Abstract
Resistance is threatening the effectiveness of insecticide-based interventions in use for malaria control. Pinpointing genes associated with resistance is crucial for evidence-based resistance management targeting the major malaria vectors. Here, a combination of RNA-seq based genome-wide transcriptional analysis and RNA-silencing in vivo functional validation were used to identify key insecticide resistance genes associated with DDT and DDT/permethrin cross-resistance across Africa. A cluster of glutathione-S-transferase from epsilon group were found to be overexpressed in resistant populations of Anopheles funestus across Africa including GSTe1 [Cameroon (fold change, FC: 2.54), Ghana (4.20), Malawi (2.51)], GSTe2 [Cameroon (4.47), Ghana (7.52), Malawi (2.13)], GSTe3 [Cameroon (2.49), Uganda (2.60)], GSTe4 in Ghana (3.47), GSTe5 [Ghana (2.94), Malawi (2.26)], GSTe6 [Cameroun (3.0), Ghana (3.11), Malawi (3.07), Uganda (3.78)] and GSTe7 (2.39) in Ghana. Validation of GSTe genes expression profiles by qPCR confirmed that the genes are differentially expressed across Africa with a greater overexpression in DDT-resistant mosquitoes. RNAi-based knock-down analyses supported that five GSTe genes are playing a major role in resistance to pyrethroids (permethrin and deltamethrin) and DDT in An. funestus, with a significant recovery of susceptibility observed when GSTe2, 3, 4, 5 and GSTe6 were silenced. These findings established that GSTe3, 4, 5 and 6 contribute to DDT resistance and should be further characterized to identify their specific genetic variants, to help design DNA-based diagnostic assays, as previously done for the 119F-GSTe2 mutation. This study highlights the role of GSTes in the development of resistance to insecticides in malaria vectors and calls for actions to mitigate this resistance.
Keywords: malaria, Anopheles funestus, metabolic resistance, glutathioneS-transferase, RNA interference
1. Introduction
Malaria is the deadliest vector-borne disease, killing more than 400,000 people every year [1]. Vector control interventions through the use of long-lasting insecticide nets and the implementation of indoor residual spray have led to a significant reduction in malaria incidence, between 2000 and 2015 [2]. This gain is under threat, as the most recent WHO World Malaria Report revealed there has been increase in annual case numbers since 2016. This malaria rebound is partly due to escalation of insecticide resistance in major malaria vectors such as An. gambiae and An. funestus. This was recently shown for a population of An. funestus for which high resistance level to pyrethroids was associated with a significant loss of efficacy of insecticide-treated nets including PBO-based nets [3]. The main mechanisms of resistance are target-site and metabolic resistance. The molecular basis of metabolic resistance is more complex and involves among others an overexpression and/or over-activity of major detoxification genes, such as cytochrome P450s (CYP450s), glutathione-S-transferases (GSTs) and the carboxylesterases [4,5,6], in addition to the recently described sensory appendages proteins [7]. Several studies, including genome-wide transcriptional analyses using microarray/qPCR and functional validation have linked GST with resistance in the major malaria vectors [8,9,10]. However, most of these studies have concentrated on GSTe2, neglecting the other GST epsilon genes, even though they have been shown to also consistently be overexpressed [11,12,13,14,15]. Indeed, among the 8 potential members of the GST epsilon class, several genes have previously been shown to be overexpressed in malaria vectors including An. gambiae [10] and An. funestus [14,15,16]. However, apart from few validations such as for GSTe4 in An. gambiae and An. arabiensis [17] little is known on the role of these genes in insecticide resistance. In the case of An. funestus, progress made has focused on the GSTe2 gene detecting a key resistance marker (L119F-GSTe2) now commonly used for resistance monitoring in An. funestus [16]. In addition to conferring pyrethroid/DDT resistance, it was also shown that the L119F-GSTe2-mediated metabolic resistance to pyrethroids/DDT is associated with negative effects on some life-history traits of field populations of An. funestus, supporting that insecticide resistance is associated with a fitness cost [18]. An experimental hut study, using the same marker in An. funestus population from Mibellon (Cameroon) had confirmed that presence of the L119F-GSTe2 was associated with resistance to DDT and pyrethroids [19] and was reducing the efficacy of bed nets [20].
RNA interference (RNAi) is one of the main approaches commonly used for in vivo validation of the role of detoxification enzymes in conferring resistance to insecticides in mosquitoes. This is done by injecting adult mosquitoes with double-stranded RNA (dsRNA) corresponding to the gene of interest. In turn, this induces mRNA degradation via the RNAi pathway and suppresses expression of the protein [21,22]. RNAi has been used to link overexpression of cytochrome P450s and GSTs to insecticide resistance in An. gambiae and Aedes mosquitoes [23,24,25]. Recently, RNAi has been used to establish the role of sensory appendage proteins in the leg of An. gambiae in conferring pyrethroid resistance [7] confirming that this method provides a robust approach to validate the contribution of specific genes such as those of the GST epsilon, to a specific phenotype.
In this study, using a genome-wide RNA-seq-based transcriptomic analysis, we detected candidate genes associated with DDT-resistance and DDT/permethrin cross-resistance across different regions of Africa revealing the pre-eminence of GST epsilon genes. The role of epsilon class of GSTs in DDT/pyrethroid resistance was investigated in An. funestus population from Mibellon (Cameroon) using RNAi-mediated gene silencing. The results suggest that several members of this class in addition to GSTe2 contribute to the overall resistance observed in the field.
2. Materials and Methods
2.1. Mosquito Collection and Rearing
Indoor-resting female An. funestus were collected early in the morning (6:00 a.m.–8:00 a.m.), using battery-powered aspirators (John. W. Hock, Gainesville, FL, USA) in Mibellon (6°46′ N, 11°70′ E, Cameroon; 2015 and 2019), Obuasi (5°56′ N, 1°37′ W, Ghana; 2014), Kpome (6°55′ N, 2°19′ E, Benin, 2014), Chikwawa (16°1′ S, 34°47′ E, Malawi; 2014), and Tororo (0°45′ N, 34°5′ E, Uganda; 2014). The collection was done from randomly selected houses, following a verbal consent from the chief of the district and the household owners. Mosquitoes collected were kept in paper cups, being transported to the insectary in the Centre for Research in Infectious Diseases (CRID), Yaoundé, Cameroon. The F1 generation was generated in the insectary from field blood-fed female mosquitoes using forced-egg laying method [26]. Briefly, F0 gravid females were transferred into 1.5 mL Eppendorf tubes containing a wet filter paper, to enable them to lay eggs. After oviposition the parents that laid eggs were removed and used for species identification. Molecular identification to species level was carried out according to the protocol described priviously [27] using genomic DNA (gDNA) extracted from F0 females identified morphologically as An. funestus. DNA extraction was done using the Livak protocol [28]. The eggs were pooled into bowls and supplemented with TetraMin™ baby fish food. The emerged F1 female progenies were mixed in cages and 2 to 5-day old females used for insecticide bioassays and double-stranded RNA (dsRNA) injection.
2.2. Transcriptomic Profiling of DDT Resistance across Africa Using RNA-Seq
Transcriptional profiling of An. funestus populations was carried out to detect key candidate genes associated with DDT resistance across Africa. This was done using mosquitoes from four African regions: Central (Mibellon/Cameroon), West (Obuasi/Ghana), Southern (Chikwawa/Malawi), and East (Tororo/Uganda). Total RNA was extracted from pools of 10 female DDT-resistant mosquitoes (alive after 24 h exposure to DDT), unexposed mosquitoes (control) and lab susceptible colony (FANG) using the Arcturus PicoPure RNA Isolation Kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The FANG is a fully insecticide susceptible laboratory colony [29]. In addition, RNA was extracted from 10 permethrin-resistant mosquitoes (alive after 24 h exposure to permethrin) to study the gene differentially expressed across Africa when comparing DDT vs. permethrin-resistant mosquitoes.
