Abstract
The potential of exosomes as biomarker resources for diagnostics and even for therapeutics has intensified research in the field, including in the context of Alzheimer´s disease (AD). The search for disease biomarkers in peripheral biofluids is advancing mainly due to the easy access it offers. In the study presented here, emphasis was given to the bioinformatic identification of putative exosomal candidates for AD. The exosomal proteomes of cerebrospinal fluid (CSF), serum and plasma, were obtained from three databases (ExoCarta, EVpedia and Vesiclepedia), and complemented with additional exosomal proteins already associated with AD but not found in the databases. The final biofluids’ proteomes were submitted to gene ontology (GO) enrichment analysis and the exosomal Aβ-binding proteins that can constitute putative candidates were identified. Among these candidates, gelsolin, a protein known to be involved in inhibiting Abeta fibril formation, was identified, and it was tested in human samples. The levels of this Aβ-binding protein, with anti-amyloidogenic properties, were assessed in serum-derived exosomes isolated from controls and individuals with dementia, including AD cases, and revealed altered expression patterns. Identification of potential peripheral biomarker candidates for AD may be useful, not only for early disease diagnosis but also in drug trials and to monitor disease progression, allowing for a timely therapeutic intervention, which will positively impact the patient’s quality of life.
Keywords: exosomes, Alzheimer’s disease, Abeta binding proteins, diagnosis, biomarker
1. Introduction
Alzheimer’s disease (AD) is the most common, irreversible and progressive neurodegenerative disorder worldwide. Its progression leads to alterations in cognitive functions, such as memory and orientation that will interfere with daily life activities and impact the patient’s quality of life. The formation of extracellular senile plaques, composed of deposits of the amyloid beta peptide (Aβ), and intracellular neurofibrillary tangles, as a result of Tau hyperphosphorylation, are two main disease histopathological hallmarks [1].
At the disease diagnostic level, a wide range of clinical cognitive tests are employed to evaluate cognitive deficits and also imaging tools, which have improved considerably with emerging techniques like positron emission topography (PET) [2,3]. In addition, the detection of the biomarker triplet, Aβ42, T-Tau and p-Tau-181 in cerebrospinal fluid (CSF) [4,5], is currently the gold standard for neurochemical-based diagnosis, employed to discriminate AD from other dementia subtypes. The clinical value of this diagnosis is unquestionable; however, a limitation arises due to the invasive procedure for CSF collection. Therefore, research trends have focused on identifying novel biomarker candidates in more accessible and peripheral biofluids such as blood [6].
Exosomes, a subclass of extracellular vesicles (EVs) with a multivesicular endosomal origin, ranging from 30 to 150 nm, are formed by a lipid bilayer membrane enclosing a free-cytosol organelle, composed by several macromolecules (also called luminal cargo). Its cargo can comprise proteins, RNA, DNA and lipids [7], which arouse interest in using these nanovesicles as novel putative diagnostic resources for several diseases, including, more recently, AD [8,9,10]. These nanovesicles are secreted by all cell types in the human organism, including neurons, and can carry disease molecular profiles. In fact, exosomes can carry specific pathogenic content linked to AD. In the context of AD, exosomes can transport both Aβ and Tau and act as active players in the spread of these pathogenic species, triggering abnormal/neurodegenerative processes that contribute to disease progression [11,12,13]. Likewise, a protective role has been attributed to these types of EVs, since exosomes can also carry Aβ degrading enzymes; and exosome infusions have proven to be useful in the clearance of Aβ deposits [14,15,16,17,18]. Several other exosomal candidates have been identified as putative biomarkers for AD diagnosis. These include, not only Aβ and Tau species, but also inflammation-related mediators, like complement effector and regulatory proteins, synaptic proteins, growth factors and insulin signaling receptors [10,19,20,21,22]. Since Aβ is transported in exosomes and their levels are altered with AD in the majority of the studies [8], this encourages the search for Aβ-binding proteins in exosomes. This group of proteins, in essence the Aβ interactome, can interact with monomeric, oligomeric and fibrillar forms of Aβ and modulate its clearance and degradation, promote amyloid aggregation and influence its transport [23].
Hence, the aim of this study was to address the exosomal proteome from three distinct biofluids, namely, serum, plasma and CSF, for comparative purposes, and to identify Aβ-binding proteins within these proteomes that may constitute putative AD biomarker candidates.
2. Results and Discussion
2.1. Exosomal Proteomes Gene Ontology (GO) Analysis
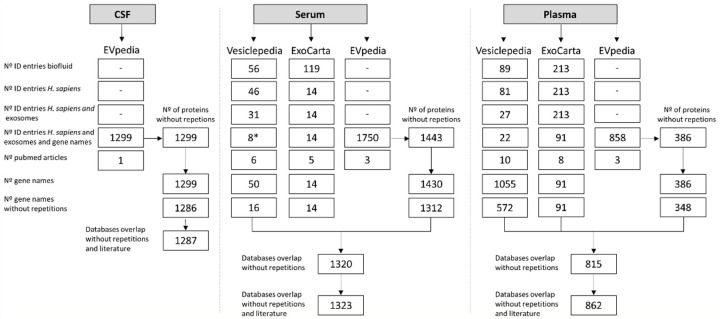
Exosomal proteomes derived from CSF, serum and plasma were obtained by accessing three online databases (ExoCarta, Vesiclepedia and EVpedia). For each database, the proteins found in exosomes were collected and a list of proteins/corresponding genes present was produced for each biofluid. The final biofluid proteome lists also include proteins described in the literature in AD-related studies and not yet entered in the databases (Supplementary Table S1). The final number of exosomal proteins in the biofluids lists was 1287 for CSF, 1323 for serum and 862 for plasma (Figure 1).
Figure 1.
Exosomal proteomes obtained for the distinct biofluids. Steps implemented to obtain the final proteomes using the three databases (Vesiclepedia, ExoCarta, EVpedia) for cerebrospinal fluid (CSF), serum and plasma. A set of exclusion filters was used in this order: number of entries for the biofluid, number of ID entries for Homo sapiens, number of ID entries for H. sapiens and exosomes, number ID entries for H. sapiens and exosomal gene names, number of corresponding PubMed articles, number of gene names and number of gene names without repetitions. Subsequently, the lists obtained for the three databases were overlapped, the repetitions deleted and, the gene names corresponding to the exosomal proteins identified in the literature for CSF, serum or plasma were included in the final lists. The EVpedia’s obtained Uniprot IDs were converted to gene names. In the case of CSF, only EVpedia had information available. * One study was excluded since it was related to urine exosomal proteome and not to serum.
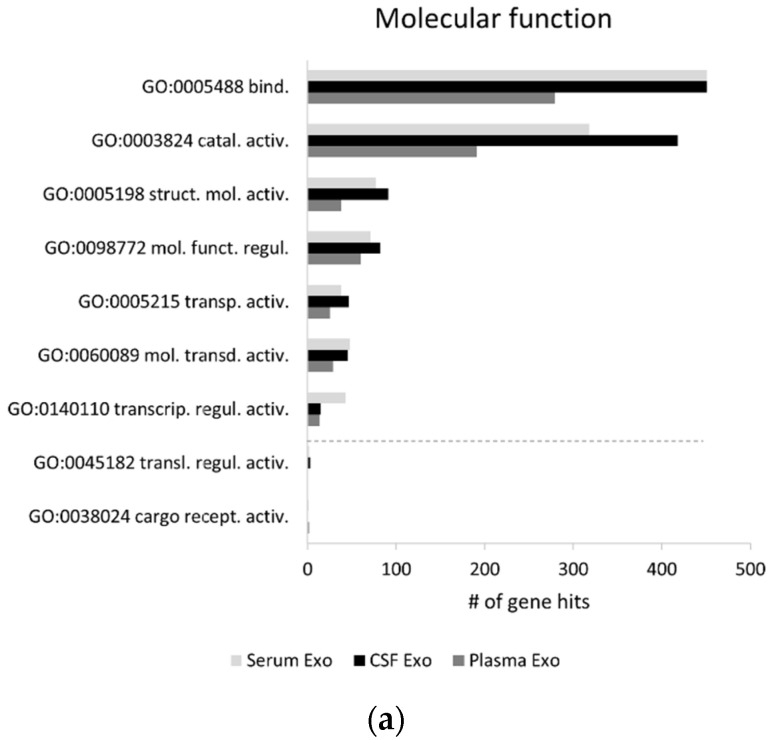
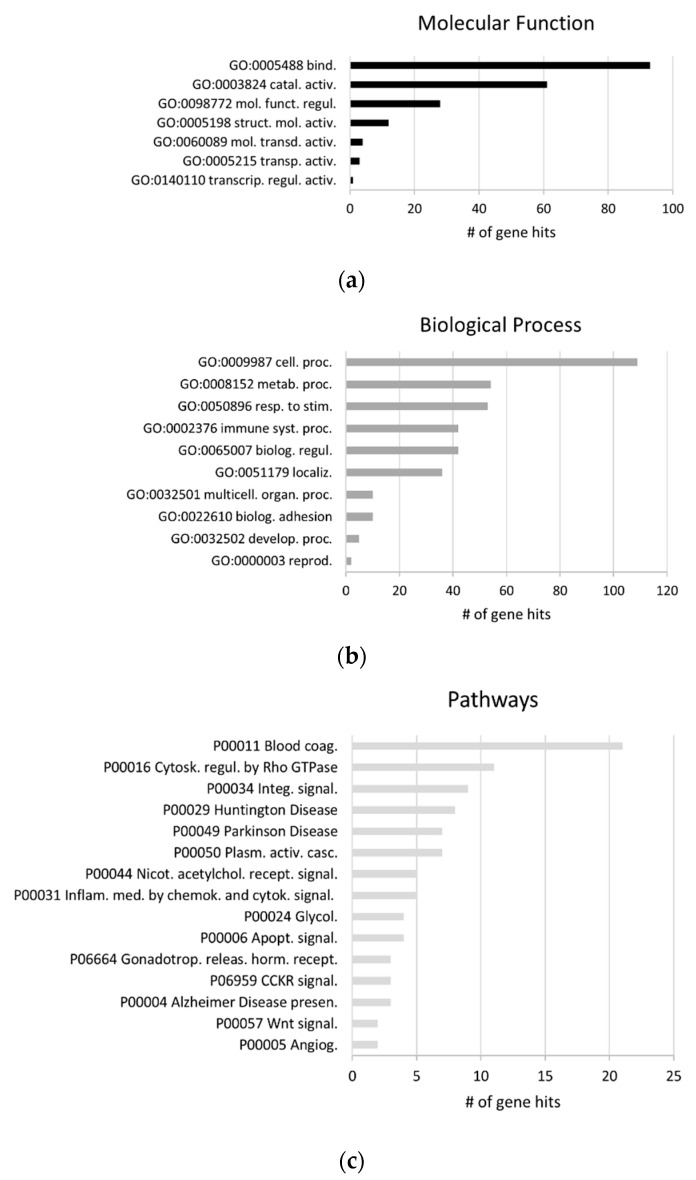
The characterization and comparison of the exosomal proteomes is relevant since it can give important clues into the main processes and pathways to which the distinct biofluid proteomes are most associated, allowing one to potentially explore new disease biomarker candidates. GO enrichment analysis was thus performed, at the level of the molecular function and biological process; and the major pathways linked to these proteomes were addressed. A global GO analysis for these biofluids’ exosomal proteomes was carried out using the Panther classification system online tool (Figure 2). At the molecular function level, the top five functions identified were the same for all exosomal proteomes and included “binding”, “catalytic activity”, “structural molecule activity”, “molecular function regulator” and “transporter activity” (Figure 2a).
Figure 2.
