Abstract
Objective
Compare paediatric COVID-19 disease characteristics, management and outcomes according to World Bank country income level and disease severity.
Design
Systematic review and meta-analysis.
Setting
Between 1 December 2019 and 8 January 2021, 3350 articles were identified. Two reviewers conducted study screening, data abstraction and quality assessment independently and in duplicate. Observational studies describing laboratory-confirmed paediatric (0–19 years old) COVID-19 were considered for inclusion.
Main outcomes and measures
The pooled proportions of clinical findings, treatment and outcomes were compared according to World Bank country income level and reported disease severity.
Results
129 studies were included from 31 countries comprising 10 251 children of which 57.4% were hospitalised. Mean age was 7.0 years (SD 3.6), and 27.1% had a comorbidity. Fever (63.3%) and cough (33.7%) were common. Of 3670 cases, 44.1% had radiographic abnormalities. The majority of cases recovered (88.9%); however, 96 hospitalised children died. Compared with high-income countries, in low-income and middle-income countries, a lower proportion of cases were admitted to intensive care units (ICUs) (9.9% vs 26.0%) yet pooled proportion of deaths among hospitalised children was higher (relative risk 2.14, 95% CI 1.43 to 3.20). Children with severe disease received antimicrobials, inotropes and anti-inflammatory agents more frequently than those with non-severe disease. Subgroup analyses showed that a higher proportion of children with multisystem inflammatory syndrome (MIS-C) were admitted to ICU (47.1% vs 22.9%) and a higher proportion of hospitalised children with MIS-C died (4.8% vs 3.6%) compared with the overall sample.
Conclusion
Paediatric COVID-19 has a favourable prognosis. Further severe disease characterisation in children is needed globally.
Keywords: COVID-19, adolescent health, epidemiology
Introduction
The coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 has spread from a local outbreak in China to a global pandemic within months. On 31 December 2019, a cluster of cases with pneumonia of unknown cause emerged from Wuhan, China. On 30 January 2020, the WHO declared the coronavirus outbreak a Public Health Emergency of International Concern, and on 11 March 2020, a pandemic. As of 21 January 2021, there have been over 95.6 million confirmed COVID-19 cases and over 2.0 million associated deaths from 216 countries, areas or territories.1 Children under-19 years of age comprise a small proportion (1%–10%) of the total reported cases2–5 with a lower risk of developing critical illness from COVID-19 infection compared with adults.6 Prior systematic reviews of paediatric COVID-19 have described a mild disease in children with good outcomes.4 7 8 Since the publication of these reviews, the pandemic has spread extensively around the globe. In addition to pulmonary manifestations of COVID-19 in children, reports from Europe, North America, Latin America and Asia have emerged, describing a multisystem inflammatory syndrome children (MIS-C) related to COVID-19 infection.9–12 COVID-19 has also disrupted essential maternal and child health interventions, including outpatient visits and vaccinations for young children in most countries, further worsening the existing burden on healthcare provision and delivery.13
The objective of this review, in addition to providing a comprehensive update of the evolving paediatric COVID-19 literature, is a unique comparison of reported cases in low-income and middle-income countries (LMICs) to high-income countries (HICs) and of children with severe versus non-severe disease. Furthermore, the review provides a subgroup analysis of children presenting with symptoms of MIS-C and neonatal cases.
Methods
The protocol of the review is registered with PROSPERO (CRD42020183134). This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).
Search methods
The review includes a comprehensive search of MEDLINE, Embase, WHO COVID-19 Database, Chinese COVID-19 Databases (CNKI and Wangfang), Latin-American and Caribbean Health Sciences Literature (LILACS) from 1 December 2019 to 8 January 2021. Complementary searches were conducted in Google Scholar, John Hopkins Health Resource, WHO news and the Chinese and US CDC Library. MedRxiv, BioRxiv and ChinaXiv were searched for preprints. No language restrictions were applied.
A search strategy was formulated and administered as shown in online supplemental table 1.
archdischild-2020-321385supp001.pdf (18.7MB, pdf)
Study selection
Observational studies reporting children (0–19 years old) with laboratory‐confirmed COVID-19 (serology or RT-PCR) were considered for inclusion. Studies with a subset of children 0–19 years were included if disaggregated data for children were provided. Studies were screened for any overlap in paediatric cases by reviewing institution details and the period reported. Review articles, case reports, commentaries and letters not presenting any original data were excluded. Case reports were excluded to reduce risk of selection bias and over-representation of extreme cases. Covidence Software (2016) was used for screening by two reviewers independently and in duplicate. Key reference lists were screened for additional studies.
Data extraction
Two reviewers conducted data extraction using a prepiloted data form. Data extracted included authors’ names, date of publication, study-design, city, country, number of cases, gender, comorbidities, travel and contact history, diagnostic tests for COVID-19, clinical details, laboratory tests, radiological findings, management and outcomes. Disaggregated data by age groups (0–5 years, 5–10 years and >10 years old) and reported disease severity was extracted where available. Criteria for severe disease were as defined within each individual study and included admission to intensive care units (ICUs), use of mechanical ventilation, multiorgan failure and presence of hypoxia (oxygen saturation <92%).
Quality assessment
Individual study quality was evaluated independently by the review authors using quality assessment tools developed by the National Heart Lung and Brain Institute (NHLBI)14 (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Study quality was scored out of 8, based on clarity of study objectives, case definition, consecutive inclusion of cases, comparability of included patients, definition and measurement of outcomes, length of follow-up, statistical methods and results. Studies with score 6–8 were considered to be good quality, 4–5 considered fair quality and <4 considered poor quality.
Data synthesis
Categorical data were summarised as counts and proportions. The pooled proportions of reported findings were calculated using Comprehensive Meta-Analysis 2.2.027 using random-effects model. I2 was calculated to examine statistical heterogeneity (I2>50% considered high heterogeneity). The clinical features and outcomes were compared according to (1) World Bank country income level (HICs versus LMICs)15 and (2) reported disease severity (severe versus non-severe) using pooled proportions and their 95% CIs, supplemented by relative-risk (RR). Subgroup analyses of children with MIS-C and neonatal cases were conducted.
Results
After removal of duplicates, 3350 citations were screened for inclusion. Full texts of 198 studies were screened and 129 studies2 3 9–12 16–138 were included (online supplemental figure 1). Sixty-nine studies were excluded as they either presented overlapping data, did not provide age-disaggregated data for children or were commentaries, editorials or reviews. In terms of study setting, 13 studies were population-based national surveillance studies, 94 studies included only children admitted to hospital and 22 studies reported patients presenting to outpatient clinics or emergency departments (hospitalisation rate of 24.2%, 385/1590).
Sixty studies were from HICs (n=6528) and 69 studies from LMICs (n=3723). Almost one-third of included studies were from China (36/129, 28.0%),2 16–33 35 37–43 45 52 53 55–57 71 74 138 one-fifth were from the USA (24/129, 18.6%)3 9 10 47 58 60 64 68–70 79 81 82 85–87 93 98 109 110 115 120 133 136 together comprising almost half of the included sample size (n=4758, 46.4.%). The country of origin of included studies and study characteristics are summarised in online supplemental figure 2, tables 2 and 3.
Demographics and epidemiology
A total of 9335 children from the 129 case series were included in the meta-analysis. Of 8455 children for whom initial disposition was reported, 4851 were hospitalised (57.4%). Among them, 55.5% were men. The patient’s ages ranged from 0 to 17 years with mean age of 7.0±3.6 years. Ninety-one of the 129 studies reported age-disaggregated data for infection incidence as shown in online supplemental table 4. Nearly half of the cases were >10 years of age. Contact exposure to COVID-19 was reported in 64.0% of cases. Travel history to an epicentre was reported in 13.0% cases. At-least one underlying comorbidity was reported in 27.1% of cases. The most common reported comorbidities were immunosuppression (15.8%) and lung disease (12.5%).
