Abstract
Cryptosporidium is a leading cause of childhood diarrhoea and associated physical and cognitive impairment in low-resource settings. Cryptosporidium-positive faecal samples (n = 190) from children aged ≤ 5 years enrolled in the Global Enteric Multicenter Study (GEMS) in Mozambique detected by ELISA (11.5%, 430/3754) were successfully PCR-amplified and sequenced at the gp60 or ssu rRNA loci for species determination and genotyping. Three Cryptosporidium species including C. hominis (72.6%, 138/190), C. parvum (22.6%, 43/190), and C. meleagridis (4.2%, 8/190) were detected. Children ≤ 23 months were more exposed to Cryptosporidium spp. infections than older children. Both C. hominis and C. parvum were more prevalent among children with diarrhoeal disease compared to those children without it (47.6% vs. 33.3%, p = 0.007 and 23.7% vs. 11.8%, p = 0.014, respectively). A high intra-species genetic variability was observed within C. hominis (subtype families Ia, Ib, Id, Ie, and If) and C. parvum (subtype families IIb, IIc, IIe, and IIi) but not within C. meleagridis (subtype family IIIb). No association between Cryptosporidium species/genotypes and child’s age was demonstrated. The predominance of C. hominis and C. parvum IIc suggests that most of the Cryptosporidium infections were anthroponotically transmitted, although zoonotic transmission events also occurred at an unknown rate. The role of livestock, poultry, and other domestic animal species as sources of environmental contamination and human cryptosporidiosis should be investigated in further molecular epidemiological studies in Mozambique.
Keywords: Cryptosporidium, gp60, ssu rRNA, genotyping, children, diarrhoea, prevalence, molecular epidemiology, Mozambique, GEMS
1. Introduction
Diarrhoeal diseases remain the second leading cause of mortality after pneumonia in children under 5 years worldwide, accounting for approximately 9% of the 5.8 million deaths associated to this condition reported in 2015 [1,2]. Most of these fatalities disproportionately occur in poor-resource settings where suboptimal hygiene conditions and sanitation prevail [3]. A recent update on diarrhoeal burden from the Global Enteric Multicenter Study (GEMS and GEMS1A) demonstrated that Rotavirus, Cryptosporidium, enterotoxigenic Escherichia coli producing heat stable toxin (ST_ETEC), and Shigella were the main pathogens associated with moderate-to-severe diarrhoea (MSD) and less severe diarrhoea (LSD) in African and Asian children [4,5]. Cryptosporidiosis in children under 5 years presents with watery diarrhoea, abdominal pain, nausea, and vomiting, being often associated to growth faltering and cognitive development impairment [6,7]. In severe cases, the disease can lead to life-threatening sequelae among malnourished and immunocompromised children [8]. Cryptosporidium spp. is also a major contributor to the burden of diarrhoeal disease in HIV-positive patients [9].
As other diarrhoea-causing pathogens, Cryptosporidium spp. are transmitted through the faecal–oral route. Humans acquire the infection through direct contact with infected hosts (person-to-person or zoonotic transmission) or by ingestion of contaminated food or water (foodborne and waterborne transmission), but the relative importance of these routes is still unclear [10]. At least 40 known Cryptosporidium species are currently recognised, and among these, more than 20 species and genotypes have been reported to cause human infections. Cryptosporidium hominis and C. parvum cause more than 90% of the human cases documented globally [11,12,13].
Molecular tools for the differentiation of Cryptosporidium species and genotypes are currently available, mostly using PCR followed by either restriction length fragment polymorphisms (RFLP) analysis or Sanger sequencing of the small subunit ribosomal ribonucleic acid (ssu rRNA) and the 60 kDa glycoprotein (gp60) genes of the parasite [10,13]. The ssu rRNA gene is largely used for the differential diagnosis of Cryptosporidium species due to its multicopy nature and associated high sensitivity. Subtype identification is primarily achieved through DNA sequence analysis of the highly polymorphic gp60 gene. Subtype assignment is based on the number of TCA, TCG, and TCT repeats in addition to other repetitive sequences, such as the ACATCA, within the gp60 tandem repeat motif region. Subtype families are named as Ia, Ib, Ic, Id, Ie, If, etc. for C. hominis and IIa, IIb, IIc, IId, etc. for C. parvum, with further species families named in ascending order [12,14].
In Africa, the epidemiology and genetic diversity of Cryptosporidium spp. remains relatively unknown. However, as noted by a recent literature review, at least 13 species and genotypes have been identified in humans, with C. hominis followed by C. parvum once again dominating the epidemiological landscape [11]. Subtyping studies support the dominance of anthroponotic over zoonotic transmission in African countries, regardless of the close contact with farm and domestic animals. Another interesting observation in Africa is the high level of subtype diversity, where at least six subtype families for C. hominis (Ia, Ib, Id, Ie, If, and Ih) have been described. For C. parvum the predominant subtypes identified in humans belong to the IIc family, in addition to IIa, IIb, IId, IIg, IIi, IIh, IIm and the rarer anthroponotically transmitted IIe subtype family [11].
In Mozambique, diarrhoea is ranked as the third cause of death in children under 14 years from the capital city Maputo [15] and fourth in children under 5 years from the Manhiça district (Maputo province) [16], being responsible for 20% of hospital paediatric admissions in this district [17]. The GEMS data support that prevention strategies targeting Rotavirus, Cryptosporidium, ST_ETEC and Shigella could contribute to reduce diarrhoeal cases by approximately 50% in infants, and hence diarrhoeal-associated mortality [18]. However, the genetic diversity of Cryptosporidium spp. in GEMS was not investigated. Two previous hospital-based studies carried out in the southern part of the country have identified IaA23R3, IIcA5G3, and IIeA12G1 subtypes among nine isolates from patients with diarrhoea in the capital city Maputo [19], and IbA10G2 and IdA22 subtypes among eight isolates in patients with HIV and tuberculosis in the Chokwe district of Gaza province [20]. However, no extensive molecular epidemiological studies have been conducted to evaluate the genetic diversity within Cryptosporidium spp. Herein, we aimed to analyse the diversity and frequency of Cryptosporidium species and subtypes detected in stools from children younger than 5 years from the Manhiça district, Mozambique, enrolled in the context of GEMS between 2007 and 2012.
2. Results
2.1. Initial Screening for the Detection of Cryptosporidium spp. by ELISA Immunoassay
During the 5-year study period (December 2007–November 2012) a total of 3754 stool samples were collected. The ELISA positivity rate for Cryptosporidium spp. was estimated at 11.5% (430/3754). The prevalence was significantly (p < 0.001) higher among diarrhoea cases (MSD and LSD cases) (16.5%, 222/1346) compared to children without diarrhoea (non-cases; 8.5%, 208/2408). Most (91.2%, 392/430) of the Cryptosporidium-positive samples by ELISA were available for molecular analyses (Figure 1). Unavailable samples were the result of the depletion of starting material as consequence of testing and analyses in previous studies [4,5,18].
Figure 1.
Flow chart summarising the diagnostic and genotyping procedures used in this study.
The distribution of the ELISA-positive Cryptosporidium infections in cases and non-cases according to sex, age group, and clinical condition is summarised in Table 1. Approximately one in two (53.6%, 210/392) children with cryptosporidiosis were aged 0–11 months. The male/female ratio was 1.8. Children with MSD and their matched controls were significantly more exposed to Cryptosporidium than their counterparts with LSD and corresponding controls (p < 0.001). HIV+ patients with diarrhoea were more likely to be infected with Cryptosporidium spp. than HIV+ patients without diarrhoea (χ2 = 9.8758, p = 0.001675). Being undernourished and having diarrhoea were also significantly associated with cryptosporidiosis (χ2 = 19.769, p ≤ 0.00001). Regarding coinfections with other intestinal pathogens, Cryptosporidium infection was more likely in children with diarrhoea and rotavirus infection (p ≤ 0.011). In contrast, coinfections by Cryptosporidium spp. and G. duodenalis were more frequent in asymptomatic (non-cases) children (p < 0.001). The full dataset showing the epidemiological, clinical, diagnostic, and genotyping data used in the analyses conducted in the present survey is presented in Table S1.
Table 1.