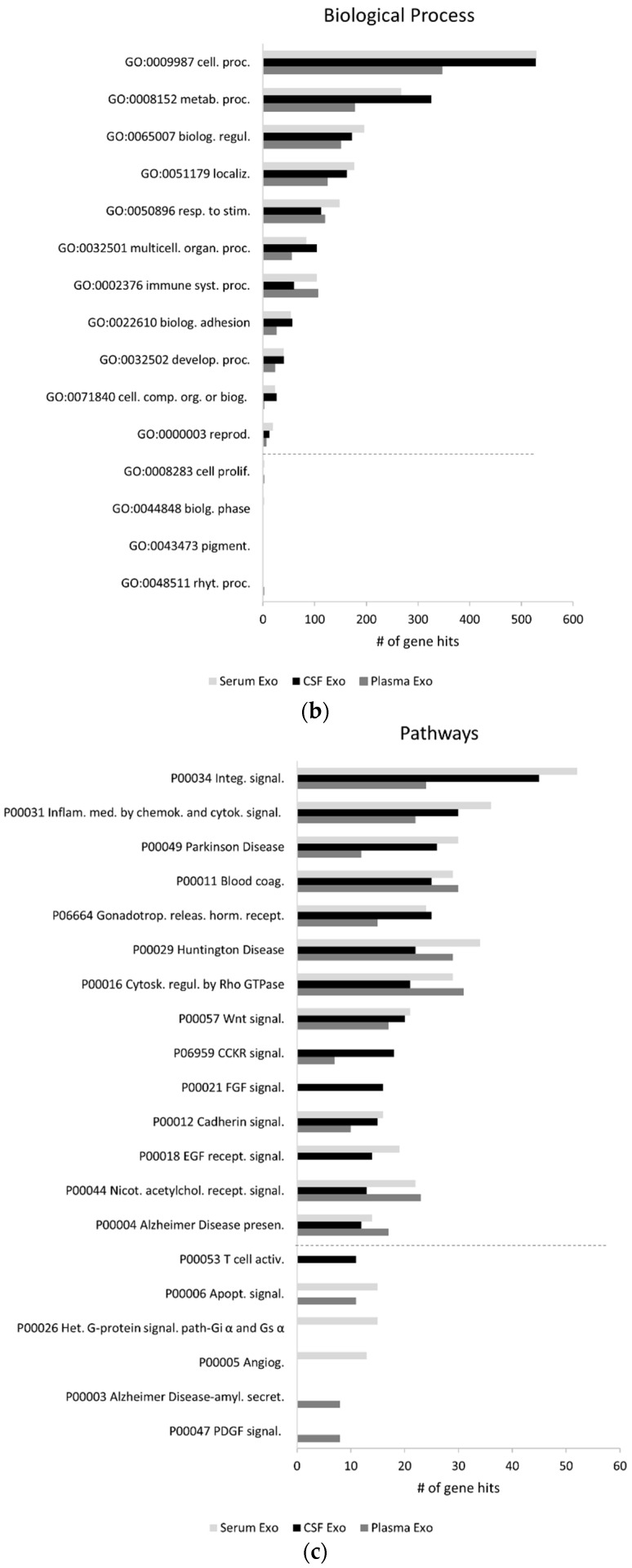
Characterization of the exosomal proteome from distinct biofluids. Exosomal proteomes associated molecular functions (a), biological processes (b) and pathways (c) analysis. Gene ontology (GO) analysis was performed for gene lists corresponding to the exosomal proteomes from serum, plasma and CSF, through the Panther classification system. Exclusive GO terms for exosomes isolated from only one biofluid or shared only by exosomes from two biofluids were represented below the dashed lines. Abbreviations of molecular function GOs terms: GO:0005488 binding; GO:0003824 catalytic activity; GO:0005198 structural molecule activity; GO:0098772 molecular function regulator; GO:0005215 transporter activity; GO:0060089 molecular transducer activity; GO:0140110 transcription regulator activity; GO:0045182 translation regulator activity; GO:0038024 cargo receptor activity. Abbreviations of biological process GOs terms: GO:0009987 cellular process; GO:0008152 metabolic process; GO:0065007 biological regulation; GO:0051179 localization; GO:0050896 response to stimulus; GO:0032501 multicellular organismal process; GO:0002376 immune system process; GO:0022610 biological adhesion; GO:0032502 developmental process; GO:0071840 cellular component organization or biogenesis; GO:0000003 reproduction; GO:0008283 cell proliferation; GO:0044848 biological phase; GO:0043473 pigmentation; GO:0048511 rhythmic process. Abbreviations of pathways GOs terms: P00034 integrin signaling pathway; P00031 inflammation mediated by chemokine and cytokine signaling pathway; P00049 Parkinson disease; P00011 blood coagulation; P06664 gonadotropin-releasing hormone receptor pathway; P00029 Huntington disease; P00016 cytoskeletal regulation by Rho GTPase; P00057 Wnt signaling pathway; P06959 CCKR signaling map; P00021 FGF signaling pathway; P00012 cadherin signaling pathway; P00018 EGF receptor signaling pathway; P00044 nicotinic acetylcholine receptor signaling pathway; P00004 Alzheimer’s disease-presenilin pathway; P00053 T cell activation; P00006 apoptosis signaling pathway; P00026 heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway; P00005 angiogenesis; P00003 Alzheimer’s disease-amyloid secretase pathway; P00047 PDGF signaling pathway. Exo, Exosomes.
Regarding the top 5 biological processes found for these three proteomes, similar processes were likewise identified, namely “cellular process”, “metabolic process”, “biological regulation”, “localization” and “response to stimulus” (Figure 2b). However, the “rhythmic process” was exclusive to plasma exosomes and “pigmentation” and “biological phase” were biological processes exclusive to serum exosomes. This is potentially relevant when directing studies aimed at addressing particular processes in distinct biofluids.
Major differences arise when addressing the top exosomal proteomes-associated pathways (Figure 2c). For CSF, the top five pathways linked to the exosomal proteome were “integrin signaling pathway”, “inflammation mediated by the chemokine and cytokine signaling pathway”, “Parkinson disease”, “blood coagulation” and “gonadotropin-releasing hormone receptor pathway”. Similar to CSF, the serum-derived exosomal proteome also associated with the “integrin signaling pathway”, “inflammation mediated by chemokine and cytokine signaling pathway”, “blood coagulation” and “Huntington disease”, but it appeared with an increased number of hits related to the “Parkinson disease” pathway.
Plasma exosomal proteome was the most distinct, being mainly associated with “cytoskeletal regulation by Rho GTPase”, “blood coagulation”, followed by “Huntington disease”, “integrin signaling pathway” and “nicotinic acetylcholine receptor signaling pathway”. In addition, if the top 15 pathways were considered, “T cell activation” and “FGF signaling pathway” appeared as being exclusive for the CSF exosome proteome, while “Alzheimer disease-amyloid secretase pathway” and “Platelet-derived growth factor (PDGF) signaling pathway” appeared as pathways exclusive to the plasma-derived proteome. Likewise, exclusive serum exosomal pathways were found, namely “angiogenesis” and “heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway”.
In general terms, the main molecular function and biological process were similar for the three biofluids’ exosomal proteomes, however, major differences arise for the pathways associated to the distinct biofluid proteomes. The data available suggest that perhaps plasma can be an ideal biofluid for biomarker studies addressing AD-related candidates, since despite being the smallest proteome, plasma exosomal proteome was associated with two AD processing pathways. However, one cannot exclude that differences in these biofluid proteomes analysis, may be due to the fact that some proteins were still not identified in the distinct biofluids to date or included in databases.
Nonetheless, taken together, data suggest that although the most abundant functions and processes are similar, variances, in particular at the pathway’s levels, can support the use of distinct biofluids for different downstream applications, depending on the research goal.
2.2. GO Analysis of the Biofluids Shared Exosomal Proteome
Cerebrospinal fluid arises as a major source of biomarkers for AD since it represents a route of communication from the brain to the rest of the body. Indeed, gold standard biomarker candidates have already been identified in this biofluid, however, the invasiveness of the CSF collection procedure limits its general use. Hence, in this study the shared proteome of the three biofluids was identified as this would represent a source of putative peripheral disease candidates.
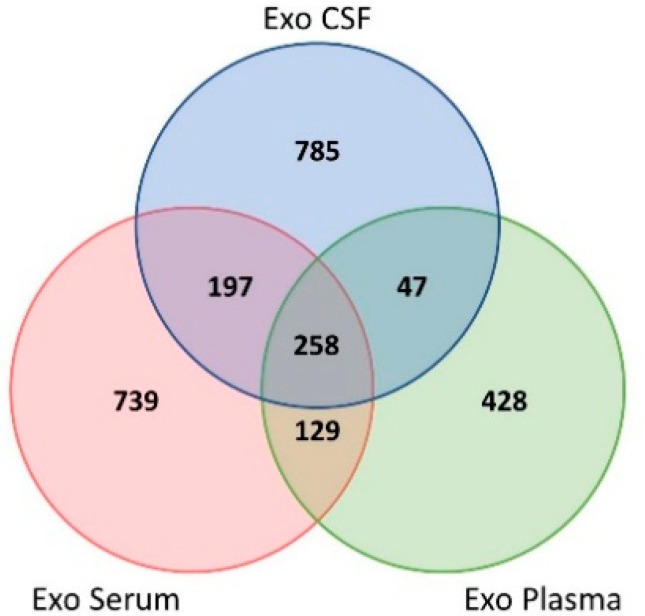
To address the shared biofluids’ proteome, the protein lists were compared by means of a Venn diagram (Figure 3). A total of 258 proteins were identified in exosomes from all three biofluids (CSF, plasma, serum). In addition, 197 proteins were shared by both CSF and serum, whereas 47 proteins were shared by both CSF and plasma.
Figure 3.
Overlap of the distinct biofluids exosomal proteomes. Venn diagram overlapping serum, plasma and CSF-derived exosomal proteomes. Abbreviations: Exo, exosomes; CSF, cerebrospinal fluid.
GO analysis for the three biofluids shared exosomal proteome was carried out (Figure 4). Comparatively to the global proteomes, differences were found in the top five molecular functions, “molecular transducer activity” arises at the top five. The most relevant biological processes associated to these proteins were “cellular process”, “metabolic process”, “response to stimulus”, “biological regulation” and “immune system process”. The latter process arises in the top five, for the shared proteome, in comparison to the general proteomes.
Figure 4.
GO analysis of the shared exosomal proteome. Shared exosomal proteome associated molecular functions (a), biological processes (b) and pathways (c). GO analysis was performed for gene lists corresponding to the exosomal proteomes from serum, plasma and CSF, through the Panther classification system. Abbreviations of molecular function GOs terms: GO:0005488 binding; GO:0003824 catalytic activity; GO:0098772 molecular function regulator; GO:0005198 structural molecule activity; GO:0060089 molecular transducer activity; GO:0005215 transporter activity; GO:0140110 transcription regulator activity. Abbreviations of biological process GOs terms: GO:0009987 cellular process; GO:0008152 metabolic process; GO:0050896 response to stimulus; GO:0002376 immune system process; GO:0065007 biological regulation; GO:0051179 localization; GO:0032501 multicellular organismal process; GO:0022610 biological adhesion; GO:0032502 developmental process; GO:0000003 reproduction. Abbreviations of pathways GOs terms: P00011 blood coagulation; P00016 cytoskeletal regulation by Rho GTPase; P00034 integrin signaling pathway; P00029 Huntington disease; P00049 Parkinson disease; P00050 plasminogen activating cascade; P00044 nicotinic acetylcholine receptor signaling pathway; P00031 inflammation mediated by chemokine and cytokine signaling pathway; P00024 glycolysis; P00006 apoptosis signaling pathway; P06664 gonadotropin-releasing hormone receptor pathway; P06959 Cholecystokinin receptor (CCKR) signaling map; P00004 Alzheimer’s disease-presenilin pathway; P00057 Wnt signaling pathway; P00005 angiogenesis.
Differences were also evident for the top five pathways, which include “blood coagulation”, “cytoskeleton regulation by Rho GTPase”, “integrin signaling pathway”, “Huntington disease”, and “Parkinson disease”. Indeed, several pathways related with distinct brain disorders, including AD, were evident in this shared proteome.
2.3. Potential Blood-Derived Exosomal Candidates for AD
Considering the interest in identifying putative biomarker candidates for AD in peripheral biofluids, a literature search was performed to identify Aβ-binding proteins in the group of the 258 exosomal proteins that were shared by the three biofluids. A total of 42 Aβ-binding proteins were identified and alterations reported for these protein levels in either biofluids, brain or both, were annotated (Supplementary Table S1 and Table 1).
Table 1.
