Abstract
Plant materials used in the production of pig feed are frequently contaminated with mycotoxins. T-2 toxin is a secondary metabolite of selected Fusarium species, and it can exert a harmful influence on living organisms. Most mycotoxins enter the body via the gastrointestinal tract, and they can modulate the gut-associated lymphoid tissue (GALT) function. However, little is known about the influence of low T-2 toxin doses on GALT. Therefore, the aim of this study was to evaluate the effect of T-2 toxin administered at 50% of the lowest-observed-adverse-effect level (LOAEL) on the percentage of CD2+ T cells, CD4+ T helper cells, CD8+ cytotoxic T cells, CD4+CD8+ double-positive T cells, TCRγδ+ cells, CD5+CD8- B1 cells, and CD21+ B2 cells, and the secretion of proinflammatory (IFN-γ, IL-1β, IL-2, IL-12/23p40, IL-17A), anti-inflammatory, and regulatory (IL-4, IL-10, TGF-β) cytokines in the porcine ileal wall. The results of the study revealed that T-2 toxin disrupts the development of tolerance to food antigens by enhancing the secretion of proinflammatory and regulatory cytokines and decreasing the production of anti-inflammatory TGF-β. T-2 toxin triggered the cellular response, which was manifested by an increase in the percentage of CD8+ T cells and a decrease in the percentage of B2 and Tγδ lymphocytes.
Keywords: T-2 toxin, prepubertal gilts, GALT, lymphocytes, cytokine
1. Introduction
Mycotoxins are secondary fungal metabolites that can exert multidirectional adverse effects on living organisms. These substances are derived from main metabolic pathways and play various roles in fungal biology. Secondary metabolism in molds is associated with fungal development. In many cases, the benefit these compounds confer on the organism is unknown [1]. Secondary metabolism is commonly associated with sporulation processes in fungi. Some mycotoxins might function as virulence factors, or their presence could give a competitive edge to the producing organism (defense and weapons against fungivorous predators) or enhance the survivability of spores or induce sporulation and enhance perithecial formation (e.g., zearalenone) [2]. Fungal secondary metabolites are harmful to humans, animals, plants, and microorganisms [3,4] because acute and chronic exposure to these substances can damage the liver and/or kidneys and impair the function of these organs [5,6].
T-2 toxin and its deacylated form, HT-2 toxin, occur in most climatic zones, and together with diacetoxyscirpenol and neosolaniol, they belong to the group of type A trichothecenes (TCHA) [7]. These mycotoxins are produced by fungi of the genera Fusarium, Myrothecium, Spicellum, Stachybotrys, Cephalosporium, and Trichothecium [8], as well as sac fungi of the phylum Ascomycota which infect field-grown corn, oats, barley, wheat, rye, sorghum, and rice [9]. T-2 toxin is accumulated mainly in cereal grain, but due to its resistance to high temperature, the toxin is not completely eliminated during high-temperature processing, and it can contaminate foodstuffs and feedstuffs [5,10].
Prolonged exposure to TCH can decrease appetite and lead to weight loss in humans and animals. Characteristic inflammatory changes, ulcers, and necrotic changes are observed in oral and esophageal mucosa of infected individuals. T-2 toxin exerts numerous adverse effects, including anorexia, vomiting, retarded growth, immunosuppression, and neuroendocrine changes [11,12]. It has been identified as an etiological factor in gastrointestinal disorders such as alimentary toxic aleukia (ATA) and inflammatory bowel disease [13]. Similarly to other TCHA, T-2 toxin inhibits protein synthesis [14] by lowering of DNA and RNA synthesis. T-2 toxin binding to peptidyl transferase enzyme generates disorders in the translation [15]. Like other TCA, T-2 toxin activates mitogen-activated protein kinase (MAPKs) by a mechanism called the “ribotoxic stress response.” This mechanism drives both cytokine gene expression and apoptosis in macrophages [16]. An important nonribosomal effect of T-2 toxin is the intensive fast free radical production. The oxidative stress induces mitochondrial DNA damage, high lipid peroxidation, and disorders in inflammatory pathways and cell signaling [17]. Impact on protein synthesis causes leukopenia, depletes cells in lymphoid organs, and inhibits erythropoiesis in bone marrow and the spleen [18,19]. The immune system is particularly sensitive to T-2 toxin due to its damaging effects on the thymus and spleen [20]. T-2 toxin has cytotoxic effects [21]; it significantly impairs the production of antibodies [22], decreases the lymphocyte proliferative response [23], and disrupts the maturation of dendritic cells [24].
Pigs are considered as the most susceptible species to TCH contamination [25]. Pigs are a particularly interesting object of research on mycotoxins because the porcine diet is rich in cereals which are natural mycotoxin sources. In addition, porcine and human immune systems share many anatomical and physiological similarities [26], and the results of research conducted on pigs can be extrapolated to human subjects. Studies of young animals provide valuable information about mycotoxin effects on the development of immune mechanisms in gut-associated lymphoid tissue (GALT) function.
GALT, the mucosal immune system that regulates the local immune response, is the largest set of immune cells in the body and comprises organized lymphoid tissues to which we can include the Peyer’s patches (PP), isolated lymphoid follicles, and mesenteric lymph nodes (MLN) [27]. PP are covered M cells which sample antigen directly from the lumen and deliver it to antigen-presenting cells [28]. Dendritic cells and macrophages can also directly sample the lumen by extending dendrites through transcellular M cell-specific pores. T cells, B cells, and memory cells are stimulated upon encountering antigens in PP. These cells then pass to the MLN where the immune response is amplified. Activated lymphocytes pass into the blood stream via the thoracic duct and travel to the gut where they carry out their final effector functions (lamina propria). The maturation of B lymphocytes takes place in the PP. The lamina propria (LP) with macrophages, mast cells, dendritic cells, neutrophils, and lymphocytes is generally considered an effector site in the GALT [29]. After immune induction, the LP performs the function as the regulator of immune responses in the intestine [30] and the LP lymphocytes, in addition to effector function, also have a role in immunoregulation [31]. Intraepithelial lymphocytes (IEL) play a similar role. IEL are a heterogeneous subclass of T cells, integrated in the epithelial layer [32] and functionally having immunoregulatory and cytolytic functions.
Most mycotoxins enter the body via the gastrointestinal tract, and they can modulate the mucosal intestinal immune system. Studies indicate that fumonisin FB1 decreases IL-8 expression [33] and thus causes a reduction in the recruitment of inflammatory cells in the intestine in response to an infection [34]. Macrophages, B and T lymphocytes, and NK cells are very sensitive to deoxynivalenol (DON) and growing evidence indicates that the DON can have a marked impact on cytokine secretion, increase cell apoptosis, and suppress the antibody response [35]. Disturbances in synthesis and secretion of cytokines by immune cells located in the Peyer’s patches after exposure to DON are the cause of an increase in IgA production and a decrease in IgG and IgM [35]. In pigs, the weaning period and the accompanying changes in rearing conditions, in particular, the transition from liquid to solid feed, induce considerable changes in GALT function. Weaning and successive stages of growth are characterized by changes in T cells, B cells, monocytes/macrophages, and antigen-presenting cells (APC cells). Cytokines play a critical role in the regulation of immune and inflammatory responses, and they are produced not only by lymphocytes, dendritic cells, and macrophages, but also by cells which, in the classical approach, are not components of the immune system, such as intraepithelial cells (IEC) [36].
Lymphocyte subpopulations differ in their susceptibility to T-2 toxin. Double-positive CD4+CD8+ T cells [37] are B lymphocyte precursors [38] and highly sensitive to T-2 toxin. Intestinal epithelial cells play an important role in the local immune response of the gastrointestinal tract. In vitro studies into IEC-6, Caco-2, and HT-28 cell lines demonstrated that intestinal epithelial cells secrete chemokines which promote communication between the epithelium, leukocytes, and the adjacent mucosal cells. According to research, T-2 toxin enhances the expression of IL-12 and TNF-α and decreases the expression of IL-1β in peritoneal macrophages and T cells in lymph nodes [39]. Previous observations also revealed that T-2 toxin increases IL-2 expression and decreases the expression of IFN-γ in pigs.