Clinical manifestations
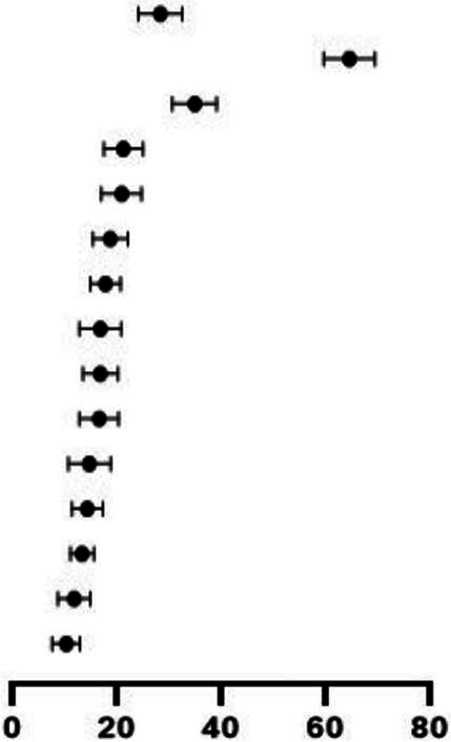
Table 1 summarises the clinical manifestations reported in the studies. There were 13.1% asymptomatic cases (95% CI 10.4% to 16.3%) who presented primarily through contact exposure in family-clusters (parents, siblings and other relatives). The most common presenting symptoms were fever (63.3%, 95% CI 58.6% to 68.4%) and cough (33.7%, 95% CI 29.6% to 38.1%) followed by nausea or vomiting (20.0%, 95% CI 16.5% to 24.0%) and diarrhoea (19.6%, 95% CI 16.1% to 23.7%). Other symptoms included dyspnoea, nasal-symptoms, rashes, kawasaki-like symptoms, conjunctivitis, fatigue, abdominal pain and neurological symptoms. Sixty-seven of the 129 studies reported age disaggregated data for clinical features (online supplemental table 5). Clinical features were similar in the three age groups: ≤5 years, >5 to ≤10 years, >10 years with higher prevalence of abdominal symptoms in children>5 years.
Table 1.
Clinical symptoms among reported paediatric COVID-19 cases
| Characteristics | Events/total patients | Mean proportion % (95% CI) | Heterogeneity I2 (%) | |
| Comorbidity | 1590/6086 |
|
27.1 (23.1 to 31.5) | 37.6 |
| Fever | 3576/6296 | 63.3 (58.6 to 68.4) | 34.9 | |
| Cough | 1807/5261 | 33.7 (29.6 to 38.1) | 34.4 | |
| Nausea/vomiting | 880/4243 | 20.0 (16.5 to 24.0) | 25.7 | |
| Diarrhoea | 796/4884 | 19.6 (16.1 to 23.7) | 13.4 | |
| Dyspnoea | 879/5332 | 17.5 (14.4 to 21.1) | 23.7 | |
| Nasal symptoms | 1080/5406 | 16.6 (13.9 to 19.7) | 10.6 | |
| Rashes | 744/4387 | 15.5 (11.9 to 19.9) | 25.9 | |
| Fatigue | 709/4474 | 15.5 (12.6 to 19.3) | 26.3 | |
| Abdominal pain | 626/4135 | 15.3 (11.9 to 19.4) | 26.5 | |
| Kawasaki shock/sign | 821/4365 | 13.3 (9.8 to 17.9) | 30.6 | |
| Asymptomatic | 1114/6084 | 13.1 (10.4 to 16.3) | 15.4 | |
| Neurological symptoms | 693/5475 | 12.1 (10.1 to 14.6) | 17.6 | |
| Conjunctivitis | 529/4998 | 10.5 (7.8 to 14.0) | 21.0 | |
| Pharyngeal erythema | 428/3638 | 9.0 (6.7 to 12.0) | 0.0 |
Radiological and laboratory findings
One thousand five hundred and thirty cases out of 3670 (44.1%, 95% CI 39.5% to 48.9%) cases had radiological abnormalities; ground glass opacities (27.4%) were the most commonly reported abnormality.
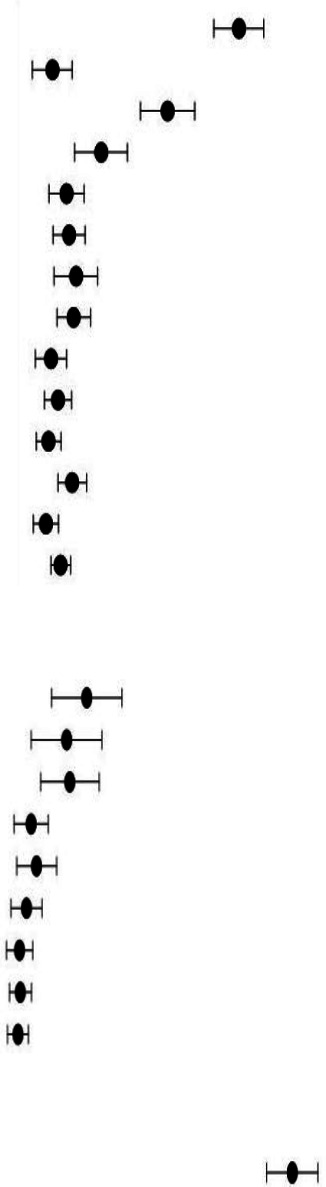
Sixty-six studies provided details on laboratory-markers (table 2). Pooled analysis revealed increased C-Reactive Protein (CRP) (54.2%, 95% CI 41.5% to 66.3%), serum-ferritin (46.7%, 95% CI 32.3% to 61.7%), lactate dehydrogenase (LDH) (36.5%, 95% CI 26.5% to 47.8%) and d-dimers (35.2%, 95% CI 22.1% to 51.0%) as the most common abnormalities. Other reported abnormalities included elevated erythrocyte sedimentation rate (ESR), lymphopaenia, procalcitonin and biomarkers for organ injury including elevated levels of pro B-type natriuretic peptide, troponin and creatine kinase-MB as shown in table 2.
Table 2.
Laboratory and radiological features among reported paediatric COVID-19 cases
| Characteristics | Events/total patients | Mean proportion % (95% CI) | Heterogeneity I2 (%) | |
| Inflammatory marker | ||||
| CRP ↑ | 556/1165 |
|
54.2 (41.5 to 66.3) | 21.4 |
| Ferritin ↑ | 247/525 | 46.7 (32.3 to 61.7) | 46.5 | |
| LDH ↑ | 356/922 | 36.5 (26.5 to 47.8) | 35.6 | |
| Procalcitonin ↑ | 137/879 | 21.3 (12.2 to 34.5) | 24.9 | |
| Leukocytes ↑ | 138/953 | 19.9 (13.3 to 28.8) | 21.4 | |
| Lymphocytes ↓ | 359/1347 | 19.0 (12.8 to 27.1) | 0.0 | |
| ESR ↑ | 248/838 | 18.9 (11.8 to 28.9) | 0.0 | |
| IL-6 ↑ | 41/341 | 13.1 (5.5 to 28.2) | 7.1 | |
| Leucopaenia (+) | 77/1037 | 10.7 (7.7 to 14.6) | 0.0 | |
| Lymphocytes ↑ | 66/1264 | 8.2 (4.9 to 13.5) | 0.0 | |
| Neutrophils ↑ | 22/574 | 7.8 (4.8 to 12.4) | 0.0 | |
| Biomarkers for organ injury | ||||
| proBNP ↑ | 211/441 | 45.5 (28.5 to 63.5) | 49.5 | |
| Troponin ↑ | 239/703 | 39.7 (24.7 to 57.0) | 30.5 | |
| LFTs ↑ | 287/816 | 29.8 (20.3 to 41.6) | 10.8 | |
| CKMB ↑ | 82/293 | 25.5 (13.4 to 43.0) | 31.1 | |
| RFTs ↑ | 86/344 | 17.6 (7.6 to 35.6) | 23.6 | |
| Coagulopathy markers | ||||
| D-dimers ↑ | 272/711 | 35.2 (22.1 to 51.0) | 19.1 | |
| Fibrinogen ↑ | 168/438 | 17.5 (7.6 to 35.4) | 0.0 | |
| Radiological test | ||||
| Abnormal CXR/CT | 1530/3670 | 44.1 (39.5 to 48.9) | 35.0 |
Management
Details of clinical management are as shown in table 3. Commonly used therapies among hospitalised children were antimicrobials (32.2%, 95% CI 25.2% to 40.1%), intravenous immunoglobulin (IVIG) (19.5%, 95% CI 13.5% to 27.2%) and systemic-steroids (19.3, 95% CI 14.9% to 24.9%). Other treatment regimens included aspirin, inotropic drugs, inhaled interferon-α (IFN‐α), antimalarials and antivirals (ribavirin, oseltamivir, lopinavir, ritonavir and litonavir). Mechanical ventilation was provided to 490 patients (12.2%, 95% CI 9.7% to 15.3%).
Table 3.