Aβ-binding proteins found in the shared exosomal proteome and their expression tendency in AD. Gene and protein names of Aβ-binding proteins identified in the exosomes isolated from CSF, serum and plasma are indicated in the table, as well as the respective alterations reported in AD.
| Gene | Protein Name | CSF | Serum | Plasma | Brain | Publication |
|---|---|---|---|---|---|---|
| A2M | Alpha-2-macroglobulin | ↑/= | ↑/= | [24,25,26] | ||
| ALB | Albumin | = | = | [26,27,28,29] | ||
| APCS | Serum amyloid P-component | = | = | ↑ | [30,31,32] | |
| APOA1 | Apolipoprotein A-I | = | ↓ | ↓/= | = | [33,34,35,36,37,38,39,40,41,42,43] |
| APOA2 | Apolipoprotein A-II | ↓ | ↓/= | [34,35,38,42] | ||
| APOA4 | Apolipoprotein A-IV | ↑ | [41] | |||
| APOB | Apolipoprotein B-100 | ↓/=/↑ | [35,43,44] | |||
| APOC1 | Apolipoprotein C-I | = | ↓ | [42,45] | ||
| APOC3 | Apolipoprotein C-III | ↓ | [43] | |||
| APOE | Apolipoprotein E | =/↑ | ↑/= | ↓/= | [33,39,40,43,46,47] | |
| BCHE | Cholinesterase | = | ↓ | ↑ | [48,49,50] | |
| C1QA, C1QB and C1QC | Complement C1q | ↓* | ↑** | [51,52,53] | ||
| C3 | Complement C3 | ↑ | ↑ | [54,55] | ||
| C4BPα | C4b-binding protein alpha chain | =* | =*/↓ | [35,56] | ||
| CAT | Catalase | = | ↑/= | [57,58,59] | ||
| CFH | Complement factor H | ↓ | = | = | [60,61,62,63] | |
| CFI | Complement factor I | = | [63] | |||
| CLU | Clusterin | ↑ | ↑/= | ↑/= | [33,35,39,46,47,64,65,66,67] | |
| CSTB | Cystatin-B | Not found | ||||
| F12 | Coagulation factor XII | ↑ | = | [68] | ||
| FGB | Fibrinogen beta chain | ↑ | [44] | |||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | ↑** | ↑ | [69,70] | ||
| GC | Vitamin D-binding protein | ↓ | [35,44] | |||
| GSN | Gelsolin | ↓ | ↓/↑ | =/↓ | [35,71,72,73,74] | |
| HP | Haptoglobin | ↑ | ↑/= | [26,75,76,77,78] | ||
| HSPB1 | Heat shock protein beta-1 | ↑ | [79] | |||
| IGHM | Immunoglobulin heavy constant mu | ↑ | [35] | |||
| L1CAM | Neural cell adhesion molecule L1 | =*** | [19,80] | |||
| LRP1 | Prolow-density lipoprotein receptor-related protein 1 | ↓ | ↑/↓ | [81,82,83,84] | ||
| NCL | Nucleolin | ↓ | [85] | |||
| PFN1 | Profilin-1 | Not found | ||||
| PZP | Pregnancy zone protein | ↑ | ↑ | [86,87] | ||
| RELN | Reelin | ↑ | = | ↑ | [88,89,90] | |
| S100A8 | Protein S100-A8 | ↓ | [91] | |||
| S100A9 | Protein S100-A9 | ↓ | ↓ | = | [91,92,93] | |
| SELENOP | Selenoprotein P | ↑ | ↑ | [94] | ||
| SERPINA1 | Alpha-1-antitrypsin | ↑/= | ↑/= | ↑/= | [26,42,67,95,96] | |
| SERPINA3 | Alpha-1-antichymotrypsin, ACT | ↑/= | ↑/= | ↑ | ↑ | [95,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114] |
| TF | Serotransferrin | = | = | [115,116] | ||
| THBS1 | Thrombospondin-1 | ↓ | ↓ | [91,117] | ||
| TTR | Transthyretin | ↓/= | ↓ | ↓/↑ | [35,118,119,120,121,122] | |
| TUBB | Tubulin | ↓* | [70] |
* For C1QA, C1QB and C1QC, articles where C1Q levels were assessed were considered. The same happened for the tubulin β chain and C4BPα chain, where tubulin or C4BP levels were considered, independently of the chain. ** Levels of S-glutathionylated GAPDH form were reported. *** Plasma-derived exosomes. ↑, increased; ↓, decreased; =, no change.
Important key players in AD pathology were found in this shared exosomal proteome. Among the Aβ-binding proteins is apolipoprotein E (APOE) that can interact and form complexes with Aβ [123,124,125], being that the ApoE4 allele was reported to promote amyloidogenesis [126]. ApoE is also involved in Aβ clearance across the blood-brain barrier and even some isoforms of APOE interact with Tau protein [127,128].
Both alpha-1-antichymotrypsin and alpha-1-antitrypsin are serine protease inhibitors found in senile plaques and neurofibrillary tangles [129], although with opposite roles in AD: alpha-1-antichymotrypsin associates with Aβ, promoting the formation of fibrils and it can also induce tau hyperphosphorylation in neurons [130,131,132] whereas alpha-1-antitrypsin exerts anti-inflammatory properties and, thus, a protective role by mediating the microglial response [133].
Alpha-2-macroglobulin (A2M), clusterin (Clu, also known as ApoJ), prolow-density lipoprotein receptor-related protein 1 (LRP1) and transthyretin (TTR) are other proteins found, that could prevent the misfolding and aggregation of soluble Aβ and/or promoting Aβ clearance. The alpha-2-macroglobulin promotes a protease-mediated Aβ-degradation and acts as a chaperone [134] whereas ApoJ and LRP1 are involved in the Aβ transport across the blood-brain barrier [135,136]. LRP1 can bind to both free Aβ, ApoE/Aβ complexes or alpha-2-macroglobulin/Aβ complexes [137].
Transthyretin is another protective element since it mediates Aβ transport across the blood-brain barrier and can proteolytically cleave Aβ, inhibiting Aβ fibrils formation [138,139].
Complement components were also evident in the proteome lists; among them C1QA subcomponent subunits, C1S, C3, C4B, CFH and CFI. In AD, the complement system is overexpressed in the brain as a response to Aβ stimulation [140]. The group of heat shock proteins represented here by the heat shock protein beta-1 (HSPB1) plays an important role in AD as chaperones due to their ability to refold proteins, acting as anti-amyloidogenic agents.
Another Abeta-binding protein found was gelsolin (GSN), which is a multifunctional actin-binding protein, involved in processes such as cytoskeletal remodeling and cell motility, and it is also considered an amateur chaperone. Several studies reported that gelsolin can prevent Aβ aggregation, exerting neuroprotective roles [141]. This protective role of gelsolin has been the focus of several studies that employed gelsolin infusions or performed its transgene expression and observed a reduction in amyloid pathology [142,143]. GSN exists in two forms, a cytoplasmic form (intracellular) and, as a secreted form in CSF or blood. These differ in length, the plasma secreted form is longer than the intracellular form, and in the disulfide structure [144]. GSN is an abundant protein in blood, present at concentrations around 150–300 µg/mL [141,145] and the levels in plasma correlated with disease progression, assessed by Mini-Mental State Examination (MMSE) [71]. The intracellular form of GSN regulates the actin assembly, and it has an anti-apoptotic and tumor suppressive role [141]. Interestingly, previous work by the group demonstrated that Aβ peptides can form complexes with both gelsolin and laminin, another Aβ-binding protein with neuroprotective impacts, and reverse the peptide intracellular effects. In addition, cell treatment with Aβ decreased the levels of GSN suggesting a potential mechanism to decrease its clearance and enhance neurodegeneration [146]. Taken together, the data prompted an assessment of the levels of gelsolin in serum-derived exosomes using two distinct study groups, one from the pcb-cohort, established at the University of Aveiro (UA), and another from the University Medical Center Goettingen (UMG) cohort. Demographics and clinical data of the UA- and the UMG-study groups is presented in Supplementary Tables S2 and S3, respectively. No differences were found for the mean age, and years of literacy in both groups. Regarding MMSE scores, differences were only found between controls and the UA-dementia group (clinical dementia rating (CDR) ≥ 1 and MMSE+). For the UMG-AD group, CSF biomarker measurements reflected cognitive alterations and, as expected, the CSF Aβ1-42/1-40 ratio was decreased in AD cases while total-Tau and P-Tau levels were increased.
2.4. Gelsolin as Putative Exosomal Biomarker Candidate
Extracellular vesicles compatible with exosome like-characteristics were isolated and characterized as previously described [9,147]. Particle concentration and mode size (Supplementary Figure S1) were assessed by nanoparticle tracking analysis (NTA) revealing no significant differences between controls and individuals with dementia. The nature of the EVs samples was further assessed by Western blot analysis for exosomal markers as TSG101 and HSP70. As expected, these were detected in the EVs preparations, while no signal was detected for calnexin.
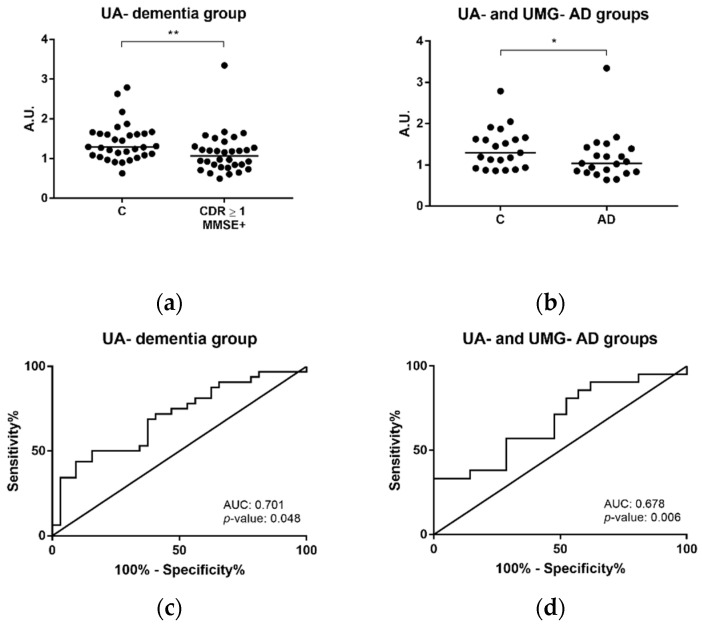
GSN levels were first evaluated by Western blot analysis, in serum-derived EVs of controls and individuals with dementia, including clinical diagnosed AD cases from the pcb-cohort (Figure 5 and Supplementary Figure S2). The antibody used recognizes both the intracellular and secreted GSN forms. Gelsolin levels were significantly decreased in serum-derived exosomes of individuals with dementia (CDR ≥ 1 and MMSE+) when compared with controls (CDR = 0 and MMSE–) (Figure 5a, p-value = 0.005). The same pattern was observed for controls and AD cases of both UA- and UMG- groups (n = 21 controls and n = 21 AD) (Figure 5b, p-value = 0.049). Receiver operating curves (ROC curves) were calculated and the area under the curve (AUC) was approximately 0.7, for both controls and individuals with dementia from the UA–dementia group (Figure 5c) and controls versus AD cases (Figure 5d).
Figure 5.
Gelsolin levels in dementia and AD determined by Western blot analysis. Immunoblot analysis of gelsolin in serum-derived exosomes from controls (CDR = 0 and MMSE–) and individuals with dementia (CDR ≥ 1 and MMSE+) from University of Aveiro (UA)-dementia group (a), including AD clinically diagnosed cases and controls (UA- and University Medical Center Goettingen (UMG)-groups) (b). ROC curves were obtained for the control and dementia group (UA-dementia group) (c) and controls and AD cases from UA and UMG (d). Each point represents the densitometry ratio obtained for each individual and the solid horizontal line shows median. Abbreviations: AD, Alzheimer’s disease; A.U., arbitrary units; AUC, area under the curve; C, controls; CDR, clinical dementia rating; MMSE, Mini-Mental State Examination; ROC, receiver operating curve. ** p ≤ 0.01, * p ≤ 0.05.