Exposure to low doses of many mycotoxins can potentially stimulate or suppress immune functions such as lymphocyte proliferation or cellular and humoral immunity, subject to the administered dose and period of exposure [40]. The no-observed-adverse-effect level (NOAEL) and lowest-observed-adverse-effect level (LOAEL) values for most mycotoxins have been identified. In pigs, the LOAEL of T-2 toxin was determined at 29 µg/kg BW, whereas the NOAEL value could not be identified [11].
The aim of this study was to determine the effect of T-2 toxin administered at 50% of the LOAEL (approx. 14.5 µg/kg BW) on: (i) the percentage of T cells with surface markers CD2+ (T cells), CD4+ (Th helper cells), CD8+ (cytotoxic T cells), CD4+CD8+ (double-positive T cells), and CD5-TCR+ (TCRγδ+ cells); (ii) the percentage of B cells with surface markers CD5+CD8- (B1 cells) and CD21+ (B2 cells); (iii) the secretion of proinflammatory (IFN-γ, IL-1β, IL-2, IL-12/23p40), anti-inflammatory, and regulatory (IL-4, IL-10, TGFβ, IL-17A) cytokines in the porcine ileal wall.
2. Results
2.1. The Effect of T-2 Toxin on the Percentage of Lymphocyte Subpopulations
2.1.1. T Lymphocyte Populations
The flow cytometric analysis was performed using a FACSCalibur flow cytometer and the Cell QuestTM program (BectonDickinson, Franklin Lakes, NJ, USA). A total of 10,000 cells of each sample were acquired. Lymphocytes were gated based on the FSC-area (FSC-H) vs. SSC-high (SSC-H) dot plots, and lymphocyte subpopulations were identified based on the fluorescence intensity of dot plot quadrant statistics. The representing dot plots depicting lymphocyte subpopulations are shown in Figure 1.
Figure 1.
Representing dot plots of lymphocyte subpopulations: (A) included lymphocytes T- CD2+ and lymphocytes B- CD21+; (B) lymphocytes Th- CD4+CD8- and Tc- CD8+CD4- are shown; (C) CD8+CD5-are NK cells; (D) CD5+TCR+ are TCRαβ lymphocytes and CD5- TCR+ are TCRγδ lymphocytes.
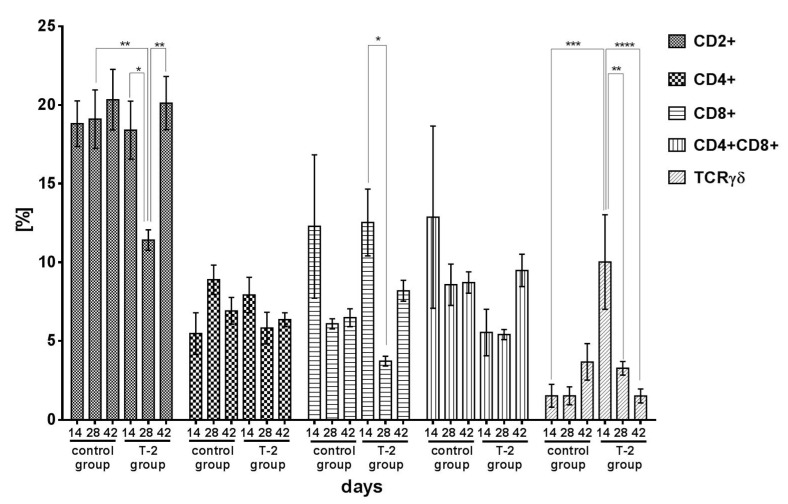
The average percentage of the CD2+ T cells subpopulation in the control increased from 18.90% (day 14) to 20.60% (day 42) during the six-week experiment. In group T-2, a significant decrease in the percentage of CD2+ T cells was observed during the first 28 days of exposure to T-2 toxin. On day 28, the percentage of CD2+ T cells was significantly (p < 0.01) higher in the control group than in group T-2. In group T-2, the percentage of CD2+ T cells was lowest on day 28 (11.40%), and it differed significantly from the values noted on days 14 and 42 (p < 0.05 and p < 0.01, respectively) (Figure 2).
Figure 2.
Cytometric analysis of CD2+, CD4+, CD8+, CD4+CD8+, TCRγδ+ cells isolated from porcine ileal wall in the control group and group T-2 on days 14, 28, and 42 (pg/mg protein; n = 5 each). The results are presented as means ± SEM. Statistically significant differences were determined at * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
The percentage of CD4+ Th helper cells did not change significantly in either group and ranged from 5.60% (day 14) to 8.98% (day 28) in the control group, and from 7.93% (day 14) to 5.82% (day 28) in group T-2 (Figure 2).
The percentage of CD8+ cytotoxic T cells (Tc) tended to decrease in the control group, but the noted differences were not statistically significant. In group T-2, the percentage of CD8+ Tc decreased significantly (p < 0.05) on day 28 relative to day 14. The percentage of CD8+ Tc increased on day 42 (Figure 2).
The percentage of double-positive CD4+CD8+ T cells in the ileal wall tended to decrease in the control group. In group T-2, the percentage of double-positive CD4+CD8+ T cells clearly decreased on days 14 and 28, and it increased rapidly on day 42, but the noted differences were not statistically significant (Figure 2).
In the control group, the percentage of TCRγδ+ lymphocytes remained low throughout the experiment, and it increased from 1.60% on day 14 to 3.65% on day 42. In group T-2, the percentage of TCRγδ+ cells was high in the first 14 days of exposure (10.00%), and it decreased significantly to 3.30% on day 28 (p < 0.01) and to 1.50% on day 42 (p < 0.001) (Figure 2).
2.1.2. B Lymphocyte Populations
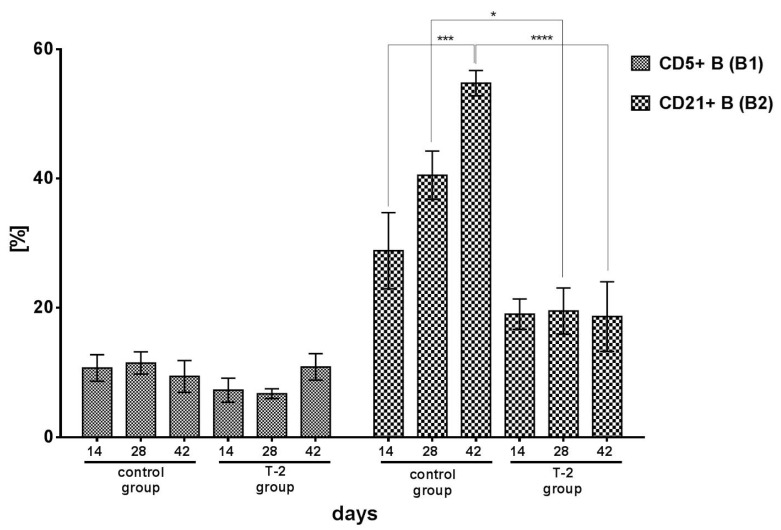
The percentage of CD5+ B (B1) cells did not differ significantly between groups or experimental days. Despite the above, the lowest percentage of CD5+ cells was noted in group T-2 on day 14 (7.28%) and day 28 (6.74%) (Figure 3).
Figure 3.
Cytometric analysis of CD5+ B (B1) cells and B CD21+ (B2) cells isolated from porcine ileal wall in the control group and group T-2 on days 14, 28, and 42 (pg/mg protein; n = 5 each). The results are presented as means ± SEM. Statistically significant differences were determined at * p < 0.05, *** p < 0.001, and **** p < 0.0001.