Clinical management and outcomes among reported paediatric COVID-19 cases
| Characteristics | Events/total patients | Mean proportion % (95% CI) | Heterogeneity I2 (%) | |
| Clinical management | ||||
| Antibiotics | 1345/3610 |

|
32.2 (25.2 to 40.1) | 41.9 |
| IVIG | 698/3522 | 19.5 (13.5 to 27.2) | 18.4 | |
| Systemic steroids | 801/4229 | 19.3 (14.9 to 24.9) | 23.7 | |
| Antiviral | 527/4019 | 15.3 (11.1 to 20.7) | 4.5 | |
| Mechanical ventilation | 490/5406 | 12.2 (9.7 to 15.3) | 15.5 | |
| Inotropes | 354/3856 | 11.8 (8.3 to 16.4) | 11.5 | |
| Antimalarial | 336/3299 | 9.9 (6.9 to 14.0) | 0.0 | |
| Aspirin | 238/2588 | 9.0 (5.9 to 13.6) | 78.1 | |
| Interferon | 138/2598 | 7.7 (4.9 to 11.8) | 0.0 | |
| Traditional medicine | 22/4229 | 4.0 (2.8 to 5.6) | 38.7 | |
| Clinical outcomes | ||||
| Recovered | 8704/9335 | 88.9 (86.0 to 91.2) | 36.3 | |
| ICU admission | 1359/9335 | 22.9 (17.6 to 29.2) | 37.2 | |
| Deaths | 96/6902 | 3.6 (2.8 to 4.5) | 24.3 | |
ICU, intensive care unit; IVIG, intravenous immunoglobulin.
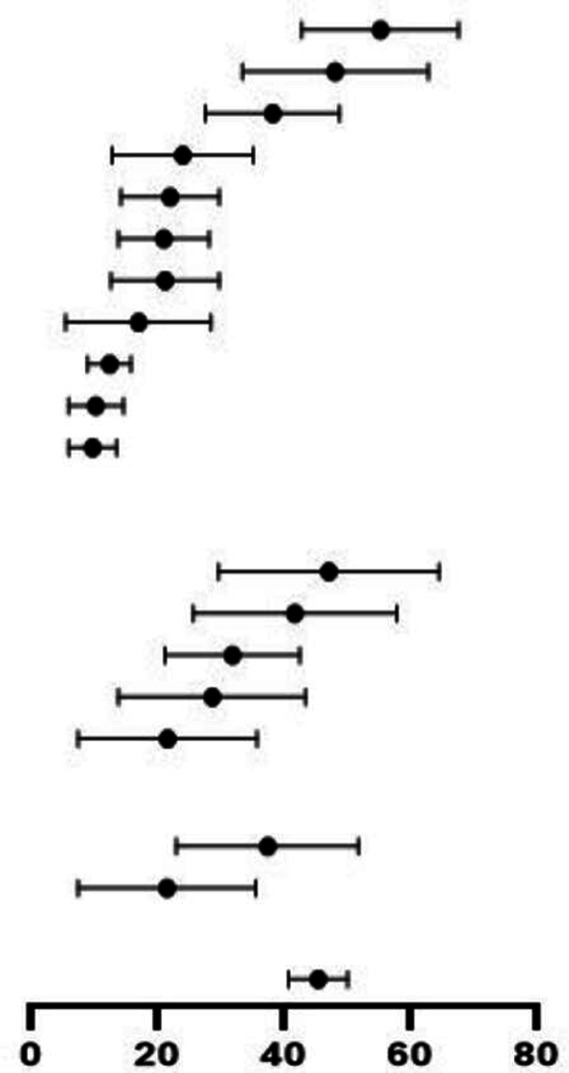
Prognosis and severe cases
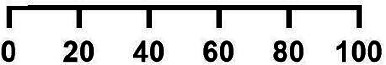
One thousand three hundred and fifty-nine patients (22.9%, 95% CI 17.6% to 29.2%) were admitted to ICUs (table 3). Thirty-eight studies provided disaggregated data for severe cases (table 4). A higher proportion of children with severe disease had symptoms consistent with MIS-C and received antimicrobials, inotropes and anti-inflammatory agents compared with those with non-severe disease. There were no deaths among children categorised as non-severe and 44 deaths among severe cases, where disaggregated data were provided. Hospital outcomes were reported for 9335 children; 8704 cases (88.9%, 95% CI 86.0% to 91.2%) were definitively discharged, 96 died and remaining children either remained hospitalised at the time of reporting or were readmitted.
Table 4.
Comparison of clinical symptoms, management and outcomes among reported paediatric COVID-19 non-severe (n=2402 cases, 64 studies) and severe (n=796 cases, 38 studies) cases
| Characteristics | Non-severe cases | Severe cases | RR severe vs non-severe (95% CI) | ||||
| Events/total patients | Mean proportion % (95% CI) | Events/total patients | Mean proportion % (95% CI) | ||||
| Clinical symptoms | |||||||
| Fever | 1394/2404 | 51.4 (45.7 to 57.0) |
|
608/756 | 80.2 (73.6 to 85.5) |
|
1.39 (1.32 to 1.46) |
| Pharyngeal erythema | 541/1149 | 8.6 (5.1 to 14.0) | 41/585 | 8.8 (5.1 to 14.8) | 0.15 (0.11 to 0.20) | ||
| Cough | 587/1521 | 35.1 (29.2 to 41.5) | 225/618 | 34.0 (24.6 to 44.9) | 0.94 (0.84 to 1.07) | ||
| Comorbidity | 541/2283 | 19.8 (14.5 to 26.4) | 351/764 | 44.1 (34.9 to 53.8) | 1.94 (1.74 to 2.16) | ||
| Nausea/vomiting | 206/1291 | 12.1 (8.7 to 16.6) | 224/632 | 41.0 (36.7 to 45.5) | 2.27 (1.93 to 2.67) | ||
| Dyspnoea | 260/1646 | 12.7 (9.5 to 16.8) | 237/701 | 36.4 (26.5 to 47.5) | 2.14 (1.84 to 2.49) | ||
| Nasal symptoms | 402/1659 | 14.1 (9.9 to 19.7) | 91/652 | 15.8 (10.6 to 23.0) | 0.58 (0.47 to 0.71) | ||
| Fatigue | 192/1319 | 13.8 (10.4 to 18.0) | 151/505 | 20.3 (15.3 to 33.4) | 2.05 (1.70 to 2.48) | ||
| Kawasaki shock/sign | 135/1243 | 8.5 (5.6 to 12.6) | 242/695 | 30.7 (19.3 to 45.0) | 3.21 (2.65 to 3.87) | ||
| Rashes | 168/1587 | 10.3 (7.6 to 13.7) | 180/660 | 32.3 (22.3 to 44.2) | 2.58 (2.13 to 3.11) | ||
| Abdominal pain | 95/1193 | 8.1 (5.8 to 11.3) | 184/621 | 28.4 (18.8 to 40.4) | 3.72 (2.96 to 4.67) | ||
| Diarrhoea | 144/1326 | 13.5 (10.6 to 17.1) | 217/632 | 35.3 (26.3 to 45.4) | 3.16 (2.62 to 3.82) | ||
| Conjunctivitis | 111/1621 | 7.5 (5.1 to 10.8) | 116/657 | 22.6 (15.1 to 32.4) | 2.58 (2.02 to 3.29) | ||
| Neurological symptoms | 200/2230 | 11.0 (9.0 to 13.4) | 118/703 | 17.4 (11.9 to 24.6) | 1.87 (1.52 to 2.31) | ||
| Clinical management | |||||||
| Mechanical ventilation | – | – | 322/735 | 43.8 (33.8 to 54.3) | – | ||
| Antiviral | 217/715 | 26.5 (17.5 to 38.1) | 136/567 | 24.1 (16.2 to 34.3) | 0.79 (0.66 to 0.95) | ||
| Interferon | 127/685 | 20.2 (11.6 to 32.3) | 4/445 | 6.8 (3.6 to 12.3) | 0.05 (0.02 to 0.13) | ||
| Antibiotics | 180/363 | 21.6 (14.2 to 31.3) | 365/566 | 59.6 (44.3 to 73.3) | 1.30 (1.15 to 1.47) | ||
| Antimalarial | 73/717 | 10.1 (6.4 to 16.4) | 123/537 | 22.9 (14.3 to 34.6) | 2.25 (1.72 to 2.94) | ||
| IVIG | 54/721 | 11.8 (7.2 to 18.8) | 202/498 | 41.1 (27.0 to 56.8) | 5.42 (4.10 to 7.15) | ||
| Systemic steroids | 46/721 | 8.9 (5.4 to 14.4) | 265/575 | 46.8 (35.7 to 58.2) | 7.22 (5.39 to 9.69) | ||
| Inotropes | 24/718 | 6.8 (4.1 to 11.7) | 171/498 | 33.6 (21.2 to 48.9) | 10.27 (6.81 to 15.50) | ||
| Traditional medicine | 18/723 | 7.4 (4.8 to 11.3) | 4/575 | 6.3 (3.8 to 10.3) | 0.28 (0.10 to 0.82) | ||
| Aspirin | 11/683 | 6.7 (4.3 to 10.4) | 83/445 | 14.9 (7.9 to 26.4) | 11.58 (6.25 to 21.47) | ||
| Clinical outcomes | |||||||
| ICU | – | – | 793/796 | 95.0 (92.1 to 96.8) | – | ||
| Recovered | 1700/1925 | 85.4 (76.5 to 91.2) | 532/796 | 77.6 (67.5 to 85.3) | 0.76 (0.72 to 0.80) | ||
| Deaths | 0/1925 | 44/796 | 8.0 (5.2 to 12.1) | ||||
|

|
||||||
ICU, intensive care unit; IVIG, intravenous immunoglobulin.