RNA libraries were pooled in equimolar amounts using the Qubit and Bioanalyzer data. The quantity and quality of each pool were assessed by Bioanalyzer and subsequently by qPCR using the Kapa Illumina library quantification kit (Kapa Biosystems, Wilmington, MA, USA), on a Light Cycler LC480II (Roche, Basel, Switzerland), according to manufacturers’ instructions. The pool of libraries was sequenced on one lane of the HiSeq 2500 (Illumina, San Diego, CA, USA) at 2 × 125 bp paired-end sequencing with v4 chemistry. Sequence library preparation, sequencing, initial processing and quality control were done by the Centre for Genomic Research, University of Liverpool, UK. Alignment to the reference sequence using the AfunF3.1 annotation. (https://vectorbase.org/vectorbase/app/record/dataset/DS_1a787d4361#pmids, accessed on 7 April 2020). Data were analysed as described previously [30,31,32]. Differential gene expression analysis was performed using edgeR and the Strand NGS program (Strand Life Sciences, version 3.0, Hebbal, Bangalore, India).
2.3. Investigation of Expression Profile of GSTe Genes in An. funestus across Africa
The expression profiles of GSTe1, GSTe2, GSTe3, GSTe4, GSTe5, GSTe6, GSTe7 and GSTe8 in DDT and permethrin-resistant mosquitoes was assessed across Africa (Cameroon, Benin, Malawi, and Uganda), using qRT-PCR. RNA was extracted from three biological replicates from each population of 10 each of DDT-resistant females (alive 24 h after exposure to DDT), permethrin-resistant (alive 24 h after exposure to permethrin), control (An. funestus mosquitoes not exposed to any insecticide), as well as susceptible laboratory colony (FANG). Briefly, 1 μg of the total RNA from each of the three biological replicates was used as the template for cDNA synthesis using Superscript III (Invitrogen, Carlsbad, CA, USA) with oligo-dT20 and RNase H, according to the manufacturer’s instructions. The qRT-PCR amplification was performed as described [15] using the primers provided in Supplementary Table S2.1. The relative expression and fold change of each GSTe gene was calculated as previously described [33] by comparing expression in resistant, susceptible and control samples. The normalization was done with the ribosomal protein S7, RPS7 (AFUN007153) and actin5C (AFUN006819) housekeeping genes.
2.4. Functional Validation of Role of GSTe Genes in Resistance Using RNA Interference
2.4.1. Double Strand RNA Synthesis
Double-stranded RNAs specific to GSTe genes of interest were synthesized for use in RNAi gene-silencing experiments. Each GSTe oligonucleotide primer was designed using specific cDNA of the corresponding genes downloaded from the Vector Base (https://vectorbase.org/vectorbase/app, accessed on 8 December 2016). The T7 RNA polymerase promoter sequence, TAATACGACTCACTATAGGGAGA, was added to the 5′ end of each primer (Supplementary Table S2.2). Specific GST2, 3, 4, 5, 6, 7 and GSTe8 fragments were amplified by PCR from plasmid clones using KAPA Taq Kit (Kapa Biosystems, Wilmington, MA USA). Double-stranded RNA (dsRNA) was synthesized using in vitro transcription MEGAscript®® T7 Kit (Ambion Inc., Austin, TX, USA) and purified using MEGAclear columns (Ambion). The purified products were concentrated by ethanol precipitation and the dsRNA was resuspended in nuclease-free water and stored at −20 °C. The successful construction of dsRNA was confirmed by running 3 μL of dsRNA-diluted products in 1.5% agarose gel in a Tris-acetate-EDTA (TAE) buffer.
2.4.2. Mosquitoes Injection and Susceptibility Bioassays
To explore the role of GSTe genes in conferring insecticide resistance, RNAi was performed on Mibellon An. funestus population, by injecting sequence-specific dsRNA to 2–3 days old F1 female mosquitoes, followed by insecticide bioassay. A Nano injector (Nanoinject; Drummond, Burton, OH, USA) was used to inject dsGSTe2, 3, 4, 5, 6, 7 and dsGSTe8 into the thorax of 2 to 3 days old female An. funestus mosquitoes as described [34]. Briefly, mosquitoes, induced to sleep with CO2, were injected with 69 nL of either aliquot of above dsGSTes or dsGFP (control). Four days after injection, four replicates of 20 mosquitoes for each dsRNA were exposed to permethrin (0.75%), deltamethrin (0.05%) and DDT (4%) for 1 h following the WHO testing protocol [35]. Mosquitoes were transferred to holding tubes after exposure, supplemented with sugar and mortalities counted 24 h after the exposure. The susceptibility test was performed in triplicate with experimental mosquitoes comprising the mosquitoes injected with dsGSTes above, whereas mosquitoes injected with dsGFP and those not injected were used as controls.
2.4.3. Quantitative RT-PCR to Confirm the Knockdown Effect
For dsGSTe-injected and non-injected mosquitoes, RNA was extracted from 3 pools of 5 mosquitoes using TRIzol reagent (Gibco BRL, Gaithersburg, MD, USA). cDNA from each of the three biological replicates was synthesized using the Super-Script III (Invitrogen, Carlsbad, CA, USA) with oligo-dT20 and RNase H, according to the manufacturer’s instructions. The cDNA from each replicate treatment was then used to assess the extent of RNAi by measuring levels of gene expression after injection by qRT-PCR. To assess the knockdown efficiency after injection and quantitative difference in the level of GSTes expression between injected and non-injected mosquitoes, a standard curve of each gene was established using a serial dilution of cDNA. The qPCR amplification was carried out in a MX3005 real-time PCR system using Brilliant III Ultra-Fast SYBR Green qPCR Master Mix (Agilent, Santa Clara, CA, USA). A total of 10 ng of cDNA from each sample was used as a template in a three-step program involving a denaturation at 95 °C for 3 min followed by 40 cycles of 10 s at 95 °C and 10 s at 60 °C and a last step of 1 min at 95 °C, 30 s at 55 °C and 30 s at 95 °C. The relative expression and fold-change of each target gene were calculated according to the 2−ΔΔCT Livak method [33], comparing expression in specific dsGSTe-injected samples to non-injected ones, after normalization with the housekeeping genes, RPS7 (AFUN007153) and actin5C (AFUN006819), as described above.
2.5. Data Analysis
All analyses were conducted using GraphPad Prism version 7.00, R 3.3.2. for Windows and Strand NGS program (Strand Life Sciences, version 3.0, Hebbal, Bangalore, India). Students’ t-test was used to assess statistical differences between experimental and control groups.
3. Results
3.1. RNAseq-Based Comparative Transcriptomic Profiling of DDT Resistance across Africa
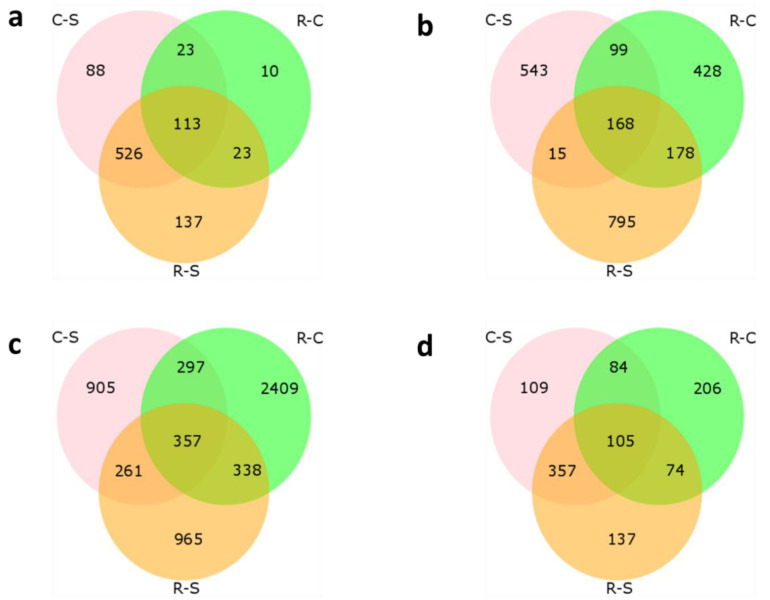
To detect genes associated with DDT resistance in An. funestus mosquitoes Africa-wide, transcriptional profiling of mosquitoes from different regions of Africa was performed. This comprised populations from southern (Malawi), East (Uganda), West (Ghana) and Central (Cameroon) Africa. Priority was given to the comparison between genes upregulated in DDT-resistant mosquitoes (R) and the control (C, unexposed mosquitoes) because this comparison directly focuses on the difference between mosquitoes having the same genetic background (accounting for potential induction of expression), but differing in treatment received. Attention was also given to genes that were commonly upregulated in R vs Susceptible FANG colony mosquitoes (S) and C vs S as these genes are the ones expressed constitutively in natural mosquitoes’ populations. The number of differentially expressed genes in each of the four populations and the FANG susceptible strain is shown in Venn diagrams (Figure 1). Raw data from RNA-seq is deposited on sequence archive, with the following link: https://www.ebi.ac.uk/ena/browser/view/PRJEB24351, accessed on 10 January 2018.