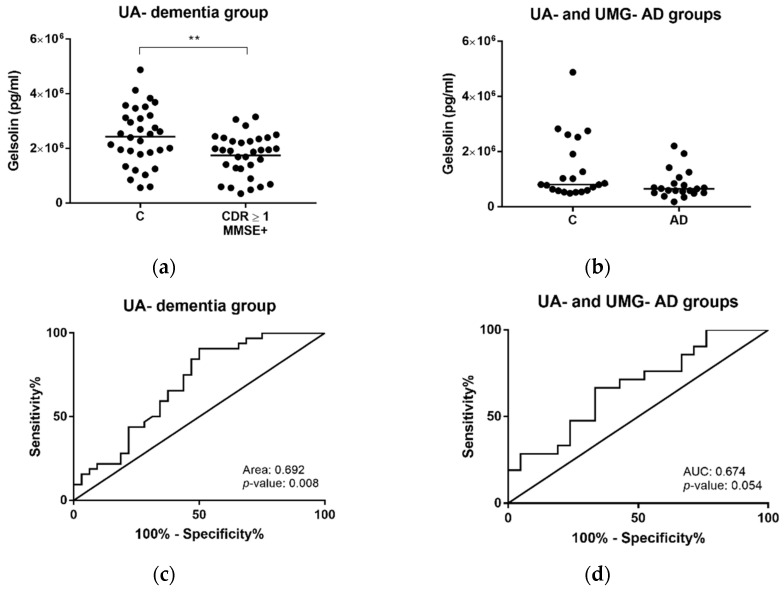
Further, results obtained by Western blot analysis were validated by a complementary approach, using an enzyme-linked immunosorbent assay (ELISA)-based assay for detection of human GSN levels. Consistently, a significant decrease in the median levels of GSN was likewise observed for serum-derived exosomes in the dementia group (CDR ≥ 1 and MMSE+) when compared with controls (CDR = 0 and MMSE+) (p-value = 0.008) (Figure 6a). In the case of controls and ADs from both UA- and UMG-groups, the same pattern of decrease was exhibited for AD cases, although without significant differences (p-value = 0.055) (Figure 6b). ROC curves were calculated and the AUC obtained was again approximately 0.7 when comparing controls and CDR ≥ 1 and MMSE+ individuals from the UA-dementia group (Figure 6c); and controls and AD cases from UA- and UMG-groups (Figure 6d).
Figure 6.
Gelsolin concentrations in dementia and AD cases determined by ELISA. Gelsolin levels were assessed through ELISA assays in serum-derived exosomes from controls (CDR = 0 and MMSE–) and individuals with dementia (CDR ≥ 1 and MMSE+) from UA-dementia group (a), and from AD clinically diagnosed cases and controls from UA- and UMG-groups (b). ROC curves were obtained for the control and dementia group (UA-dementia group) (c) and controls and AD cases from UA- and UMG-groups (d). Each point represents the gelsolin mean concentration obtained for each individual and the solid horizontal line shows median of the groups. Abbreviations: AD, Alzheimer’s disease; A.U., arbitrary units; AUC, area under the curve; C, controls; CDR, clinical dementia rating; ELISA, enzyme-linked immunosorbent assay, MMSE, Mini-Mental State Examination; ROC, receiver operating curve. ** p ≤ 0.01.
In summary, GSN levels appear to hold potential as a biomarker candidate for dementia/AD. Of note, other Aβ-binding protein candidates identified (laminin and clusterin) can represent likewise interesting targets for AD, that can be tested in future studies. In fact, the use of a combination of blood-derived exosomal biomarkers could undoubtedly increase the sensitivity and specificity of the diagnosis. To validate gelsolin´s potential, also regarding differential diagnosis, its levels should be assessed in a higher number of individuals, with distinct dementia subtypes, or even including other pathologies where GSN levels might also be altered [141,145]. Nonetheless, the results herein presented are in accordance with the reduced levels of plasma and CSF gelsolin and their correlation to the disease progression, already reported in the literature [71,72,73]. To our knowledge, this is the first time that GSN levels were assessed in serum-derived EVs, in the context of AD. GSN has been signaled as an antiamyloidogenic protein in AD, since this protein has the ability to either bind Aβ promoting its clearance, preventing its fibrillization or both. Hence, it is not surprising that if gelsolin is counteracting disease progression in the brain, its levels can be decreased to some degree in blood-derived exosomes; an aspect that deserves further clarification. It would be interesting to simultaneously compare the diagnostic sensitivity and specificity of GSN in serum/plasma and in exosomes-derived from serum/plasma. In addition, as exosomes’ cargos can reflect disease stages it is not surprising that exosomal gelsolin levels in the periphery could change during dementia progression.
3. Materials and Methods
3.1. Exosomal Proteomes Construction
Exosomal proteomes for the three biofluids (CSF, serum and plasma) were obtained from three online databases: EXOCARTA, Vesiclepedia and EVpedia (all accessed on 12 January 2020). All databases comprise protein, lipids and nucleic acids data and are all manually curated. EXOCARTA was the first of these databases to be launch and includes data only from exosomes (http://www.exocarta.org/) [148,149,150,151]. Vesiclepedia was launched later and, now, it gathers information from all classes of extracellular vesicles among them exosomes, microparticles and microvesicles (http://microvesicles.org/) [152,153]. EVpedia is the only one of the three databases which includes eukaryotic and prokaryotic extracellular vesicles data (http://student4.postech.ac.kr/evpedia2_xe/xe/) [154,155]. In the work described here, only human proteomes were considered.
The exosomal protein cargo for each biofluid was refined through several steps (Figure 1), considering the following: number of entries for the specific biofluid, number of entries for H. sapiens, number of entries for H. sapiens and exosomes, number of ID entries for gene names of H. sapiens and exosomes. Finally, repeated gene names were removed to obtain the final biofluid gene list. The EVpedia output was in the form of Uniprot IDs and thus it was necessary to convert this data to gene names. To achieve this the Uniprot Retrieve/ID mapping tool was used to convert the Uniprot IDs to gene names (https://www.uniprot.org/uploadlists/; accessed on 14 January 2020).
The proteome list for each biofluid includes every protein/corresponding gene name present in at least one of the databases. In the case of CSF, only data from EVpedia was collected since no information was available in the other databases.
Additionally, the proteomes obtained from the databases were complemented by including information from a literature search on PubMed (https://PubMed.ncbi.nlm.nih.gov/; accessed on 17 January 2020) using the keywords: “exosomes and Alzheimer´s disease and CSF or Serum or Plasma” (Supplementary Table S4). The protein name was converted to gene name, as described above. Thus, the final proteome lists for each biofluid included information collected from the databases and from the literature search (Supplementary Tables S5–S7). These final lists will be herein referred to as the distinct biofluid proteomes.
3.2. Proteome GO and Overlap Analysis
All proteomes were analyzed using the Panther classification system (http://www.pantherdb.org/), version 14. Gene ontology (GO) enrichment analysis at the molecular function, biological process was carried out on the 22 January 2020. The biofluids proteomes’ main pathways were likewise identified.
The proteomes of the different biofluids, were compared and overlapped against each other by means of a Venn diagram using the Bioinformatics and Evolutionary Genomics website (http://bioinformatics.psb.ugent.be/webtools/Venn/; accessed on 30 January 2020). This allowed identifying the shared biofluids proteome, that is also present in CSF.
3.3. Aβ-binding Proteins Literature Search
To identify the Aβ-binding proteins present in the group of 258 exosomal proteins shared by the three biofluids (CSF, serum and plasma), an individual PubMed search was performed for each protein using the following keywords “(protein name) and Abeta and (binding or clearance or aggregating or fibril formation)” or “(gene name) and Abeta and (binding or clearance or aggregating or fibril formation)”.
3.4. EVs Isolation and Characterization
Human samples were obtained from participants of the pcb-cohort, a primary care-based cohort stablished by the group, which includes controls and individuals with dementia, characterized by cognitive testing such as clinical dementia rating (CDR) and Mini-Mental State Examination (MMSE) [156,157]. CDR scale applied scores between 0 and 3 where 0 accounts for normal, 1 for mild dementia, 2 for moderate and 3 for severe dementia stages. The MMSE scale considered scores between 0 and 30 and the cutoffs were set according to the Portuguese population: 0–2 years of literacy, cutoff = 22; 3–6 years of literacy, cutoff = 24; and ≥7 years of literacy, cutoff = 27. Tests equal to or below cutoff were scored to cognitive deficits and those above were scored as normal. In this study, controls (n = 32; CDR = 0 and that scored negative for MMSE (MMSE–); mean age 76.69 ± 8.07) and demented individuals (n = 32; CDR ≥ 1 and that scored positive for MMSE (MMSE+); mean age 77.38 ± 9.17) were included. AD clinical diagnosed cases were also monitored, 8 AD patients that were CDR ≥ 1 and MMSE+, plus 1 AD patient who scored CDR = 1 (mean age 78.67 ± 5.07); and 9 age- and sex-matched controls (mean age 77.56 ± 4.83), Supplementary Table S2. The study is part of a project approved by the Ethics Committee (Comissão de Ética para a Saúde da ARS Centro, protocol No. 012804-04.04.2012) and by the National Committee for Data Protection (CNPD No. 369/2012). Serum from controls (n = 12; mean age 67.58 ± 7.74) and AD cases (n = 12; mean age 73.17 ± 10.66) were also obtained from the UMG-cohort, established at the University Medical Center of Goettingen, Germany. These individuals were highly characterized including either cognitive testing, CSF-neurochemical diagnostic biomarkers, PET imaging, or in combination, as described in [9], Supplementary Table S3. The collection of these samples and their use was approved by the ethics committee of the University of Goettingen (9/2/16). All participants gave written informed consent.
Serum-derived EVs, with exosome-like characteristics, were isolated from 250 µL of serum from controls and individuals with either dementia, AD, or both, cases using the ExoQuick Serum Exosome Precipitation Solution (System Biosciences, Palo Alto, CA, USA). The resulting exosomal pellet was resuspended in RIPA buffer (Sigma-Aldrich) with protease inhibitors for Western blot and ELISA; or in PBS for NTA analysis, as previously described [9,147].
Randomly chosen samples were used for NTA analysis and Western blot procedures. NTA analyses were performed using a Nanosight NS300 (Malvern Instruments, Malvern, UK) and NTA 3.2 software (Malvern Instruments, Malvern, UK). NTA analysis was then carried out in duplicate for each sample. Particle numbers obtained were multiplied by the dilution factor to determine particle concentration.
A set of exosomal markers was also tested for exosome characterization. After bicinchoninic acid assay (BCA) protein quantification assay, normalized samples for protein content (50 µg of protein) were used for SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by a wet electrophoretic transfer of the proteins. Nitrocellulose membranes were blocked in non-fat dry milk solution (5%) and incubated overnight with the exosomal marker antibodies, anti-TSG101 (1:500) (612,697; BD transduction laboratories, San Jose, CA, USA) and anti-HSP70 (1:200) (sc-24; Santa Cruz Biotechnology, Dallas, TX, USA) or the negative exosomal marker antibody anti-calnexin (1:500) (ADI-SPA-860-J; Enzo Life Sciences, Farmingdale, NY, USA). This was followed by incubation with the anti-mouse IgG, HRP-linked antibody (1:2000) (Cell Signaling Technology, Danvers, MA, USA) or anti-rabbit IgG, HRP-linked antibody (1:2000) (7074; Cell Signaling Technology, Danvers, MA, USA). Protein bands were detected with the Chemidoc gel imaging system (Bio-Rad, Hercules, CA, USA) with Image Lab Touch Software (Bio-Rad, Hercules, CA, USA).
3.5. Evaluation of Gelsolin Levels
For GSN level evaluation, an equal amount of total protein (50 µg) was likewise loaded from each sample. An exosomal pool was included in each membrane for sample normalization between membranes. SDS-PAGE was carried out and the proteins were electrophoretic transferred to nitrocellulose membranes in a wet system, as described above. The membranes were blocked and incubated overnight with the primary monoclonal antibody, anti-gelsolin (1:500) (G4896; Sigma-Aldrich). Then, the membranes were incubated with the secondary anti-mouse IgG, HRP-linked antibody (1:5000) (Cell Signaling Technology, Danvers, MA, USA). To detect the protein bands, chemiluminescence reagent ECL Select (GE Healthcare Life Sciences, Chicago, IL, USA) was added to the membranes and images were obtained using Chemidoc gel imaging system (Bio-Rad, Hercules, CA, USA) with Image Lab Touch Software (Bio-Rad, Hercules, CA, USA). Further, gelsolin levels were also accessed in serum-derived exosomes using the commercial Human Gelsolin ELISA Kit (ab270215; Abcam, Cambridge, UK) following the manufacturer’s instructions. Samples were diluted (1:100) and equal amounts of total protein (0.5 µg) for each sample were added for GSN quantification by ELISA.