Main epidemiological and clinical variables of Cryptosporidium-positive children under five years of age by ELISA (n = 392) with diarrhoea (cases) and without diarrhoea (non-cases) according to age group. Children were recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012.
| 0–11 Months | 12–23 Months | 24–59 Months | ||||
|---|---|---|---|---|---|---|
| Variable | Cases | Non-Cases 1 | Cases | Non-Cases 1 | Cases | Non-Cases 1 |
| n = 111 (%) | n = 99 (%) | n = 72 (%) | n = 68 (%) | n = 21 (%) | n = 21 (%) | |
| MSD 2 | 87 (78.4) | 85 (85.9) | 45 (62.5) | 57 (83.8) | 12 (57.1) | 19 (90.5) |
| LSD 2 | 24 (21.6) | 14 (14.1) | 27 (37.5) | 11 (16.2) | 9 (42.9) | 2 (9.5) |
| Mean age (months) | 7.3 | 7.1 | 15.9 | 16.9 | 31.1 | 31 |
| Sex (male) | 68 (61.3) | 68 (68.7) | 48 (66.7) | 41 (61.2) | 13 (69.9) | 14 (66.7) |
| HIV+ 3 | 10/44 (24.7) | 2/18 (11.1) | 7/37 (18.9) | 2/21 (9.5) | 2/9 (22.2) | 0 (0.0) |
| Undernutrition | 14 (12.6) | 2 (2.0) | 12 (16.7) | 1 (1.5) | 1 (4.8) | 0 (0.0) |
| Co-infections | ||||||
| Rotavirus | 33 (29.7) | 13 (13.1) | 9 (12.5) | 10 (14.7) | 4 (19.1) | 1 (4.7) |
| Shigella spp. | 0 (0.0) | 0 (0.0) | 6 (8.3) | 0 (0.0) | 1 (4.8) | 0 (0.0) |
| All ETECs | 7 (6.3) | 5 (5.1) | 13 (18.1) | 7 (10.3) | 4 (19.1) | 4 (19.1) |
| G. duodenalis | 16 (14.4) | 30 (30.3) | 18 (25.0) | 38 (55.9) | 4 (19.5) | 10 (47.6) |
| E. histolytica 4 | 6 (5.5)4 | 8 (8.1) | 7 (9.7) | 5 (7.4) | 2 (9.5) | 4 (19.1) |
1 Non-cases are asymptomatic children without diarrhoea matched by age, sex, and neighbourhood with MSD and LSD cases. 2 Only applicable to cases. 3 Only part of the participants were tested for HIV, and the numbers of participants with known HIV status are specified in the denominator. 4 Missing values: n = 1. ETEC: Enterotoxigenic Escherichia coli; HIV: Human immunodeficiency virus; LSD: Less severe diarrhoea; MSD: Moderate-to-severe diarrhoea; NA: Not applicable.
2.2. Confirmation of Cryptosporidium spp. by Nested PCR Methods
Out of the 392 samples that tested positive by ELISA, 37.2% (146/392) were successfully sub-genotyped at the gp60 locus. The remaining 250 isolates with a negative result by gp60-PCR were subsequently re-assessed at the ssu rRNA marker, allowing the confirmation of Cryptosporidium DNA in 44 additional samples. Overall, the presence of the parasite was confirmed by gp60-PCR and/or ssu-PCR in 48.5% (190/392) of the analysed samples (Table 2). Sequence alignment analyses including appropriate reference sequences allowed the identification of three Cryptosporidium species including C. hominis (72.6%, 138/190), C. parvum (22.6%, 43/190), and C. meleagridis (4.2%, 8/190). An additional isolate (0.5%, 1/190) was only identified at the genus level due to poor sequence quality (Table 2). Both C. hominis and C. parvum were more prevalent among diarrhoeal children (cases) compared to non-diarrhoeal (non-cases) children (47.6% vs. 33.3%, p = 0.007 and 23.7% vs. 11.8%, p = 0.014, respectively). Infections by Cryptosporidium spp. were most common in children younger than 24 months, with C. hominis being the Cryptosporidium species more prevalent in all age groups investigated (Table 2). Cases of cryptosporidiosis by C. hominis and C. parvum were consistently detected along the whole study period, peaking during November 2011 and March 2012, particularly in children with LSD (Figure S1).
Table 2.
Diagnostic performance of PCR methods and distribution of the Cryptosporidium species detected in children under five years of age (n = 396) with diarrhoea (cases) and without diarrhoea (non-cases) according to age group. Children were recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012.
| 0–11 Months | 12–23 Months | 24–59 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR Results | Cases | Non-Cases | p | Cases | Non-Cases | p | Cases | Non-Cases | p |
| n = 112 (%) | n = 101 (%) | n = 73 (%) | n = 67 (%) | n = 21 (%) | n = 22 (%) | ||||
| gp60 | 49 (44.1) | 24 (24.4) | 0.003 | 37 (51.4) | 30 (44.1) | 0.389 | 3 (14.3) | 3 (14.3) | 1 |
| (n = 146) | |||||||||
| ssu rRNA 1 | 12/62 (19.4) | 10/75 (13.3) | 0.339 | 9/35 (25.7) | 8/38 (21.1) | 0.638 | 4/18 (22.2) | 1/18 (5.6) | 0.148 |
| (n = 44) | |||||||||
| Both | 61 (54.9) | 34 (34.3) | 0.003 | 46 (63.9) | 38 (55.9) | 0.334 | 7 (33.3) | 4 (19.1) | 0.292 |
| (n = 190) | |||||||||
| Species 2 | |||||||||
| C. hominis | 45 (47.4) | 27 (29.4) | 0.011 | 33 (55.9) | 26 (46.4) | 0.308 | 3 (22.2) | 3 (15.0) | 0.687 |
| (n = 138) | |||||||||
| C. parvum | 14 (21.9) | 6 (8.5) | 0.028 | 12 (31.6) | 9 (23.1) | 0.402 | 2 (12.5) | 0 (0.0) | 0.229 |
| (n = 43) | |||||||||
| C. meleagridis | 1 (1.9) | 1 (1.5) | 1 | 1 (3.7) | 3 (9.1) | 0.620 | 1 (6.7) | 1 (5.6) | 1 |
| (n = 8) | |||||||||
| Unknown 3 | 1 (1.9) | 0 (0.0) | 0.440 | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| (n = 1) | |||||||||
1 Only negative samples by gp60-PCR (n = 250) were analysed by ssu-PCR. 2 Species assigned on the combination of both gp60-PCR and ssu-PCR results. 3 Poor sequence quality data only allowed subtyping at genus level. NA: Not applicable.
2.3. Genetic Variation within C. hominis and C. parvum
Sequence analysis of the 117 isolates characterised as C. hominis at the gp60 locus revealed the presence of five subtype families including Ia (35.0%, 41/117), Ib (20.5%, 24/117), Id (1.7%, 2/117), Ie (34.2%, 40/117), and If (8.6%, 10/117). The most prevalent subtypes found were IaA23R1 within family Ia, IbA13G2 within family Ib, and IdA20 within family Id. All isolates belonging to families Ie and If were identified as IeA11G3T3 and IfA12G1, respectively. Two genetic variants within IaA24R3 and IbA13G2 corresponded to novel subtypes (Table 3). Sequence analyses of the 29 isolates characterised as C. parvum at the same locus revealed the presence of four subtype families including IIb (3.4%, 1/29), IIc (86.2%, 25/29), IIe (6.9%, 2/29), and IIi (3.4%, 1/29). IIbA11 within family IIb, IIcA5G3 within family IIc, IIeA11G1 within family IIe, and IIiA6-like within family IIi were the only subtypes found. Novel genetic variants were found within IIbA11, IIcA5G3, and IIiA6-like subtypes. All four isolates assigned to C. meleagridis belonged to subtype IIIbA23G1R1 within family IIIb (Table 3).
Table 3.