In the control group, a significant (p < 0.001) increase in the percentage of CD21+ B (B2) cells was noted during the experiment, from 29.40% on day 14 to 55.20% on day 42. In group T-2, the percentage of B2 lymphocytes remained low and stable throughout the experiment at 19.00% on day 14, 19.94% on day 28, and 18.63% on day 42. The exposure to T-2 toxin significantly suppressed the percentage of CD21+ B2 cells. On days 28 and 42, the percentage of B2 lymphocytes in group T-2 was significantly lower than in the control group (p < 0.001 and p < 0.0001, respectively) (Figure 3).
2.2. The Effect of T-2 Toxin on Cytokine Secretion
Proinflammatory Cytokines
The concentration of IFN-γ in the ileal wall of control group and group T-2 animals tended to decrease throughout the experiment. The mean concentration of IFN-γ was determined at 623.50 pg/mg on day 14, and it was significantly higher than on days 28 and 42 (p < 0.05 and p < 0.01, respectively). IFN-γ levels peaked (805.90 pg/mg) in group T-2 after 14 days of exposure. On days 28 and 42, the analyzed parameter decreased significantly to 101.60 pg/mg (p < 0.001) and 318.20 pg/mg (p < 0.05), respectively (Figure 4).
Figure 4.
Proinflammatory cytokines concentrations in the porcine ileal wall in the control group and group T-2 on days 14, 28, and 42 (pg/mg protein; n = 5 each). The results are presented as means ± SEM. Statistically significant differences were determined at * p < 0.05, ** p < 0.01, and *** p < 0.001.
In the control group, the concentration of IL-1β was characterized by a linear decrease from 377.50 pg/mg on day 14 to 231.60 pg/mg on day 42. In contrast, in group T-2, the concentration of IL-1β increased from 275.10 pg/mg on day 14 to 448.60 pg/mg on day 42. On day 42, the analyzed parameter was significantly (p < 0.05) higher in group T-2 than in the control group (Figure 4).
The concentration of IL-2 in the ileal wall of control group animals continued to decrease throughout the experiment, from 12.70 pg/mg on day 14 to 8.30 pg/mg on day 42, but the differences between mean values were not significant. In group T-2, the concentration of IL-2 was significantly (p < 0.05) higher on day 14 than on day 28. The analyzed parameter increased significantly (p < 0.05) on day 42 relative to day 28 (Figure 4).
In the control group, the concentration of IL-12/23p40 in the ileal wall ranged from 229.50 pg/mg (day 28) to 397.85 pg/mg (day 14). In group T-2, the concentration of IL-12/23p40 was lowest on day 28 (231.50 pg/mg), and a significant (p < 0.001) increase was noted on day 42 (584.80 pg/mg). The above increase was also significant (p < 0.01) relative to the value noted in the control group on the last day of the experiment (Figure 4).
In the control group, the mean concentration of IL-17A in the ileal wall ranged from 0.83 pg/mg (day 14) to 1.05 pg/mg (day 28), and it was considerably lower than in group T-2 during the entire experiment. The concentration of IL-17A peaked in group T-2 on day 14 (2.37 pg/mg). In animals exposed to T-2 toxin for 14 and 42 days, IL-17A levels were significantly higher (p < 0.01 and p < 0.05, respectively) than in the control group (Figure 4).
2.3. Anti-Inflammatory and Regulatory Cytokines
In the control group, a minor decrease in the concentration of IL-4 was noted during the experiment, from 72.40 pg/mg on day 14 to 56.30 pg/mg on day 42, but no significant differences were found between mean values. IL-4 levels also decreased in group T-2. On day 14, the mean concentration of IL-4 in the ileal wall of the animals exposed to T-2 toxin was significantly (p < 0.001) higher than in the control group. In group T-2, the concentration of IL-4 decreased significantly (p < 0.0001) on day 28 relative to day 14. On day 42, the analyzed parameter was significantly (p < 0.01) lower than on day 14, but significantly (p < 0.05) higher than on day 28 (Figure 4).
In the control group, the mean concentration of IL-10 in the ileal wall decreased from 17.00 pg/mg on day 14 to 6.50 pg/mg on day 28, and a minor increase to 7.20 pg/mg was observed on day 42. A similar change trend was noted in group T-2, but on day 14, the mean concentration of IL-10 (37.50 pg/mg) was significantly (p < 0.01) higher than in the control group. In group T-2, a minor increase in IL-10 levels was observed between days 28 and 42, but the studied parameter was significantly lower on days 28 and 42 than on day 14 (p < 0.0001 and p < 0.001, respectively) (Figure 5).
Figure 5.
Anti-inflammatory and regulatory cytokines concentrations in the porcine ileal wall in the control group and group T-2 on days 14, 28, and 42 (pg/mg protein; n = 5 each). The results are presented as means ± SEM. Statistically significant differences were determined at * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
In the control group, the mean concentration of TGFβ in the ileal wall ranged from 80.95 pg/mg on day 28 to 104.00 pg/mg on day 42, and it was considerably higher than in the experimental group. After 28 days of exposure to T-2 toxin, TGFβ levels in the experimental group reached 64.69 pg/mg. The concentration of TGFβ was lower in group T-2 than in the control group on all analyzed days of the experiment (p < 0.0001, p < 0.05, p < 0.0001, respectively) (Figure 5).
3. Discussion
A strong immune system is essential for protecting living organisms against pathogens, but the same mechanisms activated in response to dietary proteins or commensal bacteria can lead to the development of chronic diseases. A complex interplay of regulatory mechanisms in the intestinal immune system generally prevents such interactions [27]. Immune responses are initiated in sites such as Peyer’s patches with T and B lymphocytes, dendritic cells, and macrophages that secrete cytokines, creating a unique cellular and cytokine environment. Intestinal epithelial cells also participate in the activation of immune system cells. Two distinct lymphocyte populations are formed in the intestines: intraepithelial lymphocytes (IEL), accounting for 10–30% of the lymphocyte population, which suppress immune responses to antigens and exert cytotoxic effects on virus-infected cells (Tc), and lamina propria lymphocytes (LPL), mostly B cells, plasma cells producing mainly dimeric IgA, and memory cells, accounting for 70–90% of the lymphocyte population.
There is a general scarcity of published research into the effects of low oral doses of T-2 toxin on the intestinal immune system. Therefore, this study evaluated the influence of T-2 toxin administered at 50% of the LOAEL (approximately 14.5 µg/kg BW) [9] on the percentage of T cells with surface markers CD2+ (total percentage of T cells), CD4+ (Th helper cells), CD8+ (cytotoxic T cells), CD4+CD8+ (double-positive T cells), and CD5-TCR+ (TCRγδ+ lymphocytes, intraepithelial lymphocytes), the percentage of B cells with surface markers CD5+CD8- (B1 cells) and CD21+ (B2 cells), and the secretion of proinflammatory (IFN-γ, IL-1β, IL-2, IL-12/23p40), anti-inflammatory, and regulatory cytokines (IL-4, IL-10, TGFβ, IL-17A) in the porcine ileal wall.
The present study was conducted on prepubertal pigs because this animal species is highly sensitive to TCH and is regarded as a model species for biomedical research due to physiological and immunological similarities to humans [41]. Moreover, the anatomy, nutritional requirements, and hypersensitive responses of young pigs are also similar to those observed in children [42], which is yet another important consideration. An attempt was made in this study to determine whether exposure to a low dose of T-2 toxin over different periods of time affects the development of the intestinal immune system, the recognition of potentially beneficial and harmful antigens, and the activation of tolerance to food antigens in prepubertal gilts.