3.6. Statistical Analysis
The Shapiro–Wilk test was used to assess the data distribution. Particle concentrations, mode sizes, cognitive scores, CSF biomarker results and median gelsolin levels between controls and the dementia group (CDR ≥ 1 and MMSE+) or controls and AD were compared, using the Mann–Whitney test, since data did not follow a normal distribution. In the case of gelsolin, an exosomal pool sample was used to normalize data across results either in Western blot or ELISA assays. For immunoblotting, the densitometry value of each individual band was divided by the densitometry value of the pool within the same membrane. These ratios were expressed as arbitrary units (A.U.). In ELISA assays, the mean concentration obtained for each individual was divided by the mean concentration of the exosomal pool within the same ELISA plate and then multiplied by the mean concentrations of all pools, from all plates. The level of significance was set at or below 5%. Analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA) or SPSS version 22 (IBM).
4. Conclusions
Exosome content can reflect physiological and pathological stages highlighting the potential of these EVs as biomarkers resources for disease diagnostics or therapeutics. This is particularly relevant in diseases such as AD, where the only molecular available methodology is based on monitoring of a biomarker triplet in the CSF, which requires a lumbar puncture, limiting its use as a widely available tool, hence identification of peripheral biomarker is of clinical value.
Biomarkers that enter the bloodstream, in particular those arising from the brain will be considerably diluted, but exosomes may represent a contained source of protein candidates. Exosomes are innovative tools with huge biomarker potential in AD, not only because these nanovesicles may represent an enclosed source of biomarker candidates, but also because exosomes arising from peripheral biofluids can be more accessible and widely available. Besides GSN, the bioinformatic analysis carried out revealed several exosomal putative biomarker candidates, some already linked to AD that can be further tested for their differential and diagnostic potential.
Acknowledgments
We thank the volunteers and their families, as well as to all the health professionals, involved in this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22083933/s1, Figure S1: Serum-derived EVs characterization; Figure S2: Western blot analysis of gelsolin in UA- and UMG-groups; Table S1: Aβ-binding proteins found in exosomal proteomes from CSF, serum and plasma; Table S2: Demographics and clinical data of UA-group participants; Table S3: Demographics and clinical data of UMG-group participants, Table S4: List of proteins found in exosomes isolated from CSF, serum and plasma and previously related to Alzheimer’s disease; Table S5: Final CSF proteome list; Table S6: Final serum proteome list; Table S7: Final plasma proteome list.
Author Contributions
Conceptualization, A.G.H. and T.S.M.; methodology, T.S.M., R.M., M.F., M.V., I.M.R., O.C.S. and A.G.H.; investigation, T.S.M., R.M., M.F. and A.G.H.; data curation, T.S.M., M.F., R.M.S. and A.G.H.; writing—original draft preparation, T.S.M., O.C.S. and A.G.H.; writing—review and editing, T.S.M., R.M., M.F., R.M.S., I.M.R., J.V., J.W., O.C.S. and A.G.H.; supervision, A.G.H.; funding acquisition, A.G.H. and O.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Alzheimer’s Association (2019-AARG-644347) and supported by iBiMED UIDB/04501/2020, FCT, COMPETE program, QREN, European Union; “pAGE” (CENTRO2020-CENTRO-01-0145-FEDER-000003), Centro 2020 program, Portugal 2020, European Union. T.S.M. is supported by the FCT through the individual PhD grant (SFRH/BD/145979/2019). J.W. is supported by an Ilídio Pinho professorship at University of Aveiro.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee (Comissão de Ética para a Saúde da ARS Centro, protocol No. 012804-04.04.2012) and by the National Committee for Data Protection (CNPD N◦ 369/2012). The collection of UMG-Cohort samples and their use was approved by the ethics committee of the University of Goettingen (9/2/16).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:1–18. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus C., Mena E., Subramaniam R.M. Brain PET in the diagnosis of Alzheimer’s disease. Clin. Nucl. Med. 2014;39:e413–e426. doi: 10.1097/RLU.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberghe R., Adamczuk K., Dupont P., Van Laere K., Chételat G. Amyloid PET in clinical practice: Its place in the multidimensional space of Alzheimer’s disease. NeuroImage Clin. 2013;2:497–511. doi: 10.1016/j.nicl.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blennow K., Zetterberg H. Cerebrospinal Fluid biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis. 2009;18:413–417. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 5.Welge V., Fiege O., Lewczuk P., Mollenhauer B., Esselmann H., Klafki H.-W., Wolf S., Trenkwalder C., Otto M., Kornhuber J., et al. Combined CSF tau, p-tau181 and amyloid-β 38/40/42 for diagnosing Alzheimer’s disease. J. Neural Transm. 2009;116:203–212. doi: 10.1007/s00702-008-0177-6. [DOI] [PubMed] [Google Scholar]
- 6.Hampel H., O’Bryant S.E., Molinuevo J.L., Zetterberg H., Masters C.L., Lista S., Kiddle S.J., Batrla R., Blennow K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018;14:639–652. doi: 10.1038/s41582-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 8.Martins T.S., Trindade D., Vaz M., Campelo I., Almeida M., Trigo G., Silva O.A.B.D.C.E., Henriques A.G. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. J. Neurochem. 2021;156:162–181. doi: 10.1111/jnc.15112. [DOI] [PubMed] [Google Scholar]
- 9.Martins T.S., Magalhães S., Rosa I.M., Vogelgsang J., Wiltfang J., Delgadillo I., Catita J., Silva O.A.D.C.E., Nunes A., Henriques A.G. Potential of FTIR Spectroscopy applied to exosomes for Alzheimer’s disease discrimination: A pilot study. J. Alzheimer’s Dis. 2020;74:391–405. doi: 10.3233/JAD-191034. [DOI] [PubMed] [Google Scholar]
- 10.Kapogiannis D., Mustapic M., Shardell M.D., Berkowitz S.T., Diehl T.C., Spangler R.D., Tran J., Lazaropoulos M.P., Chawla S., Gulyani S., et al. Association of extracellular vesicle biomarkers with Alzheimer disease in the baltimore longitudinal study of aging. JAMA Neurol. 2019;76:1340–1351. doi: 10.1001/jamaneurol.2019.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajendran L., Honsho M., Zahn T.R., Keller P., Geiger K.D., Verkade P., Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha M.S., Ansell-Schultz A., Civitelli L., Hildesjö C., Larsson M., Lannfelt L., Ingelsson M., Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi P.G., Turola E., Ruiz A., Bergami A., Libera D.D., Benussi L., Giussani P., Magnani G., Comi G., Legname G., et al. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2014;21:582–593. doi: 10.1038/cdd.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding M., Shen Y., Wang P., Xie Z., Xu S., Zhu Z., Wang Y., Lyu Y., Wang D., Xu L., et al. exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem. Res. 2018;43:2165–2177. doi: 10.1007/s11064-018-2641-5. [DOI] [PubMed] [Google Scholar]
- 15.Canales-Aguirre A.A., Reza-Zaldivar E.E., Hernández-Sapiéns M.A., Gutiérrez-Mercado Y.K., Sandoval-Ávila S., Gomez-Pinedo U., Márquez-Aguirre A.L., Vázquez-Méndez E., Padilla-Camberos E. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019;14:1626–1634. doi: 10.4103/1673-5374.255978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuyama K., Sun H., Usuki S., Sakai S., Hanamatsu H., Mioka T., Kimura N., Okada M., Tahara H., Furukawa J.-I., et al. A potential function for neuronal exosomes: Sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589:84–88. doi: 10.1016/j.febslet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Bulloj A., Leal M.C., Xu H., Castaño E.M., Morelli L. Insulin-degrading enzyme sorting in exosomes: A secretory pathway for a key brain amyloid-β degrading protease. J. Alzheimer’s Dis. 2010;19:79–95. doi: 10.3233/JAD-2010-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacheco-Quinto J., Clausen D., Pérez-González R., Peng H., Meszaros A., Eckman C.B., Levy E., Eckman E.A. Intracellular metalloprotease activity controls intraneuronal Aβ aggregation and limits secretion of Aβ via exosomes. FASEB J. 2018;33:3758–3771. doi: 10.1096/fj.201801319R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiandaca M.S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J.B., Abner E.L., Petersen R.C., Federoff H.J., Miller B.L., et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015;11:600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetzl E.J., Schwartz J.B., Abner E.L., Jicha G.A., Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018;83:544–552. doi: 10.1002/ana.25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetzl E.J., Kapogiannis D., Schwartz J.B., Lobach I.V., Goetzl L., Abner E.L., Jicha G.A., Karydas A.M., Boxer A., Miller B.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30:4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapogiannis D., Boxer A., Schwartz J.B., Abner E.L., Biragyn A., Masharani U., Frassetto L., Petersen R.C., Miller B.L., Goetzl E.J. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viola K.L., Klein W.L. amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varma V.R., Varma S., An Y., Hohman T.J., Seddighi S., Casanova R., Beri A., Dammer E.B., Seyfried N.T., Pletnikova O., et al. Alpha-2 macroglobulin in Alzheimer’s disease: A marker of neuronal injury through the RCAN1 pathway. Mol. Psychiatry. 2017;22:13–23. doi: 10.1038/mp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scacchi R., Ruggeri M., Gambina G., Martini M.C., Corbo R.M. α2-Macroglobulin Deletion Polymorphism and Plasma Levels in Late Onset Alzheimer’s disease. Clin. Chem. Lab. Med. 2002;40:333–336. doi: 10.1515/CCLM.2002.052. [DOI] [PubMed] [Google Scholar]
- 26.Giometto B., Argentiero V., Sanson F., Ongaro G., Tavolato B. Acute-Phase Proteins in Alzheimer’s disease. Eur. Neurol. 1988;28:30–33. doi: 10.1159/000116224. [DOI] [PubMed] [Google Scholar]
- 27.Can M., Varlibas F., Guven B., Akhan O., Yuksel G.A. Ischemia Modified Albumin and Plasma Oxidative Stress Markers in Alzheimer’s disease. Eur. Neurol. 2013;69:377–380. doi: 10.1159/000339006. [DOI] [PubMed] [Google Scholar]
- 28.Schirinzi T., Di Lazzaro G., Sancesario G.M., Colona V.L., Scaricamazza E., Mercuri N.B., Martorana A., Sancesario G. Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer’s disease. J. Neural Transm. 2017;124:1621–1625. doi: 10.1007/s00702-017-1786-8. [DOI] [PubMed] [Google Scholar]
- 29.Miners J.S., Kehoe P.G., Love S., Zetterberg H., Blennow K. CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimer’s Res. Ther. 2019;11:1–6. doi: 10.1186/s13195-019-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford J.R., Bjorklund N.L., Taglialatela G., Gomer R.H. Brain serum amyloid P levels are reduced in individuals that lack dementia while having Alzheimer’s disease neuropathology. Neurochem. Res. 2012;37:795–801. doi: 10.1007/s11064-011-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Human neurons generate C-reactive protein and amyloid P: Upregulation in Alzheimer’s disease. Brain Res. 2000;887:80–89. doi: 10.1016/S0006-8993(00)02970-X. [DOI] [PubMed] [Google Scholar]