Diversity, frequency, and main molecular features of Cryptosporidium-positive samples at the gp60 and ssu rRNA loci in children under 5 years of age recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012. GenBank accession numbers of representative sequences were provided.
| Locus | Species | Isolates | Family | Subtype | Reference | Stretch | Single Nucleotide Polymorphisms | GenBank ID |
|---|---|---|---|---|---|---|---|---|
| gp60 | C. hominis | 37 | Ia | IaA23R3 | KX579755 | 3–805 | None | MW480826 |
| 1 | IaA24R3 | KX579755 | 1–793 | 84_86InsTCA | MW480827 | |||
| 3 | IaA25R3 | JF927194 | 18–838 | None | MW480828 | |||
| 7 | Ib | IbA10G2 | AY262031 | 22–857 | None | MW480829 | ||
| 15 | IbA13G2 | MT053132 | 13–896 | G85A | MW480830 | |||
| 2 | IbA9G3 | DQ665688 | 14–825 | None | MW480831 | |||
| 1 | Id | IdA20 | JX088404 | 48–904 | None | MW480832 | ||
| 1 | IdA21 | MN904672 | 47–910 | None | MW480833 | |||
| 39 | Ie | IeA11G3T3 | AY738184 | 19–923 | None | MW480834 | ||
| 1 | IeA11G3T3 | AY738184 | 48–923 | T284Y, A662R | MW480835 | |||
| 10 | If | IfA12G1 | EU161655 | 1–870 | None | MW480836 | ||
| C. parvum | 1 | IIb | IIbA11 | AY166805 | 1–782 | 51_59DelTCATCATCA | MW480837 | |
| 11 | IIc | IIcA5G3 | GU214365 | 31–851 | None | MW480838 | ||
| 7 | IIcA5G3 | GU214365 | 29–854 | 38 SNPs 2 | MW480839 | |||
| 5 | IIcA5G3 | GU214365 | 29–853 | 40 SNPs 2 | MW480840 | |||
| 1 | IIcA5G3 | GU214365 | 50–853 | C110T | MW480841 | |||
| 1 | IIcA5G3 | GU214365 | 50–853 | 40 SNPs 2 | MW480842 | |||
| 1 | IIe | IIeA11G1 | MN904721 | 1–813 | None | MW480843 | ||
| 1 | IIeA13G1 | KU852716 | 7–795 | None | MW480844 | |||
| 1 | IIi | IIiA6-like | AY873782 | 26–932 | 85 SNPs 2 | MW480845 | ||
| C. meleagridis 1 | 4 | IIIb | IIIbA23G1R1 | MK331716 | 1–714 | None | MW480846 | |
| ssu rRNA | C. hominis | 17 | – | – | AF108865 | 529–954 | None | MW487256 |
| 1 | – | – | AF108865 | 587–965 | A892R | MW487257 | ||
| 2 | – | – | AF108865 | 591–969 | T795Y, A892R | MW487258 | ||
| 1 | – | – | AF108865 | 640–956 | 697delT, T795Y, A892R | MW487259 | ||
| C. parvum | 1 | – | – | AF112571 | 565–956 | A646G, T649G, 686_689DelTAAT, A691T | MW487260 | |
| 3 | – | – | AF112571 | 526–1039 | A646G, T649G, 686_689DelTAAT, T693A | MW487261 | ||
| 1 | – | – | AF112571 | 524–1039 | A646G, 647_649DelATT, T663C, 686_689DelTAAT, C795T | MW487262 | ||
| 5 | – | – | AF112571 | 539–1031 | A646G, T649G, 686_689DelTAAT, T693A, C795T | MW487263 | ||
| 1 | – | – | AF112571 | 526–965 | A646G, T649G, 686_689DelTAAT, T693A, C795Y | MW487264 | ||
| 3 | – | – | AF112571 | 539–954 | A646G, T649G, 686_689DelTAAT, A691T, C795Y, A892R | MW487265 | ||
| Unknown | 1 | – | – | – | – | – | – | |
| C. meleagridis | 8 | – | – | AF112574 | 524–1034 | None | MW487266 |
Out of the 21 sequences characterised as C. hominis at the ssu rRNA locus, 81% (17/21) showed 100% identity with reference sequence AF108865. The remaining four sequences differed from AF108865 by 1–3 single nucleotide polymorphisms (SNPs) including a deletion mutation (Table 3). All the 14 sequences assigned to C. parvum corresponded to different genetic variations of the “bovine genotype” of this Cryptosporidium species, which is characterised by the presence of a four-base deletion TAAT at positions 686 to 689 of reference sequence AF112571. Indeed, some authors have proposed this genetic variant as an independent species named C. pestis [22]. Cryptosporidium hominis and C. parvum sequences differing from reference sequences at the ssu rRNA locus included ambiguous (C/T, A/G) positions in the form of double peaks, transition (A↔G, C↔T) and transversion (T↔G, A↔T) mutations, and single- to multiple-base deletions.
Finally, all eight sequences identified as C. meleagridis at the ssu rRNA locus showed 100% identity with reference sequence AF112574. Four of these eight isolates were successfully amplified at the gp60 locus using a specific PCR protocol for this Cryptosporidium species (see Section 4.3.2.). Sanger sequencing analyses allowed the identification of subtype IIIbA23G1R1 in all four sequences, which were identical to reference sequence MK331716.
Cryptosporidium hominis was the most prevalent species in children with MSD or LSD (79.6%, 70/88), followed by C. parvum (19.3%, 17/88), and C. meleagridis (1.1%, 1/88) (Table 4). Within C. hominis, nearly three out of every four diarrhoea-associated infections were caused by subtype families Ie (30.7%, 27/88) and Ia (27.3%, 24/88). Subtype family Ie was more frequent in children with MSD (43.1%, 25/58), and subtype family Ia in children with LSD (46.7%, 14/30). No obvious differences in subtype distribution were observed among the age groups considered. Near half of the cryptosporidiosis cases identified in HIV+ patients were caused by the subtype family Ia (55.6%, 5/9) (Table 4).
Table 4.
Diversity and frequency of Cryptosporidium subtypes families within C. hominis, C. parvum, and C. meleagridis in symptomatic (cases) children under 5 years of age according to severity of the diarrhoea, age group, and HIV coinfection. Children were recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012.
| C. hominis | C. parvum | C. meleagridis | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total | Ia | Ib | Id | Ie | If | IIc | IIIb |
| n = 90 (%) | n = 25 (%) | n = 11 (%) | n = 2 (%) | n = 28 (%) | n = 6 (%) | n = 17 (%) | n = 1 (%) | |
| Diarrhoea | ||||||||
| MSD | 60 (66.7) | 11 (44.0) | 7 (63.6) | 1 (50.0) | 26 (92.9) | 5 (83.3) | 9 (52.9) | 1 (100) |
| LSD | 30 (33.3) | 14 (56.0) | 4 (36.4) | 1 (50.0) | 2 (7.1) | 1 (16.7) | 8 (47.1) | 0 (0) |
| Age (months) | ||||||||
| 0–11 | 49 (54.4) | 16 (64.0) | 8 (72.7) | 1 (50.0) | 14 (50) | 2 (33.3) | 8 (47.1) | 0 (0) |
| 12–23 | 37 (41.1) | 7 (28.0) | 3 (27.3) | 1 (50.0) | 13 (46.4) | 4 (66.7) | 9 (52.9) | 0 (0) |
| 24–59 | 4 (4.4) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 1 (100) |
| Co-infections | ||||||||
| HIV+ 1 | 9/42 (21.4) | 5/17 (29.4) | 1/4 (25) | 0/1 (0.0) | 1/5 (20) | 0/1 (0.0) | 2/14 (14.3) | NA |
1 Frequencies calculated over the total of HIV+ children only. 42 of the 90 children had an HIV test result. HIV: Human immunodeficiency virus; LSD: Less severe diarrhoea; MSD: Moderate-to-severe diarrhoea. NA: Not applicable.
In matched controls without diarrhoea (non-cases), C. hominis was also the predominant species found (75.0%, 45/60), followed by C. parvum (20.0%, 12/60) and C. meleagridis (5.0%, 3/60) (Table S2). Within C. hominis, half of the infections were attributed to subtype families Ia (26.7%, 16/60) and Ib (21.7%, 13/60). No obvious differences in subtype distribution were observed among the age groups considered. No Cryptosporidium subtype families could be determined in HIV+ patients without diarrhoea (Table S2).
Cryptosporidium hominis subtype families Ib and Ie were more frequently found during study years 1 to 4, whereas C. hominis subtype family Ia and C. parvum subtype family IIc were observed only in study year 5, suggesting variable seasonal patterns in the frequency of Cryptosporidium subtypes (Figure S2).