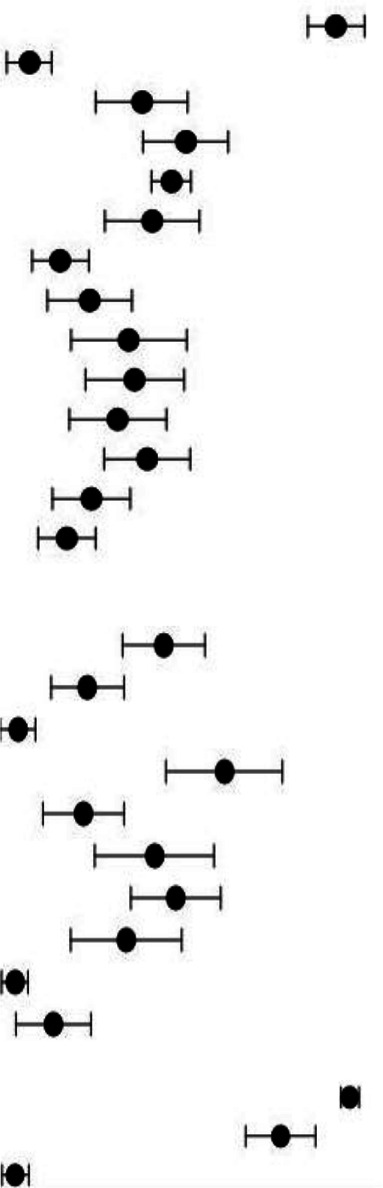
Comparison of outcomes according to World Bank Country Classification
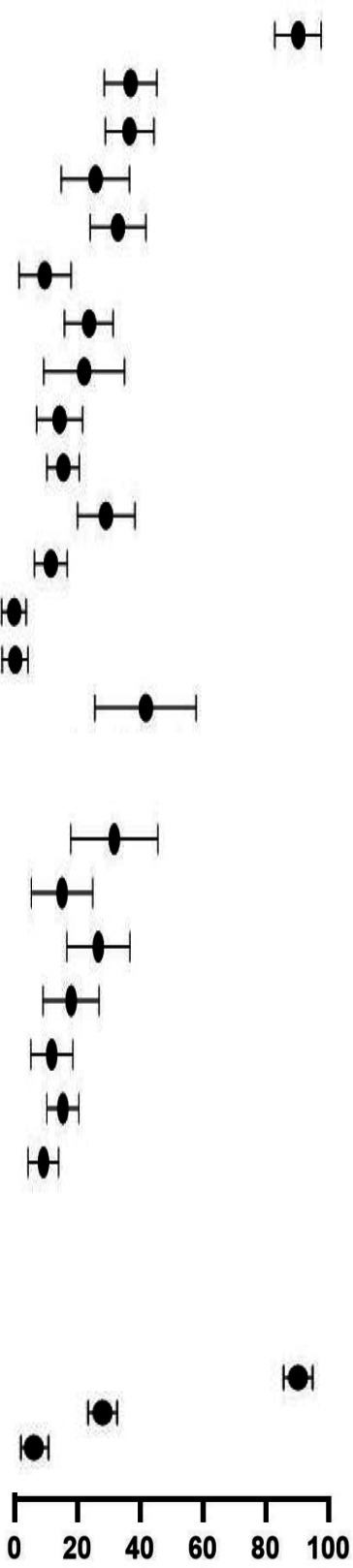
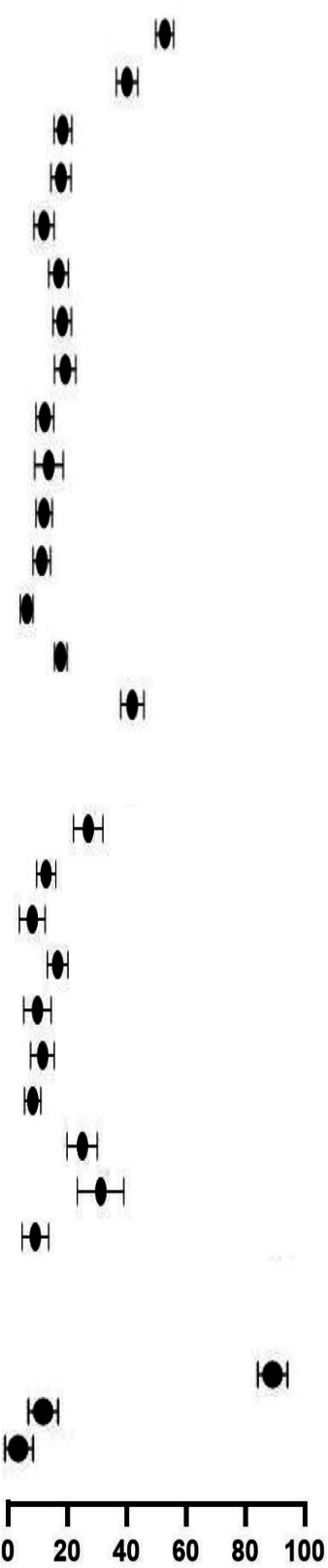
Of the included studies that reported clinical characteristics and outcomes, 60 studies were from HICs (n=6528) and 69 studies from LMICs (n=3723) as show in table 5. Studies in LMICs included a higher proportion of hospitalised children (1981/3723, 53.2%) compared with HIC studies (2897/6528, 44.4%). Abdominal symptoms and symptoms consistent with MIS-C were more frequently reported in HICs. A lower proportion of children in LMICs were admitted to the ICU (RR 0.56, 95% CI 0.50 to 0.63, p<0.05), mechanically ventilated (RR 0.32, 95% CI 0.26 to 0.39, p<0.05) and treated with different therapies; inotropes, antimicrobials, steroids, aspirin and IVIG. Only children in LMICs received inhaled IFN‐α. Among the hospitalised cases, 40 deaths were reported in HICs compared with 56 in LMICs (pooled proportion 2.9% vs 5.2%). Risk-adjusted mortality according to severity of illness could not be calculated due to lack of data (table 5).
Table 5.