Figure 1.
Venn diagrams showing the number of differentially expressed genes in each population compared to the FANG susceptible colony: (a) Cameroon population, (b) Ghana population, (c) Uganda population and (d) Malawi population. R-C: genes induced upon exposure and constitutive expression (resistant vs control unexposed), C-S: constitutive differential expression (control vs susceptible); R-S: genes induced upon exposure and constitutive expression (resistant vs susceptible).
3.1.1. The Central Africa Population of Cameroon
The major detoxification gene families found to be overexpressed in Cameroon population are cytochrome P450s, glutathione S-transferases and carboxylesterases. When comparing resistant, susceptible and control mosquito cytochrome P450 CYP325A is over-expressed, followed by CYP6P9b, CYP6P5, CYP315A1. For GSTs, the epsilon and delta family are upregulated when comparing R-S vs. C-S expression profile. The most overexpressed GSTs are GSTe2, GSTe1, GSTe3, GSTe6, GSTd3 and GSTt2. Several genes from carboxylesterase classes, e.g., an unknown COE (AFUN002514), and COEBE3C (AFUN016311, a glutathione peroxidase (AFUN022201), an ATP-binding cassette transporter (AFUN019220), a UDP-glucuronosyltransferase (AFUN011266), and sulfotransferase family (AFUN016207) (SULT1B), were also found to be overexpressed (Table 1).
Table 1.
Detoxification genes differentially expressed in Cameroon An. funestus between different comparisons at false discovery rate (FDR) < 0.05 and fold change (FC) > 1.5 for genes induced upon exposure and constitutive expression (R-C) or FC > 2 for constitutive differential expression (C-S) and genes induced upon exposure and constitutive expression (R-S).
| Gene ID | R-C | C-S | R-S | Description |
|---|---|---|---|---|
| AFUN002514 | 2.8934693 | 3.4858232 | Carboxylesterase, COEunkn | |
| AFUN019220 | 3.755253 | 4.1573305 | ABC transporter family A | |
| AFUN015966 | 15.648611 | 19.80706 | Cytochrome P450, CYP325A | |
| AFUN015889 | 2.4889274 | 3.1110048 | Cytochrome P450, CYP6P9b | |
| AFUN015888 | 5.4777937 | 8.212809 | Cytochrome P450, CYP6P5 | |
| AFUN005715 | 2.3714201 | 2.252707 | Cytochrome P450, CYP315A1 | |
| AFUN011266 | 2.67154 | 2.5182736 | UDP-glucuronosyltransferase 3A1 | |
| AFUN022201 | 10.439024 | 9.539281 | Glutathione peroxidase, Short | |
| AFUN015807 | 2.3210542 | 2.5459306 | Glutathione S-transferase, GSTE1 |
|
| AFUN015808 | 2.4095497 | 2.4903498 | Glutathione S-transferase, GSTE3 | |
| AFUN015839 | 2.004527 | 2.098784 | Glutathione S-transferase, GSTD3 |
|
| AFUN016008 | 3.0603988 | 3.182327 | Glutathione S-transferase, GSTE6 | |
| AFUN007291 | 2.4175029 | 2.2559748 | Glutathione S-transferase, GSTT2 | |
| AFUN015809 | 3.4979115 | 4.4708834 | Glutathione S-transferase epsilon 2, GSTE2 |
|
| AFUN016207 | 2.297438 | 2.2629843 | Sulfotransferase family cytosolic 1B member 1; Short |
|
| AFUN008941 | 2.1940587 | ABC transporter family C | ||
| AFUN016311 | 2.155015 | Carboxylesterase, COEBE3C | ||
| AFUN002978 | 2.058842 | Cytochrome P450, CYP314A1 | ||
| AFUN001383 | 1.6504059 | Cytochrome P450, CYP9J5 | ||
| AFUN002602 | 2.0370903 | Cytochrome b-561 domain containing protein |
||
| AFUN006858 | 2.3245227 | Cytochrome P450, CYP306A1 |
3.1.2. The West African Population of Ghana
Many cytochrome P450s were found to be overexpressed when comparing expression profiles of R, C and S in mosquitoes from Ghana. These include CYP6P4a (AFUN020895), CYP325B/C, CYP4H26, CYP6M4, CYP4C36, CYP9K1, CYP9J5, CYP6P9b, CYP6P9a, CYP6P5 and CYP4H17 (Table 2). In Ghana, almost all the GST epsilon clusters, GSTe1, GSTe2, GSTe3, GSTe4, GSTe5, GSTE6 and GSTe7, are upregulated in C and R samples. We also note the overexpression of a sulfotransferase SULT1B, D7 short form salivary protein (AFUN016458), UDP-glucuronosyltransferase (AFUN011266), carboxylesterase (AFUN016367) and ATP-binding cassette transporter (AFUN019220).
Table 2.
Detoxification genes differentially expressed in Ghana between different comparisons at FDR < 0.05 and FC > 1.5 for R-C or FC > 2 for C-S and R-S.
| Genes ID | R-C | C-S | R-S | Description |
|---|---|---|---|---|
| AFUN015830 | 1.5176085 | 2.6203227 | 3.976624 | Cytochrome P450, CYP325C |
| AFUN015894 | 1.7204243 | 4.830863 | 8.311134 | Cytochrome P450, CYP4H26 |
| AFUN019348 | 2.2147367 | 5.578066 | 12.353948 | Cytochrome P450, CYP325B |
| AFUN015767 | 1.5001847 | 2.0304153 | 3.0459979 | Glutathione S-transferase, GSTD11 |
| AFUN019220 | 3.5253153 | 3.8225787 | ABC transporter family A | |
| AFUN002796 | 2.9251132 | 2.0283942 | Sub-family G member 1 | |
| AFUN016367 | 2.291519 | 2.4250317 | Carboxylesterase, COEJHE4E | |
| AFUN019401 | 2.4856389 | 2.5770857 | Cytochrome P450, CYP6M4 | |
| AFUN004316 | 4.7323303 | 7.0621023 | Cytochrome P450, CYP4H17 | |
| AFUN006135 | 2.4041867 | 2.1305745 | Cytochrome P450, CYP4C36 | |
| AFUN007549 | 2.8564737 | 2.3530338 | Cytochrome P450, CYP9K1 | |
| AFUN020895 | 41.131523 | 30.434338 | Cytochrome P450, CYP6P4 | |
| AFUN019365 | 17.181461 | 13.95981 | Cytochrome P450, CYP6P4 | |
| AFUN001383 | 3.2154377 | 3.093064 | Cytochrome P450, CYP9J5 | |
| AFUN015792 | 3.5820096 | 5.169663 | Cytochrome P450, CYP6P9A | |
| AFUN015889 | 6.209893 | 6.1071906 | Cytochrome P450, CYP6P9b | |
| AFUN015888 | 7.485999 | 6.140407 | Cytochrome P450, CYP6P5 | |
| AFUN006858 | 2.186648 | 2.5465918 | Cytochrome P450, CYP306A1 | |
| AFUN015795 | 2.3312643 | 2.0709553 | Cytochrome P450, CYP6M3 | |
| AFUN005715 | 2.0942264 | 2.0190682 | Cytochrome P450, CYP315A1 | |
| AFUN019567 | 6.2830515 | 6.3453665 | Cytochrome P450, CYP4H18 | |
| AFUN016456 | 2.2823555 | 2.137907 | D7 short form salivary protein | |
| AFUN011266 | 2.815869 | 3.8286955 | UDP-glucuronosyltransferase 3A1 | |
| AFUN015807 | 3.92929 | 4.207333 | Glutathione S-transferase, GSTE1 | |
| AFUN015808 | 2.9914625 | 2.6008918 | Glutathione S-transferase, GSTE3 | |
| AFUN015810 | 3.4769716 | 2.789998 | Glutathione S-transferase, GSTE4 | |
| AFUN015811 | 2.9477298 | 2.0025258 | Glutathione S-transferase, GSTE5 | |
| AFUN015839 | 4.0754375 | 3.4843476 | Glutathione S-transferase, GSTD3 | |
| AFUN015840 | 3.6762526 | 4.7960463 | Glutathione S-transferase, GSTD10 | |
| AFUN016008 | 3.3691344 | 3.113351 | Glutathione S-transferase, GSTE6 | |
| AFUN001774 | 2.3985367 | 2.0373375 | Glutathione s-transferase, GSTE7 | |
| AFUN015809 | 9.269093 | 7.5253835 | Glutathione S-transferase, GSTE2 | |
| AFUN008239 | 2.6680195 | 2.1065671 | Sulfotransferase | |
| AFUN016207 | 2.6827705 | 2.3955698 | Sulfotransferase | |
| AFUN010696 | 2.5507598 | 3.926304 | Cytochrome b5 domain-containing protein 1 |