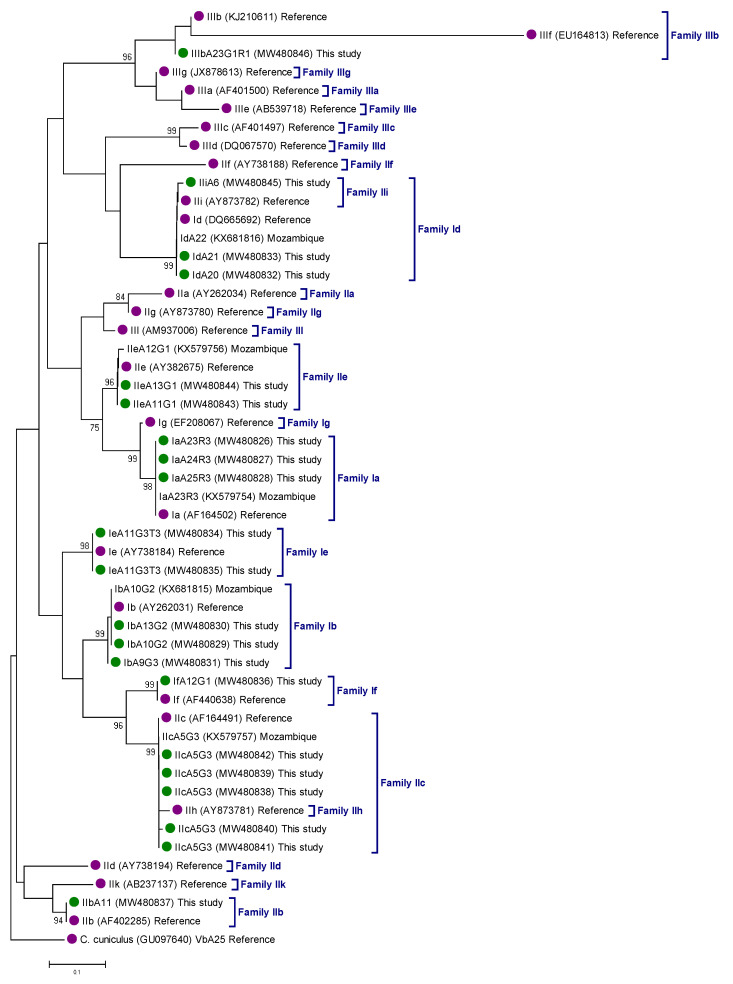
The genetic relationships among gp60 gene sequences generated in the present study, as inferred by a neighbor-joining analysis, are shown in Figure 2. All Cryptosporidium sequences clustered together (monophyletic groups) with different well-supported clades (≥93% of bootstrap) corresponding to appropriate reference sequences for Cryptosporidium subtype families.
Figure 2.
Phylogenetic relationships among Cryptosporidium hominis (family I), C. parvum (family II), and C. meleagridis (family III) genotypes identified in children under 5 years of age recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012. The analysis was conducted by a neighbor-joining method of the gp60 gene. Genetic distances were calculated using the Kimura two-parameter model. Green filled dots represent sequences generated in the present study. Purple filled dots represent reference sequences. Bootstrap values lower than 75% are not displayed. Cryptosporidium cuniculus was used as outgroup taxon to root the tree.
3. Discussion
This is the most comprehensive molecular epidemiological study conducted in Mozambique to date investigating the genetic diversity of the diarrhoea-causing enteric protozoan parasite Cryptosporidium spp. The analysis took advantage of the large sample repository generated by the GEMS in children younger than five years of age with and without diarrhoea in Maputo province [4,5]. Consequently, a total of 392 stool samples with a positive result by ELISA were available for molecular investigations, of which 190 were successfully genotyped at the gp60 or ssu rRNA loci.
A preliminary assessment of the Cryptosporidium-positive samples by ELISA corroborated results obtained in previous epidemiological studies carried out in sub-Saharan African countries [11]. For instance, cryptosporidiosis was confirmed as a serious public health concern in children younger than 2 years old, particularly if immunocompromised by other infections (e.g., HIV/AIDS) or malnourished. Young children are more susceptible to intestinal parasites and other infectious pathogens due to their low level of immunity [23]. The level of exposure and risk of infections increase in poor settings with limited access to safe drinking water, sanitation, and hygiene [24,25]. A significant association between Cryptosporidium infection and malnutrition (stunting, wasting, underweight) has been documented in children from several African countries, including Kenya [26], Mozambique [27], Tanzania [28], and Uganda [29], among others. Similarly, Cryptosporidium infection was more frequently found in HIV-positive than in HIV-negative children and patients in Mozambique [20,30] and other African endemic regions [28,31,32].
Our molecular analyses revealed the presence of three Cryptosporidium species (C. hominis, C. parvum, and C. meleagridis) in the studied paediatric population. Mostly anthroponotic C. hominis and zoonotic C. parvum were previously known to be circulating in Mozambique [19,20,33], but this is the first report of C. meleagridis in the country. An additional two species, C. felis and C. viatorum, have also been recently described in adult patients with diarrhoea in the Maputo province and in asymptomatic children in the Zambézia province [19,34]. Cryptosporidium meleagridis and C. felis are adapted to infect birds and domestic cats, respectively, as primary host species, but they are also responsible for a significant number of human infections globally, particularly the former [10,12]. These data seem to indicate that direct contact with cats, poultry, and other avian species (or their faecal material) may be a risk factor for human cryptosporidiosis in Mozambique. Following the same line of reasoning, the fact that all the C. parvum isolates characterised at the ssu rRNA gene belonged to the “bovine genotype” of C. parvum supported the notion that an unknown number of human cases of cryptosporidiosis are indeed of zoonotic nature. This is without precluding that some of the infections caused by this genetic variant of C. parvum may be also transmitted through person-to-person contact. The extent of the exact contribution of each potential transmission pathway (zoonotic, anthropic, direct contact, or indirect through ingestion of contaminated water or food) remains to be elucidated. Finally, C. viatorum was initially thought to be a human-adapted species [35], but recent epidemiological surveys conducted in Australia and China have demonstrated that this Cryptosporidium species can successfully infect rodents and therefore may have zoonotic potential [36,37]. Overall, these data agree with those reported in the African continent, where C. hominis was the most prevalent (2–100%) Cryptosporidium species in humans, followed by C. parvum (3–100%) and C. meleagridis (up to 75%), the latter species found mainly in immunocompromised individuals [11].
Subtyping analyses identified Ia and Ie as the most prevalent subtype families within C. hominis, being responsible for nearly 70% of the infections attributable to this Cryptosporidium species in both diarrhoeal and non-diarrhoeal children. Similar results have been reported in Kenyan young children with and without HIV infection [38], mostly HIV-positive patients in Nigeria [39], children younger than 10 years in São Tomé and Príncipe [40], and children younger than five years in South Africa [41]. As already described in most previous epidemiological studies conducted in the continent, subtype family Ib was also underrepresented in the paediatric population surveyed here. Indeed, Ib has been shown as the predominant subtype family only in Nigerian children [42]. Subtype families If and Id are typically documented at low frequencies in African human populations. Subtype family If has been identified in Kenyan patients with and without HIV/AIDS [38,43], children of young age in South Africa [41], and individuals from rural areas in Tanzania [44]. Finally, members of the subtype family Id have been found circulating in HIV-positive patients in Ethiopia, Equatorial Guinea, and Mozambique [20,45,46], in children with diarrhoea in Ghana and Madagascar [47,48], in Kenyan children with and without HIV infection [38], and in paediatric populations from Nigeria and South Africa [41,42].
Mainly transmitted anthroponotically, IIc was the predominant (86%) C. parvum subtype family circulating in the children investigated here. This is in agreement with previous results obtained in diverse human populations from other sub-Saharan countries including Kenya [38], Madagascar [48], Nigeria [42], South Africa [41], and Uganda [49]. In contrast, subtype families IIb, IIe, and IIi were only sporadically detected, and subtype family IIa was absent. It should be noted that IIa was the most prevalent C. parvum subtype family circulating in HIV-positive and diarrhoeal disease patients in Ethiopia and Kenya [43,45,50] and also in Tunisian young children [51]. Taken together, these geographically segregated patterns of C. parvum genetic variants may be indicative of differences in sources of infections and transmission pathways.