Comparison of clinical symptoms, management and outcomes among reported paediatric COVID-19 cases in HICs (n=5641 cases, 60 studies) and LMICs (n=3694, 69 studies)
| Characteristics | HICs | LMICs | RR LMICs vs HICs, (95% CI) |
||||
| Events/total patients | Mean proportion % (95% CI) | Events/total patients | Mean proportion % (95% CI) | ||||
| Clinical symptoms | |||||||
| Fever | 2276/3332 | 72.0 (66.3 to 77.0) |
|
1300/2964 | 50.0 (47.4 to 52.6) |
|
0.64 (0.61 to 0.67) |
| Cough | 995/2730 | 33.2 (27.5 to 39.5) | 812/2531 | 39.2 (36.2 to 42.3) | 0.88 (0.82 to 0.95) | ||
| Comorbidity | 1069/3357 | 33.7 (27.4 to 38.5) | 521/2729 | 20.8 (18.3 to 23.4) | 0.60 (0.55 to 0.66) | ||
| Rashes | 491/2109 | 24.9 (17.9 to 33.5) | 253/2278 | 20.2 (17.5 to 23.2) | 0.48 (0.41 to 0.55) | ||
| Nausea/vomiting | 668/2374 | 30.3 (24.3 to 37.0) | 212/1869 | 15.3 (12.6 to 18.3) | 0.40 (0.35 to 0.46) | ||
| Conjunctivitis | 309/2732 | 13.1 (8.4 to 20.1) | 220/2266 | 19.5 (16.9 to 22.5) | 0.86 (0.73 to 1.01) | ||
| Dyspnoea | 543/2454 | 23.6 (18.5 to 29.6) | 336/2878 | 20.6 (18.1 to 23.4) | 0.53 (0.47 to 0.60) | ||
| Kawasaki shock/sign | 583/2087 | 21.9 (14.1 to 32.5) | 238/2278 | 21.4 (18.5 to 24.6) | 0.37 (0.33 to 0.43) | ||
| Fatigue | 394/1943 | 16.8 (12.2 to 22.6) | 315/2531 | 15.6 (13.3 to 18.3) | 0.61 (0.54 to 0.70) | ||
| Abdominal pain | 457/2266 | 22.7 (16.4 to 30.5) | 169/1869 | 16.6 (12.9 to 21.1) | 0.45 (0.38 to 0.53) | ||
| Nasal symptoms | 425/2549 | 17.8 (14.4 to 21.8) | 269/2519 | 15.4 (13.2 to 17.8) | 0.64 (0.56 to 0.74) | ||
| Diarrhoea | 527/2365 | 27.5 (21.5 to 34.6) | 125/1105 | 14.7 (12.4 to 17.3) | 0.51 (0.42 to 0.61) | ||
| Neurological symptoms | 493/3197 | 15.0 (11.6 to 19.0) | 200/2278 | 10.4 (8.8 to 12.3) | 0.57 (0.49 to 0.67) | ||
| Asymptomatic | 263/2428 | 6.4 (4.2 to 9.7) | 851/3656 | 20.2 (18.4 to 22.1) | 2.15 (1.89 to 2.44) | ||
| Pharyngeal erythema | 73/1494 | 6.7 (4.3 to 10.1) | 519/2531 | 40.7 (37.4 to 44.0) | 4.20 (3.31 to 5.32) | ||
| Clinical management | |||||||
| Antibiotics | 908/1875 | 36.4 (25.6 to 48.7) | 437/1735 | 27.0 (22.9 to 31.5) | 0.52 (0.47 to 0.57) | ||
| IVIG | 504/1867 | 31.6 (20.3 to 45.5) | 194/1655 | 14.7 (12.1 to 17.7) | 0.43 (0.37 to 0.51) | ||
| Aspirin | 187/985 | 16.0 (9.1 to 26.8) | 51/1603 | 10.4 (7.2 to 14.7) | 0.17 (0.12 to 0.23) | ||
| Systemic steroids | 566/2523 | 27.2 (19.0 to 37.3) | 235/1706 | 18.0 (15.2 to 21.2) | 0.61 (0.53 to 0.71) | ||
| Inotropes | 309/2309 | 19.1 (12.3 to 28.5) | 45/1547 | 11.9 (8.5 to 16.5) | 0.22 (0.16 to 0.30) | ||
| Antimalarial | 241/1696 | 13.6 (8.7 to 20.8) | 95/1603 | 13.5 (10.5 to 17.3) | 0.42 (0.33 to 0.52) | ||
| Mechanical ventilation | 387/2930 | 17.2 (13.1 to 22.3) | 103/2476 | 10.8 (8.6 to 13.4) | 0.32 (0.26 to 0.39) | ||
| Antiviral | 230/2372 | 11.4 (7.7 to 16.6) | 297/1647 | 25.2 (21.1 to 29.9) | 1.86 (1.58 to 2.18) | ||
| Interferon | 0/995 | – | 138/1603 | 30.5 (24.1 to 37.7) | – | ||
| Traditional medicine | 0/2523 | – | 22/1706 | 11.3 (8.0 to 15.7) | – | ||
| Clinical outcomes | |||||||
| Recovered | 5269/5641 | 91.0 (87.7 to 93.4) | 3435/3694 | 83.9 (81.2 to 86.2) | 0.99 (0.98 to 1.01) | ||
| ICU admission | 993/5641 | 26.0 (24.0 to 28.0) | 366/3694 | 9.9 (8.5 to 11.6) | 0.56 (0.50 to 0.63) | ||
| Deaths | 40/4710 | 2.9 (2.1 to 4.1) | 56/2192 | 5.2 (4.1 to 6.7) | 2.14 (1.43 to 3.20) | ||
HICs, high-income countries; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LMICs, low-income and middle-income countries; RR, relative risk.
Subgroup analyses of children presenting with MIS-C, and COVID-19 in neonates
Thirty-one studies (n=1208) with 22 from HIC (n=602), reported series of children presenting with MIS-C. Fever, abdominal pain and diarrhoea were the most common symptoms. Nearly half of children (638/1208) who met criteria for MIS-C were admitted to ICU (449/638, 70.3% of which were from HIC) compared with 22.9% in the overall analysis (online supplemental tables 6 and 7).
Disaggregated data were available on 184 neonates with fever; inability to feed/lethargy and dyspnoea were the most commonly reported symptoms. Twenty-one neonates (16.6%, 95% CI 11.2% to 23.9%) were asymptomatic at the time of diagnosis.
Quality assessment of included studies
One hundred and twenty-one studies were determined to be of good quality while eight were of fair quality (online supplemental table 8). Studies were primarily downgraded for incomplete case definition,29 31 44 46 48–50 130 135 138 incomplete case follow-up,10 23 24 26 29 32 35 44 51 53 77 82 85 90 94 99 106 112–114 120 130 135–137 missing data2 3 19–21 29 35 42–44 52 57 74 89 92–94 117 128 and non-consecutive patient enrolment,9 11 12 16 18–26 28 30–35 37–39 50 57 61–73 80 82 87 89–91 93 95 102 109 110 112 114 123 125 126 129 134–136 which raises concern that the included sample could be biased towards more severe presentations.
Discussion
Global knowledge of COVID-19 epidemiology, clinical characteristics and management has continued to evolve since the onset of the pandemic. Children have been noted to have relatively lower rates of severe illness and low mortality; however, they have been impacted by MIS-C.4 139
The findings of our review, the largest in terms of published systematic reviews on paediatric COVID-19, are consistent with previous reviews that identified predominance of infection in school-age children, slight male predisposition, prevalence of comorbidities among children with COVID-19 and low hospitalisation and mortality rates.2 4 140 The clinical presentation in children is heterogeneous, including a wide spectrum of clinical features. Fever and cough were the most commonly reported presenting symptoms, in line with the previously published systematic reviews.4 141A U-shaped curve of severity has been demonstrated in children diagnosed with COVID-19 with infants under 1 year of age and adolescents 10–14 years of age at higher risk of developing severe COVID-19.3 47 75 Due to lack of age-disaggregated data, we could not reliably compare the frequency of severe cases by age group in this review. Reported risk factors for severe disease among children include age, viral load142 and presence of comorbidities.3 There is a possibility that children with comorbidities may have been hospitalised related to their underlying chronic condition and incidentally determined to have COVID-19 infection or investigated more extensively. Some of the common comorbidities reported in children with COVID-19 infection include asthma, immunosuppression, congenital heart disease, kidney disease and obesity.3 4 47
Regional differences were identified in the comparison of clinical features, treatment and outcomes between HICs and LMICs. Pooled estimates of hospital mortality were higher in LMICs compared with HICs. Given that it was not possible to calculate risk-adjusted mortality rates for COVID-19, it is unclear whether observed differences in mortality are related to selection bias (eg, differences in severity of illness of included patients or differences in case definitions and inclusion criteria) or differences in available hospital resources. Nevertheless, there is ongoing concern that, in LMICs with high burden of illness and health system limitations, children with severe disease and MIS-C may be at greater risk for adverse outcomes and death than perceived to date. The differences in frequency of observed clinical features may be related to increasing recognition of MIS-C over the course of the pandemic and their inclusion in more recent COVID-19 case series, but is likely similar between HICs and LMICs.
Comparisons of clinical features and outcomes according to severity of illness were limited by heterogeneous reporting across the included case series. A higher proportion of children with severe disease demonstrated symptoms consistent with MIS-C (fever, abdominal symptoms, rash, neurological symptoms, conjunctivitis) and received IVIG, steroids and inotropes.
Compared with previous reviews, several at an earlier stage of the pandemic,4 7 8 140 143 this review has several strengths. Using a broad search strategy implemented in English, Chinese and Spanish databases, we summarise evidence from 129 studies from 31 different countries, the largest sample to-date. We excluded case reports to minimise selective reporting of extreme and atypical cases. We also attempted to reduce possible overlap in cases to prevent duplications. We identified differences in features from studies in HICs compared with LMICs, and between severe and non-severe cases, although with limited available data. Finally, we report subgroup analyses for neonates, and children presenting with MIS-C.
The review is limited primarily by the small sample sizes of individual studies, limitations in study reporting, and study quality limitations due to non-consecutive patient enrolment, unclear case definition and incomplete follow-up to hospital discharge. Our approach of pooling proportions is subject to bias and wide confidence-intervals due to small study sample size. We could not undertake multivariate analysis to identify risk factors for severe infection or adverse outcome in children due to lack of individual-patient-data. The inclusion of asymptomatic cases could have contributed to underestimation of the prevalence of clinical characteristics and optimism in the reporting of outcomes. Finally, it should be noted that a large number (36/129, 28.0%) of the included studies were from China. While the Chinese healthcare system is well-resourced in certain regions, many of the Chinese studies included were conducted in the city of Wuhan or in Hubei Province (n=9, 32.1%), where the gross domestic product per capita is less than half of that of Beijing and Shanghai.144 Therefore, the findings of studies from China may be generalisable to the socioeconomic and health development status of other middle-income countries.