Exposure to T-2 toxin increased the secretion of cytokines, in particular, proinflammatory cytokines, in the ileal wall. The similar effect causes a low level of the deoxynivalenol (DON) member of the TCH type B, which induces mRNAs for TNF-α and IL-6 in macrophages [43]. DON also induces secretion of IL-2, IL-4, IL-6, IL-8, and TNFα by lymphocytes [44]. In our study, the concentration of IFN-γ increased over time and peaked on day 42 (Figure 4). The in vivo effect of FB1 on the production of IFN-γ has also been reported by Taranu et al. [45]. They found increased expression of IFN-γ in the mesenteric lymph node and spleen of pigs. An increase was also noted in the levels of IL-1β, IL-2, IL-12/23p40, and IL-17A. Interestingly, a growing trend was also observed in the concentrations of anti-inflammatory cytokines, including IL-10 and IL-4. What is important is that IL-10 is produced by both Tregs and rTh17 to regulate inflammation [46]. The proinflammatory effects of T-2 toxin were confirmed by a decrease in the secretion of TGFβ (Figure 5), whose concentration continued to decrease on successive days of the experiment relative to the control group. It should be noted that TGFβ has immunosuppressive properties, and low levels of this cytokine induce the Th17 cell response and the secretion of IL-17A [47]. Other authors also reported correlations between the dose of T-2 toxin and other TCH and the concentrations of secreted cytokines. According to Schuhmacher-Wolz et al. [48], unlike high doses of TCH (mg/kg BW), low doses (µg/kg BW) generally stimulate the immune system. Similar effects were noted by Ahmadi and Riazipour [39] in in vitro cultures of mouse peritoneal macrophages and lymph node cells exposed to T-2 toxin doses of 0.001 µg/mL to 100 µg/mL. DON in low concentrations induces expression of early response and the mRNA proinflammatory genes levels [49]. The concentrations of IL-1β, IL-2, IL-4, IL-10, IL-12, TNFα, and IFN-γ increased in response to T-2 toxin doses lower than 0.1 µg/mL, but not under exposure to doses higher than 1 µg/mL [28]. Li et al. also observed an increase in the secretion of proinflammatory cytokines in an avian macrophage cell line in response to T-2 toxin doses of 1–5 ng/mL [50].
In addition to immunocompetent cells, enterocytes also play a very important role in the induction of tolerance to food antigens in the intestinal lumen [51]. Intestinal epithelial cells secrete cytokines which suppress the immune system and increase tolerance to food antigens, including thymic stromal lymphopoietin (TSLP), TGFβ, and retinol metabolites (retinoids) that induce the development of dendritic cells [52]. Dendritic cells trigger the activation of antigen-specific regulatory T cells (Tregs) [53,54,55] and promote immunoglobulin class switching to IgA in B2 lymphocytes regardless of CD4+ Th cells. TGFβ plays a key role in the induction of IgA synthesis by B2 cells, and the induction of CD4+ regulatory (iTreg) T cells with the FoxP3 (CD4+CD25+FoxP3+) transcription factor from CD4+CD25- FoxP3 naive T cells [56].
In the group of animals exposed to T-2 toxin, the decrease in TGFβ secretion could be one of the factors that triggered the production of proinflammatory cytokines and impair natural tolerance to food antigens in the intestine. Dendritic cells, which exhibit tolerogenic properties, migrate to mesenteric lymph nodes and induce the production of iTreg cells and TGFβ secretion [57]. The proinflammatory properties of T-2 toxin suppress the activity of tolerogenic dendritic cells. In addition to macrophages, these cells are the main source of IL-12/23 p40 and IL-1β. In this study, dendritic cells were secreted in large amounts in the ileal wall of gilts exposed to T-2 toxin, and their concentrations continued to increase during the experiment. The above cytokines, similarly to IL-17A, regulate the body’s response to infections, including to lipopolysaccharides (LPS) in Gram-negative bacteria. Similar activity is demonstrated by B1 lymphocytes (CD5+CD8-) (Figure 3) which produce immunoglobulin M. One of the negative effects exerted by a low dose of T-2 toxin on GALT was a decrease in the percentage of B2 lymphocytes (CD21+), which could decrease the synthesis of IgA, compromise the integrity of the intestinal epithelial barrier, and impair tolerance to food antigens.
The results of the present study indicate that T-2 toxin administered at 50% of the LOAEL exerts stimulatory and proinflammatory effects on the immune system. Similar observations were made by Ahmadi and Riazipour [39], who found that low doses of T-2 toxin increase the synthesis of interleukin-12 and TNF-α in mice peritoneal macrophages. However, our study investigated young animals whose tolerance to food antigens should increase during the development of immunity. Unfortunately, this process was compromised in the group of animals exposed to T-2 toxin. The above led to inflammation which impaired nutrient absorption. Similar responses have been observed under exposure to other trichothecene mycotoxins. Satrartoxin G, roridin A, verrucarin A, and T-2 toxin can activate caspase-3 as well as caspase-1, whose activation is mediated by the inflammasome protein complex (e.g., in macrophages). The above can increase the secretion of IL-1β and IL-18 [58]. Trichothecenes, including T-2 toxin, interact with nucleic acids at the cellular level. They trigger the ribotoxic stress response, which leads to depurination and cleavage of 28S and 18S rRNA [59], and activation of mitogen-activated protein kinase (MAPK), protein kinase R (PKR), and JNK/p38 kinase [60]. These kinases increase the expression of genes encoding proinflammatory cytokines IL-1β, IL-6, and TNFα, as well as chemokines IL-8 and the macrophage inflammatory protein-2 (MIP-2) in monocytes/macrophages [61]. The antigens present in the intestinal lumen and those reaching the intestine indirectly via the blood stream were effectively absorbed in the jejunum under exposure to deoxynivalenol (DON) [62]. A reduction in the number of goblet cells in the intestines and a decrease in the expression of tight junction proteins (claudin-3, claudin-4, zonula occludens-1 (ZO-1) protein, and occludin) were also noted [33]. The expression of tight junction proteins is related to the activity of MAPK. The above correlations were found mostly in studies of DON, which is less toxic than T-2 toxin [63]. In the current study, similar conclusions can be formulated based on the increase in the concentration of IFNγ (Figure 4), which also stimulates extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinases (JNK) [64]. The activation of MAPK also decreases the expression of tight junction proteins claudin-3 and claudin-4 [33].
In the studied pigs, the immune proinflammatory response of the ileal wall was similar to that noted in humans with inflammatory bowel disease (IBD). Mycotoxins are environmental factors that are often associated with Crohn’s disease and ulcerative colitis [65]. The concentrations of proinflammatory cytokines also increase in other autoimmune disorders, including celiac disease. In this study, IL-17A and IFNγ continued to increase on successive days of exposure to T-2 toxin (relative to day 28), and they were secreted by immunocompetent cells at a similar rate. Inversely proportional changes in the concentrations of TGFβ and IFNγ (decrease in TGFβ secretion and increase in IFNγ production) corroborate the above observations. An increase in the percentage of CD8+ cytotoxic T cells which may secrete IFNγ as well as IL-17A [55] also leads to various types of hypersensitivity in autoimmune disorders [66]. Crohn’s disease and celiac disease are type IV hypersensitivity reactions which involve IFNγ and cytotoxic lymphocytes. Similar effects are observed in contact hypersensitivity induced by haptens, which are nonimmunogenic, low-molecular-weight chemicals (below 1 kDa). Haptens can interact with the immune system when they are covalently attached to carrier molecules, including other proteins [67].