Very limited information is currently available on the intra-species molecular diversity of C. meleagridis in African isolates of human origin. In the present study, all the C. meleagridis isolates identified belonged to the subtype family IIIb, and no genetic heterogeneity was observed among their sequences. This very same subtype family has also been reported in an urban population in Tunisia [52], whereas IIId has been described in diarrhoeic paediatric patients in South Africa [41]. Interestingly, a wide range of C. meleagridis subtype families including IIIb (but not IIId) has been recently identified in river water and its sediment in South Africa [53]. This finding has important public health implications, as it demonstrated that the consumption of contaminated, non-treated surface waters might lead to waterborne cryptosporidiosis by C. meleagridis.
The main strength of this study is the large number of Cryptosporidium-positive samples of human origin molecularly characterised by Sanger sequencing. However, certain methodological and study design issues may have hampered its accuracy. For instance, the fact that only half (48.6%, 190/392) of the ELISA-positive samples were amplified at the gp60 or ssu rRNA loci may have biased the actual proportion of Cryptosporidium species and genotypes reported here. This may be due to the suboptimal preservation of parasitic DNA through time (stool samples were collected during the period 2007–2012), or to potential false-positive results in the ELISA immunoassay, or to amplification failure associated to suboptimal removal of PCR inhibitors (e.g., proteases, DNases, polysaccharides, bile salts). We cannot completely rule out the possibility that the ELISA immunoassay initially used for screening purposes yielded an unknown number of false-negative results, particularly for Cryptosporidium species less frequently found in humans (e.g., C. felis, C. viatorum, C. ubiquitum, among others). Additionally, no attempts were carried out to analyse in depth the potential associations between Cryptosporidium species/genotypes and the sociodemographic, epidemiological, and clinical features of the participating children, as this task will be specifically tackled in an independent study.
Overall, the high level of genetic diversity observed within Cryptosporidium isolates reveals an epidemiological scenario where infection and re-infection events seem common and environmental contamination high. In this regard, a recent risk association study conducted in the province of Zambézia revealed that drinking untreated water and having regular contact with domestic animals were major risks for acquiring protist infections including cryptosporidiosis [25]. Additionally, a recent quantitative microbial risk assessment analysis has estimated that the consumption of unsafe water causes 2 million cryptosporidiosis cases and 1.6 × 105 disability-adjusted life years in Mozambique annually [54]. These results highlight the relevance of improving access to safe drinking water and sanitary conditions to minimise the risk of environmental contamination and the waterborne and foodborne transmission of diarrhoea-causing enteric pathogens.
4. Materials and Methods
4.1. Study Context
In Mozambique, the GEMS was conducted by the Centro de Investigação em Saúde de Manhiça (CISM), in six health facilities in the Manhiça District [55], which is located approximately 80 km north of the capital city Maputo in the country’s Southern region. The district covers 2380 km2 and has a subtropical climate with two distinct seasons: a warm, rainy season from November to April, and the cool and dry season during the rest of the year [17,56]. Since 1996, CISM has been conducting a continuous Health and Demographic Surveillance System (HDSS) with regular update of demographic events for all surveyed population (current population followed: 203,132 uniquely identified individuals; 46,851 enumerated and geo-positioned households; 27,504 are children under 5 years). During the study period, the HDSS was covering approximately 95,000 inhabitants [17].
The rationale, study design, and methodology of the GEMS have been previously described elsewhere [57]; the study comprised three years of recruitment of acute moderate-to-severe diarrhoea cases (MSD, GEMS1) and one additional year including less-severe diarrhoea (LSD, GEMS1A) [4,5]. In Manhiça, the GEMS collected samples uninterruptedly over 5 years, from December 2007 to October 2011, and GEMS1A from November 2011 to November 2012. The standardised epidemiological and clinical methods for the case-control study as well as the full definitions have been previously described elsewhere [18,55]. Briefly, all children aged 0–59 months (stratified in three age groups: 0–11 months, 12–23 months, and 24–59 months), presenting in the six sentinel health facilities with diarrhoea meeting inclusion criteria for the study were invited to participate. Community controls (up to three for MSD cases and one for LSD cases) matched to the index case by age, sex, and neighbourhood were identified using the HDSS databases and enrolled within 14 days after enrolment of the case, and the stool samples were collected and sent to the laboratory at CISM [58].
4.2. Stool Collection and Initial Testing
The stool samples collection and processing protocols were also standardised across GEMS sites as described elsewhere [55,58]. Samples were collected in sterile flasks and placed in a refrigerator or in a cool-box with a cooler block (2–8 °C) for up to 6 h until transported to the laboratory. Sample aliquots without preservatives were frozen at −80 °C for further testing. The CRYPTOSPORIDIUM II™ ELISA immunoassay (TECHLAB®, Blacksburg, VA, USA) was used as screening method for the specific detection of Cryptosporidium spp. following the manufacturer’s instructions.
4.3. Molecular Study
4.3.1. DNA Extraction and Purification
Molecular analyses were performed only on stool samples that were Cryptosporidium-positive by immunoassay. Genomic DNA was isolated from about 200 mg of faecal material by using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, except that samples mixed with ASL lysis buffer were incubated for 10 min at 95 °C. Resulting eluates (200 μL in PCR-grade water) were stored at −20 °C and shipped to the Spanish National Centre for Microbiology at Majadahonda (Spain) for downstream molecular analysis.
4.3.2. Molecular Detection and Characterisation of Cryptosporidium spp.
As this study was based on Cryptosporidium-positive samples by ELISA, to optimise time and resources the following diagnostic and genotyping algorithm was implemented. A nested PCR protocol was initially used to amplify an 870-bp fragment of the gp60 gene of the parasite as previously described [59]. This approach allowed for the differential diagnosis of C. hominis and C. parvum (the two Cryptosporidium species more prevalent in humans), and for the identification of subtype families within these two species. The outer primers were AL-3531_F (5’-ATAGTCTCCGCTGTATTC-3’) and AL-3535_R (5’-GGAAGGAACGATGTATCT-3’), and the inner primers were AL-3532_F (5’-TCCGCTGTATTCTCAGCC-3’) and AL-3534_R (5’-GCAGAGGAACCAGCATC-3’). Reaction mixtures (50 µL) contained 200 nM of each primer and 2–3 μL of template DNA. Cycling conditions included one step of 94 °C for 5 min, followed by 35 cycles of amplification (denaturation at 94 °C for 45 s, annealing at 59 °C for 45 s, and elongation at 72 °C for 1 min), concluding with a final extension of 72 °C for 10 min. The same conditions were used in the secondary reaction, except that the annealing temperature was 50 °C.
Samples with a negative result by gp60-PCR were re-analysed by a nested PCR to amplify a 587-bp fragment of the ssu rRNA gene of the parasite [60]. This approach allowed for the detection of low burdens of Cryptosporidium infections and for the identification of Cryptosporidium species other than C. hominis or C. parvum. The outer primers were CR-P1 (5’-CAGGGAGGTAGTGACAAGAA-3’) and CR-P2 (5’-TCAGCCTTGCGACCATACTC-3’), and the inner primers were CR-P3 (5’-ATTGGAGGGCAAGTCTGGTG-3’) and CPB-DIAGR (5’-TAAGGTGCTGAAGGAGTAAGG-3’). In all cases, reaction mixtures (50 µL) contained 300 nM of each primer and 3 μL of template DNA. Cycling conditions consisted of one step of 94°C for 5 min, followed by 35 cycles of amplification (denaturation at 94 °C for 40 s, annealing at 50 °C for 40 s, and elongation at 72 °C for 1 min), finalising with a final extension at 72 °C for 10 min.
Samples that were identified by ssu-PCR (and Sanger sequencing, see below) as C. meleagridis were re-analysed at the gp60 locus by a nested PCR specifically developed for this Cryptosporidium species [21]. This protocol amplifies a 900 bp fragment of the gp60 gene. The outer primers were CRSout115F (5´-GATGAGATTGTCGCTCGTTATC-3´) and CRSout1328R (5´-AACCTGCGGAACCTGTG-3´), and the inner primers were ATGFmod (5´-GAGATTGTCGCTCGTTATCG-3´) and GATR2 (5´-GATTGCAAAAACGGAAGG-3´). Reaction mixtures (50 µL) contained 250 nM of each primer and 2–3 μL of template DNA. Cycling conditions included one step of 95 °C for 4 min, followed by 35 cycles of amplification (denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 1 min), concluding with a final extension of 72 °C for 7 min. The same conditions were used in the secondary reaction, except that the annealing temperature was 58 °C.