|
| AFUN015963 | 1.5521032 | 2.153224 | Cytochrome P450, CYP6R1 | |
| AFUN016458 | 2.4133177 | 3.0593035 | D7 short form salivary protein | |
| AFUN008338 | 2.4747007 | 3.1143987 | Sulfotransferase 1C4 | |
| AFUN008852 | 2.1302986 | Glycosyltransferase | ||
| AFUN008941 | 2.0767095 | ABC transporter family C | ||
| AFUN021098 | 2.351223 | Cytochrome P450, CYP4H19 | ||
| AFUN015909 | 2.0092266 | Cytochrome P450, CYP305A3 | ||
| AFUN015776 | 2.0201716 | Cytochrome P450, CYP12F1 | ||
| AFUN001382 | 2.1402965 | Cytochrome P450, CYP9J5 | ||
| AFUN019845 | 2.144599 | Glucosyl/glucuronosyl transferases |
||
| AFUN016010 | 2.0161536 | Glutathione S-transferase, GSTD1 | ||
| AFUN008819 | 2.0289357 | Glutathione transferase microsomal, GSTMS3 |
||
| AFUN008941 | 2.0767095 | ABC transporter family C | ||
| AFUN021098 | 2.351223 | Cytochrome P450, CYP4H19 | ||
| AFUN015909 | 2.0092266 | Cytochrome P450, CYP305A3 | ||
| AFUN015776 | 2.0201716 | Cytochrome P450, CYP12F1 | ||
| AFUN001382 | 2.1402965 | Cytochrome P450, CYP9J5 | ||
| AFUN019845 | 2.144599 | UDP-glucuronosyltransferase 2C1 | ||
| AFUN016010 | 2.0161536 | Glutathione-S-transferase, GSTD1 | ||
| AFUN008819 | 2.0289357 | Glutathione-S-transferase microsomal, GSTMS3 |
3.1.3. The Southern Africa Population of Malawi
The most overexpressed genes in the Malawi population when comparing R to S are the two P450s CYP6P9a and b (Table 3). Besides those two genes, many other P450s, including CYP325J1, CYP6M4, CYP6P2, CYP9K1, CYP314A1, CYP6N1 and cytochrome b5, are upregulated. For GSTs, the most overexpressed genes are from delta family, e.g., GDTD1 and GSTD11. The GST epsilon family include GSTe1, GSTe2, GSTe5 and GSTe6. In addition, theta family GSTt1 was also found to be overexpressed in exposed mosquitoes. As for Central and West Africa, we also have the overexpression of carboxylesterase (AFUN016265), sulfotransferase (AFUN016207) and ATP-binding cassette transporter (AFUN019220).
Table 3.
Detoxification genes differentially expressed in Malawi between different comparisons at FDR < 0.05 and FC > 1.5 for R-C or FC > 2 for C-S and R-S.
| Genes ID | R-C | C-S | R-S | Description |
|---|---|---|---|---|
| AFUN019523 | 1.507577 | 4.439413 | 6.692756 | Cytochrome P450, CYP325J1 |
| AFUN015889 | 2.5220747 | 16.651278 | 41.99576 | Cytochrome P450, CYP6P9b |
| AFUN019220 | 3.1381407 | 2.659554 | ABC transporter family A | |
| AFUN016265 | 3.7209604 | 4.703569 | Carboxylic ester hydrolase | |
| AFUN019401 | 2.8624895 | 2.7167373 | Cytochrome P450, CYP6M4 | |
| AFUN015801 | 2.299937 | 2.2996929 | Cytochrome P450, CYP6P2 | |
| AFUN020895 | 6.031834 | 6.101497 | Cytochrome P450, CYP6P4 | |
| AFUN015792 | 53.420876 | 49.825886 | Cytochrome P450, CYP6P9a | |
| AFUN002978 | 2.048785 | 2.5891387 | Cytochrome P450, CYP314A1 | |
| AFUN010918 | 2.2058907 | 2.3527682 | Cytochrome P450, CYP6N1 | |
| AFUN011266 | 2.2034643 | 3.0369558 | UDP-glucuronosyltransferase 3A1 | |
| AFUN015839 | 5.0719476 | 3.63867 | Glutathione S-transferase, GSTD3 | |
| AFUN016008 | 2.8427892 | 3.0745156 | Glutathione S-transferase, GSTE6 | |
| AFUN016207 | 2.7247274 | 2.1642609 | Sulfotransferase | |
| AFUN015907 | 2.2404246 | Cytochrome P450, CYP305A3 | ||
| AFUN015785 | 2.1118221 | Cytochrome P450, CYP6AA2 | ||
| AFUN015807 | 2.5705426 | Glutathione S-transferase, GSTE1 | ||
| AFUN004322 | 2.2281806 | Cytochrome b5 | ||
| AFUN006135 | 2.3192496 | Cytochrome P450, CYP4C36 | ||
| AFUN007549 | 2.2119997 | Cytochrome P450, CYP9K1 | ||
| AFUN016010 | 2.1173494 | Glutathione S-transferase, GSTD1 | ||
| AFUN015767 | 2.0815623 | Glutathione S-transferase, GSTD11 | ||
| AFUN015811 | 2.2649431 | Glutathione S-transferase, GSTE5 | ||
| AFUN015809 | 2.1379018 | Glutathione S-transferase epsilon 2, GSTE2 | ||
| AFUN007291 | 2.0031114 | Glutathione S-transferase, GSTT2 | ||
| AFUN008239 | 2.3338351 | Sulfotransferase | ||
| AFUN016367 | 1.8275834 | Carboxylesterase, COEJHE4E | ||
| AFUN016209 | 1.5197136 | Sulfotransferase |
3.1.4. The East African Population of Uganda
Here, the most overexpressed genes are cytochrome P450s, with CYP4C26, CYP6P5, CYP6P4a, CYP306A1, CYP305A3 and CYP315A1. Some GST families are also significantly over-expressed when comparing R to C mosquitoes. These include GSTD1, GSTD3 and GSTe6. However, not many GST epsilon genes are overexpressed in Uganda compared to other Africa countries, e.g., Cameroon and Ghana. Many other genes families, such as carboxyesterases, sulfotransferase, NADHP, and ATP-binding cassette transporter, are expressed in Uganda (Table 4).
Table 4.
Detoxification genes differentially expressed in Uganda between different comparisons at FDR < 0.05 and FC > 1.5 for R-C or FC > 2 for C-S and R-S.
| Genes ID | R-C | C-S | R-S | Description |
|---|---|---|---|---|
| AFUN019220 | 2.9239964 | 2.9306972 | ABC transporter family A | |
| AFUN000421 | 2.0856595 | 2.4146874 | Carboxylesterase, COEBE4C | |
| AFUN015777 | 3.8722458 | 3.1892729 | Cytochrome P450, CYP4C26 | |
| AFUN015888 | 3.0184207 | 3.1936443 | Cytochrome P450, CYP6P5 | |
| AFUN015839 | 2.9561374 | 2.1264198 | Glutathione S-transferase, GSTD3 | |
| AFUN016008 | 3.5037928 | 2.787403 | Glutathione S-transferase, GSTE6 | |
| AFUN016207 | 2.6702209 | 2.6851285 | Sulfotransferase | |
| AFUN002796 | 2.711219 | ABC transporter sub-family G member 1; | ||
| AFUN019365 | 2.3760457 | Cytochrome P450, CYP6P4 | ||
| AFUN006858 | 2.0747128 | Cytochrome P450, CYP306A1 | ||
| AFUN011518 | 2.2290916 | Cytochrome c oxidase assembly factor 5 | ||
| AFUN015735 | 2.302202 | Cytochrome P450, CYP49A1 | ||
| AFUN007537 | 1.6417327 | ABC transporter ABCF3 | ||
| AFUN019517 | 1.9148197 | Cytochrome P450, CYP325J1 | ||
| AFUN019708 | 1.8381125 | Sulfotransferase 2 | ||
| AFUN019950 | 1.8083609 | Carboxylesterase, COE2580 | ||
| AFUN020232 | 1.5087312 | Metalloproteinase domain-containing protein | ||
| AFUN020202 | 1.7750088 | NADPH oxidase 5 | ||
| AFUN015907 | 2.350208 | Cytochrome P450, CYP305A3 | ||
| AFUN005715 | 2.1279395 | Cytochrome P450, CYP315A1 | ||
| AFUN016010 | 2.034498 | Glutathione S-transferase, GSTD1 |
Noticeably, the analysis of gene expression across Africa between DDT-resistant mosquito population revealed that GSTs are upregulated across the continent. The level of expression of GSTs is variable across the continent and three major families, the epsilon, the delta and the theta family, are the most overexpressed. Regarding the GST epsilon cluster specifically, we observed that all genes are up-regulated across Africa when comparing control mosquitoes versus DDT-resistant population, except for GSTe8.