This review contributes to the global understanding of paediatric COVID-19 disease and supports priority setting in research for current pandemic and future outbreaks. This body of literature would be improved by complete reporting of larger series with consecutive recruitment of patients, specific case definitions and complete long-term follow-up to determine global epidemiological trends, age‐specific burden of disease and illness trajectory following COVID-19 infection. Improved characterisation of disease severity and increased reports from low-income countries are needed to better understand differences in clinical manifestations, resource utilisation and outcome by region, which can be integrated in future updated analyses. The concern for selection bias remains as it is possible that in LMICs, the population of hospitalised children was sicker and at higher baseline risk of death, independent of resources. Individual-patient-data meta-analysis would be of benefit to characterise risk factors for severe disease, clinical features in different age groups and account for observed differences in outcome. With respect to clinical management, none of the therapies instituted in the treatment of children with severe COVID-19 disease have been demonstrated to improve outcome in randomised trials; therefore, a recommendation regarding their use is challenging. Given that children appear less likely to develop severe respiratory disease, but are at risk of multiorgan dysfunction due to MIS-C, further studies are needed to characterise the clinical trajectory of this novel syndrome and determine the optimal treatment for it. Finally, there remains paucity of studies reporting long-term prognosis of COVID-19 in children.145
Conclusion
Our review suggests that children predominantly contracted mild form of infection but could be at risk of more severe outcomes. It is crucial to take into consideration risk factors including contact-exposure, underlying comorbidities, young age and male sex which may increase the risk of severe disease. While we have identified several elements that highlight the disease spectrum and higher risk of adverse outcomes in certain settings, such as LMICs, there is the need for much closer scrutiny of this illness globally with individual patient data analysis.
Footnotes
Contributors: ZB conceptualised the study and secured funding. ZSL and OI drafted the study protocol, conducted the literature search, study screening, selection and data extraction and drafted the manuscript. LJ and KT designed the data collection instruments, collected data, carried out data analyses and reviewed and revised the manuscript. FM drafted the initial manuscript and reviewed and revised the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript as submitted. ZB is the guarantor.
Funding: This review was funded in part by a grant from UNICEF (Headquarters) in partnership with the International Pediatric Association and with core support from the Centre for Global Child Health (Toronto) and the Center of Excellence in Women & Child Health, The Aga Khan University, Karachi, Pakistan
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon request
References
- 1. WHO . Who coronavirus disease (COVID-19) Dashboard data last updated: 2021/1/21. Available: https://covid19.who.int/
- 2. Dong Y, Mo X, Hu Y. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. J Emerg Med 2020;58:712–3. 10.1016/j.jemermed.2020.04.006 [DOI] [Google Scholar]
- 3. Covid CD CC, COVID C, Bialek S, et al. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine 2020;24:100433. 10.1016/j.eclinm.2020.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AAP . Children and COVID19: state data report 28/8/2020, 2020. [Google Scholar]
- 6. Wang E, Brar K. COVID-19 in children: an epidemiology study from China. J Allergy Clin Immunol 2020;8:2118–20. 10.1016/j.jaip.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Souza TH, Nadal JA, Nogueira RJN, et al. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol 2020;55:1892–9. 10.1002/ppul.24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents. JAMA Pediatr 2020;174:882. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 9. Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020;324:294. 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med Overseas Ed 2020;383:334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology 2020;297:E283–8. 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020;79:999–1006. 10.1136/annrheumdis-2020-217960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Global Financing Facility for Women, Children and Adolescents (GFF)- September 18, 2020. Available: https://www.globalfinancingfacility.org/new-findings-confirm-global-disruptions-essential-health-services-women-and-children-covid-19
- 14. National Heart L, and Blood Institute . Quality assessment tool for observational cohort and cross-sectional studies. Bethesda: National Institutes of health, department of health and human services, 2014. [Google Scholar]
- 15. Group WB . World bank country and lending groups (country classification), 2020. [Google Scholar]
- 16. Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689–96. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang D, Ju XL, Xie F, et al. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China]. Zhonghua Er Ke Za Zhi 2020;58:E011–E. 10.3760/cma.j.cn112140-20200225-00138 [DOI] [PubMed] [Google Scholar]
- 18. Zheng F, Liao C, Fan Q-H, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci 2020;40:275–80. 10.1007/s11596-020-2172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng K, Yun YX, Wang XF, et al. [Analysis of CT features of 15 Children with 2019 novel coronavirus infection]. Zhonghua Er Ke Za Zhi 2020;58:E007. 10.3760/cma.j.issn.0578-1310.2020.0007 [DOI] [PubMed] [Google Scholar]
- 21. Jiehao C, Jin X, Daojiong L, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 2020;71:1547–51. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei M, Yuan J, Liu Y, et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 2020;323:1313–4. 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect 2020;9:707–13. 10.1080/22221751.2020.1744483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou Y, Yang G-D, Feng K, et al. [Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children]. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun D, Li H, Lu X-X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr 2020;16:251–9. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W, Zhang Q, Chen J, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med 2020;382:1370–1. 10.1056/NEJMc2003717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020;63:706–11. 10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu M, Song Z, Xiao K. High-Resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr 2020;44:311–3. 10.1097/RCT.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect 2020;80:e7-e13. 10.1016/j.jinf.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lou XX, Shi CX, Zhou CC, et al. Three children who recovered from novel coronavirus 2019 pneumonia. J Paediatr Child Health 2020;56:650-651. 10.1111/jpc.14871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang T, Cui X, Zhao X, et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol 2020;92:909–14. 10.1002/jmv.25795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Guo F, Cao Y, et al. Insight into COVID-2019 for pediatricians. Pediatr Pulmonol 2020;55:E1–4. 10.1002/ppul.24734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ji L-N, Chao S, Wang Y-J, et al. Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J Pediatr 2020;16:267–70. 10.1007/s12519-020-00356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr 2020. 10.1001/jamapediatrics.2020.1346. [Epub ahead of print: 08 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen A, Huang J-xiang, Liao Y, et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiology 2020;2:e200117. 10.1148/ryct.2020200117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. See KC, Liew SM, Ng DCE, et al. COVID-19: four paediatric cases in Malaysia. Int J Infect Dis 2020;94:125–7. 10.1016/j.ijid.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang G-X, Zhang A-M, Huang L, et al. [Twin girls infected with SARS-CoV-2]. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng . A case report of two children with corona virus disease 2019 complicated with diffuse intravascular coagulation, 2020. [Google Scholar]
- 39. Tang A, Xu W, Chen P. A retrospective study of the clinical characteristics of COVID-19 infection in 26 children. medRxiv 2020. [Google Scholar]
- 40. Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol 2020;55:1430–2. 10.1002/ppul.24767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan X, Huang J, Zhao F, et al. [Clinical features of children with SARS-CoV-2 infection: an analysis of 13 cases from Changsha, China]. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma H, Shao J, Wang Y. High resolution CT features of COVID-19 in children. Chinese Journal of Radiology 2020;54. [Google Scholar]
- 43. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol 2020;55:1424–9. 10.1002/ppul.24762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwierzeck V, König JC, Kühn J, et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis 2020. 10.1093/cid/ciaa491. [Epub ahead of print: 27 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang J, Duan L, Xiong D. Epidemiological and clinical characteristics of novel coronavirus infection in children: thoughts on the diagnostic criteria of suspected cases outside Hubei Province. Chinese Pediatric Emergency Medicine 2020. [Google Scholar]
