 2015;2015:497980. doi: 10.1155/2015/497980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clinical and Laboratory Standards Institute—CLSI . Performance Standards for Antimicrobial Susceptibility Testing. CLSI; Wayne, PA, USA: 2019. [(accessed on 18 May 2020)]. pp. 1–320. Available online: http://www.emeraldinsight.com/doi/10.1108/08876049410065598. [Google Scholar]
- 85.Moffat J., Chalmers G., Reid-Smith R., Mulvey M.R., Agunos A., Calvert J., Cormier A., Ricker N., Weese J.S., Boerlin P. Resistance to extended-spectrum cephalosporins in Escherichia coli and other Enterobacterales from Canadian turkeys. PLoS ONE. 2020;15:e0236442. doi: 10.1371/journal.pone.0236442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamza E., Dorgham S.M., Hamza D.A. Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. Integr. Med. Res. 2016;7:8–10. doi: 10.1016/j.jgar.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 87.WHO . Integrated Surveilance of Antimicrobial Resistance. WHO; Geneva, Switzerland: 2013. [(accessed on 3 September 2018)]. pp. 1–100. Guidance from WHO Advisory Group. Available online: http://apps.who.int/iris/bitstream/10665/91778/1/9789241506311_eng.pdf?ua=1. [Google Scholar]
- 88.Magiorakos A., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An internatiojnal expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 89.Moremi N., Manda E.V., Falgenhauer L., Ghosh H., Imirzalioglu C., Matee M., Chakraborty T., Mshana S.E. Predominance of CTX-M-15 among ESBL producers from environment and fish gut from the shores of Lake Victoria in Mwanza, Tanzania. Front. Microbiol. 2016;7:1862. doi: 10.3389/fmicb.2016.01862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valat C., Forest K., Billet M., Polizzi C., Saras E., Madec J.Y., Haenni M. Absence of co-localization between pathovar-associated virulence factors and extended-spectrum β-lactamase (blaCTX-M) genes on a single plasmid. Vet. Microbiol. 2016;192:163–166. doi: 10.1016/j.vetmic.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Reich F., Atanassova V. Extended-Spectrum Enterobacteria in Healthy Broiler. Emerg Infect. Dis. 2013;19:1253–1259. doi: 10.3201/eid1908.120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moawad A.A., Hotzel H., Awad O., Tomaso H., Neubauer H., Hafez H.M., El-Adawy H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017;9:1–13. doi: 10.1186/s13099-017-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park C.H., Robicsek A., Jacoby G.A., Sahm D., Hooper D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006;50:3953–3955. doi: 10.1128/AAC.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thong K.L., Lim K.T., Yasin R., Yeo C.C., Puthucheary S. Characterization of multidrug resistant ESBL-Producing Escherichia coli isolates from hospitals in Malaysia. J. Biomed. Biotechnol. 2009;2009:1–10. doi: 10.1155/2009/165637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated is contained within the article.