3.1.5. Genes Differentially Expressed across Africa when Comparing DDT vs. Permethrin-Resistant Mosquitoes
Cameroon (Central Africa):
Analysis of the genes significantly upregulated in DDT-resistant mosquitoes when comparing to permethrin-resistant mosquitoes revealed the presence of CYP325A (FC: 1.56), some eukaryotic large and small subunit ribosomal RNA (AFUN017765) (FC: 24), secretory phospholipase A2 (AFUN022209) (FC: 1.7), and D7 short form salivary protein (AFUN016457) (FC: 1.6). Some genes including the probable prefoldin subunit PFD6 (FC: −2.07), odorant-binding protein (AFUN002277) (FC: −2.6) and Hexamerin (AFUN018859) (FC: −2.51) are rather significantly downregulated in permethrin-resistant mosquitoes (Supplementary Table S1.1).
Ghana (West Africa):
Analysis has shown that no upregulated detoxification enzyme family such as cytochrome P450s, glutathione-S-transferase or carboxylesterase is significantly over-expressed in DDT-resistant mosquitoes when compared to permethrin-resistant. However, some other genes with no known detoxification role including haemolymph protein (AFUN021325) P27K (FC 1.5), heat shock protein (AFUN019847) HSP90 (FC: 1.62), and transcription initiation factor (AFUN019720) TAF13 (FC 1.56) are upregulated in DDT-resistant mosquitoes. However, CYP4H19 is downregulated in permethrin-resistant mosquitoes (FC: −1.54) (Supplementary Table S1.2).
Malawi (Southern Africa):
When comparing DDT vs. permethrin-resistant populations in Malawi some detoxification genes including CYP6P9b (FC: 1.99), GSTU3 (FC: 1.67), glutathione peroxidase (AFUN022201) (FC: 1.8), as well as serine proteinase (AFUN022250) (FC: 5.9) and the probable prefoldin subunit 6 (AFUN021561) (FC: 3.4) are overexpressed in DDT-resistant mosquitoes. Other genes including carboxylic ester hydrolase COEBE2C (AFUN016052) (FC: −3.21), CYP4H25 (FC: −1.74), GSTD11 (FC: −1.80) and GSTU2 (FC: −1.64) are downregulated in permethrin-resistant mosquitoes (Supplementary Table S1.3).
Uganda (East Africa):
Comparison shows that some genes such as carboxylesterase (AFUN016265) (FC 1.77), Putative serine protease (AFUN016153) (FC 2.87), and the eukaryotic large and small subunit ribosomal RNA (AFUN017404, AFUN017328,) (with, respectively, fold change 47.04 and 3.20) are upregulated in DDT-resistant mosquitoes. Some detoxification genes including cytochromes P450 (CYP325J1, CYP325Z1, CYP6Z4), Zinc metalloproteinase nas-1(AFUN021369) (FC 2.91), UDP-glucuronosyltransferase (AFUN000679) (FC: −1.75), Lactosylceramide 4-α-galactosyltransferase (AFUN020965) (FC: −3.27), and Heat shock protein 70 B2 (AFUN019513) (FC: −4.58) (Supplementary Table S1.4) are rather downregulated in permethrin-resistant mosquitoes.
3.2. qPCR Transcriptional Profiling of GSTe Genes in An. funestus across Africa
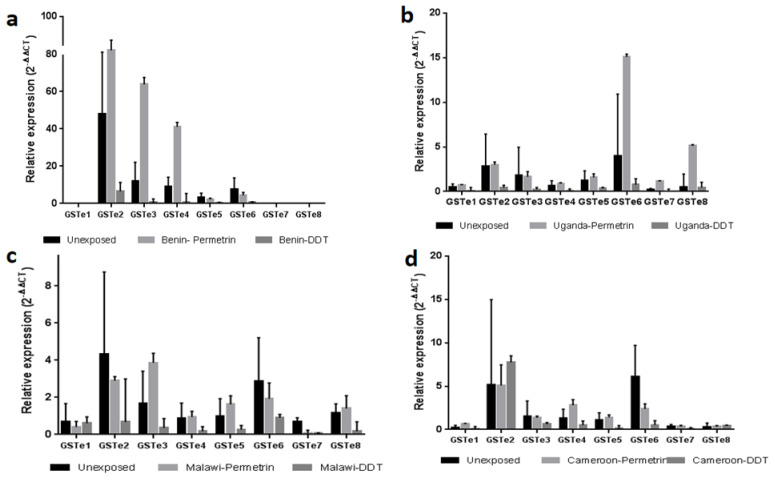
The validation of the expression profile of genes of the GSTe cluster across the continent was carried out using qPCR, comparing the expression level of GSTes between FANG, unexposed (control), permethrin-alive and DDT-alive mosquitoes after 24 h exposure. In Benin, the level of expression of GSTe2, GSTe3 and GSTe4 was significantly higher in mosquitoes resistant to DDT compared to the unexposed group and the permethrin-resistant mosquitoes (Figure 2). In Uganda, all the GSTes were expressed but at comparatively the same level, with the most over-expressed one being GSTe6, followed by GSTe8. As observed with Benin samples, these two genes were more expressed in mosquitoes surviving DDT exposure. In Malawi, it was observed in ascending order that the most expressed GSTes were GSTe2, GSTe3, GSTe6, GSTe5 and GSTe8. However, except for the GSTe6, which was more overexpressed in mosquitoes resistant to permethrin, the level of expression of other GSTes was higher in DDT-resistant mosquitoes. For Cameroon, GSTe2, GSTe4 and GSTe3 were more overexpressed in DDT-alive females. Overall, these results showed that the GSTes are overexpressed mainly in DDT-resistant mosquitoes compared to permethrin and unexposed mosquitoes.
Figure 2.
Evaluation of GSTe expression profile across Africa by qPCR. (a) GSTe expression profiles in Benin: GSTe2, 3 and 4 are the more expressed (b) GSTes expression profile in Uganda: GSTe6, 8, 2 and 3 are the more expressed; (c) GSTes expression profile in Malawi: GSTe2, 3, 6, 5 and 8 are the more expressed; (d) GSTes expression profile in Cameroon: GSTe2 6, 4, 3 and GSTe5 are the more expressed. Consistent with RNA-seq data, GST epsilons are differentially expressed, and expression level is higher in DDT-resistant mosquitoes than the control in general.