- 46. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet 2020;395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020;174:868. 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet 2020;395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rahimzadeh G, Ekrami Noghabi M, Kadkhodaei Elyaderani F, et al. COVID-19 infection in Iranian children: a case series of 9 patients. J Pediatr Rev 2020;8:139–44. 10.32598/jpr.8.2.139 [DOI] [Google Scholar]
- 50. Tullie L, Ford K, Bisharat M, et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health 2020;4:e19–20. 10.1016/S2352-4642(20)30165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sajid MI, Altaf S, Mushtaq N. A wrinkle in time: how has management of pediatric cancers changed during COVID-19 in Pakistan? 2020. [Google Scholar]
- 52. Zhang Z-J, Yu X-J, Fu T, et al. Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J 2020;55:2000697. 10.1183/13993003.00697-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr 2020;174:722. 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salvatori G, De Rose DU, Concato C, et al. Managing COVID-19-Positive maternal-infant dyads: an Italian experience. Breastfeed Med 2020;15:347–8. 10.1089/bfm.2020.0095 [DOI] [PubMed] [Google Scholar]
- 55. Zhang XMY, Xiao J, Zhang Z. Clinical characteristics of novel coronavirus pneumonia in children in Jinan. 10.21203/rs.3.rs-17119/v1 [DOI]
- 56. Wu H-P, Li B-F, Chen X, et al. [Clinical features of coronavirus disease 2019 in children aged <18 years in Jiangxi, China: an analysis of 23 cases]. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang H-Y, Zhang Y. [Coronavirus disease 2019 and hypertension in 2 children]. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr 2020;223:14–19. 10.1016/j.jpeds.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eghbali ASS, Sadat N, et al. COVID-19 in pediatric patients: a case series. J Cell Mol Med 2020;5:3–5. [Google Scholar]
- 60. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc 2020;9:393–8. 10.1093/jpids/piaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Flores V, Miranda R, Merino L, et al. SARS-CoV-2 infection in children with febrile neutropenia. Ann Hematol 2020;99:1–2. 10.1007/s00277-020-04115-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. García-Salido A, Leoz-Gordillo I, Martínez de Azagra-Garde A, et al. Children in critical care due to severe acute respiratory syndrome coronavirus 2 infection: experience in a Spanish hospital. Pediatr Crit Care Med 2020;21:e576-e580. 10.1097/PCC.0000000000002475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cabrero-Hernández M, García-Salido A, Leoz-Gordillo I, et al. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr Infect Dis J 2020;39:e195–8. 10.1097/INF.0000000000002777 [DOI] [PubMed] [Google Scholar]
- 64. Waltuch T, Gill P, Zinns LE, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med 2020;38:2246.e3–2246.e6. 10.1016/j.ajem.2020.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kakuya F, Okubo H, Fujiyasu H, et al. The first pediatric patients with coronavirus disease 2019 (COVID-19) in Japan: risk of co-infection with other respiratory viruses. Jpn J Infect Dis 2020;73:377–80. 10.7883/yoken.JJID.2020.181 [DOI] [PubMed] [Google Scholar]
- 66. Soltani J, Sedighi I, Shalchi Z, et al. Pediatric coronavirus disease 2019 (COVID-19): an insight from West of Iran. North Clin Istanb 2020;7:284. 10.14744/nci.2020.90277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094. 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Foster CE, Moulton EA, Munoz FM, et al. Coronavirus disease 2019 in children Cared for at Texas children's Hospital: initial clinical characteristics and outcomes. J Pediatric Infect Dis Soc 2020;9:373–7. 10.1093/jpids/piaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. White A, Mukherjee P, Stremming J, et al. Neonates hospitalized with community-acquired SARS-CoV-2 in a Colorado neonatal intensive care unit. Neonatology 2021;117:641–5. 10.1159/000508962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parri N, Lenge M, Cantoni B, et al. COVID-19 in 17 Italian pediatric emergency departments. Pediatrics 2020;146. 10.1542/peds.2020-1235. [Epub ahead of print: 23 Sep 2020]. [DOI] [PubMed] [Google Scholar]
- 71. Gao Y, Zhang D, Sui S, et al. Clinical features and treatment protocol in eleven Chinese children with mild COVID-19. Indian J Pediatr 2020;87:748. 10.1007/s12098-020-03352-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harman K, Verma A, Cook J, et al. Ethnicity and COVID-19 in children with comorbidities. Lancet Child Adolesc Health 2020;4:e24–5. 10.1016/S2352-4642(20)30167-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lu X, Zhang L, Du H. SARS-CoV-2 infection in children. N Engl J Med 2000;382. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020;4:653–61. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parri N, Lenge M, Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med Overseas Ed 2020;383:187–90. 10.1056/NEJMc2007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abdel-Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol 2020. 10.1001/jamaneurol.2020.2687. [Epub ahead of print: 01 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Banerjee S, Guha A, Das A, et al. A preliminary report of COVID-19 in children in India. Indian Pediatr 2020;57:963–4. 10.1007/s13312-020-2004-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhumbra S, Malin S, Kirkpatrick L, et al. Clinical features of critical coronavirus disease 2019 in children. Pediatr Crit Care Med 2020;21:e948-e953. 10.1097/PCC.0000000000002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bisogno G, Provenzi M, Zama D. Clinical characteristics and outcome of SARS-CoV-2 infection in Italian pediatric oncology patients: a study from the infectious diseases Working group of the AIEOP. J Pediatric Infect Dis Soc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Blumfield E, Levin TL. COVID-19 in pediatric patients: a case series from the Bronx, NY. Pediatr Radiol 2020;50:1369–74. 10.1007/s00247-020-04782-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Derespina KR, Kaushik S, Plichta A, et al. Clinical manifestations and outcomes of critically ill children and adolescents with coronavirus disease 2019 in New York City. J Pediatr 2020;226 10.1016/j.jpeds.2020.07.039. [Epub ahead of print: 16 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. George S, Ansari MS, Kalliath A. COVID‐19 in children in Brunei Darussalam: higher incidence but mild manifestations. J Med Virol 2020. 10.1002/jmv.26310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. González Cortés R, García-Salido A, Roca Pascual D, et al. A multicenter national survey of children with SARS-CoV-2 infection admitted to Spanish pediatric intensive care units. Intensive Care Med 2020;46:1774–6. 10.1007/s00134-020-06146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kainth MK, Goenka PK, Williamson KA, et al. Early experience of COVID-19 in a US children's Hospital. Pediatrics 2020;146:e2020003186. 10.1542/peds.2020-003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr 2020;224:24–9. 10.1016/j.jpeds.2020.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kest H, Kaushik A, DeBruin W. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr 2020;2020:1–4. 10.1155/2020/8875987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Korkmaz MF, Türe E, Dorum BA, et al. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in turkey: an observational cohort study. J Korean Med Sci 2020;35:e236. 10.3346/jkms.2020.35.e236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Meslin P, Guiomard C, Chouakria M, et al. Coronavirus disease 2019 in newborns and very young infants: a series of six patients in France. Pediatr Infect Dis J 2020;39:e145–7. 10.1097/INF.0000000000002743 [DOI] [PubMed] [Google Scholar]
- 90. Moraleda C, Serna-Pascual M, Soriano-Arandes A, et al. Multi-Inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin Infect Dis 2020. 10.1093/cid/ciaa1042. [Epub ahead of print: 25 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. KF N, Kothari T, Bandi S. COVID‐19 multisystem inflammatory syndrome in three teenagers with confirmed SARS‐CoV‐2 infection. J Med Virol 2020;92. 10.1002/jmv.26206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Paquette D, Bell C, Roy M, et al. Laboratory-confirmed COVID-19 in children and youth in Canada, January 15-April 27, 2020. Can Commun Dis Rep 2020;46:121–4. 10.14745/ccdr.v46i05a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perez A, Kogan-Liberman D, Sheflin-Findling S. Presentation of severe acute respiratory syndrome-coronavirus 2 infection as cholestatic jaundice in two healthy adolescents. J Pediatr 2020. 10.1016/j.jpeds.2020.07.054. [Epub ahead of print: 23 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sarangi B, Reddy VS, Oswal JS, et al. Epidemiological and clinical characteristics of COVID-19 in Indian children in the initial phase of the pandemic. Indian Pediatr 2020;57:914–7. 10.1007/s13312-020-1994-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sadiq M, Aziz OA, Kazmi U, et al. Multisystem inflammatory syndrome associated with COVID-19 in children in Pakistan. Lancet Child Adolesc Health 2020;4:e36–7. 10.1016/S2352-4642(20)30256-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ 2020;370:m3249. 10.1136/bmj.m3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Antúnez-Montes OY, Escamilla MI, Figueroa-Uribe AF, et al. COVID-19 and multisystem inflammatory syndrome in Latin American children. Pediatr Infect Dis J 2020;Publish Ahead of Print. 10.1097/INF.0000000000002949 [DOI] [PubMed] [Google Scholar]