The proinflammatory properties of T-2 toxin can also be attributed to its ability to attach protein sulfhydryl groups, including enzymes of the oxidoreductase family. Trichothecenes can affect mitochondrial metabolism, in particular, enzymes that participate in the tricarboxylic acid cycle and the oxidative phosphorylation (OXPHOS) process. T-2 toxin inhibits succinate dehydrogenase, which catalyzes the conversion of succinic acid to fumaric acid [68]. This process leads to the accumulation of succinic acid, an inflammatory marker [69,70] that influences the expression of hypoxia-inducible factor-1α (HIF1α). Macrophages that are classically activated by LPS (M1 macrophages) as well as Th1 and Th17 lymphocytes undergo metabolic reprogramming during which the Krebs cycle is disrupted by deficiencies in succinate dehydrogenase and isocitrate dehydrogenase, which leads to the accumulation of succinate and isocitrate. The described organic acids in the structure of tricarboxylic acid stabilize HIF-1α. Macrophages secrete IL-1β and alter the phenotype characterized by high activity of the glycolytic pathway and the pentose phosphate pathway. In Th1 and Th17 lymphocytes, metabolic reprogramming towards glycolysis is also associated with the secretion of IFN-γ and IL-17A. The presence of proinflammatory cytokines in the ileal wall is also observed in IBD, including Crohn’s disease. T-2 toxin inhibits mitochondrial metabolism, disrupts the electron transport chain, and promotes the release of reactive oxygen species that increase oxidative stress and further contribute to intestinal inflammation.
4. Conclusions
In prepubertal gilts orally administered T-2 toxin at 50% of the LOAEL, immune homeostasis in the ileal wall was compromised between day 14 and day 42 of exposure. The observed increase in the concentrations of proinflammatory cytokines IL-1β, IL-2, IL-12/23p40, IL-17A, and IFNγ and regulatory cytokines IL-4 and IL-10, and the decrease in the secretion of anti-inflammatory cytokine TGF-β indicate that T-2 toxin disrupted the development of tolerance to food antigens. When administered at a dose of approximately 14.5 µg/kg BW, T-2 toxin stimulated the cellular response by increasing the percentage of CD8+ cytotoxic T cells and decreasing the concentration of CD21+ B2 cells. An increase in IL-1β and IL-12/23p40 levels in the ileal wall of gilts and ongoing inflammation suggest that T-2 toxin compromises the integrity of the intestinal mucosal barrier. The decrease in the percentage of Tγδ intraepithelial lymphocytes indirectly corroborates the above observation.
5. Materials and Methods
5.1. Animals and Procedures
All experimentation procedures involving animals were consistent with the Polish law and had been approved by the Local Bioethics Committee on Animal Research in Olsztyn, Poland, under decision No. 14/2008 of 22 January 2008.
The experiment was conducted on 30 Polish Large White prepubertal gilts aged eight weeks, with a body weight of 18–20 kg (Research project No. N N308 237936 of 24 April 2009, financed by the Polish National Science Centre). The animals were randomly divided into two groups and were kept in separate pens with ad libitum access to water. The adaptation period lasted seven days. Gilts were fed a complete commercial cereal-based diet whose composition is presented in Table 1. To account for the changes in nutrient requirements during growth, the animals were fed Diet 1 until 25 kg BW, followed by Diet 2 until the end of the experiment. The experimental group (n = 15) was administered T-2 toxin at a daily dose of 14.5 µg/kg BW (T-2 Toxin, T4887, Sigma-Aldrich, Poznań, Poland). The daily dose of T-2 toxin was modified to account for the increase in the body weight of animals. The toxin was administered orally in gel capsules, once a day, before morning feeding. At the same time, control group gilts (n = 15) were administered empty gel capsules to maintain identical levels of handling stress in all animals.
Table 1.
Composition of diets fed to gilts (per 1 kg of feed).
| Ingredients | Unit | Diet 1 | Diet 2 |
|---|---|---|---|
| Metabolizable energy | MJ | 13.80 | 13.00 |
| Total protein | % | 6.00 | 16.00 |
| Crude fiber | max% | 2.63 | 4.94 |
| Sodium | % | 0.21 | 0,18 |
| Calcium | min% | 0.73 | 0.58 |
| Total phosphorus | min% | 0.65 | 0.73 |
| Lysine | % | 1.27 | 1.04 |
| Methionine + cystine | % | 0.83 | 0.64 |
| Threonine | % | 0.72 | 0.59 |
| Tryptophan | % | 0.26 | 0.20 |
| Vitamin A | IU | 15,000 | 13,500 |
| Vitamin D3 | IU | 2000 | 2000 |
| Vitamin E | IU | 100 | 60 |
| Phytase | present | + | + |
| Enzymes | present | + | + |
| Flavor | present | + | - |
| Acidifier | present | + | + |
To rule out the presence of mycotoxins in the administered diets, every feed batch was analyzed for the presence of T-2 toxin, aflatoxin B1, ochratoxin A, zearalenone, alpha-zearalenone, and deoxynivalenol with the use of high-performance liquid chromatography (HPLC) with FLD and/or UV detection (Agilent Technologies 1100, Santa Clara, CA, USA). None of the above mycotoxins were identified above the limit of detection in the administered feeds.
The experiment lasted 42 days. Cytokine secretion and the percentage of lymphocyte subpopulations were determined on experimental days 14, 28, and 42. Every two weeks, five gilts selected randomly from each group were administered azaperone by IM injection at 4 mg/kg BW (Stresnil, Beerse, Belgium) and were euthanized with an IV-administered lethal dose of sodium pentobarbital (0.6 mL/kg BW) (Morbital, Biowet Puławy, Poland) after 15 min.
5.2. Tissue Sampling and Specimen Preparation
Immediately after euthanasia, 5 cm long segments of the ileal wall were sampled approximately 2 cm before the ileocecal valve. Tissue samples for the cytokine analysis were stored at a temperature of −80 °C.
Ileal samples for the cytometric analysis of selected lymphocyte populations (Table 2) were rinsed in phosphate-buffered saline (PBS, pH 7.4, 0.1 M) and finely chopped to remove lymphatic cells with PBS. The cells were centrifuged to remove excess PBS, the pellet was distributed into test tubes, and the cells were counted in the Fuchs-Rosenthal counting chamber. Cell suspensions containing 106 cells/μL were prepared for immunophenotyping. Lymphocyte subpopulations were labeled by dual staining with a combination of primary antibodies (Table 3). The antibodies labeled with fluorescent dyes fluorescein (FITC) and phycoerythrin (PE) were the secondary antibodies (Table 4). Three controls were used in immunophenotyping: without fluorochromes, and with PE and FITC fluorochromes, but without primary antibodies. Stained cells were fixed in 1% formalin to preserve the samples for 24 h. The prepared specimens were immediately transported to the Flow Cytometry Laboratory of the Institute of Laboratory Diagnostics at the Department of Pathology and Veterinary Diagnostics, Faculty of Veterinary Medicine of the Warsaw University of Life Sciences. In the laboratory, the specimens were analyzed in the FACScalibur flow cytometer, and data were processed in the Cell QuestTM program (Becton Dickinson, San Jose, CA, USA).
Table 2.
Analyzed lymphocyte subpopulations.
| Antigen | Subpopulation |
|---|---|
| CD2+ | T cells |
| CD4+ | Th cells |
| CD8+ | Cytotoxic T cells |
| CD4+CD8+ | Double-positive T cells |
| CD5-TCR+ | TCRγδ+ cells, intraepithelial lymphocytes |
| CD5+CD8- | B1 cells |
| CD21+ | B2 cells |
All reagents for immunophenotyping were supplied by Pharmingen (San Diego, CA, USA).
Table 3.
Primary antibodies used in flow cytometry.
| Symbol | Detected Antigen | Immunoglobulin Class | Catalog Number |
|---|---|---|---|
| P1 | CD2 | IgG2a | MSA4 |
| P2 | CD4 | IgG2b | 74-12-4 |
| P3 | CD5 | IgG1 | PG114A |
| P4 | CD8α | IgG2a | 76-2-11 |
| P5 | CD21 | IgG1 | BB6-11C9 |
| P6 | TCR1-N7 | IgG1 | 86D |
All reagents for immunophenotyping were supplied by Pharmingen (San Diego, CA, USA).