- 32.Verwey N.A., Schuitemaker A., Van Der Flier W.M., Mulder S.D., Mulder C., Hack C.E., Scheltens P., Blankenstein M.A., Veerhuis R. Serum amyloid p component as a biomarker in mild cognitive impairment and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008;26:522–527. doi: 10.1159/000178756. [DOI] [PubMed] [Google Scholar]
- 33.Song F., Poljak A., Crawford J., Kochan N.A., Wen W., Cameron B., Lux O., Brodaty H., Mather K., Smythe G.A., et al. Plasma Apolipoprotein Levels Are Associated with Cognitive Status and Decline in a Community Cohort of Older Individuals. PLoS ONE. 2012;7:e34078. doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawano M., Kawakami M., Otsuka M., Yashima H., Yaginuma T., Ueki A. Marked decrease of plasma apolipoprotein AI and AII in Japanese patients with late-onset non-familial Alzheimer’s disease. Clin. Chim. Acta. 1995;239:209–211. doi: 10.1016/0009-8981(95)06115-T. [DOI] [PubMed] [Google Scholar]
- 35.Song F., Poljak A., Kochan N.A., Raftery M., Brodaty H., Smythe G.A., Sachdev P.S. Plasma protein profiling of Mild Cognitive Impairment and Alzheimer’s disease using iTRAQ quantitative proteomics. Proteome Sci. 2014;12:5. doi: 10.1186/1477-5956-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merched A., Xia Y., Visvikis S., Serot J., Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging. 2000;21:27–30. doi: 10.1016/S0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 37.Liu H.-C., Hu C.-J., Chang J.-G., Sung S.-M., Lee L.-S., Yuan R.-Y., Leu S.-J. Proteomic Identification of Lower Apolipoprotein A-I in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2006;21:155–161. doi: 10.1159/000090676. [DOI] [PubMed] [Google Scholar]
- 38.Kuriyama M., Takahashi K., Yamano T., Hokezu Y., Togo S., Osame M., Igakura T. Low Levels of Serum Apolipoprotein AI and AII in Senile Dementia. Psychiatry Clin. Neurosci. 1994;48:589–593. doi: 10.1111/j.1440-1819.1994.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 39.Harr S.D., Uint L., Hollister R., Hyman B.T., Mendez A.J. Brain Expression of Apolipoproteins E, J, and A-I in Alzheimer’s disease. J. Neurochem. 2002;66:2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- 40.Song H., Saito K., Seishima M., Noma A., Urakami K., Nakashima K. Cerebrospinal fluid apo E and apo A-I concentrations in early- and late-onset Alzheimer’s disease. Neurosci. Lett. 1997;231:175–178. doi: 10.1016/S0304-3940(97)00558-2. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura Y., Usami R., Ichihara S., Kida H., Satoh M., Tomimoto H., Murata M., Oikawa S. Plasma protein profiling for potential biomarkers in the early diagnosis of Alzheimer’s disease. Neurol. Res. 2017;39:231–238. doi: 10.1080/01616412.2017.1281195. [DOI] [PubMed] [Google Scholar]
- 42.Kim W.S., He Y., Phan K., Ahmed R.M., Rye K.-A., Piguet O., Hodges J.R., Halliday G.M. Altered High Density Lipoprotein Composition in Behavioral Variant Frontotemporal Dementia. Front. Neurosci. 2018;12:847. doi: 10.3389/fnins.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih Y.-H., Tsai K.-J., Lee C.-W., Shiesh S.-C., Chen W.-T., Pai M.-C., Kuo Y.-M. Apolipoprotein C-III is an amyloid-β-Binding Protein and an Early Marker for Alzheimer’s disease. J. Alzheimer’s Dis. 2014;41:855–865. doi: 10.3233/JAD-140111. [DOI] [PubMed] [Google Scholar]
- 44.Muenchhoff J., Poljak A., Song F., Raftery M., Brodaty H., Duncan M., McEvoy M., Attia J., Schofield P.W., Sachdev P.S. Plasma Protein Profiling of Mild Cognitive Impairment and Alzheimer’s disease Across Two Independent Cohorts. J. Alzheimer’s Dis. 2014;43:1355–1373. doi: 10.3233/JAD-141266. [DOI] [PubMed] [Google Scholar]
- 45.Petit-Turcotte C., Stohl S.M., Beffert U., Cohn J.S., Aumont N., Tremblay M., Dea D., Yang L., Poirier J., Shachter N.S. Apolipoprotein C-I Expression in the Brain in Alzheimer’s disease. Neurobiol. Dis. 2001;8:953–963. doi: 10.1006/nbdi.2001.0441. [DOI] [PubMed] [Google Scholar]
- 46.Rezeli M., Zetterberg H., Blennow K., Brinkmalm A., Laurell T., Hansson O., Marko-Varga G. Quantification of total apolipoprotein E and its specific isoforms in cerebrospinal fluid and blood in Alzheimer’s disease and other neurodegenerative diseases. EuPA Open Proteom. 2015;8:137–143. doi: 10.1016/j.euprot.2015.07.012. [DOI] [Google Scholar]
- 47.Bertrand P., Poirier J., Oda T., Finch C.E., Pasinetti G.M. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Mol. Brain Res. 1995;33:174–178. doi: 10.1016/0169-328X(95)00097-C. [DOI] [PubMed] [Google Scholar]
- 48.Marksteiner J., Pirchl M., Ullrich C., Oberbauer H., Blasko I., Lederer W., Hinterhuber H., Humpel C. Analysis of Cerebrospinal Fluid of Alzheimer Patients. Pharmacology. 2008;82:214–220. doi: 10.1159/000156487. [DOI] [PubMed] [Google Scholar]
- 49.Zhuravin I.A., Nalivaeva N.N., Kozlova D.I., Kochkina E.G., Fedorova Y.B., Gavrilova S.I. The activity of blood serum cholinesterases and neprilysin as potential biomarkers of mild-cognitive impairment and Alzheimer’s disease. Zhurnal Nevrol. Psikhiatrii Im. Korsakova. 2015;115:110–117. doi: 10.17116/jnevro2015115112110-117. [DOI] [PubMed] [Google Scholar]
- 50.Macdonald I.R., Maxwell S.P., Reid G.A., Cash M.K., DeBay D.R., Darvesh S. Quantification of Butyrylcholinesterase Activity as a Sensitive and Specific Biomarker of Alzheimer’s disease. J. Alzheimer’s Dis. 2017;58:491–505. doi: 10.3233/JAD-170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyth M.D., Cribbs D.H., Tenner A.J., Shankle W., Dick M., Kesslak J., Cotman C.W. Decreased levels of C1q in cerebrospinal fluid of living Alzheimer patients correlate with disease state. Neurobiol. Aging. 1994;15:609–614. doi: 10.1016/0197-4580(94)00055-7. [DOI] [PubMed] [Google Scholar]
- 52.Khoonsari P.E., Häggmark A., Lönnberg M., Mikus M., Kilander L., Lannfelt L., Bergquist J., Ingelsson M., Nilsson P., Kultima K., et al. Analysis of the Cerebrospinal Fluid Proteome in Alzheimer’s disease. PLoS ONE. 2016;11:e0150672. doi: 10.1371/journal.pone.0150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winston C.N., Goetzl E.J., Schwartz J.B., Elahi F.M., Rissman R.A. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019;11:61–66. doi: 10.1016/j.dadm.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu T., Dejanovic B., Gandham V.D., Gogineni A., Edmonds R., Schauer S., Srinivasan K., Huntley M.A., Wang Y., Wang T.-M., et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 2019;28:2111–2123.e6. doi: 10.1016/j.celrep.2019.07.060. [DOI] [PubMed] [Google Scholar]
- 55.Litvinchuk A., Wan Y.-W., Cole A., Chen F., Liu Z., Zheng H. The role of complement c3 and c3ar receptor in tau pathology in Alzheimer’s disease. Alzheimer’s Dement. 2017;13:P229. doi: 10.1016/j.jalz.2017.07.115. [DOI] [Google Scholar]
- 56.Trouw L.A., Nielsen H.M., Minthon L., Londos E., Landberg G., Veerhuis R., Janciauskiene S., Blom A.M. C4b-binding protein in Alzheimer’s disease: Binding to Aβ1–42 and to dead cells. Mol. Immunol. 2008;45:3649–3660. doi: 10.1016/j.molimm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Puertas M., Martínez-Martos J., Cobo M., Carrera M., Mayas M., Ramírez-Expósito M. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp. Gerontol. 2012;47:625–630. doi: 10.1016/j.exger.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Zabel M., Nackenoff A., Kirsch W.M., Harrison F.E., Perry G., Schrag M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2018;115:351–360. doi: 10.1016/j.freeradbiomed.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovell M.A., Ehmann W.D., Butler S.M., Markesbery W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45:1594–1601. doi: 10.1212/WNL.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 60.Lu G., Liu W., Huang X., Zhao Y. Complement factor H levels are decreased and correlated with serum C-reactive protein in late-onset Alzheimer’s disease. Arq. Neuro-Psiquiatr. 2020;78:76–80. doi: 10.1590/0004-282x20190151. [DOI] [PubMed] [Google Scholar]
- 61.Gezen-Ak D., Dursun E., Hanağası H., Bilgiç B., Lohman E., Araz Ö.S., Atasoy I.L., Alaylıoğlu M., Önal B., Gürvit H., et al. BDNF, TNFα, HSP90, CFH, and IL-10 Serum levels in patients with early or late onset alzheimer’s disease or mild cognitive impairment. J. Alzheimer’s Dis. 2013;37:185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- 62.Williams M.A., Haughton D., Stevenson M., Craig D., Passmore A.P., Silvestri G. Plasma Complement factor H in Alzheimer’s disease. J. Alzheimer’s Dis. 2015;45:369–372. doi: 10.3233/JAD-142742. [DOI] [PubMed] [Google Scholar]
- 63.Strohmeyer R., Shen Y., Rogers J. Detection of complement alternative pathway mRNA and proteins in the Alzheimer’s disease brain. Mol. Brain Res. 2000;81:7–18. doi: 10.1016/S0169-328X(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 64.Nilselid A.-M., Davidsson P., Nägga K., Andreasen N., Fredman P., Blennow K. Clusterin in cerebrospinal fluid: Analysis of carbohydrates and quantification of native and glycosylated forms. Neurochem. Int. 2006;48:718–728. doi: 10.1016/j.neuint.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Schürmann B., Wiese B., Bickel H., Weyerer S., Riedel-Heller S.G., Pentzek M., Bachmann C., Williams J., Bussche H.V.D., Maier W., et al. Association of the Alzheimer’s disease clusterin risk allele with plasma clusterin concentration. J. Alzheimer’s Dis. 2011;25:421–424. doi: 10.3233/JAD-2011-110251. [DOI] [PubMed] [Google Scholar]
- 66.Thambisetty M., Simmons A., Velayudhan L., Hye A., Campbell J., Zhang Y., Wahlund L.-O., Westman E., Kinsey A., Güntert A., et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch. Gen. Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao P.-C., Yu L., Kuo C.-C., Lin C., Kuo Y.-M. Proteomics analysis of plasma for potential biomarkers in the diagnosis of Alzheimer’s disease. Proteom. Clin. Appl. 2007;1:506–512. doi: 10.1002/prca.200600684. [DOI] [PubMed] [Google Scholar]
- 68.Suidan G.L., Singh P.K., Patel-Hett S., Chen Z.-L., Volfson D., Yamamoto-Imoto H., Norris E.H., Bell R.D., Strickland S. Abnormal clotting of the intrinsic/contact pathway in Alzheimer disease patients is related to cognitive ability. Blood Adv. 2018;2:954–963. doi: 10.1182/bloodadvances.2018017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai C.W., Tsai C.F., Lin K.H., Chen W.J., Lin M.S., Hsieh C.C., Lin C.C. An investigation of the correlation between the S-glutathionylated GAPDH levels in blood and Alzheimer’s disease progression. PLoS ONE. 2020;15:e0233289. doi: 10.1371/journal.pone.0233289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sultana R., Boyd-Kimball D., Cai J., Pierce W.M., Klein J.B., Merchant M., Butterfield D.A. Proteomics Analysis of the Alzheimer’s disease Hippocampal Proteome. J. Alzheimer’s Dis. 2007;11:153–164. doi: 10.3233/JAD-2007-11203. [DOI] [PubMed] [Google Scholar]
- 71.Güntert A., Campbell J., Saleem M., O’Brien D.P., Thompson A.J., Byers H.L., Ward M.A., Lovestone S. Plasma gelsolin is decreased and correlates with rate of decline in Alzheimer’s disease. J. Alzheimer’s Dis. 2010;21:585–596. doi: 10.3233/JAD-2010-100279. [DOI] [PubMed] [Google Scholar]