- 98. Capone CA, Subramony A, Sweberg T, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr 2020;224:141–5. 10.1016/j.jpeds.2020.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cura Yayla BC, Özsürekçi Y, Aykaç K, et al. Characteristics and management of children with COVID-19 in turkey. Balkan Med J 2020;37:341–7. 10.4274/balkanmedj.galenos.2020.2020.7.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health 2020;4:669–77. 10.1016/S2352-4642(20)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de Farias ECF, Pedro Piva J, de Mello MLFMF, et al. Multisystem inflammatory syndrome associated with coronavirus disease in children: a Multi-centered study in Belém, Pará, Brazil. Pediatr Infect Dis J 2020;39:e374-e376. 10.1097/INF.0000000000002865 [DOI] [PubMed] [Google Scholar]
- 102. Dima M, Enatescu I, Craina M, et al. First neonates with severe acute respiratory syndrome coronavirus 2 infection in Romania: three case reports. Medicine 2020;99:e21284. 10.1097/MD.0000000000021284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA 2020. 10.1001/jama.2020.12458 [DOI] [PubMed] [Google Scholar]
- 104. Ibrahim LF, Tosif S, McNab S, et al. SARS-CoV-2 testing and outcomes in the first 30 days after the first case of COVID-19 at an Australian children's hospital. Emerg Med Australas 2020;32:801–8. 10.1111/1742-6723.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kilani MM, Odeh MM, Shalabi M, et al. Clinical and laboratory characteristics of SARS-CoV2-infected paediatric patients in Jordan: serial RT-PCR testing until discharge. Paediatr Int Child Health 2020:1–10. 10.1080/20469047.2020.1804733 [DOI] [PubMed] [Google Scholar]
- 106. Okarska-Napierała M, Ludwikowska KM, Szenborn L, et al. Pediatric inflammatory multisystem syndrome (PIMS) did occur in Poland during months with low COVID-19 prevalence, preliminary results of a nationwide register. J Clin Med 2020;9. 10.3390/jcm9113386. [Epub ahead of print: 22 Oct 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mahmoudi S, Mehdizadeh M, Shervin Badv R, et al. The coronavirus disease 2019 (COVID-19) in children: a study in an Iranian children's referral hospital. Infect Drug Resist 2020;13:2649–55. 10.2147/IDR.S259064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mamishi S, Movahedi Z, Mohammadi M, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect 2020;148:e196. 10.1017/S095026882000196X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mithal LB, Machut KZ, Muller WJ, et al. SARS-CoV-2 Infection in Infants Less than 90 Days Old. J Pediatr 2020;224:150–2. 10.1016/j.jpeds.2020.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Newman AM, Jhaveri R, Patel AB, et al. Trisomy 21 and coronavirus disease 2019 in pediatric patients. J Pediatr 2021;228:294–6. 10.1016/j.jpeds.2020.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Torres JP, Izquierdo G, Acuña M, et al. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis 2020;100:75–81. 10.1016/j.ijid.2020.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saha S, Ahmed ANU, Sarkar PK, et al. The direct and indirect impact of SARS-CoV-2 infections on neonates: a series of 26 cases in Bangladesh. Pediatr Infect Dis J 2020;39:e398-e405. 10.1097/INF.0000000000002921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health 2021;5:113–21. 10.1016/S2352-4642(20)30342-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schwartz DA, Mohagheghi P, Beigi B. Spectrum of neonatal COVID-19 in Iran: 19 infants with SARS-CoV-2 perinatal infections with varying test results, clinical findings and outcomes. The Journal of Maternal-Fetal & Neonatal Medicine 2020;2:1–10. 10.1080/14767058.2020.1797672 [DOI] [PubMed] [Google Scholar]
- 115. Schwartz SP, Thompson P, Smith M, et al. Convalescent plasma therapy in four critically ill pediatric patients with coronavirus disease 2019: a case series. Crit Care Explor 2020;2:e0237. 10.1097/CCE.0000000000000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Almoosa ZA, Al Ameer HH, AlKadhem SM, et al. Multisystem Inflammatory Syndrome in Children, the Real Disease of COVID-19 in Pediatrics - A Multicenter Case Series From Al-Ahsa, Saudi Arabia. Cureus 2020;12:e11064. 10.7759/cureus.11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alsharrah DY, Al-Haddad F, Aljamaan S, et al. 441. clinical characteristics of pediatric SARS-CoV-2 infection and coronavirus disease 2019 (COVID-19) in Kuwait. Open Forum Infect Dis 2020;7:S288. 10.1093/ofid/ofaa439.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Prata-Barbosa A, Lima-Setta F, Santos GRD, et al. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr 2020;96:582–92. 10.1016/j.jped.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bayesheva D, Boranbayeva R, Turdalina B, et al. COVID-19 in the paediatric population of Kazakhstan. Paediatr Int Child Health 2020:1–7. 10.1080/20469047.2020.1857101 [DOI] [PubMed] [Google Scholar]
- 120. Bhavsar SM, Clouser KN, Gadhavi J, et al. COVID-19 in pediatrics: characteristics of hospitalized children in New Jersey. Hosp Pediatr 2021;11:79–87. 10.1542/hpeds.2020-001719 [DOI] [PubMed] [Google Scholar]
- 121. Bustos-Cordova E, Castillo-García D, Cerón-Rodríguez M, et al. Clinical spectrum of COVID-19 in a Mexican pediatric population. Indian Pediatr 2020. [Epub ahead of print: 19 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fernández Colomer B, Sánchez-Luna M, de Alba Romero C, et al. Neonatal infection due to SARS-CoV-2: an epidemiological study in Spain. Frontiers in Pediatrics 2020;8:670. 10.3389/fped.2020.580584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Storch-de-Gracia P, Leoz-Gordillo I, Andina D, et al. Clinical spectrum and risk factors for complicated disease course in children admitted with SARS-CoV-2 infection. Anales de Pediatría 2020;93:323–33. 10.1016/j.anpede.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dhanalakshmi K, Venkataraman A, Balasubramanian S, et al. Epidemiological and Clinical Profile of Pediatric Inflammatory Multisystem Syndrome - Temporally Associated with SARS-CoV-2 (PIMS-TS) in Indian Children. Indian Pediatr 2020;57:1010–4. 10.1007/s13312-020-2025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Falah NU, Hashmi S, Ahmed Z, et al. Kawasaki disease-like features in 10 pediatric COVID-19 cases: a retrospective study. Cureus 2020;12:e11035. 10.7759/cureus.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care 2020;24:1–13. 10.1186/s13054-020-03332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jain S, Sen S, Lakshmivenkateshiah S, et al. Multisystem inflammatory syndrome in children with COVID-19 in Mumbai, India. Indian Pediatr 2020;57:1015–9. 10.1007/s13312-020-2026-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kalamdani P, Kalathingal T, Manerkar S, et al. Clinical profile of SARS-CoV-2 infected neonates from a tertiary government hospital in Mumbai, India. Indian Pediatr 2020;57:1143–6. 10.1007/s13312-020-2070-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lima-Setta F, Magalhães-Barbosa MCde, Rodrigues-Santos G, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatr 2020. 10.1016/j.jped.2020.10.008. [Epub ahead of print: 09 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Montoya J, Ugaz C, Alarcon S, et al. COVID-19 in pediatric cancer patients in a resource-limited setting: national data from Peru. Pediatr Blood Cancer 2021;68:e28610. 10.1002/pbc.28610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Önal P, Kılınç AA, Aygün F. COVID‐19 in turkey: a tertiary center experience. Pediatr Int 2020. 10.1111/ped.14549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rabha AC, Oliveira Junior FIde, Oliveira TAde, et al. Clinical manifestations of children and adolescents with covid-19: report of the first 115 cases from sabará hospital infantil. Revista Paulista de Pediatria 2021;39. 10.1590/1984-0462/2021/39/2020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sethuraman U, Kannikeswaran N, Ang J, et al. Multisystem inflammatory syndrome in children associated with novel coronavirus SARS-CoV-2: presentations to a pediatric emergency department in Michigan. Am J Emerg Med 2021;39:164–7. 10.1016/j.ajem.2020.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shahbaznejad L, Navaeifar MR, Abbaskhanian A, et al. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr 2020;20:1–12. 10.1186/s12887-020-02415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Webb K, Abraham DR, Faleye A, et al. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health 2020;4:e38. 10.1016/S2352-4642(20)30272-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr 2020;174:e202430-e. 10.1001/jamapediatrics.2020.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van der Zalm MM, Lishman J, Verhagen LM. Clinical experience with SARS CoV-2 related illness in children-hospital experience in Cape town, South Africa. Clinical infectious diseases: an official publication of the infectious diseases Society of America, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zheng G, Wang B, Zhang H, et al. Clinical characteristics of acute respiratory syndrome with SARS-CoV-2 infection in children in South China. Pediatr Pulmonol 2020;55:2419–26. 10.1002/ppul.24921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020;20:e276–88. 10.1016/S1473-3099(20)30651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109:1088–95. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Terry C, Mühlemann B, Veith T, et al. An analysis of SARS-CoV-2 viral load by patient age 2020. 10.1101/2020.06.08.20125484 [DOI]
- 143. Chang T-H, Wu J-L, Chang L-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc 2020;119:982-989. 10.1016/j.jfma.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. NBS . National Bureau of statistics of China, 2019. [Google Scholar]
- 145. Ludvigsson JF. Case report and systematic review suggest that children may experience similar long‐term effects to adults after clinical COVID‐19. Acta Paediatr 2020;370. 10.1111/apa.15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2020-321385supp001.pdf (18.7MB, pdf)
Data Availability Statement
Data are available upon request