Table 4.
Secondary antibodies used in flow cytometry.
| Symbol | Immunoglobulin or Ligand Class | Dye | Catalog Number |
|---|---|---|---|
| S1 | Mouse IgG1 | Phycoerythrin (PE) | 550083 |
| S2 | Mouse IgG2a | Fluorescein isothiocyanate (FITC) | 553390 |
| S3 | Mouse IgG2b | Biotin | 550333 |
| S-PE | Biotin | PE | 554061 |
All reagents for immunophenotyping were supplied by Pharmingen (San Diego, CA, USA).
5.3. Determination of Protein Levels and Cytokine Concentrations
Ileal samples of 1 g each were homogenized in an extraction buffer containing PBS (Sigma-Aldrich, Saint Louis, MO, USA), 0.5% sodium citrate (POCH, Poland), 0.05% Tween 20 (Sigma Aldrich, Saint Louis, MO, USA) and protease inhibitors (Ref 11 697 498 001, Roche, Poland) with the use of Omni TipsTM plastic disposable homogenizer probes (Omni International). The homogenate was centrifuged (8600× g) for 1 h in the Eppendorf 5804R centrifuge, and the supernatant was distributed into test tubes and stored at a temperature of −80 °C until analysis.
The concentration of protein in the extract was determined according to the Bradford method with minor modifications [71], and the results were used as a reference in the cytokine analysis. Cytokine concentrations were expressed in pg/mg protein.
The content of IFN-γ, IL-1β, IL-2, IL-12/23p40, IL-4, IL-10, TGFβ, and IL-17A was determined with the use of commercial ELISA kits (Table 5) in a microplate spectrophotometer (TECAN Infinite M200, Männedorf, Switzerland).
Table 5.
ELISA kits for determining cytokine concentrations in the porcine ileum.
| Antigen | ELISA Kit and Catalog Number | Manufacturer, Country | Assay Range pg/mL |
|---|---|---|---|
| IFN-γ | Porcine IFN-gamma DuoSet ELISA, DY985 |
R&D Systems Inc., Minneapolis, MN, USA | 62.5–4.000 Intra-assay CV < 3.4% Inter-assay CV < 4.6% |
| IL-1β | Porcine IL-1 beta/IL-1F2 DuoSet ELISA, DY681 | R&D Systems Inc., Minneapolis, MN, USA | 62.5–4.000 Intra-assay CV < 1.1% Inter-assay CV < 3.2% |
| IL-2 | Swine IL-2 CytoSet™ CSC124 |
Invitrogens™, Camarillo, CA, USA |
35.6–570 Intra-assay CV < 4.48% Inter-assay CV < 5.02% |
| IL-12/23p40 | Porcine IL-12/IL-23 p40 DuoSet ELISA, DY912 | R&D Systems Inc., Minneapolis, MN, USA | 78.1–5.000 Intra-assay CV < 3.67% Inter-assay CV < 4.25% |
| IL-4 | Porcine IL-4 DuoSet ELISA, DY654 | R&D Systems Inc., Minneapolis, MN, USA | 156.0–10.000 Intra-assay CV < 5% Inter-assay CV < 6.69% |
| IL-10 | Porcine IL-10 DuoSet ELISA, DY693B |
R&D Systems Inc., Minneapolis, MN, USA | 23.4–1.500 Intra-assay CV <3% Inter-assay CV <4.64% |
| TGFβ | TGF beta-1 Multispecies Matched Antibody Pair, CHC1683 | Thermo Fisher Scientific, Waltham, MA, USA | 62.5–4.000 Intra-assay CV < 2.9% Inter-assay CV < 5% |
| IL-17A | Porcine IL-17 (IL-17A) ELISA Kit, ESIL17A | Thermo Fisher Scientific, Waltham, MA, USA | 16.38–4.000 Intra-assay CV < 10% Inter-assay CV < 12% |
5.4. Statistical Analysis
The results were processed in Excel (Microsoft, Redmond, WA, USA) and Graph Pad Prism 5 (GraphPad Software, San Diego, CA, USA) programs. Mean values and standard error of the mean (SEM) were calculated for all studied groups. The distribution of the population was determined with the Kolmogorov–Smirnov test. The results were analyzed statistically using an unpaired t-test and one-way ANOVA at a significance level of p < 0.05.
Abbreviations
18S RNA: 18S ribosomal RNA; 28S RNA: 28S ribosomal RNA; B1 cells: subclass of CD5+CD8- B lymphocytes; B2 cells: subclass of CD21+ B lymphocytes; Caco-2: CACO-2 Cell Line human; CD4+: CD4+ helper T cells; CD8+: cytotoxic T cells; CD4+CD8+: double-positive T cells; CD44low: T cell populations with low expression of CD44; CD45low: T cell populations with low expression of CD45; CD5-TCR+: CD5-positive T cells; DON: Deoxynivalenol; ELISA: Enzyme-linked immunosorbent assay; ERK: Extracellular signal-regulated kinase; FITC: Fluorescein isothiocyanate; FoxP3: Forkhead box P3; GALT: Gut-associated lymphoid tissue; HIF-1α: Hypoxia-inducible factor 1-alpha; HPLC-FLD: High-performance liquid chromatography with fluorescence detection; HPLC-UV: High-performance liquid chromatography with ultraviolet detection; HT-2: HT-2 toxin; HT-28: HT-28 cells; IBD: Inflammatory bowel disease; IEC-6: Intestinal Epithelioid Cell line No. 6; IEL: Intraepithelial lymphocytes; IFN-γ: Interferon gamma; IgA: Immunoglobulin A; IL-1: Interleukin 1; IL-10: Interleukin 10; IL-12/23p40—Interleukin 12/23 with 40 subunits; IL-15: Interleukin 15; IL-17A: Interleukin 17A; IL-18: Interleukin 18; IL-1β: Interleukin 1 beta; IL-2: Interleukin 2; IL-4: Interleukin 4; IL-6: Interleukin 6; IL-8: Interleukin 8; JNK: C-Jun N-terminal kinase; LOAEL: Lowest-observed-adverse-effect level; LPL: Lamina propria lymphocytes; LPS: Lipopolysaccharides; MAP: Multiple antigenic peptide; MAPK: Mitogen-activated protein kinase; MIP-2: Macrophage inflammatory protein-2; NOAEL: No-observed-adverse-effect level; OXPHOS: Oxidative phosphorylation; PBS: Phosphate-buffered saline; PE: Phycoerythrin; PKR: Protein kinase R; RNA: Ribonucleic acid: sIgA: Secretory IgA; SLA-DQ+: SLA-DQ+ cells; SWC3+: SWC3 + cells; CD4+CD8+ T cells: Double-positive T Cells; CD8+ T cells: T cells with CD8 co-receptors; T-2: T-2 toxin; TCH: Trichothecenes; TCHA: Type A trichothecenes; TCRγδ: T cell receptor formed by gamma-delta chains; TGF-α: Transforming growth factor alpha; TGFβ: Transforming growth factor beta; Th1: Type 1 helper T cells; Th17: Th17 helper cells; TNF-α: Tumor necrosis factor α; Tδγ: Gamma delta T cells; ZO-1: Zonula occludens-1.