Nested PCR protocols described above were conducted on a 2720 Thermal Cycler (Applied Biosystems, CA, USA). Reaction mixes always included 2.5 units of MyTAQTM DNA polymerase (Bioline GmbH, Luckenwalde, Germany), and 5× MyTAQTM Reaction Buffer containing 5 mM dNTPs and 15 mM MgCl2. Laboratory-confirmed positive and negative DNA samples of human origin were routinely used as controls and included in each round of PCR. PCR amplicons were visualised on 2% D5 agarose gels (Conda, Madrid, Spain) stained with Pronasafe nucleic acid staining solution (Conda) and recorded using the MiniBIS Pro system controlled by GelCapture version 7.5.2 software (DNR Bio-Imaging Systems, Jerusalem, Israel). A 100 bp DNA ladder (Boehringer Mannheim GmbH, Baden-Wurttemberg, Germany) was used for the sizing of obtained amplicons. Positive-PCR products were directly sequenced in both directions using the internal primer sets described above. DNA sequencing was conducted by capillary electrophoresis using the BigDye® Terminator chemistry (Applied Biosystems) on an on ABI PRISM 3130 automated DNA sequencer at the Core Genomic Facility of the Spanish National Centre for Microbiology, Majadahonda (Spain). Sequencing reactions were repeated on samples for which genotyping was unsuccessful in the first instance.
The Cryptosporidium sequences obtained in this study have been deposited in GenBank under accession numbers MW480826–MW480846 (gp60 locus) and MW487256–MW487266 (ssu rRNA locus).
4.4. Data Analysis
4.4.1. Epidemiological Analysis
PCR data were entered in a Microsoft Excel spreadsheet (Redmond, WA, USA) and then checked for accuracy and consistency by independent laboratory personnel. Clinical and demographic data were extracted from the original GEMS dataset. Cryptosporidium spp., study groups (diarrhoeal vs. non-diarrhoeal, MSD vs. LSD) age groups, and study years were treated as categorical variables. Differences in frequencies were compared using Chi-squared test or Fisher’s exact test as appropriate. A p-value < 0.05 was considered statistically significant. Missing values were excluded from the analyses; thus, denominators for some comparisons may differ. Data analyses were performed in Stata version 14 (StataCorp LP, College Station, TX, USA).
4.4.2. Sequence and Phylogenetic Analysis
Raw sequencing data in both forward and reverse directions were visually inspected using the Chromas Lite version 2.1 sequence analysis program [61]. Special attention was paid to the detection and recording of ambiguous (double peak) positions. The BLAST tool was used to search for identity among sequences deposited in the National Center for Biotechnology Information (NCBI) public repository database [62]. Multiple sequence alignment analyses with appropriate reference sequences were conducted using MEGA 6 to identify Cryptosporidium species and to annotate the presence of single nucleotide polymorphisms (SNPs) [63]. Cryptosporidium hominis and C. parvum subtypes were assigned according to the number of TCA (A), TCG (G), ACATCA/ACATCG (R), and TCTT (T) fragment repeats in the microsatellite region of the gp60 gene, in accordance with the established nomenclature, as previously described [14].
The evolutionary relationships among the identified Cryptosporidium species and subtypes were inferred by a phylogenetic analysis using the neighbor-joining method in MEGA 6 [64]. Only sequences with unambiguous (no double peak) positions were used in the analyses. The evolutionary distances were computed using the Kimura 2-parameter method and modelled with a gamma distribution. The reliability of the phylogenetic analyses at each branch node was estimated by the bootstrap method using 1000 replications. Representative sequences of different Cryptosporidium species and subtypes were retrieved from the NCBI database and included in the phylogenetic analysis for reference and comparative purposes.
5. Conclusions
This study provides the most comprehensive description of the molecular diversity of the enteric protozoan parasite Cryptosporidium spp. in Mozambique to date. Our findings revealed the circulation of at least three Cryptosporidium species in young Mozambican children primarily affected with diarrhoea. A high intra-species genetic variability was observed within C. hominis (subtype families Ia, Ib, Id, Ie, and If) and C. parvum (subtype families IIb, IIc, IIe, and IIi), but not within C. meleagridis (subtype family IIIb). No associations between Cryptosporidium species/genetic variants and age-related patterns could be demonstrated. The predominance of mainly anthroponotically transmitted C. hominis and C. parvum IIc strongly suggests that most of the Cryptosporidium infections detected in the surveyed paediatric population are of human origin. However, a significant proportion of the infections were caused by host-adapted Cryptosporidium species (e.g., C. meleagridis) or genetic variants (e.g., C. parvum “bovine genotype”) suggesting the occurrence of zoonotic transmission events at an unknown rate. Further molecular epidemiological studies are warranted to assess the actual contribution of livestock, poultry, and other domestic animal species to the environmental (including surface waters intended for human consumption and soils) burden of Cryptosporidium oocysts in Mozambique and other African endemic areas.
Acknowledgments
We thank the children and their caretakers who participated in the study, as well as the clinical, field, and laboratory staff who worked tirelessly to ensure the data collection and laboratory testing was performed according to the standardized protocol. We also thank all the local government authorities (district Administration and Health Directorate) and all community leaders for supporting and collaborating in the study. CISM is supported by the Government of Mozambique and the Spanish Agency for International Development Cooperation (AECID). ISGlobal receives support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10040452/s1, Figure S1: Seasonal distribution and temporal clustering of Cryptosporidium species in children under 5 years of age, with and without diarrhoea, recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012. Figure S2: Seasonal distribution and temporal clustering of Cryptosporidium subtype families in children under 5 years of age, with and without diarrhoea, recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012. Table S1: PCR and sequencing data. Table S2: Diversity and frequency of Cryptosporidium family subtypes within C. hominis (subtype family I), C. parvum (subtype family II) and C. meleagridis (subtype family III) in asymptomatic (non-cases) children under 5 years of age according to severity of clinical manifestations, age group, and HIV coinfection. Children were recruited during the Global Enteric Multicenter Study at the Manhiça district (Maputo, southern Mozambique), 2007–2012. Figures between brackets represent relative frequencies.