3.3. Functional Validation of the Role of GSTe Genes in Insecticide Resistance
3.3.1. Confirmation of GSTe Knockdown Effect by qRT-PCR
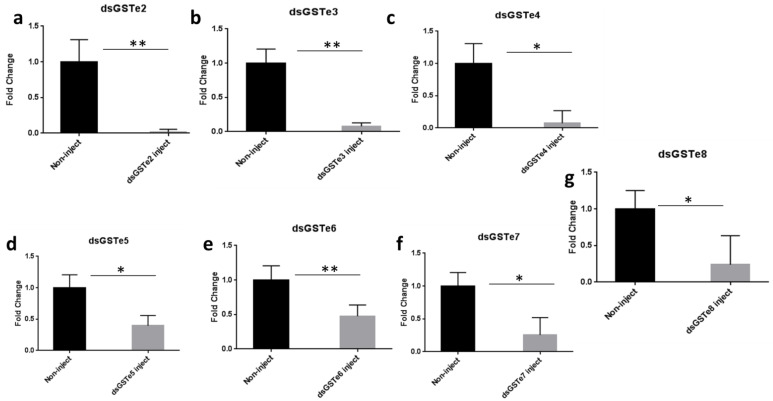
To confirm whether the injection of dsGSTes did knock-down the expression of GSTe genes in vivo in mosquitoes, qPCR was performed using the cDNA from unexposed dsGSTes (injected) and non-injected mosquitoes with the primers of each GSTes cluster, using actin5C and RPS7 as housekeeping genes. As shown in Figure 3, we noticed a significant partial reduction in GSTes gene expression when comparing control non-exposed and double-strand GSTe-injected mosquitoes 4 days post-injection p = 0.0024 (GSTe2), 0.0014 (GSTe3), 0.0377 (GSTe4), 0.0422 (GSTe5), 0.0014 (GSTe6), 0.0387 (GSTe7) and 0.0241 (GSTe8). This low expression of all the GSTes in mosquitoes injected compared to the non-injected supports that in vivo injection of dsRNA significantly reduces the expression of GST epsilon genes in the Mibellon An. funestus mosquitoes.
Figure 3.
Confirmation of GSTe knockdown effect by quantitative RT-PCR between non-exposed double-strand injected and non-injected mosquitoes of the same age (a) dsGSTe2, (b) dsGSTe3, (c) dsGSTe4, (d) dsGSTe5, (e) dsGSTe6, (f) dsGSTe7, (g) dsGSTe8,. There is low expression of all GSTe injected mosquitoes compared to the non-injected mosquitoes. dsRNA injection significantly reduces the expression of the GST epsilon genes. * = p < 0.05, and ** = p < 0.01.
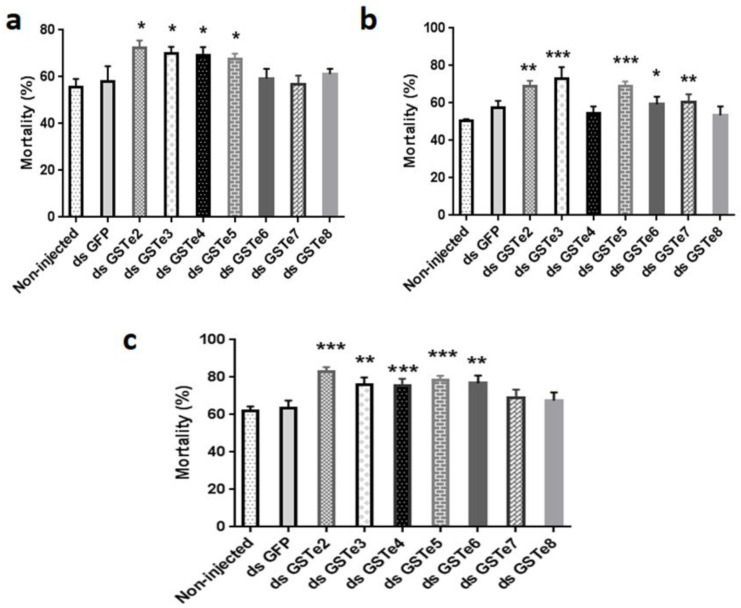
3.3.2. GSTe Knockdown Increases Susceptibility to Permethrin
Significantly higher mortalities were observed in dsGSTe2-injected mosquitoes (Mibellon An. funestus) exposed to permethrin (72.46% ± 3.09; p = 0.0052), compared with non-injected control mosquitoes, with mortalities of 55.50% ± 3.59 (Figure 4a). Similar patterns were seen with GSTe3 (mortality in dsGSTe3 = 70.09 ± 2.75%; p = 0.009), GSTe4 (69.01 ± 3.70%; p = 0.017) and GSTe5 (67.61 ± 4.41%; p = 0.018). Mortality with control mosquitoes injected with dsGFP is also 58.09% ± 6.41. No significant difference was obtained between the mortality of mosquitoes injected with dsGSTe6 (59.28 ± 4.14%; p = 0.25), dsGSTe7 (56.93 ± 3.63%; p = 0.39) and dsGSTe8 (61.25 ± 2.16; p = 0.13) compared to control mosquitoes exposed to permethrin. The mortality rate did not vary significantly between non-injected and mosquitoes injected with dsGFP, indicating that injection did not affect the survival of mosquitoes. These findings showed that GSTe2 3, 4 and 5 are playing a major role in permethrin resistance in this An. funestus population.
Figure 4.
GSTe knockdown increases susceptibility to insecticides; bioassay result of mosquitoes after injection and exposure to (a) permethrin, (b) deltametrin and (c) DDT. * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
3.3.3. GSTe Knockdown Increases Susceptibility to Deltamethrin
Exposure of dsGSTes-injected mosquitoes to deltamethrin (Figure 4b) revealed that the mortality rate was higher in dsGSTe2 (69.06 ± 2.92%; p = 0.026), dsGSTe3 (72.87 ± 6.29%; p = 0.0001), dsGSTe5 (68.92 ± 2.58%; p = 0.000038), dsGSTe6 (59.28 ± 4.14%; p = 0.018) and dsGSTe7-injected mosquitoes (60.53 ± 1.63%; p = 0.00039) compared to non-injected (50.25% ± 1.18) and dsGFP-injected mosquitoes (57.40 ± 3.78%). No significant differences were observed in GSTe4 (54.41 ± 3.68%; p = 0.11) and GSTe8-injected mosquitoes (53.49 ± 4.73%; p = 0.22) compared to the control non-injected ones. This result showed that knock-down of GSTes increased mosquito susceptibility to deltamethrin except for GSTe4 and GSTe8 where no impact was observed.
3.3.4. GSTe Knockdown Increases Susceptibility to DDT
By exposing double-strand GSTes-injected mosquitoes to DDT, we observed a significant difference between the mortality rates of GSTe2 (83.05 ± 2.39%; p = 0.0001), GSTe3 (75.87 ± 3.99%; p = 0.0036), GSTe4 (75.45 ± 3.72%; p = 0.00052), GSTe5 (78.68 ± 2.11%; p = 0.00038) and GSTe6 (76.78 ± 4.19%; p = 0.003) knocked-down mosquitoes compared to the non-injected mosquitoes (62.21 ± 2.16%) and dsGFP-injected mosquitoes (63.52 ± 4.01%) (Figure 4c). Knockdowns of GSTes genes family in Mibellon mosquitoes increased resistance to DDT excepted for GSTe7 and GSTe8. From these results, we concluded that GST epsilon genes are playing a role in An. funestus insecticide resistance.
4. Discussion
Understanding of the dynamics of resistance development, the potential of some candidate genes to confer cross-resistance between insecticide classes and designing suitable diagnostic tools are crucial for malaria control. The major genes linked to metabolic insecticide resistance in the major malaria vector An. funestus, include CYP450s, GSTs and carboxylesterase. The characterisation of these major genes provides important information enabling the understanding and the dynamic of resistance development and how and where it spreads, facilitating its management.
In this study, comparative RNAseq-based transcriptomic profiling across Africa showed key differences in the level of expression of GSTs across Africa, including the epsilon, delta and the theta class. These genes are highly expressed in West and Central Africa, in contrast to southern Africa where GSTs are found to be less overexpressed, contrary to P450s, especially the duplicated CYP6P9a and -b genes, which are highly overexpressed in this region [32]. The greater over-expression of P450s genes observed when comparing transcriptomic profiling of DDT resistance across Africa do not necessarily indicate that this enzyme family plays the major role in DDT resistance but could be a result of the multiple resistance observed in these mosquito population with pyrethroids and carbamate resistance as reported. This is supported by the fact that previous studies, such as in An. gambiae, have showed little difference in mortality between bioassays done with DDT alone or after pre-exposure to the synergist PBO [36], showing that P450s may not be the main drivers of DDT resistance but more likely, GSTs in the absence of kdr as seen in An. funestus [37]. The difference in gene expression pattern observed between An. funestus populations is in line with previous studies done in An. funestus [15,32,38,39]. Transcriptional profiling using microarrays/qPCR has established higher overexpression of GSTs in populations from West Africa (Benin) compared with populations from Uganda and Malawi [15]. Among GSTs, the epsilon and delta families are the most expressed as seen also in other mosquito species [13,40], but the GST epsilon cluster is more consistently over-expressed across Africa as previously reported in An. funestus permethrin resistance [32]. All genes of the GST epsilon cluster are observed to be overexpressed in Africa in resistant population of An. funestus, but at different levels, except for GSTe8. This association between overexpression of GSTes and DDT resistance could be explained by the fact that GST plays a role in oxidative stress and its expression is elevated in the response of oxidative damage caused by xenobiotic [41]. Besides cytochrome P450 and GSTs, other gene families were involved in DDT resistance including carboxylesterases, sulfotransferase, ATP-binding factor, UDP-glucuronosyltransferase, and metalloproteinase, and the same pattern were observed in An. gambiae using microarray [42,43].