- 72.Peng M., Jia J., Qin W. Plasma gelsolin and matrix metalloproteinase 3 as potential biomarkers for Alzheimer disease. Neurosci. Lett. 2015;595:116–121. doi: 10.1016/j.neulet.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Antequera D., Vargas T., Ugalde C., Spuch C., Molina J.A., Ferrer I., Bermejo-Pareja F., Carro E. Cytoplasmic gelsolin increases mitochondrial activity and reduces Aβ burden in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2009;36:42–50. doi: 10.1016/j.nbd.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Yao F., Zhang K., Zhang Y., Guo Y., Li A., Xiao S., Liu Q., Shen L., Ni J. Identification of blood biomarkers for Alzheimer’s disease through computational prediction and experimental validation. Front. Neurol. 2019;9 doi: 10.3389/fneur.2018.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elovaara I., Icen A., Palo J., Erkinjuntti T. CSF in Alzheimer’s disease. J. Neurol. Sci. 1985;70:73–80. doi: 10.1016/0022-510X(85)90189-3. [DOI] [PubMed] [Google Scholar]
- 76.Ayton S., Janelidze S., Roberts B., Palmqvist S., Kalinowski P., Diouf I., Belaidi A.A., Stomrud E., Bush A.I., Hansson O. Acute phase markers in CSF reveal inflammatory changes in Alzheimer’s disease that intersect with pathology, APOE ε4, sex and age. Prog. Neurobiol. 2021;198:101904. doi: 10.1016/j.pneurobio.2020.101904. [DOI] [PubMed] [Google Scholar]
- 77.Song I.-U., Kim Y.-D., Chung S.-W., Cho H.-J. Association between serum haptoglobin and the pathogenesis of Alzheimer’s disease. Intern. Med. 2015;54:453–457. doi: 10.2169/internalmedicine.54.2876. [DOI] [PubMed] [Google Scholar]
- 78.Zhu C.-J., Jiang G.-X., Chen J.-M., Zhou Z.-M., Cheng Q. Serum haptoglobin in chinese patients with Alzheimer’s disease and mild cognitive impairment: A case-control study. Brain Res. Bull. 2018;137:301–305. doi: 10.1016/j.brainresbull.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Björkdahl C., Sjögren M.J., Zhou X., Concha H., Avila J., Winblad B., Pei J.-J. Small heat shock proteins Hsp27 or αB-crystallin and the protein components of neurofibrillary tangles: Tau and neurofilaments. J. Neurosci. Res. 2007;86:1343–1352. doi: 10.1002/jnr.21589. [DOI] [PubMed] [Google Scholar]
- 80.Goetzl E.J., Boxer A.L., Schwartz J.B., Abner E.L., Petersen R.C., Miller B.L., Kapogiannis D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zetterberg H. Identification of a β-amyloid-binding plasma protein, LRP1: Implications for biomarker research and therapy in Alzheimer’s disease. Biomarker. Med. 2007;1:347–348. doi: 10.2217/17520363.1.3.347. [DOI] [PubMed] [Google Scholar]
- 82.Kang D.E., Pietrzik C.U., Baum L., Chevallier N., Merriam D.E., Kounnas M.Z., Wagner S.L., Troncoso J.C., Kawas C.H., Katzman R., et al. Modulation of amyloid β-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor–related protein pathway. J. Clin. Investig. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamanaka Y., Faghihi M.A., Magistri M., Alvarez-Garcia O., Lotz M., Wahlestedt C. Antisense RNA controls LRP1 sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 2015;11:967–976. doi: 10.1016/j.celrep.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsui T., Ingelsson M., Fukumoto H., Ramasamy K., Kowa H., Frosch M.P., Irizarry M.C., Hyman B.T. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007;1161:116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 85.Dranovsky A., Vincent I., Gregori L., Schwarzman A., Colflesh D., Enghild J., Strittmatter W., Davies P., Goldgaber D. Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer’s disease pathology. Neurobiol. Aging. 2001;22:517–528. doi: 10.1016/S0197-4580(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 86.Ijsselstijn L., Dekker L.J.M., Stingl C., Van Der Weiden M.M., Hofman A., Kros J.M., Koudstaal P.J., Smitt P.A.E.S., Ikram M.A., Breteler M.M.B., et al. Serum levels of pregnancy zone protein are elevated in presymptomatic Alzheimer’s disease. J. Proteome Res. 2011;10:4902–4910. doi: 10.1021/pr200270z. [DOI] [PubMed] [Google Scholar]
- 87.Nijholt D.A., Ijsselstijn L., Van Der Weiden M.M., Zheng P.-P., Smitt P.A.E.S., Koudstaal P.J., Luider T.M., Kros J.M. Pregnancy zone protein is increased in the Alzheimer’s disease brain and associates with senile plaques. J. Alzheimer’s Dis. 2015;46:227–238. doi: 10.3233/JAD-131628. [DOI] [PubMed] [Google Scholar]
- 88.Sáez-Valero J., Costell M., Sjögren M., Andreasen N., Blennow K., Luque J.M. Altered levels of cerebrospinal fluid reelin in frontotemporal dementia and Alzheimer’s disease. J. Neurosci. Res. 2003;72:132–136. doi: 10.1002/jnr.10554. [DOI] [PubMed] [Google Scholar]
- 89.Botella-López A., Burgaya F., Gavín R., García-Ayllón M.S., Gómez-Tortosa E., Peña-Casanova J., Ureña J.M., Del Río J.A., Blesa R., Soriano E., et al. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5573–5578. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mata-Balaguer T., Cuchillo-Ibañez I., Calero M., Ferrer I., Sáez-Valero J. Decreased generation of C-terminal fragments of ApoER2 and increased reelin expression in Alzheimer’s disease. FASEB J. 2018;32:3536–3546. doi: 10.1096/fj.201700736RR. [DOI] [PubMed] [Google Scholar]
- 91.Shen L., Liao L., Chen C., Guo Y., Song D., Wang Y., Chen Y., Zhang K., Ying M., Li S., et al. Proteomics analysis of blood serums from Alzheimer’s disease patients using iTRAQ labeling technology. J. Alzheimer’s Dis. 2017;56:361–378. doi: 10.3233/JAD-160913. [DOI] [PubMed] [Google Scholar]
- 92.Chang K.-A., Shin K.Y., Nam E., Lee Y.-B., Moon C., Suh Y.-H., Lee S.H. Plasma soluble neuregulin-1 as a diagnostic biomarker for Alzheimer’s disease. Neurochem. Int. 2016;97:1–7. doi: 10.1016/j.neuint.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 93.Horvath I., Jia X., Johansson P., Wang C., Moskalenko R., Steinau A., Forsgren L., Wågberg T., Svensson J., Zetterberg H., et al. Pro-inflammatory S100A9 protein as a robust biomarker differentiating early stages of cognitive impairment in Alzheimer’s disease. ACS Chem. Neurosci. 2015;7:34–39. doi: 10.1021/acschemneuro.5b00265. [DOI] [PubMed] [Google Scholar]
- 94.Rueli R.H., Parubrub A.C., Dewing A.S., Hashimoto A.C., Bellinger M.T., Weeber E.J., Uyehara-Lock J.H., White L.R., Berry M.J., Bellinger F.P. Increased selenoprotein P in choroid plexus and cerebrospinal fluid in Alzheimer’s disease brain. J. Alzheimer’s Dis. 2015;44:379–383. doi: 10.3233/JAD-141755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nielsen H.M., Minthon L., Londos E., Blennow K., Miranda E., Perez J., Crowther D.C., Lomas D.A., Janciauskiene S.M. Plasma and CSF serpins in Alzheimer disease and dementia with Lewy bodies. Neurology. 2007;69:1569–1579. doi: 10.1212/01.wnl.0000271077.82508.a0. [DOI] [PubMed] [Google Scholar]
- 96.Shinohara Y., Ohsuga H., Yamamoto M., Ohsuga S., Akiyama K., Yoshii F., Tsuda M., Kamiguchi H., Yamamura M. CSF α1-Antichymotrypsin and antitrypsin in multi-infarct dementia and Alzheimer’s disease. Cereb. Ischemia Dement. 1991:334–337. doi: 10.1007/978-3-642-76208-6_40. [DOI] [Google Scholar]
- 97.Abraham C.R., Selkoe D.J., Potter H. Immunochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-X. [DOI] [PubMed] [Google Scholar]
- 98.Furby A., Leys D., Delacourte A., Buee L., Soetaert G., Petit H. Are alpha-1-antichymotrypsin and inter-alpha-trypsin inhibitor peripheral markers of Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry. 1991;54:469. doi: 10.1136/jnnp.54.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pirttila T., Mehta P., Frey H., Wisniewski H. α1-Antichymotrypsin and IL-1β are not increased in CSF or serum in Alzheimer’s disease. Neurobiol. Aging. 1994;15:313–317. doi: 10.1016/0197-4580(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 100.Lanzrein A.-S., Johnston C.M., Perry V.H., Jobst K.A., King E.M., Smith A.D. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998;12:215–227. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 101.Lawlor B.A., Swanwick G.R., Feighery C., Walsh J.B., Coakley D. Acute phase reactants in Alzheimer’s disease. Biol. Psychiatry. 1996;39:1051–1052. doi: 10.1016/0006-3223(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 102.Matsubara E., Amari M., Shoji M., Harigaya Y., Yamaguchi H., Okamoto K., Hirai S. Serum concentration of alpha 1-antichymotrypsin is elevated in patients with senile dementia of the Alzheimer type. Prog. Clin. Boil. Res. 1989;317:707–714. [PubMed] [Google Scholar]
- 103.Brugge K., Katzman R., Hill L.R., Hansen L.A., Saitoh T. Serological α1-Antichymotrypsin in Down’s syndrome and Alzheimer’s disease. Ann. Neurol. 1992;32:193–197. doi: 10.1002/ana.410320211. [DOI] [PubMed] [Google Scholar]
- 104.Altstiel L.D., Lawlor B., Mohs R.C., Schmeidler J., Dalton A.J., Mehta P.D., Davis K.L. Elevated Alpha1-Antichymotrypsin serum levels in a subset of nondemented first-degree relatives of Alzheimer’s disease patients. Dement. Geriatr. Cogn. Disord. 1995;6:17–20. doi: 10.1159/000106917. [DOI] [PubMed] [Google Scholar]
- 105.Lieberman J., Schleissner L., Tachiki K.H., Kling A.S. Serum α1-antichymotrypsin level as a marker for Alzheimer-type dementia. Neurobiol. Aging. 1995;16:747–753. doi: 10.1016/0197-4580(95)00056-K. [DOI] [PubMed] [Google Scholar]
- 106.Licastro F., Pedrini S., Caputo L., Annoni G., Davis L.J., Ferri C., Casadei V., Grimaldi L.M.E. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer’s disease: Peripheral inflammation or signals from the brain? J. Neuroimmunol. 2000;103:97–102. doi: 10.1016/S0165-5728(99)00226-X. [DOI] [PubMed] [Google Scholar]
- 107.Licastro F., Mallory M., Hansen L., Masliah E. Increased levels of α-1-antichymotrypsin in brains of patients with Alzheimer’s disease correlate with activated astrocytes and are affected by APOE 4 genotype. J. Neuroimmunol. 1998;88:105–110. doi: 10.1016/S0165-5728(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 108.Padmanabhan J., Levy M., Dickson D.W., Potter H. Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer’s disease brain, induces tau phosphorylation in neurons. Brain. 2006;129:3020–3034. doi: 10.1093/brain/awl255. [DOI] [PubMed] [Google Scholar]
- 109.Vanni S., Moda F., Zattoni M., Bistaffa E., De Cecco E., Rossi M., Giaccone G., Tagliavini F., Haïk S., Deslys J.P., et al. Differential overexpression of SERPINA3 in human prion diseases. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-15778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Licastro F., Parnetti L., Morini M.C., Davis L.J., Cucinotta D., Gaiti A., Senin U. Acute phase reactant α1Antichymotrypsin is increased in cerebrospinal fluid and serum of patients with probable Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1995;9:112–118. doi: 10.1097/00002093-199509020-00009. [DOI] [PubMed] [Google Scholar]
- 111.Porcellini E., Davis E., Chiappelli M., Ianni E., Di Stefano G., Forti P., Ravaglia G., Licastro F. Elevated plasma levels of α -1-anti-chymotrypsin in age-related cognitive decline and Alzheimers disease: A potential therapeutic target. Curr. Pharm. Des. 2008;14:2659–2664. doi: 10.2174/138161208786264151. [DOI] [PubMed] [Google Scholar]
- 112.DeKosky S.T., Ikonomovic M.D., Wang X., Farlow M., Wisniewski S., Lopez O.L., Becker J.T., Saxton J., Klunk W.E., Sweet R., et al. Plasma and cerebrospinal fluid α1-antichymotrypsin levels in Alzheimer’s disease: Correlation with cognitive impairment. Ann. Neurol. 2002;53:81–90. doi: 10.1002/ana.10414. [DOI] [PubMed] [Google Scholar]