Author Contributions
Conceptualisation, A.M.J., K.K., M.M.L., P.L.A., D.C. and I.M.; methodology, K.K., M.M.L., P.L.A., D.C. and I.M.; software, A.M.J. and P.C.K.; validation, D.C. and I.M.; formal analysis, A.M.J., P.C.K., M.G., D.C. and I.M.; investigation, A.M.J., P.C.K., M.G., T.N., S.M., A.C. and Q.B.; resources, K.K., M.M.L., P.L.A., D.C. and I.M.; data curation, D.C. and I.M.; writing—original draft preparation, A.M.J., D.C. and I.M.; writing—review and editing, A.M.J., P.C.K., M.G., T.N., Q.B., A.C., K.K., M.M.L., P.L.A., D.C. and I.M.; visualisation, A.M.J., K.K., M.M.L., P.L.A., D.C. and I.M.; supervision, D.C. and I.M.; project administration, D.C. and I.M.; funding acquisition, K.K., M.M.L., P.L.A., D.C. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bill and Melinda Gates Foundation through the Center for Vaccine Development at the University of Maryland, School of Medicine who coordinated GEMS, grant number 38874 (GEMS) and OPP1033572 (GEMS1A). Additional funding was obtained from the Health Institute Carlos III (ISCIII), Ministry of Economy and Competitiveness (Spain), grant number PI16CIII/00024, from the Fundo Nacional de Investigacão, Ministry of Science and Technology (Mozambique), grant number 245-INV, and from the USAID Country Office of Mozambique, grant number AID-656-F-16-00002.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Review Board of the Mozambican National Bioethics Committee for Health, Mozambique (Ref. 11/CNBS/07), the Ethics Committee of the Hospital Clinic of Barcelona, Spain (Ref. 2006/3260) and the Institutional Review Board for Human Subject Research at University of Maryland Baltimore, USA.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the paper and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Child Mortality Collaborators Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1725–1774. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD Diarrhoeal Diseases Collaborators Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017;17:909–948. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 5.Kotloff K.L., Nasrin D., Blackwelder W.C., Wu Y., Farag T., Panchalingham S., Sow S.O., Sur D., Zaidi A.K.M., Faruque A.S.G., et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS) Lancet Glob. Health. 2019;7:e568–e584. doi: 10.1016/S2214-109X(19)30076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner K.L., Ahmed S., Gilchrist C.A., Burkey C., Cook H., Ma J.Z., Korpe P.S., Ahmed E., Alam M., Kabir M., et al. Species of Cryptosporidia causing subclinical infection associated with growth faltering in rural and urban Bangladesh: A birth cohort study. Clin. Infect. Dis. 2018;67:1347–1355. doi: 10.1093/cid/ciy310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platts-Mills J.A., Babji S., Bodhidatta L., Gratz J., Haque R., Havt A., McCormick B.J., McGrath M., Olortegui M.P., Samie A., et al. MAL-ED Network Investigators. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED) Lancet Glob. Health. 2015;3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouzid M., Hunter P.R., Chalmers R.M., Tyler K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013;26:115–134. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehmadpour E., Safarpour H., Xiao L., Zarean M., Hatam-Nahavandi K., Barac A., Picot S., Rahimi M.T., Rubino S., Mahami-Oskouei M., et al. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite. 2020;27:27. doi: 10.1051/parasite/2020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Squire S.A., Ryan U. Cryptosporidium and Giardia in Africa: Current and future challenges. Parasit. Vectors. 2017;10:195. doi: 10.1186/s13071-017-2111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan U., Fayer R., Xiao L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology. 2014;141:1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y., Ryan U., Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Strong W.B., Gut J., Nelson R.G. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 2000;68:4117–4134. doi: 10.1128/IAI.68.7.4117-4134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dgedge M., Novoa A., Macassa G., Sacarlal J., Black J., Michaud C., Cliff J. The burden of disease in Maputo City, Mozambique: Registered and autopsied deaths in 1994. Bull. World Health Organ. 2001;79:546–552. [PMC free article] [PubMed] [Google Scholar]
- 16.Sacarlal J., Nhacolo A.Q., Sigaúque B., Nhalungo D.A., Abacassamo F., Sacoor C.N., Aide P., Machevo S., Nhampossa T., Macete E.V., et al. A 10 year study of the cause of death in children under 15 years in Manhiça, Mozambique. BMC Public Health. 2009;9:67. doi: 10.1186/1471-2458-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacoor C., Nhacolo A., Nhalungo D., Aponte J.J., Bassat Q., Augusto O., Mandomando I., Sacarlal J., Lauchande N., Sigaúque B., et al. Profile: Manhiça Health Research Centre (Manhiça HDSS) Int. J. Epidemiol. 2013;42:1309–1318. doi: 10.1093/ije/dyt148. [DOI] [PubMed] [Google Scholar]
- 18.Nhampossa T., Mandomando I., Acacio S., Quintó L., Vubil D., Ruiz J., Nhalungo D., Sacoor C., Nhabanga A., Nhacolo A., et al. Diarrheal disease in rural Mozambique: Burden, risk factors and etiology of diarrheal disease among children aged 0–59 months seeking care at health facilities. PLoS ONE. 2015;10:e0119824. doi: 10.1371/journal.pone.0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casmo V., Lebbad M., Maungate S., Lindh J. Occurrence of Cryptosporidium spp. and Cystoisospora belli among adult patients with diarrhoea in Maputo, Mozambique. Heliyon. 2018;4:1–13. doi: 10.1016/j.heliyon.2018.e00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irisarri-Gutiérrez M.J., Mingo M.H., de Lucio A., Gil H., Morales L., Seguí R., Nacarapa E., Muñoz-Antolí C., Bornay-Llinares F.J., Esteban J.G., et al. Association between enteric protozoan parasites and gastrointestinal illness among HIV- and tuberculosis-infected individuals in the Chowke district, southern Mozambique. Acta Trop. 2017;170:197–203. doi: 10.1016/j.actatropica.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Stensvold C.R., Beser J., Axén C., Lebbad M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J. Clin. Microbiol. 2014;52:2311–2319. doi: 10.1128/JCM.00598-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slapeta J. Cryptosporidium species found in cattle: A proposal for a new species. Trends Parasitol. 2006;22:469–474. doi: 10.1016/j.pt.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Valiathan R., Ashman M., Asthana D. Effects of ageing on the immune system: Infants to elderly. Scand. J. Immunol. 2016;83:255–266. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- 24.Local Burden of Disease Diarrhoea Collaborators Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000–2017: Analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:1779–1801. doi: 10.1016/S0140-6736(20)30114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muadica A.S., Balasegaram S., Beebeejaun K., Köster P.C., Bailo B., Hernández-de-Mingo M., Dashti A., Dacal E., Saugar J.M., Fuentes I., et al. Risk associations for intestinal parasites in symptomatic and asymptomatic schoolchildren in central Mozambique. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.05.031. in press. [DOI] [PubMed] [Google Scholar]
- 26.Delahoy M.J., Omore R., Ayers T.L., Schilling K.A., Blackstock A.J., Ochieng J.B., Moke F., Jaron P., Awuor A., Okonji C., et al. Clinical, environmental, and behavioral characteristics associated with Cryptosporidium infection among children with moderate-to-severe diarrhea in rural western Kenya, 2008-2012: The Global Enteric Multicenter Study (GEMS) PLoS Negl. Trop. Dis. 2018;12:e0006640. doi: 10.1371/journal.pntd.0006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acácio S., Mandomando I., Nhampossa T., Quintó L., Vubil D., Sacoor C., Kotloff K., Farag T., Nasrin D., Macete E., et al. Risk factors for death among children 0–59 months of age with moderate-to-severe diarrhea in Manhiça district, southern Mozambique. BMC Infect. Dis. 2019;19:322. doi: 10.1186/s12879-019-3948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tellevik M.G., Moyo S.J., Blomberg B., Hjøllo T., Maselle S.Y., Langeland N., Hanevik K. Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among young children with and without diarrhea in Dar es Salaam, Tanzania. PLoS Negl. Trop. Dis. 2015;9:e0004125. doi: 10.1371/journal.pntd.0004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumwine J.K., Kekitiinwa A., Nabukeera N., Akiyoshi D.E., Rich S.M., Widmer G., Feng X., Tzipori S. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am. J. Trop. Med. Hyg. 2003;68:710–715. doi: 10.4269/ajtmh.2003.68.710. [DOI] [PubMed] [Google Scholar]