Silencing of An. funestus GSTe genes supported the role that these genes played in DDT, as well as cross-resistance they confer to pyrethroids. This is in agreement with the findings of Riveron and colleagues [16] who also revealed a cross-resistance between these insecticide classes for GSTe2 using GAL4/UAS transgenic expression in Drosophila and also the association studies of the L119F-GSTe2 genotypes and resistance phenotypes [14,16]. While the work of Riveron and other researchers [18] characterized GSTe2, in particular, in this study, other epsilon GSTs were investigated, confirming their role in DDT and pyrethroid resistance. This cross-resistance could be either by directly metabolising the insecticides or conferring protection from oxidative stress induced by pyrethroids using a mechanism of sequestration [44]. It could also act by detoxifying/scavenging the secondary product generated by reactive oxygen species or by directly metabolizing 4-hydroxy-nonenal, an end product of lipid peroxidation, through conjugation [45].
This study has shown that knockdown of GSTes in An. funestus significantly increase susceptibility to type I and II pyrethroids, suggesting that the overexpression of GSTes could confer permethrin and deltamethrin resistance. This observation agrees with the results of Lumjuan in 2011, where they proved that partial knockdown of GSTe2 and GSTe7 in Aedes aegypti significantly increased susceptibility to DDT and deltamethrin [46]. This study has also supported that gene silencing through RNAi technique is a good tool to validate the role of candidate genes in insecticide resistance notably for GSTes.
5. Conclusions
The results have identified genes associated with DDT resistance in the major malaria vector An. funestus Africa-wide. The gene families identified as overexpressed include the cytochrome P450s commonly known to confer resistance to wide range of public health insecticide in malaria vector, carboxylesterase and glutathione-S-transferase, which are the major focus of this study. This has established that GSTe genes are differentially over-expressed in resistant populations of An. funestus across Africa, with the genes consistently more overexpressed in Western and Central Africa compared to East and Southern Africa, consistent with the higher DDT resistance known in the An. funestus populations from West Africa. In addition, the GSTe2, GSTe3, GSTe4, GSTe5 and GSTe6 genes were shown to confer cross-resistance to permethrin, deltamethrin and DDT explaining the multiple resistance observed in the field, highlighting the complexity of resistance and challenges associated with malaria vector control. Further studies need to be performed to detect the genetic variants associated with the resistance conferred by the GST epsilon genes as done previously for GSTe2.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12040561/s1. Supplementary file 1: Genes differentially expressed when comparing DDT (dichlo-ro-diphenyl-trichloroethane) vs permethrin-resistant individuals using RNA-seq (RNA sequenc-ing). Table S1.1: Genes differentially expressed in Cameroon when comparing DDT vs Perm resistant mosquitoes, Table S1.2: Genes differentially expressed in Ghana when comparing DDT vs Perm resistant mosquitoes, Table S1.3: Genes differentially expressed in Uganda when comparing DDT vs Perm resistant mosquitoes, Table S1.4: Genes differentially expressed in Malawi when comparing DDT vs Perm resistant mosquitoes, Table S1.5: List of genes differentially over-expressed across Africa when comparing resistant population against Permethrin and DDT using RNAseq. Supplementary file 2: List of primers used. Table S2.1: list of primers used to evaluate GSTes expression profile across Africa, Table S2.2: list of primers used for double-stranded RNA synthesis.
Author Contributions
Conceptualization, M.F.M.K. and C.S.W.; data curation, S.S.I., J.H. and C.S.W.; formal analysis, M.F.M.K. and J.M.R.; funding acquisition, C.S.W.; investigation, M.F.M.K., M.K., T.E., W.T., M.J.W. and H.I.; methodology, M.F.M.K. and C.S.W.; software, M.F.M.K., J.H. and C.S.W.; supervision, J.M.R., M.K., T.B., F.F.B. and C.S.W.; validation, S.S.I. and J.H.; writing–original draft, M.F.M.K.; writing–review and editing, M.F.M.K., S.S.I., J.H., M.K., M.T., T.B., F.F.B. and C.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Wellcome Trust Senior Fellowship in Biomedical Sciences to CSW, grant number 217188/Z/19/Z.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data from RNAseq is deposited on sequence archive, with the following link: https://www.ebi.ac.uk/ena/browser/view/PRJEB24351, accessed on 11 February 2021. All other data is present in the manuscript and supplemental files.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World Malaria Report 2020: 20 Years of Global Progress and Challenges. WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 2.Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., Battle K.E., Moyes C.L., Henry A., Eckhoff P.A., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riveron J.M., Huijben S., Tchapga W., Tchouakui M., Wondji M.J., Tchoupo M., Irving H., Cuamba N., Maquina M., Paaijmans K., et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles funestus Induces a Loss of Efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019;220:467–475. doi: 10.1093/infdis/jiz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu N., Li T., Reid W.R., Yang T., Zhang L. Multiple Cytochrome P450 Genes: Their Constitutive Overexpression and Permethrin Induction in Insecticide Resistant Mosquitoes, Culex quinquefasciatus. PLoS ONE. 2011;6:e23403. doi: 10.1371/journal.pone.0023403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muleya V., Hayeshi R., Ranson H., Abegaz B., Bezabih M.-T., Robert M., Ngadjui B.T., Ngandeu F., Mukanganyama S. Modulation of Anopheles gambiae Epsilon glutathione transferase activity by plant natural products in vitro. J. Enzym. Inhib. Med. Chem. 2008;23:391–399. doi: 10.1080/14756360701546595. [DOI] [PubMed] [Google Scholar]
- 6.Riveron J.M., Tchouakui M., Mugenzi L., Menze B.D., Chiang M.-C., Wondji C.S. Towards Malaria Elimination-A Leap Forward. IntechOpen; London, UK: 2018. Insecticide Resistance in Malaria Vectors: An Update at a Global Scale. [Google Scholar]
- 7.Ingham V.A., Anthousi A., Douris V., Harding N.J., Lycett G., Morris M., Vontas J., Ranson H. A sensory appendage protein protects malaria vectors from pyrethroids. Nat. Cell Biol. 2020;577:376–380. doi: 10.1038/s41586-019-1864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranson H., Rossiter L., Ortelli F., Jensen B., Wang X., Roth C.W., Collins F., Hemingway J. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem. J. 2001;359:295–304. doi: 10.1042/bj3590295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y., Ortelli F., Rossiter L.C., Hemingway J., Ranson H. The Anopheles gambiae glutathione transferase supergene family: Annotation, phylogeny and expression profiles. BMC Genom. 2003;4:35. doi: 10.1186/1471-2164-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell S.N., Rigden D.J., Dowd A.J., Lu F., Wilding C.S., Weetman D., Dadzie S., Jenkins A.M., Regna K., Boko P., et al. Metabolic and Target-Site Mechanisms Combine to Confer Strong DDT Resistance in Anopheles gambiae. PLoS ONE. 2014;9:e9266. doi: 10.1371/journal.pone.0092662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas E.R., Rockett K.A., Lynd A., Essandoh J., Grisales N., Kemei B., Njoroge H., Hubbart C., Rippon E.J., Morgan J., et al. A high throughput multi-locus insecticide resistance marker panel for tracking resistance emergence and spread in Anopheles gambiae. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-49892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca-González I., Quiñones M.L., Lenhart A., Brogdon W.G. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag. Sci. 2011;67:430–437. doi: 10.1002/ps.2081. [DOI] [PubMed] [Google Scholar]
- 13.Ranson H., Hemingway J. Mosquito Glutathione Transferases. Methods Enzymol. 2005;401:226–241. doi: 10.1016/S0076-6879(05)01014-1. [DOI] [PubMed] [Google Scholar]