- 113.Matsubara E., Hirai S., Amari M., Shoji M., Yamaguchi H., Okamoto K., Ishiguro K., Harigaya Y., Wakabayashi K. α1-Antichymotrypsin as a possible biochemical marker for Alzheimer-type dementia. Ann. Neurol. 1990;28:561–567. doi: 10.1002/ana.410280414. [DOI] [PubMed] [Google Scholar]
- 114.Delamarche C., Berger F., Gallard L., Pouplard-Barthelaix A. Aging and Alzheimer’s disease: Protease inhibitors in cerebrospinal fluid. Neurobiol. Aging. 1991;12:71–74. doi: 10.1016/0197-4580(91)90042-I. [DOI] [PubMed] [Google Scholar]
- 115.Loeffler D.A., DeMaggio A.J., Juneau P.L., Brickman C.M., Mashour G.A., Finkelman J.H., Pomara N., LeWitt P.A. Ceruoplasmin is increased in cerebrospinal fluid in Alzheimer’s disease but not Parkinson’s disease. Alzheimer Dis. Assoc. Disord. 1994;8:190–197. doi: 10.1097/00002093-199408030-00005. [DOI] [PubMed] [Google Scholar]
- 116.Hare D.J., Doecke J.D., Faux N.G., Rembach A., Volitakis I., Fowler C.J., Grimm R., Doble P.A., Cherny R.A., Masters C.L., et al. Decreased plasma iron in Alzheimer’s disease is due to transferrin desaturation. ACS Chem. Neurosci. 2015;6:398–402. doi: 10.1021/cn5003557. [DOI] [PubMed] [Google Scholar]
- 117.Son S.M., Nam D.W., Cha M.-Y., Kim K.H., Byun J., Ryu H., Mook-Jung I. Thrombospondin-1 prevents amyloid beta–mediated synaptic pathology in Alzheimer’s disease. Neurobiol. Aging. 2015;36:3214–3227. doi: 10.1016/j.neurobiolaging.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Merched A., Serot J.-M., Visvikis S., Aguillon D., Faure G., Siest G. Apolipoprotein E, transthyretin and actin in the CSF of Alzheimer’s patients: Relation with the senile plaques and cytoskeleton biochemistry. FEBS Lett. 1998;425:225–228. doi: 10.1016/S0014-5793(98)00234-8. [DOI] [PubMed] [Google Scholar]
- 119.Han S.-H., Jung E.S., Sohn J.-H., Hong H.S., Kim J.W., Na D.L., Kim M., Kim H., Ha H.J., Kim Y.H., et al. Human serum transthyretin levels correlate inversely with Alzheimer’s disease. J. Alzheimer’s Dis. 2011;25:77–84. doi: 10.3233/JAD-2011-102145. [DOI] [PubMed] [Google Scholar]
- 120.Schultz K., Nilsson K., Nielsen J.E., Lindquist S.G., Hjermind L.E., Andersen B.B., Wallin A., Petersén Å. Transthyretin as a potential CSF biomarker for Alzheimer’s disease and dementia with Lewy bodies: Effects of treatment with cholinesterase inhibitors. Eur. J. Neurol. 2010;17:456–460. doi: 10.1111/j.1468-1331.2009.02841.x. [DOI] [PubMed] [Google Scholar]
- 121.Ribeiro C.A., Santana I., Oliveira C., Baldeiras I., Moreira J., Saraiva M.J., Cardoso I. Transthyretin decrease in plasma of MCI and AD patients: Investigation of mechanisms for disease modulation. Curr. Alzheimer Res. 2012;9:881–889. doi: 10.2174/156720512803251057. [DOI] [PubMed] [Google Scholar]
- 122.Velayudhan L., Killick R., Hye A., Kinsey A., Güntert A., Lynham S., Ward M., Leung R., Lourdusamy A., To A.W., et al. Plasma transthyretin as a candidate marker for Alzheimer’s disease. J. Alzheimer’s Dis. 2012;28:369–375. doi: 10.3233/JAD-2011-110611. [DOI] [PubMed] [Google Scholar]
- 123.Haas C., Cazorla P., De Miguel C., Valdivieso F., Vázquez J. Apolipoprotein E forms stable complexes with recombinant Alzheimer’s disease β-amyloid precursor protein. Biochem. J. 1997;325:169–175. doi: 10.1042/bj3250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S., Roses A.D. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vogelgsang J., Vukovich R., Wedekind D., Wiltfang J. Higher level of mismatch in APOEε4 carriers for amyloid-beta peptide Alzheimer’s disease biomarkers in cerebrospinal fluid. ASN Neuro. 2019;11 doi: 10.1177/1759091419845524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu C.-C., Zhao N., Fu Y., Wang N., Linares C., Tsai C.-W., Bu G. ApoE4 Accelerates early seeding of amyloid pathology. Neuron. 2017;96:1024–1032.e3. doi: 10.1016/j.neuron.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bachmeier C., Paris D., Beaulieu-Abdelahad D., Mouzon B., Mullan M., Crawford F. A multifaceted role for apoE in the clearance of Beta-amyloid across the blood-brain barrier. Neurodegener. Dis. 2013;11:13–21. doi: 10.1159/000337231. [DOI] [PubMed] [Google Scholar]
- 128.Strittmatter W.J., Saunders A.M., Goedert M., Weisgraber K.H., Dong L.M., Jakes R., Huang D.Y., Pericak-Vance M., Schmechel D., Roses A.D. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gollin P.A., Kalaria R.N., Eikelenboom P., Rozemuller A., Perry G. α1-Antitrypsin and α1-antichymotrypsin are in the lesions of Alzheimer’s disease. NeuroReport. 1992;3:201–203. doi: 10.1097/00001756-199202000-00020. [DOI] [PubMed] [Google Scholar]
- 130.Eriksson S., Janciauskiene S., Lannfelt L. Alpha 1-antichymotrypsin regulates Alzheimer beta-amyloid peptide fibril formation. Proc. Natl. Acad. Sci. USA. 1995;92:2313–2317. doi: 10.1073/pnas.92.6.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tyagi E., Fiorelli T., Norden M., Padmanabhan J. Alpha 1-Antichymotrypsin, an inflammatory protein overexpressed in the brains of patients with Alzheimer’s disease, induces Tau hyperphosphorylation through c-Jun N-terminal kinase activation. Int. J. Alzheimer’s Dis. 2013;2013:1–11. doi: 10.1155/2013/606083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nilsson L.N.G., Bales K.R., Dicarlo G., Gordon M.N., Morgan D., Paul S.M., Potter H. α-1-Antichymotrypsin promotes β-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2001;21:1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gold M., Dolga A.M., Koepke J., Mengel D., Culmsee C., Dodel R., Koczulla A.R., Bach J.-P. α 1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-β-induced toxicity. J. Neuroinflamm. 2014;11:165. doi: 10.1186/s12974-014-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wyatt A.R., Constantinescu P., Ecroyd H., Dobson C.M., Wilson M.R., Kumita J.R., Yerbury J.J. Protease-activated alpha-2-macroglobulin can inhibit amyloid formation via two distinct mechanisms. FEBS Lett. 2013;587:398–403. doi: 10.1016/j.febslet.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bell R.D., Sagare A.P., Friedman A.E., Bedi G.S., Holtzman D.M., Deane R., Zlokovic B.V. Transport pathways for clearance of human alzheimer’s amyloid β-Peptide and apolipoproteins E and J in the mouse central nervous system. Br. J. Pharmacol. 2006;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Storck S.E., Meister S., Nahrath J., Meißner J.N., Schubert N., Di Spiezio A., Baches S., Vandenbroucke R.E., Bouter Y., Prikulis I., et al. Endothelial LRP1 transports amyloid-β1–42 across the blood-brain barrier. J. Clin. Investig. 2015;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tachibana M., Holm M.-L., Liu C.-C., Shinohara M., Aikawa T., Oue H., Yamazaki Y., Martens Y.A., Murray M.E., Sullivan P.M., et al. APOE4-mediated amyloid-β pathology depends on its neuronal receptor LRP1. J. Clin. Investig. 2019;129:1272–1277. doi: 10.1172/JCI124853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silva C.S., Eira J., Ribeiro C.A., Oliveira Â., Sousa M.M., Cardoso I., Liz M.A. Transthyretin neuroprotection in Alzheimer’s disease is dependent on proteolysis. Neurobiol. Aging. 2017;59:10–14. doi: 10.1016/j.neurobiolaging.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 139.Alemi M., Gaiteiro C., Ribeiro C.A., Santos L.M., Gomes J.R., Oliveira S.M., Couraud P.-O., Weksler B., Romero I., Saraiva M.J., et al. Transthyretin participates in beta-amyloid transport from the brain to the liver- involvement of the low-density lipoprotein receptor-related protein 1? Sci. Rep. 2016;6:20164. doi: 10.1038/srep20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tenner A.J. Complement-mediated events in Alzheimer’s disease: Mechanisms and potential therapeutic targets. J. Immunol. 2020;204:306–315. doi: 10.4049/jimmunol.1901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Piktel E., Levental I., Durnaś B., Janmey P.A., Bucki R. Plasma Gelsolin: Indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 2018;19:2516. doi: 10.3390/ijms19092516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hirko A.C., Meyer E.M., King M.A., Hughes J. Peripheral transgene expression of plasma gelsolin reduces amyloid in transgenic mouse models of Alzheimer’s disease. Mol. Ther. 2007;15:1623–1629. doi: 10.1038/sj.mt.6300253. [DOI] [PubMed] [Google Scholar]
- 143.Matsuoka Y., Saito M., Lafrancois J., Saito M., Gaynor K., Olm V., Wang L., Casey E., Lu Y., Shiratori C., et al. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to β-amyloid. J. Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wen D., Corina K., Chow E.P., Miller S., Janmey P.A., Pepinsky R.B. The plasma and cytoplasmic forms of human gelsolin differ in disulfide structure. Biochem. 1996;35:9700–9709. doi: 10.1021/bi960920n. [DOI] [PubMed] [Google Scholar]
- 145.Solomon J.P., Page L.J., Balch W.E., Kelly J.W. Gelsolin amyloidosis: Genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Crit. Rev. Biochem. Mol. Biol. 2012;47:282–296. doi: 10.3109/10409238.2012.661401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Henriques A.G., Oliveira J.M., Gomes B., Ruivo R., Silva E.F.D.C.E., Silva O.A.B.D.C.E. Complexing Aβ prevents the cellular anomalies induced by the Peptide alone. J. Mol. Neurosci. 2014;53:661–668. doi: 10.1007/s12031-014-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martins T.S., Catita J., Rosa I.M., da Cruz e Silva A.B., Henriques A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE. 2018;13:e0198820. doi: 10.1371/journal.pone.0198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mathivanan S., Fahner C.J., Reid G.E., Simpson R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keerthikumar S., Chisanga D., Ariyaratne D., Al Saffar H., Anand S., Zhao K., Samuel M., Pathan M., Jois M., Chilamkurti N., et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simpson R.J., Kalra H., Mathivanan S. ExoCarta as a resource for exosomal research. J. Extracell. Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mathivanan S., Simpson R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 152.Kalra H., Simpson R.J., Ji H., Aikawa E., Altevogt P., Askenase P.W., Bond V.C., Borràs F.E., Breakefield X.O., Budnik V., et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pathan M., Fonseka P., Chitti S.V., Kang T., Sanwlani R., Van Deun J., Hendrix A., Mathivanan S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516–D519. doi: 10.1093/nar/gky1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim D.-K., Kang B., Kim O.Y., Choi D.-S., Lee J., Kim S.R., Go G., Yoon Y.J., Kim J.H., Jang S.C., et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim D.-K., Lee J., Simpson R.J., Lötvall J., Gho Y.S. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin. Cell Dev. Biol. 2015;40:4–7. doi: 10.1016/j.semcdb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 156.Rosa I.M., Henriques A.G., Carvalho L., Oliveira J., Silva O.A.D.C.E. Screening younger individuals in a primary care setting flags putative dementia cases and correlates gastrointestinal diseases with poor cognitive performance. Dement. Geriatr. Cogn. Disord. 2016;43:15–28. doi: 10.1159/000452485. [DOI] [PubMed] [Google Scholar]
- 157.Rosa I.M., Henriques A.G., Wiltfang J., Silva O.A.D.C.E. Putative dementia cases fluctuate as a function of mini-mental state examination cut-off points. J. Alzheimer’s Dis. 2017;61:157–167. doi: 10.3233/JAD-170501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.