- 30.Acácio S., Nhampossa T., Quintó L., Vubil D., Sacoor C., Kotloff K., Farag T., Dilruba N., Macete E., Levine M.M., et al. The role of HIV infection in the etiology and epidemiology of diarrheal disease among children aged 0–59 months in Manhiça District, Rural Mozambique. Int. J. Infect. Dis. 2018;73:10–17. doi: 10.1016/j.ijid.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlinac P.B., John-Stewart G.C., Naulikha J.M., Onchiri F.M., Denno D.M., Odundo E.A., Singa B.O., Richardson B.A., Walson J.L. High-risk enteric pathogens associated with HIV infection and HIV exposure in Kenyan children with acute diarrhoea. AIDS. 2014;28:2287–2296. doi: 10.1097/QAD.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mengist H.M., Taye B., Tsegaye A. Intestinal parasitosis in relation to CD4+T cells levels and anemia among HAART initiated and HAART naive pediatric HIV patients in a Model ART center in Addis Ababa, Ethiopia. PLoS ONE. 2015;10:e0117715. doi: 10.1371/journal.pone.0117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sow S.O., Muhsen K., Nasrin D., Blackwelder W.C., Wu Y., Farag T.H., Panchalingam S., Sur D., Zaidi A.K., Faruque A.S., et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS) PLoS Negl. Trop. Dis. 2016;10:e0004729. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muadica A.S., Köster P.C., Dashti A., Bailo B., Hernández-de-Mingo M., Balasegaram S., Carmena D. Molecular diversity of Giardia duodenalis, Cryptosporidium spp., and Blastocystis sp. in symptomatic and asymptomatic school children in Zambézia province (Mozambique) Pathogens. 2021;10:255. doi: 10.3390/pathogens10030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elwin K., Hadfield S.J., Robinson G., Crouch N.D., Chalmers R.M. Cryptosporidium viatorum n. sp. (Apicomplexa: Cryptosporidiidae) among travellers returning to Great Britain from the Indian subcontinent, 2007–2011. Int. J. Parasitol. 2012;42:675–682. doi: 10.1016/j.ijpara.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Koehler A.V., Wang T., Haydon S.R., Gasser R.B. Cryptosporidium viatorum from the native Australian swamp rat Rattus lutreolus—An emerging zoonotic pathogen? Int. J. Parasitol. Parasites Wildl. 2018;7:18–26. doi: 10.1016/j.ijppaw.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao W., Zhou H., Huang Y., Xu L., Rao L., Wang S., Wang W., Yi Y., Zhou X., Wu Y., et al. Cryptosporidium spp. in wild rats (Rattus spp.) from the Hainan Province, China: Molecular detection, species/genotype identification and implications for public health. Int. J. Parasitol. Parasites Wildl. 2019;9:317–321. doi: 10.1016/j.ijppaw.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mbae C., Mulinge E., Waruru A., Ngugi B., Wainaina J., Kariuki S. Genetic diversity of Cryptosporidium in children in an urban informal settlement of Nairobi, Kenya. PLoS ONE. 2015;10:e0142055. doi: 10.1371/journal.pone.0142055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinbo F.O., Okaka C.E., Omoregie R., Dearen T., Leon E.T., Xiao L. Molecular characterization of Cryptosporidium spp. in HIV-infected persons in Benin City, Edo State, Nigeria. Fooyin J. Health Sci. 2010;2:85–89. doi: 10.1016/S1877-8607(11)60003-9. [DOI] [Google Scholar]
- 40.Lobo M.L., Augusto J., Antunes F., Ceita J., Xiao L.H., Codices V., Matos O. Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and other intestinal parasites in young children in Lobata province, Democratic Republic of São Tomé and Príncipe. PLoS ONE. 2014;9:e97708. doi: 10.1371/journal.pone.0097708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu Samra N., Thompson P.N., Jori F., Frean J., Poonsamy B., du Plessis D., Mogoye B., Xiao L. Genetic characterization of Cryptosporidium spp. in diarrhoeic children from four provinces in South Africa. Zoonoses Public Health. 2013;60:154–159. doi: 10.1111/j.1863-2378.2012.01507.x. [DOI] [PubMed] [Google Scholar]
- 42.Molloy S.F., Smith H.V., Kirwan P., Nichols R.A., Asaolu S.O., Connelly L., Holland C.V. Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am. J. Trop. Med. Hyg. 2010;82:608–613. doi: 10.4269/ajtmh.2010.09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanyiri J.W., Kanyi H., Maina S., Wang D.E., Steen A., Ngugi P., Kamau T., Waithera T., O’Connor R., Gachuhi K., et al. Cryptosporidiosis in HIV/AIDS patients in Kenya: Clinical features, epidemiology, molecular characterization and antibody responses. Am. J. Trop. Med. Hyg. 2014;91:319–328. doi: 10.4269/ajtmh.13-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons M.B., Travis D., Lonsdorf E.V., Lipende I., Roellig D.M., Collins A., Kamenya S., Zhang H., Xiao L., Gillespie T.R. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015;9:e0003529. doi: 10.1371/journal.pntd.0003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamu H., Petros B., Zhang G., Kassa H., Amer S., Ye J., Feng Y., Xiao L. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Negl. Trop. Dis. 2014;8:e2831. doi: 10.1371/journal.pntd.0002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanco M.A., Montoya A., Iborra A., Fuentes I. Identification of Cryptosporidium subtype isolates from HIV-seropositive patients in Equatorial Guinea. Trans. R. Soc. Trop. Med. Hyg. 2014;108:594–596. doi: 10.1093/trstmh/tru108. [DOI] [PubMed] [Google Scholar]
- 47.Eibach D., Krumkamp R., Al-Emran H.M., Sarpong N., Hagen R.M., Adu-Sarkodie Y., Tannich E., May J. Molecular characterization of Cryptosporidium spp. among children in rural Ghana. PLoS Negl. Trop. Dis. 2015;9:e0003551. doi: 10.1371/journal.pntd.0003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Areeshi M., Dove W., Papaventsis D., Gatei W., Combe P., Grosjean P., Leatherbarrow H., Hart C.A. Cryptosporidium species causing acute diarrhoea in children in Antananarivo, Madagascar. Ann. Trop. Med. Parasitol. 2008;102:309–315. doi: 10.1179/136485908X278793. [DOI] [PubMed] [Google Scholar]
- 49.Akiyoshi D.E., Tumwine J.K., Bakeera-Kitaka S., Tzipori S. Subtype analysis of Cryptosporidium isolates from children in Uganda. J. Parasitol. 2006;92:1097–1100. doi: 10.1645/GE-843R.1. [DOI] [PubMed] [Google Scholar]
- 50.Adamu H., Petros B., Hailu A., Petry F. Molecular characterization of Cryptosporidium isolates from humans in Ethiopia. Acta Trop. 2010;115:77–83. doi: 10.1016/j.actatropica.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Rahmouni I., Essid R., Aoun K., Bouratbine A. Glycoprotein 60 diversity in Cryptosporidium parvum causing human and cattle cryptosporidiosis in the rural region of Northern Tunisia. Am. J. Trop. Med. Hyg. 2014;90:346–350. doi: 10.4269/ajtmh.13-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essid R., Menotti J., Hanen C., Aoun K., Bouratbine A. Genetic diversity of Cryptosporidium isolates from human populations in an urban area of Northern Tunisia. Infect. Genet. Evol. 2018;58:237–242. doi: 10.1016/j.meegid.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Grace Mphephu M., Deogratias Ekwanzala M., Ndombo Benteke Momba M. Cryptosporidium species and subtypes in river water and riverbed sediment using next-generation sequencing. Int. J. Parasitol. 2021 doi: 10.1016/j.ijpara.2020.10.005. in press. [DOI] [PubMed] [Google Scholar]
- 54.Limaheluw J., Medema G., Hofstra N. An exploration of the disease burden due to Cryptosporidium in consumed surface water for sub-Saharan Africa. Int. J. Hyg. Environ. Health. 2019;222:856–863. doi: 10.1016/j.ijheh.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Kotloff K.L., Blackwelder W.C., Nasrin D., Nataro J.P., Farag T.H., van Eijk A., Adegbola R.A., Alonso P.L., Breiman R.F., Faruque A.S., et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: Epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 2012;55(Suppl. 4):S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alonso P.L., Saúte F., Aponte J.J., Gómez-Olivé F.X., Nhacolo A., Thomson R., Macete E., Abacassamo F., Ventura P.J., Bosch X., et al. Population and Health in Developing Countries. Volume 1. International Development Research Centre; Otawa, ON, Canada: 2002. Manhiça DSS, Mozambique; pp. 189–195. [Google Scholar]
- 57.Levine M.M., Kotloff K.L., Nataro J.P., Muhsen K. The Global Enteric Multicenter Study (GEMS): Impetus, rationale, and genesis. Clin. Infect. Dis. 2012;55(Suppl. 4):S215–S224. doi: 10.1093/cid/cis761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panchalingam S., Antonio M., Hossain A., Mandomando I., Ochieng B., Oundo J., Ramamurthy T., Tamboura B., Zaidi A.K., Petri W., et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin. Infect. Dis. 2012;55(Suppl. 4):S294–S302. doi: 10.1093/cid/cis754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feltus D.C., Giddings C.W., Schneck B.L., Monson T., Warshauer D., McEvoy J.M. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J. Clin. Microbiol. 2006;44:4303–4308. doi: 10.1128/JCM.01067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiangtip R., Jongwutiwes S. Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop. Med. Int. Health. 2002;7:357–364. doi: 10.1046/j.1365-3156.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- 61.Chromas Lite 2.1—Software Informer. [(accessed on 8 April 2021)]; Available online: http://chromaslite.software.informer.com/2.1/
- 62.Agarwala R., Barrett T., Beck J., Benson D.A., Bollin C., Bolton E., Bourexis D., Brister J.R., Bryant S.H., Canese K., et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44:D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supplementary Materials.