Author Contributions
Conceptualization, P.W., W.T., P.P. and K.O.; investigation, P.W., P.P., M.Ż., J.T., E.J., T.B., M.B. and K.O.; methodology, P.W., P.P., M.Ż. and K.O.; visualization, M.Ż. and Ł.Z.; writing—original draft, P.W., W.T. and K.O.; writing—review & editing, M.P.-Ś., E.J. and K.Ż.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was a part of a research project No. NN 308 237936 funding by the National Science Centre in Poland. Project financially co-supported by Minister of Science and Higher Education in the range of the program entitled “Regional Initiative of Excellence” for the years 2019-2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This is the first study to investigate the effect of T-2 toxin administered per os at a dose below the LOAEL on the maintenance of homeostasis in the intestinal immune system of prepubertal gilts. The results indicate that T-2 toxin impairs the induction of food tolerance in GALT by enhancing the secretion of CD8+ T cells; decreasing B2 lymphocyte counts; increasing the concentrations of proinflammatory and regulatory cytokines; and decreasing the levels of the anti-inflammatory cytokine TGF-β.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calvo A.M., Wilson R.A., Bok J.W., Keller N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002;66:447–459. doi: 10.1128/MMBR.66.3.447-459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo A.M., Cary J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015;6:62. doi: 10.3389/fmicb.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinotti L., Ottoboni M., Giromini C., Dell’Orto V., Cheli F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins. 2016;8:45. doi: 10.3390/toxins8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos Pereira C., Cunha C.S., Fernandes J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins. 2019;11:290. doi: 10.3390/toxins11050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari M., Negi B., Kaushik N., Adhikari A., Al-Khedhairy A.A., Kaushik N.K., Choi E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget. 2017;8:33933–33952. doi: 10.18632/oncotarget.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moretti A., Logrieco A.F., Susca A. Mycotoxins: An underhand food problem. Methods Mol. Biol. 2017;1542:3–12. doi: 10.1007/978-1-4939-6707-0_1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J., Zhang H., Liu S., Wu W., Zhang H. Comparison of Anorectic Potencies of Type A Trichothecenes T-2 Toxin, HT-2 Toxin, Diacetoxyscirpenol, and Neosolaniol. Toxins. 2018;10:179. doi: 10.3390/toxins10050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick S.P., Stanley A.M., Stover N.A., Alexander N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins. 2011;3:802–814. doi: 10.3390/toxins3070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Kuchenbuch H.S., Becker S., Schulz M., Cramer B., Humpf H.U. Thermal stability of T-2 and HT-2 toxins during biscuit- and crunchy muesli-making and roasting. Food Addit. Contam. Part A. 2018;35:2158–2167. doi: 10.1080/19440049.2018.1530456. [DOI] [PubMed] [Google Scholar]
- 11.EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011;9:2481. [Google Scholar]
- 12.EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific opinion on the appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA J. 2017;15:4655. doi: 10.2903/j.efsa.2017.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makowska K., Obremski K., Zielonka L., Gonkowski S. The Influence of Low Doses of Zearalenone and T-2 Toxin on Calcitonin Gene Related Peptide-Like Immunoreactive (CGRP-LI) Neurons in the ENS of the Porcine Descending Colon. Toxins. 2017;9:98. doi: 10.3390/toxins9030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meissonnier G.M., Laffitte J., Raymond I., Benoit E., Cossalter A.M., Pinton P., Bertin G., Oswald I.P., Galtier P. Subclinical doses of T-2 toxin impair acquired immune response and liver cytochrome P450 in pigs. Toxicology. 2008;247:46–54. doi: 10.1016/j.tox.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Rocha O., Ansari K., Doohan F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005;22:369–378. doi: 10.1080/02652030500058403. [DOI] [PubMed] [Google Scholar]
- 16.Pestka J.J. Deoxynivalenol-Induced Proinflammatory Gene Expression: Mechanisms and Pathological Sequelae. Toxins. 2010;2:1300–1317. doi: 10.3390/toxins2061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Q.H., Wang X., Yang W., Nussler A.K., Xiong L.Y., Kuca K., Dohnal V., Zhang X.J., Yuan Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014;88:1309–1326. doi: 10.1007/s00204-014-1280-0. [DOI] [PubMed] [Google Scholar]
- 18.Nagata T., Suzuki H., Ishigami N., Shinozuka J., Uetsuka K., Nakayama H., Doi K. Development of apoptosis and changes in lymphocyte subsets in thymus, mesenteric lymph nodes and Peyer’s patches of mice orally inoculated with T-2 toxin. Exp. Toxicol. Pathol. 2001;53:309–315. doi: 10.1078/0940-2993-00196. [DOI] [PubMed] [Google Scholar]
- 19.Grizzle J.M., Kersten D.B., McCracken M.D., Houston A.E., Saxton A.M. Determination of the acute 50% lethal dose T-2 toxin in adult bobwhite quail: Additional studies on the effect of T-2 mycotoxin on blood chemistry and the morphology of internal organs. Avian Dis. 2004;48:392–399. doi: 10.1637/7100. [DOI] [PubMed] [Google Scholar]
- 20.Makowska K., Gonkowski S., Zielonka L., Dabrowski M., Calka J. T2 Toxin-Induced Changes in Cocaineand Amphetamine-Regulated Transcript (CART)-Like Immunoreactivity in the Enteric Nervous System Within Selected Fragments of the Porcine Digestive Tract. Neurotox. Res. 2017;31:136–147. doi: 10.1007/s12640-016-9675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidner M., Huwel S., Ebert F., Schwerdtle T., Galla H.J., Humpf H.U. Influence of T-2 and HT-2 toxin on the blood-brain barrier in vitro: New experimental hints for neurotoxic effects. PLoS ONE. 2013;8:e60484. doi: 10.1371/journal.pone.0060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M., Cuff C.F., Pestka J.J. T-2 toxin impairment of enteric reovirus clearance in the mouse associated with suppressed immunoglobulin and IFN-gamma responses. Toxicol. Appl. Pharmacol. 2006;214:318–325. doi: 10.1016/j.taap.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafai P., Tuboly S., Bata A., Tilly P., Ványi A., Papp Z., Jakab L., Túry E. Effect of various levels of T-2 toxin in the immune system of growing pigs. Vet. Rec. 1995;136:511–514. doi: 10.1136/vr.136.20.511. [DOI] [PubMed] [Google Scholar]
- 24.Hymery N., Sibiril Y., Parent-Massin D. In vitro effects of trichothecenes on human dendritic cells. Toxicol. In Vitro. 2006;20:899–909. doi: 10.1016/j.tiv.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Murtaugh M.P. Porcine cytokines. Vet. Immunol. Immunopathol. 1994;43:37–44. doi: 10.1016/0165-2427(94)90118-X. [DOI] [PubMed] [Google Scholar]
- 26.Verma N., Rettenmeier A.W., Schmitz-Spanke S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics. 2011;11:776–793. doi: 10.1002/pmic.201000320. [DOI] [PubMed] [Google Scholar]
- 27.Mowat A.M. Anatomical Basis of Tolerance and Immunity to Intestinal Antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 28.Brandtzaeg P., Pabst R. Let’s Go Mucosal: Communication on Slippery Ground. Trends. Immunol. 2004;25:570–577. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 2001;1:59–67. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- 30.Makala L.H., Kamada T., Nagasawa H., Igarashi I., Fujisaki K., Suzuki N., Mikami T., Haverson K., Bailey M., Stokes C.R., et al. Ontogeny of pig discrete Peyer’s patches: Expression of surface antigens. J. Vet. Med. Sci. 2001;63:625–636. doi: 10.1292/jvms.63.625. [DOI] [PubMed] [Google Scholar]
- 31.Bailey M., Plunkett F.J., Rothkotter H.J., Vega-Lopez M.A., Haverson K., Stokes C.R. Regulation of mucosal immune responses in effector sites. Proc. Nutr. Soc. 2001;60:427–435. doi: 10.1079/PNS2001118. [DOI] [PubMed] [Google Scholar]