- 14.Riveron J.M., Ibrahim S.S., Mulamba C., Djouaka R., Irving H., Wondji M.J., Ishak I.H., Wondji C.S. Genome-Wide Transcription and Functional Analyses Reveal Heterogeneous Molecular Mechanisms Driving Pyrethroids Resistance in the Major Malaria Vector Anopheles funestus across Africa. G3: Genes Genomes Genet. 2017;7:1819–1832. doi: 10.1534/g3.117.040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riveron J.M., Irving H., Ndula M., Barnes K.G., Ibrahim S.S., Paine M.J.I., Wondji C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA. 2012;110:252–257. doi: 10.1073/pnas.1216705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riveron J.M., Yunta C., Ibrahim S.S., Djouaka R., Irving H., Menze B.D., Ismail H.M., Hemingway J., Ranson H., Albert A., et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014;15:R27. doi: 10.1186/gb-2014-15-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilding C.S., Weetman D., Rippon E.J., Steen K., Mawejje H.D., Barsukov I., Donnelly M.J. Parallel evolution or purifying selection, not introgression, explains similarity in the pyrethroid detoxification linked GSTE4 of Anopheles gambiae and An. arabiensis. Mol. Genet. Genom. 2014;290:201–215. doi: 10.1007/s00438-014-0910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchouakui M., Riveron J.M., Djonabaye D., Tchapga W., Irving H., Takam P.S., Njiokou F., Wondji C.S. Fitness Costs of the Glutathione S-Transferase Epsilon 2 (L119F-GSTe2) Mediated Metabolic Resistance to Insecticides in the Major African Malaria Vector Anopheles funestus. Genes. 2018;9:645. doi: 10.3390/genes9120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menze B.D., Wondji M.J., Tchapga W., Tchoupo M., Riveron J.M., Wondji C.S. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar. J. 2018;17:317. doi: 10.1186/s12936-018-2467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menze B.D., Kouamo M.F., Wondji M.J., Tchapga W., Tchoupo M., Kusimo M.O., Mouhamadou C.S., Riveron J.M., Wondji C.S. An Experimental Hut Evaluation of PBO-Based and Pyrethroid-Only Nets against the Malaria Vector Anopheles funestus Reveals a Loss of Bed Nets Efficacy Associated with GSTe2 Metabolic Resistance. Genes. 2020;11:143. doi: 10.3390/genes11020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal N., Dasaradhi P.V.N., Mohmmed A., Malhotra P., Bhatnagar R.K., Mukherjee S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003;67:657–685. doi: 10.1128/MMBR.67.4.657-685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Baker N., Amdam G.V. RNAi-mediated Double Gene Knockdown and Gustatory Perception Measurement in Honey Bees (Apis mellifera) J. Vis. Exp. 2013;2013:e50446. doi: 10.3791/50446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lycett G.J., McLaughlin L.A., Ranson H., Hemingway J., Kafatos F.C., Loukeris T.G., Paine M.J.I. Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol. 2006;15:321–327. doi: 10.1111/j.1365-2583.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez S.B.G., Guimarães-Ribeiro V., Rodriguez J.V.G., Dorand F.A.P.S., Salles T.S., Sá-Guimarães T.E., Alvarenga E.S.L., Melo A.C.A., Almeida R.V., Moreira M.F. RNAi-based bioinsecticide for Aedes mosquito control. Sci. Rep. 2019;9:4038. doi: 10.1038/s41598-019-39666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isoe J., Koch L.E., Isoe Y.E., Rascón A.A., Jr., Brown H.E., Massani B.B., Miesfeld R.L. Identification and characterization of a mosquito-specific eggshell organizing factor in Aedes aegypti mosquitoes. PLoS Biol. 2019;17:e3000068. doi: 10.1371/journal.pbio.3000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan J.C., Irving H., Okedi L.M., Steven A., Wondji C.S. Pyrethroid Resistance in an Anopheles funestus Population from Uganda. PLoS ONE. 2010;5:e11872. doi: 10.1371/journal.pone.0011872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koekemoer L.L., Kamau L., Coetzee M., Hunt R.H. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 28.Livak K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt R.H., Brooke B.D., Pillay C., Koekemoer L.L., Coetzee M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Veter. Entomol. 2005;19:271–275. doi: 10.1111/j.1365-2915.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 30.Weedall G.D., Irving H., Hughes M.A., Wondji C.S. Molecular tools for studying the major malaria vector Anopheles funestus: Improving the utility of the genome using a comparative poly(A) and Ribo-Zero RNAseq analysis. BMC Genom. 2015;16:931. doi: 10.1186/s12864-015-2114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mugenzi L.M.J., Menze B.D., Tchouakui M., Wondji M.J., Irving H., Tchoupo M., Hearn J., Weedall G.D., Riveron J.M., Wondji C.S. Cis-regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus. Nat. Commun. 2019;10:4652. doi: 10.1038/s41467-019-12686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weedall G.D., Mugenzi L.M.J., Menze B.D., Tchouakui M., Ibrahim S.S., Amvongo-Adjia N., Irving H., Wondji M.J., Tchoupo M., Djouaka R., et al. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019;11:eaat7386. doi: 10.1126/scitranslmed.aat7386. [DOI] [PubMed] [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Blandin S., Moita L.F., Köcher T., Wilm M., Kafatos F.C., Levashina E.A. Reverse genetics in the mosquito Anopheles gambiae: Targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO . Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. WHO; Geneva, Switzerland: 2016. [Google Scholar]
- 36.Djouaka R., Riveron J.M., Yessoufou A., Tchigossou G., Akoton R., Irving H., Djegbe I., Moutairou K., Adeoti R., Tamò M., et al. Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasites Vectors. 2016;9:1–12. doi: 10.1186/s13071-016-1723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irving H., Wondji C.S. Investigating knockdown resistance (kdr) mechanism against pyrethroids/DDT in the malaria vector Anopheles funestus across Africa. BMC Genet. 2017;18:1–11. doi: 10.1186/s12863-017-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahim S.S., Riveron J.M., Bibby J., Irving H., Yunta C., Paine M.J.I., Wondji C.S. Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector. PLoS Genet. 2015;11:e1005618. doi: 10.1371/journal.pgen.1005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nardini L., Hunt R.H., Dahan-Moss Y.L., Christie N., Christian R.N., Coetzee M., Koekemoer L.L. Malaria vectors in the Democratic Republic of the Congo: The mechanisms that confer insecticide resistance in Anopheles gambiae and Anopheles funestus. Malar. J. 2017;16:1–15. doi: 10.1186/s12936-017-2099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lumjuan N., McCarroll L., Prapanthadara L.-A., Hemingway J., Ranson H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Insect Biochem. Mol. Biol. 2005;35:861–871. doi: 10.1016/j.ibmb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Qiu L., Ranson H., Lumjuan N., Hemingway J., Setzer W.N., Meehan E.J., Chen L. Structure of an insect epsilon class glutathione S-transferase from the malaria vector Anopheles gambiae provides an explanation for the high DDT-detoxifying activity. J. Struct. Biol. 2008;164:228–235. doi: 10.1016/j.jsb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Vontas J., Blass C., Koutsos A.C., David J.-P., Kafatos F.C., Louis C., Hemingway J., Christophides G.K., Ranson H. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol. Biol. 2005;14:509–521. doi: 10.1111/j.1365-2583.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell S.N., Stevenson B.J., Müller P., Wilding C.S., Egyir-Yawson A., Field S.G., Hemingway J., Paine M.J.I., Ranson H., Donnelly M.J. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. USA. 2012;109:6147–6152. doi: 10.1073/pnas.1203452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vontas J.G., Small G.J., Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001;357:65–72. doi: 10.1042/bj3570065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassan F., Singh K.P., Ali V., Behera S., Shivam P., Das P., Dinesh D.S. Detection and functional characterization of sigma class GST in Phlebotomus argentipes and its role in stress tolerance and DDT resistance. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-56209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lumjuan N., Rajatileka S., Changsom D., Wicheer J., Leelapat P., Prapanthadara L.-A., Somboon P., Lycett G., Ranson H. The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochem. Mol. Biol. 2011;41:203–209. doi: 10.1016/j.ibmb.2010.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data from RNAseq is deposited on sequence archive, with the following link: https://www.ebi.ac.uk/ena/browser/view/PRJEB24351, accessed on 11 February 2021. All other data is present in the manuscript and supplemental files.