- 32.Hayday A., Theodoridis E., Ramsburg E., Shires J. Intraepithelial lymphocytes: Exploring the third way in immunology. Nat. Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 33.Pinton P., Nougayrede J.P., del Rio J.C., Moreno C., Marin D.E., Ferrier L., Bracarense A.P., Kolf-Clauw M., Oswald I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009;237:41–48. doi: 10.1016/j.taap.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Maresca M., Yahi N., Younes-Sakr L., Boyron M., Caporiccio B., Fantini J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1beta effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008;228:84–92. doi: 10.1016/j.taap.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Maresca M. From the gut to the brain: Journey and pathophysiological effects of the food associated trichothecene mycotoxin deoxynivalenol. Toxins. 2013;5:784–820. doi: 10.3390/toxins5040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onyiah J.C., Colgan S.P. Cytokine responses and epithelial function in the intestinal mucosa. Cell. Mol. Life Sci. 2016;73:4203–4212. doi: 10.1007/s00018-016-2289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam Z., Nagase M., Yoshizawa T., Yamauchi K., Sakato N. T-2 toxin induces thymic apoptosis in vivo in mice. Toxicol. Appl. Pharmacol. 1998;148:205–214. doi: 10.1006/taap.1997.8338. [DOI] [PubMed] [Google Scholar]
- 38.Holladay S.D., Smith B.J., Luster M.I. B lymphocyte precursor cells represent sensitive targets of T2 mycotoxin exposure. Toxicol. Appl. Pharmacol. 1995;131:309–315. doi: 10.1006/taap.1995.1073. [DOI] [PubMed] [Google Scholar]
- 39.Ahmadi K., Riazipour M. Effects of T-2 toxin on cytokine production by mice peritoneal macrophages and lymph node T-cells. Iran. J. Immunol. 2008;5:177–180. [PubMed] [Google Scholar]
- 40.Edite Bezerra da Rocha M., Freire F.D.C.O., Erlan F.M.F., Izabel F.G.M., Rondina D. Mycotoxins and their effects on human and animal health. Food Control. 2014;36:159–165. doi: 10.1016/j.foodcont.2013.08.021. [DOI] [Google Scholar]
- 41.Murtaugh M.P., Johnson C.R., Xiao Z., Scamurra R.W., Zhou Y. Species specialization in cytokine biology: Is interleukin-4 central to the T(H)1-T(H)2 paradigm in swine? Dev. Comp. Immunol. 2009;33:344–352. doi: 10.1016/j.dci.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Helm R.M., Furuta G.T., Stanley J.S., Ye J., Cockrell G., Connaughton C., Simpson P., Bannon G.A., Burks A.W. A neonatal swine model for peanut allergy. J. Allergy Clin. Immunol. 2002;109:136–142. doi: 10.1067/mai.2002.120551. [DOI] [PubMed] [Google Scholar]
- 43.Chung Y.J., Zhou H.R., Pestka J.J. Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin) Toxicol. Appl. Pharmacol. 2003;193:188–201. doi: 10.1016/S0041-008X(03)00299-0. [DOI] [PubMed] [Google Scholar]
- 44.Meky F.A., Hardie L.J., Evans S.W., Wild C.P. Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production. Food Chem. Toxicol. 2001;39:827–836. doi: 10.1016/S0278-6915(01)00029-1. [DOI] [PubMed] [Google Scholar]
- 45.Taranu I., Marin D.E., Bouhet S., Pascale F., Bailly J.D., Miller J.D., Pinton P., Oswald I.P. Mycotoxin fumonisin B1 alters the cytokine profile and decreases the vaccinal antibody titer in pigs. Toxicol. Sci. 2005;84:301–307. doi: 10.1093/toxsci/kfi086. [DOI] [PubMed] [Google Scholar]
- 46.McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 47.Kuwabara T., Ishikawa F., Kondo M., Kakiuchi T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuhmacher-Wolz U., Heine K., Schneider K. Report on toxicity data on trichothecene mycotoxins HT-2 and T-2 toxins. EFSA Support. Publ. 2010;7 doi: 10.2903/sp.efsa.2010.EN-65. [DOI] [Google Scholar]
- 49.Pestka J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- 50.Li S.J., Pasmans F., Croubels S., Verbrugghe E., Van Waeyenberghe L., Yang Z., Haesebrouck F., Martel A. T-2 toxin impairs antifungal activities of chicken macrophages against Aspergillus fumigatus conidia but promotes the pro-inflammatory responses. Avian Pathol. 2013;42:457–463. doi: 10.1080/03079457.2013.822958. [DOI] [PubMed] [Google Scholar]
- 51.Chistiakov D.A., Bobryshev Y.V., Kozarov E., Sobenin I.A., Orekhov A.N. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front. Microbiol. 2015;5:781. doi: 10.3389/fmicb.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pabst O., Mowat A.M. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iliev I.D., Spadoni I., Mileti E., Matteoli G., Sonzogni A., Sampietro G.M., Foschi D., Caprioli F., Viale G., Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 55.Spadoni I., Iliev I.D., Rossi G., Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–193. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 56.Shevach E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Müller W., Sparwasser T., Förster R., Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 58.Kankkunen P., Rintahaka J., Aalto A., Leino M., Majuri M.L., Alenius H., Wolff H., Matikainen S. Trichothecene mycotoxins activate inflammatory response in human macrophages. J. Immunol. 2009;182:6418–6425. doi: 10.4049/jimmunol.0803309. [DOI] [PubMed] [Google Scholar]
- 59.Li M., Pestka J.J. Comparative Induction of 28S Ribosomal RNA Cleavage by Ricin and the Trichothecenes Deoxynivalenol and T-2 Toxin in the Macrophage. Toxicol. Sci. 2008;105:67–78. doi: 10.1093/toxsci/kfn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sergent T., Parys M., Garsou S., Pussemier L., Schneider Y.J., Larondelle Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006;164:167–176. doi: 10.1016/j.toxlet.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Leyva-Illades D., Cherla R.P., Lee M.S., Tesh V.L. Regulation of cytokine and chemokine expression by the ribotoxic stress response elicited by Shiga toxin type 1 in human macrophage-like THP-1 cells. Infect. Immun. 2012;80:2109–2120. doi: 10.1128/IAI.06025-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avantaggiato G., Havenaar R., Visconti A. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food Chem. Toxicol. 2004;42:817–824. doi: 10.1016/j.fct.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Behrens M., Hüwel S., Galla H.J., Humpf H.U. Blood-Brain Barrier Effects of the Fusarium Mycotoxins Deoxynivalenol, 3 Acetyldeoxynivalenol, and Moniliformin and Their Transfer to the Brain. PLoS ONE. 2015;10:e0143640. doi: 10.1371/journal.pone.0143640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucioli J., Pinton P., Callu P., Laffitte J., Grosjean F., Kolf-Clauw M., Oswald I.P., Bracarense A.P. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon. 2013;66:31–36. doi: 10.1016/j.toxicon.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 65.Abraham C., Cho J.H. IL-23 and autoimmunity: New insights into the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- 66.Hamada H., Hiroi T., Nishiyama Y., Takahashi H., Masunaga Y., Hachimura S., Kaminogawa S., Takahashi-Iwanaga H., Iwanaga T., Kiyono H., et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 67.Nowak D., Panaszek B. Anaphylactic reactions to low-molecular weight chemicals. Postepy Hig. I Med. Dosw. (Online) 2015;69:197–206. [PubMed] [Google Scholar]
- 68.Ngampongsa S., Hanafusa M., Ando K., Ito K., Kuwahara M., Yamamoto Y., Yamashita M., Tsuru Y., Tsubone H. Toxic effects of T-2 toxin and deoxynivalenol on the mitochondrial electron transport system of cardiomyocytes in rats. J. Toxicol. Sci. 2013;38:495–502. doi: 10.2131/jts.38.495. [DOI] [PubMed] [Google Scholar]
- 69.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills E.L., O’Neill L.A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 2016;46:13–21. doi: 10.1002/eji.201445427. [DOI] [PubMed] [Google Scholar]
- 71.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.